Abstract
The helicene, pyrido[3,2-f]quinolino[6,5-c]cinnoline 5-oxide, was prepared by treatment of 6-hydroxylaminoquinoline with xanthine oxidase or treatment of 6-nitroquinoline with glucose in 30% NaOH and the product characterized using NMR, high resolution mass spectrometry, and X-ray crystallography. The hydrogens on carbons 7 and 12 of the terminal aromatic rings are separated by 2.495 Å creating an angle of 25.0° between the planes of the two quinoline ring systems. In the crystal, water molecules serve to link the helicenes into a one dimensional chain structure forming a hydrogen bonded bridge between N2 of one molecule and N4 of another. The molecule (C18H10N4O•H2O) crystallized in the monoclinic P21/n space group. Unit cell parameters for pyrido[3,2-f]quinolino[6,5-c]cinnoline 5-oxide monohydrate: a = 7.0829(12), b = 18.559(3), c = 11.0985(19) Å, β = 107.736(2)°, and Z = 4.
Keywords: Crystal structure; helicene; azoxy; pyrido[3,2-f]quinolino[6,5-c]cinnoline 5-oxide
Introduction
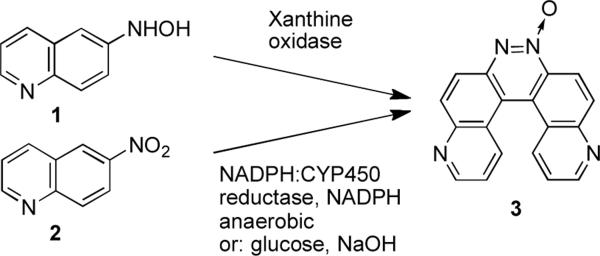
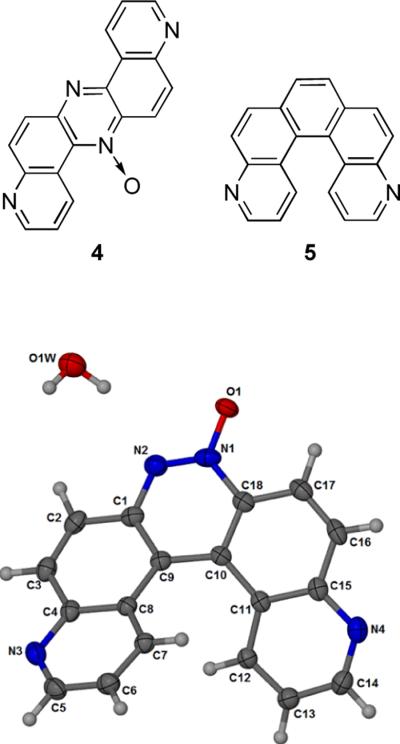
We have a longstanding interest in the enzymatic bioactivation of nitrogen-containing aromatic molecules.[1–4] During the course of recent studies on the enzymatic metabolism of 6-nit roquinoline, we observed formation of an unexpected product when 6-hydroxylaminoquinoline (1, Scheme 1) was treated with xanthine oxidase or when 6-nitroquinoline (2) was treated with NADPH:cytochrome P450 reductase and NADPH under anaerobic conditions. Characterization of the molecule by NMR, high resolution mass spectrometry, and X-ray crystallography revealed this product to be the helicene, pyrido[3,2-f]quinolino[6,5-c]cinnoline 5-oxide (3). This structure was first proposed in 1948 for the so-called azoxydichinyl product arising from the reaction of 6-nitroquinoline with sodium methoxide,[5,6] but subsequent studies provided evidence that the product of this reaction was actually 4.[7,8] However, as part of his work, Farrar revised an original structure assignment by Galbraith et al.,[9] suggesting that 3 was produced in good yield by a different reaction involving reduction of 6-nitroquinoline in a solution of alkaline glucose,[7] but the structure of the product generated in this reaction has not been characterized by modern spectroscopic or crystallographic methods. We carried out the alkaline glucose reduction of 6-nitroquinoline by the method of Galbraith[9] and Farrar[7] and showed that the product of this reaction is, in fact, compound 3, identical to the material obtained from our enzymatic reactions. To the best of our knowledge, the work described here provides the first modern characterization of the azoxydichinyl helicene (3). Our work further shows that the 1951 method of Galbraith,[9] as suggested by Farrar,[7] represents a remarkably simple synthesis of a structurally interesting helicene in one step from commercially available starting materials.
Scheme 1.
Synthesis of compounds 3.
Experimental
The compound, 6-hydroxylaminoquinoline (100 mg, 0.625 mmol), was dissolved in DMF (1 mL) and sprayed into warm water (300 mL) while stirring vigorously. To this solution, warm sodium phosphate buffer (100 mL of pH 7.4, 500 mM) was added with stirring. To this mixture, an aliquot of xanthine oxidase (100 μL of 0.005 U/mL) was added every 12 h over the course of 3 d. The mixture was then extracted with ethyl acetate (3 × 100 mL), the organic extracts combined and extracted with brine (15 mL) and the organic layer then dried over magnesium sulfate. The ethyl acetate was removed by rotary evaporation and the products isolated by flash column chromatography on silica gel eluted with ethyl acetate and methanol. Compound 3 was obtained as a yellow powder (2 mg, Rf = 0.1; 4% MeOH:EtOAc) 1H NMR (300 MHz, CDCl3) δ 9.15 (d, J = 4.5 Hz 1H), 9.05 (m, 2H), 8.87 (d, J = 9.0 Hz, 1H), 8.67 (d, J = 9.0 Hz, 1H), 8.43 (dd, J = 9.0 Hz & J = 9.0 Hz, 2H), 8.23 (d, J = 9.0 Hz, 1H), 7.46 (dd, J = 9.0 Hz, J = 4.5 Hz, 1H), 7.41 (dd, J = 9.0 Hz, J = 4.5 Hz, 1H). 13C-NMR (500 MHz, CDCl3) δ 153.34, 151.47, 149.70, 148.53, 144.07, 137.08, 136.01, 134.79, 134.27, 133.40, 128.00, 127.44, 123.52, 123.34, 121.74, 120.56, 120.46, 114.21; HRMS (ESI, M+H+) m/z calcd for C18H10N4O 299.0933, found 299.0934. Material prepared by the method of Galbraith et al.[9] and isolated by column chromatography as described above was identical in all regards to the material 3 produced in our enzymatic reaction described above.
Crystallography
Crystals of the monohydrate 3a were obtained by dissolving 3 in a minimum amount of warm methanol, followed by slow evaporation over 3 d in a 2 mL glass vial. Data was collected on a Bruker APEX II system at 173 K. The methanol used in crystallization was not dried. Presumably adventitious water present in the methanol crystallized with compound 3 to yield the monohydrate 3a in the crystals. Crystal structures were solved and refined using the SHELX programs[10] with the aid of X-Seed.[11] Conditions for crystal structure data collection and structure refinement are given in Table 1. Hydrogen atoms on the water were located in a difference map and included in the model but not refined. All other hydrogen atoms were placed at calculated positions and included using a riding model. A residual electron density peak of 1.05 e remains 1.1 Å from N2, but not in a reasonable position for a hydrogen atom. It may represent some minor whole body disorder. It persisted through two additional data sets collected with different crystals.
Table 1.
Crystallographic data for 3a.
| Empirical formula | C18 H10 N4 O•H2O |
|---|---|
| Formula weight | 316.32 |
| Crystal system | Monoclinic |
| Space group | P21/n |
| Temperature | 173(2) K |
| Unit cell dimensions | a = 7.0829(12) (Å) |
| b = 18.559(3) (Å) | |
| c = 11.0985(19)(Å) | |
| β = 107.736(2)0 | |
| Volume(Å3) | 1389.6 (4) |
| Z | 4 |
| Densitycalc(Mg/m3) | 1.512 |
| Absorption coefficient | 0.103 mm−1 |
| Total data | 15979 |
| Unique data collected | 3339 |
| Observed data | 2700 |
| Internal agreement of data(Rint) | 0.029 |
| Number of parameters refined | 217 |
| Goodness of fit(S) | 1.032 |
| R 1 | 0.0592 |
| wR 2 | 0.1653 |
Results and discussion
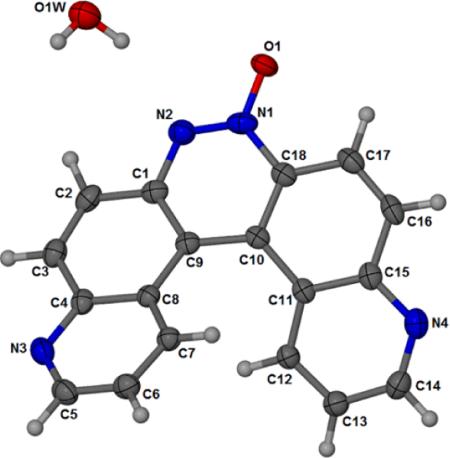
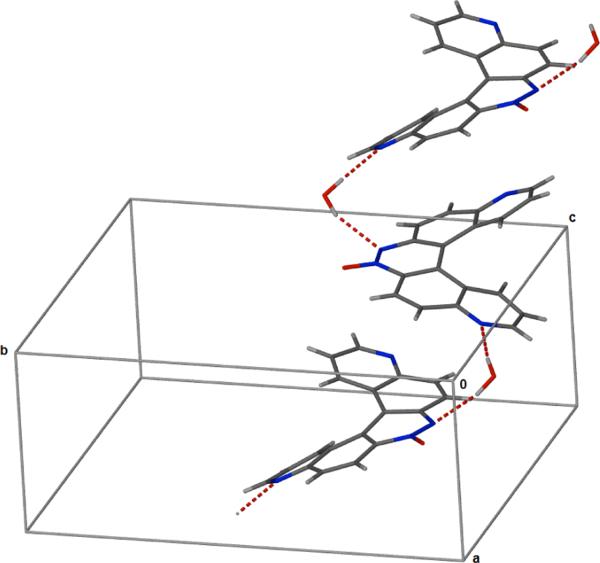
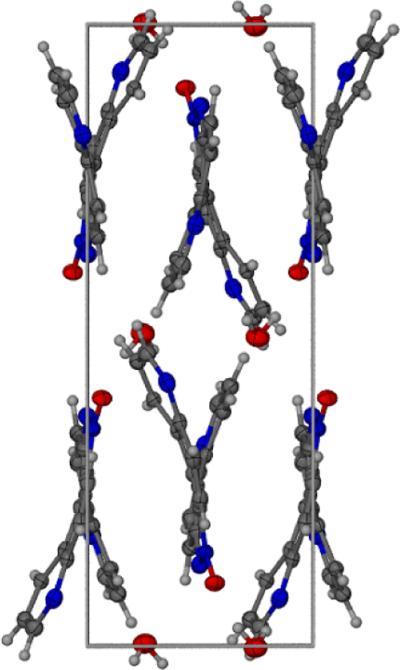
The monohydrate form of the helicene 3a was crystallized in the monoclinic space group P21/n and the crystallographic data are given in Table 1. The crystal structure of 3a is shown in Figure 1. Atomic coordinates and equivalent isotopic displacement parameters for non hydrogen atoms of compound 3a is given in Table 2. Bond distances, bond angles, and hydrogen bond lengths are given in Tables 3, 4, and 5 respectively. The terminal aromatic rings in the wings of 3a are offset in a manner typical of helicenes.[12,13] The hydrogens on carbons 7 and 12 of the terminal aromatic rings are separated by 2.495 Å creating an angle of 25.0° between the least squares planes of the two quinoline ring systems (Figs. 2 and 3). Compound 3a displays a pattern of alternating short and long carbon-carbon bonds analogous to 4,11-diazo[5]helicene (5),[12] perhaps induced by the strain inherent in the helical molecular geometry.[12] Thermal parameters are consistent with the assigned locations of the quinoline nitrogens. The asymmetric crystal structure of 3a possesses a pseudo two-fold axis that bisects the azoxy bond between N1 and N2. Molecules of 3a pack as anti-parallel pairs driven by stacking between the external aromatic rings to yield racemic columns (Fig. 2 and 3). The arrangement is analogous to the crystal structure solved previously for 5.[12] In the crystal, water molecules serve to link the glide-related molecules through N2 and N4i giving one-dimensional chains which extend along the c axis, forming a hydrogen bonded bridge between N2 of one molecule and N4 of another.
Figure 1.
Atom numbering scheme and thermal ellipsoid for 3a drawn at the 50% probability level.
Table 2.
Atomic coordinates and equivalent isotopic displacement parameters of the non-hydrogen atoms for compound 3a.
| Atom | X | y | z | U(eq) (Å) |
|---|---|---|---|---|
| O1 | 4352(3) | 1012(1) | 847(2) | 40(1) |
| N1 | 4756(2) | 1505(1) | 1690(2) | 30(1) |
| C1 | 5185(3) | 1834(1) | 3727(2) | 27(1) |
| N2 | 5023(3) | 1304(1) | 2850(2) | 31(1) |
| C2 | 5453(3) | 1581(1) | 4987(2) | 30(1) |
| N3 | 4805(3) | 3220(1) | 6573(2) | 31(1) |
| C3 | 5450(3) | 2053(1) | 5905(2) | 30(1) |
| N4 | 6475(3) | 4241(1) | 278(2) | 31(1) |
| C4 | 5022(3) | 2797(1) | 5621(2) | 26(1) |
| C5 | 4176(3) | 3885(1) | 6283(2) | 33(1) |
| C6 | 3671(3) | 4172(1) | 5057(2) | 31(1) |
| C7 | 3981(3) | 3766(1) | 4106(2) | 25(1) |
| C8 | 4760(3) | 3061(1) | 4379(2) | 23(1) |
| C9 | 5102(3) | 2576(1) | 3450(2) | 24(1) |
| C10 | 5341(3) | 2768(1) | 2253(2) | 23(1) |
| C11 | 6022(3) | 3458(1) | 1933(2) | 23(1) |
| C12 | 7059(3) | 3966(1) | 2846(2) | 24(1) |
| C13 | 7774(3) | 4584(1) | 2462(2) | 29(1) |
| C14 | 7358(3) | 4711(1) | 1165(2) | 32(1) |
| C15 | 5900(3) | 3601(1) | 655(2) | 26(1) |
| C16 | 5319(3) | 3039(1) | −267(2) | 30(1) |
| C17 | 4994(3) | 2362(1) | 80(2) | 30(1) |
| O(1W) | 2543(3) | −32(1) | 3254(2) | 44(1) |
| C18 | 5027(3) | 2224(1) | 1336(2) | 26(1) |
Table 3.
Bond distances (Å) for compound 3a.
| 3a | |
|---|---|
| O1-N1 | 1.277(2) |
| N1-N2 | 1.298(3) |
| N1-C18 | 1.420(3) |
| C1-N2 | 1.363(3) |
| C1-C9 | 1.408(3) |
| C1-C2 | 1.432(3) |
| C2-C3 | 1.344(3) |
| C2-H2 | 0.9500 |
| N3-C5 | 1.319(3) |
| N3-C4 | 1.362(3) |
| C3-C4 | 1.427(3) |
| C3-H3 | 0.9500 |
| N4-C14 | 1.321(3) |
| N4-C15 | 1.363(3) |
| C4-C8 | 1.422(3) |
| C5-C6 | 1.402(3) |
| C5-H5 | 0.9500 |
| C6-C7 | 1.368(3) |
| C6-H6 | 0.9500 |
| C7-C8 | 1.416(3) |
| C7-H7 | 0.9500 |
| C8-C9 | 1.444(3) |
| C9-C10 | 1.434(3) |
| C10-C18 | 1.402(3) |
| C10-C11 | 1.451(3) |
| C11-C12 | 1.415(3) |
| C11-C15 | 1.419(3) |
| C12-C13 | 1.373(3) |
| C12-H12 | 0.9500 |
| C13-C14 | 1.398(3) |
| C13-H13 | 0.9500 |
| C14-H14 | 0.9500 |
| C15-C16 | 1.431(3) |
| C16-C17 | 1.354(3) |
| C16-H16 | 0.9500 |
| C17-C18 | 1.410(3) |
| C17-H17 | 0.9500 |
| O1W-H2W | 0.8952 |
| O1W-H1W | 0.9406 |
Table 4.
Bond Angles (°) for compound 3a.
| 3a | |
|---|---|
| O1-N1-N2 | 116.94(19) |
| O1-N1-C18 | 119.28(19) |
| N2-N1-C18 | 123.62(18) |
| N2-C1-C9 | 124.0(2) |
| N2-C1-C2 | 114.7(2) |
| C9-C1-C2 | 121.2(2) |
| N1-N2-C1 | 117.12(19) |
| C3-C2-C1 | 119.8(2) |
| C3-C2-H2 | 120.1 |
| C1-C2-H2 | 120.1 |
| C5-N3-C4 | 117.41(19) |
| C2-C3-C4 | 120.8(2) |
| C2-C3-H3 | 119.6 |
| C4-C3-H3 | 119.6 |
| C14-N4-C15 | 117.75(19) |
| N3-C4-C8 | 122.8(2) |
| N3-C4-C3 | 116.94(19) |
| C8-C4-C3 | 120.2(2) |
| N3-C5-C6 | 124.0(2) |
| N3-C5-H5 | 118.0 |
| C6-C5-H5 | 118.0 |
| C7-C6-C5 | 119.1(2) |
| C7-C6-H6 | 120.4 |
| C5-C6-H6 | 120.4 |
| C6-C7-C8 | 119.2(2) |
| C6-C7-H7 | 120.4 |
| C8-C7-H7 | 120.4 |
| C7-C8-C4 | 116.92(19) |
| C7-C8-C9 | 124.27(19) |
| C4-C8-C9 | 118.48(19) |
| C1-C9-C10 | 115.93(17) |
| C1-C9-C8 | 117.29(18) |
| C10-C9-C8 | 126.77(19) |
| C18-C10-C9 | 117.12(19) |
| C18-C10-C11 | 116.66(18) |
| C9-C10-C11 | 126.04(18) |
| C12-C11-C15 | 116.87(19) |
| C12-C11-C10 | 123.49(18) |
| C15-C11-C10 | 119.06(19) |
| C13-C12-C11 | 119.63(19) |
| C13-C12-H12 | 120.2 |
| C11-C12-H12 | 120.2 |
| C12-C13-C14 | 118.6(2) |
| C12-C13-H13 | 120.7 |
| C14-C13-H13 | 120.7 |
| N4-C14-C13 | 124.0(2) |
| N4-C14-H14 | 118.0 |
| C13-C14-H14 | 118.0 |
| N4-C15-C11 | 122.4(2) |
| N4-C15-C16 | 117.68(19) |
| C11-C15-C16 | 119.7(2) |
| C17-C16-C15 | 120.6(2) |
| C17-C16-H16 | 119.7 |
| C15-C16-H16 | 119.7 |
| C16-C17-C18 | 119.8(2) |
| C16-C17-H17 | 120.1 |
| C18-C17-H17 | 120.1 |
| H2W-O1W-H1W | 104.2 |
| C10-C18-C17 | 122.6(2) |
| C10-C18-N1 | 118.69(19) |
| C17-C18-N1 | 118.74(19) |
Table 5.
Hydrogen bonds for 3a.
| D-H-A | d(D-H) (Å) | d(H…A) (Å) | d(D…A) (Å) | DHA (deg) | symm |
|---|---|---|---|---|---|
| O1W-H1W…N2 | 0.94 | 2.26 | 3.149(3) | 158.1 | |
| O1W-H2W…N4 | 0.90 | 2.09 | 2.967(2) | 168.0 | x−1/2,−y+1/2, z+1/2. |
Figure 2.
View along the a axis showing antiparallel stacking of 3a
Figure 3.
Packing view of 3a along the c axis
Acknowledgements
We thank National Institutes of Health (CA 100757) for partial support of this work.
Footnotes
Supplementary material X-ray crystallographic data reported in this paper is deposited with the Cambridge Crystallographic Data Center as supplementary publication numbers CCDC 819338. Copies of available material can be obtained, free of charge, on application to the Director, CCDC, 12 Union Road, Cambridge CB21EZ, UK.
References
- 1.Kotandeniya D, Ganley B, Gates KS. Bioorg. Med. Chem. Lett. 2002;12:2325. doi: 10.1016/s0960-894x(02)00468-7. [DOI] [PubMed] [Google Scholar]
- 2.Solano B, Junnotula V, Marin A, Villar R, Burguete A, Vicente E, Perez-Silanes S, Monge A, Dutta S, Sarkar U, Gates KS. J. Med. Chem. 2007;50:5485. doi: 10.1021/jm0703993. [DOI] [PubMed] [Google Scholar]
- 3.Junnotula V, Sarkar U, Sinha S, Gates KS. J. Am. Chem. Soc. 2009;131:1015. doi: 10.1021/ja8049645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs T, Gates KS, Hwang J-T, Greenberg MM. Chem. Res. Toxicol. 1999;12:1190. doi: 10.1021/tx990149s. [DOI] [PubMed] [Google Scholar]
- 5.Knueppel CA. Liebigs Ann. 1899;310:75. [Google Scholar]
- 6.Huisgen R. Liebigs Ann. 1948;559:127. [Google Scholar]
- 7.Farrar WV. J. Chem. Soc. 1965;799 [Google Scholar]
- 8.Rummens FHA, Bellaart AC. Tetrahedron. 1967;23:2735. [Google Scholar]
- 9.Galbraith HW, Degering EF, Hitch EF. J. Am. Chem. Soc. 1951;73:1323. [Google Scholar]
- 10.Sheldrick G. Acta Cryst. 2008;A64:112. [Google Scholar]
- 11.Barbour LJ. J. Supramol. Chem. 2001;1:189. [Google Scholar]
- 12.Bazzini C, Caronna T, Fontana F, Macchi P, Mele A, Sora IN, Panzeri W, Sironi A. New J. Chem. 2008;32:1710. [Google Scholar]
- 13.Staab HA, Zirnstein MA, Krieger C. Angew. Chem. Int. Ed. Eng. 1989;28:86. [Google Scholar]