Abstract
We investigated the influence of elevated CO2 and O3 on soil N cycling within the soybean growing season and across soil environments (i.e., rhizosphere and bulk soil) at the Soybean Free Air Concentration Enrichment (SoyFACE) experiment in Illinois, USA. Elevated O3 decreased soil mineral N likely through a reduction in plant material input and increased denitrification, which was evidenced by the greater abundance of the denitrifier gene nosZ. Elevated CO2 did not alter the parameters evaluated and both elevated CO2 and O3 showed no interactive effects on nitrifier and denitrifier abundance, nor on total and mineral N concentrations. These results indicate that elevated CO2 may have limited effects on N transformations in soybean agroecosystems. However, elevated O3 can lead to a decrease in soil N availability in both bulk and rhizosphere soils, and this likely also affects ecosystem productivity by reducing the mineralization rates of plant-derived residues.
Keywords: Nitrification, Denitrification, Real-time quantitative PCR, FACE, Soil N cycling
1. Introduction
Crop productivity is in part limited by soil nitrogen (N) (Vitousek and Howarth, 1991), which influences plant growth, quality, and yield. Many ecosystem processes, including C and nutrient cycling, are driven by soil microorganisms, which depend on N availability to carry out their activities (Hallin et al., 2009). One of the natural sources of N in the soil is via N2 fixation, whereby atmospheric N2 is converted into organic forms. Furthermore, soil N levels depend on plant N uptake, quality and quantity of the plant residue input, and soil moisture content (Alcoz et al., 1993).
The current and predicted increases of atmospheric CO2 concentrations (IPCC, 2007) are likely to modify the factors affecting the transformations of soil N (Kanerva et al., 2006). Elevated CO2 generally enhances above- and belowground plant productivity by increasing photosynthetic rates (Ainsworth and Long, 2005; de Graaff et al., 2006a; Zak et al., 1993) and water use efficiency (Tyree and Alexander, 1993). Both of these factors have the potential to modify N dynamics at the ecosystem level. Several studies have focused on understanding the influence of elevated atmospheric CO2 on the acquisition, accumulation, and losses of N by measuring changes in plant N uptake, N mineralization, microbial N immobilization, nitrification, and denitrification (de Graaff et al., 2006b; Hungate et al., 1997; Luo et al., 2006; Zak et al., 1993). Previous studies have mainly concentrated in grass and forestland systems, with only limited data collected from croplands.
In contrast to CO2, the elevation of tropospheric O3 concentration inhibits plant productivity (Morgan et al., 2003). The atmospheric concentration of O3 is predicted to increase 20% by 2050 (Prather et al., 2001), reaching levels that can reduce photosynthetic rates of sensitive plant species (Morgan et al., 2003). In soybean (Glycine max L. Merr), elevated O3 has been shown to reduce plant growth and seed yield (Morgan et al., 2006), and accelerate leaf senescence (Dermody et al., 2006). However, little is known about the effects of elevated O3 on soil N dynamics, and most studies have focused on forest species (Holmes et al., 2003, 2006). Since elevated CO2 and O3 alter plant growth and development in opposing ways (Ainsworth and Long, 2005; Morgan et al., 2006), an understanding of how the concomitant increase in concentration of both these atmospheric gases will affect N transformations in the soil beneath annual plants needs further investigation. The effects of elevated CO2 and O3 on belowground N dynamics have been reported to be mediated, indirectly, through altered plant processes and C allocation (Andersen, 2003; Zak et al., 2000a). Therefore, we propose that the effects of elevated CO2 and O3 may alter ecosystem N balances by leading to changes in soil N availability, through the increase or decrease of plant N uptake, microbial N immobilization and/or denitrification.
Elevated CO2 and O3 may affect soil N dynamics differently at different plant phenological stages in distinct soil environments (rhizosphere vs. bulk soil) through changes in substrate plant input. The plant rhizosphere is a unique environment because of direct inputs of substrate through the sloughing off of root cells and root exudation (Lynch and Whipps, 1990). Because there is greater substrate for decomposition in the rhizosphere than bulk soil (Cheng et al., 2003), there will be more mineral N cycling in the rhizosphere than bulk soil. Therefore, we hypothesized that the rhizosphere would be a microenvironment where the effects of elevated CO2 and O3 would be most pronounced.
In this study, we investigated the effects of elevated CO2 and O3 on soil N availability and N-transforming microorganisms (nitrifiers and denitrifiers) across different soil environments (i.e., rhizosphere and bulk soil) during the soybean growing season at the SoyFACE (Soybean Free Air Concentration Enrichment) experiment.
2. Materials and methods
2.1. Site description and sampling
This study was conducted at the Soybean Free Air Concentration Enrichment (SoyFACE) facility, located in Champaign, IL, USA at the South Farms, University of Illinois at Urbana-Champaign; 40° 03′21.3″N 88° 12′3.4″W (http://soyface.illinois.edu). The 32-ha facility is located on farmland that is cultivated with an annual rotation of soybean (Glycine max (L.) Merr.) and corn (Zea mays L.) for more than 25 years. The soil at the site is a Drummer fine-silty, mixed, mesic Typic Endoaquoll, and is typical of wet, dark-colored “prairie soils” in northern and central Illinois.
The target concentration of elevated CO2 treatment was 550 μL L−1 while the elevated O3 treatment was +20% ambient, and these concentrations are based on the Intergovernmental Panel on Climate Change estimates for the year 2050. Fumigation with CO2 started in 2001 in the 16-ha half of the western side of the field. In 2002, the FACE treatments were resumed on the eastern side of the field, with 12 treatment rings established for the ambient, elevated CO2, and elevated O3 treatments. The combined elevated CO2 and O3 treatment was started in 2003 (Ort et al., 2006). Since then, the crops within the rings have been fumigated only during the growing seasons. Nitrogen fertilization has been used only for the cultivation of corn, while phosphorus and potassium were applied as needed based on soil test for both crops.
The study reported here was performed during the growing season of 2008 on the 16 ha – eastern side of the field, where soybeans were exposed to factorial treatments of elevated CO2 and O3 in a randomized complete block design (n = 4). In 2008, the eastern side of the field had been under elevated CO2 and elevated O3 treatments for four growing seasons, and under combined elevated CO2 and O3 treatment for three growing seasons.
Soil samples were collected at different phenological stages of soybean: the fourth trifoliolate leaf (V4), full pod (R4), and full maturity (R8) stages, following the phenological system of Ritchie et al. (1997). Soil samples (6 cm dia. × 15 cm depth) were collected from the rhizosphere and bulk soil. To collect rhizosphere soils, the soybean aboveground biomass was removed, and a soil core was inserted over the root crown to obtain the root system and associated soil. Bulk soil samples were collected from soils between soybean rows. Four soil cores were taken per ring, two cores from the rhizosphere and two from the bulk soils; and each soil type was pooled into a single sample. Soil samples were cooled to 4 °C and transported overnight on ice to the University of California at Davis, where all analyses were carried out.
2.2. Soil properties
Soil samples were homogenized through an 8-mm sieve and analyzed for their gravimetric soil moisture content. Approximately 100 g aliquots of soil were frozen for molecular analyses of the microorganisms and the remaining soil was air-dried. Rhizosphere soil and bulk soil were ground and analyzed for total N and C concentrations using a PDZ Europa 20–20 Stable Isotope Analyzer (Europa Scientific, Crewe, UK) at the University of California-Davis Stable Isotope Facility (http://stableisotopefacility.ucdavis.edu/). Since no carbonates were present in these soils, carbon associated with the samples was considered to be entirely soil organic carbon (SOC).
2.3. Determination of soil ammonium and nitrate
For soil and analyses, 10 g aliquots of soil were shaken for 1 h with 50 ml of 2 M KCl, and filtered through Whatman, No. 42, ashless, filters. The concentration of N in samples were determined colorimetrically, by using the Berthelot reaction for (Forster, 1995) and the vanadium(III) chloride reduction method for (Doane and Horwath, 2003).
2.4. Bacterial community abundance
DNA was extracted from 0.5 g aliquots of soil using the FastDNA Spin Kit for Soil (MP Biomedicals, Illkirch, France). Final DNA extracts were stored at −20 °C before analyzing the extracts using real-time (RT) PCR. The concentration of DNA in the extracts was determined using Qubit with Quant-iT dsDNA HS Assay Kits (Invitrogen, Carlsbad, CA, USA).
The abundance of total eubacterial DNA in rhizosphere and bulk soils was quantified by using a real-time TaqMan qPCR assay targeting the universal bacterial 16S rRNA gene (Suzuki et al., 2000). The quantification of the 16S rRNA gene was performed using 4 μl of template DNA, 10 μl of TaqMan Universal PCR Master Mix (Applied Biosystems, NJ, USA), 4 μl of H2O, 0.8 μl each of forward (BACT1369F: 5′-CGG TGA ATA CGT TCY CGG-3′; 800 nM) and reverse primers (PROK1492R: 5′-GGW TAC CTT GTT ACG ACTT-3′; 800 nM), and 0.4 μl of the probe (TM1389: 5′-CTT GTA CAC ACC GCC CGTC-3′; 200 nM) (Suzuki et al., 2000). The PCR conditions were as follows: 2 min at 50 °C, 10 min at 95 °C, and 40 cycles consisting of 15 s at 95 °C, and 1 min at 56 °C.
Real-time quantitative PCR of the ammonia monooxygenase gene amoA was used to quantify the abundance of nitrifier populations. RT-PCR was performed using 5 μl of template DNA, 1.2 μl of the A189 forward (5′-GNG ACT GGG ACT TCT GG-3′; 0.3 μM) and 3.6 μl of the amoA-2R′ reverse (5′-CCC CTC KGS AAA GCC TTC TTC-3′; 0.9 μM) primers. The PCR conditions were as follows: 15 s at 95 °C, and then 40 cycles consisting of 15 s at 95 °C, 30 s at 55 °C, and 31 s at 72 °C, followed by a dissociation stage of 15 s at 95 °C, 30 s at 60 °C, and 15 s at 95 °C (Okano et al., 2004).
The nitrous oxide reductase gene (nosZ) was used to quantify the abundance of denitrifier populations. The nosZ gene abundance has been shown to correlate well with the denitrifying activity (Ruyters et al., 2010) and other denitrifier genes, such the nitrite reductase gene nirS (Morales et al., 2010), and thus it can be used as a marker for denitrifying bacteria (Rich et al., 2003). RT-PCR reactions contained 5 μl of template DNA, 10 μl of 2× ABI Power SYBR Green PCR Master Mix, and 0.8 μl each of forward (nosZ2F: 5′-CGC RAC GGC AAS AAG GTS MSS GT-3′; 0.3 μM) and reverse (nosZ2R: 5′-CAK RTG CAK SGC RTG GCA GAA-3′, 0.3 μM) primers. The PCR conditions were as follows: an initial cycle of 95 °C for 10 min, followed by 6 cycles of 95 °C for 15 s, 65 °C for 30 s, 72 °C for 30 s, then 40 cycles of 95 °C for 15 s, 60 °C for 15 s, 72 °C for 30 s, and 83 °C for 30 s (data acquisition step). Reactions were completed with one cycle at 95 °C for 15 s and 60 °C for 30 s, to 95 °C for 15 s (Henry et al., 2006).
The amoA, nosZ, and 16S rDNA gene abundance was quantified with Applied Biosystems 7300 Real-Time PCR system (Foster City, CA, USA), using triplicate samples. Standard curves were generated for each gene by using serial dilutions of a standard containing a known number of the target sequences. DNA was extracted with a Plasmid Mini Kit (Qiagen) from three plasmids containing amoA (GenBank: Z97833), nosZ (GenBank: AF197468), and 16S rRNA gene fragments amplified from Nitrosomonas europaea (ATCC 19718), Bradyrhizobium japonicum (strain USDA 110), and Escherichia coli (strain K-12), respectively. The concentration of plasmid DNA was quantified spectrofluorometrically using the Quant-iT fluorescent dye method (Molecular Probes, Invitrogen, Paisley, UK). Standard curves were linear over six orders of magnitude and the detection limit was approximately 100 copies for the amoA and nosZ real-time qPCRs and 1000 copies for the 16S rRNA real-time qPCR (data not shown). The number of copies of amoA, nosZ, and 16S rRNA in soil extracts were calculated from the respective concentrations of extracted plasmid DNA.
2.5. Statistical analysis
The analysis of variance (ANOVA) for a randomized block design was performed for each variable. The analyses were performed using the mixed procedures in the SAS statistical package (SAS, 2002), and the blocks were considered as a random factor. Pairwise comparisons were performed using the Tukey–Kramer method, and significance was accepted at α = 0.05.
3. Results
3.1. General soil properties
The greatest soil moisture content was measured at V4, followed by R8, and R4 and soil moisture content was higher in the rhizosphere than in the bulk soil at all plant stages studied (data not shown). No changes in soil moisture were associated with elevated CO2 or O3 across the three plant phenological stages.
In contrast, total soil N was 11% lower under ambient O3 than under elevated O3 conditions and did not differ over the growing season (Tables 2 and 3). Similarly, SOC was significantly higher under elevated O3 compared to ambient O3. Elevated CO2 had no effect on soil N. SOC concentrations were not affected by elevated CO2, soil environment, or plant phenological stages.
Table 2.
Soil nitrogen (Total N) and soil organic carbon (SOC) concentration under elevated CO2 and O3 treatments in the rhizosphere and bulk soil during the 2008 growing season. Values in parentheses are standard errors (n = 12). Means followed by the same letter within a column are not statistically different (P > 0.05).
| Treatment | Total N (g N kg−1 soil) |
SOC (g C kg−1 soil) |
||
|---|---|---|---|---|
| Rhizosphere | Bulk | Rhizosphere | Bulk | |
| Ambient | 1.76 (0.09) B | 1.83 (0.07) B | 19.66 (1.14) B | 20.86 (1.00) B |
| Elevated CO2 | 1.82 (0.06) B | 1.83 (0.05) B | 20.45 (1.18) B | 20.48 (0.84) B |
| Elevated O3 | 2.07 (0.06) A | 2.06 (0.08) A | 24.25 (1.27) A | 23.91 (0.99) A |
| Elevated CO2 + O3 | 2.01 (0.05) A | 2.01 (0.06) A | 22.89 (0.91) A | 22.9 (0.82) A |
Table 3.
Total bacterial abundance (16S rRNA gene copies per gramof soil) under elevated CO2 and O3 treatments during the 2008 growing season at three sampling times (V4 = Fourth trifoliate leaf; R4=Full pod;R8 = Full maturity). Values are means with standard errorsinparentheses. Means withina column followed bythe same lowercase letterorwithin a row followed by the same uppercase letter are not significantly different (P > 0.05).
| 16S rRNA (copies g−1 soil) |
|||
|---|---|---|---|
| V4 | R4 | R8 | |
| Ambient | 1.7 × 108 (1.8 × 107) Bb | 2.2 × 108 (1.9 × 107) Aa | 2.4 × 108 (3.2 × 107) Ab |
| CO2 | 1.8 × 108 (1.8 × 107) Bb | 1.9 × 108 (1.9 × 107) Ba | 3.0 × 108 (3.3 × 107) Aab |
| O3 | 2.2 × 108 (1.6 × 107) Aa | 2.3 × 108 (1.1 × 107)Aa | 2.8 × 108 (1.8 × 107) Aab |
| CO2 + O3 | 2.3 × 108 (1.7 × 107) Ba | 2.2 × 108 (1.3 × 107) Ba | 3.2 × 108 (2.6 × 107) Aa |
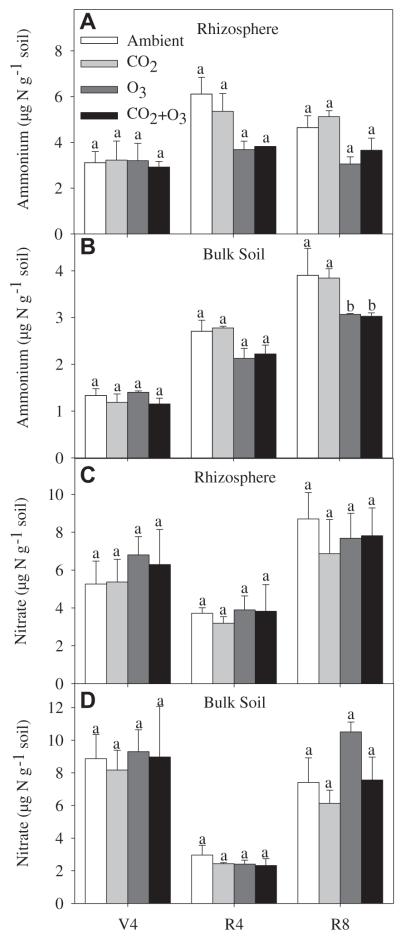
Soil concentration was significantly greater in the rhizosphere than in bulk soil. With the exception of the R8 stage in the rhizosphere, increased significantly over the growing season in both rhizosphere and bulk soil (Fig. 1). Elevated O3 significantly decreased soil concentrations by the end of the season, relative to that observed under ambient O3 (Fig. 1). In contrast, elevated CO2 did not alter soil content (Fig. 1). On average, the soil concentration decreased by 59% at R4 compared to the V4 and R8 plant stages. The soil content was similar at the V4 and R8 stages and ranged from 5.26 to 9.29 μg N g−1 soil (Fig. 1). Soil concentration did not differ between the rhizosphere and the bulk soil. In addition, the elevated CO2 or O3 treatments had no significant effect on the soil concentration.
Fig. 1.
Concentrations of ammonium () in the rhizosphere (a) and bulk soil (b) and concentrations of nitrate () in the rhizosphere (c) and bulk soil (d) under elevated CO2 and O3 treatments (V4 = Fourth trifoliate leaf; R4 = Full pod; R8 = Full maturity).
3.2. Quantification of the 16S rRNA, amoA, and nosZ gene abundance
The 16S rRNA gene abundance ranged from 1.54 × 108 to 3.77 × 108 copies g−1 soil (Table 3). Elevated CO2 increased bacterial populations at the R8 stage, but not V4 and R4 (Table 3). At the V4 stage, elevated O3 increased the soil bacterial abundance when compared to that under ambient O3. The 16S rRNA gene abundance under both elevated CO2 and O3 was similar to that under elevated O3.
The amoA gene abundance ranged from 6.5 × 106 to 1.5 × 107 copies g−1 soil (Table 4). Elevated CO2 and O3 had no significant effects on amoA abundance. Furthermore, amoA abundance was not different across plant developmental stages, nor in bulk and rhizosphere soil environments (Table 2).
Table 4.
Abundance of nitrifier (amoA) and denitrifier (nosZ) genes under elevated CO2 and O3 treatments in the rhizosphere and bulk soil during the 2008 growing season at three sampling times (V4 = Fourth trifoliate leaf; R4 = Full pod; R8 = Full maturity). Values are means with standard errors in parentheses (n = 4).
| Soil environment |
Treatment |
amoA (copies g−1 soil) |
nosZ (copies g−1 soil) |
||||
|---|---|---|---|---|---|---|---|
| V4 | R4 | R8 | V4 | R4 | R8 | ||
| Rhizosphere | Ambient | 1.2 × l07 (2.3 × 106) | 9.2 × 106 (3.8 × 105) | 7.6 × 106 (1.3 × 106) | 2.6 × 106 (6.2 × 105) | 3.7 × 106 (8.3 × 105) | 7.2 × 105 (1.4 × 105) |
| CO2 | 1.3 × 107 (1.1 × 106) | 6.5 × 106 (4.3 × 10s) | 9.5 × 106 (2.4 × 106) | 1.9 × 106 (6.3 × 105) | 1.6 × 106 (5.5 × 105) | 8.9 × 105 (1.1 × 105) | |
| O3 | 1.5 × 107 (2.0 × 106) | 1.1 × 107 (9.5 × 105) | 8.8 × 106 (9.4 × 105) | 4.2 × 106 (7.9 × 105) | 1.2 × 106 (8.3 × 104) | 1.3 × 106 (2.0 × 105) | |
| CO2 + O3 | 1.3 × 107 (1.5 × 106) | 7.6 × 106 (1.0 × 106) | 1.2 × 107 (2.5 × 106) | 2.2 × 106 (5.3 × 105) | 1.4 × 106 (1.9 × 105) | 1.2 × 106 (1.8 × 105) | |
| Bulk soil | Ambient | 1.2 × 107 (6.5 × 105) | 8.4 × 106 (1.2 × 106) | 5.1 × 106 (9.7 × 105) | 1.0 × 106 (2.3 × 105) | 1.2 × 106 (2.1 × 105) | 7.0 × 105 (2.2 × 105) |
| CO2 | 8.0 × 106 (6.5 × 105) | 1.4 × 107 (1.0 × 106) | 1.1 × 107 (1.3 × 106) | 2.9 × 105 (8.8 × 104) | 1.9 × 106 (5.8 × 105) | 9.1 × 105 (1.3 × 105) | |
| O3 | 1.0 × 107 (2.1 × 105) | 1.0 × 107 (1.3 × 106) | 6.7 × 106 (6.8 × 105) | 1.8 × 106 (5.3 × 105) | 2.5 × 106 (9.2 × 105) | 7.5 × 105 (1.1 × 105) | |
| CO2 + O3 | 1.1 × 107 (1.6 × 106) | 7.3 × 106 (7.6 × 105) | 8.0 × 106 (2.4 × 105) | 2.8 × 106 (7.3 × 105) | 2.3 × 106 (4.5 × 105) | 7.7 × 105 (1.0 × 105) | |
The abundance of the nosZ gene in soils ranged from 2.87 × 105 to 4.18 × 106 copies g−1 soil, and this was less than that found for amoA (Table 4). The nosZ gene was significantly more abundant in the rhizosphere than the bulk soil, and the rhizosphere effect was much more pronounced at the V4 stage of plant growth. Elevated O3 tended to increase the abundance of nosZ gene, and this was marginally significant. On the other hand, elevated CO2 had no impact on the abundance of nosZ (Table 4).
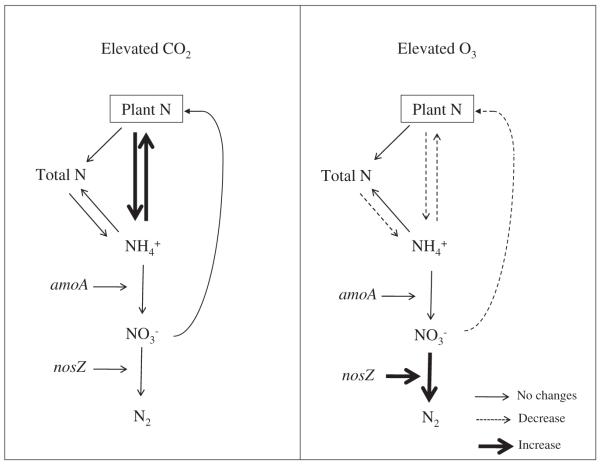
The flows of N between the plants and soil under elevated CO2 and O3 observed in this study are shown in the conceptual diagram in the Fig. 2.
Fig. 2.
Flows of N between the plants and soil under elevated CO2 and O3. Even though elevated CO2 increases plant biomass production and thus increases the plant demands of N, the mineral N released in the decomposition process is quickly taken up by the plants. In contrast, elevated O3 decreases plant biomass production and thus the demand of N by the plant. Since the input of organic material is reduced under elevated O3, the mineralization process is also decreased, which leads to an accumulation of total N. Lastly, possibly due to the availability of organic material under elevated O3, a higher abundance of the denitrifier gene was observed compared to ambient O3 plots.
4. Discussion
4.1. The effect of elevated CO2 and O3 on total N, SOC, and mineral N
Our results indicated that elevated CO2 had no effects, whereas elevated O3 increased total N and SOC in soil. One explanation for this finding is that decomposition processes were reduced under elevated O3 due to the lower amount of plant material input compared to the ambient and elevated CO2 plots, and this, in turn, may have reduced microbial activities (Singh and Gupta, 1977).
The concentrations of and at three plant phenological stages in the rhizosphere and bulk soil were quantified to better understand the influence of elevated atmospheric CO2 and O3 on plant- and microbial-available N. Since the deposition of plant-derived-material to soils increases during the growing season, we hypothesized that elevated CO2 increases the abundance of soil mineral N in the later stages and that these effects are more prominent in rhizosphere, due to higher root inputs, than bulk soil. Since elevated O3 has been observed to decrease the amount of plant residue added to soils in the SoyFACE experiment (Morgan et al., 2006), we expected the opposite effects under elevated O3 conditions with respect to interactions with plant phenological stages and soil environments. Surprisingly, no interactions were observed between the FACE treatments and plant phenological stage or soil environment (Table 1), which indicates that the effects of elevated CO2 and O3 were uniform across plant phenological stages and soil environments.
Table 1.
P-values for soil moisture (dθ), total N (TN), soil organic carbon (SOC), ammonium (), nitrate (), total bacteria (16S rRNA), nitrifying (amoA), and denitrifying bacteria (nosZ) by analysis of variance (ANOVA).
| Sourcea | dθ | TN | SOC | 16S rRNA | amoA | nosZ | ||
|---|---|---|---|---|---|---|---|---|
| CO2 | – | – | – | – | – | – | – | |
| O3 | – | 0.01 | 0.01 | 0.03 | – | 0.01 | – | 0.06 |
| Soil environment (SE) | 0.001 | – | – | <0.0001 | – | – | – | 0.01 |
| Plant phenological stage (PS) | <0.0001 | – | – | <0.0001 | <0.0001 | <0.0001 | – | 0.06 |
| PS × CO2 | – | – | – | – | – | 0.04 | – | – |
| PS × SE | <0.0001 | – | – | 0.01 | 0.04 | – | – | – |
(–) Not significant at P < 0.05.
CO2 × O3, SE × CO2, SE × O3, SE CO2 + Oxl, PS × O3, PS × CO2 × O3, PS × SE × CO2, PS × SE × O3, PS × SE × CO2 × O3 interactions wrer not significant for these variables.
The lack of observed interactions between the FACE treatments and plant phenological stage or soil environment might be due to simultaneously occurring, but counterbalancing, changes in N transformations and plant N uptake across the season and soil environments. For example, while there is an increase in plant material input under elevated CO2, compared to ambient CO2 conditions, there is also an increasing demand for N by plants and microorganisms across the growing season, and within the rhizosphere (Zak et al., 2000b). In contrast, such interactions may not have been observed under elevated O3 because plant-derived inputs for N mineralization are lower than in other treatments (Kanerva, 2006). At the same time, the demand for N by the plant is lower in the rhizosphere and across the growing season. Hence, the separation of soil environments across the growing season were likely not of sufficient sensitivity to be able to detect the effect of elevated CO2 and O3 on soil N dynamics.
With exception of the V4 stage, soil decreased in both rhizosphere and bulk soil under elevated compared to ambient O3 (Fig.1a and b), possibly explained by lower substrate inputs. Kanerva et al. (2006) also observed that elevated O3 decreased soil concentration in meadow soil, which was associated with a 34 and 40% reduction in aboveground and root biomass, respectively. Elevated O3 decreased shoot and root dry biomass by about 21% in the SoyFACE experiment (Morgan et al., 2003), and it is possible that the decrease in in our study was similarly due to reductions in residue inputs under elevated O3. Another possible reason for decrease in soil under elevated O3 may be related to the decrease in symbiotic N2 fixation by the soybean plants. Although the N concentration in soybean organs and tissues were not affected by elevated O3, N2 fixation has been shown to decrease due to the reduced photosynthate translocation to nodules (Pausch et al., 1996). With less N being supplied via symbiosis under elevated O3, soybean plants would use the soil mineral N available.
Since plant biomass production, N2 fixation, and microbial decomposition generally increase under elevated CO2 (Tarnawski and Aragno, 2006), it was expected that soil mineral N content would also increase. However, no significant differences in soil and in the elevated CO2 treatments were found relative to the control plots (Table 1). A lack of response in soil mineral N following an increase in CO2 has similarly been observed in other systems (Barnard et al., 2004; Kanerva et al., 2006; Niklaus et al., 1998). Specific mechanisms that may have counterbalanced the expected increase in N include increased plant N uptake (King et al., 2003) and increased microbial N immobilization (de Graaff et al., 2006a). Increased shortgrass biomass production under elevated CO2 conditions resulted in increases in plant N uptake in northeastern Colorado steppe ecosystem (King et al., 2003). Elevated CO2 also was reported to enhance soil N mineralization and consequently increase plant N uptake (Hungate et al., 1997). Greater N immobilization under elevated CO2 may also have no measurable effect on soil mineral N (Holmes et al., 2006; Mosier et al., 2002). Overall, however, a meta-analysis of 117 studies indicated that elevated CO2 increased gross N immobilization and microbial N content by 22% and 5.8% respectively (de Graaff et al., 2006a).
Plants often exhibit increased water use efficiency under elevated CO2, which reduces plant water loss, but might also increase water loss through the soil profile. Johnson et al. (2001) measured a reduction of soil N under elevated CO2 in a scrub-oak ecosystem and attributed this effect to increased leaching of mineral N. However, in our studies we did not observe higher soil moisture content under elevated CO2 (data not shown) and thus, increased leaching of mineral N was unlikely. Therefore, we attribute the lack of differences in soil mineral N content between the elevated and ambient CO2 plots to the enhanced plant N uptake and microbial immobilization. These processes can counterbalance the increased N input in the elevated CO2 plots to the stimulated output of N caused by elevated CO2, thereby we concluded that elevated CO2 conditions do not lead to an accumulation of mineral N at the SoyFACE.
We detected no apparent interactive effect between elevated CO2 and O3 on mineral N content. Soil and content under elevated CO2 + O3 were similar to values measured under elevated O3. This suggests that any amelioration of elevated CO2 on the inhibitory effect of O3 on plant photosynthesis (Fiscus et al., 1997) did not offset the O3 effects on the mineralization of organic N. Thus, elevated O3 may lead to changes in the chemical composition of plant material returned to the soil. For example, elevated O3 can affect leaf residue decomposition by decreasing nonstructural carbohydrate and increasing ash-free lignin concentrations, which, in turn, can reduce N mineralization when substrate quantity is not the key factor limiting mineral N release (Booker et al., 2005). The hypothesis that N mineralization is reduced is supported by higher concentrations of total soil N under elevated O3 than ambient O3 treatments (Table 2). For woody species, Holmes et al. (2006) observed that elevated O3 significantly decreased gross N mineralization under both elevated CO2 and O3. They concluded that these changes were caused by a modification of the CO2 effect by O3 on plant litter production, either by decreasing root turnover or chemical changes in the belowground litter input.
4.2. Responses of N-transforming microorganisms to elevated CO2 and O3
While elevated O3 did not affect the abundance of the amoA gene, it increased the abundance of nosZ in both rhizosphere and bulk soil. The latter response was likely driven by the higher SOC observed under elevated O3 providing a carbon source for the reaction (Table 4). Denitrifying microorganisms are dependent on organic C as their source of energy (Wallenstein et al., 2006), and thus the observed increase in the nosZ gene abundance, is consistent with the higher SOC observed under elevated O3.
We hypothesized that the higher plant residue inputs to the elevated CO2 soil would increase availability and, in turn, nitrifier populations, as well as favor the heterotrophic denitrifier community. Our measurements of amoA and nosZ gene copy numbers, however, suggest that elevated CO2 has little influence on abundances of either nitrifier and denitrifier populations (Table 4), although it is not appropriate to rule out that some group members may not be detected by the primer sets currently used for qPCR. The abundance of amoA gene in the soil is governed by factors that control availability in the soil, such as N mineralization, microbial N immobilization, and plant uptake (Forbes et al., 2009). In this study, soil content under elevated CO2 was similar to the amount found under ambient CO2 (Fig. 1), even though the input of plant substrate into the soil was greater under CO2 enrichment. This suggests that the was not available for the nitrifying bacteria, but rather may have been taken up by plants and heterotrophic microorganisms (Bowatte et al., 2008; Hungate et al., 1999). Similarly, Horz et al. (2004) investigated the response of soil bacteria to multi-factorial global change parameters and observed that the abundance of amoA decreased in response to elevated CO2. Elevated CO2 stimulated growth of heterotrophic microorganisms and autotrophic nitrifying microorganisms were poor competitors for common resources. Thus, Horz et al. (2004) postulated that the nitrifiers’ inability to effectively compete explained the decreases in amoA gene abundance under elevated CO2.
The abundance of denitrifying bacteria is controlled by soil O2, the availability of C substrates, and concentrations (Barnard et al., 2005). Thus, the lack of response of nosZ gene abundance may be expected from the lack of change in soil moisture and content under elevated CO2 conditions. Elevated CO2 failed to increase the abundance of nosZ genes and other genes involved in the denitrification process in the rhizosphere of Phaseolus vulgaris L. under two levels of N (Haase et al., 2008). Although elevated CO2 stimulates C deposition through root exudates, it apparently has only a small effect on the denitrifier community.
We found neither any interactive effect between elevated CO2 and O3 on populations of nitrifiers and denitrifiers, nor any interactions between the FACE treatments and plant phenological stage or soil environment on either microbial population (Table 4). The abundance of the nitrifier populations is driven, in part, by the amount of in the soil. Consequently, the lack of interaction between elevated CO2 and O3, and plant phenological stage or soil environment on the concentration of , is mirrored in the abundance of amoA genes. The abundance of denitrifiers is controlled by SOC, soil moisture and concentration in the soil (Wallenstein et al., 2006). No changes in the abundance of nosZ were expected because of the absence of any interactions between elevated CO2 and O3, and plant phenological stage or soil environment affecting these variables.
5. Conclusion
N transformations at the SoyFACE site were less impacted by elevated CO2 than elevated O3, and any differences were unaffected by the plant phenological stage or the presence of a plant rhizosphere. Although elevated CO2 increases plant biomass production, this increase had limited effects on belowground N processes. Also, though increases of tropospheric O3 can diminish plant-available N by decreasing plant inputs and mineralization, and by increasing denitrification, we observed an accumulation of total N. To explore further if elevated O3 limits N availability, research should focus on changes in specific components of plant residues (e.g., cellulose, lignin), to more carefully track decomposition patterns under elevated O3.
Acknowledgements
This research was funded by the National Science Foundation (grant # NSF-DEB 0543218). Additional support was provided by Award Number P42ES004699 from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Footnotes
Our findings indicate that although elevated CO2 increases plant biomass, N transformations were minimally affected. In contrast, elevated O3 decreased soil mineral N likely through a reduction in plant material input and increased denitrification as indicated by the greater abundance of the denitrifier gene nosZ.
References
- Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytologist. 2005;165:351–371. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Alcoz MM, Hons FM, Haby VA. Nitrogen fertilisation, timing effect on wheat production, nitrogen uptake efficiency, and residual soil nitrogen. Agronomy Journal. 1993;85:1198–1203. [Google Scholar]
- Andersen CP. Sourceesink balance and carbon allocation below ground in plants exposed to ozone. New Phytologist. 2003;157:213–228. doi: 10.1046/j.1469-8137.2003.00674.x. [DOI] [PubMed] [Google Scholar]
- Barnard R, Barthes L, Le Roux X, Harmens H, Raschi A, Soussana JF, Winkler B, Leadley PW. Atmospheric CO2 elevation has little effect on nitrifying and denitrifying enzyme activity in four European grasslands. Global Change Biology. 2004;10:488–497. [Google Scholar]
- Barnard R, Leadley PW, Lensi R, Barthes L. Plant, soil microbial and soil inorganic nitrogen responses to elevated CO2: a study in microcosms of Holcus lanatus. Acta Oecologica-International Journal of Ecology. 2005;27:171–178. [Google Scholar]
- Booker FL, Miller JE, Fiscus EL, Pursley WA, Stefanski LA. Comparative responses of container- versus ground-grown soybean to elevated carbon dioxide and ozone. Crop Science. 2005;45:883–895. [Google Scholar]
- Bowatte S, Carran RA, Newton PCD, Theobald P. Does atmospheric CO2 concentration influence soil nitrifying bacteria and their activity? Australian Journal of Soil Research. 2008;46:617–622. [Google Scholar]
- Cheng WX, Johnson DW, Fu SL. Rhizosphere effects on decomposition: controls of plant species, phenology, and fertilization. Soil Science Society of America Journal. 2003;67:1418–1427. [Google Scholar]
- de Graaff MA, van Groeningen KJ, Six J, Hungate BA, van Kessel C. Interactions between plant growth and nutrient dynamics under elevated CO2: a meta analysis. Global Change Biology. 2006a;12:1–15. [Google Scholar]
- de Graaff MA, Six J, Blim H, van Kessel C. Prolonged elevated atmo-spheric CO2 does not affect decomposition of plant material. Soil Biology and Biochemistry. 2006b;38:187–190. [Google Scholar]
- Dermody O, Long SP, DeLucia EH. How does elevated CO2 or ozone affect the leaf-area index of soybean when applied independently? New Phytologist. 2006;169:145–155. doi: 10.1111/j.1469-8137.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- Doane TA, Horwath WR. Spectrophotometric determination of nitrate with a single reagent. Analytical Letters. 2003;36:2713–2722. [Google Scholar]
- Fiscus EL, Reid CD, Miller JE, Heagle AS. Elevated CO2 reduced O3 flux and O3 - induced yield losses in soybeans: possible implications for elevated CO2 studies. Journal of Experimental Botany. 1997;48:307–313. [Google Scholar]
- Forbes MS, Broos K, Baldock JA, Gregg AL, Wakelin SA. Environmental and edaphic drivers of bacterial communities involved in soil N-cycling. Australian Journal of Soil Research. 2009;47:380–388. [Google Scholar]
- Forster JC. Soil nitrogen. In: Alef K, Nannipieri P, editors. Methods in Applied Soil Microbiology and Biochemistry. Academic Press; San Diego, CA: 1995. pp. 79–87. [Google Scholar]
- Haase J, Brandl R, Scheu S, Schadler M. Above- and belowground interactions are mediated by nutrient availability. Ecology. 2008;89:3072–3081. doi: 10.1890/07-1983.1. [DOI] [PubMed] [Google Scholar]
- Hallin S, Jones CM, Schloter M, Philippot L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. The ISME Journal. 2009;3:597–605. doi: 10.1038/ismej.2008.128. [DOI] [PubMed] [Google Scholar]
- Henry S, Bru D, Stres B, Hallet S, Philippot L. Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Applied and Environmental Microbiology. 2006;72:5181–5189. doi: 10.1128/AEM.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes WE, Zak DR, Pregitzer KS, King JS. Soil nitrogen transformations under Populus tremuloides, Betula papyrifera and Acer saccharum following 3 years exposure to elevated CO2 and O3. Global Change Biology. 2003;9:1743–1750. [Google Scholar]
- Holmes WE, Zak DR, Pregitzer KS, King JS. Elevated CO2 and O3 alter soil nitrogen transformations beneath trembling aspen, paper birch, and sugar maple. Ecosystems. 2006;9:1354–1363. [Google Scholar]
- Horz HP, Barbrook A, Field CB, Bohannan BJM. Ammonia-oxidizing bacteria respond to multifactorial global change. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15136–15141. doi: 10.1073/pnas.0406616101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungate BA, Chapin FS, Zhong H, Holland EA, Field CB. Stimulation of grassland nitrogen cycling under carbon dioxide enrichment. Oecologia. 1997;109:149–153. doi: 10.1007/s004420050069. [DOI] [PubMed] [Google Scholar]
- Hungate BA, Dijkstra P, Johnson DW, Hinkle CR, Drake BG. Elevated CO2 increases nitrogen fixation and decreases soil nitrogen mineralization in Florida scrub oak. Global Change Biology. 1999;5:781–789. [Google Scholar]
- Intergovernamental Panel on Climate Change (IPCC) Contribution of Working Group I to the Fourth Assessment Report of the Intergovernamental Panel on Climate Change The physical Sciences Basis. C. C; 2007.2007. [Google Scholar]
- Johnson DW, Hungate BA, Dijkstra P, Hymus G, Drake B. Effects of elevated carbon dioxide on soils in a Florida scrub oak ecosystem. Journal of Environmental Quality. 2001;30:501–507. doi: 10.2134/jeq2001.302501x. [DOI] [PubMed] [Google Scholar]
- Kanerva T. Ph.D. Thesis. Department of Biological and Environmental Sciences; University of Helsinki, Finland,Yliopistopaino, Helsinki: 2006. Below-ground processes in meadow soil under elevated ozone and carbon dioxide. [Google Scholar]
- Kanerva T, Palojarvi A, Ramo K, Ojanpera K, Esala M, Manninen S. A 3-year exposure to CO2 and O3 induced minor changes in soil N cycling in a meadow ecosystem. Plant and Soil. 2006;286:61–73. [Google Scholar]
- King JY, Milchunas DG, Mosier AR, Moore JC, Quirk MH, Morgan JA, Slusser JR. Initial impacts of altered UVB radiation on plant growth and decomposition in shortgrass steppe. In: Herman JR, Gao W, editors. International Soc. Optical Eng; Ultraviolet Ground- and Space-Based Measurements, Models, and Effects III. SPIE Proceedings, vol. 5156. Slusser; Bellingham, WA. 2003.pp. 384–395. [Google Scholar]
- Lynch JM, Whipps JM. Substrate flow in the rhizosphere. Plant and Soil. 1990;129:1–10. [Google Scholar]
- Luo YQ, Hui DF, Zhang DQ. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: a meta-analysis. Ecology. 2006;87:53–63. doi: 10.1890/04-1724. [DOI] [PubMed] [Google Scholar]
- Morales SE, Cosart T, Holben WE. Bacterial gene abundances as indicators of greenhouse gas emission in soils. The ISME Journal. 2010;4:799–808. doi: 10.1038/ismej.2010.8. [DOI] [PubMed] [Google Scholar]
- Morgan PB, Ainsworth EA, Long SP. How does elevated ozone impact soybean? A meta-analysis of photosynthesis, growth and yield. Plant, Cell and Environment. 2003;26:1317–1328. [Google Scholar]
- Morgan PB, Mies TA, Bollero GA, Nelson RL, Long SP. Season-long elevation of ozone concentration to projected 2050 levels under fully open-air conditions substantially decreases the growth and production of soybean. New Phytologist. 2006;170:333–343. doi: 10.1111/j.1469-8137.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- Mosier AR, Morgan JA, King JY, LeCain D, Milchunas DG. Soil–eatmosphere exchange of CH4, CO2, NOx, and N2O in the Colorado shortgrass steppe under elevated CO2. Plant and Soil. 2002;240:201–211. [Google Scholar]
- Niklaus PA, Leadley PW, Stocklin J, Korner C. Nutrient relations in calcareous grassland under elevated CO2. Oecologia. 1998;116:67–75. doi: 10.1007/s004420050564. [DOI] [PubMed] [Google Scholar]
- Okano Y, Hristova KR, Leutenegger CM, Jackson LE, Denison RF, Gebreyesus B, Lebauer D, Scow KM. Application of real-time PCR to study effects of ammonium on population size of ammonia-oxidizing bacteria in soil. Applied and Environmental Microbiology. 2004;70:1008–1016. doi: 10.1128/AEM.70.2.1008-1016.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Ainsworth EA, Aldea M, Allen DJ, Bernacchi CJ, Berenbaum MR, Bollero GA, Cornic G, Davey PA, Dermody O, Dohleman FG, Hamilton JG, Heaton EA, Leakey ADB, Mahoney J, Morgan PB, Nelson RL, O’Neill B, Rogers A, Zangerl AR, Zhu XG, DeLucia EH, Long SP. SoyFACE: the effects and interactions of elevated [CO2] and [O3] on soybean. In: Nosberger J, Long SP, Stitt GR, Hendrey GR, Blum H, editors. Managed Ecosystems and CO2: Case Studies, Processes and Perspectives. Springer; Berlin: 2006. pp. 71–85. [Google Scholar]
- Pausch RC, Mulchi CL, Lee EH, Meisinger JJ. Use of 13C and 15N isotopes to investigate O3 effects on C and N metabolism in soybeans. Part II. Nitrogen uptake, fixation, and partitioning. Agriculture, Ecosystems and Environment. 1996;60:61–69. [Google Scholar]
- Prather M, Ehhalt D, Dentener F, Derwent R, Dlugokencky E, Holland E, Isaksen I, Katima J, Kirchhoff V, Matson P, Midgley PM, Wang M. Atmospheric chemistry and greenhouse gases. In: Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linder PJ, Dai X, Maskell K, Johnson CA, editors. Climate Change 2001: The Scientific Basis; Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press, Cambridge, UK. 2001.pp. 239–287. [Google Scholar]
- Rich JJ, Heichen RS, Bottomley PJ, Cromack K, Jr., Myrold DD. Community composition and functioning of denitrifying bacteria from adjacent meadow and forest soils. Applied and Environmental Microbiology. 2003:5974–5982. doi: 10.1128/AEM.69.10.5974-5982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SW, Hanway JJ, Thompson HE, Benson GO. How a Soybean Plant Develops. Cooperative Extension Service; Iowa State University of Science and Technology: 1997. [Google Scholar]
- Ruyters S, Mertens J, T’Seyen I, Springael D, Smolders E. Dynamics of the nitrous oxide reducing community during adaptation to Zn stress in soil. Soil Biology & Biochemistry. 2010;42:1581–1587. [Google Scholar]
- SAS Institute . Statistical Analysis System, v.8.2. SAS Institute; Cary, NC: 2002. [Google Scholar]
- Singh JS, Gupta SR. Plant decomposition and soil respiration in terrestrial ecosystems. The Botanical Review. 1977;44:449–528. [Google Scholar]
- Suzuki MT, Taylor LT, DeLong EF. Quantitative analysis of small-subunit rRNA genes in mi′-nuclease assays. Applied and Environmental Microbiology. 2000;66:4605–4614. doi: 10.1128/aem.66.11.4605-4614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnawski S, Aragno M. The influence of elevated CO2 on diversity, activity and biogeochemical function of rhizosphere and soil bacterial communities. In: Nosberger J, Long SP, Stitt GR, Hendrey GR, Blum H, editors. Managed Ecosystems and CO2: Case Studies, Processes and Perspectives. Springer; Berlin: 2006. pp. 393–409. [Google Scholar]
- Tyree MT, Alexander JD. Plant water relations and the effects of elevated CO2 – a review and suggestions for future research. Vegetation. 1993;104:47–62. [Google Scholar]
- Vitousek PM, Howarth RW. Nitrogen limitation on land and in the sea e how can it occur. Biogeochemistry. 1991;13:87–115. [Google Scholar]
- Wallenstein MD, Myrold DD, Firestone M, Voytek M. Environmental controls on denitrifying communities and denitrification rates: insights from molecular methods. Ecological Applications. 2006;16:2143–2152. doi: 10.1890/1051-0761(2006)016[2143:ecodca]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Zak DR, Pregitzer KS, Curtis PS, Teeri JA, Fogel R, Randlett DL. Elevated atmospheric CO2 and feedback between carbon and nitrogen cycles. Plant and Soil. 1993;151:105–117. [Google Scholar]
- Zak DR, Pregitzer KS, King JS, Holmes WE. Elevated atmospheric CO2, fine roots and the response of soil microorganisms: a review and hypothesis. New Phytologist. 2000a;147:201–222. [Google Scholar]
- Zak DR, Pregitzer KS, Curtis PS, Holmes WE. Atmospheric CO2 and the composition and function of soil microbial communities. Ecological Applications. 2000b [Google Scholar]