Abstract
Adiponectin is an important adipocyte-derived hormone that regulates metabolism of lipids and glucose, and its receptors (AdipoR1, AdipoR2, T-cadherin) appear to exert actions in peripheral tissues by activating the AMP-activated protein kinase, p38-MAPK, PPARα and NF-kappa B. Adiponectin has been shown to exert a wide range of biological functions that could elicit different effects, depending on the target organ and the biological milieu. There is substantial evidence to suggest that adiponectin receptors are expressed widely in the brain. Their expression has been detected in regions of the mouse hypothalamus, brainstem, cortical neurons and endothelial cells, as well as in whole brain and pituitary extracts. While there is now considerable evidence for the presence of adiponectin and its receptors in the brain, their precise roles in brain diseases still remain unclear. Only a few research studies have looked at this facet of adiponectins in brain disorders. This brief review will describe the evidence for important functions by adiponectin, its structure and known actions, evidence for expression of AdipoRs in the brain, their involvement in brain disorders and the therapeutic potential of agents that could modify AdipoR signalling.
Keywords: adiponectin, adiponectin receptors, Alzheimer's disease, brain diseases, cell death, ischaemic stroke, multiple sclerosis, neuronal cells, NF-κB
Introduction
Adipose tissue has long been regarded as paramount for maintenance of life and exerting various physiological functions in mammals (Gesta et al., 2008). In addition to regulating cold adaptation and non-shivering thermogenesis (Redinger, 2009), it is also an important passive fuel storage site, assisting in generation of ATP. Furthermore, research over the past two decades has shown that adipose tissue is not merely an inert tissue devoted to energy storage, but it is also a metabolically active organ capable of regulating immunological and inflammatory processes under physiological and pathological states (Fantuzzi, 2005). In the last decade, the epidemic of obesity and its related pathologies such as hypertension, diabetes and strokes has prompted further research into this multitasking tissue. These studies have yielded the intriguing finding that adipose tissue can function as an endocrine organ because of its ability to secrete various hormones, tissue factors and cytokines, collectively known as adipokines (Trayhurn and Beattie, 2001; Trayhurn and Wood, 2004; Wood and Trayhurn, 2007; Vazquez-Vela et al., 2008; Bastard et al., 2009; Li et al., 2009).
Adiponectin
One important adipocyte-derived hormone is adiponectin, which was first discovered by Scherer and colleagues in 1995 in 3T3-L1 adipocytes. Since then, other research groups have identified adiponectin in murine and human adipocytes (Scherer et al., 1995; Hu et al., 1996; Maeda et al., 1996). Adiponectin was initially thought to be produced exclusively by adipose tissue. However, recent studies have shown it to be expressed, both at mRNA and protein levels, in other tissues such as human and murine osteoblasts (Berner et al., 2004), liver parenchyma cells (Yoda-Murakami et al., 2001; Jonsson et al., 2005; Kaser et al., 2005), myocytes (Delaigle et al., 2004), epithelial cells (Shimada et al., 2004; Patel et al., 2008) and placental tissue (Caminos et al., 2005; Chen et al., 2006).
Human adiponectin is a 28–30 kDa protein that comprises 244 amino acids and structurally belongs to the soluble defence collagen superfamily (Berg et al., 2002). It bears 82% amino acid homology with its murine counterpart and its structure comprises multiple domains (Scherer et al., 1995; Maeda et al., 1996; Nakano et al., 1996; Berg et al., 2002). It has a signalling peptide region and a species-specific variable domain at the N-terminal, followed by a collagenous domain and a globular domain at the C-terminal (Figure 1). The N-terminal is quite unique, as it shows no structural homology to any other proteins and is structurally diverse among different species. On the contrary, the globular domain at the C-terminal bears the same amino acid sequence as that of the complement factor, C1q, and bears a striking structural similarity to the globular domains of other proteins such as collagens VIII and X, mannose-binding protein, TNF-α and pulmonary surfactant proteins A and D (Brass et al., 1992; Hu et al., 1996; Shapiro and Scherer, 1998; Fruebis et al., 2001; Yamauchi et al., 2003). This domain facilitates the binding of adiponectin to its receptors. The collagenous domain in the centre comprises 22 G-X-Y repeats and facilitates the triple-helix formation of the protein structure (Berg et al., 2002).
Figure 1.
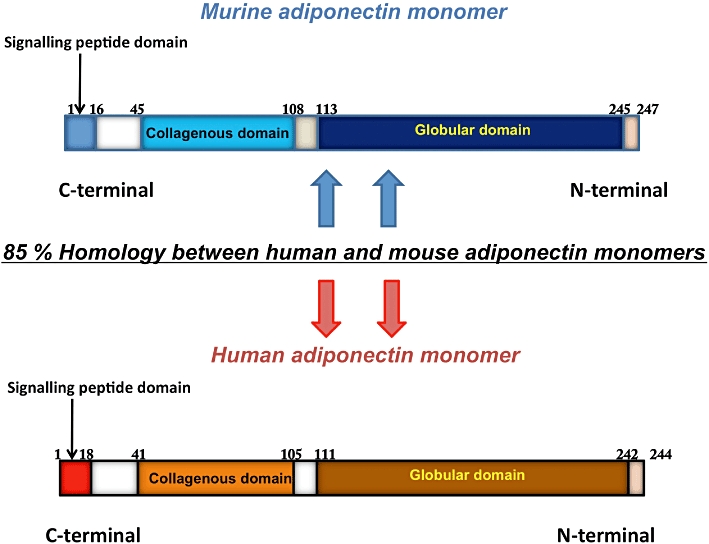
Schematic representation of adiponectin isoforms. The human and murine isoforms of adiponectin have 85% structural homology. Each of the adiponectin isoforms is composed of an N-terminal collagenous domain and a C-terminal globular region.
Adiponectin is a relatively abundant protein that accounts for about 0.01% of total serum proteins in humans and 0.05% in rodents (Matsuzawa, 2005). In humans, its serum concentration is 2–20 ug·mL−1, and it has been shown to undergo oligomerization in the circulation (Shimada et al., 2004) (Figure 2). Adiponectin is released into the circulation as full-length trimers, hexamers, high molecular weight (HMW) multimers and a globular fraction called globular adiponectin (gAD), generated by proteolytic cleavage of the full-length adiponectin monomer (Waki et al., 2003). Collectively, trimers and hexamers are referred to as low molecular weight (LMW) multimers, while larger complexes (18-mers and above) are known as HMW forms. The trimeric form is the basic form of circulating adiponectin, and it is formed through non-covalent disulphide bond linkages in its collagenous domains (Pajvani et al., 2003). The hydrophobic interactions in the globular head help to maintain trimer stability (Waki et al., 2003). The trimers undergo further post-translational modifications that include disulphide bond linkage between the cysteine residues located in the N-terminal variable region (Cys36 in human and Cys39 in mouse adiponectin, respectively), hydroxylation and glycosylation of four conserved proline and lysine residues in the collagenous domain of the trimers, to oligomerize into hexamers and larger complexes. (Tsao et al., 2002; 2003; Pajvani et al., 2003; Waki et al., 2003; Richards et al., 2006; Wang et al., 2006a). In addition, a small fraction of adiponectin is cleaved by leukocyte elastase and circulates as a proteolytic fragment that comprises the globular domain of the protein, known as gAd (Fruebis et al., 2001) (Figure 2). Hexamers and HMW species are the two major oligomeric forms of circulating adiponectin (Nakano et al., 1996). The lower plasma concentrations of trimeric and globular forms may be explained by their shorter half-lives (Pajvani et al., 2003). Thus, adiponectin is a complex protein that circulates in the body as different oligomers.
Figure 2.
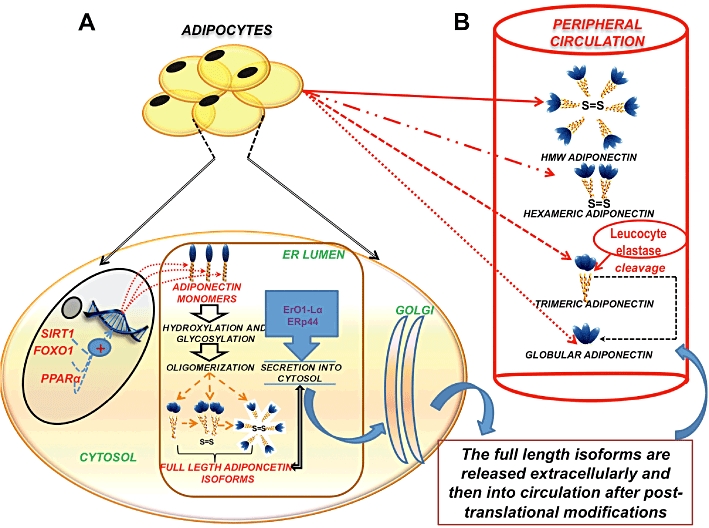
Regulation of synthesis, secretion and circulation of adiponectin. (A) Adipocytes synthesize adiponectin mRNA in its monomeric form within the nucleus. This transcription is regulated and promoted by SIRT1/FOXO-1 and PPARα. Once transcribed, the adiponectin protein monomer is released into the ER, where it undergoes various post-translational modifications, regulated by ER chaperones like ERp44 and ErO1- Lα to form trimers, hexamers and HMW (full-length adiponectin) isoforms. (B) Following their packaging in the golgi, the adiponectin isomers are released into the peripheral circulation. The HMW isomer is the most abundant and biologically active form of adioponectin. Another form of circulating adiponectin is the gAD leukocyte elastase-mediated cleavage of the globular domain of the trimeric adiponectin.
The regulation of adiponectin expression and secretion of its oligomers into the circulation is not yet well understood. A sexual dimorphism is seen in the levels of circulating adiponectin, with males having lower adiponectin levels than females (Arita et al., 1999). In addition, circulating adiponectin levels can be modified by various hormonal, nutritional, pharmacological factors, circulating cytokines, inflammatory conditions and disease states (Fasshauer et al., 2002; Maeda et al., 2002; Nishizawa et al., 2002; Combs et al., 2003; Bottner et al., 2004; Tsou et al., 2004; Brochu-Gaudreau et al., 2010). Certain proteins, such as silent mating type information regulation 2 homologue 1 (SIRT1), NAD+-dependent protein deacetylase and forkhead transcription factor O1 (FOXO1)-C enhancer-binding protein alpha (EBPα) transcription complex, have also been shown to influence adiponectin release (Picard et al., 2004; Qiao and Shao, 2006). Furthermore, a recently published study showed that the secretion of these oligomers is tightly regulated by a pair of molecular chaperones in the endoplasmic reticulum, namely ERp44 (an ER protein of 44 kDa) and Ero1-Lα (ER oxidoreductase 1-Lα) (Wang et al., 2008). While ERp44 was shown to inhibit the secretion of adiponectin oligomers through thiol-mediated retention, Ero1-Lα promoted the release of HMW adiponectin trapped by ERp44.
Adiponectin receptors (AdipoRs)
With obesity being a major factor in morbidities such as diabetes, hypertension and atherosclerosis, the discovery of adiponectin has led to a paradigm shift in the approach of research undertaken in this area. As a result of the extensive work examining adiponectin and its role in obesity, it is now regarded as an important protein, exerting a myriad of potentially beneficial effects ranging from body weight regulation, modulation of endothelial function, insulin-sensitization and anti-atherogenic and anti-inflammatory actions (Ouchi et al., 1999; Berg et al., 2001; Fruebis et al., 2001; Yamauchi et al., 2001; Okamoto et al., 2002; Whitehead et al., 2006; Kang et al., 2009). Adiponectin exerts these effects by activating numerous signalling molecules, including adenosine monophosphate-activated protein kinase (AMPK), p38-MAPK, JNK, PPARα transcription factor and NF-κB in multiple tissues (Figure 3). These activating signals are transduced via the AdipoRs (Yamauchi et al., 2003; Tang et al., 2005), which include two seven transmembrane domain (7TM) receptors, AdipoR1 and AdipoR2, and a recently discovered cell surface protein called T-cadherin (Hug et al., 2004). The first AdipoR to be discovered was AdipoR1, by Kadowaki and colleagues (Yamauchi et al., 2003). This finding was followed by a search of human and mouse databases for AdipoR1-homologous genes, leading to the identification of a protein that exhibited a 68% identity to AdipoR1, which was termed AdipoR2 (Yamauchi et al., 2003; Vasseur, 2006). The human AdipoR1 gene is located on chromosome 1(q32.1) and encodes a 375 amino acid 42 kDa protein. The human AdipoR2 gene is found on chromosome 12(p13.33), and it encodes a 43 kDa protein comprising 386 amino acids (Narasimhan et al., 2005).
Figure 3.
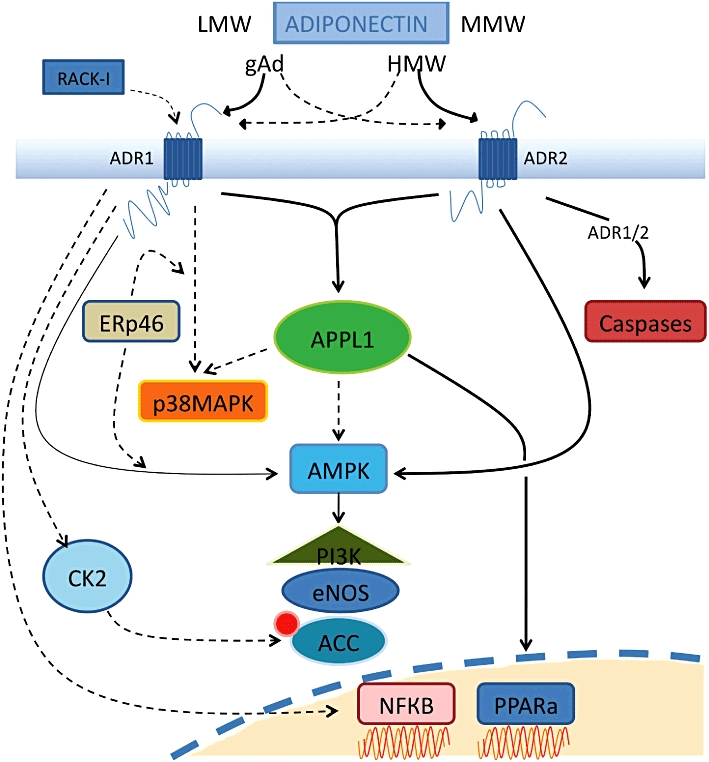
Representation of signalling transduction via adiponectin receptor activation. Adiponectin exists in full-length, globular, high molecular and low molecular weight forms. The binding of the different forms of adiponectin to the two known adiponectin receptors, AdipoR1 and AdipoR2, can lead to stimulation of AMPK, p38-MAPK, JNK and PPARα. Interacting directly with the N-terminal of adiponectin receptors, APPL1 elicits signalling through PPARα and along with AMPK modulates the PI3K pathway and eNOS levels. ACC phosphorylation occurs from CK2 adaptor protein at the AdipoR1 N-terminus whilst interaction of the scaffold protein RACK-I at AdipoR1 can mediate glucose metabolism. The ER chaperone, ERp46 helps mediate AdipoR1 signalling to p38 and AMPK. Signalling to NF-κB is solely through AdipoR1, but evidence exists for both its activation and inhibition upon gAD binding. Activation of both receptors results in activation of the caspase cascade and alterations in cell survival.
While the two main AdipoRs, AdipoR1 and R2, belong to the 7TM receptor family, they are structurally and functionally distinct from other 7TM receptor proteins (e.g. the GPCRs), one of the striking differences is the reversed membrane topology that they possess (Yamauchi et al., 2003). Hence, contrary to the conventional 7TM receptor protein structure, AdipoRs possess an intracellular amino terminus and an extracellular carboxyl-terminus. In addition to this structural variation, their downstream signalling mechanism bear no similarity to G-protein receptor-mediated signalling. Owing to these major differences, AdipoRs have now been categorised into a niche subset of the 7TM protein family, called the progestin and AdipoQ receptor (PAQR) family, which comprises 11 mammalian proteins that also includes the progestin receptors (Tang et al., 2005).
AdipoRs are expressed in liver (Neumeier et al., 2005; Bonnard et al., 2008; Felder et al., 2010), muscle (Civitarese et al., 2004; Staiger et al., 2004; Beylot et al., 2006; Dai et al., 2006), heart (Neumeier et al., 2005; Ding et al., 2007; Palanivel et al., 2007), adipose tissue (Fasshauer et al., 2004; Bluher et al., 2005; Dai et al., 2006; Rasmussen et al., 2006; Liu et al., 2008a, b), osteoblasts (Berner et al., 2004; Kanazawa et al., 2007), pancreas (Kharroubi et al., 2003; Staiger et al., 2005; Gu et al., 2006), leukocytes (Chinetti et al., 2004; Alberti et al., 2007; Weigert et al., 2008) and the brain (Kubota et al., 2007) of humans, rodents and various other mammals. The expression profile of AdipoR1 is quite ubiquitous and is most abundant in skeletal muscle. On the other hand, the expression of AdipoR2 is most abundant in liver (Coope et al., 2008). Recently, a third AdipoR, T-cadherin, was discovered. It bears no structural semblance to AdipoR1 and R2 as it lacks the trans-membrane and intracellular domains (Ranscht and Dourszimmermann, 1991; Takeuchi et al., 2007). T-cadherin is highly expressed in the vasculature and to a lesser degree in muscle (Takeuchi et al., 2007). However, the liver, a primary target tissue of adiponectin, does not show a high degree of T-cadherin expression. This raises an intriguing possibility that T-cadherin may not be a functional receptor of adiponectin but could be an adiponectin-binding protein.
Mechanisms regulating the expression of AdipoRs appear to be complex and are governed by numerous factors. Physiologically, a circadian expression profile of these receptors is seen in adipose tissue (Berner et al., 2004) that appears to be regulated by feeding states (Ding et al., 2004; Liu et al., 2008a), levels of free fatty acids and insulin (Tsuchida et al., 2004; Beylot et al., 2006; Bullen et al., 2007; Bonnard et al., 2008; Liu et al., 2008b). Growth hormone has been shown to selectively up-regulate AdipoR2 (Fasshauer et al., 2004; Liu et al., 2008b), while long-term exercise can specifically up-regulate AdipoR1 expression (Chang et al., 2006; Zeng et al., 2007; Christiansen et al., 2009). Additionally, various nuclear receptors like liver X receptor (LXR), PPARα and drugs such as PPARγ agonists, metformin and fibrates may alter expression levels of AdipoRs (Chinetti et al., 2004; Ding et al., 2007; Shimizu et al., 2007; Liu et al., 2008b). The AdipoRs display differential binding affinities for the various adiponectin multimers. While AdipoR1 binds to gAd with high affinity, AdipoR2 has an intermediate binding affinity for both gAd and full-length adiponectin. On the other hand, T-cadherin only has a putative interaction with hexameric and HMW multimeric forms (Yamauchi et al., 2003).
AdipoR signalling
Signalling molecules activated by adiponectin include AMPK, p38-MAPK and PPARα (Matsuzawa, 2005) (Figure 3). Of these, AMPK acts as a major downstream component of adiponectin signalling. AMPK generally acts as the cellular energy sensor in the body and is normally activated when there is an increase in the intracellular AMP/ ATP ratio (Snehalatha et al., 2003; Wang et al., 2005a). Numerous studies have shown that metabolic effects mediated by adiponectin in the liver and muscle are due to the activation of AMPK (Yamauchi et al., 2002; Civitarese et al., 2005; 2006; Kola et al., 2006; Viollet et al., 2006). AMPK activation is also involved in adiponectin-mediated actions in vascular endothelial cells and the heart, in a manner that is reported to be beneficial in protection against cardiovascular diseases (Kobayashi et al., 2004; Ouchi et al., 2004). For example, adiponectin-mediated activation of AMPK in endothelial cells stimulates production of NO, and it reduces myocardial infarct size and apoptosis in a mouse model of heart ischaemia–reperfusion (Chen et al., 2003; Shibata et al., 2005; Guerre-Millo, 2008). Thus, there is evidence that adiponectin-dependent AMPK signalling may mediate beneficial metabolic and cardiovascular effects of adiponectin (Kahn et al., 2005).
Recently, an adaptor protein containing a pleckstrin homology domain, a phosphotyrosine domain and a leucine zipper motif (APPL1) has been shown to both bind to AdipoRs and acts as a link between the receptors and its downstream signalling molecules (Mao et al., 2006; Cheng et al., 2007). APPL1 is required for adiponectin-induced activation of AMPK, p38-MAPK and ERK 1/2–MAPK pathways. APPL1 has been shown to mediate adiponectin-regulated insulin, sensitizing actions in the periphery and modulating endothelial NOS (eNOS) function in endothelial cells (Deepa and Dong, 2009). In addition, Chandrasekar et al. (2008) reported that the anti-inflammatory and cytoprotective effects of adiponectin are mediated, at least in part, through APPL1-dependent AMPK activation of the phosphatidylinositol 3-kinase (PI3K)-v-akt (Akt) signalling pathway. Hence, until recently, APPL1 was the only AdipoR-interacting protein identified, through which AdipoR activation could modulate and possibly integrate its downstream signalling responses (Mao et al., 2006). However, over the past two to three years, various interacting and adapter proteins for AdipoRs have been discovered. These include the regulatory subunit of the protein kinase casein kinase (CK) 2 that binds to the AdipoR1 N-terminus and appears to be vital in mediating the crucial step of acetyl-CoA carboxylase (ACC) phosphorylation in adiponectin-mediated fatty acid oxidation (Heiker et al., 2009). In addition, the receptors for activated C-kinase-I (RACK-I) and the endoplasmic reticulum protein 46 (ERp46) have been reported as two other potential binding partners for AdipoR1. While RACK-I appeared to act as a scaffold protein in mediating adiponectin-induced glucose uptake in hepatocytes, the ER chaperone ERp46 appears to be vital in adiponectin-mediated AMPK- and p38-MAPK signalling, through AdipoR1 (Xu et al., 2009; Charlton et al., 2010). Another important facet of AdipoR signalling is its arrangement at the cell surface during adiponectin-mediated signalling because of the possibility of its dimerization and internalization. It has been shown that AdipoR1 and AdipoR2 are capable of forming homo- and hetero-oligomeric complexes in vitro (Yamauchi et al., 2003), with homodimerization more common with AdipoR1 than AdipoR2. In addition, AdipoR1 can internalize constitutively and in an agonist-dependent manner (Kosel et al., 2010), whereas little information is available regarding these processes in AdipoR2 (Ding et al., 2009). Since information regarding the oligomerization, internalization and desensitization of cell surface receptors during downstream signal modulation are vital to ascertain the receptor accessibility and the prospects of its use as therapeutic targets (Dalrymple et al., 2008; Hanyaloglu and von Zastrow, 2008), the presence of such features in AdipoR-mediated signalling could be instrumental in exploring their therapeutic potentials.
While adiponectin seems to exert several positive effects via multiple signalling mechanisms, it seems possible that adiponectin may also exert many unfavourable effects. For example, the view that adiponectin may be an anti-inflammatory cytokine could be challenged in light of recent reports of elevated levels of this protein in various inflammatory disease states. Adiponectin has been shown to be elevated in arthritis, preeclampsia and end-stage renal diseases (Shoji et al., 2005; D'Anna et al., 2006; Haugen et al., 2006; Otero et al., 2006; Senolt et al., 2006; Haugen and Drevon, 2007). Also, it was shown that adiponectin induces production of the pro-inflammatory mediator IL-6 and activation of NF-κB in human synovial fibroblasts and adhesion molecule expression in endothelial cells (Hattori et al., 2006; Tomizawa et al., 2008; Smith et al., 2009; Liao et al., 2010). One plausible explanation for this pleiotropy of effects demonstrated by adiponectin could be the presence of various circulating oligomers of adiponectin. Although HMW multimers appear to be the most bioactive form of adiponectin in the circulation, which mediate the actions of adiponectin on liver, muscle and the vasculature, other isomeric forms of adiponectin like hexamers and gAd could modulate different signalling molecules at various other sites in the body and exert quite different effects (Wang et al., 2005b; Hada et al., 2007; Palanivel et al., 2007; Hattori et al., 2008). Thus, the question of whether or not adiponectin has an overall anti- or pro-inflammatory role still needs to be clarified, as this may be dependent on the specific tissue it acts on and the presence or absence of a disease state.
Adiponectin in the brain
In stark contrast to available information regarding adiponectin and its actions in the periphery, still very little is known about the effects of adiponectin in the CNS. The presence of adiponectin and its role in the brain still remains controversial and a matter of debate. It had been initially suggested that adiponectin was not present in the brain, as it was unable to cross the blood–brain barrier (BBB). These conclusions were based on the results of some studies that could not detect adiponectin in the CSF (Pan et al., 2006; Spranger et al., 2006). However, this initial view conflicted with a report that CSF adiponectin was detectable after an i.v. injection of full-length adiponectin in C57Bl/6J mice, and that both systemic and i.c.v. administration of adiponectin reduced serum glucose and lipid levels and decreased body weight (Qi et al., 2004). This latter study was therefore instrumental for suggesting that adiponectin could be a centrally acting signalling molecule. This proposition was further substantiated by Kubota et al. (2007), who showed that peripherally administered adiponectin stimulated AMPK in the hypothalamus of mice to increase food intake and decrease energy expenditure. However, whether endogenous adiponectin might have a similar effect in humans is not clear. This is because the physiological levels of adiponectin in human CSF is 1000-fold lower than its concentration in serum (Pan et al., 2006; Kos et al., 2007; Kusminski et al., 2007) (Figure 4). In mice too, the CSF adiponectin corresponds to only 1–4% of plasma adiponectin in mice (Ahima et al., 2006). As regards the presence of endogenous adiponectin in the brain in addition to CSF, adiponectin mRNA has been detected in chicken and murine brain extracts (Maddineni et al., 2005; Hoyda et al., 2007). Adiponectin mRNA was not detected in human brain extract, but it has been reported to be expressed in human pituitary gland (Rodriguez-Pacheco et al., 2007; Wilkinson et al., 2007). In the pituitary gland, adiponectin seems to assume a putative role in the autocrine/paracrine control and regulation of the release of somatotrophs and gonadotrophs (Qi et al., 2004; Malagon et al., 2006; Pan et al., 2006; Psilopanagioti et al., 2009).
Figure 4.
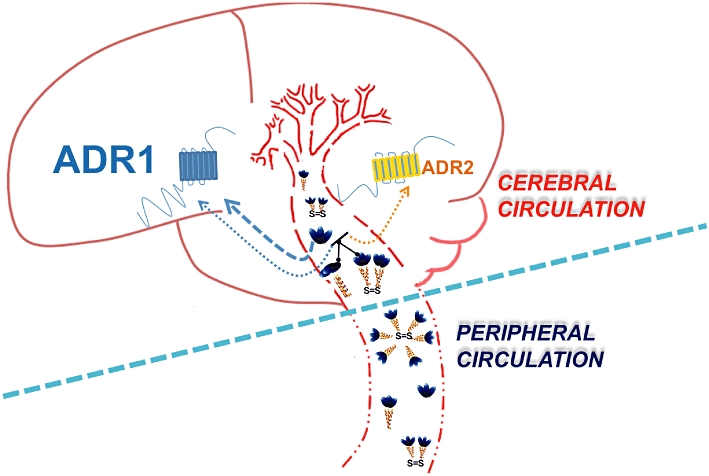
Differences between the peripheral and cerebral circulations regarding adiponectin signalling. The tight regulated membrane permeability of the cerebral blood vessels, as compared with their peripheral counterparts, permits the selective passage of only the trimers, hexamers and possibly globular forms of adiponectin into the CNS. Both AdipoR1and AdipoR2 are present in the brain, with AdipoR1 being more pronounced. Different adiponectin isomers bind to the AdipoRs with different binding affinities, as demonstrated by the thickness of the arrows.
There is substantial evidence to suggest that AdipoRs are expressed widely in the brain. Their expressions have been detected in regions of the mouse hypothalamus (area postrema and paraventricular nuclei), brainstem and endothelial cells, as well as in whole brain and pituitary extracts (Yamauchi et al., 2003; Fry et al., 2006; Spranger et al., 2006; Hoyda et al., 2007; Neumeier et al., 2007; Rodriguez-Pacheco et al., 2007; Wilkinson et al., 2007) (Figures 4 and 5). We recently showed that mouse cortical neurons also express both AdipoR1 and AdipoR2, with AdipoR1 expression being more pronounced than AdipoR2 (Thundyil et al., 2010). Our results were consistent with those of another study that mapped the distribution of AdipoR2 mRNA to various cortical and subcortical regions of the rat brain (Repunte-Canonigo et al., 2010). In addition to the expression in the murine nervous system, moderate to strong expressions of AdipoR1 and AdipoR2 have been shown in the pars distalis area of the pituitary gland, and in humans, AdipoR1 expression was localized to neurons in the lateral hypothalamic area and nucleus basalis of Meynert (Psilopanagioti et al., 2009). Although there is still some ambiguity regarding the bioactive oligomer of adiponectin in the brain, growing evidence suggests that LMW multimers, particularly trimers, may be the active forms. LMW multimers have been detected in the CSF of both humans and mice (Escobar-Morreale et al., 2006; Aroda et al., 2008; Glintborg et al., 2008). Hence, it appears that while adiponectin's peripheral effects are mediated predominantly by HMW forms, LMW multimers may be responsible for its central effects.
Figure 5.
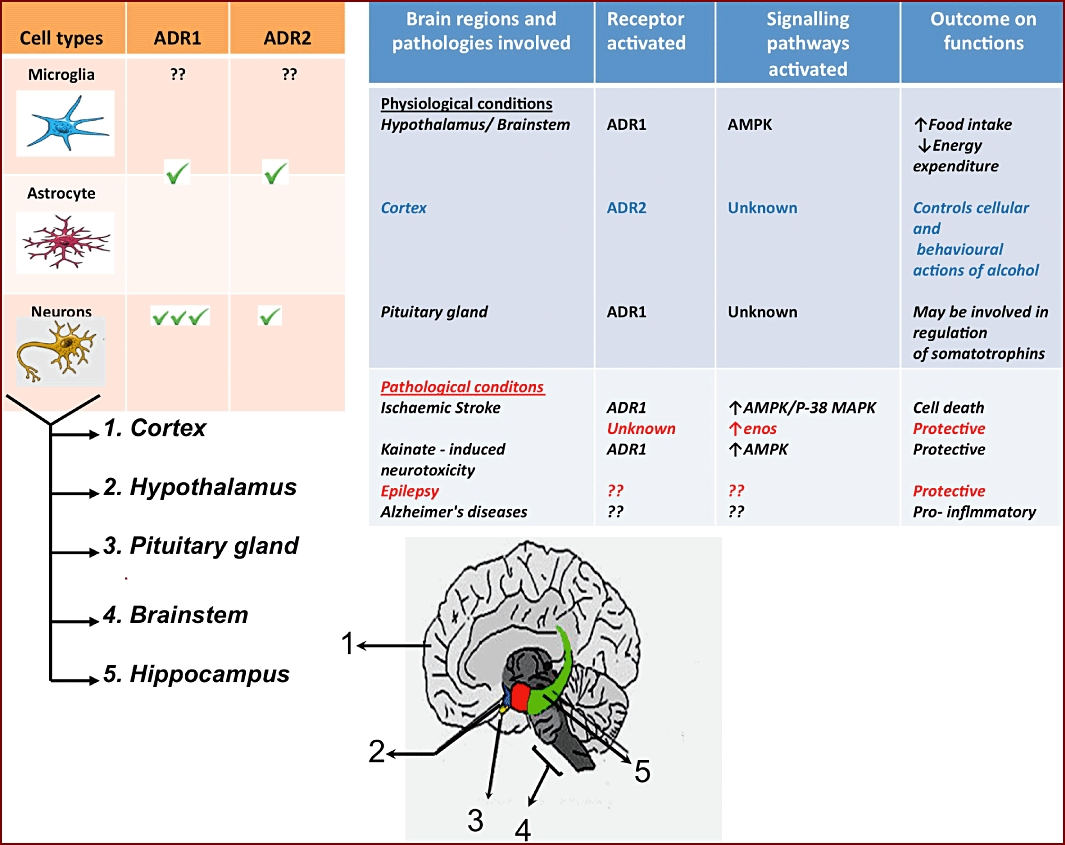
The CNS distribution of adiponectin receptors and their roles in adiponectin-mediated signalling in normal and disease states. Table A indicates the different cells of the CNS and their AdipoR receptor expression patterns. Expression of AdipoRs in isolated microglia is yet to be ascertained. The neuronal expression of both AdipoR1 and AdipoR2 has been demonstrated in different brain regions as indicated in the figure. Table B indicates the roles of adiponectin-mediated signalling.
Contrary to its counterpart leptin, the role of adiponectin in the brain has not yet been well elucidated (Schulz et al., 2010). However, the discovery of leptin in the brain and its precise roles in food intake and regulating energy expenditure prompted more researchers to explore the potential cross-talk between brain and adipose tissue. In addition, the inverse co-relations of plasma leptin and adiponectin levels with that of body weight led to hypotheses that adiponectin could exert a central control of energy homeostasis. This speculation led to various studies that suggested that adiponectin does indeed exert central actions, especially as regards regulating energy homeostasis. One such study has showed that an i.c.v. injection of adiponectin increased energy expenditure, without any effect on food intake (Qi et al., 2004). Contrary to this result, other publications showed that adiponectin reduced food intake (Shklyaev et al., 2003; Coope et al., 2008) via AdipoR1-mediated activation of IRS1/2, ERK, Akt, FOXO1, JAK2 and STAT3. Peripheral administration of adiponectin was shown to decrease body weight by enhancing fatty acid oxidation, without any apparent change in food intake (Berg et al., 2001; Yamauchi et al., 2001; Tomas et al., 2002). However, Kubota et al. (2007) reported that peripherally administered adiponectin rather exerted an orexigenic effect and decreased energy expenditure. These findings strongly suggest that this adipose-derived hormone plays a vital role in the physiological neuromodulation of food intake, energy expenditure and possibly even neuroendocrine and autonomic functions in the brain. While there is now considerable evidence for the presence of adiponectin and its receptors in the brain, the precise molecular and signalling mechanisms activated during the neural regulation of food intake and energy homeostasis still remain unclear. Moreover, given the broad distribution of AdipoRs in different regions of the brain, the role of adiponectin may not be limited to regulation of energy homeostasis, but this important adipokine could perhaps also be a major contributor to ‘adipocyte-brain cross-talk’.
Although its anti-inflammatory properties have been questioned by some (Garaulet et al., 2007; Fantuzzi, 2008), the anti-diabetic and anti-atherosclerotic effects of adiponectin in various in vitro and in vivo studies have prompted researchers to explore its use, or the targeting of its receptors, for therapy in these disease conditions (Iwaki et al., 2003; Shimada et al., 2004; Axelsson et al., 2005; Mendez-Sanchez et al., 2006). Another facet of adiponectin actions that has attracted attention in recent years is its effect on apoptosis. Physiological apoptosis is well known to regulate many pivotal biological processes like organ development and differentiation. However, dysfunction or dysregulation of apoptosis under pathological conditions like cancer, ischaemia–reperfusion injury and cardiovascular and cerebrovascular conditions is detrimental (Kockx and Herman, 2000; Ghavami et al., 2009; Guo and Wang, 2010). A few studies have reported anti-apoptotic effects of adiponectin (Lin et al., 2004; Masaki et al., 2004; Rakatzi et al., 2004; Shibata et al., 2005; 2007), whereas many others have found adiponectin to promote apoptosis under both in vitro and in vivo conditions (Yokota et al., 2000; Brakenhielm et al., 2004; Kang et al., 2005; Dieudonne et al., 2006; Wang et al., 2006b; Cong et al., 2007; Ishikawa et al., 2007; Korner et al., 2007; Akifusa et al., 2008; Dos Santos et al., 2008; Kawai et al., 2008; Konturek et al., 2008; Thundyil et al., 2010). Hence, caution must be exercised in characterizing the role of adiponectin on apoptosis in different organ systems. This may be particularly important in the case of the brain, and since adiponectin and its receptors have been shown to be present in the brain, its role in mediating apoptosis in brain cells is of considerable interest for physiology and especially in disease.
Adiponectin in CNS pathologies
A few studies have investigated the role of adiponectin in cerebrovascular diseases. The objectives of these studies ranged from establishing whether hypoadiponectinaemia is a predictor of cerebrovascular disease to ascertaining if adiponectin has a neuroprotective function during stroke. The results from these studies have so far been conflicting with no clear correlation between serum adiponectin levels and stroke onset (Soderberg et al., 2004; Chen et al., 2005). Recently, Nishimura et al. (2008) reported that adiponectin has a cerebroprotective action mediated through the eNOS signalling pathway. They showed that adiponectin-KO mice developed larger brain infarction and exhibited greater neurological deficits after ischaemia–reperfusion compared with wild-type (WT) mice. Moreover, systemic administration of adenoviral vectors expressing full-length murine adiponectin significantly reduced cerebral infarct size in WT and adiponectin-KO mice (Nishimura et al., 2008). However, that study did not investigate the effect of gAD, which has a higher binding affinity towards AdipoR1. We recently published that mouse cortical neurons express AdipoR1 and AdipoR2, and that the levels of AdipoR1 increase in response to ischaemic conditions in vitro or in vivo (Thundyil et al., 2010). Furthermore, we observed that neurons treated with either globular or trimeric adiponectin exhibited increased vulnerability to oxygen and glucose deprivation, which was associated with increased activation of a pro-apoptotic signalling cascade involving p38-MAPK and AMPK. We propose an underlying mechanism initiated by the complex pathophysiological processes that disrupt the BBB in stroke (Arumugam et al., 2005), whereby the microvasculature assumes an inflammatory phenotype characterized by leukocyte–endothelial cell adhesion, leukocyte capillary plugging, endothelial barrier dysfunction and activation of resident leukocytes including neutrophils (Ishikawa et al., 2005). Although the brain is normally isolated from the immune system by the BBB, activated leukocytes can readily infiltrate the brain once the BBB is disrupted during ischaemic stroke. This disruption could also facilitate the penetration of full-length adiponectin into injured brain tissues, which could then be further cleaved into gAD by leukocyte elastase at the site of injury (Waki et al., 2005).
The pathophysiological importance of adiponectin cleavage by leukocyte elastase in vivo and especially during stroke still remains unclear. However, various studies in different cell types have reported a pro-inflammatory role for gAD (Haugen and Drevon, 2007; Hattori et al., 2008; Tomizawa et al., 2008). These studies showed gAD to be a potent stimulator of NF-κB and other pro-inflammatory genes, which could be detrimental during an inflammatory pathology such as ischaemic stroke. Since physiological levels of adiponectin in both human and mouse serum have been reported to range from 2 to 17 µg·mL−1, and that they are elevated during inflammatory disease conditions such as preeclampsia and arthritis (Senolt et al., 2006; Toussirot et al., 2007; Fantuzzi, 2008; Brochu-Gaudreau et al., 2010), the concentration of adiponectin used in our study (10 µg·mL−1) was well within the physiological range.
A recent study by Une et al. (2010) demonstrated the presence of adiponectin in mild cognitive impairment and Alzheimer's disease (AD) patients. They analysed adiponectin in plasma and CSF from cognitively normal controls (NC), subjects with mild cognitive impairment (MCI) and patients with AD. They found that plasma adiponectin was significantly higher in MCI and AD compared with NC, whereas CSF adiponectin was significantly higher in MCI compared to NC. These higher adiponectin levels in plasma and CSF in MCI and AD patients is intriguing and could suggest that this molecule plays a role in the onset of AD. The authors also suggested that the weight loss and lower body mass index seen in elderly AD patients, and the correspondingly high level of adiponectin during the same period, could point to a role for adiponectin during the pro-dromal state of AD by altering the CNS control of energy homeostatsis and body weight (Aziz et al., 2008; Luchsinger, 2008; Luchsinger and Gustafson, 2009a,b) (Figure 5).
From observations obtained so far regarding its pro-apoptotic role, it could be that adiponectin promotes neuronal apoptosis even in conditions such as stroke and AD. Furthermore, an epemiological study by Hietaharju et al. (2010) showed that multiple sclerosis (MS) patients had elevated adiponectin levels in CSF that could be related to immune reactions responsible for inciting MS relapses. In another study published by Jeon et al. (2009), they showed that adiponectin treatment protected hippocampal neurons against kainic acid (KA)-induced neurotoxicity and thus had neuroprotective effects in an animal model of seizures. The decreased serum adiponectin levels and increased hippocampal AdipoR1 expression levels in KA-treated mice were shown to be protected by adiponectin pretreatment. The up-regulation of hippocampal VEGF, eNOS and NF-κB levels in the seizure mice were reduced following adiponectin treatment. Although that study shows a beneficial effect of adiponectin and is in stark contrast to our studies, the up-regulation of AdipoR1 in this model, too, suggests the possibility that adiponectin and its receptors may not be mere spectators in neuronal regulation during physiological and pathological processes, but they may have a larger and more definitive role in the functioning and maintenance of the brain.
Although it is now established that that adiponectin is present in the CSF, further investigations will be needed to clarify where CSF adiponectin is produced and how it circulates in the brain. The issue of adiponectin transport from the periphery into the CNS and how this occurs is still a matter of contention (Qi et al., 2004; Pan et al., 2006; Spranger et al., 2006). One plausible explanation is that adiponectin enters the brain via receptor-mediated transcytosis (Kos et al., 2007). Another potential site of entry is via the circumventricular organs (CVOs) (Ahima et al., 2006; Fry et al., 2006). However, this view has been challenged because the low surface area of the CVOs cannot account for the levels of adiponectin detectable in CSF (Begley, 1994). Also, an intrathecal synthesis of adiponectin is possible because the level of adiponectin in CSF does not appear to correlate with the level in plasma (Hietaharju et al., 2010). This capacity for endogenous production of adiponectin within the CNS has been shown in rodents and chicken (Maddineni et al., 2005; Wilkinson et al., 2007). Hence, more work is required to clarify whether adiponectin is synthesized intrathecally or whether it flows into the intrathecal space from plasma passing through BBB. Second, it will be important to define the role(s) of AdipoR-mediated signalling in the brain in normal physiology and in disease states. It appears that the confounding findings have arisen at least partly from the fact that adiponectin circulates as different oligomers. Although, recently developed elisas are more sensitive for detecting adiponectin in the CSF, their large-scale use has been curtailed primarily due to the high costs involved. Furthermore, the complications arising from the measurement of different adiponectin oligomers versus total adiponectin, together with its pleiotropic roles, has limited the usefulness of adiponectin as a biomarker of human diseases. Hence, the prospect of devising a co-relation of adiponectin between peripheral and CNS is still premature and requires much deliberation.
Concluding remarks
It has become evident in recent years that AdipoR expression and functionality extends to the CNS. Neurons express different subsets of AdipoRs, and it is not surprising, therefore, that AdipoRs play important roles in neurodegenerative processes. AdipoRs appear to be intimately involved in several neurodegenerative disorders including AD, MS, epilepsy and ischaemic stroke, which implies a broad functionality of these receptors in neurological disease. We predict that AdipoRs may emerge as a target for intervention in the relentless efforts to find new therapies for neurodegenerative disorders.
Acknowledgments
This work was supported by the National Heart Foundation of Australia and by an ARC Future Fellowship awarded to TVA.
Glossary
- ACC
acetyl-CoA carboxylase
- AD
Alzheimer's disease
- AdipoR
adiponectin receptor
- AMPK
adenosine monophosphate-activated protein kinase
- APPL
adaptor protein containing a pleckstrin homology domain, a phosphotyrosine domain and a leucine zipper motif
- EBP
enhancer-binding protein
- eNOS
endothelial NOS
- FOXO1
forkhead transcription factor O1
- gAD
globular adiponectin
- MS
multiple sclerosis
- PAQR
progestin and AdipoQ receptor
- PI3K
phosphatidyl inositol 3-kinase
- RACK
receptor for activated C-kinase
- SIRT1
silent mating type information regulation 2 homologue 1
Conflict of Interest
On behalf of all the named authors, I declare that there is no conflict of interest.
References
- Ahima RS, Qi Y, Singhal NS, Jackson MB, Scherer PE. Brain adipocytokine action and metabolic regulation. Diabetes. 2006;55(Suppl. 2):S145–S154. doi: 10.2337/db06-s018. [DOI] [PubMed] [Google Scholar]
- Akifusa S, Kamio N, Shimazaki Y, Yamaguchi N, Yamashita Y. Regulation of globular adiponectin-induced apoptosis by reactive oxygen/nitrogen species in RAW264 macrophages. Free Radic Biol Med. 2008;45:1326–1339. doi: 10.1016/j.freeradbiomed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Alberti L, Gilardini L, Girola A, Moro M, Cavagnini F, Invitti C. Adiponectin receptors gene expression in lymphocytes of obese and anorexic patients. Diabetes Obes Metab. 2007;9:344–349. doi: 10.1111/j.1463-1326.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Aroda V, Ciaraldi TP, Chang SA, Dahan MH, Chang RJ, Henry RR. Circulating and cellular adiponectin in polycystic ovary syndrome: relationship to glucose tolerance and insulin action. Fertil Steril. 2008;89:1200–1208. doi: 10.1016/j.fertnstert.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Granger DN, Mattson MP. Stroke and T-cells. Neuromolecular Med. 2005;7:229–242. doi: 10.1385/NMM:7:3:229. [DOI] [PubMed] [Google Scholar]
- Axelsson J, Heimburger O, Lindholm B, Stenvinkel P. Adipose tissue and its relation to inflammation: the role of adipokines. J Ren Nutr. 2005;15:131–136. doi: 10.1053/j.jrn.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Aziz NA, van der Marck MA, Pijl H, Olde Rikkert MG, Bloem BR, Roos RA. Weight loss in neurodegenerative disorders. J Neurol. 2008;255:1872–1880. doi: 10.1007/s00415-009-0062-8. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Feve B, Bruckert E. Adipose tissue: an endocrine gland and organ of immunity. Sang Thromb Vaiss. 2009;21:343–346. [Google Scholar]
- Begley DJ. Peptides and the blood-brain barrier: the status of our understanding. Ann NY Acad Sci. 1994;739:89–100. doi: 10.1111/j.1749-6632.1994.tb19810.x. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, et al. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–849. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Beylot M, Pinteur C, Peroni O. Expression of the adiponectin receptors AdipoR1 and AdipoR2 in lean rats and in obese Zucker rats. Metabolism. 2006;55:396–401. doi: 10.1016/j.metabol.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Bluher M, Fasshauer M, Kralisch S, Krohn K, Paschke R. Regulation of adiponectin receptor R1 and R2 gene expression in adipocytes of C57BL/6 mice. Biochem Biophys Res Commun. 2005;329:1127–1132. doi: 10.1016/j.bbrc.2005.02.081. [DOI] [PubMed] [Google Scholar]
- Bonnard C, Durand A, Vidal H, Rieusset J. Changes in adiponectin, its receptors and AMPK activity in tissues of diet-induced diabetic mice. Diabetes Metab. 2008;34:52–61. doi: 10.1016/j.diabet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Bottner A, Kratzsch J, Muller G, Kapellen TM, Bluher S, Keller E, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89:4053–4061. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- Brakenhielm E, Veitonmaki N, Cao RH, Kihara S, Matsuzawa YJ, Zhivotovsky B, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass A, Kadler KE, Thomas JT, Grant ME, Boothandford RP. The fibrillar collagens, collagen-Viii, collagen-X and the C1q complement proteins share a similar domain in their c-terminal noncollagenous regions. FEBS Lett. 1992;303:126–128. doi: 10.1016/0014-5793(92)80503-9. [DOI] [PubMed] [Google Scholar]
- Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, Palin MF. Adiponectin action from head to toe. Endocrine. 2010;37:11–32. doi: 10.1007/s12020-009-9278-8. [DOI] [PubMed] [Google Scholar]
- Bullen JW, Jr, Bluher S, Kelesidis T, Mantzoros CS. Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. Am J Physiol Endocrinol Metab. 2007;292:E1079–E1086. doi: 10.1152/ajpendo.00245.2006. [DOI] [PubMed] [Google Scholar]
- Caminos JE, Nogueiras R, Gallego R, Bravo S, Tovar S, Garcia-Caballero T, et al. Expression and regulation of adiponectin and receptor in human and rat placenta. J Clin Endocrinol Metab. 2005;90:4276–4286. doi: 10.1210/jc.2004-0930. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Boylston WH, Venkatachalam K, Webster NJG, Prabhu SD, Valente AJ. Adiponectin blocks interleukin-18-mediated endothelial cell death via APPL1-dependent AMP-activated Protein Kinase (AMPK) activation and IKK/NF-kappa B/PTEN suppression. J Biol Chem. 2008;283:24889–24898. doi: 10.1074/jbc.M804236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SP, Chen YH, Chang WC, Liu IM, Cheng JT. Increase of adiponectin receptor gene expression by physical exercise in soleus muscle of obese Zucker rats. Eur J Appl Physiol. 2006;97:189–195. doi: 10.1007/s00421-006-0163-3. [DOI] [PubMed] [Google Scholar]
- Charlton HK, Webster J, Kruger S, Simpson F, Richards AA, Whitehead JP. ERp46 binds to AdipoR1, but not AdipoR2, and modulates adiponectin signalling. Biochem Biophys Res Commun. 2010;392:234–239. doi: 10.1016/j.bbrc.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- Chen MP, Tsai JC, Chung FM, Yang SS, Hsing LL, Shin SJ, et al. Hypoadiponectinemia is associated with ischemic cerebrovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:821–826. doi: 10.1161/01.ATV.0000157784.25920.a7. [DOI] [PubMed] [Google Scholar]
- Chen J, Tan B, Karteris E, Zervou S, Digby J, Hillhouse EW, et al. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia. 2006;49:1292–1302. doi: 10.1007/s00125-006-0194-7. [DOI] [PubMed] [Google Scholar]
- Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–1394. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Zawadski C, Fruchart JC, Staels B. Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPARalpha, PPARgamma, and LXR. Biochem Biophys Res Commun. 2004;314:151–158. doi: 10.1016/j.bbrc.2003.12.058. [DOI] [PubMed] [Google Scholar]
- Christiansen T, Paulsen SK, Bruun JM, Ploug T, Pedersen SB, Richelsen B. Diet-induced weight loss and exercise alone and in combination enhance the expression of adiponectin receptors in adipose tissue and skeletal muscle, but only diet-induced weight loss enhanced circulating adiponectin. J Clin Endocrinol Metab. 2009;95:911–919. doi: 10.1210/jc.2008-2505. [DOI] [PubMed] [Google Scholar]
- Civitarese AE, Jenkinson CP, Richardson D, Bajaj M, Cusi K, Kashyap S, et al. Adiponectin receptors gene expression and insulin sensitivity in non-diabetic Mexican Americans with or without a family history of Type 2 diabetes. Diabetologia. 2004;47:816–820. doi: 10.1007/s00125-004-1359-x. [DOI] [PubMed] [Google Scholar]
- Civitarese AE, Ukropcova B, Hulver M, Defronzo R, Mandarino L, Scherer P, et al. Adiponectin receptors mediated regulation of mitochondrial bioenergetics in skeletal-muscle. Diabetes. 2005;54:A91–A91. [Google Scholar]
- Civitarese AE, Ukropcova B, Carling S, Hulver M, DeFronzo RA, Mandarino L, et al. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab. 2006;4:75–87. doi: 10.1016/j.cmet.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- Cong L, Gasser J, Zhao J, Yang B, Li F, Zhao AZ. Human adiponectin inhibits cell growth and induces apoptosis in human endometrial carcinoma cells, HEC-1-A and RL95-2. Endocr Relat Cancer. 2007;14:713–720. doi: 10.1677/ERC-07-0065. [DOI] [PubMed] [Google Scholar]
- Coope A, Milanski M, Araujo EP, Tambascia M, Saad MJ, Geloneze B, et al. AdipoR1 mediates the anorexigenic and insulin/leptin-like actions of adiponectin in the hypothalamus. FEBS Lett. 2008;582:1471–1476. doi: 10.1016/j.febslet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Dai MH, Xia T, Zhang GD, Chen XD, Gan L, Feng SQ, et al. Cloning, expression and chromosome localization of porcine adiponectin and adiponectin receptors genes. Domest Anim Endocrinol. 2006;30:117–125. doi: 10.1016/j.domaniend.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Dalrymple MB, Pfleger KDG, Eidne KA. G protein-coupled receptor dimers: functional consequences, disease states and drug targets. Pulm Pharmacol Ther. 2008;118:359–371. doi: 10.1016/j.pharmthera.2008.03.004. [DOI] [PubMed] [Google Scholar]
- D'Anna R, Baviera G, Corrado F, Giordano D, De Vivo A, Nicocia G, et al. Adiponectin and insulin resistance in early- and late-onset pre-eclampsia. BJOG. 2006;113:1264–1269. doi: 10.1111/j.1471-0528.2006.01078.x. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Dong LQ. APPL1: role in adiponectin signaling and beyond. Am J Physiol Endocrinol Metab. 2009;296:E22–E36. doi: 10.1152/ajpendo.90731.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM. Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology. 2004;145:5589–5597. doi: 10.1210/en.2004-0503. [DOI] [PubMed] [Google Scholar]
- Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2006;345:271–279. doi: 10.1016/j.bbrc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- Ding ST, Liu BH, Ko YH. Cloning and expression of porcine adiponectin and adiponectin receptor 1 and 2 genes in pigs. J Anim Sci. 2004;82:3162–3174. doi: 10.2527/2004.82113162x. [DOI] [PubMed] [Google Scholar]
- Ding GL, Qin QH, He N, Francis-David SC, Hou J, Liu J, et al. Adiponectin and its receptors are expressed in adult ventricular cardiomyocytes and upregulated by activation of peroxisome proliferator-activated receptor gamma. J Mol Cell Cardiol. 2007;43:73–84. doi: 10.1016/j.yjmcc.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding QR, Wang ZZ, Chen Y. Endocytosis of adiponectin receptor 1 through a clathrin- and Rab5-dependent pathway. Cell Res. 2009;19:317–327. doi: 10.1038/cr.2008.299. [DOI] [PubMed] [Google Scholar]
- Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, et al. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep. 2008;20:971–977. [PubMed] [Google Scholar]
- Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Alvarez-Blasco F, Sanchon R, Luque-Ramirez M, et al. Adiponectin and resistin in PCOS: a clinical, biochemical and molecular genetic study. Hum Reprod. 2006;21:2257–2265. doi: 10.1093/humrep/del146. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121:326–330. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Klein J, Kralisch S, Klier M, Lossner U, Bluher M, et al. Growth hormone is a positive regulator of adiponectin receptor 2 in 3T3-L1 adipocytes. FEBS Lett. 2004;558:27–32. doi: 10.1016/S0014-5793(03)01525-4. [DOI] [PubMed] [Google Scholar]
- Felder TK, Hahne P, Soyal SM, Miller K, Hoffinger H, Oberkofler H, et al. Hepatic adiponectin receptors (ADIPOR) 1 and 2 mRNA and their relation to insulin resistance in obese humans. Int J Obes. 2010;34:846–851. doi: 10.1038/ijo.2010.7. [DOI] [PubMed] [Google Scholar]
- Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M, Smith PM, Hoyda TD, Duncan M, Ahima RS, Sharkey KA, et al. Area postrema neurons are modulated by the adipocyte hormone adiponectin. J Neurosci. 2006;26:9695–9702. doi: 10.1523/JNEUROSCI.2014-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet M, Hernandez-Morante JJ, de Heredia FP, Tebar FJ. Adiponectin, the controversial hormone. Public Health Nutr. 2007;10:1145–1150. doi: 10.1017/S1368980007000638. [DOI] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source (vol 131, pg 242, 2007) Cell. 2008;135:366–366. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, et al. Apoptosis and cancer: mutations within caspase genes. J Med Genet. 2009;46:497–510. doi: 10.1136/jmg.2009.066944. [DOI] [PubMed] [Google Scholar]
- Glintborg D, Frystyk J, Hojlund K, Andersen KK, Henriksen JE, Hermann AP, et al. Total and high molecular weight (HMW) adiponectin levels and measures of glucose and lipid metabolism following pioglitazone treatment in a randomized placebo-controlled study in polycystic ovary syndrome. Clin Endocrinol (Oxf) 2008;68:165–174. doi: 10.1111/j.1365-2265.2007.03015.x. [DOI] [PubMed] [Google Scholar]
- Gu WQ, Li XY, Liu CQ, Yang J, Ye L, Tang JF, et al. Globular adiponectin augments from pancreatic islet beta cells at insulin secretion high glucose concentrations. Endocrine. 2006;30:217–221. doi: 10.1385/ENDO:30:2:217. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M. Adiponectin: an update. Diabetes Metab. 2008;34:12–18. doi: 10.1016/j.diabet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Guo Z, Wang JP. Blockade of spinal nerves attenuates myocardial apoptosis in acute myocardial ischaemia/infarction in rats. Eur J Anaesthesiol. 2010;27:146–152. doi: 10.1097/EJA.0b013e32832e08f8. [DOI] [PubMed] [Google Scholar]
- Hada Y, Yamauchi T, Waki H, Tsuchida A, Hara K, Yago H, et al. Selective purification and characterization of adiponectin multimer species from human plasma. Biochem Biophys Res Commun. 2007;356:487–493. doi: 10.1016/j.bbrc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by Endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Hattori S, Kasai K. Globular adiponectin activates nuclear Factor-kappa B in vascular endothelial cells, which in turn induces expression of proinflammatory and adhesion molecule genes. Diabetes Care. 2006;29:139–141. doi: 10.2337/diacare.29.1.139. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Nakano Y, Hattori S, Tomizawa A, Inukai K, Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-kappa B activation in vascular endothelial cells. FEBS Lett. 2008;582:1719–1724. doi: 10.1016/j.febslet.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Haugen F, Drevon CA. Activation of nuclear factor-kappaB by high molecular weight and globular adiponectin. Endocrinology. 2007;148:5478–5486. doi: 10.1210/en.2007-0370. [DOI] [PubMed] [Google Scholar]
- Haugen F, Ranheim T, Harsem NK, Lips E, Staff AC, Drevon CA. Increased plasma levels of adipokines in preeclampsia: relationship to placenta and adipose tissue gene expression. Am J Physiol Endocrinol Metab. 2006;290:E326–E333. doi: 10.1152/ajpendo.00020.2005. [DOI] [PubMed] [Google Scholar]
- Heiker JT, Wottawah CM, Juhl C, Kosel D, Morl K, Beck-Sickinger AG. Protein kinase CK2 interacts with adiponectin receptor 1 and participates in adiponectin signaling. Cell Signal. 2009;21:936–942. doi: 10.1016/j.cellsig.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Hietaharju A, Kuusisto H, Nieminen R, Vuolteenaho K, Elovaara I, Moilanen E. Elevated cerebrospinal fluid adiponectin and adipsin levels in patients with multiple sclerosis: a Finnish co-twin study. Eur J Neurol. 2010;17:332–334. doi: 10.1111/j.1468-1331.2009.02701.x. [DOI] [PubMed] [Google Scholar]
- Hoyda TD, Fry M, Ahima RS, Ferguson AV. Adiponectin selectively inhibits oxytocin neurons of the paraventricular nucleus of the hypothalamus. J Physiol. 2007;585:805–816. doi: 10.1113/jphysiol.2007.144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Vowinkel T, Stokes KY, Arumugam TV, Yilmaz G, Nanda A, et al. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005;111:1690–1696. doi: 10.1161/01.CIR.0000160349.42665.0C. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Kitayama J, Yamauchi T, Kadowaki T, Maki T, Miyato H, et al. Adiponectin inhibits the growth and peritoneal metastasis of gastric cancer through its specific membrane receptors AdipoR1 and AdipoR2. Cancer Sci. 2007;98:1120–1127. doi: 10.1111/j.1349-7006.2007.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- Jeon BT, Shin HJ, Kim JB, Kim YK, Lee DH, Kim KH, et al. Adiponectin protects hippocampal neurons against kainic acid-induced excitotoxicity. Brain Res Rev. 2009;61:81–88. doi: 10.1016/j.brainresrev.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Jonsson JR, Moschen AR, Hickman IJ, Richardson MM, Kaser S, Clouston AD, et al. Adiponectin and its receptors in patients with chronic hepatitis C. J Hepatol. 2005;43:929–936. doi: 10.1016/j.jhep.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biol. 2007;8:1–12. doi: 10.1186/1471-2121-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Lee YY, Yu BY, Yang BS, Cho KH, Yoon DK, et al. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch Pharm Res. 2005;28:1263–1269. doi: 10.1007/BF02978210. [DOI] [PubMed] [Google Scholar]
- Kang KH, Higashino A, Kim HS, Lee YT, Kageyama T. Molecular cloning, gene expression, and tissue distribution of adiponectin and its receptors in the Japanese monkey, Macaca fuscata. J Med Primatol. 2009;38:77–85. doi: 10.1111/j.1600-0684.2008.00298.x. [DOI] [PubMed] [Google Scholar]
- Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, et al. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117–121. doi: 10.1136/gut.2003.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K, Kageyama A, Tsumano T, Nishimoto S, Fukuda K, Yokoyama S, et al. Effects of adiponectin on growth and differentiation of human keratinocytes – implication of impaired wound healing in diabetes. Biochem Biophys Res Commun. 2008;374:269–273. doi: 10.1016/j.bbrc.2008.07.045. [DOI] [PubMed] [Google Scholar]
- Kharroubi I, Rasschaert J, Eizirik DL, Cnop M. Expression of adiponectin receptors in pancreatic beta cells. Biochem Biophys Res Commun. 2003;312:1118–1122. doi: 10.1016/j.bbrc.2003.11.042. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:E27–E31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockx MM, Herman AG. Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovasc Res. 2000;45:736–746. doi: 10.1016/s0008-6363(99)00235-7. [DOI] [PubMed] [Google Scholar]
- Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M. Expanding role of AMPK in endocrinology. Trends Endocrinol Metab. 2006;17:205–215. doi: 10.1016/j.tem.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Konturek PC, Burnat G, Rau T, Hahn EG, Konturek S. Effect of adiponectin and ghrelin on apoptosis of barrett adenocarcinoma cell line. Dig Dis Sci. 2008;53:597–605. doi: 10.1007/s10620-007-9922-1. [DOI] [PubMed] [Google Scholar]
- Korner A, Pazaitou-Panayiotou K, Kelesidis T, Kelesidis I, Wiliams CJ, Kaprara A, et al. Human adiponectin inhibits cell growth and induces apoptosis in human endometrial carcinoma cells, HEC-1-A and RL95-2 (vol 14, pg 713, 2007) Endocr Relat Cancer. 2007;14:1127–1127. doi: 10.1677/ERC-07-0065. [DOI] [PubMed] [Google Scholar]
- Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S, et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab. 2007;92:1129–1136. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- Kosel D, Heiker JT, Juhl C, Wottawah CM, Bluher M, Morl K, et al. Dimerization of adiponectin receptor 1 is inhibited by adiponectin. J Cell Sci. 2010;123:1320–1328. doi: 10.1242/jcs.057919. [DOI] [PubMed] [Google Scholar]
- Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kusminski CM, McTernan PG, Schraw T, Kos K, O'Hare JP, Ahima R, et al. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007;50:634–642. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- Li P, Sun F, Cao HM, Ma QY, Pan CM, Ma JH, et al. Expression of adiponectin receptors in mouse adrenal glands and the adrenocortical Y-1 cell line: adiponectin regulates steroidogenesis. Biochem Biophys Res Commun. 2009;390:1208–1213. doi: 10.1016/j.bbrc.2009.10.122. [DOI] [PubMed] [Google Scholar]
- Liao WQ, Yu CA, Wen JY, Jia W, Li G, Ke YA, et al. Adiponectin induces interleukin-6 production and activates STAT3 in adult mouse cardiac fibroblasts. Circulation. 2010;122:E193–E193. doi: 10.1042/BC20080117. [DOI] [PubMed] [Google Scholar]
- Lin LY, Lin CY, Su TC, Liau CS. Angiotensin II-induced apoptosis in human endothelial cells is inhibited by adiponectin through restoration of the association between endothelial nitric oxide synthase and heat shock protein 90. FEBS Lett. 2004;574:106–110. doi: 10.1016/j.febslet.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Liu BH, Wang PH, Wang YC, Cheng WM, Mersmann HJ, Ding ST. Fasting regulates the expression of adiponectin receptors in young growing pigs. J Anim Sci. 2008a;86:3377–3384. doi: 10.2527/jas.2008-0971. [DOI] [PubMed] [Google Scholar]
- Liu BH, Wang YC, Wu SC, Mersmann HJ, Cheng WT, Ding ST. Insulin regulates the expression of adiponectin and adiponectin receptors in porcine adipocytes. Domest Anim Endocrinol. 2008b;34:352–359. doi: 10.1016/j.domaniend.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA. Adiposity, hyperinsulinemia, diabetes and Alzheimer's disease: an epidemiological perspective. Eur J Pharmacol. 2008;585:119–129. doi: 10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Gustafson DR. Adiposity and Alzheimer's disease. Curr Opin Clin Nutr Metab Care. 2009a;12:15–21. doi: 10.1097/MCO.0b013e32831c8c71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer's disease. J Alzheimers Dis. 2009b;16:693–704. doi: 10.3233/JAD-2009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddineni S, Metzger S, Ocon O, Hendricks G, Ramachandran R. Adiponectin gene is expressed in multiple tissues in the chicken: food deprivation influences adiponectin messenger ribonucleic acid expression. Endocrinology. 2005;146:4250–4256. doi: 10.1210/en.2005-0254. [DOI] [PubMed] [Google Scholar]
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. CDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (Adipose most abundant gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Malagon MM, Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, et al. Regulation of pituitary cell function by the adipokine adiponectin. Front Neuroendocrinol. 2006;27:35–35. [Google Scholar]
- Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, et al. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. Adiponectin: identification, physiology and clinical relevance in metabolic and vascular disease. Atherosclerosis Suppl. 2005;6:7–14. doi: 10.1016/j.atherosclerosissup.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Mendez-Sanchez N, Chavez-Tapia NC, Zamora-Valdes D, Uribe M. Adiponectin, structure, function and pathophysiological implications in non-alcoholic fatty liver disease. Mini Rev Med Chem. 2006;6:651–656. doi: 10.2174/138955706777435689. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- Narasimhan ML, Coca MA, Jin JB, Yamauchi T, Ito Y, Kadowaki T, et al. Osmotin is a homolog of mammalian adiponectin and controls apoptosis in yeast through a homolog of mammalian adiponectin receptor (vol 17, pg 171, 2005) Mol Cell. 2005;17:611–611. doi: 10.1016/j.molcel.2004.11.050. [DOI] [PubMed] [Google Scholar]
- Neumeier M, Weigert J, Schaffler A, Weiss T, Kirchner S, Laberer S, et al. Regulation of adiponectin receptor 1 in human hepatocytes by agonists of nuclear receptors. Biochem Biophys Res Commun. 2005;334:924–929. doi: 10.1016/j.bbrc.2005.06.187. [DOI] [PubMed] [Google Scholar]
- Neumeier M, Weigert J, Buettner R, Wanninger J, Schaffler A, Muller AM, et al. Detection of adiponectin in cerebrospinal fluid in humans. Am J Physiol Endocrinol Metab. 2007;293:E965–E969. doi: 10.1152/ajpendo.00119.2007. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, et al. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008;117:216–223. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–2741. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules – Adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajvani UB, Du XL, Combs TP, Berg AH, Rajala MW, Schulthess T, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin – Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- Palanivel R, Fang XP, Park M, Eguchi M, Pallan S, De Girolamo S, et al. Globular and full-length forms of adiponectin mediate specific changes in glucose and fatty acid uptake and metabolism in cardiomyocytes. Cardiovasc Res. 2007;75:148–157. doi: 10.1016/j.cardiores.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Pan WH, Tu H, Kastin AJ. Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides. 2006;27:911–916. doi: 10.1016/j.peptides.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Patel JV, Abraheem A, Dotsenko O, Creamer J, Gunning M, Hughes EA, et al. Circulating serum adiponectin levels in patients with coronary artery disease: relationship to atherosclerotic burden and cardiac function. J Int Med. 2008;264:593–598. doi: 10.1111/j.1365-2796.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psilopanagioti A, Papadaki H, Kranioti EF, Alexandrides TK, Varakis JN. Expression of adiponectin and adiponectin receptors in human pituitary gland and brain. Neuroendocrinology. 2009;89:38–47. doi: 10.1159/000151396. [DOI] [PubMed] [Google Scholar]
- Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281:39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- Rakatzi I, Mueller H, Ritzeler O, Tennagels N, Eckel J. Adiponectin counteracts cytokine- and fatty acid-induced apoptosis in the pancreatic beta-cell line INS-1. Diabetologia. 2004;47:249–258. doi: 10.1007/s00125-003-1293-3. [DOI] [PubMed] [Google Scholar]
- Ranscht B, Dourszimmermann MT. T-cadherin, a novel cadherin cell-adhesion molecule in the nervous-system lacks the conserved cytoplasmic region. Neuron. 1991;7:391–402. doi: 10.1016/0896-6273(91)90291-7. [DOI] [PubMed] [Google Scholar]
- Rasmussen MS, Lihn AS, Pedersen SB, Bruun JM, Rasmussen M, Richelsen B. Adiponectin receptors in human adipose tissue: effects of obesity, weight loss, and fat depots. Obesity. 2006;14:28–35. doi: 10.1038/oby.2006.5. [DOI] [PubMed] [Google Scholar]
- Redinger RN. Fat storage and the biology of energy expenditure. Transl Res. 2009;154:52–60. doi: 10.1016/j.trsl.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Berton F, Cottone P, Reifel-Miller A, Roberts AJ, Morales M, et al. A potential role for adiponectin receptor 2 (AdipoR2) in the regulation of alcohol intake. Brain Res. 2010;1339:11–17. doi: 10.1016/j.brainres.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards AA, Stephens T, Charlton HK, Jones A, MacDonald GA, Prins JB, et al. Adiponectin multimerization is dependent on conserved lysines in the collagenous domain: evidence for regulation of multimerization by alterations in posttranslational modifications. Mol Endocrinol. 2006;20:1673–1687. doi: 10.1210/me.2005-0390. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C, et al. Regulation of pituitary cell function by adiponectin. Endocrinology. 2007;148:401–410. doi: 10.1210/en.2006-1019. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- Schulz C, Paulus K, Lehnert H. Adipocyte-brain: crosstalk. Results Probl Cell Differ. 2010;52:189–201. doi: 10.1007/978-3-642-14426-4_16. [DOI] [PubMed] [Google Scholar]
- Senolt L, Pavelka K, Housa D, Haluzik M. Increased adiponectin is negatively linked to the local inflammatory process in patients with rheumatoid arthritis. Cytokine. 2006;35:247–252. doi: 10.1016/j.cyto.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol. 1998;8:335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2 dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, et al. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–1074. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Miyazaki T, Daida H. Adiponectin and atherosclerotic disease. Clin Chim Acta. 2004;344:1–12. doi: 10.1016/j.cccn.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Shimizu A, Takamura T, Matsuzawa N, Nakamura S, Nabemoto S, Takeshita Y, et al. Regulation of adiponectin receptor expression in human liver and a hepatocyte cell line. Metabolism. 2007;56:1478–1485. doi: 10.1016/j.metabol.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, et al. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci USA. 2003;100:14217–14222. doi: 10.1073/pnas.2333912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Shinohara K, Hatsuda S, Kimoto E, Fukumoto S, Emoto M, et al. Altered relationship between body fat and plasma adiponectin in end-stage renal disease. Metabolism. 2005;54:330–334. doi: 10.1016/j.metabol.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Smith BW, Hebbard L, George J. Adiponectin induces pro-inflammatory cytokines in kupffer cells and tolerance to itself via the generation of IL-10. J Gastroenterol Hepatol. 2009;24:A274–A274. [Google Scholar]
- Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian Indians. Diabetes Care. 2003;26:3226–3229. doi: 10.2337/diacare.26.12.3226. [DOI] [PubMed] [Google Scholar]
- Soderberg S, Stegmayr B, Stenlund H, Sjostrom LG, Agren A, Johansson L, et al. Leptin, but not adiponectin, predicts stroke in males. J Int Med. 2004;256:128–136. doi: 10.1111/j.1365-2796.2004.01351.x. [DOI] [PubMed] [Google Scholar]
- Spranger J, Verma S, Gohring I, Bobbert T, Seifert J, Sindler AL, et al. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55:141–147. [PubMed] [Google Scholar]
- Staiger H, Kaltenbach S, Staiger K, Stefan N, Fritsche A, Guirguis A, et al. Expression of adiponectin receptor mRNA in human skeletal muscle cells is related to in vivo parameters of glucose and lipid metabolism. Diabetes. 2004;53:2195–2201. doi: 10.2337/diabetes.53.9.2195. [DOI] [PubMed] [Google Scholar]
- Staiger K, Stefan N, Staiger H, Brendel MD, Brandhorst D, Bretzel RG, et al. Adiponectin is functionally active in human islets but does not affect insulin secretory function or beta-cell lipoapoptosis. J Clin Endocrinol Metab. 2005;90:6707–6713. doi: 10.1210/jc.2005-0467. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Adachi Y, Ohtsuki Y, Furihata M. Adiponectin receptors, with special focus on the role of the third receptor, T-cadherin, in vascular disease. Med Mol Morphol. 2007;40:115–120. doi: 10.1007/s00795-007-0364-9. [DOI] [PubMed] [Google Scholar]
- Tang YT, Hu TH, Arterburn M, Boyle B, Bright JM, Emtage PC, et al. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–380. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- Thundyil J, Tang SC, Okun E, Shah K, Karamyan VT, Li YI, et al. Evidence that adiponectin receptor 1 activation exacerbates ischemic neuronal death. Exp Transl Stroke Med. 2010;2:1–8. doi: 10.1186/2040-7378-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa A, Hattori Y, Kasai K, Nakano Y. Adiponectin induces NF-kappa B activiation that leads to suppression of cytokine-induced NF-kappa B activation in vascular endothelial cells: globular adiponectin vs. high molecular weight adiponectin. Diab Vasc Dis Res. 2008;5:123–127. doi: 10.3132/dvdr.2008.020. [DOI] [PubMed] [Google Scholar]
- Toussirot E, Streit G, Wendling D. The contribution of adipose tissue and adipokines to inflammation in joint diseases. Curr Med Chem. 2007;14:1095–1100. doi: 10.2174/092986707780362826. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–339. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) J Biol Chem. 2002;277:29359–29362. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, et al. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity – Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- Tsou PL, Jiang YD, Chang CC, Wei JN, Sung FC, Lin CC, et al. Sex-related differences between adiponectin and insulin resistance in schoolchildren. Diabetes Care. 2004;27:308–313. doi: 10.2337/diacare.27.2.308. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279:30817–30822. doi: 10.1074/jbc.M402367200. [DOI] [PubMed] [Google Scholar]
- Une K, Takei YA, Tomita N, Asamura T, Ohrui T, Furukawa K, et al. Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer's disease. Eur J Neurol. 2010;18:1006–1009. doi: 10.1111/j.1468-1331.2010.03194.x. [DOI] [PubMed] [Google Scholar]
- Vasseur F. Adiponectin and its receptors: partners contributing to the ‘vicious circle’ leading to the metabolic syndrome? Pharmacol Res. 2006;53:478–481. doi: 10.1016/j.phrs.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Vazquez-Vela MEF, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Arch Med Res. 2008;39:715–728. doi: 10.1016/j.arcmed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, et al. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J Physiol. 2006;574:41–53. doi: 10.1113/jphysiol.2006.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Diabetes. 2003;52:A1–A1. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- Waki H, Yamauchi T, Kamon J, Kita S, Ito Y, Hada Y, et al. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790–796. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- Wang AYH, Hickman IJ, Richards AA, Whitehead JP, Prins JB, MacDonald GA. High molecular weight adiponectin correlates with insulin sensitivity in patients with hepatitis C genotype 3, but not genotype 1 infection. Am J Gastroenterol. 2005a;100:2717–2723. doi: 10.1111/j.1572-0241.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KSL, Xu JY, Lu G, Xu LY, Cooper GJS, et al. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005b;280:18341–18347. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KSL, Chan L, Chan KW, Lam JBB, Lam MC, et al. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem. 2006a;281:16391–16400. doi: 10.1074/jbc.M513907200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam JB, Lam KSL, Liu J, Lam MC, Hoo RLC, et al. Adiponectin modulates the glycogen synthase kinase-3 beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006b;66:11462–11470. doi: 10.1158/0008-5472.CAN-06-1969. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409:623–633. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- Weigert J, Neumeier M, Wanninger J, Wurm S, Kopp A, Schober F, et al. Reduced response to adiponectin and lower abundance of adiponectin receptor proteins in type 2 diabetic monocytes. FEBS Lett. 2008;582:1777–1782. doi: 10.1016/j.febslet.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin – a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson M, Brown R, Imran SA, Ur E. Adipokine gene expression in brain and pituitary gland. Neuroendocrinology. 2007;86:191–209. doi: 10.1159/000108635. [DOI] [PubMed] [Google Scholar]
- Wood IS, Trayhurn P. Adipokines and the signaling role of adipose tissue in inflammation and obesity. Future Lipidol. 2007;1:81–89. [Google Scholar]
- Xu YZ, Wang NF, Ling F, Li PZ, Gao Y. Receptor for activated C-kinase 1, a novel binding partner of adiponectin receptor 1. Biochem Biophys Res Commun. 2009;378:95–98. doi: 10.1016/j.bbrc.2008.11.026. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yoda-Murakami M, Taniguchi M, Takahashi K, Kawamata S, Saito K, Choi-Miura NH, et al. Change in expression of GBP28/adiponectin in carbon tetrachloride-administrated mouse liver. Biochem Biophys Res Commun. 2001;285:372–377. doi: 10.1006/bbrc.2001.5134. [DOI] [PubMed] [Google Scholar]
- Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- Zeng Q, Isobe K, Fu L, Ohkoshi N, Ohmori H, Takekoshi K, et al. Effects of exercise on adiponectin and adiponectin receptor levels in rats. Life Sci. 2007;80:454–459. doi: 10.1016/j.lfs.2006.09.031. [DOI] [PubMed] [Google Scholar]


