Abstract
The blood-brain barrier (BBB) is formed by tightly connected cerebrovascular endothelial cells, but its normal function also depends on paracrine interactions between the brain endothelium and closely located glia. There is a growing consensus that brain injury, whether it is ischemic, hemorrhagic, or traumatic, leads to dysfunction of the BBB. Changes in BBB function observed after injury are thought to contribute to the loss of neural tissue and to affect the response to neuroprotective drugs. New discoveries suggest that considering the entire gliovascular unit, rather than the BBB alone, will expand our understanding of the cellular and molecular responses to traumatic brain injury (TBI). This review will address the BBB breakdown in TBI, the role of blood-borne factors in affecting the function of the gliovascular unit, changes in BBB permeability and post-traumatic edema formation, and the major pathophysiological factors associated with TBI that may contribute to post-traumatic dysfunction of the BBB. The key role of neuroinflammation and the possible effect of injury on transport mechanisms at the BBB will also be described. Finally, the potential role of the BBB as a target for therapeutic intervention through restoration of normal BBB function after injury and/or by harnessing the cerebrovascular endothelium to produce neurotrophic growth factors will be discussed.
Keywords: Blood-brain barrier, Gliovascular unit, Traumatic brain injury
Introduction
The brain is from many vantage points—anatomy, cellular organization, and function—an intricate and complex organ. When it is injured, the response is also complex and multifaceted. The initial injury forces, whether resulting from a direct blow to the skull, penetrating injury, or acceleration/deceleration and rotational forces, produce an array of tissue and cellular injury patterns which are not always consistent in patients with similar mechanisms of injury. Common pathoanatomical consequences of traumatic brain injury (TBI) include hematoma, subarachnoid hemorrhage (SAH), contusion, and diffuse axonal injury [1]. Mechanical stresses associated with TBI injure blood vessels in the brain, producing smaller cortical hemorrhages and contusions, and larger hemorrhages, such as SAH, and subdural and epidural hematomas, which may result in cerebral ischemia in the later stages of injury. The formation of post-traumatic brain edema can raise intracranial pressure inside the unyielding cranial cavity, and this can reduce cerebral perfusion pressure and cause ischemia. Diffuse axonal injury is predominantly associated with acceleration/deceleration and rotational forces acting on the head, which may cause excessive axonal stretching and the structural damage.
While neurons have been the major focus of translational research in all types of brain injury, growing experimental evidence supports the shift from neuron-oriented studies to investigations in the less explored area of blood-brain barrier (BBB) dysfunction in the injured central nervous system (CNS). The BBB plays an instrumental role in creating a highly restricted environment in the brain as it relates to the entry of blood-borne factors and circulating immune cells. Generally, the BBB is formed by the brain endothelial cells connected by tight junctions. However, astrocytes, whose processes make an intimate contact with the cerebrovascular endothelium of parenchymal blood microvessels, are critical for normal function of the BBB and for the BBB phenotype of brain endothelial cells [2, 3]. In addition, there is evidence that not only astrocytes, but also microglia are closely associated with the brain endothelium [4], and that glial and endothelial cells functionally interact with each other in a paracrine manner [2]. This anatomical and functional relationship has led to a concept that goes beyond the BBB to the gliovascular unit [2, 3], which will be the subject of this review.
In TBI, both immediate and delayed dysfunction of the BBB/gliovascular unit is observed. The disruption of the tight junction complexes and the integrity of the basement membranes result in increased paracellular permeability. Injury causes oxidative stress, and the increased production of proinflammatory mediators and an upregulation of expression of cell adhesion molecules on the surface of brain endothelium promote the influx of inflammatory cells into the traumatized brain parenchyma. There is also evidence suggesting that brain injury can change the expression and/or activity of BBB-associated transporters. These pathophysiological processes alter the normal functional interactions between glial cells and the cerebrovascular endothelium, which may further contribute to dysfunction of the BBB. There is a growing consensus that post-traumatic changes in function of the BBB are one of the major factors determining the progression of injury [5]. Dysfunction of the BBB observed after injury is implicated in the loss of neurons, altered brain function (impaired consciousness, memory, and motor impairment), and is believed to alter the response to therapy. Post-traumatic dysfunction of the BBB has also been proposed to affect the time course and the extent of neuronal repair.
TBI and the breakdown of the BBB
Biomechanically, the brain is a highly heterogeneous organ, with various brain structures having distinctive viscoelastic properties and a different degree of attachment to each other and to the skull. Therefore, in response to a direct impact or acceleration-deceleration forces applied to the head, certain brain structures move faster than others, which may generate considerable shear, tensile, and compressive forces within the brain. The two most commonly used animal models of TBI are the fluid percussion and controlled cortical impact models. These models produce the same structural abnormalities as observed in TBI patients, such as focal contusions, petechial intraparenchymal hemorrhages, SAH, and axonal injury [6, 7]. Careful light and electron microscopic analysis of the lateral fluid percussion model in rats [8] has demonstrated evolving hemorrhagic contusions at the gray-white interface underlying the somatosensory cortex and within the ambient cistern at the level of the superior colliculus and lateral geniculate body. This indicates that impact-induced shearing stresses result in primary vascular damage leading to the leakage of blood-borne proteins and extravasation of red blood cells. In addition to these specific areas, isolated petechial hemorrhages were scattered throughout the brain and were sometimes located contralaterally to injury. At the ultrastructural level, disrupted endothelial lining and endothelial vacuolation was observed together with extravasation of red blood cells, especially around small venules coursing within the subcortical white matter and lower layers of the cerebral cortex.
The disruption of integrity of the walls of brain blood microvessels caused by the impact rapidly activates the coagulation cascade. Extensive intravascular coagulation within the areas of pericontusional brain tissue has been reported, with intravascular thrombi predominantly occluding venules and, to a lesser extent, arterioles [9, 10]. The formation of platelet and leukocyte-platelet aggregates was observed within pial and parenchymal venules with both intravital and electron microscopy [8, 10]. This post-traumatic intravascular coagulation resembles the so-called no-reflow phenomenon occurring after cerebral ischemia [11], and results in a significant reduction in blood flow in the pericontusional brain tissue [10, 12, 13]. Studies in humans [12, 13] indicate that it is intravascular coagulation rather than vasospasm of the large conductance vessels (potentially caused by accompanying SAH) that lowers cerebral blood flow in pericontusional area, and suggest that a pericontusional zone of low blood flow represents the potential risk of secondary ischemic injury.
The effect of blood-borne factors (including the components of the coagulation cascade) on the function of the gliovascular unit in the injured brain
Brain parenchymal cells are normally shielded from periphery by the tight and selective BBB. However, mechanical disruption of vascular integrity and/or increased permeability of the BBB associated with functional changes at the BBB occurring after trauma allow blood-borne factors, such as albumin and fibrinogen, to enter the brain in non-selective manner. The coagulation process triggered by vascular damage also generates thrombin through the Factor Xa-mediated enzymatic cleavage of its circulating precursor prothrombin. An increasing body of evidence indicates that these factors exert profound biological effects on the function of astrocytes and microglia (Fig. 1), the integral components of the gliovascular unit. Recent studies [14] using in vivo two-photon confocal microscopy imaging of cerebral cortex have shown that the localized laser-mediated disruption of the BBB at the level of individual brain microvessels results in an immediate response of microglia characterized by targeted movement of nearby microglial processes toward the site of injury. Although the physiological significance of this phenomenon is not yet clear, the rapid time frame of microglial response to microvascular injury suggests the involvement of blood-borne factors. Unlike microglia, astrocytes showed no morphological response to the laser-produced disruption of the BBB [14].
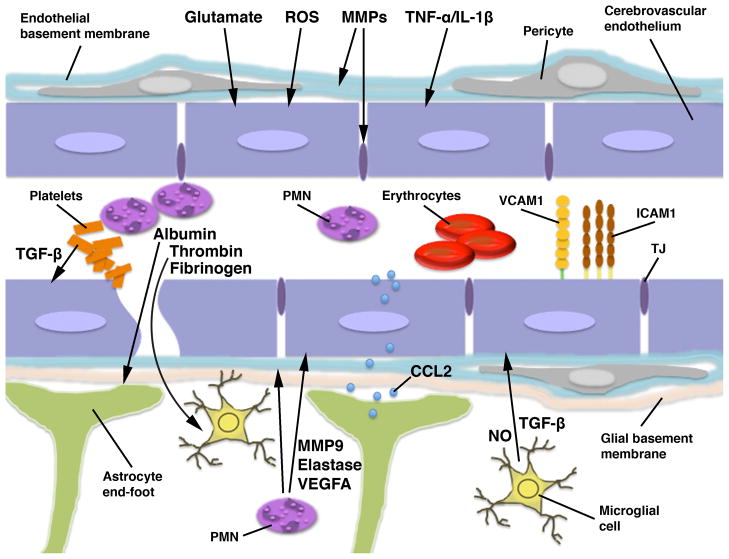
Figure 1.
Schematic representation of the blood-brain barrier (BBB)/gliovascular unit in the injured brain. The BBB is formed by the brain endothelial cells connected by tight junctions (TJs). However, astrocytes, whose end-feet make an intimate contact with the cerebrovascular endothelium, are critical for normal function of the BBB. Microglial processes are also closely associated with the brain endothelium in 4–13% of cerebral microvessels, and glial and endothelial cells functionally interact with each other in a paracrine manner. Brain parenchymal cells are normally shielded from periphery by the BBB. However, the disruption of vascular integrity occurring after neurotrauma allows blood-borne factors, such as albumin and fibrinogen, as well as thrombin, which is cleaved from prothrombin by Factor Xa, to enter the brain. Fibrinogen, after its conversion to fibrin, acts on microglia, causing the rearrangement of microglial cytoskeleton and increased phagocytosis. Thrombin predominantly acts on microglia, stimulating their proliferation and inducing the production of nitric oxide (NO). It also increases the microglial synthesis of proinflammatory mediators, such as tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 and -12. In addition, thrombin may target the cerebrovascular endothelium and increase the permeability of the BBB. Similar to thrombin, albumin promotes the proliferation of microglial cells and increases the production of NO and proinflammatory mediators, such as IL-1β. Albumin also acts on astrocytes by binding to transforming growth factor-β (TGF-β) receptor II, which may play a role in post-traumatic cortical epileptogenesis. Among the putative factors contributing to post-traumatic dysfunction of the BBB are glutamate, reactive oxygen species (ROS), matrix metalloproteinases (MMPs), proinflammatory cytokines TNF-α and IL-1β, and vascular endothelial growth factor A (VEGFA). After injury, glutamate is released from various parenchymal cells and from invading neutrophils (polymorphonuclear leukocytes; PMN). It increases the permeability of the BBB and has been shown to promote apoptosis of brain endothelial cells, although this latter action of glutamate has been questioned. ROS not only increase the permeability of brain endothelium, but also play an important role in promoting post-traumatic invasion of inflammatory cells by, for example, upregulating the endothelial expression of cell adhesion molecules, such as intercellular adhesion molecule-1 (ICAM1). MMPs are produced by multiple types of parenchymal cells and can also be released from invading leukocytes. MMPs disrupt the integrity of the BBB by attacking basal lamina proteins and degrading tight junctions. TNF-α and IL-1β increase the permeability of the BBB, but, more importantly, they also play a key role in progression of post-traumatic neuroinflammation. These cytokines increase the endothelial expression of cell adhesion molecules, such as E-selectin, ICAM1, and vascular cell adhesion molecule-1 (VCAM1). They also induce the endothelial and astrocytic production of CXC and CC chemokines, including CXCL1, -2, and CCL2, which attract circulating inflammatory cells and facilitate their migration across the BBB. Astrocyte-derived CCL2 can be transported across the cerebrovascular endothelium and then presented on its luminal surface. This chemokine can also increase the permeability of the BBB. VEGFA increases the permeability of brain endothelium by changing the distribution and downregulating the expression of tight junction proteins. After injury, VEGFA is predominantly produced by astrocytes, but is also carried by invading neutrophils. The major source of TGF-β is the aggregating platelets, but TGF-β is also produced by microglia and, to a lesser extent, by astrocytes. TGF-β has been shown to increase the permeability of the cerebrovascular endothelium; however, other investigators postulated that this growth factor has an opposite effect on the BBB, which is to enhance and maintain the barrier properties of brain endothelium. Invading neutrophils exert an adverse effect on BBB function. These inflammatory cells not only produce proinflammatory cytokines, such as TNF-α, and generate large amounts of ROS, but they also release various proteolytic enzymes, including MMP9 and neutrophil elastase.
Fibrinogen
One possible candidate blood-borne factor involved in the activation of microglia observed after microvascular injury is fibrinogen. Immobilized fibrinogen has been shown to have a dramatic effect on microglia, causing a significant rearrangement of their cytoskeleton and an increase in cell size and phagocytic activity [15]. In contrast to immobilized fibrinogen, soluble fibrinogen did not activate microglial cells. Fibrinogen acts by binding to the CD11b/CD18 (αMβ2, Mac-1, or CR3) integrin receptor, a member of the β2 integrin family, expressed on microglia. Fibrinogen recognizes CD11b/CD18 through the C-terminal cryptic binding epitope, which is only exposed after the immobilization of the fibrinogen molecule [16]. Binding of fibrinogen to CD11b/CD18 activates Rho and the Akt (protein kinase B) signaling pathway, which leads to cytoskeletal rearrangement and increased phagocytosis [15]. CD11b/CD18 is not only present on the surface of microglial cells, but is also ubiquitously expressed on leukocytes of both lymphoid and myelomonocytic lineages. Not surprisingly, fibrinogen was found to bind to inflammatory cells, such as neutrophils and monocytes [17], suggesting that such fibrinogen-leukocyte interactions may play a role in promoting post-traumatic neuroinflammation.
The activation of microglia observed in response to the disruption of the BBB or after exposure to immobilized fibrinogen could also be triggered by lipopolysaccharide (LPS) [14, 15]. Although this suggests the involvement of toll-like receptor (TLR) 4, the fibrinogen-induced phagocytic activity of microglia could not be inhibited by the blockade of TLR4 [15]. Acting through TLR4, fibrinogen can however stimulate the macrophage production of CXC and CC chemokines [18]. In addition to LPS, TLR4 has been shown to be activated by a number of endogenous factors released in response to injury, the so-called damage-associated molecular patterns (DAMPs), such as, for instance, high mobility group box 1 (HMGB1) protein, a DNA-binding protein [19]. This suggests that not only fibrinogen, but also DAMPs released in response to injury, may play a role in the activation of microglia observed after disruption of the BBB.
Fibrinogen may affect the post-traumatic processes of neuronal repair. It has been demonstrated that fibrinogen inhibits neurite outgrowth by acting as a ligand for αVβ3 integrin and transactivating the epidermal growth factor receptor in neurons [20]. Recent studies [21] have also shown that fibrinogen promotes astroglial scar formation by acting as a carrier for latent transforming growth factor-β (TGF-β). Upon its activation, TGF-β potently induces the astrocytic production of axonal regeneration-inhibiting chondroitin sulfate proteoglycans. The effect of TGF-β on BBB function will be discussed later.
Thrombin
Thrombin is a multifunctional serine protease and, as mentioned above, is cleaved from prothrombin by activated Factor X. Blood is the major source of prothrombin; however, it has been demonstrated that the transcripts for both prothrombin and Factor X are present in the CNS [22, 23]. It has also been shown that in rat models of spinal cord injury and global cerebral ischemia, mRNA for prothrombin is upregulated [24, 25]. Although these observations suggest that thrombin could potentially be produced by neural tissue, it remains unclear whether this serine protease could be generated from its precursor protein in the CNS. Thrombin receptors, which are protease-activated receptors (PARs), belong to the superfamily of G protein-coupled receptors (GPCRs) [26]. However, unlike the classical GPCRs, they are not activated by ligand binding, but rather by the proteolytic cleavage. Three PARs, PAR1, -3, and -4, are activated by thrombin, whereas PAR2 is activated by trypsin and tryptase, as well as by coagulation Factors VIIa and Xa [26]. All four PARs are expressed in the CNS, and the expression of PAR1–3 has been shown to be upregulated after ischemia [27]. The biological effects of thrombin on brain parenchymal cells are complex, and could be both detrimental and protective, depending on the concentration of thrombin [28]. For example, thrombin can induce apoptosis of astrocytes and neurons through the activation of Rho [29]. On the other hand, studies using PAR1-deficient mice and selective peptide PAR1 activator have demonstrated that by stimulating astrocyte proliferation, thrombin plays an important role in promoting astrogliosis in the injured brain [30]. This thrombin action is associated with sustained activation of extracellular signal-regulated kinase (ERK) and involves the Rho signaling pathway. Thrombin also has a significant effect on the function of microglia. It rapidly increases [Ca2+]i in microglial cells and activates mitogen-activated protein kinases (MAPKs) ERK, p38, and c-Jun N-terminal kinase (JNK), the actions in part mediated by PAR1 [31–33]. Thrombin stimulates the proliferation of microglial cells, with its mitogenic effect being also in part dependent on the activation of PAR1. Studies of primary cultures of microglial cells suggest that thrombin may be one of the factors initiating the post-traumatic brain inflammatory response as it has the ability to stimulate the microglial synthesis of proinflammatory mediators, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and -12, and a neutrophil chemoattractant CXCL1 [31]. Thrombin may also play a role in augmenting oxidative stress, which commonly accompanies brain injury, by increasing the microglial expression of inducible nitric oxide (NO) synthase (iNOS) and inducing the release of NO [31, 32]. These thrombin actions do not appear to be mediated by PAR1.
There is evidence that thrombin is involved in early edema formation after intracerebral hemorrhage [28], but the underlying cellular and molecular mechanisms are not fully understood. Interestingly, the cerebrovascular endothelium itself is a target for thrombin. It has been demonstrated that under in vitro conditions, thrombin induces the contraction of brain endothelial cells [34], suggesting that this thrombin action may lead to increased paracellular permeability of the endothelial barrier. Three PARs, PAR1–3, were identified to be expressed on rat brain capillary endothelial cells [35]. Similar to microglia, in the cerebrovascular endothelium, thrombin causes a significant increase in [Ca2+]i [35]. This increase in [Ca2+]i is in part mediated by PAR1 and is completely abrogated by plasmin.
Thrombin actions on the gliovascular unit could be modulated by thrombin inhibitors, such as serine protease inhibitors or serpins [28]. An immunohistochemical analysis of human cerebral cortex [36] has demonstrated that a potent thrombin inhibitor, protease nexin-1 (PN-1, SERPINE2), is expressed in capillaries and in the smooth muscle cells of arteries and arterioles. In addition, PN-1 was shown to be highly expressed in astrocyte end-feet making a close contact with the cerebrovascular endothelium. This anatomical localization of PN-1 suggests that this serpin may play a protective role against the deleterious effects of thrombin on the function of the gliovascular unit.
Albumin
Albumin is a major plasma protein, which is normally excluded from contact with brain tissue by the presence of the BBB. This raises an important question about the possible effect of albumin on the function of brain parenchymal cells once the integrity of the BBB is breached. Similar to thrombin, albumin was found to increase [Ca2+]i in microglial cells and to promote microglial proliferation, the latter effect being dependent on changes in the level of cytosolic free Ca2+ [37]. In both microglia and astrocytes, albumin was shown to activate the MAPK pathways and induce the synthesis of proinflammatory cytokine IL-1β [38]. It has been proposed that, at least in astrocytes, albumin binds to TGF-β receptor II (TGFBR2) and activates the Smad signaling cascade [39], although the activation of Smad proteins did not appear to be involved in the albumin-dependent production of IL-1β by astroglia [40]. In a series of elegant studies [39, 41, 42], Friedman, Kaufer, and colleagues have demonstrated that the albumin-dependent activation of TGF-β signaling in astrocytes may play a critical role in post-traumatic cortical epileptogenesis. Similar to thrombin, albumin may also be an initiator of post-traumatic neuroinflammation. In addition to increasing the synthesis of IL-1β, it augments the microglial production of TNF-α [43, 44]. Furthermore, transcriptome profiling of cortical tissue exposed to albumin demonstrated an upregulation of expression of a number of genes associated with inflammation [39]. Cell culture studies also suggest that albumin may play a role in promoting oxidative stress observed after TBI. This protein induces the expression of iNOS in microglial cells and increases the production of NO, the actions mediated, at leas in part, by the ERK signaling pathway [44]. Albumin has also been shown to augment the microglial production of reactive oxygen species (ROS), and a minimum fragment of the amino acid sequence of albumin responsible for this biological effect of this protein has been identified [45].
Post-traumatic increase in the permeability of the BBB
Disruption of vascular integrity caused by initial injury forces triggers the coagulation cascade, which, as described above, leads to a rapid intravascular coagulation and significant reduction in blood flow in the areas of pericontusional brain tissue. Therefore, the post-traumatic opening of the BBB to high-molecular-weight markers consistently observed in animal models of TBI appears to be predominantly associated with functional changes occurring at the BBB rather than mechanical disruption of cerebrovascular walls. Studies of rat models of TBI have demonstrated a biphasic increase in the BBB permeability to albumin and other high-molecular-weight proteins peaking at 4–6 hours and 2–3 days after injury [46–49]. Whereas the first peak in post-traumatic increase in the BBB permeability generally coincides with increased production of a number of putative factors that may contribute to dysfunction of the BBB and with the influx of neutrophils, which may have a similar effect (to be discussed below), the mechanisms underlying the delayed increase in BBB permeability are presently unclear. The post-traumatic increase in the permeability of the BBB to high-molecular-weight molecules could result from increased paracellular permeability of endothelial barrier associated with changes in expression, distribution, and/or function of tight junction proteins, and from augmented transport of pinocytotic vesicles across the BBB. There is evidence for increased pinocytotic activity in the cerebrovascular endothelium of TBI patients [50, 51]; however, the results from experiments in animal models of cerebral ischemic injury [52] raise the question about the quantitative importance of this pathophysiological process. Additional studies of post-traumatic changes in the permeability of the BBB to inert, low-molecular-weight markers would provide a better insight into the mechanisms underlying the opening of the BBB occurring after TBI.
The formation of vasogenic and cytotoxic edema in the injured brain
Vasogenic edema is caused by a pathological increase in the permeability of the BBB, leading to interstitial accumulation of plasma-derived, osmotically active molecules (such as plasma proteins) and water. By comparison, cytotoxic or cellular edema is associated with changes in cell metabolism and malfunction of membrane-associated pumps and ion transporters, which result in the cellular accumulation of osmotically active molecules and water. Among brain parenchymal cells, both the cerebrovascular endothelium and astrocytes appear to be most affected by post-traumatic cytotoxic edema [51], the features of which closely resemble those of cytotoxic edema observed after cerebral ischemia [53]. In the controlled cortical impact model of TBI in rats, the histological features of cytotoxic edema are apparent as early as 2–4 hours after injury (Szmydynger-Chodobska and Chodobski, unpublished observations). Studies of rodent models of TBI [46–49], in which an increase in the BBB permeability to high-molecular-weight markers has been shown, suggest the formation of vasogenic edema early after injury. In a rat model of diffuse TBI uncomplicated by contusion and hemorrhage, a transient increase in the BBB permeability to albumin has also been reported [54]. However, the significance of vasogenic edema in the overall process of post-traumatic brain swelling, especially in patients with TBI, has been questioned [55]. This may be, at least in part, related to the relatively small and heterogeneous groups of TBI patients studied, and it is likely that both forms of edema coexist in TBI. It is worth noting that the long-term increase in the permeability of the BBB, which correlated well with the development of post-traumatic epilepsy, has been observed in TBI patients [56].
Factors contributing to post-traumatic dysfunction of the BBB
Functional interactions among the components of the gliovascular unit are complex and include (but are not limited to) paracrine signaling between glial cells and the cerebrovascular endothelium [2], and between glia themselves [57]. As we mentioned above, both astrocytes and microglia can rapidly respond to injury by increasing the production of multiple factors that may have a profound effect on BBB function. There is a large amount of information covering this topic, which would be difficult to thoroughly analyze in this review. We will therefore focus our discussion on several selected factors (Fig. 1) and their pathophysiological roles in promoting dysfunction of the BBB in the injured brain.
TGF-β
Transforming growth factor-β has been recognized for some time as an important regulator of many cellular functions, including proliferation, differentiation, and survival, in various cell types. There are three isoforms of TGF-β, TGF-β1, -β2, and -β3, which are the products of separate genes. This growth factor is synthesized and sequestrated in tissues as a latent high-molecular-weight complex and is activated by a number of factors/mechanisms, including thrombospondin-1, integrins, ROS, and proteolysis [58]. Platelets are among the richest sources of TGF-β, which suggests that large amounts of TGF-β are released after injury when platelet aggregation is triggered by the mechanical damage of vascular walls [8, 10]. In platelets, however, TGF-β is predominantly stored in its latent form [59], and therefore requires the activation to exert its biological effects. This growth factor can also be produced by brain parenchymal cells, such as astrocytes and microglia. All three isoforms of TGF-β are synthesized in astrocytes, whereas TGF-β1 is predominantly produced by microglia, but the level of its microglial expression is considerably higher than that found in astroglia [60]. A rapid increase (within 6–12 hours) in cortical and hippocampal expression of TGF-β1 was observed after cryogenic brain injury [61], and we have also noted a rapid (within hours) increase in TGF-β1 expression in the injured cortex in the controlled cortical impact model of TBI in rats (Szmydynger-Chodobska and Chodobski, unpublished observations). Transforming growth factor-β receptor I (TGFBR1) and TGFBR2 are expressed on the cerebrovascular endothelium, and the level of endothelial expression of TGFBR2 in the cerebral cortex was shown to be upregulated in response to cryogenic brain injury, albeit with a significant delay [62]. Cell culture studies involving bovine retinal vascular endothelial cells and the human brain endothelial cell line, hCMEC/D3, have demonstrated that TGF-β dose-dependently increases the paracellular permeability of endothelial monolayers [63], suggesting that TGF-β may play a mediatory role in post-traumatic increase in the permeability of the BBB. This action of this growth factor was attributed to increased tyrosine phosphorylation and reduced expression of tight junction protein claudin-5 (CLDN5) and adherence junction protein VE-cadherin. In contrary to these results, other authors using neutralizing antibodies to TGF-β and an inhibitor of TGFBR1 have shown that the astrocyte- and pericyte-derived TGF-β plays an important role in enhancing and maintaining the barrier properties of brain endothelium [64, 65]. Furthermore, the targeted disruption of Smad4 in brain endothelial cells, causing the breakdown of the BBB, provided evidence that TGF-β is a critical factor in stabilizing the N-cadherin dependent interactions between the cerebrovascular endothelium and pericytes [66]. The reasons for these discrepant results are not clear.
Glutamate
Glutamate excitotoxicity has been considered as one of the major mechanisms of secondary injury leading to the post-traumatic loss of neural tissue. However, multiple clinical trials in TBI targeting glutamate, and specifically its N-methyl-D-aspartate (NMDA) receptor, have failed to demonstrate a beneficial effect [67, 68]. One possible reason for these disappointing results of clinical implementation of NMDA receptor antagonists may be the time course of post-traumatic changes in interstitial glutamate concentration in the injured brain parenchyma. In animal experiments [69–72], the interstitial concentration of glutamate increases rapidly after injury, but elevated glutamate levels are only maintained for a very short period of time. It has also been proposed that in the later stage post-injury, glutamate may actually promote neuronal survival [73]. This means that the potential therapeutic window for targeting glutamate excitotoxicity associated with TBI may be unrealistically short, especially in the clinical setting.
It should be emphasized when analyzing the function of the gliovascular unit in the injured brain that under normal conditions, astrocytes play a critical role in maintaining the optimal levels of glutamate in brain interstitial fluid through the sodium- and ATP-dependent glutamate uptake mechanisms [74]. After injury, astrocytes can release glutamate via uptake reversal resulting from ATP depletion and through other mechanisms [74]. One of the most important consequences of increased glutamate release is swelling of astroglia [75], which may contribute to the formation of post-traumatic cytotoxic edema. In addition to astrocytes, glutamate can be released from microglia in response to albumin entering the brain from the blood through the leaky BBB [44], and from neutrophils [76], which invade the traumatized brain parenchyma within hours after TBI [77]. The plasma levels of glutamate are relatively high compared to those found in the interstitial fluid of the intact brain (100 versus 3 μM, respectively) [71, 72], and blood-borne glutamate may therefore enter the brain through a leaky BBB, especially in the areas of brain contusion [72]. However, the measurements of glutamate levels in the injured brain suggest that the post-traumatic increase in interstitial concentration of this amino acid is not caused by the influx of glutamate from the blood stream, but rather results from its release from brain parenchymal cells [71].
The glutamate receptors are divided into two groups, ionotropic (iGluRs) and metabotropic (mGluRs) glutamate receptors [78]. Ionotropic receptors are ligand-gated ion channels and there are three known types of iGluRs based on their pharmacological properties, the NMDA receptor, the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, and the kainate receptor. Metabotropic receptors belong to the superfamily of GPCRs and are divided into three groups (I–III) based on their signal transduction mechanisms. The expression of NMDA and AMPA receptors, and of several members of the family of mGluRs on the rat and/or human cerebrovascular endothelium has been reported [76, 79–81]. However, based on their functional studies, one group [82] has questioned the presence of glutamate receptors on the cerebrovascular endothelium and suggested that the effect of glutamate on BBB function observed in vivo is indirect and is the result of interaction of this amino acid with its receptors expressed on parenchymal cells located closely to the brain endothelium. Although glutamate may have an indirect effect on BBB function, this hypothesis does not explain the results from cell culture experiments that we will now describe.
Using primary cultures of human brain endothelial cells, Collard et al. [76] have shown that glutamate acting through its mGluRs significantly increases the permeability of endothelial monolayers. These authors also demonstrated that selective antagonists of group I and III mGluRs reduced the permeability of the BBB in hypoxic mice, whereas selective agonists of group I and III mGluRs slightly augmented an increase in the BBB permeability caused by hypoxia. It has also been shown that glutamate acting through its NMDA receptor can increase the permeability of human brain endothelial monolayers [81], although unlike Collard et al. [76], these authors did not observe any changes in the permeability of endothelial monolayers in response to a group I/II mGluR agonist. The NMDA receptor-mediated increase in endothelial permeability was dependent on changes in [Ca2+]i and was associated with increased production of ROS [81, 83]. These observations are supported by in vivo studies, in which a selective NMDA receptor antagonist was found to reduce the permeability of the BBB and the formation of cerebral edema in a rat model of TBI [84].
Glutamate excitotoxicity is associated with increased production of NO and with oxidative stress [78]. It has been demonstrated that glutamate promotes apoptosis of brain endothelial cells through the increased production of ROS [85]. Interestingly, glutamate also stimulates the heme oxygenase (HO) activity in endothelial cells [86], and both HO1 and -2 were found to be protective against glutamate toxicity [85]. However, more recent studies [87] have questioned the glutamate-induced death of brain endothelial cells.
ROS
Similar to glutamate, oxidative stress has been placed on the top of the list of pathophysiological mechanisms responsible for secondary injury in neurotrauma. However, clinical trials in TBI testing the efficacy of antioxidant drugs have generated mixed results [67, 68]. The lack of efficacy in these pharmacological studies may have been related to inappropriate timing of administration of drugs and/or the failure to achieve sufficient brain levels of antioxidant agents. It has also been proposed that a combination therapy involving antioxidants targeting complementary mechanisms of oxidative stress rather than a single-target strategy would be a more effective therapeutic approach in TBI [88].
One of the consequences of post-traumatic oxidative stress is the peroxidation of membrane polyunsaturated fatty acids, which may affect the function of the BBB [88]. Hydroxyl radicals (•OH), whose brain interstitial levels increase rapidly after TBI [89], may play a particularly important role in peroxidation of membrane lipids, eventually giving rise to highly active aldehydes, such as 4-hydroxynonenal (4-HNE) [88]. Exogenous 4-HNE was shown to significantly increase the permeability of endothelial monolayers in an in vitro model of the BBB [90], and the administration of an inhibitor of lipid peroxidation attenuated a post-traumatic increase in the permeability of the BBB in a rat model of TBI [89].
Normal BBB function is highly dependent on the ability of brain endothelial cells to defend themselves from noxious effects of free radicals. In fact, the pharmacological depletion of glutathione (GSH), an important endogenous antioxidant, in brain or brain endothelial cells in vitro, results in a significant increase in the paracellular permeability of the BBB to low-molecular-weight markers [90, 91]. Interestingly, GSH depletion does not change the BBB permeability to high-molecular-weight markers [91]. The exposure of brain endothelial monolayers to a mixture of ROS predominantly containing superoxide anion radicals (•O2−) and to a lesser extent H2O2 and •OH was shown to rapidly increase the permeability of endothelial monolayers, which was associated with the formation of actin stress fibers and redistribution and degradation of tight junction proteins occludin and CLDN5 [92]. These ROS actions were mediated by Rho and the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway. Hydrogen peroxide on its own was found to exert similar effects on brain endothelial cells [93]. In a dose-dependent manner, H2O2 increased the paracellular permeability of endothelial monolayers, which was associated with the redistribution of occludin and the tight junction-associated proteins zonula occludens (ZO)-1 and ZO2. These H2O2 actions required the activation of the ERK signal transduction pathway, but did not appear to involve the PI3K/Akt signaling cascade.
Nitric oxide is a potent vasodilatory factor, but it can also contribute to oxidative stress by rapidly reacting with •O2− to yield a variety of free radicals [88]. There are a number of NO sources in the injured brain, including microglia, which, as described above, can produce NO in response to thrombin and albumin [31, 32, 44]. The cerebrovascular endothelium itself has the ability to produce NO in small quantities, and NO has been shown to affect BBB function. Using either the primary cultures of human brain microvascular endothelial cells or a rat brain endothelial cell line RBE4, two groups [94, 95] have demonstrated that the exposure to moderate to high micromolar concentrations of NO or NO donors significantly increases the paracellular permeability of endothelial monolayers. Interestingly, low micromolar concentrations of NO were found to actually attenuate the hypoxia/reoxygenation-induced increase in the permeability of endothelial barrier [95]. The mechanisms underlying this protective action of low concentrations of NO are unclear. Nitric oxide and ROS, such as H2O2, have also been shown to promote the endothelial synthesis of matrix metalloproteinases (MMPs) [94], the zinc-dependent endopeptidases, whose activity may considerably affect the integrity of the BBB. The effect of MMPs on BBB function will be reviewed below.
It is also important to note that ROS may play a significant role in promoting post-traumatic neuroinflammation. In the RBE4 cell line, •O2− was found to increase the adhesion and migration of monocytic cells across the endothelial monolayers [96]. Although these investigators did not study the mechanisms underlying the observed phenomena, their results suggest the ROS-dependent upregulation of cell adhesion molecules on the cerebrovascular endothelium. Indeed, hydrogen peroxide at non-cytotoxic concentrations was shown to increase the expression of intercellular adhesion molecule-1 (ICAM1) on peripheral vascular endothelium [97]. By conducting the proteomic analysis of RBE4 cells exposed to ROS, Schreibelt et al. [96] have also identified peroxiredoxin-1 (PRDX1) as an important antioxidant protective protein in the brain endothelium. The expression of PRDX1 was found to be upregulated by •O2− and H2O2 in both the RBE4 cells and the primary cultures of brain endothelial cells from the rat. Furthermore, overexpression of PRDX1 in RBE4 cells was shown to be protective against the H2O2-induced cytotoxicity and to affect the interactions of the cerebrovascular endothelium with inflammatory cells.
MMPs
Matrix metalloproteinases are divided into two general classes, the secreted MMPs and membrane-type MMPs [98]. Thirteen MMPs are secreted as latent zymogens and, therefore, need to undergo a complex process of activation to exhibit full enzymatic activity [98]. There is a growing body of evidence indicating that MMPs can disrupt the integrity of the BBB by attacking basal lamina proteins and degrading the components of the tight junction complexes, leading to the formation of vasogenic edema [99–101]. In the injured brain, MMPs, such as MMP2 (gelatinase A), -3 (stromelysin-1), and -9 (gelatinase B), appear to be produced by multiple types of parenchymal cells, including the cerebrovascular endothelium, astrocytes, microglia, and neurons [102]. Matrix metalloproteinases can also be released from invading leukocytes. Neutrophils predominantly carry MMP9 [103], whereas monocytes can produce multiple MMPs [104]. A study of the rat model of TBI has demonstrated a rapid (within 4 hours post-TBI) increase in MMP9 activity in the traumatized brain parenchyma, whereas a delayed (at 24 hours post-TBI) increase in the activity of MMP2 was observed [105]. The augmented activity of these MMPs persisted for 5 days after injury (the longest observation period in this study). Consistent with these findings, the upregulation of synthesis of MMP2 and -9 was shown in specimens of human brain tissue from TBI patients [106]. The increased levels of MMP2 and -9 in the plasma and brain interstitial fluid (monitored using the microdialysis technique) of TBI patients were also reported [107]. Genetic deletion of the Mmp9 gene or the pharmacological inhibition of activity of MMPs was found to significantly reduce the extent of brain tissue damage and to improve functional outcome in murine models of cerebral ischemia and TBI [108, 109].
The activities of MMPs are normally regulated by endogenous inhibitors—the tissue inhibitors of metalloproteinases (TIMPs) [98]. The expression of TIMP1, but not TIMP2–4, has been found to be rapidly upregulated after ischemic brain injury [110]. In a mouse model of focal cerebral ischemia, the deletion of the Timp1 gene resulted in increased expression and activity of MMP9, and was accompanied by increased permeability of the BBB and augmented loss of neural tissue when compared to wild-type animals [110]. Interestingly, Timp2−/− mice also had the leakier BBB compared to wild-type animals, but no increase in MMP9 expression or exacerbation of neuronal loss was observed in TIMP2 knockouts when compared to wild-type mice. By contrast, when transgenic mice overexpressing the human TIMP1 gene under the control of the metallothionein-1 promoter were subjected to TBI or transient cerebral ischemia, reduced levels of MMP9 synthesis and the less leaky BBB, as well as the decreased brain tissue damage, were observed compared to control animals [111]. While these observations, collectively, suggest that MMPs could represent the potential targets for pharmacological intervention in TBI, it should be emphasized that MMPs may also play an important role in the neuronal repair processes at the later stage post-injury, and the inhibition of their activity later after injury may actually have adverse therapeutic effects [112].
Vascular endothelial growth factor (VEGF) A
The VEGF family members are secreted, dimeric glycoproteins. In mammals, there are five members of the VEGF family, VEGFA, -B, -C, -D, and placenta growth factor. We will focus our discussion on VEGFA, which was previously referred to as vascular permeability factor, because of its ability to increase the microvascular permeability to low-molecular-weight markers and macromolecules. Vascular endothelial growth factor A is a potent mitogen for vascular endothelial cells, and is not only indispensable for vasculogenesis, as evidenced by vascular abnormalities and embryonic lethality of VEGFA-deficient mice, but is also critical for angiogenesis associated with reproduction, wound healing, ischemia, and tumorigenesis [113]. Five major isoforms of human VEGFA exist, with the number of amino acid residues ranging between 121 and 206 (VEGFA121, VEGFA145, VEGFA165, VEGFA189, and VEGFA206). Rodent isoforms of VEGFA are shorter by one amino acid. Although VEGFA165 is the predominant isoform of VEGFA, the transcripts for VEGFA121 and VEGFA189 are present in the majority of cells expressing the VEGFA gene. The VEGFA isoforms differ in their bioavailability, which is related to their heparin-binding abilities [114–116]. For example, VEGFA121 does not bind to heparin and is secreted as a freely diffusible protein, whereas a significant fraction of synthesized VEGFA165 is retained within the extracellular matrix (ECM) and/or bound to the cell surface. In contrast, VEGFA189 and VEGFA206 are almost completely sequestered within the ECM and/or tightly bound to the cell surface. Heparin-binding isoforms of VEGFA can be released from their bound state by heparin or heparinases, suggesting that VEGFA binds to the ECM- and/or cell membrane-associated heparan sulfate proteoglycans (HSPGs). In addition to being released from their bound state, VEGFA189 and VEGFA206 appear to require the enzymatic processing by plasmin or urokinase-type plasminogen activator to acquire biological activity [115, 117]. It is also important to note that the bioavailability of VEGFA could be regulated by MMPs [118]. The diverse biochemical properties of VEGFA isoforms and their post-translational enzymatic processing may play key roles in the fine-tuned regulation of biological activity of this growth factor.
The two major receptors that VEGFA binds to are receptor tyrosine kinases VEGFR1 (also known as Flt1) and VEGFR2 or KDR/Flk1 [113, 119]. Interestingly, soluble VEGFR1 and -2, which may act as endogenous competitive inhibitors of their ligand, have also been discovered [120, 121]. The intracerebroventricular administration of adenoviral vector encoding soluble VEGFR1 was shown to significantly reduce the permeability of the BBB, the extent of cerebral edema, and the magnitude of neuronal loss in a rat model of cerebral ischemia [122].
In the normal brain, VEGFA is expressed in the cells positioned close to the cerebrospinal fluid (CSF) space, such as astrocytes located under the ependyma and the pial-glial lining [77]. It is also highly expressed by the choroid plexus epithelium. Furthermore, VEGFA is constitutively expressed in neurons in various brain regions, including the cerebral cortex and hippocampus. It has been proposed that the choroid plexus-derived VEGFA plays an important role in inducing the fenestrated phenotype of choroidal microvessels [123]. However, other factors must also play a part in this putative VEGFA action on the choroidal microvessels, as the neuronally produced VAGFA apparently does not have a similar effect on the BBB. In animal models, neurotrauma has been shown to result in a rapid (within 2–4 hours post-TBI) upregulation of VEGFA synthesis in astrocytes and to some extent (and also with some delay) in the cerebrovascular endothelium [77]. Consistent with these findings, an increase in astrocytic expression of VEGFA was demonstrated in specimens of brain tissue obtained from TBI patients [124]. In addition to brain parenchymal cells, VEGFA is expressed by invading neutrophils [77]. It appears that upon its release, VEGFA is, at least in part, deposited within the ECM, possibly through the binding to HSPGs, from where this growth factor may be gradually released during the early phase post-injury.
In primary cultures of brain and retinal endothelial cells, the VEGFA-induced increase in the paracellular permeability of endothelial monolayers was found to be associated with the redistribution and downregulation of expression of tight junction protein occludin, which was phosphorylated on Ser490 and then ubiquitinated [125, 126]. In addition, upon the exposure to VEGFA, the tight junction-associated protein ZO1, which is normally expressed along the cell boundaries, becomes clustered in patchy aggregates in the cytoplasm of endothelial cells. The VEGFA-induced downregulation of expression of another tight junction protein CLDN5 in brain endothelial cell cultures was also reported, and the microinjection of VEGFA into a mouse cerebral cortex was shown to result in the disruption of normal pattern of immunostaining for occludin and CLDN5, and the opening of the BBB [127]. The VEGFA-dependent increase in the permeability of brain endothelial cells is mediated by VEGFR1, and not VEGFR2, and requires the activation of the PI3K/Akt signaling cascade [128]. Studies involving primary cultures of human peripheral vascular endothelial cells have also demonstrated that VEGFA causes the ROS-dependent tyrosine phosphorylation of the components of the adherens junction complexes, such as VE-cadherin, β-catenin, plakoglobin, and p120 [129, 130].
BBB and post-traumatic neuroinflammation
Increasing evidence indicates that the brain inflammatory response to injury is a major part of the pathophysiology of TBI, especially when the injury is complicated by contusions and hemorrhages. Shortly after trauma, there is a surge in production of proinflammatory cytokines, such as TNF-α and IL-1β, by brain parenchymal cells, followed by increased synthesis of chemokines and expression of cell adhesion molecules on the surface of the cerebrovascular endothelium, which eventually leads to the influx of inflammatory cells from the blood into the brain. In animal studies, neutrophil invasion has been observed within hours after injury, whereas monocytes/macrophages infiltrate the traumatized parenchyma within days post-TBI [131, 132]. There is also evidence for the influx of peripheral inflammatory cells into the injured brain parenchyma of TBI patients [133]. The mechanical disruption of the vascular walls, which may occur after the impact, causes the extravasation of red blood cells, but is not accompanied by any significant influx of leukocytes [8]. It is because the recruitment of leukocytes to the injured brain parenchyma requires a coordinated upregulation or induction of expression on the brain endothelium of cell adhesion molecules, which then interact with their counterparts expressed on the surface of white blood cells. This occurs in conjunction with an increase in the production of chemokines that attract inflammatory cells and regulate the process of their migration across the endothelial barrier [134]. Another reason for the limited initial post-injury migration of white blood cells across the damaged vascular walls is that the mechanical disruption of integrity of brain vasculature rapidly activates the coagulation cascade [9, 10], which results in a significant reduction in blood flow within the pericontusional brain tissue [12, 13]. The time frame of influx of inflammatory cells into the injured brain suggests that there is a potentially extended window of opportunity (compared for example to that available for targeting glutamate excitotoxicity) for therapeutic intervention directed against post-traumatic neuroinflammation. In preclinical studies involving rodent models of TBI, a reduction in the magnitude of post-traumatic influx of inflammatory cells, a decrease in the extent of post-traumatic loss of neural tissue, or an improvement in recovery after injury has been reported after treatment with monoclonal antibodies to CD11b/CD18 and CD11d/CD18 integrins or to ICAM1 [135–138]. On the other hand, studies of ICAM1 and ICAM1/P-selectin knockout mice have shown no difference in brain neutrophil accumulation or histopathological brain tissue damage when compared to wild-type animals, although the reduction in post-traumatic brain edema was found in ICAM1/P-selectin–deficient mice compared to control group [139, 140]. These latter studies not only underscore the complexity, but also a certain degree of redundancy, of the pathophysiological mechanisms underlying neuroinflammation. This suggests that combination therapies (for instance, directed against both chemokines and cell adhesion molecules [141]) must be applied to effectively target the multiple pathological processes associated with post-traumatic brain inflammatory response.
Signals initiating post-traumatic inflammation
The pathophysiological roles of proinflammatory cytokines, chemokines, and immune cells in post-traumatic neuroinflammation have been intensely studied, but much less effort has been directed to identify the molecules that initiate this pathological process. Although these early post-traumatic events would be difficult to target therapeutically, it is nevertheless important to understand how the neuroinflammatory cascade originates. As we discussed above, the disruption of vascular integrity resulting from injury forces creates the conditions for blood-borne factors to enter the brain parenchyma. Among such factors, thrombin has been shown to stimulate the microglial synthesis of proinflammatory mediators, including various cytokines and the chemokine CXCL1 [31]. The cellular damage causes the release of a number of endogenous factors, collectively called DAMPs, such as RNA, DNA, heat shock proteins, and HMGB1, a DNA-binding protein, which are the ligands for TLRs [142]. Binding of DAMPs to TLRs causes the activation of the nuclear factor-κB (NF-κB) and MAPK signaling cascades, leading to increased synthesis of proinflammatory cytokines and chemokines [142]. Several members of the TLR family have been shown to be expressed on the cerebrovascular endothelium, as well as on astrocytes and microglia [143, 144]. Other endogenous factors that may also play a part in initiating neuroinflammation are nucleotides, such ATP, UTP, or their analogues, released from brain parenchymal cells after injury [142]. They bind to P2 purinoceptors, leading to increased production of proinflammatory mediators in a manner similar to that observed in response to the activation of TLRs. There are two classes of P2 receptors—P2Y metabotropic receptors, which belong to the superfamily of GPCRs, and ionotropic P2X receptors [145]. There is functional evidence that P2Y2 receptor is expressed on brain endothelium [146]. The members of both the P2Y and P2X families of P2 receptors were shown to be expressed on astrocytes and microglia [147], and specifically, P2Y2 and P2Y4 were found to be highly expressed on astrocyte end-feet making a close contact with the cerebrovascular endothelium [148].
Mechanical stress caused by the initial injury forces may also affect the gene expression in individual parenchymal cells. It has been demonstrated in astrocyte cell cultures that the exposure of these glial cells to mechanical deformation results in a rapid increase in [Ca2+]i and in the cytoplasmic levels of inositol trisphosphate, which is followed by a substantial increase in the synthesis of endothelin-1 [149]. Astrocytes subjected to mechanical stress and exposed to proinflammatory cytokine IL-1β with one-hour delay were found to produce increased amounts of MMP9 [150]. This increase in astrocytic MMP9 synthesis was dependent on the activation of the ERK signaling pathway.
The effect of proinflammatory cytokines on BBB function
Studies of rodent models of TBI have shown that the synthesis of proinflammatory cytokines, such as TNF-α and IL-1β, is rapidly upregulated in the injured cortex and subcortical structures, including the hippocampus and thalamus. In the injured cortex, the high levels of message for TNF-α are observed as early as one hour after TBI, followed by a rather precipitous decline in expression of this cytokine [151, 152]. In comparison, the message for IL-1β increases gradually to reach a peak at 6–8 hours post-TBI and then decreases quite abruptly at one day after injury [152, 153]. Both TNF-α and IL-1β are produced as precursor proteins, and the analysis of cortical levels of biologically active forms of these cytokines showed that they peak between 3 and 8 hours after TBI [154]. Proinflammatory cytokines have multiple effects on the function of the BBB. Cell culture experiments involving rat and bovine brain endothelial cells have demonstrated that both TNF-α and IL-1β can increase the permeability of endothelial monolayers [155, 156]. Transgenic mice, in which the overexpression of the human IL1B gene was driven by the glial fibrillary acidic protein promoter, have also been found to have a leaky BBB [157]. More detailed studies of TNF-α action on vascular endothelium conducted in primary cultures of human peripheral vascular endothelial cells have shown that TNF-α promotes the formation of actin stress fibers, followed by cell retraction and formation of intercellular gaps [158]. The formation of intercellular gaps was found to be mediated by Rho and myosin light chain kinase. The TNF-α dependent increase in the permeability of the endothelial barrier may also, at least in part, be mediated by ROS [159]. Furthermore, it is worth noting that TNF-α has the ability to downregulate the expression of the tight junction protein occludin [160].
Although proinflammatory cytokines may have an effect on the BBB permeability in the injured brain, it is their ability to induce chemokine synthesis and induce or increase the expression of cell adhesion molecules on the surface of the cerebrovascular endothelium that play critical roles in progression of post-traumatic neuroinflammation. The post-traumatic production of chemokines will be discussed below, whereas here we will analyze the effect of proinflammatory cytokines on the endothelial expression of cell adhesion molecules. Using the primary cultures of human brain endothelial cells, several groups have demonstrated that the exposure to TNF-α or IL-1β results in a significant increase in expression of E-selectin, ICAM1, and vascular cell adhesion molecule-1 (VCAM1) on the surface of endothelial cells [161–164]. The mechanisms underlying the transcriptional regulation of expression of these adhesion molecules are complex and involve the activation of various signal transduction pathways, including the NF-κB and JNK signaling cascades [165]. Consistent with in vitro observations, animal studies have shown a rapid induction of endothelial expression of E-selectin and an increase in expression of ICAM1 after injury, although, surprisingly, no change in endothelial expression of VCAM1 was reported [137, 166, 167]. It is also important to note that the clinical studies of patients with TBI have demonstrated a positive correlation between the CSF or serum levels of soluble ICAM1 and the severity of injury and neurological outcome [168, 169].
Post-traumatic production of chemokines: a role of the gliovascular unit
There is an increasing interest in chemokines as potential therapeutic targets in inflammatory diseases [141]. Studies of rodent models of cerebral ischemia and TBI involving anti-chemokine intervention or the use of mice deficient in CXCR2 and CCR2 chemokine receptors have demonstrated a significant reduction in the magnitude of influx of inflammatory cells and the formation of edema, decreased loss of neural tissue, and an improvement in functional recovery when compared to untreated or wild-type animals, respectively [170–174]. In contrast, the adenovirus-mediated overexpression of the rat Cxcl2 gene in a mouse brain was found to cause a massive recruitment of neutrophils and an increase in the permeability of the BBB [175]. Similarly, transgenic mice overexpressing the murine Ccl2 gene driven by the myelin basic protein promoter showed significant accumulation of mononuclear cells within the perivascular spaces, meninges, and the choroid plexus stroma [176]. These transgenic mice, when subjected to the permanent occlusion of the middle cerebral artery, also had larger brain infarct volumes when compared to control animals [177].
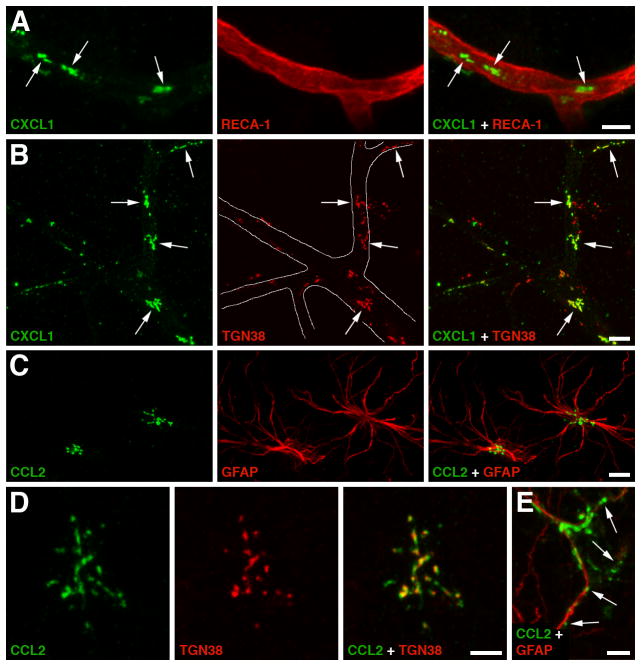
In the injured brain, at least at the early stage after the impact, chemokines appear to be predominantly synthesized by the cerebrovascular endothelium and astrocytes [178]; however, later on, invading neutrophils and monocytes may also contribute to chemokine production in the traumatized brain parenchyma [179, 180]. Interestingly, the immunohistochemical analysis of injured rat brains showed that the immunoreactive products for neutrophil chemoattractants, such as CXCL1 and CXCL2, and for the major monocyte chemoattractant CCL2 are associated with microvessels, whereas only anti-CCL2 antibody stained astrocytes (Fig. 2). This contrasts with the results from cell culture experiments, in which both the cerebrovascular endothelium and astrocytes were found to produce CXC and CC chemokines in response to proinflammatory cytokines [178, 181]. Similar to the peripheral vascular endothelium [182], the brain endothelium has the ability to transport chemokines, such as CCL2, in the abluminal-to-luminal direction, which is the receptor- and caveolae-mediated process [183]. This means that not only the endothelium-derived chemokines, but also those produced by other brain parenchymal cells or invading leukocytes, could be presented on the luminal surface of cerebrovascular endothelium. Chemokines bind to glycosaminoglycans expressed on the surface of endothelial cells forming the haptotactic or immobilized chemokine gradients that direct the recruitment of inflammatory cells [182].
Figure 2.
Production of CXC and CC chemokines by the cerebrovascular endothelium and astrocytes in a rat model of traumatic brain injury (TBI). A The immunoreactive product for neutrophil chemoattractant CXCL1 (arrows) associated with a microvessel in the traumatized brain parenchyma at 6 hours post-TBI. Double immunostaining with anti-CXCL1 antibody and an antibody to RECA-1, a marker for vascular endothelium, is shown. Similar results were obtained for CXCL2 and CCL2, a major chemoattractant for monocytes. This distinct pattern of immunopositive staining for CXC and CC chemokines was not associated with pericytes, perivascular macrophages, or smooth muscle cells, suggesting that these chemokines are produced by the brain endothelium. B Double immunostaining with anti-CXCL1 antibody and an antibody to TGN38, a Golgi marker, demonstrates that the CXCL1-immunoreactive product in cerebral microvessels (outlined) is predominantly associated with the Golgi complex (arrows). The localization of CXC and CC chemokines to the Golgi complex is consistent with increased synthesis of these secreted proteins occurring after injury. C Post-traumatic expression of CCL2 in astrocytes. Double immunostaining with anti-CCL2 antibody and an antibody to glial fibrillary acidic protein (GFAP), an astrocyte marker, is shown. Neither CXCL1 nor CXCL2 were expressed in astrocytes after injury. D CCL2 expressed in a cortical astrocyte at 6 hours post-TBI co-localizes with the Golgi complex. E Astrocytic expression of CCL2 at 24 hours after TBI. Note that at this time after injury, the CCL2-immunopositive product is predominantly localized extracellularly along astrocyte processes (arrows). This pattern of CCL2-immunopositive staining likely reflects the binding of this chemokine to heparan sulfate proteoglycans expressed on the surface of astrocytic cells and/or within the extracellular matrix. Scale bars: panels A–C, 10 μm; panels D, E 5 μm. Reprinted with permission from [178].
An intriguing new discovery is the ability of neuropeptides, such as arginine vasopressin (AVP), to act synergistically with proinflammatory cytokines to amplify the post-traumatic production of CXC and CC chemokines [178]. These synergistic interactions between cytokines and AVP occur in the cerebrovascular endothelium and astrocytes, and are mediated by the JNK signal transduction pathway. Neurotrauma results in increased AVP synthesis not only in the hypothalamus, where the paraventricular and supraoptic nuclei are the major source of peripheral AVP, but also in perivascular macrophages and the cerebrovascular endothelium in the injured brain parenchyma [184]. Studies of AVP-deficient Brattleboro rats have demonstrated a significant reduction in post-traumatic production of neutrophil and monocyte chemoattractants, the magnitude of influx of inflammatory cells, and the extent of loss of neural tissue when compared to wild-type animals [178].
It is important to note that chemokines not only play a key role in post-traumatic recruitment of leukocytes, but may also change the permeability of the BBB. There is evidence that CCL2 increases the permeability of the BBB, leading to the formation of vasogenic edema [185]. Experiments involving the primary cultures of murine brain endothelial cells have shown that CCL2 increases the paracellular permeability of endothelial monolayers by inducing the formation of actin stress fibers and causing the redistribution of tight junction proteins occludin and CLDN5, as well as the tight junction-associated proteins ZO1 and ZO2 [186]. These CCL2 actions are mediated by the Rho signaling cascade.
Post-traumatic invasion of inflammatory cells
Several studies [173, 174, 178, 187] of rodent models of TBI have demonstrated that there is an association between the magnitude of post-traumatic influx of neutrophils and monocytes, and the formation of edema and the extent of brain tissue damage. However, some studies [188, 189], have reported different results. These discrepancies may, at least in part, be related to the experimental design. For example, Uhl et al. [188] used a microtubule inhibitor vinblastine to induce neutropenia in rats. However, vinblastine has been shown to significantly stimulate the activity of JNK [190], the MAPK playing an important role in augmenting the brain inflammatory response to injury [178]. In the study by Whalen et al. [189], the permeability of the BBB to albumin was compared between neutropenic and control rats at 4 hours after TBI. However, as the authors showed in the same paper, the extent of influx of neutrophils into the inured brain parenchyma of control animals was insignificant at 4 hours post-TBI. Further studies will be needed to clarify the discrepancies in the published reports.
Neutrophils are highly toxic to neurons, especially those stressed by hypoxia and glucose deprivation [191, 192]. There is also a large body of evidence indicating that neutrophils can increase the permeability of the endothelial barrier [193]. These inflammatory cells not only have the ability to produce proinflammatory cytokines, such as TNF-α, and generate large amounts of ROS, but they also carry various proteolytic enzymes, including neutrophil elastase (ELANE) and MMP9 [193]. In in vivo studies [194], intracerebral injection of ELANE has been shown to increase the permeability of the BBB and produce multifocal perivascular and intraparenchymal hemorrhages. More recent work [195] has demonstrated that both pharmacological inhibition of ELANE activity and genetic deletion of the Elane gene in a mouse model of transient middle cerebral artery occlusion reduced the permeability of the BBB, the extent of formation of cerebral edema, and the magnitude of post-ischemic neuronal death. Similar results were obtained in Mmp9−/− mice or in chimeras, in which bone marrow from MMP9-deficient mice was transplanted into wild-type animals [196]. These findings suggest that neutrophil-derived proteases play a part in post-traumatic disruption of the BBB, although it remains unclear whether they are released from adherent or transmigrating cells. It is also important to note that, as discussed above, neutrophils may increase the permeability of the BBB by releasing VEGFA, which likely occurs when these inflammatory cells invade the traumatized brain parenchyma [77].
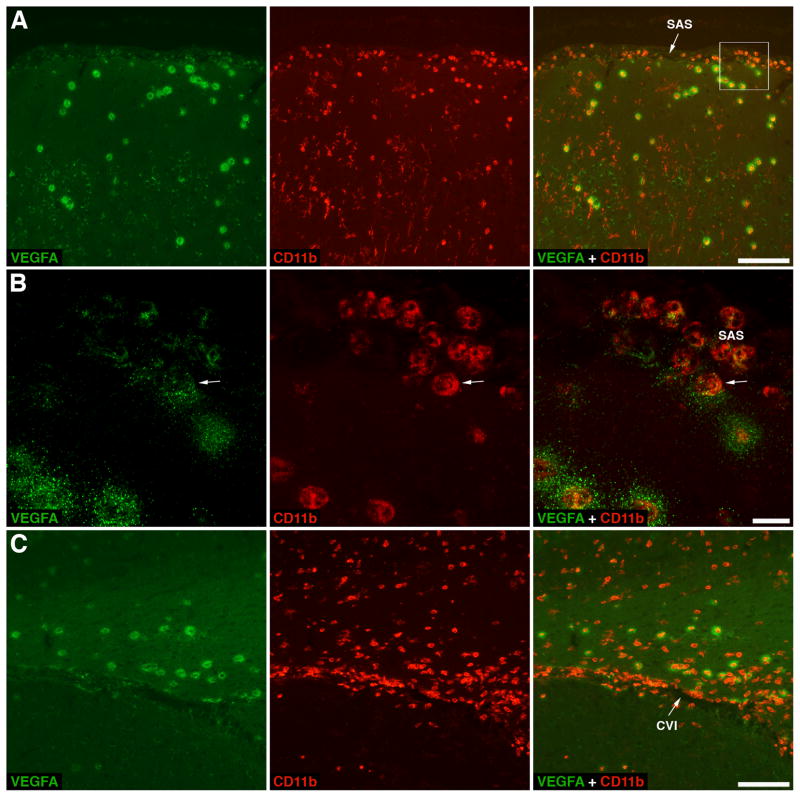
Although after injury, leukocytes largely migrate across the brain parenchymal microvessels, there are some areas of the brain, which, at least in rodent models of TBI, are particularly active with regard to influx of inflammatory cells from the blood into the CNS [77, 166]. These are the pericontusional leptomeninges and the subarachnoid CSF space near the injury site, as well as the cistern of velum interpositum, a slit-shaped space above the third ventricle with highly vascularized pia mater (Fig. 3). Not coincidentally, these are also the areas where the mechanical disruption of vascular integrity and the extravasation of plasma proteins and red blood cells frequently occur [8]. However, it is not only the mechanical stress caused by the impact that triggers the cascade of events culminating in massive influx of inflammatory cells in these vulnerable brain regions. Rather, it is the functional differences between the brain parenchymal and pial microvessels that may be responsible for this phenomenon. Indeed, peripheral administration of TNF-α or LPS in mice has been shown to induce a strong expression of cell adhesion molecule P-selectin on the endothelial surface of pial microvessels, but much weaker expression on brain parenchymal microvessels [197]. Consistent with this finding, the intraparenchymal injection of IL-1β caused acute myelomonocytic cell recruitment to meninges without leukocyte influx at the site of injection [198]. The reasons for these functional differences between these two types of microvessels are not known, but may be related, at least in part, to the differences in anatomy. Pial microvessels lack the perivascular ensheathment of astrocyte end-feet that is normally present in parenchymal microvessels [199]. Interestingly, the influx of inflammatory cells across the BBB residing in pial microvessels has also been observed in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis [200, 201].
Figure 3.
Migration of neutrophils across pial microvessels in a rat model of traumatic brain injury (TBI). Upon invasion of traumatized brain parenchyma, neutrophils release vascular endothelial growth factor A (VEGFA). The VEGFA-immunoreactive product appears as haloes surrounding neutrophils, which likely represent the growth factor sequestered within the extracellular matrix due to the VEGFA binding to heparan sulfate proteoglycans. A The cerebral cortex adjacent to the post-traumatic lesion at 8 hours post-TBI. Double immunostaining with anti-VEGFA antibody and an antibody to CD11b, a subunit of integrin receptor expressed on neutrophils, monocytes/macrophages, and microglia, is shown. Note that neutrophils not only invade the traumatized cortex after crossing the blood-brain barrier (BBB) in brain parenchymal microvessels, but also accumulate in the subarachnoid space (SAS) after crossing the BBB in pial microvessels. B A higher magnification view of selected area from panel A. This confocal microscopy image shows neutrophils accumulating in the SAS, from where these inflammatory cells appear to invade the brain parenchyma. An arrow points at a neutrophil that is in the initial stage of crossing the pial/glial lining of the cerebral cortex and starts releasing VEGFA. Note that other neutrophils accumulating in the SAS that are located further from the cortical surface clearly carry preformed VEGFA, but do not release it. C A coronal brain section cut at the level of the hippocampus and stained with anti-VEGFA and anti-CD11b antibodies. The rat was sacrificed at 24 hours after TBI. Similar to their accumulation in the SAS, neutrophils enter the cistern of velum interpositum (CVI), a slit-shaped space above the third ventricle with highly vascularized pia mater, from where these leukocytes also appear to invade the injured brain parenchyma. Scale bars: panels A, C, 100 μm; panel B, 10 μm. Reprinted with permission from [77].
The role of the blood-CSF barrier (BCSFB) in post-traumatic influx of leukocytes
The BBB is the major route for inflammatory cells to invade the injured brain. However, it has been recently recognized that the BCSFB also plays a role in this pathophysiological process [152, 202]. The BCSFB primarily resides in the choroid plexus, a highly vascularized tissue located in all four cerebral ventricles, but it also includes the arachnoid membrane. Unlike the BBB, the BCSFB is formed by tight junctions connecting adjacent cells in a single layer of cuboidal epithelium enclosing the leaky choroidal blood microvessels [203]. Similar to other types of epithelial cells, the choroid plexus epithelium has the ability to produce CXC and CC chemokines when stimulated with proinflammatory cytokines, and a rapid increase in choroidal synthesis of CXCL1–3 and CCL2 was observed in response to neurotrauma [152, 202]. Studies of primary cultures of rat choroid plexus epithelial cells demonstrated that the chemokines are secreted both apically (toward the CSF) and basolaterally (toward the choroidal stroma or blood) from the choroidal epithelium, which is a prerequisite for leukocyte migration across epithelial barriers. There is also electron microscopic evidence for trafficking of neutrophils and monocytes (sometimes in tandem) across the BCSFB [152, 202]. Confocal microscopic studies suggest that the inflammatory cells can migrate to traumatized brain parenchyma from the CSF space (see Fig. 3B); however, it remains to be determined where and how they invade the brain parenchyma after crossing the BCSFB.
TBI and the transport systems at the BBB
Na+-K+-2Cl− cotransporter and Na+/H+ exchanger
Preclinical studies involving rodent models of traumatic and ischemic brain injury suggest that targeting certain ion transporters associated with the cerebrovascular endothelium may be therapeutically beneficial. The Na+-K+-2Cl− cotransporter isoform 1 (NKCC1; also known as BSC2 or SLC12A2) and the Na+/H+ exchanger isoform 1 (NHE1 or SLC9A1) and NHE2 (SLC9A2) are expressed at the luminal surface of brain endothelium [204, 205]. Being located at the BBB, both NKCC1 and NHE1/2 may play a part in the formation of post-traumatic vasogenic edema by functioning in vectorial transport of Na+ from the blood into the CNS [206]. NKCC1 and NHE1 are also the major regulators of the cell volume [206], and may therefore play important roles in promoting post-traumatic swelling of brain endothelium [51]. The pharmacological inhibition of activity of NKCC1 in rat models of TBI and cerebral ischemia was found to significantly reduce the formation of brain edema and decrease the extent of post-traumatic and post-ischemic loss of neural tissue [204, 207]. Similarly, the use of a highly selective inhibitor of Na+/H+ exchange was reported to decrease the brain water content and attenuate brain tissue damage in ischemic rats [208]. Interestingly, both NKCC1 and Na+/H+ exchange activities in brain endothelial cells are stimulated by AVP [205, 209]. Furthermore, in the cerebrovascular endothelium, AVP increases the levels of expression and phosphorylation of NKCC1 [209, 210]. These results are consistent with previously discussed observations that AVP promotes the formation of post-traumatic brain edema and exacerbates the loss of neural tissue in the injured brain [178]. In this context, it is also important to note that TBI is associated with a rapid upregulation of expression of AVPR1A receptors on astrocytes and, with some delay, on the cerebrovascular endothelium in the traumatized brain parenchyma [211].
ATP-binding cassette transporter C8 (ABCC8)
ABCC8, also known as sulfonylurea receptor 1 (SUR1), is an atypical ATP-binding cassette protein, which has no identified transport function [212, 213]. Instead, it acts as a regulatory subunit of ATP-sensitive K+ channels best known for their role in the control of insulin secretion from pancreatic β-cells. It has also been demonstrated that SUR1 regulates the activity of Ca2+-activated, ATP-sensitive nonselective cation (NCCa-ATP) channel [214]. This SUR1-regulated NCCa-ATP channel was found to be activated by ATP depletion and to conduct inorganic monovalent cations, while being impermeable to Ca2+ and Mg2+ [215]. Given its functional properties and based on the results obtained in a rodent model of spinal cord injury [215, 216], it has been proposed that TRPM4, a member of the mammalian transient receptor potential superfamily of nonselective cation channels, represents the pore-forming subunit of the SUR1-regulated NCCa-ATP channel. Unlike NKCC1 and NHE1 and -2, both ABCC8 and TRPM4 are not constitutively expressed in the cerebrovascular endothelium, but their expression is upregulated in response to various forms of CNS injury, including spinal cord injury, cerebral ischemia, and SAH [216–219]. In vitro studies have also shown that the expression of ABCC8 in brain endothelium is increased after exposure to proinflammatory cytokine TNF-α [219]. Simard and colleagues have postulated that sustained opening of SUR1-regulated TRPM4 channel causes the swelling of the cerebrovascular endothelium, and eventually leads to death of endothelial cells, disintegration of vascular integrity, and progressive secondary hemorrhage in the injured CNS [220]. Consistent with this hypothesis, an antidiabetic drug glibenclamide, a potent blocker of SUR1-regulated TRPM4 channel, has been shown to significantly reduce the formation of edema and loss of neural tissue, and to improve functional outcome in diverse forms of CNS injury [217, 218, 221, 222]. It has also been proposed that NKCC1 and SUR1-regulated TRPM4 channel act synergistically in the injured CNS, and that combination therapy directed against these two targets should be more efficacious than a single-target approach [220].
ATP-binding cassette transporter A1 (ABCA1)
Neurotrauma is thought to be one of the risk factors for the development of Alzheimer’s disease [223]. A post-mortem analysis of brains of TBI patients has demonstrated the deposition of amyloid-β (Aβ) peptides in 30% of examined individuals [224]. The deposits of Aβ in the injured brain are found within 24 hours after the impact [225], and studies of a mouse model of TBI have shown that the genetic ablation of β-secretase or pharmacological inhibition of activity of γ-secretase, the two enzymes required for production of Aβ from its amyloid precursor protein, reduces the extent of neuronal loss and improves functional recovery after injury [226]. More recent studies have also demonstrated that the pharmacological upregulation of expression of ABCA1 by a liver X receptor agonist decreases the levels of Aβ in the injured brain, reduces the post-traumatic brain tissue damage, and improves functional outcome after injury [227].
ABCA1 functions as a cholesterol efflux pump. It is expressed in brain parenchymal cells [228], including the cerebrovascular endothelium [229, 230], and its brain endothelial expression levels can be pharmacologically upregulated by agonists of liver X receptors [229]. The experiments conducted in Abca1−/− mice have shown that ABCA1 acts as a cholesterol efflux transporter at the BBB [230], but, unlike the BBB-associated low-density lipoprotein receptor-related protein 1 [231], it does not transport Aβ peptides from the brain into the blood [232]. This suggests the indirect effect of ABCA1 on Aβ deposition in the CNS. Although the upregulation of ABCA1 expression reduces the Aβ levels in the injured brain [227], it is presently unclear as to whether the brain endothelium-associated ABCA1 plays a role in this regulation of Aβ deposition. Targeted disruption of the Abca1 gene in the cerebrovascular endothelium may help answer this question.
ATP-binding cassette transporter B1 (ABCB1)
The bioavailability of neuroprotective drugs to the brain depends, among other factors, on the activity of xenobiotic efflux transporters residing at the BBB [233]. The molecular mechanisms underlying the regulation of expression/activity of these transporters have not been fully characterized. However, a number of factors playing a role in this regulation, including proinflammatory mediators and ROS (both produced in the injured brain), have been identified [234, 235]. It has recently been demonstrated [236] that ischemic brain injury is associated with a significant upregulation of cerebrovascular expression of ABCB1, a member of the superfamily of ATP-binding cassette transporters, also known as multidrug resistance protein 1 or P-glycoprotein. In a mouse model of cerebral ischemia, the pharmacological inhibition of ABCB1 activity or genetic deletion of the Abcb1a/Abcb1b genes increased the brain levels and enhanced the efficacy of neuroprotective drugs known to be the substrates for ABCB1 [236]. These results suggest that the improved efficacy of neuroprotective treatment in brain injury may be achieved by concomitant targeting the xenobiotic efflux transporters at the BBB.
When discussing the role of ABCB1 in brain injury it is also worth noting that a well-known substrate and inhibitor of ABCB1, cyclosporine A (CsA), has demonstrated a significant neuroprotective effect in experimental TBI [237–239]. The rationale for using CsA for neuroprotective treatment in TBI was the ability of this immunosuppressant to potently inhibit the Ca2+-induced permeability transition in mitochondria [240], a hallmark of mitochondrial dysfunction observed in TBI [241]. CsA has moved to initial clinical trials for TBI and some encouraging therapeutic effects were observed [241]. However, certain concerns about its clinical applicability, such as insufficient brain penetration (likely related to the fact that CsA is an ABCB1 substrate) and variable effectiveness in inhibiting the mitochondrial permeability transition, were also raised [241].
BBB as a target for therapeutic intervention in TBI
As has been presented above, previous research has established the integral and expansive role of the BBB/gliovascular unit in the pathological processes of TBI. However, despite teasing out many of the cellular and molecular aspects of the injury cascade, few investigations have considered the BBB as a target for therapeutic intervention. There is a strong rationale to do so. The weakness of many laboratory and clinical studies in TBI is that targeting an isolated molecule or pathological mechanism with a therapy ignores the complexity of pathophysiological processes associated with TBI. Focusing on just one mediator/mechanism of injury, for example the increased production of ROS, omits many other processes that contribute to the pathology of TBI. The logical response to this problem has been to move to combination therapies that could target multiple complementary pathways and/or pathological processes in TBI [242]. Another problem with previous strategies has been the crucial aspect of time and the secondary injury processes. As noted above, the release of glutamate and the production of ROS, proinflammatory cytokines, and other mediators of injury may be increased at various time points after TBI, with possible harmful or beneficial effects depending on the timing. Therefore, therapeutic agents must be delivered at the appropriate time after injury. In clinical trials of TBI, the window of opportunity must match the reality of when the patient is available for intervention. Thus, pathological processes that are activated within minutes of TBI may not be good targets for post-injury intervention.
Therapies targeting the BBB to restore its normal function after injury may represent another solution to these dilemmas. Normally functioning BBB is key to restore brain homeostasis and to create an optimal microenvironment for neuronal repair. It may also allow for more reliable delivery of neuroprotective drugs. The evidence presented above suggests that particularly with neuroinflammation, there may be a longer time window during which the restoration of normal BBB function would be effective. Candidate therapies may for example be directed to reduce the expression of cell adhesion molecules and/or interfere with signaling of chemokines presented on the luminal surface of brain endothelium. There has been some progress made to selectively target the cerebrovascular endothelium and not the endothelium of peripheral blood vessels [243], which would reduce the risk of interference with the peripheral immune system.
Strategies to therapeutically engage the BBB in neuronal repair processes after TBI could also have many beneficial downstream effects. Future investigations might determine how to effectively harness the cerebrovascular endothelium to produce neuroprotective growth factors that can act on nearby neurons. This would be a new approach based on the evidence that neurotrophic factors, such as brain-derived neurotrophic factor and artemin/neublastin, endogenously produced by the cerebrovascular endothelium, can potentially have beneficial effect on functional recovery after injury [244, 245]. This approach would also be devoid of the problems associated with delivering exogenous neuroprotectants across the BBB. However, just at it was never very likely that a drug targeting a specific mechanism would have huge therapeutic benefits in the outcome from TBI, it is unlikely that a single growth factor produced by the brain endothelium will be the “silver bullet” in brain recovery from TBI. As the understanding of the complex cellular and molecular interactions within the gliovascular unit grows, it is likely that more opportunities for interventions that are directed at the BBB will arise.
Acknowledgments
We thank Ms. Julie Sarri for her secretarial help in preparing this manuscript. This work was supported by grant NS49479 from the NIH and by funds from the Department of Emergency Medicine at the Alpert Medical School of Brown University.
Footnotes
Financial and competing interests disclosure
The authors have no financial and/or competing interests to disclose.
References
- 1.Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25(7):719–38. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 3.Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009;335(1):75–96. doi: 10.1007/s00441-008-0658-9. [DOI] [PubMed] [Google Scholar]
- 4.Lassmann H, Zimprich F, Vass K, Hickey WF. Microglial cells are a component of the perivascular glia limitans. J Neurosci Res. 1991;28(2):236–43. doi: 10.1002/jnr.490280211. [DOI] [PubMed] [Google Scholar]
- 5.Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, Engelhardt B, Grammas P, Nedergaard M, Nutt J, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7(1):84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- 6.Gennarelli TA. Animate models of human head injury. J Neurotrauma. 1994;11(4):357–68. doi: 10.1089/neu.1994.11.357. [DOI] [PubMed] [Google Scholar]
- 7.Povlishock JT, Hayes RL, Michel ME, McIntosh TK. Workshop on animal models of traumatic brain injury. J Neurotrauma. 1994;11(6):723–32. doi: 10.1089/neu.1994.11.723. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich WD, Alonso O, Halley M. Early microvascular and neuronal consequences of traumatic brain injury: a light and electron microscopic study in rats. J Neurotrauma. 1994;11(3):289–301. doi: 10.1089/neu.1994.11.289. [DOI] [PubMed] [Google Scholar]
- 9.Stein SC, Chen XH, Sinson GP, Smith DH. Intravascular coagulation: a major secondary insult in nonfatal traumatic brain injury. J Neurosurg. 2002;97(6):1373–7. doi: 10.3171/jns.2002.97.6.1373. [DOI] [PubMed] [Google Scholar]
- 10.Schwarzmaier SM, Kim SW, Trabold R, Plesnila N. Temporal profile of thrombogenesis in the cerebral microcirculation after traumatic brain injury in mice. J Neurotrauma. 2010;27(1):121–30. doi: 10.1089/neu.2009.1114. [DOI] [PubMed] [Google Scholar]
- 11.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23(8):879–94. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 12.Schröder ML, Muizelaar JP, Fatouros PP, Kuta AJ, Choi SC. Regional cerebral blood volume after severe head injury in patients with regional cerebral ischemia. Neurosurgery. 1998;42(6):1276–80. doi: 10.1097/00006123-199806000-00042. discussion 1280–1. [DOI] [PubMed] [Google Scholar]
- 13.von Oettingen G, Bergholt B, Gyldensted C, Astrup J. Blood flow and ischemia within traumatic cerebral contusions. Neurosurgery. 2002;50(4):781–8. doi: 10.1097/00006123-200204000-00019. discussion 788–90. [DOI] [PubMed] [Google Scholar]
- 14.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 15.Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, Degen JL, Akassoglou K. The fibrin-derived γ377–395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med. 2007;204(3):571–82. doi: 10.1084/jem.20061931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lishko VK, Kudryk B, Yakubenko VP, Yee VC, Ugarova TP. Regulated unmasking of the cryptic binding site for integrin αMβ2 in the γC-domain of fibrinogen. Biochemistry. 2002;41(43):12942–51. doi: 10.1021/bi026324c. [DOI] [PubMed] [Google Scholar]
- 17.Altieri DC, Plescia J, Plow EF. The structural motif glycine 190-valine 202 of the fibrinogen γ chain interacts with CD11b/CD18 integrin (αMβ2, Mac-1) and promotes leukocyte adhesion. J Biol Chem. 1993;268(3):1847–53. [PubMed] [Google Scholar]
- 18.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167(5):2887–94. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 19.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol. 2006;290(3):C917–24. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 20.Schachtrup C, Lu P, Jones LL, Lee JK, Lu J, Sachs BD, Zheng B, Akassoglou K. Fibrinogen inhibits neurite outgrowth via β3 integrin-mediated phosphorylation of the EGF receptor. Proc Natl Acad Sci USA. 2007;104(28):11814–9. doi: 10.1073/pnas.0704045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-β after vascular damage. J Neurosci. 2010;30(17):5843–54. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dihanich M, Kaser M, Reinhard E, Cunningham D, Monard D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron. 1991;6(4):575–81. doi: 10.1016/0896-6273(91)90060-d. [DOI] [PubMed] [Google Scholar]
- 23.Shikamoto Y, Morita T. Expression of factor X in both the rat brain and cells of the central nervous system. FEBS Lett. 1999;463(3):387–9. doi: 10.1016/s0014-5793(99)01657-9. [DOI] [PubMed] [Google Scholar]
- 24.Citron BA, Smirnova IV, Arnold PM, Festoff BW. Upregulation of neurotoxic serine proteases, prothrombin, and protease-activated receptor 1 early after spinal cord injury. J Neurotrauma. 2000;17(12):1191–203. doi: 10.1089/neu.2000.17.1191. [DOI] [PubMed] [Google Scholar]
- 25.Riek-Burchardt M, Striggow F, Henrich-Noack P, Reiser G, Reymann KG. Increase of prothrombin-mRNA after global cerebral ischemia in rats, with constant expression of protease nexin-1 and protease-activated receptors. Neurosci Lett. 2002;329(2):181–4. doi: 10.1016/s0304-3940(02)00645-6. [DOI] [PubMed] [Google Scholar]
- 26.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407(6801):258–64. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 27.Striggow F, Riek-Burchardt M, Kiesel A, Schmidt W, Henrich-Noack P, Breder J, Krug M, Reymann KG, Reiser G. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur J Neurosci. 2001;14(4):595–608. doi: 10.1046/j.0953-816x.2001.01676.x. [DOI] [PubMed] [Google Scholar]
- 28.Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J Neurochem. 2003;84(1):3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 29.Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. J Neurosci. 1997;17(14):5316–26. doi: 10.1523/JNEUROSCI.17-14-05316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicole O, Goldshmidt A, Hamill CE, Sorensen SD, Sastre A, Lyuboslavsky P, Hepler JR, McKeon RJ, Traynelis SF. Activation of protease-activated receptor-1 triggers astrogliosis after brain injury. J Neurosci. 2005;25(17):4319–29. doi: 10.1523/JNEUROSCI.5200-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Möller T, Hanisch UK, Ransom BR. Thrombin-induced activation of cultured rodent microglia. J Neurochem. 2000;75(4):1539–47. doi: 10.1046/j.1471-4159.2000.0751539.x. [DOI] [PubMed] [Google Scholar]
- 32.Ryu J, Pyo H, Jou I, Joe E. Thrombin induces NO release from cultured rat microglia via protein kinase C, mitogen-activated protein kinase, and NF-κB. J Biol Chem. 2000;275(39):29955–9. doi: 10.1074/jbc.M001220200. [DOI] [PubMed] [Google Scholar]
- 33.Suo Z, Wu M, Ameenuddin S, Anderson HE, Zoloty JE, Citron BA, Andrade-Gordon P, Festoff BW. Participation of protease-activated receptor-1 in thrombin-induced microglial activation. J Neurochem. 2002;80(4):655–66. doi: 10.1046/j.0022-3042.2001.00745.x. [DOI] [PubMed] [Google Scholar]
- 34.Nagy Z, Kolev K, Csonka E, Pék M, Machovich R. Contraction of human brain endothelial cells induced by thrombogenic and fibrinolytic factors. An in vitro cell culture model. Stroke. 1995;26(2):265–70. doi: 10.1161/01.str.26.2.265. [DOI] [PubMed] [Google Scholar]
- 35.Bartha K, Dömötör E, Lanza F, Adam-Vizi V, Machovich R. Identification of thrombin receptors in rat brain capillary endothelial cells. J Cereb Blood Flow Metab. 2000;20(1):175–82. doi: 10.1097/00004647-200001000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Choi BH, Suzuki M, Kim T, Wagner SL, Cunningham DD. Protease nexin-1. Localization in the human brain suggests a protective role against extravasated serine proteases. Am J Pathol. 1990;137(4):741–7. [PMC free article] [PubMed] [Google Scholar]
- 37.Hooper C, Taylor DL, Pocock JM. Pure albumin is a potent trigger of calcium signalling and proliferation in microglia but not macrophages or astrocytes. J Neurochem. 2005;92(6):1363–76. doi: 10.1111/j.1471-4159.2005.02982.x. [DOI] [PubMed] [Google Scholar]
- 38.Ralay Ranaivo H, Wainwright MS. Albumin activates astrocytes and microglia through mitogen-activated protein kinase pathways. Brain Res. 2010;1313:222–31. doi: 10.1016/j.brainres.2009.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cacheaux LP, Ivens S, David Y, Lakhter AJ, Bar-Klein G, Shapira M, Heinemann U, Friedman A, Kaufer D. Transcriptome profiling reveals TGF-β signaling involvement in epileptogenesis. J Neurosci. 2009;29(28):8927–35. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ralay Ranaivo H, Patel F, Wainwright MS. Albumin activates the canonical TGF receptor-smad signaling pathway but this is not required for activation of astrocytes. Exp Neurol. 2010;226(2):310–9. doi: 10.1016/j.expneurol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, Seiffert E, Heinemann U, Friedman A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130(2):535–47. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- 42.David Y, Cacheaux LP, Ivens S, Lapilover E, Heinemann U, Kaufer D, Friedman A. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J Neurosci. 2009;29(34):10588–99. doi: 10.1523/JNEUROSCI.2323-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao TZ, Xia YZ, Li L, Li J, Zhu G, Chen S, Feng H, Lin JK. Bovine serum albumin promotes IL-1β and TNF-α secretion by N9 microglial cells. Neurol Sci. 2009;30(5):379–83. doi: 10.1007/s10072-009-0123-x. [DOI] [PubMed] [Google Scholar]
- 44.Hooper C, Pinteaux-Jones F, Fry VA, Sevastou IG, Baker D, Heales SJ, Pocock JM. Differential effects of albumin on microglia and macrophages; implications for neurodegeneration following blood-brain barrier damage. J Neurochem. 2009;109(3):694–705. doi: 10.1111/j.1471-4159.2009.05953.x. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura Y, Si QS, Takaku T, Kataoka K. Identification of a peptide sequence in albumin that potentiates superoxide production by microglia. J Neurochem. 2000;75(6):2309–15. doi: 10.1046/j.1471-4159.2000.0752309.x. [DOI] [PubMed] [Google Scholar]
- 46.Shapira Y, Setton D, Artru AA, Shohami E. Blood-brain barrier permeability, cerebral edema, and neurologic function after closed head injury in rats. Anesth Analg. 1993;77(1):141–8. doi: 10.1213/00000539-199307000-00028. [DOI] [PubMed] [Google Scholar]
- 47.Baldwin SA, Fugaccia I, Brown DR, Brown LV, Scheff SW. Blood-brain barrier breach following cortical contusion in the rat. J Neurosurg. 1996;85(3):476–81. doi: 10.3171/jns.1996.85.3.0476. [DOI] [PubMed] [Google Scholar]
- 48.Hicks RR, Baldwin SA, Scheff SW. Serum extravasation and cytoskeletal alterations following traumatic brain injury in rats. Comparison of lateral fluid percussion and cortical impact models. Mol Chem Neuropathol. 1997;32(1–3):1–16. doi: 10.1007/BF02815164. [DOI] [PubMed] [Google Scholar]
- 49.Başkaya MK, Rao AM, Doğan A, Donaldson D, Dempsey RJ. The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci Lett. 1997;226(1):33–6. doi: 10.1016/s0304-3940(97)00239-5. [DOI] [PubMed] [Google Scholar]
- 50.Castejón OJ. Formation of transendothelial channels in traumatic human brain edema. Pathol Res Pract. 1984;179(1):7–12. doi: 10.1016/S0344-0338(84)80054-0. [DOI] [PubMed] [Google Scholar]
- 51.Vaz R, Sarmento A, Borges N, Cruz C, Azevedo I. Ultrastructural study of brain microvessels in patients with traumatic cerebral contusions. Acta Neurochir (Wien) 1997;139(3):215–20. doi: 10.1007/BF01844754. [DOI] [PubMed] [Google Scholar]
- 52.Preston E, Webster J. Differential passage of [14C]sucrose and [3H]inulin across rat blood-brain barrier after cerebral ischemia. Acta Neuropathol. 2002;103(3):237–42. doi: 10.1007/s004010100458. [DOI] [PubMed] [Google Scholar]
- 53.Liu KF, Li F, Tatlisumak T, Garcia JH, Sotak CH, Fisher M, Fenstermacher JD. Regional variations in the apparent diffusion coefficient and the intracellular distribution of water in rat brain during acute focal ischemia. Stroke. 2001;32(8):1897–905. doi: 10.1161/01.str.32.8.1897. [DOI] [PubMed] [Google Scholar]
- 54.Kelley BJ, Lifshitz J, Povlishock JT. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J Neuropathol Exp Neurol. 2007;66(11):989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- 55.Marmarou A, Signoretti S, Fatouros PP, Portella G, Aygok GA, Bullock MR. Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. J Neurosurg. 2006;104(5):720–30. doi: 10.3171/jns.2006.104.5.720. [DOI] [PubMed] [Google Scholar]
- 56.Tomkins O, Shelef I, Kaizerman I, Eliushin A, Afawi Z, Misk A, Gidon M, Cohen A, Zumsteg D, Friedman A. Blood-brain barrier disruption in post-traumatic epilepsy. J Neurol Neurosurg Psychiatry. 2008;79(7):774–7. doi: 10.1136/jnnp.2007.126425. [DOI] [PubMed] [Google Scholar]
- 57.Liu W, Tang Y, Feng J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci. 2011;89(5–6):141–6. doi: 10.1016/j.lfs.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 58.Koli K, Myllärniemi M, Keski-Oja J, Kinnula VL. Transforming growth factor-β activation in the lung: focus on fibrosis and reactive oxygen species. Antioxid Redox Signal. 2008;10(2):333–42. doi: 10.1089/ars.2007.1914. [DOI] [PubMed] [Google Scholar]
- 59.Pircher R, Jullien P, Lawrence DA. β-transforming growth factor is stored in human blood platelets as a latent high molecular weight complex. Biochem Biophys Res Commun. 1986;136(1):30–7. doi: 10.1016/0006-291x(86)90872-7. [DOI] [PubMed] [Google Scholar]
- 60.Constam DB, Philipp J, Malipiero UV, ten Dijke P, Schachner M, Fontana A. Differential expression of transforming growth factor-β1, -β2, and -β3 by glioblastoma cells, astrocytes, and microglia. J Immunol. 1992;148(5):1404–10. [PubMed] [Google Scholar]
- 61.Cook JL, Marcheselli V, Alam J, Deininger PL, Bazan NG. Temporal changes in gene expression following cryogenic rat brain injury. Mol Brain Res. 1998;55(1):9–19. doi: 10.1016/s0169-328x(97)00350-1. [DOI] [PubMed] [Google Scholar]
- 62.Fee DB, Sewell DL, Andresen K, Jacques TJ, Piaskowski S, Barger BA, Hart MN, Fabry Z. Traumatic brain injury increases TGFβRII expression on endothelial cells. Brain Res. 2004;1012(1–2):52–9. doi: 10.1016/j.brainres.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 63.Shen W, Li S, Chung SH, Zhu L, Stayt J, Su T, Couraud PO, Romero IA, Weksler B, Gillies MC. Tyrosine phosphorylation of VE-cadherin and claudin-5 is associated with TGF-β1-induced permeability of centrally derived vascular endothelium. Eur J Cell Biol. 2011;90(4):323–32. doi: 10.1016/j.ejcb.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 64.Garcia CM, Darland DC, Massingham LJ, D’Amore PA. Endothelial cell-astrocyte interactions and TGFβ are required for induction of blood-neural barrier properties. Dev Brain Res. 2004;152(1):25–38. doi: 10.1016/j.devbrainres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 65.Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-β production. Brain Res. 2005;1038(2):208–15. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 66.Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A, Yang X. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell. 2011;20(3):291–302. doi: 10.1016/j.devcel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Tolias CM, Bullock MR. Critical appraisal of neuroprotection trials in head injury: what have we learned? NeuroRx. 2004;1(1):71–9. doi: 10.1602/neurorx.1.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marklund N, Bakshi A, Castelbuono DJ, Conte V, McIntosh TK. Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr Pharm Des. 2006;12(13):1645–80. doi: 10.2174/138161206776843340. [DOI] [PubMed] [Google Scholar]
- 69.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73(6):889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 70.Nilsson P, Hillered L, Ponten U, Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J Cereb Blood Flow Metab. 1990;10(5):631–7. doi: 10.1038/jcbfm.1990.115. [DOI] [PubMed] [Google Scholar]
- 71.Palmer AM, Marion DW, Botscheller ML, Swedlow PE, Styren SD, DeKosky ST. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J Neurochem. 1993;61(6):2015–24. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- 72.Koizumi H, Fujisawa H, Ito H, Maekawa T, Di X, Bullock R. Effects of mild hypothermia on cerebral blood flow-independent changes in cortical extracellular levels of amino acids following contusion trauma in the rat. Brain Res. 1997;747(2):304–12. doi: 10.1016/s0006-8993(96)01240-1. [DOI] [PubMed] [Google Scholar]
- 73.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1(6):383–6. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 74.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32(1):1–14. [PubMed] [Google Scholar]
- 75.Maxwell WL, Bullock R, Landholt H, Fujisawa H. Massive astrocytic swelling in response to extracellular glutamate a possible mechanism for post-traumatic brain swelling? Acta Neurochir Suppl (Wien) 1994;60:465–7. doi: 10.1007/978-3-7091-9334-1_127. [DOI] [PubMed] [Google Scholar]
- 76.Collard CD, Park KA, Montalto MC, Alapati S, Buras JA, Stahl GL, Colgan SP. Neutrophil-derived glutamate regulates vascular endothelial barrier function. J Biol Chem. 2002;277(17):14801–11. doi: 10.1074/jbc.M110557200. [DOI] [PubMed] [Google Scholar]
- 77.Chodobski A, Chung I, KoŸniewska E, Ivanenko T, Chang W, Harrington JF, Duncan JA, Szmydynger-Chodobska J. Early neutrophilic expression of vascular endothelial growth factor after traumatic brain injury. Neuroscience. 2003;122(4):853–67. doi: 10.1016/j.neuroscience.2003.08.055. [DOI] [PubMed] [Google Scholar]
- 78.Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch. 2010;460(2):525–42. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 79.Krizbai IA, Deli MA, Pestenácz A, Siklós L, Szabó CA, András I, Joó F. Expression of glutamate receptors on cultured cerebral endothelial cells. J Neurosci Res. 1998;54(6):814–9. doi: 10.1002/(SICI)1097-4547(19981215)54:6<814::AID-JNR9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 80.Gillard SE, Tzaferis J, Tsui HC, Kingston AE. Expression of metabotropic glutamate receptors in rat meningeal and brain microvasculature and choroid plexus. J Comp Neurol. 2003;461(3):317–32. doi: 10.1002/cne.10671. [DOI] [PubMed] [Google Scholar]
- 81.Sharp CD, Hines I, Houghton J, Warren A, Jackson THt, Jawahar A, Nanda A, Elrod JW, Long A, Chi A, et al. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am J Physiol. 2003;285(6):H2592–8. doi: 10.1152/ajpheart.00520.2003. [DOI] [PubMed] [Google Scholar]
- 82.Morley P, Small DL, Murray CL, Mealing GA, Poulter MO, Durkin JP, Stanimirovic DB. Evidence that functional glutamate receptors are not expressed on rat or human cerebromicrovascular endothelial cells. J Cereb Blood Flow Metab. 1998;18(4):396–406. doi: 10.1097/00004647-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 83.Sharp CD, Houghton J, Elrod JW, Warren A, Jackson THt, Jawahar A, Nanda A, Minagar A, Alexander JS. N-methyl-D-aspartate receptor activation in human cerebral endothelium promotes intracellular oxidant stress. Am J Physiol. 2005;288(4):H1893–9. doi: 10.1152/ajpheart.01110.2003. [DOI] [PubMed] [Google Scholar]
- 84.Dempsey RJ, Başkaya MK, Doğan A. Attenuation of brain edema, blood-brain barrier breakdown, and injury volume by ifenprodil, a polyamine-site N-methyl-D-aspartate receptor antagonist, after experimental traumatic brain injury in rats. Neurosurgery. 2000;47(2):399–404. doi: 10.1097/00006123-200008000-00024. discussion 404–6. [DOI] [PubMed] [Google Scholar]
- 85.Parfenova H, Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: contributions of HO-1 and HO-2 to cytoprotection. Am J Physiol. 2006;290(5):C1399–410. doi: 10.1152/ajpcell.00386.2005. [DOI] [PubMed] [Google Scholar]
- 86.Parfenova H, Fedinec A, Leffler CW. Ionotropic glutamate receptors in cerebral microvascular endothelium are functionally linked to heme oxygenase. J Cereb Blood Flow Metab. 2003;23(2):190–7. doi: 10.1097/01.WCB.000004823561824.C4. [DOI] [PubMed] [Google Scholar]
- 87.Domoki F, Kis B, Gáspár T, Bari F, Busija DW. Cerebromicrovascular endothelial cells are resistant to L-glutamate. Am J Physiol. 2008;295(4):R1099–108. doi: 10.1152/ajpregu.90430.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hall ED, Vaishnav RA, Mustafa AG. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7(1):51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith SL, Andrus PK, Zhang JR, Hall ED. Direct measurement of hydroxyl radicals, lipid peroxidation, and blood-brain barrier disruption following unilateral cortical impact head injury in the rat. J Neurotrauma. 1994;11(4):393–404. doi: 10.1089/neu.1994.11.393. [DOI] [PubMed] [Google Scholar]
- 90.Mertsch K, Blasig I, Grune T. 4-Hydroxynonenal impairs the permeability of an in vitro rat blood-brain barrier. Neurosci Lett. 2001;314(3):135–8. doi: 10.1016/s0304-3940(01)02299-6. [DOI] [PubMed] [Google Scholar]
- 91.Agarwal R, Shukla GS. Potential role of cerebral glutathione in the maintenance of blood-brain barrier integrity in rat. Neurochem Res. 1999;24(12):1507–14. doi: 10.1023/a:1021191729865. [DOI] [PubMed] [Google Scholar]
- 92.Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Romero IA, Couraud PO, Piontek J, et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007;21(13):3666–76. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- 93.Fischer S, Wiesnet M, Renz D, Schaper W. H2O2 induces paracellular permeability of porcine brain-derived microvascular endothelial cells by activation of the p44/42 MAP kinase pathway. Eur J Cell Biol. 2005;84(7):687–97. doi: 10.1016/j.ejcb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 94.Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem. 2007;101(2):566–76. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- 95.Utepbergenov DI, Mertsch K, Sporbert A, Tenz K, Paul M, Haseloff RF, Blasig IE. Nitric oxide protects blood-brain barrier in vitro from hypoxia/reoxygenation-mediated injury. FEBS Lett. 1998;424(3):197–201. doi: 10.1016/s0014-5793(98)00173-2. [DOI] [PubMed] [Google Scholar]
- 96.Schreibelt G, van Horssen J, Haseloff RF, Reijerkerk A, van der Pol SM, Nieuwenhuizen O, Krause E, Blasig IE, Dijkstra CD, Ronken E, et al. Protective effects of peroxiredoxin-1 at the injured blood-brain barrier. Free Radic Biol Med. 2008;45(3):256–64. doi: 10.1016/j.freeradbiomed.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 97.Bradley JR, Johnson DR, Pober JS. Endothelial activation by hydrogen peroxide. Selective increases of intercellular adhesion molecule-1 and major histocompatibility complex class I. Am J Pathol. 1993;142(5):1598–609. [PMC free article] [PubMed] [Google Scholar]
- 98.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 99.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50(4):329–39. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 100.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27(4):697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 101.Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22(5):E4. doi: 10.3171/foc.2007.22.5.5. [DOI] [PubMed] [Google Scholar]
- 102.Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893(1–2):104–12. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- 103.Borregaard N, Sørensen OE, Theilgaard-Mönch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28(8):340–5. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 104.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol. 2008;28(12):2108–14. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- 105.Truettner JS, Alonso OF, Dalton Dietrich W. Influence of therapeutic hypothermia on matrix metalloproteinase activity after traumatic brain injury in rats. J Cereb Blood Flow Metab. 2005;25(11):1505–16. doi: 10.1038/sj.jcbfm.9600150. [DOI] [PubMed] [Google Scholar]
- 106.Vilalta A, Sahuquillo J, Poca MA, De Los Rios J, Cuadrado E, Ortega-Aznar A, Riveiro M, Montaner J. Brain contusions induce a strong local overexpression of MMP-9. Results of a pilot study. Acta Neurochir Suppl. 2008;102:415–9. doi: 10.1007/978-3-211-85578-2_81. [DOI] [PubMed] [Google Scholar]
- 107.Vilalta A, Sahuquillo J, Rosell A, Poca MA, Riveiro M, Montaner J. Moderate and severe traumatic brain injury induce early overexpression of systemic and brain gelatinases. Intensive Care Med. 2008;34(8):1384–92. doi: 10.1007/s00134-008-1056-1. [DOI] [PubMed] [Google Scholar]
- 108.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20(12):1681–9. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 109.Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20(18):7037–42. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fujimoto M, Takagi Y, Aoki T, Hayase M, Marumo T, Gomi M, Nishimura M, Kataoka H, Hashimoto N, Nozaki K. Tissue inhibitor of metalloproteinases protect blood-brain barrier disruption in focal cerebral ischemia. J Cereb Blood Flow Metab. 2008;28(10):1674–85. doi: 10.1038/jcbfm.2008.59. [DOI] [PubMed] [Google Scholar]
- 111.Tejima E, Guo S, Murata Y, Arai K, Lok J, van Leyen K, Rosell A, Wang X, Lo EH. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J Neurotrauma. 2009;26(11):1935–41. doi: 10.1089/neu.2009.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12(4):441–5. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 113.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 114.Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5(12):1806–14. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 115.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267(36):26031–7. [PubMed] [Google Scholar]
- 116.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4(12):1317–26. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Plouët J, Moro F, Bertagnolli S, Coldeboeuf N, Mazarguil H, Clamens S, Bayard F. Extracellular cleavage of the vascular endothelial growth factor 189-amino acid form by urokinase is required for its mitogenic effect. J Biol Chem. 1997;272(20):13390–6. doi: 10.1074/jbc.272.20.13390. [DOI] [PubMed] [Google Scholar]
- 118.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169(4):681–91. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 120.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA. 1993;90(22):10705–9. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ebos JM, Bocci G, Man S, Thorpe PE, Hicklin DJ, Zhou D, Jia X, Kerbel RS. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol Cancer Res. 2004;2(6):315–26. [PubMed] [Google Scholar]
- 122.Kumai Y, Ooboshi H, Ibayashi S, Ishikawa E, Sugimori H, Kamouchi M, Kitazono T, Egashira K, Iida M. Postischemic gene transfer of soluble Flt-1 protects against brain ischemia with marked attenuation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2007;27(6):1152–60. doi: 10.1038/sj.jcbfm.9600420. [DOI] [PubMed] [Google Scholar]
- 123.Maharaj AS, Walshe TE, Saint-Geniez M, Venkatesha S, Maldonado AE, Himes NC, Matharu KS, Karumanchi SA, D’Amore PA. VEGF and TGF-β are required for the maintenance of the choroid plexus and ependyma. J Exp Med. 2008;205(2):491–501. doi: 10.1084/jem.20072041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suzuki R, Fukai N, Nagashijma G, Asai JI, Itokawa H, Nagai M, Suzuki T, Fujimoto T. Very early expression of vascular endothelial growth factor in brain oedema tissue associated with brain contusion. Acta Neurochir Suppl. 2003;86:277–9. doi: 10.1007/978-3-7091-0651-8_60. [DOI] [PubMed] [Google Scholar]
- 125.Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am J Physiol. 2001;280(1):H434–40. doi: 10.1152/ajpheart.2001.280.1.H434. [DOI] [PubMed] [Google Scholar]
- 126.Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem. 2009;284(31):21036–46. doi: 10.1074/jbc.M109.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci USA. 2009;106(6):1977–82. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vogel C, Bauer A, Wiesnet M, Preissner KT, Schaper W, Marti HH, Fischer S. Flt-1, but not Flk-1 mediates hyperpermeability through activation of the PI3-K/Akt pathway. J Cell Physiol. 2007;212(1):236–43. doi: 10.1002/jcp.21022. [DOI] [PubMed] [Google Scholar]
- 129.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111 (13):1853–65. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- 130.Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem. 2009;284(38):25602–11. doi: 10.1074/jbc.M109.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Soares HD, Hicks RR, Smith D, McIntosh TK. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci. 1995;15(12):8223–33. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Royo NC, Wahl F, Stutzmann JM. Kinetics of polymorphonuclear neutrophil infiltration after a traumatic brain injury in rat. Neuroreport. 1999;10(6):1363–7. doi: 10.1097/00001756-199904260-00038. [DOI] [PubMed] [Google Scholar]
- 133.Holmin S, Söderlund J, Biberfeld P, Mathiesen T. Intracerebral inflammation after human brain contusion. Neurosurgery. 1998;42(2):291–8. doi: 10.1097/00006123-199802000-00047. discussion 298–9. [DOI] [PubMed] [Google Scholar]
- 134.Worthylake RA, Burridge K. Leukocyte transendothelial migration: orchestrating the underlying molecular machinery. Curr Opin Cell Biol. 2001;13(5):569–77. doi: 10.1016/s0955-0674(00)00253-2. [DOI] [PubMed] [Google Scholar]
- 135.Clark RS, Carlos TM, Schiding JK, Bree M, Fireman LA, DeKosky ST, Kochanek PM. Antibodies against Mac-1 attenuate neutrophil accumulation after traumatic brain injury in rats. J Neurotrauma. 1996;13(6):333–41. doi: 10.1089/neu.1996.13.333. [DOI] [PubMed] [Google Scholar]
- 136.Weaver KD, Branch CA, Hernandez L, Miller CH, Quattrocchi KB. Effect of leukocyte-endothelial adhesion antagonism on neutrophil migration and neurologic outcome after cortical trauma. J Trauma. 2000;48(6):1081–90. doi: 10.1097/00005373-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 137.Knoblach SM, Faden AI. Administration of either anti-intercellular adhesion molecule-1 or a nonspecific control antibody improves recovery after traumatic brain injury in the rat. J Neurotrauma. 2002;19(9):1039–50. doi: 10.1089/089771502760341956. [DOI] [PubMed] [Google Scholar]
- 138.Utagawa A, Bramlett HM, Daniels L, Lotocki G, Dekaban GA, Weaver LC, Dietrich WD. Transient blockage of the CD11d/CD18 integrin reduces contusion volume and macrophage infiltration after traumatic brain injury in rats. Brain Res. 2008;1207:155–63. doi: 10.1016/j.brainres.2008.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Whalen MJ, Carlos TM, Dixon CE, Schiding JK, Clark RS, Baum E, Yan HQ, Marion DW, Kochanek PM. Effect of traumatic brain injury in mice deficient in intercellular adhesion molecule-1: assessment of histopathologic and functional outcome. J Neurotrauma. 1999;16(4):299–309. doi: 10.1089/neu.1999.16.299. [DOI] [PubMed] [Google Scholar]
- 140.Whalen MJ, Carlos TM, Dixon CE, Robichaud P, Clark RS, Marion DW, Kochanek PM. Reduced brain edema after traumatic brain injury in mice deficient in P-selectin and intercellular adhesion molecule-1. J Leukoc Biol. 2000;67(2):160–8. doi: 10.1002/jlb.67.2.160. [DOI] [PubMed] [Google Scholar]
- 141.Mackay CR. Moving targets: cell migration inhibitors as new anti-inflammatory therapies. Nat Immunol. 2008;9(9):988–98. doi: 10.1038/ni.f.210. [DOI] [PubMed] [Google Scholar]
- 142.Pineau I, Lacroix S. Endogenous signals initiating inflammation in the injured nervous system. Glia. 2009;57(4):351–61. doi: 10.1002/glia.20763. [DOI] [PubMed] [Google Scholar]
- 143.Nagyőszi P, Wilhelm I, Farkas AE, Fazakas C, Dung NT, Haskó J, Krizbai IA. Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem Int. 2010;57(5):556–64. doi: 10.1016/j.neuint.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 144.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28(3):138–45. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 145.Barnard EA, Simon J, Webb TE. Nucleotide receptors in the nervous system. An abundant component using diverse transduction mechanisms. Mol Neurobiol. 1997;15(2):103–29. doi: 10.1007/BF02740631. [DOI] [PubMed] [Google Scholar]
- 146.Albert JL, Boyle JP, Roberts JA, Challiss RA, Gubby SE, Boarder MR. Regulation of brain capillary endothelial cells by P2Y receptors coupled to Ca2+, phospholipase C and mitogen-activated protein kinase. Br J Pharmacol. 1997;122(5):935–41. doi: 10.1038/sj.bjp.0701453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abbracchio MP, Verderio C. Pathophysiological roles of P2 receptors in glial cells. Novartis Found Symp. 2006;276:91–103. discussion 103–12, 275–81. [PubMed] [Google Scholar]
- 148.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23(27):9254–62. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ostrow LW, Langan TJ, Sachs F. Stretch-induced endothelin-1 production by astrocytes. J Cardiovasc Pharmacol. 2000;36(Suppl 1):S274–7. doi: 10.1097/00005344-200036051-00081. [DOI] [PubMed] [Google Scholar]
- 150.Ralay Ranaivo H, Zunich S, Choi N, Hodge J, Wainwright M. Mild stretch-induced injury increases susceptibility to interleukin-1β induced release of matrix metalloproteinase-9 from astrocytes. J Neurotrauma. 2011;28(9):1757–66. doi: 10.1089/neu.2011.1799. [DOI] [PubMed] [Google Scholar]
- 151.Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces differential expression of tumor necrosis factor-α mRNA in the CNS. Mol Brain Res. 1996;36(2):287–91. doi: 10.1016/0169-328x(95)00274-v. [DOI] [PubMed] [Google Scholar]
- 152.Szmydynger-Chodobska J, Strazielle N, Zink BJ, Ghersi-Egea JF, Chodobski A. The role of the choroid plexus in neutrophil invasion after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29(9):1503–16. doi: 10.1038/jcbfm.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kinoshita K, Chatzipanteli K, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD. Interleukin-1β messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurgery. 2002;51(1):195–203. doi: 10.1097/00006123-200207000-00027. discussion 203. [DOI] [PubMed] [Google Scholar]
- 154.Taupin V, Toulmond S, Serrano A, Benavides J, Zavala F. Increase in IL-6, IL-1 and TNF levels in rat brain following traumatic lesion. Influence of pre- and post-traumatic treatment with Ro5 4864, a peripheral-type (p site) benzodiazepine ligand. J Neuroimmunol. 1993;42(2):177–85. doi: 10.1016/0165-5728(93)90008-m. [DOI] [PubMed] [Google Scholar]
- 155.de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64(1):37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 156.Mark KS, Miller DW. Increased permeability of primary cultured brain microvessel endothelial cell monolayers following TNF-α exposure. Life Sci. 1999;64(21):1941–53. doi: 10.1016/s0024-3205(99)00139-3. [DOI] [PubMed] [Google Scholar]
- 157.Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK. Chronic interleukin-1β expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007;27(35):9301–9. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wójciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-α-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176(1):150–65. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 159.Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, et al. NF-κB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22(15):3898–909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD. Expression from the human occludin promoter is affected by tumor necrosis factor α and interferon γ. J Cell Sci. 2000;113 (11):2085–90. doi: 10.1242/jcs.113.11.2085. [DOI] [PubMed] [Google Scholar]
- 161.Hess DC, Bhutwala T, Sheppard JC, Zhao W, Smith J. ICAM-1 expression on human brain microvascular endothelial cells. Neurosci Lett. 1994;168(1–2):201–4. doi: 10.1016/0304-3940(94)90450-2. [DOI] [PubMed] [Google Scholar]
- 162.Wong D, Dorovini-Zis K. Expression of vascular cell adhesion molecule-1 (VCAM-1) by human brain microvessel endothelial cells in primary culture. Microvasc Res. 1995;49(3):325–39. doi: 10.1006/mvre.1995.1028. [DOI] [PubMed] [Google Scholar]
- 163.Wong D, Dorovini-Zis K. Regualtion by cytokines and lipopolysaccharide of E-selectin expression by human brain microvessel endothelial cells in primary culture. J Neuropathol Exp Neurol. 1996;55(2):225–35. doi: 10.1097/00005072-199602000-00011. [DOI] [PubMed] [Google Scholar]
- 164.Stanimirovic DB, Wong J, Shapiro A, Durkin JP. Increase in surface expression of ICAM-1, VCAM-1 and E-selectin in human cerebromicrovascular endothelial cells subjected to ischemia-like insults. Acta Neurochir Suppl. 1997;70:12–6. doi: 10.1007/978-3-7091-6837-0_4. [DOI] [PubMed] [Google Scholar]
- 165.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66(6):876–88. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 166.Carlos TM, Clark RS, Franicola-Higgins D, Schiding JK, Kochanek PM. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J Leukoc Biol. 1997;61(3):279–85. doi: 10.1002/jlb.61.3.279. [DOI] [PubMed] [Google Scholar]
- 167.Balabanov R, Goldman H, Murphy S, Pellizon G, Owen C, Rafols J, Dore-Duffy P. Endothelial cell activation following moderate traumatic brain injury. Neurol Res. 2001;23(2–3):175–82. doi: 10.1179/016164101101198514. [DOI] [PubMed] [Google Scholar]
- 168.McKeating EG, Andrews PJ, Mascia L. The relationship of soluble adhesion molecule concentrations in systemic and jugular venous serum to injury severity and outcome after traumatic brain injury. Anesth Analg. 1998;86(4):759–65. doi: 10.1097/00000539-199804000-00016. [DOI] [PubMed] [Google Scholar]
- 169.Pleines UE, Stover JF, Kossmann T, Trentz O, Morganti-Kossmann MC. Soluble ICAM-1 in CSF coincides with the extent of cerebral damage in patients with severe traumatic brain injury. J Neurotrauma. 1998;15(6):399–409. doi: 10.1089/neu.1998.15.399. [DOI] [PubMed] [Google Scholar]
- 170.Yamasaki Y, Matsuo Y, Zagorski J, Matsuura N, Onodera H, Itoyama Y, Kogure K. New therapeutic possibility of blocking cytokine-induced neutrophil chemoattractant on transient ischemic brain damage in rats. Brain Res. 1997;759(1):103–11. doi: 10.1016/s0006-8993(97)00251-5. [DOI] [PubMed] [Google Scholar]
- 171.Beech JS, Reckless J, Mosedale DE, Grainger DJ, Williams SC, Menon DK. Neuroprotection in ischemia-reperfusion injury: an antiinflammatory approach using a novel broad-spectrum chemokine inhibitor. J Cereb Blood Flow Metab. 2001;21(6):683–9. doi: 10.1097/00004647-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 172.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38(4):1345–53. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- 173.Semple BD, Bye N, Ziebell JM, Morganti-Kossmann MC. Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration and cortical damage following closed head injury. Neurobiol Dis. 2010;40(2):394–403. doi: 10.1016/j.nbd.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 174.Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J Cereb Blood Flow Metab. 2010;30(4):769–82. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bell MD, Taub DD, Kunkel SJ, Strieter RM, Foley R, Gauldie J, Perry VH. Recombinant human adenovirus with rat MIP-2 gene insertion causes prolonged PMN recruitment to the murine brain. Eur J Neurosci. 1996;8(9):1803–11. doi: 10.1111/j.1460-9568.1996.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 176.Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, Bravo R, Lira SA. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995;155(12):5769–76. [PubMed] [Google Scholar]
- 177.Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, Vogel SN. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003;23(6):748–55. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- 178.Szmydynger-Chodobska J, Fox LM, Lynch KM, Zink BJ, Chodobski A. Vasopressin amplifies the production of proinflammatory mediators in traumatic brain injury. J Neurotrauma. 2010;27(8):1449–61. doi: 10.1089/neu.2010.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 180.Colotta F, Borré A, Wang JM, Tattanelli M, Maddalena F, Polentarutti N, Peri G, Mantovani A. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J Immunol. 1992;148(3):760–5. [PubMed] [Google Scholar]
- 181.Zhang W, Smith C, Shapiro A, Monette R, Hutchison J, Stanimirovic D. Increased expression of bioactive chemokines in human cerebromicrovascular endothelial cells and astrocytes subjected to simulated ischemia in vitro. J Neuroimmunol. 1999;101(2):148–60. doi: 10.1016/s0165-5728(99)00137-x. [DOI] [PubMed] [Google Scholar]
- 182.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100(12):3853–60. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 183.Ge S, Song L, Serwanski DR, Kuziel WA, Pachter JS. Transcellular transport of CCL2 across brain microvascular endothelial cells. J Neurochem. 2008;104(5):1219–32. doi: 10.1111/j.1471-4159.2007.05056.x. [DOI] [PubMed] [Google Scholar]
- 184.Szmydynger-Chodobska J, Zink BJ, Chodobski A. Multiple sites of vasopressin synthesis in the injured brain. J Cereb Blood Flow Metab. 2011;31(1):47–51. doi: 10.1038/jcbfm.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25(5):593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- 186.Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci. 2003;116(22):4615–28. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- 187.Schoettle RJ, Kochanek PM, Magargee MJ, Uhl MW, Nemoto EM. Early polymorphonuclear leukocyte accumulation correlates with the development of posttraumatic cerebral edema in rats. J Neurotrauma. 1990;7(4):207–17. doi: 10.1089/neu.1990.7.207. [DOI] [PubMed] [Google Scholar]
- 188.Uhl MW, Biagas KV, Grundl PD, Barmada MA, Schiding JK, Nemoto EM, Kochanek PM. Effects of neutropenia on edema, histology, and cerebral blood flow after traumatic brain injury in rats. J Neurotrauma. 1994;11(3):303–15. doi: 10.1089/neu.1994.11.303. [DOI] [PubMed] [Google Scholar]
- 189.Whalen MJ, Carlos TM, Kochanek PM, Clark RS, Heineman S, Schiding JK, Franicola D, Memarzadeh F, Lo W, Marion DW, et al. Neutrophils do not mediate blood-brain barrier permeability early after controlled cortical impact in rats. J Neurotrauma. 1999;16(7):583–94. doi: 10.1089/neu.1999.16.583. [DOI] [PubMed] [Google Scholar]
- 190.Osborn MT, Chambers TC. Role of the stress-activated/c-Jun NH2-terminal protein kinase pathway in the cellular response to adriamycin and other chemotherapeutic drugs. J Biol Chem. 1996;271(48):30950–5. doi: 10.1074/jbc.271.48.30950. [DOI] [PubMed] [Google Scholar]
- 191.Dinkel K, Dhabhar FS, Sapolsky RM. Neurotoxic effects of polymorphonuclear granulocytes on hippocampal primary cultures. Proc Natl Acad Sci USA. 2004;101(1):331–6. doi: 10.1073/pnas.0303510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Neumann J, Sauerzweig S, Rönicke R, Gunzer F, Dinkel K, Ullrich O, Gunzer M, Reymann KG. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J Neurosci. 2008;28(23):5965–75. doi: 10.1523/JNEUROSCI.0060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009;30(11):547–56. doi: 10.1016/j.it.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Armao D, Kornfeld M, Estrada EY, Grossetete M, Rosenberg GA. Neutral proteases and disruption of the blood-brain barrier in rat. Brain Res. 1997;767(2):259–64. doi: 10.1016/s0006-8993(97)00567-2. [DOI] [PubMed] [Google Scholar]
- 195.Stowe AM, Adair-Kirk TL, Gonzales ER, Perez RS, Shah AR, Park TS, Gidday JM. Neutrophil elastase and neurovascular injury following focal stroke and reperfusion. Neurobiol Dis. 2009;35(1):82–90. doi: 10.1016/j.nbd.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol. 2005;289(2):H558–68. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 197.Gotsch U, Jäger U, Dominis M, Vestweber D. Expression of P-selectin on endothelial cells is upregulated by LPS and TNF-α in vivo. Cell Adhes Commun. 1994;2(1):7–14. doi: 10.3109/15419069409014198. [DOI] [PubMed] [Google Scholar]
- 198.Andersson PB, Perry VH, Gordon S. Intracerebral injection of proinflammatory cytokines or leukocyte chemotaxins induces minimal myelomonocytic cell recruitment to the parenchyma of the central nervous system. J Exp Med. 1992;176(1):255–9. doi: 10.1084/jem.176.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Allt G, Lawrenson JG. Is the pial microvessel a good model for blood-brain barrier studies? Brain Res Rev. 1997;24(1):67–76. doi: 10.1016/s0165-0173(97)00011-8. [DOI] [PubMed] [Google Scholar]
- 200.Bartholomäus I, Kawakami N, Odoardi F, Schläger C, Miljkovic D, Ellwart JW, Klinkert WE, Flügel-Koch C, Issekutz TB, Wekerle H, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462(7269):94–8. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 201.Kivisäkk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, Ransohoff RM, Khoury SJ. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65(4):457–69. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Szmydynger-Chodobska J, Strazielle N, Gandy JR, Keefe TH, Zink BJ, Ghersi-Egea JF, Chodobski A. Posttraumatic invasion of monocytes across the blood-cerebrospinal fluid barrier. J Cereb Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2011.111. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Strazielle N, Ghersi-Egea JF. Choroid plexus in the central nervous system: biology and physiopathology. J Neuropathol Exp Neurol. 2000;59(7):561–74. doi: 10.1093/jnen/59.7.561. [DOI] [PubMed] [Google Scholar]
- 204.O’Donnell ME, Tran L, Lam TI, Liu XB, Anderson SE. Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metab. 2004;24(9):1046–56. doi: 10.1097/01.WCB.0000130867.32663.90. [DOI] [PubMed] [Google Scholar]
- 205.Lam TI, Wise PM, O’Donnell ME. Cerebral microvascular endothelial cell Na/H exchange: evidence for the presence of NHE1 and NHE2 isoforms and regulation by arginine vasopressin. Am J Physiol. 2009;297(2):C278–89. doi: 10.1152/ajpcell.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pedersen SF, O’Donnell ME, Anderson SE, Cala PM. Physiology and pathophysiology of Na+/H+ exchange and Na+-K+-2Cl− cotransport in the heart, brain, and blood. Am J Physiol. 2006;291(1):R1–25. doi: 10.1152/ajpregu.00782.2005. [DOI] [PubMed] [Google Scholar]
- 207.Lu KT, Cheng NC, Wu CY, Yang YL. NKCC1-mediated traumatic brain injury-induced brain edema and neuron death via Raf/MEK/MAPK cascade. Crit Care Med. 2008;36(3):917–22. doi: 10.1097/CCM.0B013E31816590C4. [DOI] [PubMed] [Google Scholar]
- 208.Suzuki Y, Matsumoto Y, Ikeda Y, Kondo K, Ohashi N, Umemura K. SM-20220, a Na+/H+ exchanger inhibitor: effects on ischemic brain damage through edema and neutrophil accumulation in a rat middle cerebral artery occlusion model. Brain Res. 2002;945(2):242–8. doi: 10.1016/s0006-8993(02)02806-8. [DOI] [PubMed] [Google Scholar]
- 209.O’Donnell ME, Duong V, Suvatne J, Foroutan S, Johnson DM. Arginine vasopressin stimulation of cerebral microvascular endothelial cell Na-K-Cl cotransporter activity is V1 receptor and [Ca] dependent. Am J Physiol. 2005;289(2):C283–92. doi: 10.1152/ajpcell.00001.2005. [DOI] [PubMed] [Google Scholar]
- 210.Foroutan S, Brillault J, Forbush B, O’Donnell ME. Moderate-to-severe ischemic conditions increase activity and phosphorylation of the cerebral microvascular endothelial cell Na+-K+-Cl− cotransporter. Am J Physiol. 2005;289(6):C1492–501. doi: 10.1152/ajpcell.00257.2005. [DOI] [PubMed] [Google Scholar]
- 211.Szmydynger-Chodobska J, Chung I, KoŸniewska E, Tran B, Harrington FJ, Duncan JA, Chodobski A. Increased expression of vasopressin V1a receptors after traumatic brain injury. J Neurotrauma. 2004;21(8):1090–102. doi: 10.1089/0897715041651033. [DOI] [PubMed] [Google Scholar]
- 212.Burke MA, Mutharasan RK, Ardehali H. The sulfonylurea receptor, an atypical ATP-binding cassette protein, and its regulation of the KATP channel. Circ Res. 2008;102(2):164–76. doi: 10.1161/CIRCRESAHA.107.165324. [DOI] [PubMed] [Google Scholar]
- 213.Bryan J, Muñoz A, Zhang X, Düfer M, Drews G, Krippeit-Drews P, Aguilar-Bryan L. ABCC8 and ABCC9: ABC transporters that regulate K+ channels. Pflügers Arch. 2007;453(5):703–18. doi: 10.1007/s00424-006-0116-z. [DOI] [PubMed] [Google Scholar]
- 214.Chen M, Dong Y, Simard JM. Functional coupling between sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J Neurosci. 2003;23(24):8568–77. doi: 10.1523/JNEUROSCI.23-24-08568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Simard JM, Tarasov KV, Gerzanich V. Non-selective cation channels, transient receptor potential channels and ischemic stroke. Biochim Biophys Acta. 2007;1772(8):947–57. doi: 10.1016/j.bbadis.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gerzanich V, Woo SK, Vennekens R, Tsymbalyuk O, Ivanova S, Ivanov A, Geng Z, Chen Z, Nilius B, Flockerzi V, et al. De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat Med. 2009;15(2):185–91. doi: 10.1038/nm.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Simard JM, Tsymbalyuk O, Ivanov A, Ivanova S, Bhatta S, Geng Z, Woo SK, Gerzanich V. Endothelial sulfonylurea receptor 1-regulated NCCa-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest. 2007;117(8):2105–13. doi: 10.1172/JCI32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, Tsymbalyuk N, West GA, Gerzanich V. Newly expressed SUR1-regulated NCCa-ATP channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12(4):433–40. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Simard JM, Geng Z, Woo SK, Ivanova S, Tosun C, Melnichenko L, Gerzanich V. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2009;29(2):317–30. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Simard JM, Kahle KT, Gerzanich V. Molecular mechanisms of microvascular failure in central nervous system injury—synergistic roles of NKCC1 and SUR1/TRPM4. J Neurosurg. 113(3):622–9. doi: 10.3171/2009.11.JNS081052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Simard JM, Yurovsky V, Tsymbalyuk N, Melnichenko L, Ivanova S, Gerzanich V. Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke. 2009;40(2):604–9. doi: 10.1161/STROKEAHA.108.522409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Patel AD, Gerzanich V, Geng Z, Simard JM. Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J Neuropathol Exp Neurol. 69(12):1177–90. doi: 10.1097/NEN.0b013e3181fbf6d6. [DOI] [PubMed] [Google Scholar]
- 223.Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24(6):439–51. doi: 10.1097/HTR.0b013e3181c15600. [DOI] [PubMed] [Google Scholar]
- 224.Roberts GW, Gentleman SM, Lynch A, Murray L, Landon M, Graham DI. β amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1994;57(4):419–25. doi: 10.1136/jnnp.57.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM, Clark RS, Marion DW, Wisniewski SR, DeKosky ST. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190(1):192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 226.Loane DJ, Pocivavsek A, Moussa CE, Thompson R, Matsuoka Y, Faden AI, Rebeck GW, Burns MP. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med. 2009;15(4):377–9. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Loane DJ, Washington PM, Vardanian L, Pocivavsek A, Hoe HS, Duff KE, Cernak I, Rebeck GW, Faden AI, Burns MP. Modulation of ABCA1 by an LXR agonist reduces beta-amyloid levels and improves outcome after traumatic brain injury. J Neurotrauma. 2011;28(2):225–36. doi: 10.1089/neu.2010.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kim WS, Guillemin GJ, Glaros EN, Lim CK, Garner B. Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. Neuroreport. 2006;17(9):891–6. doi: 10.1097/01.wnr.0000221833.41340.cd. [DOI] [PubMed] [Google Scholar]
- 229.Panzenboeck U, Kratzer I, Sovic A, Wintersperger A, Bernhart E, Hammer A, Malle E, Sattler W. Regulatory effects of synthetic liver X receptor- and peroxisome-proliferator activated receptor agonists on sterol transport pathways in polarized cerebrovascular endothelial cells. Int J Biochem Cell Biol. 2006;38(8):1314–29. doi: 10.1016/j.biocel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 230.Do TM, Ouellet M, Calon F, Chimini G, Chacun H, Farinotti R, Bourasset F. Direct evidence of abca1-mediated efflux of cholesterol at the mouse blood-brain barrier. Mol Cell Biochem. 2011;357(1–2):397–404. doi: 10.1007/s11010-011-0910-6. [DOI] [PubMed] [Google Scholar]
- 231.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, et al. LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron. 2004;43(3):333–44. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 232.Akanuma S, Ohtsuki S, Doi Y, Tachikawa M, Ito S, Hori S, Asashima T, Hashimoto T, Yamada K, Ueda K, et al. ATP-binding cassette transporter A1 (ABCA1) deficiency does not attenuate the brain-to-blood efflux transport of human amyloid-β peptide (1–40) at the blood-brain barrier. Neurochem Int. 2008;52(6):956–61. doi: 10.1016/j.neuint.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 233.Löscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76(1):22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 234.Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60(2):196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Potschka H. Targeting regulation of ABC efflux transporters in brain diseases: a novel therapeutic approach. Pharmacol Ther. 2010;125(1):118–27. doi: 10.1016/j.pharmthera.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 236.Spudich A, Kilic E, Xing H, Kilic U, Rentsch KM, Wunderli-Allenspach H, Bassetti CL, Hermann DM. Inhibition of multidrug resistance transporter-1 facilitates neuroprotective therapies after focal cerebral ischemia. Nat Neurosci. 2006;9(4):487–8. doi: 10.1038/nn1676. [DOI] [PubMed] [Google Scholar]
- 237.Scheff SW, Sullivan PG. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J Neurotrauma. 1999;16(9):783–92. doi: 10.1089/neu.1999.16.783. [DOI] [PubMed] [Google Scholar]
- 238.Sullivan PG, Thompson M, Scheff SW. Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp Neurol. 2000;161(2):631–7. doi: 10.1006/exnr.1999.7282. [DOI] [PubMed] [Google Scholar]
- 239.Sullivan PG, Rabchevsky AG, Hicks RR, Gibson TR, Fletcher-Turner A, Scheff SW. Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience. 2000;101(2):289–95. doi: 10.1016/s0306-4522(00)00380-8. [DOI] [PubMed] [Google Scholar]
- 240.Hansson MJ, Persson T, Friberg H, Keep MF, Rees A, Wieloch T, Elmér E. Powerful cyclosporin inhibition of calcium-induced permeability transition in brain mitochondria. Brain Res. 2003;960(1–2):99–111. doi: 10.1016/s0006-8993(02)03798-8. [DOI] [PubMed] [Google Scholar]
- 241.Merenda A, Bullock R. Clinical treatments for mitochondrial dysfunctions after brain injury. Curr Opin Crit Care. 2006;12(2):90–6. doi: 10.1097/01.ccx.0000216573.26686.27. [DOI] [PubMed] [Google Scholar]
- 242.Margulies S, Hicks R The Combination Therapies for Traumatic Brain Injury Workshop Leaders. Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma. 2009;26(6):925–39. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Work LM, Büning H, Hunt E, Nicklin SA, Denby L, Britton N, Leike K, Odenthal M, Drebber U, Hallek M, et al. Vascular bed-targeted in vivo gene delivery using tropism-modified adeno-associated viruses. Mol Ther. 2006;13(4):683–93. doi: 10.1016/j.ymthe.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 244.Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, Stins MF, Wang X, Dedhar S, Lo EH. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc Natl Acad Sci USA. 2008;105(21):7582–7. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sawada N, Kim HH, Moskowitz MA, Liao JK. Rac1 is a critical mediator of endothelium-derived neurotrophic activity. Sci Signal. 2009;2(61):ra10. doi: 10.1126/scisignal.2000162. [DOI] [PMC free article] [PubMed] [Google Scholar]