Abstract
Aims
To evaluate midlife risk factors of developing type 2 diabetes mellitus (T2DM) in late life in a population-based study of older persons.
Methods
A cohort of 2251 persons, aged 65-96, participated in AGES-Reykjavik in 2002-2004; all attended the Reykjavik Study 26 years earlier, at the mean age of 50. Based on glucometabolic status in 2002-2004 the participants are divided into a normoglycemic control group (n=1695), an impaired fasting glucose (IFG) group (n=313) and T2DM group (n=243). Change in risk parameters from midlife is evaluated in these three groups.
Results
Since examined earlier 14.3% of men and 8.2% of women developed T2DM. A family history of diabetes was reported in 39.5% of T2DM compared to 19.3% in both IFG and normoglycemics. The T2DM and IFG groups currently have higher levels of fasting triglycerides, greater BMI and higher systolic blood pressure than normoglycemics and this difference was already apparent in midlife. In late life, two or more metabolic syndrome criteria are present in 60% of the T2DM groups compared to 25% in normoglycemic groups. T2DM with impaired cardiovascular health is more marked in women than men when compared with controls.
Conclusions
Family history and higher levels of BMI, TG and systolic blood pressure in midlife are associated with the development of T2DM in late life, suggesting risk can be evaluated long before onset. A continued rise in risk factors throughout life allows a scope for more aggressive measures in preventing or delaying development of T2DM and its effect on cardiovascular health.
Keywords: Cohort study, epidemiology, Type 2 diabetes, older persons, long term risk evaluation of T2DM
Introduction
Type 2 diabetes is currently recognized as one of the main threats to human health worldwide [1] and the prevalence of the disease keeps increasing in an epidemic fashion. It is well recognized that development of the T2DM is influenced by both genetic and environmental factors [2] and that onset can be prevented or delayed by lifestyle modification [3] [4]. Identifying people at risk of developing T2DM as early as possible is therefore of great importance in effective primary prevention strategies.
Several predictive models have been designed for detecting short term risk of developing T2DM, some of these models are highly sophisticated [5], whereas others are relatively simple and easy to use in general practice [6]. The metabolic syndrome has also been used to predict both T2DM [7] and CVD [8] as in the cross-sectional data from the NHANES III where the prevalence of metabolic syndrome (NCEP criteria) was very high among people, 50 years and older, with diabetes and those with diabetes and metabolic syndrome had the highest prevalence of CHD [9]. Most of the prediction models have taken into account risk factors such as family history, age, BMI, hypertension, high blood glucose and some also use high TG and low HDL cholesterol levels. Recent studies have in addition focused on the association between T2DM and low-grade systemic inflammation [10], where the pathophysiology seems to be largely attributable to insulin resistance with high blood levels of fatty acids [11].
Not many studies have, however, analysed long term risk of developing T2DM, before glucometabolic impairment is detected. In the present work we have assessed changes in risk parameters, some of which are also known risk factors for cardiovascular disease, associated with the development of diabetes over a mean period of 26 years. The following questions regarding the development of T2DM in the Age, Gene/Environment Susceptibility - Reykjavik Study will be addressed:
Can the risk of developing T2DM in late life be detected already in midlife?
Is cardiovascular disease more common in older persons with T2DM or IFG than in normoglycemics?
Patients and methods
Participants
The men and women participated in the AGES-Reykjavik Study between 2002-2004 [12]. The AGES-Reykjavik is a continuation of the population-based Reykjavik Study, which started in 1967 by the Icelandic Heart Association and has been described in detail elsewhere [13].
As part of the AGES-Reykjavik examination, a comprehensive questionnaire is administered. It includes questions regarding diabetes in close relatives as well as their own health history, including cardiovascular events and strokes. Participants were asked to bring all medications and supplements used in the previous 2 weeks to the clinic. All participants also had a fasting blood specimen drawn and an analyzed as documented below. They also brought urinary morning samples to the clinic.
We divided the study cohort into three subgroups for both genders according to their glycemic status, based on the WHO recommendations from 1999 [14]. The first group, used as reference, were normoglycemic (fasting glucose ≤ 6.0mmol/l). The second group had impaired fasting glucose values at the AGES-Reykjavik examination (6.1-6.9mmol/l). The third group, the T2DM, was either diagnosed upon their visit to the clinic with fasting serum glucose of ≥ 7mmol/l, or identified by self-reported diabetes in the questionnaire, use of diabetes medication, or being on a special diet for T2DM and under regular observation of health professionals. From the original study cohort of 2300 individuals, 21 were excluded as they either gave conflicting information about having diabetes, or the questionnaire related to diabetes was not completed. We also excluded 28 subjects who were already diagnosed with T2DM when first attending the Reykjavik Study. The study population therefore consists of 2251 individuals, 956 men and 1395 women, aged 65-96, with an overall response rate of 75% for men and 68% for women. The mean age of the AGES-Reykjavik cohort when first entering the Reykjavik Study was 50 years.
Risk factor ascertainment
In the AGES-Reykjavik Study, glucose, total cholesterol, HDL cholesterol, triglycerides, high sensitivity CRP, HbA1c as well as urinary albumin and creatinine were measured on a Hitachi 912, using reagents from Roche Diagnostics and following the manufacturer's instructions. Insulin was measured by an electrochemiluminescence immunoassay on a Roche Elecsys 2010 instrument, using two monoclonal antibodies and a sandwich principle. The method was standardized using the 1st IRP WHO Reference Standard 66/304 (NIBSC). The analytical methods used in the Reykjavik Study have been described elsewhere [13]. HOMAIR, a measure of the insulin resistance, is calculated using the formula [insulin(mU/l) × glucose(mmol/l)]/22.5 [15].
Blood pressure was measured with a mercury sphygmomanometer, with a large cuff, the mean value of two consecutive blood pressure measurements being used. A 12 lead resting ECG was evaluated according to the Minnesota code [16]. The coronary events are verified myocardial infarcts, coronary artery bypass graft and percutaneous transluminal coronary intervention, collected from National Health System Records by the Icelandic Heart Association.
Metabolic syndrome criteria, as defined by WHO, are used. As current glucometabolic status is used to divide the cohort into study groups we decided to omit insulin resistance and glycemic status, and include two or more of the following criteria: BMI>30kg/m2; triglycerides≥1.7mmol/l or HDL<0.9, in males, and <1.0mmol/l, in females; hypertension >140/90mmHg; U-albumin/creatinine ≥3.5mg/mmol [17].
Informed consent was obtained from all participants and the study was approved by the National Bioethics Committee in Iceland (VSN 00-063) as well as the Institutional Review Board of the Intramural Research Program of the National Institute on Aging and the Data Protection Authority in Iceland.
Statistical analysis
Response variables on a continuous scale were analysed with linear regression models by sex, adjusted for age and a categorical predictor for glycemic status. Binary variables were analysed similarly with logistic regression models. TG and CRP were log-transformed. Analyses of change in continuous variables were performed, using mixed effects regression models, adjusted for age and time between visits and a random effect for subjects. The change in BMI, blood pressure, lipids and inflammatory markers over a mean period of over 26 years was calculated. All parameters were adjusted to the age of 50 in midlife and a 26-year follow-up. The association of late life T2DM with midlife risk parameters was estimated in a multivariable logistic regression model. We analyzed the data using SAS/STAT® software, version 9.1.
Results
The study cohort is categorized into subgroups by gender and present glycemic status as described above. Mean age when entering the AGES-Reykjavik is similar in all subgroups (Table 1) with an age range of 65-96 in both genders. From the mean age of 50, 14.3% of men developed T2DM in over the 26 year study period, compared with 8.2% in women. The mean age of onset based on self report was 69 (SD 10) years in both genders. Out of 243 in the total T2DM group, 174 had been diagnosed with the disease between their examination in midlife and participation in the AGES-Reykjavik; those diagnosed by screening as part of the study (n=69) were about a year older than the mean age of the cohort. Despite this the undiagnosed T2DM group did not show any distinct characteristics different from those already diagnosed so all T2DM participants are treated as one group in this study. A positive family history of diabetes is reported by 39.5% of participants with T2DM, compared with 19.3% in the IFG and normoglycemic groups (Table 1).
Table 1. AGES – Reykjavik participants by glycemic status and family history of diabetes.
| Glycemic status | mean age | % (n) in groups | % family history of T2DM | % (n) in groups | |
|---|---|---|---|---|---|
| both genders | both genders | men (n) | women (n) | ||
| Normoglycemic | 76 ± 6 | 75.3 (1695) | 19.3 | 70.6 (675) | 78.8 (1022) |
| IFG | 76 ± 6 | 13.9 (313) | 19.3 | 15.1 (144) | 13.1 (169) |
| Total T2DM | 76 ± 5 | 10.8 (243) | 39.5 | 14.3 (137) | 8.2 (106) |
| (uT2DM) | 77 ± 5 | 3.1 (69) | 40.6 | 4.0 (38) | 2.4 (31) |
| (dT2DM) | 76 ± 5 | 7.7 (174) | 39.1 | 10.4 (99) | 5.8 (75) |
|
| |||||
| Total number | 2251 | 956 | 1295 | ||
The study cohort divided by glycemic status at the AGES-Reykjavik into subgroups, as well as by gender.
Total T2DM is also shown divided into diagnosed (dT2DM) and undiagnosed (uT2DM) groups.
The age is given in years ± SD and the % reporting family history of T2DM for both genders.
Change in risk parameters from midlife to late life
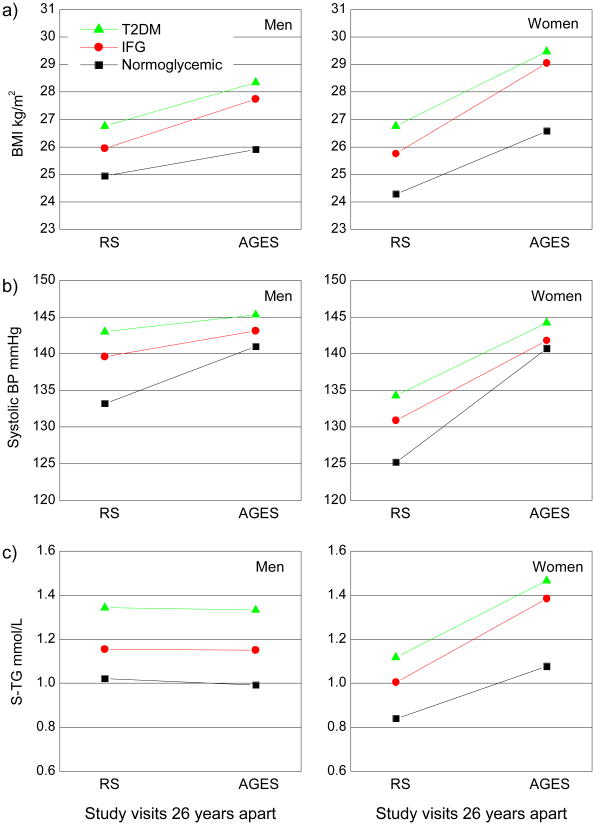
Based on glycemic groups present at the AGES-Reykjavik baseline, we examined retrospectively the 26 year change in BMI, blood pressure and fasting serum lipids measured in midlife at a mean age of 50 in the Reykjavik Study to the AGES-Reykjavik examination, as illustrated in Figure 1. In midlife the mean BMI in both men and women that develop T2DM is significantly higher than in normoglycemics. A significant increase in BMI with age is observed in all study groups (p<.0001), with a greater weight gain seen in the IFG and T2DM study groups than in the normoglycemics (p<.0001 for men and <.0005 for women). In men the increase of mean BMI in the IFG and T2DM groups was 1.8 and 1.6 kg/m2 but 3.3 and 2.7 kg/m2 in respective groups of women. Mean BMI in the normoglycemic group of men increased by 1.0 but in women by 2.3 kg/m2 over the study period.
Figure 1. Change in age-adjusted mean of BMI (a) mean Systolic Blood Pressure (b) and geometric mean of S-Triglycerides (c) from the time of first visit in RS to the AGES visit, from the mean age of 50 to 76 years. Grouped by glycemic status and gender.
Note: The increase in mean BMI is significant (p<.0001) in all subgroups of men and women over the period of 26 years. Increase in mean Systolic Blood Pressure is also significant (p<.0001) in all subgroups of men and women. The change in the geometric mean of TG in men over the period of 26 years is not significant. In women the increase of geometric mean levels in TG is highly significant in all subgroups (p<.0001) over the period of 26 years.
Men that developed IFG and T2DM had significantly higher mean systolic blood pressure (p<.0001) in midlife than men remaining normoglycemic throughout the study period (Figure 1b). All groups of women have a significantly lower systolic blood pressure in midlife than men, but women who developed IFG and T2DM also had higher mean systolic blood pressure in midlife than women remaining normoglycemic.
Both men and women developing IFG and T2DM in late life have significantly higher TG levels in midlife than the respective normoglycemics (p<.0001) as shown in Figure 1c. All subgroups of women show a highly significant increase (p<.0001) in TG levels over the 26 years, but this is not so in men.
Odds ratios for the assocation of T2DM in late life with midlife risk parameters are summarized in Table 2; they are estimated using a multivariable logistic regression model with adjustment for sex and age at entry into the Reykjavik Study. Variables are listed by the strength of the assocation.
Table 2. Odds ratios for the assocation of T2DM in late life with midlife risk parameters.
| Risk parameter | Odds Ratio | Lower | Upper | P-value |
|---|---|---|---|---|
| Glucose (1 mmol/l) | 2.94 | 2.15 | 4.04 | <0.0001 |
| TG (0.1 mmol/l) | 1.10 | 1.06 | 1.13 | <0.0001 |
| BMI (1 unit, kg/m2) | 1.10 | 1.05 | 1.14 | <0.0001 |
| Systolic BP (10 mmHg) | 1.15 | 1.06 | 1.24 | 0.0002 |
| Family history | 1.85 | 1.17 | 2.91 | 0.0083 |
| Smoking | 1.30 | 0.96 | 1.76 | 0.0846 |
Odds ratios are estimated using a multivariable logistic regression model with adjustment for sex and age at entry into the Reykjavik Study. Variables are listed by the strength of the assocation.
No difference is seen in cholesterol levels in midlife between study groups. In late life cholesterol has decreased significantly (p<.0001) in all subgroups (not shown). However in line with greater proportion of men taking statins (Table 3) the drop is considerably greater in men than women. In both genders the mean late life cholesterol of the T2DM group is significantly lower (p<.001) than in the normoglycemic group (Table 3).
Table 3. Cardiovascular measures by glycemic status in men and women at entry in AGES-Reykjavik.
| Variables | men | women | ||||
|---|---|---|---|---|---|---|
| (mean ± SD, median & quartile range or %) | Normoglycemic | IFG | T2DM | Normoglycemic | IFG | T2DM |
| (number) | (675) | (144) | (137) | (1022) | (169) | (106) |
| Glucose mmol/l | 5.4 ± 0.4 | 6.4 ± 0.2 *** | 7.9 ± 1.8 *** | 5.3 ± 0.4 | 6.4 ± 0.2 *** | 7.5 ± 1.8 *** |
| Insulin mU/l a) | 8.6 ± 5.8 | 12.9 ± 6.1 *** | 15.7 ± 10.3 *** | 8.3 ± 5.4 | 14.6 ± 9.2 *** | 14.9 ± 9.2 *** |
| HOMA-IR | 2.2 ± 1.8 | 4.3 ± 4.6 *** | 6.7 ± 7.4 *** | 2.0 ± 1.5 | 4.2 ± 2.9 *** | 5.3 ± 4.1 *** |
| HbA1c % | 5.6 ± 0.3 | 5.8 ± 0.3 *** | 6.6 ± 0.8 *** | 5.7 ± 0.3 | 5.9 ± 0.4 *** | 6.3 ± 0.7 *** |
| % on diabetic medication (only the dT2DM) | - | - | 82.8 | - | - | 52.0 |
| Systolic BP mmHg | 141.2 ± 20.7 | 143.0 ± 20.3 | 145.1 ± 20.0 | 140.9 ± 20.9 | 141.7 ± 22.7 | 144.2 ± 20.8 * |
| Diastolic BP mmHg | 75.8 ± 9.3 | 77.4 ± 9.4 | 74.6 ± 9.4 | 72.1 ± 9.3 | 72.4 ± 8.7 | 72.3 ± 10.1 |
| % on HT medication | 56.2 | 66.7 * | 78.1 *** | 61.3 | 68.1 | 84.9 *** |
|
| ||||||
| BMI kg/m2 | 26.0 ± 3.5 | 27.9 ± 3.6 *** | 28.5 ± 4.0 *** | 26.5 ± 4.7 | 29.0 ± 4.9 *** | 29.5 ± 5.4 *** |
| Hypertension % | 63.9 | 70.1 | 76.6 ** | 67.8 | 80.5 ** | 84.0 ** |
| Triglycerides mmol/lb) | 0.97 (0.58) | 1.13 (0.61) *** | 1.34 (0.91) *** | 1.04 (0.64) | 1.35 (0.81) *** | 1.47 (0.98) *** |
|
| ||||||
| Cholesterol mmol/l | 5.3 ± 1.0 | 5.3 ± 1.1 | 5.0 ± 1.1 *** | 6.1 ± 1.1 | 6.2 ± 1.1 | 5.7 ± 1.1 *** |
| HDL-Chol mmol/l | 1.43 ± 0.39 | 1.36 ± 0.39 * | 1.25 ± 0.32 *** | 1.75 ± 0.43 | 1.59 ± 0.40 *** | 1.50 ± 0.43 *** |
| LDL-Chol mmol/l | 3.40 ± 0.94 | 3.34 ± 0.97 | 3.06 ± 0.99 *** | 3.86 ± 0.99 | 3.93 ± 1.05 | 3.41 ± 0.99 *** |
| % on statin medication | 23.6 | 29.2 | 37.2 ** | 14.8 | 10.7 | 24.5 ** |
| % with ≥ 2 MS criteriac) | 24.4 | 47.9 *** | 58.4 *** | 28.1 | 49.1 *** | 63.2 *** |
| U-Albumin/creatinine mg/mmol % ≥3.5 | 9.6 | 13.2 | 26.1 *** | 5.3 | 8.0 | 12.5 ** |
| Smoking % current | 14.2 | 9.1 | 13.1 | 12.2 | 13.8 | 12.3 |
| Smoking % never | 23.0 | 14.8 * | 19.0 | 53.7 | 44.9 * | 50.0 |
Glycemic groups, based on WHO recommendations from 1999 [11]. All values shown are mean ± SD unless otherwise indicated.
For insulin the truncated mean is used, excluding values >60 mU/L.
Median and (quartile range).
With two or more of the metabolic syndrome criteria, other than insulin resistance and glycemic status, as listed in materials and methods; ie. BMI, TG, HDL, HT, U-albumin/creatinine [15].
Significance estimates:
p<.05;
p<.01;
p<.001 for age-adjusted comparison among the study groups.
Glucometabolic status and cardiovascular measures in late life
The glucometabolic status in each study group at the AGES-Reykjavik baseline is assessed by mean values of fasting serum glucose and insulin, with the calculated HOMAIR and blood HbA1c (Table 3). Diabetic medication in the groups with diagnosed T2DM is also shown in the table. A highly significant difference is found in fasting insulin levels between the normoglycemic and the groups with impaired glucose regulation, but the level in the diabetics is not significantly different from the IFG groups. As expected a stepwise increase is observed in HbA1c values, increasing from the normoglycemic to the IFG and the diabetic groups.
In late life the T2DM groups have significantly higher BMI, TG and a higher proportion are classified as hypertensive than are normoglycemics. The same is true for the IFG groups although they do not reach the same level as the T2DM groups. These results are reflected in the WHO metabolic syndrome criteria (with glucose level and insulin resistance omitted), where about 25% of persons in the normoglycemic subgroups have 2 or more MS criteria, compared to about 60% of the T2DM groups and almost 50% of the IFG groups (Table 3).
Selected indicators of cardiovascular impairment along with CRP levels are shown in Table 4. No significant difference is seen in CRP levels in the three study groups of men. Over 35% of men report a CVD event (cardiovascular disease and stroke) with no significant difference between study groups. Significantly higher proportion of T2DM men report heart failure (p<.001) than normoglycemics and similarly significantly higher proportion have an abnormal ECG (p<.01). A similar trend is seen for the adjudicated CE from health records. In women there is a significant increase in CRP levels going from normoglycemics to IFG and T2DM groups. Women report a considerably lower prevalence of CVD than men in all subgroups, but women with T2DM have a significantly greater risk (p<0.05) of both reported CVD and adjudicated CE from health records compared to IFG and normoglycemic women. No significant difference is seen in abnormal ECG in the three study groups of women.
Table 4. CRP and indicators of cardiovascular disease by glycemic status, reported as %.
| Variables | Normoglycemic | IFG | dT2DM |
|---|---|---|---|
| men (n) | (675) | (144) | (137) |
|
| |||
| CRP mg/La) | 1.90 (2.40) | 2.10 (3.00) | 1.90 (2.60) |
| CVDb) questionnaire | 35.4 | 35.4 | 38.0 |
| Heart failure questionnaire | 3.5 | 5.6 | 11.9 *** |
| Abnormal ECG | 58.5 | 64.3 | 70.9 ** |
|
| |||
| women (n) | (1020) | (169) | (106) |
|
| |||
| CRP mg/La) | 1.80 (2.80) | 2.40 (3.10) * | 2.75 (4.30) *** |
| CVDb) questionnaire | 16.8 | 13.0 | 25.5 * |
| Heart failure questionnaire | 2.7 | 5.4 | 3.9 |
| Abnormal ECG | 44.1 | 42.8 | 48.1 |
Median and (quartile range).
CVD : stroke, transient ischemic attack (TIA) and cardiac event.
Note. Glycemic groups based on WHO recommendations from 1999 [11].
Significance estimates:
p<.05;
p<.01;
p<.001 for age-adjusted comparison among the study groups using age-adjusted logistic regression and Chi2 analysis for MI and AF in women.
Discussion
The data shown here reflect the status of T2DM in older persons in AGES-Reykjavik, a population-based study in Iceland. The major findings are that family history and high levels of BMI, TG and systolic blood pressure in midlife are associated with the development of T2DM in late life. As levels of these parameters continue to rise with age, the differences between the three study groups, T2DM, IFG and normoglycemics, observed in midlife are maintained throughout the study period. Short term prediction studies have shown that high BMI and high systolic blood pressure along with high blood glucose are risk factors for T2DM, but TG levels have not been greatly emphasized. TG levels have, however, recently been considered as a risk factor in the development of T2DM and are linked to insulin resistance [17]. High TG levels in this study are seen to precede any impairment in glucose regulation, an observation that could be used to predict a risk of developing T2DM earlier than before as TG levels have not been a major risk factor included in earlier prediction models. In midlife men have higher levels of TG than women, but over time the TG levels in women rise and reach about the same level as men at age 65 years [18]. Classification of persons with IFG and T2DM is of course based on measurement of fasting glucose or a glucose tolerance test, but people at risk could be identified earlier based on levels of the three observed risk parameters and monitored more closely thereafter and possibly directed towards lifestyle modifications.
Other findings are that prevalence of T2DM in the study cohort as a whole is 11.6%, including those attending that were diagnosed with T2DM between the mean ages of 40-49 (omitted in other data presentation). This is low for the age group 65-96, compared with prevalence of over 20% in adults older than 65 years in US [19] [20]. Less than one third of the diabetic group was undiagnosed prior to entering the study, which also is low compared with US estimates, where equivalent numbers are under medical treatment for diabetes as those undiagnosed in the community. Another finding is that men in AGES-Reykjavik show both higher prevalence and receive more intensive glucose lowering treatment judged from the medication profile of hypoglycaemic drugs than women. Similar observations have been reported from the Icelandic diabetic clinics [21]. In the DECODE study [22] the prevalence of diagnosed diabetes did not differ between men and women except in people over the age of 80 where the prevalence in women was higher. When compared with prevalence of diabetes in Europe, Icelanders are shown to be at the lower end of the scale [22] [23]. Prevalence of T2DM has, however, been rising in the general population of Iceland over the last several years [24] as in other populations, mostly because of increase in incidence in the lower age groups, but in the 70-88 years old the prevalence has remained the same for at least 10 years, or 12% as calculated from the Reykjavik Study in 1993. A similar situation has been observed in southern Sweden, where the incidence and age at diagnosis remained constant at the same time as prevalence increased considerably [25].
Cardiovascular disease is strongly associated with diabetes and is one of the more severe co morbidities of the disease. CRP is a marker for low systemic inflammation and one of the cardiovascular risk factors, but has recently also been linked to T2DM, more so in women than in men [10]. Men in the AGES-Reykjavik cohort that develop IFG or T2DM do not, however, report higher incidence of CVD than normoglycemics, nor is there any significant difference in CRP levels between the three study groups. T2DM women on the other hand report significantly higher CVD incidence (p<0.05) and have higher CRP levels than normoglycemic women. A possible explanation is that men with the most severe CVD and T2DM have not survived. Recent studies have shown that diabetes has a greater impact on CHD mortality risk in women than in men [26]; others have found that diabetic patients have an equal risk of myocardial infarction as non-diabetic patients with previous MI [27], showing diabetic women with a 50% higher relative risk for fatal coronary heart disease than diabetic men [28].
The participation rate of 75% among men and 68% for women in the AGES-Reykjavik is high and the nonparticipants when asked, claimed they were not up to attend. Midlife data show that nonparticipants had a higher mean value of systolic blood pressure (men 143 vs. 136 and women 133 vs. 127 mmHg), but similar values are seen in glucose, BMI and TG [12].
In summary family history of diabetes, high levels of BMI, TG, and high systolic blood pressure in midlife are associated with the development of IFG and T2DM in late life. Our data show a continued rise in these risk parameters over the study period opening a possibility of predicting the risk of developing T2DM decades before onset. The campaign for health promotion and prevention strategies might accordingly start quite early in life in the hope of preventing or delaying development of T2DM and its detrimental effect on cardiovascular health.
Acknowledgments
The study is supported by National Institutes of Health contract NO1-AG-1-2100, the National Institute on Aging Intramural Research Program, the Icelandic Government and the Icelandic Heart Association.
Abbreviations
- AGES-Reykjavik
Age, Gene/Environment Susceptibility - Reykjavik Study
- CE
coronary events
- CHD
coronary heart disease
- CRP
C-reactive protein
- CVD
cardiovascular disease
- ECG
electrocardiograph
- HbA1c
haemoglobin A1c
- HOMAIR
homeostasis model assessment: insulin resistance
- IFG
impaired fasting glucose
- MI
myocardial infarction
- MS
metabolic syndrome
- NCEP
National Cholesterol Education Program
- NHANES
National Health and Nutrition Examination Survey
- RS
Reykjavik Study
- dT2DM
diagnosed type 2 diabetes mellitus
- uT2DM
undiagnosed type 2 diabetes mellitus
- TG
triglycerides
- WHO
World Health Organization
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy MI. Progress in defining the molecular basis of type 2 diabetes mellitus through susceptibility-gene identification. Hum Mol Genet. 2004;13 Spec No 1:R33–41. doi: 10.1093/hmg/ddh057. [DOI] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eddy DM, Schlessinger L. Archimedes: a trial-validated model of diabetes. Diabetes Care. 2003;26:3093–3101. doi: 10.2337/diacare.26.11.3093. [DOI] [PubMed] [Google Scholar]
- 6.Lindstrom J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26:725–731. doi: 10.2337/diacare.26.3.725. [DOI] [PubMed] [Google Scholar]
- 7.Meigs JB, Williams K, Sullivan LM, Hunt KJ, Haffner SM, Stern MP, et al. Using metabolic syndrome traits for efficient detection of impaired glucose tolerance. Diabetes Care. 2004;27:1417–1426. doi: 10.2337/diacare.27.6.1417. [DOI] [PubMed] [Google Scholar]
- 8.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 9.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 10.Dehghan A, van Hoek M, Sijbrands EJ, Stijnen T, Hofman A, Witteman JC. Risk of type 2 diabetes attributable to C-reactive protein and other risk factors. Diabetes Care. 2007;30:2695–2699. doi: 10.2337/dc07-0348. [DOI] [PubMed] [Google Scholar]
- 11.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 12.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonsdottir LS, Sigfusson N, Gudnason V, Sigvaldason H, Thorgeirsson G. Do lipids, blood pressure, diabetes, and smoking confer equal risk of myocardial infarction in women as in men? The Reykjavik Study J Cardiovasc Risk. 2002;9:67–76. [PubMed] [Google Scholar]
- 14.WHO. Report of a WHO consultation, part 1: diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 1999. Expert Committee Definition, diagnosis and classification of diabetes mellitus and its complications. [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Rose GA, Blackburn H. Cardiovascular survey methods. Geneva: World Health Organization; 1968. [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 18.Rifai N, Warnick GR, Dominiczak MH. Handbook of Lipoprotein Testing. Washington DC: AACC Press; 1997. [Google Scholar]
- 19.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 20.Selvin E, Coresh J, Brancati FL. The burden and treatment of diabetes in elderly individuals in the u.s. Diabetes Care. 2006;29:2415–2419. doi: 10.2337/dc06-1058. [DOI] [PubMed] [Google Scholar]
- 21.Bjornsdottir S, Rossberger J, Gudbjornsdottir HS, Hreidarsson AB. Treatment pattern and results in an outpatient population with type 2 diabetes in Iceland. Laeknabladid. 2004;90:623–627. [PubMed] [Google Scholar]
- 22.Decode SG. Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care. 2003;26:61–69. doi: 10.2337/diacare.26.1.61. [DOI] [PubMed] [Google Scholar]
- 23.Forouhi NG, Merrick D, Goyder E, Ferguson BA, Abbas J, Lachowycz K, et al. Diabetes prevalence in England, 2001--estimates from an epidemiological model. Diabet Med. 2006;23:189–197. doi: 10.1111/j.1464-5491.2005.01787.x. [DOI] [PubMed] [Google Scholar]
- 24.Bergsveinsson J, Aspelund T, Gudnason V, Benediktsson R. Prevalence of Type 2 Diabetes Mellitus in Iceland 1967-2002. Laeknabladid. 2007;93:397–402. [PubMed] [Google Scholar]
- 25.Berger B, Stenstrom G, Sundkvist G. Incidence, prevalence, and mortality of diabetes in a large population. A report from the Skaraborg Diabetes Registry. Diabetes Care. 1999;22:773–778. doi: 10.2337/diacare.22.5.773. [DOI] [PubMed] [Google Scholar]
- 26.Natarajan S, Liao Y, Sinha D, Cao G, McGee DL, Lipsitz SR. Sex differences in the effect of diabetes duration on coronary heart disease mortality. Arch Intern Med. 2005;165:430–435. doi: 10.1001/archinte.165.4.430. [DOI] [PubMed] [Google Scholar]
- 27.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 28.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]