Abstract
Inflammation results in heightened mitochondrial ceramide levels, which cause electron transport chain dysfunction, elevates reactive oxygen species, and increases apoptosis. As mitochondria in aged hearts also display many of these characteristics, we hypothesized that mitochondrial decay stems partly from an age-related ceramidosis that heretofore has not been recognized for the heart. Intact mitochondria or their purified inner membranes (IMM) were isolated from young (4-6 mo) and old (26-28 mo) rats and analyzed for ceramides by LC-MS/MS. Results showed that ceramide levels increased by 32% with age and three ceramide isoforms, found primarily in the IMM (e.g. C16-, C18-, and C24:1-ceramide), caused this increase. The ceramidosis may stem from enhanced hydrolysis of sphingomyelin, as neutral sphingomyelinase (nSMase) activity doubled with age but with no attendant change in ceramidase activity. Because (R)-α-lipoic acid (LA) improves many parameters of cardiac mitochondrial decay in aging and lowers ceramide levels in vascular endothelial cells, we hypothesized that LA may limit cardiac ceramidosis and thereby improve mitochondrial function. Feeding LA [0.2% wt/wt] to old rats for two weeks prior to mitochondrial isolation reversed the age-associated decline in glutathione levels and concomitantly improved Complex IV activity. This improvement was associated with lower nSMase activity and a remediation in mitochondrial ceramide levels. In summary, LA treatment lowers ceramide levels to that seen in young rat heart mitochondria and restores Complex IV activity which otherwise declines with age.
Supplementary key words: electron transport, Complex IV, sphingomyelinase, LC-mass spectrometry
1. Introduction
Mitochondria from aged tissue undergo a systematic decline in overall function, which manifests in the heart as an increased rate of reactive oxygen species (ROS) formation, concomitant oxidative damage, and impaired electron transport [1-8]. All of these factors limit the ability of mitochondria to meet cellular energy needs. Interestingly, these mitochondrial traits of aging are also evident, albeit more severely, in inflammatory pathologies [9-11]. As it is recognized that the aging heart is subjected to a low-grade chronic inflammation, it is reasonable to argue that inflammatory bio-factors may be involved in both the initiation and progression of mitochondrial decay. One such bio-factor that appears to be a hallmark of pro-inflammatory conditions is ceramide, a pro-apoptotic and growth arrest sphingolipid [12-20], which increases ROS formation, oxidative stress, and altered energy metabolism upon its accumulation in membranes [21,22].
Generally, acute inflammatory stimuli generate ceramide at the plasma membrane or endoplasmic reticulum by sphingomyelin hydrolysis or de novo synthesis, respectively [23-29]. Recent evidence shows that mitochondria may also be an important site of sphingolipid action [22,30-33]. Our laboratory recently showed that cardiac mitochondria normally contain a variety of sphingolipids, including sphingomyelin and ceramide [34]. Mitochondria from other organs also contain ceramide as well as neutral sphingomyelinase (nSMase), which hydrolyzes sphingomyelin to ceramide [35-37]. This suggests that mitochondria have the means to alter ceramide levels in response to pro-inflammatory stimuli. Moreover, in vitro experiments suggest that even small elevations of mitochondrial ceramide is able to adversely affect electron transport chain (ETC) activity, heighten ROS appearance, and also initiate mitochondrial-mediated apoptosis [21,22,31-33]. Thus, age-associated inflammation of the heart and mitochondrial decay may be connected via ceramidosis (i.e., the accumulation of ceramide). If so, this would provide a novel target for therapies to improve cardiac mitochondrial function and bioenergetics, which otherwise decline with age.
Despite this potential association, the role that ceramide plays in age-related mitochondrial decay has not been studied. Because many of the phenotypes of mitochondrial dysfunction can be plausibly linked to ceramidosis, the goal of the current study was to determine ceramide levels in interfibrillary mitochondria isolated from young and old rat hearts. Moreover, as mitochondria are double-membraned organelles, we further pursued the hypothesis that ceramide accumulation would be evident in the inner mitochondrial membrane (IMM) and adversely affect ETC activity. Lastly, if mitochondrial ceramides were indeed found to become elevated with age, a contingent goal was to determine whether anti-inflammatory agents could remediate any ceramide accumulation, thereby ameliorating the mitochondrial aging phenotype.
With regard to this latter contingent goal, our laboratory and others showed that the dithiol compound, (R)-α-lipoic acid (LA) may act as a potent anti-inflammatory and anti-oxidant agent at pharmacological doses [38-42]. Moreover, we recently reported that when old rats were treated with LA, age-associated increases in nSMase activity were limited and ceramide imbalance in aortic endothelia was remediated [43]. We have also previously shown that LA lowers indices of mitochondrial dysfunction [43-45], thus providing a rationale that LA may reverse at least certain aspects of mitochondrial decay by opposing ceramidosis.
2. Materials and Methods
2.1 Chemicals and antibodies
Digitonin, genistein, Subtilisin A (type VIII), Triton X-100, and Tween 20 were from Sigma-Aldrich (St. Louis, MO). Bovine serum albumin (fraction V, fatty acid free) was obtained from EMD Biosciences (La Jolla, CA). Purified ceramide standards were purchased from Avanti Polar Lipids (Alabaster, AL). NBD-sphingomyelin and NBD-ceramide were purchased from Life Technologies (Carlsbad, CA). Rabbit polyclonal antibody to the voltage-dependent anion channel protein (VDAC) and mouse monoclonal antibody to protein disulfide isomerase (PDI) were purchased from Abcam, Inc. (Cambridge, MA). All other compounds were reagent grade or of the highest purity obtainable.
2.2 Ethical treatment of vertebrate animals
Young (4-6 mo) and old (26-28 mo) Fischer 344 male rats were obtained from the National Institute on Aging animal colonies. Animals were housed in approved facilities in Weniger Hall, Oregon State University, and maintained by the Department of Laboratory Animal Resources and Care. All animal procedures were performed in accordance with the Oregon State University guidelines for animal experimentation.
2.3 Lipoic acid supplementation
Rats were fed an AIN-93M diet (Dyets Inc., Bethlehem, PA) ± 0.2% (w/w) LA (MAK Wood Inc., Grafton, WI) for two weeks prior to sacrifice. Because a two-week LA-treatment results in a mild hypophagia, animals were pair-fed. LA-treated animals were fed ad libitum and food consumption was measured every 24 hours. Rats on the unsupplemented diet were given the same amount of food as the supplemented ones had consumed the previous day. By this staggered protocol of pair-feeding, the possibility of a caloric intake difference affecting the data was eliminated. Although food consumption decreased over the course of the two-week treatment (18.75 g and 23.69 g consumed on the first day of treatment decreased to 13.18 and 16.23 g on day thirteen for young and old rats, respectively), all animals maintained a consistent body weight.
Animals were sacrificed between 8:00 AM and 12:00 PM. Rats were first anesthetized by diethyl ether inhalation, and heparin [0.2% (w/v); 1.0 ml/kg b.w.] was injected into the iliac artery to prevent blood clotting. The animal was then sacrificed by cutting through the diaphragm and exposing the heart. The heart was perfused with ice-cold phosphate buffered saline, pH 7.4, immediately excised, and placed in ice-cold buffer for a few minutes until mitochondrial isolation.
2.4 Mitochondrial isolation
Cardiac mitochondria were isolated using differential centrifugation as described by Palmer et al. [46] with modifications as in Monette et al. [34]. This procedure resulted in an enriched interfibrillary mitochondrial fraction. All steps of the isolation were performed on ice or at 4°C. Protein values were determined using the BCA protein assay kit (Thermo Scientific; Rockford, IL).
2.5 Inner mitochondrial membrane (IMM) isolation
The outer mitochondrial membrane (OMM) was selectively removed using digitonin [47]. Six mg/ml digitonin at 37°C in isotonic buffer (225 mM mannitol, 75 mM sucrose, 10 mM KCl, 10 mM tris-HCl, 5 mM KH2PO4, pH 7.2) was optimal for removing the OMM. This procedure resulted in a highly purified IMM fraction with less than 2% contamination from OMM as determined by immunoblotting for VDAC. The IMM could not be further purified by Percoll density centrifugation as yields were too low to allow for LC-MS/MS analysis.
2.6 Mitochondrial Complex IV activity
Cytochrome c oxidase (Complex IV) activity was measured using a commercially available kit from Sigma-Aldrich that follows the oxidation of cytochrome c.
2.7 Activity assay for neutral sphingomyelinase and ceramidase
Fluorescently-labeled sphingomyelin and ceramide (NBD-sphingomyelin and NBD-ceramide, respectively) were used as substrates to determine the activities of ceramide metabolizing enzymes by the method of Nikolova-Karakashian [48].
2.8 Measurement of glutathione (GSH)
GSH was conjugated to dansyl chloride and measured by using HPLC and fluorescence detection as described by Dixon et al. [49].
2.9 Lipid extraction
All samples were prepared as in Merrill et al. [50] except that samples were not saponified in KOH.
2.10 LC-tandem mass spectrometry
Lipids were separated by HPLC using a Supelco Discovery column (2 mm × 50 mm; Sigma-Aldrich). The flow rate was set at 300 μl per minute. Mobile phase A contained methanol:water (60:40) while mobile phase B was composed of methanol:chloroform (60:40). Both solvents contained 0.2% (v/v) formic acid and 10 mM ammonium acetate. The pump schedule was as follows: the column was pre-equilibrated at 100% mobile phase A followed by sample injection (5 μl); 100% mobile phase A was maintained for one minute, followed by a linear increase to 40% mobile phase B over a 7 minute period; followed by a linear increase to 70% mobile phase B over the next 6 minutes; 70% mobile phase B was maintained for the remainder (6 minutes) of the 20 minute run.
Analytes were detected on a triple-quadrupole mass spectrometer operated in positive mode (Applied Biosystems/MDS Sciex, API 3000) using multiple reaction monitoring, which selectively detects fragment ions from the collision-induced dissociation of the parent molecular ion. For a list of molecular ion transitions, please see Monette et al. [34]. Quantitation was based on comparison to synthetic sphingolipid standards.
2.11 Statistics
Data are presented as means ± SEM. Samples were assessed for statistical significance using a one-way ANOVA test. Multiple comparisons were made using a Tukey's post hoc test or the Student's t-test. A p value ≤ 0.05 was considered statistically significant.
3. Results
3.1 Profile of cardiac mitochondrial sphingolipids
In keeping with our previous work [34], both intact cardiac mitochondria as well as purified IMM contained six ceramide isotypes with N-acyl-chain lengths varying from 16- to 24-carbon units (Figure 1). The ceramides were predominantly saturated, with only one species, C24:1-ceramide, containing an unsaturated N-acyl side-chain. C24-ceramide was the predominant isoform found in cardiac mitochondria and comprised 38% of the total ceramide pool. Quantifying ceramides in the IMM showed that C16-, C18-, and C24:1-ceramide were in nearly equal concentrations as in whole mitochondria. However, the IMM contained only 35 to 50% of C20-, C22-, and C24-ceramide versus intact mitochondria, suggesting that these particular ceramides are enriched in the OMM fraction. However, further attempts to measure relative levels of ceramide isotypes in the OMM were not successful because of extra-mitochondrial membrane contamination. Thus, cardiac mitochondria contain a variety of ceramide isoforms, and it appears that IMM ceramides are particularly enriched in C16-, C18-, and C24:1-ceramide.
Figure 1. Asymmetric distribution of cardiac mitochondrial ceramides.
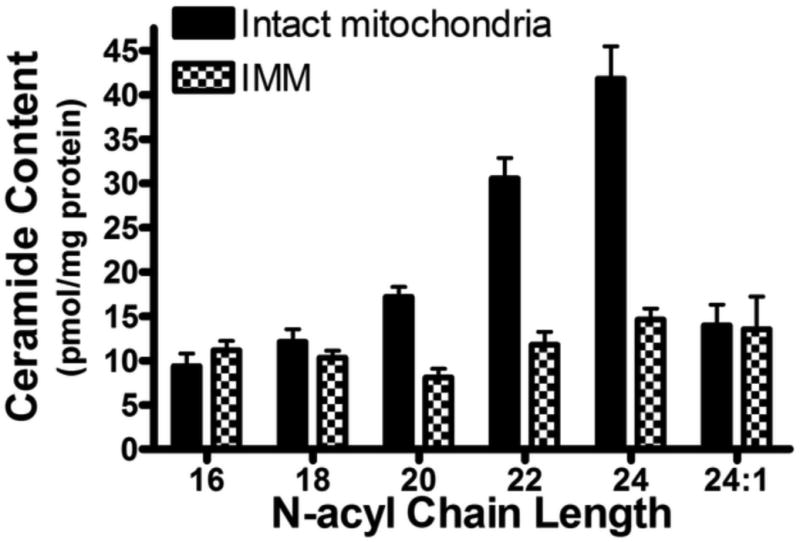
Ceramide content of purified intact mitochondria and inner mitochondrial membrane (IMM) as quantified by LC-MS/MS. Both fractions contain six ceramide isoforms ranging from 16- to 24- carbon units in length, with C24-ceramide being the predominant species in intact mitochondria. C16-, C18-, and C24:1- ceramide are found in near-equivalent quantities in the IMM as compared to intact mitochondria, whereas C20-, C22, and C24-ceramide are present in much lower quantities. This suggests that these latter ceramides are primarily found in the outer mitochondrial membrane. Data represent the means ± SEM, n = 4.
3.2 Age-related mitochondrial ceramide accumulation
On an age basis, total mitochondrial ceramide increased by 32% (Figure 2). Analysis of individual isoforms showed that the age-related elevation in ceramide stemmed from increases in all ceramide species (Table I). For analysis of significance of ceramide levels, the Student's t-test was employed in place of a one-way ANOVA test because of small sample size and unequal variances in the LA groups versus non-treated animals. The largest elevations were those of C16-, C18-, and C24:1-ceramide, which increased by ≥ 70% when compared to young controls. Despite the relatively large apparent accumulation in C24:1-ceramide, statistical significance was not reached because of high variability. There was a trend for C20-, C22-, and C24-ceramide to increase modestly (20% or less), but once again changes in these particular ceramide isoforms did not reach statistical significance (Table I). It is interesting to note that the ceramide species that are found in greater abundance in the IMM (C16-, C18-, and C24:1-ceramide, see section 3.1) are the ones that increased the most with age. This suggests that the age-related ceramide accumulation primarily occurs in the IMM.
Figure 2. LA treatment decreases mitochondrial ceramides.
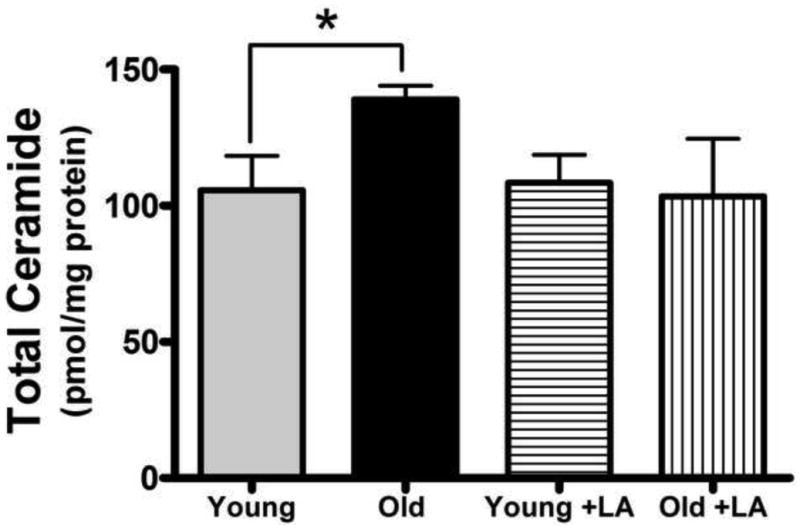
Young and old rats were fed (R)-α-lipoic acid (LA; 0.2% [w/w]) or a control diet for two weeks. Mitochondria were isolated, lipids extracted and analyzed by LC-MS/MS. Total ceramide was increased in cardiac mitochondria from aged rats; LA restored ceramides to levels seen in young animals but resulted in no alteration of ceramide levels in young rats. Data represent the means ± SEM, n = 4; an asterisk (*) denotes a significant difference by Student's t-test between old and young control animals, p < 0.03.
Table I. Cardiac mitochondrial ceramide levels with and without lipoic acid supplementation.
| Ceramide Species | Young Control | Old Control | Young +LA | Old +LA | ||||
|---|---|---|---|---|---|---|---|---|
| pmol/mg protein | pmol/mg protein | Δ% from Young Control | pmol/mg protein | Δ% from Young Control | pmol/mg protein | Δ% from Young Control | Δ% from Old Control | |
| C16-ceramide | 7.4 ± 0.6 | 12.7 ± 1.9* | 72.3 | 8.0 ± 0.6 | 8.2 | 8.7 ±2.1 | 18.3 | −31.3 |
| C18-ceramide | 7.8 ± 1.3 | 13.5 ± 1.2* | 73.4 | 8.2 ± 1.0 | 4.7 | 8.7 ± 1.5# | 11.4 | −35.7 |
| C20-ceramide | 12.5 ± 1.5 | 14.8 ± 1.1 | 18.1 | 13.2 ± 2.2 | 5.8 | 10.3 ± 1.9 | −17.5 | −30.1 |
| C22-ceramide | 27.7 ± 2.6 | 30.0 ± 0.9 | 8.0 | 31.7 ± 2.7 | 14.2 | 25.6 ±6.1 | −7.6 | −14.4 |
| C24-ceramide | 40.6 ± 6.4 | 48.8 ± 5.6 | 20.0 | 38.2 ± 3.1 | −6.0 | 35.9 ± 7.4 | −11.6 | −26.4 |
| C24:1-ceramide | 10.9 ± 1.8 | 19.4 ± 4.2 | 77.7 | 9.3 ± 1.4 | −15.1 | 14.2 ± 2.5 | 30.2 | −26.7 |
All data are represented as means ± SEM, n = 4.
p < 0.05 vs. young control
p < 0.05 vs. old control
+LA, denotes the lipoic acid supplemented groups
3.3 Lipoic acid supplementation reverses age-related mitochondrial ceramide accumulation
In young rats, LA treatment yielded no apparent changes in overall levels of mitochondrial ceramides (Figure 2). Furthermore, only modest changes in ceramide isoforms were noted when comparing young LA-treated animals to the age-matched controls (Table I). Specifically, C22-ceramide increased by 14.2%, while C24:1-ceramide decreased by 15%. All other species were altered by less than 10%. Overall, this suggests that treatment with LA had minimal effect on mitochondrial ceramides from young animals, indicating that LA does not modulate ceramide metabolism directly.
For old rats, however, LA lowered general cardiac ceramide levels such that they were no longer different than that seen in hearts from young animals (Figure 2). When specific ceramide isoforms were examined, we observed that LA treatment caused a significant decrease in C18-ceramide (p < 0.05) when compared to old control animals (Table I). All other species, though not reaching statistical significance, showed a trend for a decrease of approximately 30%, with the exception of C22-ceramide (Table I). The near uniform decrease evident in all species of ceramide found in LA-treated old animals suggests that the mechanism of LA-dependent mitochondrial ceramide reduction occurs, not by lowering specific ceramide species, but by decreasing ceramides in general.
3.4 Mitochondrial ceramide accumulation in vitro leads to electron transport inhibition
Because the age-related ceramide accumulation appears to occur in the IMM and previous reports show that short chain ceramides (e.g. C2- and C6-ceramide) inhibit ETC activity [21,22], we hypothesized that there was an association between the age-dependent decline in ETC activity and ceramide accumulation. Using Complex IV activity as a surrogate for overall flux of electrons through the ETC, we found its activity declined by 28% (p < 0.05) on an age basis (Figure 3), which is in keeping with previous literature reports [3,51]. As this result does not prove that ceramide accumulation is responsible for ETC inhibition, IMM were incubated with bacterial sphingomyelinase (bSMase) to acutely elevate ceramide levels so that a cause-and-effect relationship between ETC inhibition and ceramide might be discerned. Incubation of bSMase with mitochondria caused ceramide levels to increase by 13.8-fold versus controls (Supplemental Figure 1A). Elevation of ceramide levels via bSMase resulted in a marked 62% loss in Complex IV activity versus controls (Supplemental Figure 1B). Thus, increases in IMM ceramides correlate with a decline in ETC function, thereby suggesting that the age-related accumulation of IMM ceramides may be linked to the loss of Complex IV activity evident in aging heart mitochondria.
Figure 3. LA treatment restores Complex IV activity in cardiac mitochondria from aged animals.
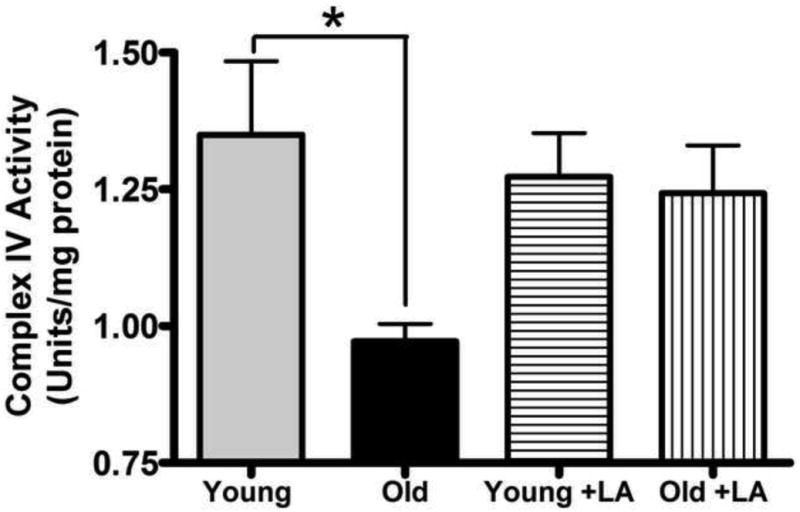
Isolated cardiac mitochondria from LA-supplemented or non-supplemented rats were assayed for Complex IV activity. Enzymatic activity declined with age and was restored by LA to the levels seen in young animals. Data represent the means ± SEM, n = 4; an asterisk (*) denotes a significant difference from old controls, p ≤ 0.05.
3.5 Lipoic acid treatment reverses age-related deficiency in Complex IV activity
Mitochondria from young LA-supplemented rats exhibited no treatment-related changes in Complex IV activity when compared to mitochondria from non-supplemented animals (Figure 3). This indicates that LA does not directly modify cytochrome oxidase per se. However, for old rats, LA reversed the loss of Complex IV function where its activity was no longer different from young animals (Figure 3). We therefore conclude that LA modulates Complex IV activity only in mitochondria from aged tissue where elevated IMM ceramides are evident.
3.6 Lipoic acid treatment restores proper neutral sphingomyelinase activity in mitochondria from old animals
While there is a positive association between LA-induced reversal of ceramide accumulation and improved ETC activity, these results do not provide a potential mechanism by which LA causes these remediative effects. As pro-inflammatory stimuli induce ceramidosis by activating sphingomyelinases, we hypothesized that LA may at least partially work through modulating nSMase activity to limit age-related increases in mitochondrial ceramides. Mitochondrial nSMase activity significantly increased by 103% with age (p < 0.05) (Figure 4); however, mitochondria from LA-supplemented old rats displayed no age-related elevations in nSMase activity. In keeping with its action on ceramide levels, LA did not alter nSMase activity in young rats.
Figure 4. LA restores neutral sphingomyelinase (nSMase) activity in mitochondria from old animals to youthful levels.
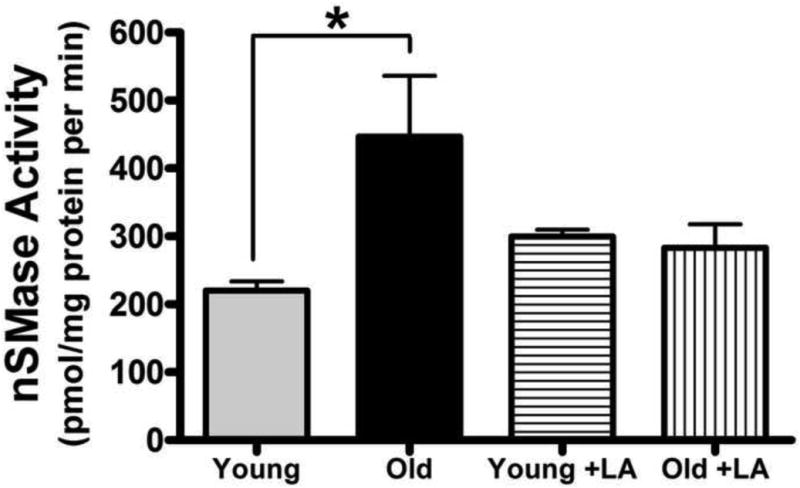
Cardiac mitochondria from young and old rats fed LA or the control diet for two weeks were assayed for nSMase activity. nSMase activity significantly increased with age and was restored to youthful levels by LA treatment. Data represent the means ± SEM, n = 4; an asterisk (*) denotes a significant difference, p < 0.01.
Because mitochondria reportedly also contain ceramidases, which catabolize ceramide to sphingomyelin [52], further experiments were performed to determine whether an age-related loss of ceramidase activity might also contribute to the mechanism by which LA reverses age-dependent mitochondrial ceramidosis. Even though cardiac mitochondrial ceramidase was detectable, no age-associated change in its activity was noted (data not shown). Furthermore, LA supplementation had no effect on ceramidase activity in either young or old animals. This indicates that aging causes an imbalance between elevated nSMase-induced ceramide production and ceramidase-mediated catabolism, which could contribute to the ceramide accumulation observed in aging rat heart mitochondria. Combined, these results suggest that pharmacological doses of LA reverse ceramide accumulation in old rat heart mitochondria, potentially through limiting elevations in nSMase activity.
3.7 Lipoic acid treatment partially restores the deficit in cardiac mitochondrial glutathione levels evident with age
Because previous reports have shown nSMase activity is inversely proportional to GSH status [27,53,54] and GSH levels decline markedly in the aging heart, we hypothesized that the LA-mediated improvement in mitochondrial ceramide status was through its means for restoring mitochondrial GSH levels [2,20]. As anticipated, LA-treatment did not alter GSH levels in young versus controls. However, LA reversed the 43% age-associated decline in mitochondrial GSH levels, such that the loss was no longer statistically different than in young untreated rats (Figure 5). These results indicate that the LA-mediated reduction in mitochondrial ceramidosis may ultimately stem from its means of controlling nSMase activity by maintenance of mitochondrial GSH.
Figure 5. LA markedly increases mitochondrial glutathione levels that otherwise decrease with age.
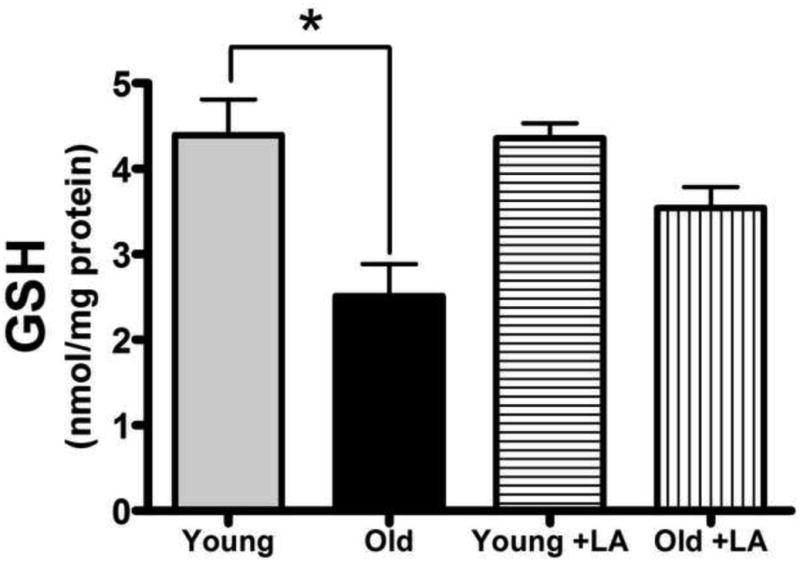
GSH levels were monitored in mitochondria from young and old rats fed LA or the control diet. GSH content significantly decreased with age and was restored to youthful levels by LA treatment. Data represent the means ± SEM, young, n = 3; old, n = 4; an asterisk (*) denotes a significant difference, p ≤ 0.05.
4. Discussion
To our knowledge, this is the first study showing that cardiac mitochondrial ceramides increase with age. Even though the accumulation was seemingly modest, nevertheless, it may be sufficient to adversely affect mitochondrial function. Tissue analysis following ischemia/reperfusion injury [55], myocardial infarct [56,57], Type II diabetes [58], as well as in vitro experiments where isolated mitochondria were treated with ceramide-laden liposomes [21,22], reinforce the view that perturbing normal mitochondrial sphingolipid status, even slightly above the norm, significantly alters mitochondrial function. For example, Yi et al. showed that when rat mesangial cells were incubated with homocysteine, a common marker for cardiovascular disease, there was a 47% increase in ceramides, which also increased ROS formation [59]. Also, Straczkowski et al. reported that men at risk for diabetes have a 50 to 200% increase in type II muscle ceramide levels [15]. Even though the age-related mitochondrial ceramide accumulation reported here is not as elevated as that found in acute pathologies, this ceramidosis may be sufficient to adversely affect mitochondrial function. Thus, we contend that increased mitochondrial ceramide should be recognized as one of the underlying factors leading to mitochondrial dysfunction with age.
A key result of the present study was the discovery of the asymmetric nature of the evident ceramidosis. C16-, C18-, and C24:1-ceramide were elevated the highest with age (Table 1); moreover, most of this accumulation occurred in the IMM. These intriguing results may be highly significant as there is growing evidence suggesting that both the acyl chain length and the degree of its unsaturation are important for specific ceramide action in cells. For example, Senkal et al. showed that squamous cell carcinomas were specifically killed by an increase in C18-ceramide, but not by orthologs containing even one site of unsaturation [60]. C16- and C18-ceramide appear to be pro-apoptogenic ceramides and commonly increase during pathological conditions [60-62]. In this regard, our current evidence supports the concept that specific ceramide species may be important for apoptotic signaling and inflammation. Indeed, the finding that three isoforms are mainly responsible for mitochondrial ceramidosis is significant as these isoforms could not only promote decline in ETC function as we have observed, but also may play a role in increasing ROS and/or promoting apoptosis, and resulting myocyte loss. We are currently conducting research aimed at understanding the roles played by individual ceramide species in mitochondria.
Another highlight of this report is the identification of LA as an agent to restore both mitochondrial ceramide levels and Complex IV activity to that seen in mitochondria from young rats. Although the pharmacological mechanism by which LA reverses the evident ceramidosis and ETC dysfunction solely in old rats is not fully understood, our data suggest that LA works by limiting age-dependent increases in mitochondrial nSMase activity. This most likely occurs through LA-dependent restoration of GSH levels, as there is an inverse correlation between nSMase activity and GSH, where low GSH status activates the enzyme by increasing its Vmax [53,54]. We previously showed that the GSH redox status of the myocardium and cardiac interfibrillary mitochondria is altered with age, and that LA treatment corrects these changes [63-67]. This is consistent with our current results showing that feeding LA markedly improved the mitochondrial GSH status in old rat hearts, strengthening the concept that age-associated decline in mitochondrial GSH may be responsible for the elevated nSMase activity.
Finally, our results clearly showed that LA did not affect mitochondrial ceramide levels in young rats, but merely restored ceramide values in aged animals to the norm. Thus, its general use as a prophylactic to prevent conditions that may lead to mitochondrial ceramidosis would appear to have few adverse consequences in young healthy subjects. This is in keeping with results from human clinical trials where the use of LA, even at relatively high pharmacological doses (1800 mg/day), resulted in only few side-effects, the predominant one being gastric upset [68]. On a cellular level, this also suggests that LA would not inappropriately cause loss in membrane ceramides. Maintenance of ceramides at normal levels is vital to preserve their role in membrane fluidity, and as a modulator of many kinases and phosphatases [19]. Thus, LA may be an appropriate adjunct to limit age-related mitochondrial ceramidosis and the adverse cardiac effects that its accumulation causes.
5. Conclusions
In conclusion, this paper shows that the age-related decay in cardiac mitochondria may stem from an accumulation of ceramide, a pro-apoptotic signaling lipid shown to induce mitochondrial dysfunction. Furthermore, we show that a two-week feeding of LA is able to restore ceramide levels to that seen in cardiac mitochondria from young animals. These results thus highlight the role of LA as an age-essential micronutrient, which may be an effective adjunct in preventing or reversing mitochondrial-associated pathologies.
Supplementary Material
Isolated mitochondria were incubated with a bacterially-derived sphingomyelinase, bSMase, followed by lipid extraction and ceramide quantification by LC-MS/MS. (A) Total ceramide levels were markedly increased by a 20-min bSMase incubation. (B) A time-course of Complex IV activity following incubation with bSMase. Results show a rapid decline in Complex IV activity. Data represent the means ± SEM, n = 3.
Acknowledgments
The authors would like to thank Alan W. Taylor of the Oxidative and Nitrosative Stress Core Lab, Linus Pauling Institute, for expert analysis of mitochondrial ceramides. We would also like to thank Alix Gitelman, Ph.D., for help with the statistical analysis. The research was funded by the National Institute on Aging, grant number 2R01AG017141. We also acknowledge the facilities service cores of the NIEHS (NIEHS ES00240).
Abbreviations
- LA
(R)-alpha-lipoic acid
- GSH
glutathione
- LC-MS/MS
LC-tandem mass spectrometry
- ETC
electron transport chain
- nSMase
neutral sphingomyelinase
- bSMase
bacterial sphingomyelinase
- ER
endoplasmic reticulum
- OMM
outer mitochondrial membrane
- ROS
reactive oxygen species
- IMM
inner mitochondrial membrane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeffrey S. Monette, Email: monettej@onid.orst.edu.
Luis A. Gómez, Email: gomezral@onid.orst.edu.
Régis F. Moreau, Email: regis.moreau@oregonstate.edu.
Kevin C. Dunn, Email: dunnk@onid.orst.edu.
Judy A. Butler, Email: judy.a.butler@oregonstate.edu.
Liam A. Finlay, Email: liamfin@gmail.com.
Alexander J. Michels, Email: michelsa@onid.orst.edu.
Kate Petersen Shay, Email: kate.shay@oregonstate.edu.
Eric J. Smith, Email: ericsmith.lpi@gmail.com.
References
- 1.Conley KE, Marcinek DJ, Villarin J. Mitochondrial dysfunction and age. Curr Opin Clin Nutr Metab Care. 2007;10:688–692. doi: 10.1097/MCO.0b013e3282f0dbfb. [DOI] [PubMed] [Google Scholar]
- 2.Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: Implications for the mitochondrial theory of aging. Faseb J. 2005;19:419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- 3.Suh JH, Heath SH, Hagen TM. Two subpopulations of mitochondria in the aging rat heart display heterogenous levels of oxidative stress. Free Radic Biol Med. 2003;35:1064–1072. doi: 10.1016/s0891-5849(03)00468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ames BN, Shigenaga MK, Hagen TM. Mitochondrial decay in aging. Biochim Biophys Acta. 1995;1271:165–170. doi: 10.1016/0925-4439(95)00024-x. [DOI] [PubMed] [Google Scholar]
- 5.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandy B, Davison AJ. Mitochondrial mutations may increase oxidative stress: Implications for carcinogenesis and aging? Free radical biology & medicine. 1990;8:523–539. doi: 10.1016/0891-5849(90)90152-9. [DOI] [PubMed] [Google Scholar]
- 7.Hagen TM, Yowe DL, Bartholomew JC, Wehr CM, Do KL, Park JY, Ames BN. Mitochondrial decay in hepatocytes from old rats: Membrane potential declines, heterogeneity and oxidants increase. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3064–3069. doi: 10.1073/pnas.94.7.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagley P, Mackay IR, Baumer A, Maxwell RJ, Vaillant F, Wang ZX, Zhang C, Linnane AW. Mitochondrial DNA mutation associated with aging and degenerative disease. Annals of the New York Academy of Sciences. 1992;673:92–102. doi: 10.1111/j.1749-6632.1992.tb27440.x. [DOI] [PubMed] [Google Scholar]
- 9.Bismuth J, Lin P, Yao Q, Chen C. Ceramide: A common pathway for atherosclerosis? Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ipatova OM, Torkhovskaya TI, Zakharova TS, Khalilov EM. Sphingolipids and cell signaling: Involvement in apoptosis and atherogenesis. Biochemistry (Mosc) 2006;71:713–722. doi: 10.1134/s0006297906070030. [DOI] [PubMed] [Google Scholar]
- 11.Kinnunen PK, Holopainen JM. Sphingomyelinase activity of ldl: A link between atherosclerosis, ceramide, and apoptosis? Trends Cardiovasc Med. 2002;12:37–42. doi: 10.1016/s1050-1738(01)00143-8. [DOI] [PubMed] [Google Scholar]
- 12.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Levin MC, Monetti M, Watt MJ, Sajan MP, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, Farese RV., Jr Increased lipid accumulation and insulin resistance in transgenic mice expressing dgat2 in glycolytic (type ii) muscle. Am J Physiol Endocrinol Metab. 2007;293:E1772–1781. doi: 10.1152/ajpendo.00158.2007. [DOI] [PubMed] [Google Scholar]
- 14.Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- 15.Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, Adamska A, Blachnio A, Gorski J, Gorska M. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50:2366–2373. doi: 10.1007/s00125-007-0781-2. [DOI] [PubMed] [Google Scholar]
- 16.Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patil S, Melrose J, Chan C. Involvement of astroglial ceramide in palmitic acid-induced alzheimer-like changes in primary neurons. Eur J Neurosci. 2007;26:2131–2141. doi: 10.1111/j.1460-9568.2007.05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang PA, Schenck M, Nicolay JP, Becker JU, Kempe DS, Lupescu A, Koka S, Eisele K, Klarl BA, Rubben H, Schmid KW, Mann K, Hildenbrand S, Hefter H, Huber SM, Wieder T, Erhardt A, Haussinger D, Gulbins E, Lang F. Liver cell death and anemia in wilson disease involve acid sphingomyelinase and ceramide. Nature medicine. 2007;13:164–170. doi: 10.1038/nm1539. [DOI] [PubMed] [Google Scholar]
- 19.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith AR, Visioli F, Frei B, Hagen TM. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: Evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2a. Aging Cell. 2006;5:391–400. doi: 10.1111/j.1474-9726.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- 21.Gudz TI, Tserng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex iii by cell-permeable ceramide. J Biol Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 22.Di Paola M, Cocco T, Lorusso M. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry. 2000;39:6660–6668. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- 23.Bao HF, Zhang ZR, Liang YY, Ma JJ, Eaton DC, Ma HP. Ceramide mediates inhibition of the renal epithelial sodium channel by tumor necrosis factor-alpha through protein kinase c. Am J Physiol Renal Physiol. 2007;293:F1178–1186. doi: 10.1152/ajprenal.00153.2007. [DOI] [PubMed] [Google Scholar]
- 24.Clarke CJ, Truong TG, Hannun YA. Role for neutral sphingomyelinase-2 in tumor necrosis factor alpha-stimulated expression of vascular cell adhesion molecule-1 (vcam) and intercellular adhesion molecule-1 (icam) in lung epithelial cells: P38 mapk is an upstream regulator of nsmase2. J Biol Chem. 2007;282:1384–1396. doi: 10.1074/jbc.M609216200. [DOI] [PubMed] [Google Scholar]
- 25.Dbaibo GS, Obeid LM, Hannun YA. Tumor necrosis factor-alpha (tnf-alpha) signal transduction through ceramide. Dissociation of growth inhibitory effects of tnf-alpha from activation of nuclear factor-kappa b. J Biol Chem. 1993;268:17762–17766. [PubMed] [Google Scholar]
- 26.Fernandez-Checa JC, Colell A, Mari M, Garcia-Ruiz C. Ceramide, tumor necrosis factor and alcohol-induced liver disease. Alcohol Clin Exp Res. 2005;29:151S–157S. [PubMed] [Google Scholar]
- 27.Liu B, Andrieu-Abadie N, Levade T, Zhang P, Obeid LM, Hannun YA. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. J Biol Chem. 1998;273:11313–11320. doi: 10.1074/jbc.273.18.11313. [DOI] [PubMed] [Google Scholar]
- 28.Nikolova-Karakashian M, Karakashian A, Rutkute K. Role of neutral sphingomyelinases in aging and inflammation. Subcell Biochem. 2008;49:469–486. doi: 10.1007/978-1-4020-8831-5_18. [DOI] [PubMed] [Google Scholar]
- 29.Pettus BJ, Chalfant CE, Hannun YA. Sphingolipids in inflammation: Roles and implications. Curr Mol Med. 2004;4:405–418. doi: 10.2174/1566524043360573. [DOI] [PubMed] [Google Scholar]
- 30.Novgorodov SA, Gudz TI. Ceramide and mitochondria in ischemia/reperfusion. J Cardiovasc Pharmacol. 2009;53:198–208. doi: 10.1097/FJC.0b013e31819b52d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 33.Birbes H, El Bawab S, Obeid LM, Hannun YA. Mitochondria and ceramide: Intertwined roles in regulation of apoptosis. Adv Enzyme Regul. 2002;42:113–129. doi: 10.1016/s0065-2571(01)00026-7. [DOI] [PubMed] [Google Scholar]
- 34.Monette JS, Gómez LA, Moreau RF, Bemer BA, Taylor AW, Hagen TM. Characteristics of the rat cardiac sphingolipid pool in two mitochondrial subpopulations. Biochem Biophys Res Commun. 2010 doi: 10.1016/j.bbrc.2010.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ardail D, Popa I, Alcantara K, Pons A, Zanetta JP, Louisot P, Thomas L, Portoukalian J. Occurrence of ceramides and neutral glycolipids with unusual long-chain base composition in purified rat liver mitochondria. FEBS Lett. 2001;488:160–164. doi: 10.1016/s0014-5793(00)02332-2. [DOI] [PubMed] [Google Scholar]
- 36.Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: Mam (mitochondria-associated membrane) and/or mitochondria? Biochem J. 2004;382:527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu BX, Rajagopalan V, Roddy PL, Clarke CJ, Hannun YA. Identification and characterization of murine mitochondrial-associated neutral sphingomyelinase (ma-nsmase), the mammalian sphingomyelin phosphodiesterase 5. J Biol Chem. doi: 10.1074/jbc.M110.102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen Shay K, Hagen TM. Age-associated impairment of akt phosphorylation in primary rat hepatocytes is remediated by alpha-lipoic acid through pi3 kinase, pten, and pp2a. Biogerontology. 2008 doi: 10.1007/s10522-008-9187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suh JH, Wang H, Liu RM, Liu J, Hagen TM. (r)-alpha-lipoic acid reverses the age-related loss in gsh redox status in post-mitotic tissues: Evidence for increased cysteine requirement for gsh synthesis. Arch Biochem Biophys. 2004;423:126–135. doi: 10.1016/j.abb.2003.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bitar MS, Ayed AK, Abdel-Halim SM, Isenovic ER, Al-Mulla F. Inflammation and apoptosis in aortic tissues of aged type ii diabetes: Amelioration with alpha-lipoic acid through phosphatidylinositol 3-kinase/akt- dependent mechanism. Life Sci. 86:844–853. doi: 10.1016/j.lfs.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Maczurek A, Hager K, Kenklies M, Sharman M, Martins R, Engel J, Carlson DA, Munch G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for alzheimer's disease. Adv Drug Deliv Rev. 2008;60:1463–1470. doi: 10.1016/j.addr.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Salinthone S, Yadav V, Bourdette DN, Carr DW. Lipoic acid: A novel therapeutic approach for multiple sclerosis and other chronic inflammatory diseases of the cns. Endocr Metab Immune Disord Drug Targets. 2008;8:132–142. doi: 10.2174/187153008784534303. [DOI] [PubMed] [Google Scholar]
- 43.Smith AR, Visioli F, Frei B, Hagen TM. Lipoic acid significantly restores, in rats, the age-related decline in vasomotion. Br J Pharmacol. 2008;153:1615–1622. doi: 10.1038/bjp.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tardif JC, Rheaume E. Lipoic acid supplementation and endothelial function. Br J Pharmacol. 2008;153:1587–1588. doi: 10.1038/bjp.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790:1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 47.Nair JR, McGuire JJ. Submitochondrial localization of the mitochondrial isoform of folylpolyglutamate synthetase in ccrf-cem human t-lymphoblastic leukemia cells. Biochim Biophys Acta. 2005;1746:38–44. doi: 10.1016/j.bbamcr.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Lightle SA, Oakley JI, Nikolova-Karakashian MN. Activation of sphingolipid turnover and chronic generation of ceramide and sphingosine in liver during aging. Mech Ageing Dev. 2000;120:111–125. doi: 10.1016/s0047-6374(00)00191-3. [DOI] [PubMed] [Google Scholar]
- 49.Dixon BM, Heath SH, Kim R, Suh JH, Hagen TM. Assessment of endoplasmic reticulum glutathione redox status is confounded by extensive ex vivo oxidation. Antioxid Redox Signal. 2008;10:963–972. doi: 10.1089/ars.2007.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merrill AH, Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: High-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods. 2005;36:207–224. doi: 10.1016/j.ymeth.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- 52.El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. Molecular cloning and characterization of a human mitochondrial ceramidase. J Biol Chem. 2000;275:21508–21513. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- 53.Rutkute K, Asmis RH, Nikolova-Karakashian MN. Regulation of neutral sphingomyelinase-2 by gsh: A new insight to the role of oxidative stress in aging-associated inflammation. J Lipid Res. 2007;48:2443–2452. doi: 10.1194/jlr.M700227-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu B, Hannun YA. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J Biol Chem. 1997;272:16281–16287. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]
- 55.Yue TL, Bao W, Jucker BM, Gu JL, Romanic AM, Brown PJ, Cui J, Thudium DT, Boyce R, Burns-Kurtis CL, Mirabile RC, Aravindhan K, Ohlstein EH. Activation of peroxisome proliferator-activated receptor-alpha protects the heart from ischemia/reperfusion injury. Circulation. 2003;108:2393–2399. doi: 10.1161/01.CIR.0000093187.42015.6C. [DOI] [PubMed] [Google Scholar]
- 56.Kusunoki M, Hara T, Tsutsumi K, Nakamura T, Miyata T, Sakakibara F, Sakamoto S, Ogawa H, Nakaya Y, Storlien LH. The lipoprotein lipase activator, no-1886, suppresses fat accumulation and insulin resistance in rats fed a high-fat diet. Diabetologia. 2000;43:875–880. doi: 10.1007/s001250051464. [DOI] [PubMed] [Google Scholar]
- 57.Hendrickson SC, St Louis JD, Lowe JE, Abdel-aleem S. Free fatty acid metabolism during myocardial ischemia and reperfusion. Mol Cell Biochem. 1997;166:85–94. doi: 10.1023/a:1006886601825. [DOI] [PubMed] [Google Scholar]
- 58.Knuuti J, Takala TO, Nagren K, Sipila H, Turpeinen AK, Uusitupa MI, Nuutila P. Myocardial fatty acid oxidation in patients with impaired glucose tolerance. Diabetologia. 2001;44:184–187. doi: 10.1007/s001250051597. [DOI] [PubMed] [Google Scholar]
- 59.Yi F, Zhang AY, Janscha JL, Li PL, Zou AP. Homocysteine activates nadh/nadph oxidase through ceramide-stimulated rac gtpase activity in rat mesangial cells. Kidney Int. 2004;66:1977–1987. doi: 10.1111/j.1523-1755.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- 60.Senkal CE, Ponnusamy S, Rossi MJ, Bialewski J, Sinha D, Jiang JC, Jazwinski SM, Hannun YA, Ogretmen B. Role of human longevity assurance gene 1 and c18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol Cancer Ther. 2007;6:712–722. doi: 10.1158/1535-7163.MCT-06-0558. [DOI] [PubMed] [Google Scholar]
- 61.Kroesen BJ, Pettus B, Luberto C, Busman M, Sietsma H, de Leij L, Hannun YA. Induction of apoptosis through b-cell receptor cross-linking occurs via de novo generated c16-ceramide and involves mitochondria. J Biol Chem. 2001;276:13606–13614. doi: 10.1074/jbc.M009517200. [DOI] [PubMed] [Google Scholar]
- 62.Panjarian S, Kozhaya L, Arayssi S, Yehia M, Bielawski J, Bielawska A, Usta J, Hannun YA, Obeid LM, Dbaibo GS. De novo n-palmitoylsphingosine synthesis is the major biochemical mechanism of ceramide accumulation following p53 up-regulation. Prostaglandins Other Lipid Mediat. 2008;86:41–48. doi: 10.1016/j.prostaglandins.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem. 2004;11:1135–1146. doi: 10.2174/0929867043365387. [DOI] [PubMed] [Google Scholar]
- 65.Packer L. Alpha-lipoic acid: A metabolic antioxidant which regulates nf-kappa b signal transduction and protects against oxidative injury. Drug Metab Rev. 1998;30:245–275. doi: 10.3109/03602539808996311. [DOI] [PubMed] [Google Scholar]
- 66.Kondo T, Higashiyama Y, Goto S, Iida T, Cho S, Iwanaga M, Mori K, Tani M, Urata Y. Regulation of gamma-glutamylcysteine synthetase expression in response to oxidative stress. Free Radic Res. 1999;31:325–334. doi: 10.1080/10715769900300891. [DOI] [PubMed] [Google Scholar]
- 67.Shenvi SV, Smith EJ, Hagen TM. Transcriptional regulation of rat gamma-glutamate cysteine ligase catalytic subunit gene is mediated through a distal antioxidant response element. Pharmacol Res. 2009;60:229–236. doi: 10.1016/j.phrs.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ziegler D, Hanefeld M, Ruhnau KJ, Hasche H, Lobisch M, Schutte K, Kerum G, Malessa R. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: A 7-month multicenter randomized controlled trial (aladin iii study). Aladin iii study group. Alpha-lipoic acid in diabetic neuropathy. Diabetes Care. 1999;22:1296–1301. doi: 10.2337/diacare.22.8.1296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Isolated mitochondria were incubated with a bacterially-derived sphingomyelinase, bSMase, followed by lipid extraction and ceramide quantification by LC-MS/MS. (A) Total ceramide levels were markedly increased by a 20-min bSMase incubation. (B) A time-course of Complex IV activity following incubation with bSMase. Results show a rapid decline in Complex IV activity. Data represent the means ± SEM, n = 3.


