Abstract
Chlorogenic acid (CGA) possesses various biological activities such as anti-oxidant, anti-inflammatory, and anti-diabetic activities. In the present study, we examined the effect of CGA on the transduction efficiency of PEP-1-ribosomal protein S3 (PEP-1-rpS3) into cells and brain tissues, and its neuroprotective potential against ischemia/reperfusion. We found that, in the presence of CGA, the transduction efficiency of PEP-1-rpS3 into astrocytes and the CA1 region of the hippocampus was enhanced, compared to its transduction in the absence of CGA. Also, cell viability data demonstrated that the sample treated with CGA + PEP-1-rpS3 exhibited improved cell viability against hydrogen peroxide (H2O2)-induced toxicity more significantly than the sample treated with PEP-1-rpS3 alone. Also, in a gerbil ischemia model, data demonstrated that following the ischemic insult, the group treated with PEP-1-rpS3 + CGA showed markedly enhanced protection of neuron cells in CA1 region of hippocampus, compared to those treated with CGA or PEP-1-rpS3 alone. Taken together, these results suggest that CGA may improve the transduction efficiency of protein transduction domain (PTD) fusion proteins into target cells or tissues, thereby enhancing their therapeutic potential against various diseases.
Keywords: chlorogenic acid, PEP-1-ribosomal protein S3, protein transduction, ischemic insult
INTRODUCTION
Numerous studies have demonstrated that the interruption and restoration of the blood flow to the forebrain lead to the neuronal death in the CA1 region of the hippocampus, or transient ischemia [1, 2]. During ischemia, a series of events, such as the depletion of ATP, production of reactive oxygen species (ROS), an imbalance of intracellular calcium concentration, and changes in cellular pH, cause cellular damage, apoptosis and/or necrosis [3, 4]. Previous studies have suggested that anti-oxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase can function as a defense mechanism against ROS-mediated cellular damage, thereby proposing that these anti-oxidant enzymes may be useful as a therapeutic agent against ROS-related diseases [5, 6].
Ribosomal proteins are essential components of ribosomes and highly conserved during evolution. Recently, it was reported that many ribosomal proteins could carry out extra-ribosomal functions in apoptosis, mRNA processing, DNA repair, and development [7, 8]. Ribosomal protein S3 (rpS3) knockdown demonstrated that rpS3 is involved in nuclear factor-kappaB (NF-κB)-mediated gene regulations [9, 10]. Also, rpS3 has potential roles in processes such as DNA repair and an apoptosis [11]. PEP-1-rpS3 was reported to protect the CA1 region of the hippocampus against cerebral ischemic damage [12].
Chlorogenic acid (CGA), a phenolic compound derived from herbs, is well-known for having various biological properties. For example, it was shown that CGA, as a natural anti-oxidant, effectively prevents iron-induced radical formation and exerts strong antimicrobial activity [13, 14]. Also, several studies revealed that CGA is an effective anti-inflammatory, analgesic and antipyretic agent in in vitro experiments and in vivo animal models [15-17]. Recently, numerous bio-molecules such as peptides and proteins, having therapeutic abilities, have been developed as new drug candidates. However, their large molecular sizes and poor hydrophilicities reduce and/or restrict their bioavailabilities or delivery to cells or tissues. Therefore, protein transduction domains (PTD), including trans-activator of transcription (Tat) (RKKRRQRRR) from human immunodeficiency virus-1 and PEP-1 (KETWWET WWTEWSQPKKKRKV) derived from the nuclear localization sequence of simian virus 40, were developed to enhance the delivery of poor bioavailable drugs to cells or tissues [18-20]. In previous studies we reported that PTD fused proteins, such as PEP-1-rpS3, PEP-1-Frataxin and Tat-sensitive to apoptosis gene (Tat-SAG), can rapidly cross the blood-brain barrier (BBB) and provide neuronal protection against cerebral ischemic insults in ischemic animal models [21-23]. In addition, we demonstrated that several molecules, such as bog blueberry anthocyanins (BBA) and pergolide mesylate, have the potential to enhance the transduction efficiency of PTD-fused proteins into cells and animal tissues, thereby leading to improved therapeutic activity [24, 25]. Therefore, in this study, we examined whether CGA can enhance the transduction efficiency of PEP-1-rpS3 into astrocytes and brain tissues, and, consequently, can increase the potential of PEP-1-rpS3 to protect neuronal cells against ischemic insult.
MATERIALS AND METHODS
Materials and cell culture
CGA and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Primary antibodies against histidine and β-actin, and peroxidase conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other chemicals and reagents, unless otherwise stated, were obtained from Sigma-Aldrich (St. Louis, MO, USA). The astrocytes were grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum, 20 mM Hepes/NaOH (pH 7.4), 5 mM NaHCO3 and antibiotics (100 µg/ml streptomycin and 100 U/ml penicillin) and kept at 37℃ under a humidified atmosphere of 95% air and 5% CO2.
Transductions of PEP-1 fusion proteins into astrocytes
PEP-1-rpS3 and PEP-1-green fluorescence protein (PEP-1-GFP) were purified as described previously [21]. To assess the concentration or time dependent transduction of PEP-1-rpS3 and PEP-1-GFP, cells were grown to confluence in wells of a 6-well plate, pretreated with or without CGA (100 ng/ml) for 1 h, and then exposed to various concentrations (0.5~2 µM) of each protein for an additional 1 h or exposed to each protein (2 µM) for various times (10~60 min). The cells were then harvested and cell extracts were prepared for Western blot analysis.
Western blot analysis
Equal quantities of β-actin normalized proteins from cell lysate were resolved by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred to a nitrocellulose membrane, which was then blocked with 5% non-fat dry milk in PBS. To detect both PEP-1-rpS3 and PEP-1-GFP, which contain histidine sequence and PEP-1 domain, the membrane was probed with a rabbit anti-histidine polyclonal antibody (diluted at 1 : 10,000), followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (diluted at 1 : 10,000). The membrane was visualized by enhanced chemiluminescence according to the manufacturer's instructions (Amersham, Piscataway, NJ, USA).
Viability assay
An established assay based on MTT was used to assess cell viability [23]. Astrocytes were grown to 70% confluence in wells of a 96-well plate and pretreated with CGA (100 ng/ml) for 1 h. The cells were incubated with various concentrations of PEP-1-rpS3 (0.5~2 µM) for an additional 1 h and cell toxicity was induced by treatment with H2O2 (1 mM) for 4 h. Cellular viability was expressed as a percentage of a H2O2 untreated control.
Animals
Mongolian gerbils (Meriones unguiculatus) were purchased from the Hallym University Experimental Animal Center. They were permitted free access to food and water, ad libitum. All experimental procedures involving animals and their care conformed to the Guide for the Care and Use of Laboratory Animals of the National Veterinary Research & Quarantine Service of Korea and were approved by the Hallym Medical Center Institutional Animal Care and Use Committee.
In vivo transduction of PEP-1-rpS3 into brain tissues
To examine the effect of CGA on the transduction of PEP-1-rpS3 into brain tissues, CGA (100 µg/kg) was i.p. administered, followed by an i.p. injection of PEP-1-rpS3 (150 µg/kg) 1 h later. Brain biopsy samples were immunostained using a rabbit anti-histidine (1 : 400) and biotinylated goat anti-rabbit secondary antibodies (1 : 200). The sections were visualized with 3,3'-diaminobenzidine (DAB) in 0.1 M Tris buffer and observed under a microscope (Olympus DP72 digital camera, Tokyo, Japan).
Induction of cerebral forebrain ischemia in experimental model
To determine whether CGA affects the protective effect of PEP-1-rpS3 against ischemic damage, male Mongolian gerbils were randomly divided into 5 groups (n=7); sham-operated, vehicle treated, CGA-treated, PEP-1-rpS3-treated, and PEP-1-rpS3 + CGA treated groups. CGA dissolved in PBS was i.p. injected (100 µg/kg). Then, PEP-1-rpS3 (150 µg/kg) was administered i.p. 30 min prior to the occlusion of common carotid arteries. For the occlusion of common carotid arteries, gerbils were placed under general anesthesia with a mixture of 2.5% isoflurane (Abbott Laboratories, North Chicago, IL, USA) in 33% oxygen and 67% nitrous oxide. A midline ventral incision was made in the neck, and the common carotid arteries were isolated, freed of nerve fibers, and occluded with nontraumatic aneurysm clips. Complete interruption of blood flow was confirmed by observing the central artery in the eyeball using an ophthalmoscope. After 5 min occlusion, the aneurysm clips were removed. The restoration of blood flow (reperfusion) was observed directly under the ophthalmoscope. Sham-operated animals (n=7) were subjected to the same surgical procedures except that the common carotid arteries were not occluded. Rectal temperature was monitored and maintained at 37±0.5℃ before, during, and after the surgery, until the animals had recovered fully from anesthesia. Immediately following ischemia-reperfusion, animals from each group were euthanized for cresyl violet staining.
Tissue processing for histology
All animals from each group were anesthetized with sodium pentobarbital and transcardially perfused with 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Brain tissues were post-fixed in the same fixative for 4 h, followed by infiltration with 30% sucrose overnight. The tissues were then frozen and sectioned with a cryostat at 50 µm. Cresyl violet staining was performed as described previously [22,23].
Statistical analysis
Data are expressed as the means±SD. Comparison between groups was performed by Student's t test. Values of p<0.01 and p<0.05 were considered to be statistically significant.
RESULTS
CGA enhanced the transduction of PEP-1 fused proteins into astrocytes
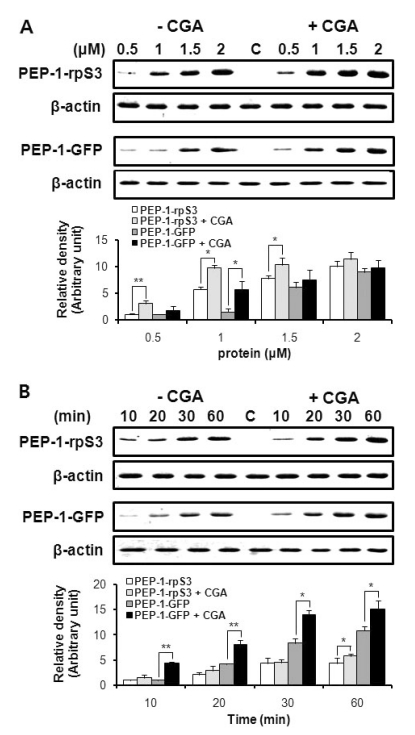
We previously reported that PEP-1-rpS3 can be effectively transduced into cells and animal tissues, thereby providing protection against UV damage or ischemic insult [12, 21]. To assess the effect of CGA on the transduction efficiency of PEP-1-fused proteins, we compared the extent of transduction of PEP-1-rpS3 and PEP-1-GFP into astrocytes in the presence or absence of CGA using Western blot analysis. The chemical structure of CGA and schematic sequences of PEP-1-rpS3 and PEP-1-GFP were shown in Fig. 1. As shown in Fig. 2, both PEP-1-rpS3 and PEP-1-GFP were delivered in a dose- and time- dependent manner to the cells without treatment of CGA. Particularly, when pretreated with CGA, a clear increase in the transduced amount of PEP-1-rpS3 and PEP-1-GFP was observed, compared to the samples without CGA pretreatment. These results demonstrated that CGA has the ability to improve the in vitro transduction of PEP-1 fused proteins.
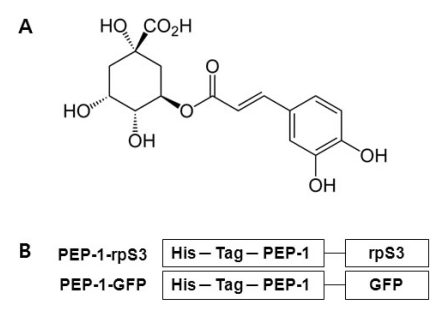
Fig. 1.
(A) The chemical structure of chlorogenic acid. (B) The schematic sequences of PEP-1-rpS3 and PEP-1-GFP. His-Tag in both PEP-1-rpS3 and PEP-1-GFP is a sequence of six histidines used for purification and detection of PEP-1 fusion proteins.
Fig. 2.
CGA enhances the transduction efficiency of PEP-1 fusion proteins. In the presence or absence of CGA (100 ng/ml), cells were (A) treated with various concentrations of PEP-1-rpS3 and PEP-1-GFP or (B) treated with each protein (2 µM) for 10~60 min. The band intensity was measured by a densitometer. The asterisk indicates a statistically significant difference between CGA-untreated and treated groups (*p<0.05, **p<0.01).
CGA produced no cellular toxicity
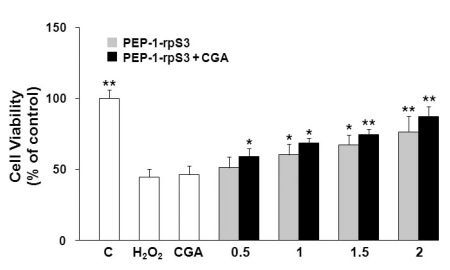
We next examined whether the enhanced transduction of PEP-1-fusion proteins is ascribed to altered membrane permeability induced by CGA. As illustrated in Fig. 3, H2O2 (1 mM) produced apparent cell death to 44% of control cells. Although CGA is known to have anti-oxidant properties, CGA alone at the concentration (100 ng/ml) which was employed in our experiments failed to recover the cell viability against H2O2-induced oxidative stress. Cell viability did, however, increase with increasing concentrations of PEP-1-rpS3 (0.5~2 µM). Moreover, co-treatment with PEP-1-rpS3 and CGA demonstrated further increases in cell viability, relative to the samples treated with PEP-1-rpS3 alone. At the maximal concentration of PEP-1-rpS3, CGA pretreatment enhanced cell viability, by approximately 11%. These data suggest that CGA can enhance the anti-oxidant activity of PEP-1-rpS3 without displaying any additional toxicity or anti-oxidative effects to astrocytes.
Fig. 3.
CGA increases the protective effect of PEP-1-rpS3 against hydrogen peroxide-induced cellular death. Cells were pretreated with CGA (100 ng/ml) for 1 h and then treated with PEP-1-rpS3 for additional 1 h. After induction of cellular toxicity by H2O2 (1 mM) for 4 h, cell viability was assessed using a MTT assay and is expressed as a percentage of a H2O2 untreated control. Statistical significance (*p<0.05, **p<0.01), compared with H2O2-treated group.
In vivo transduction of PEP-1-rpS3 into the brain tissues was enhanced in the presence of CGA
Next to verify whether CGA could increase the in vivo transduction of PEP-1-rpS3 as well, CGA was i.p. injected at a dose of 100 µg/kg 1 h prior to injection of PEP-1-rpS3 (150 µg/kg) and the distribution of PEP-1-rpS3 in the brain was identified by immunohistological staining. Fig. 4 shows results consistent with in vitro transduction of PEP-1-rpS3. rpS3 is not found in the brain tissues at all. The significant transduction of PEP-1-rpS3 was found in the CA1 regions of the hippocampus. In addition, much stronger signals in the same region were identified after combinational treatment with PEP-1-rpS3 + CGA than the sample treated with PEP-1-rpS3 alone, suggesting the effectiveness of CGA in the transduction of this fusion proteins.
Fig. 4.
PEP-1-rpS3 was effectively delivered to brain tissues of Mongolian gerbils. CGA (100 µg/kg) was administered i.p. to gerbils and then PEP-1-rpS3 (150 µg/kg) was i.p. injected 1 h later. Brain tissue was removed and immunhistological anlaysis was performed using a rabbit anti-histidine polyclonal antibody and biotinylated goat anti-rabbit secondary antibody.
CGA apparently increased the neuroprotective effect of PEP-1-rpS3 against ischemic damage
Transient ischemia induces the neuronal loss in the CA1 region of the hippocampus. We evaluated protection in each group against ischemia induction after the administration of CGA, PEP-1-rpS3, and CGA + PEP-1-rpS3 using cresyl violet staining and histological analysis. As shown in Fig. 5, most of cells in CA1 region in the vehicle-treated group were dead. Treatment with CGA slightly increased the number of viable cells in the CA1 region and also treatment with PEP-1-rpS3 clearly exhibited enhanced protection against cell death in the CA1 region. Above all, the extent of neuronal protection in the sample treated with CGA + PEP-1-rpS3 was similar to that in the sham control, demonstrating that combinational treatment with CGA + PEP-1-rpS3 was much more protective against neuronal cell death induced by transient ischemia than that of CGA or PEP-1-rpS3 alone.
Fig. 5.
CGA enhances the protective activity of PEP-1-rpS3 on hippocampus brain damage after ischemic insult. (A) CGA (100 µg/kg) was administered i.p. to gerbils and then PEP-1-rpS3 (150 µg/kg) was i.p. injected 30 min prior to ischemic damage. Hippocampi were removed after ischemia-reperfusion and stained with cresyl violet. The damaged area of brain tissue was CA1 of hippocampus. (B) Relative density of viable cells in CA1 region. Data were expressed as mean±S.D. (n=5/group). The asterisk indicates a statistically significant difference between vehicle-treated and other groups (*p<0.05, **p<0.01).
DISCUSSION
CGA is a polyphenol, which is abundantly found in coffee, fruits and vegetables. Several studies have provided evidence showing that CGA has various biological activities such as anti-oxidant, anti-inflammatory and analgesic. CGA has the ability to suppress 12-O-Tetradecanoylphorbol-13-acetate (TPA)-induced tumor promotion in a mouse model [26] and to induce p38 mitogenactivated protein kinase (MAPK)-dependent apoptosis in chronic myelogenous leukemic cells [27]. Also, the mechanism for antitumor activity of CGA suggests that, following treatment with UVB and TPA, CGA up-regulates cellular phase II anti-oxidant enzymes and suppresses activation of nuclear factor-kappa B (NF-κB), activator protein-1 (AP-1) and MAPK signaling pathways mediated by ROS [28]. CGA inhibits synthesis and release of inflammatory mediators, such as TNF-α and NO, and provides anti-inflammatory and analgesic activities in animal models of carrageenin-induced inflammation and formalin-induced pain [16]. Therefore, up-regulating levels of anti-oxidant enzymes in target cells or tissues can be proportional to the enhancement of therapeutic potentials of these enzymes. Increasing the intracellular levels of therapeutic proteins is also proposed as an effective therapy against several ROS-related diseases. Previously, we reported that, through fusion of rpS3 with PEP-1, one PTD, PEP-1-rpS3 can be effectively transduced into cells or brain tissues, suppress ROS generation and ischemic damage induced by oxidative stress, and functions as a neuroprotective agent [12].
In this study, we investigated a novel function of CGA to enhance the delivery of PEP-1 fusion proteins to cells and tissues, thereby increasing their cellular bioavailability and therapeutic potential. Our cell viability data demonstrated that, although CGA is wellknown as an anti-oxidant molecule, CGA alone, at 100 ng/ml concentration, had no influence on H2O2-induced toxicity in astrocytes (Fig. 3) and exhibited similar cell viability to the sample treated with H2O2. However, the existence of CGA improves the neuroprotective effect of PEP-1-rpS3 against H2O2, compared to the samples treated with PEP-1-rpS3 alone. Therefore, as we did not observe any clear neuroprotective effect of CGA alone, in this experiment we excluded the possibility that CGA provided any direct neuronal protection against H2O2 in the sample co-treated with CGA+PEP-1-rpS3. Next, to investigate the reason for the enhanced neuroprotective activity in the sample co-treated with CGA and PEP-1-rpS3, we examined whether treatment with CGA could affect transduction of PEP-1-rpS3 into astrocytes and subsequently enhance its neuroprotective effect against ischemic insult. As shown in Fig. 2, the transduction data show that treatment with CGA can considerably improve the transduction of PEP-1-rpS3 into astrocytes. Moreover, in vivo transduction data confirmed that PEP-1-rpS3, which has the ability to spontaneously cross the BBB, exhibited enhanced transduction into brain tissues in the presence of CGA (Fig. 4), as evidenced by in vitro transduction results. As a result of the enhanced transduction of PEP-1-rpS3 by CGA, an animal transient ischemia model provides the evidence that, even if CGA alone showed slight neuroprotection in the CA1 region of the hippocampus, CGA has the ability to definitely improve the transduction of PEP-1-rpS3 into brain tissues, resulting in improved neuroprotective effects of PEP-1-rpS3 against ischemic insult (Fig. 5).
Various biological activities of polyphenol such as CGA have been drawing much attention from many research groups. Because of the characteristic physicochemical properties of polyphenol, they chelate metals, scavenge ROS and exert a broad spectrum of biological activities [16,29]. On the other hand, our present study demonstrates that CGA also has the novel function of enhancing the transduction of PEP-1-fusion proteins beyond its already wellknown anti-oxidant function. One reason for the different results may be that the function of CGA as an anti-oxidant in other literature was evaluated at the higher concentrations than that we used in this study. Moreover, others have suggested that there is a correlation between biological activity and the concentration of biomolecules. For instance, although (-)-epigallocatechin-3-gallate (EGCG), one of polyphenols, exhibited effective in vitro anti-cancer activity in the concentration of 10~100 µM, it is possible that its concentration in plasma or tissues was observed as very low by oral administration because of very poor bioavailabilty [29, 30]. Thus, it is considered that the concentration of CGA, cell lines, experimental conditions and assay methods may contribute to absolutely different results.
Many research groups have tried to explain how PTD could traverse the cellular membrane and the BBB. Although the mechanisms of PTD transduction are suggested such as a direct penetration via electrostatic interaction with negatively charged phospholipids, endocytosis-mediated pathways, and a formation of inverted micelles or pores structure, these mechanisms are still controversial [31-34]. Besides CGA, several molecules such as DMSO, BBA, and pergolide mesylate were reported to have the potential to enhance the transduction of PTD fusion proteins [24, 25, 30]. Even if, until now, it is still not clear which molecular events mediated by CGA or other molecules are responsible for enhancing the transduction of PTD fusion proteins, it is presumable that CGA might be related with one of the mechanism suggested above or increase permeability of the cellular membranes, subsequently facilitating transduction of PTD into cells and BBB. Therefore, additional studies are required to investigate molecules and pathways to take part in the process of transduction of PTD fusion proteins. Also, it is undoubtedly necessary to develop agents which could enhance transduction efficiency for therapeutic molecules having poor transduction efficiency.
Taken our results together, we demonstrate that CGA has the novel activity to significantly enhance transduction efficiency of PEP-1-fused therapeutic proteins into target cells or tissues, and, consequently, lead to improvement of their therapeutic potential.
ACKNOWLEDGEMENTS
This work was supported by the Mid-career Researcher Program grant (2009-0086319) and by the research grant from the Brain Research Center of the 21st Century Frontier Research Program (2010K000808) and by the Priority Research Centers Program grant (2009-0093812) through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology.
References
- 1.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 2.Petito CK, Torres-Munoz J, Roberts B, Olarte JP, Nowak TS, Jr, Pulsinelli WA. DNA fragmentation follows delayed neuronal death in CA1 neurons exposed to transient global ischemia in the rat. J Cereb Blood Flow Metab. 1997;17:967–976. doi: 10.1097/00004647-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990;4:2587–2597. [PubMed] [Google Scholar]
- 4.Martone ME, Hu BR, Ellisman MH. Alterations of hippocampal postsynaptic densities following transient ischemia. Hippocampus. 2000;10:610–616. doi: 10.1002/1098-1063(2000)10:5<610::AID-HIPO12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 5.Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 6.Muzykantov VR. Targeting of superoxide dismutase and catalase to vascular endothelium. J Control Release. 2001;71:1–21. doi: 10.1016/s0168-3659(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 7.Lindström MS. Emerging functions of ribosomal proteins in gene-specific transcription and translation. Biochem Biophys Res Commun. 2009;379:167–170. doi: 10.1016/j.bbrc.2008.12.083. [DOI] [PubMed] [Google Scholar]
- 8.Wool IG. Extraribosomal functions of ribosomal proteins. Trends Biochem Sci. 1996;21:164–165. [PubMed] [Google Scholar]
- 9.Wan F, Anderson DE, Barnitz RA, et al. Ribosomal protein S3: a KH domain subunit in NF-kappaB complexes that mediates selective gene regulation. Cell. 2007;131:927–939. doi: 10.1016/j.cell.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Wan F, Lenardo MJ. The nuclear signaling of NF-kappaB: current knowledge, new insights, and future perspectives. Cell Res. 2010;20:24–33. doi: 10.1038/cr.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang CY, Lee JY, Kim J. RpS3, a DNA repair endonuclease and ribosomal protein, is involved in apoptosis. FEBS Lett. 2004;560:81–85. doi: 10.1016/S0014-5793(04)00074-2. [DOI] [PubMed] [Google Scholar]
- 12.Hwang IK, Yoo KY, Kim DW, et al. Ischemia-induced ribosomal protein S3 expressional changes and the neuroprotective effect against experimental cerebral ischemic damage. J Neurosci Res. 2008;86:1823–1835. doi: 10.1002/jnr.21621. [DOI] [PubMed] [Google Scholar]
- 13.Almeida AA, Farah A, Silva DA, Nunan EA, Glória MB. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J Agric Food Chem. 2006;54:8738–8743. doi: 10.1021/jf0617317. [DOI] [PubMed] [Google Scholar]
- 14.Kono Y, Kashine S, Yoneyama T, Sakamoto Y, Matsui Y, Shibata H. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci Biotechnol Biochem. 1998;62:22–27. doi: 10.1271/bbb.62.22. [DOI] [PubMed] [Google Scholar]
- 15.Jin XH, Ohgami K, Shiratori K, et al. Effects of blue honeysuckle (Lonicera caerulea L.) extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp Eye Res. 2006;82:860–867. doi: 10.1016/j.exer.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 16.dos Santos MD, Almeida MC, Lopes NP, de Souza GE. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol Pharm Bull. 2006;29:2236–2240. doi: 10.1248/bpb.29.2236. [DOI] [PubMed] [Google Scholar]
- 17.Sheu MJ, Chou PY, Cheng HC, et al. Analgesic and anti-inflammatory activities of a water extract of Trachelospermum jasminoides (Apocynaceae) J Ethnopharmacol. 2009;126:332–338. doi: 10.1016/j.jep.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Dietz GP. Cell-penetrating peptide technology to deliver chaperones and associated factors in diseases and basic research. Curr Pharm Biotechnol. 2010;11:167–174. doi: 10.2174/138920110790909731. [DOI] [PubMed] [Google Scholar]
- 19.Egleton RD, Davis TP. Bioavailability and transport of peptides and peptide drugs into the brain. Peptides. 1997;18:1431–1439. doi: 10.1016/s0196-9781(97)00242-8. [DOI] [PubMed] [Google Scholar]
- 20.Wadia JS, Dowdy SF. Protein transduction technology. Curr Opin Biotechnol. 2002;13:52–56. doi: 10.1016/s0958-1669(02)00284-7. [DOI] [PubMed] [Google Scholar]
- 21.Choi SH, Kim SY, An JJ, et al. Human PEP-1-ribosomal protein S3 protects against UV-induced skin cell death. FEBS Lett. 2006;580:6755–6762. doi: 10.1016/j.febslet.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 22.Kim DW, Lee SH, Jeong MS, et al. Transduced Tat-SAG fusion protein protects against oxidative stress and brain ischemic insult. Free Radic Biol Med. 2010;48:969–977. doi: 10.1016/j.freeradbiomed.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Kim MJ, Kim DW, Yoo KY, et al. Protective effects of transduced PEP-1-Frataxin protein on oxidative stress-induced neuronal cell death. J Neurol Sci. 2010;298:64–69. doi: 10.1016/j.jns.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Jeong HJ, Kim DW, et al. Enhancement of HIV-1 Tat fusion protein transduction efficiency by bog blueberry anthocyanins. BMB Rep. 2010;43:561–566. doi: 10.5483/bmbrep.2010.43.8.561. [DOI] [PubMed] [Google Scholar]
- 25.Sohn EJ, Kim DW, Kim YN, et al. Effects of pergolide mesylate on transduction efficiency of PEP-1-catalase protein. Biochem Biophys Res Commun. 2011;406:336–340. doi: 10.1016/j.bbrc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 26.Huang MT, Smart RC, Wong CQ, Conney AH. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48:5941–5946. [PubMed] [Google Scholar]
- 27.Bandyopadhyay G, Biswas T, Roy KC, et al. Chlorogenic acid inhibits Bcr-Abl tyrosine kinase and triggers p38 mitogen-activated protein kinase-dependent apoptosis in chronic myelogenous leukemic cells. Blood. 2004;104:2514–2522. doi: 10.1182/blood-2003-11-4065. [DOI] [PubMed] [Google Scholar]
- 28.Feng R, Lu Y, Bowman LL, Qian Y, Castranova V, Ding M. Inhibition of activator protein-1, NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J Biol Chem. 2005;280:27888–27895. doi: 10.1074/jbc.M503347200. [DOI] [PubMed] [Google Scholar]
- 29.Yang CS, Sang S, Lambert JD, Hou Z, Ju J, Lu G. Possible mechanisms of the cancer-preventive activities of green tea. Mol Nutr Food Res. 2006;50:170–175. doi: 10.1002/mnfr.200500105. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Zhong CY, Wu JF, Huang YB, Liu CB. Enhancement of TAT cell membrane penetration efficiency by dimethyl sulphoxide. J Control Release. 2010;143:64–70. doi: 10.1016/j.jconrel.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 32.Richard JP, Melikov K, Vives E, et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 33.Deshayes S, Plénat T, Aldrian-Herrada G, Divita G, Le Grimellec C, Heitz F. Primary amphipathic cell-penetrating peptides: structural requirements and interactions with model membranes. Biochemistry. 2004;43:7698–7706. doi: 10.1021/bi049298m. [DOI] [PubMed] [Google Scholar]
- 34.Deshayes S, Gerbal-Chaloin S, Morris MC, et al. On the mechanism of non-endosomial peptide-mediated cellular delivery of nucleic acids. Biochim Biophys Acta. 2004;1667:141–147. doi: 10.1016/j.bbamem.2004.09.010. [DOI] [PubMed] [Google Scholar]