Abstract
The essential function of the lung, gas exchange, is dependent on adequate matching of ventilation and perfusion, where air and blood are delivered through complex branching systems exposed to regionally varying transpulmonary and transmural pressures. Structure and function in the lung are intimately related, yet computational models in pulmonary physiology usually simplify or neglect structure. The geometries of the airway and vascular systems and their interaction with parenchymal tissue have an important bearing on regional distributions of air and blood, and therefore on whole lung gas exchange, but this has not yet been addressed by modelling studies. Models for gas exchange have typically incorporated considerable detail at the level of chemical reactions, with little thought for the influence of structure. To date, relatively little attention has been paid to modelling at the cellular or subcellular level in the lung, or to linking information from the protein structure/interaction and cellular levels to the operation of the whole lung. We review previous work in developing anatomically based models of the lung, airways, parenchyma and pulmonary vasculature, and some functional studies in which these models have been used. Models for gas exchange at several spatial scales are briefly reviewed, and the challenges and benefits from modelling cellular function in the lung are discussed.
Keywords: perfusion, ventilation, gas exchange, ciliated epithelial cell
1. Introduction
The human lung is primarily an organ of gas exchange, but its ability to perform this function is intimately dependent on its mechanical behaviour: ventilation is driven by expansion and recoil of the lung tissue, and perfusion is affected by regional tissue recoil pressures. Indeed, an important feature of the major respiratory diseases (asthma and chronic obstructive pulmonary disease (COPD)) is that they affect the mechanical function of the lung, with asthma having a direct effect on the mechanics of the airways, and COPD deteriorating the elastic properties of the lung parenchymal tissue. The lung's structural subsystems (lobes, airways, vasculature) are mechanically coupled, and this leads to a highly interdependent, integrated system.
The lung with its branching airway and vascular structures suspended in an elastic tissue is arguably far more complex to model than other organ systems. The functional components of the lung span multiple spatial scales, starting at the larger scale from the separate lobes (five within the human lung), to the conducting conduits within the lobes (airways and blood vessels; largest diameters of approximately 25 mm), which bifurcate into ever smaller branches and eventually terminate in the alveolar capillary plexus (diameters of approximately 0.3 and 0.008 mm for the alveoli and capillaries, respectively), hence ranging across several orders of magnitude. Distributions of cartilage, smooth muscle and cell type vary with airway location. The lung is also exposed to the environment, which results in cyclic changes of temperature and humidity in the most proximal airways. An example of function that spans many orders of spatial scale is the effect of airflow dynamics on airway surface evaporation. Ventilation distribution and levels of turbulence affect the level of shear stress that is transmitted to epithelial cells on the airway surface. The level of shear directly affects the release of ATP from the cells, and mechanosensitive ion channels, both of which control intracellular calcium levels. Intracellular calcium in turn controls transcellular water movement, which feeds back to airway humidity by controlling the amount of airway surface liquid (ASL) available for evaporation.
Over the past several decades, our understanding of dynamic biological phenomena within the lung has increased substantially. This has occurred over many scales of interest, but certainly not in a systematic manner, nor proportionately at each scale. For example, focus at the protein level has resulted in considerable information about structure and the effect of mutations to specific subunits, whereas intracellular signalling via receptors or complex networked biochemical pathways is still not well understood despite having been known to exist for some time. Advances in the visualization of intracellular processes have substantially increased our understanding of the molecular events within the cells. Paradoxically, these advances have exposed further complexities, particularly in cellular regulatory interdependencies. Considering the other end of the spatial spectrum, increased resolution in medical imaging has contributed to our understanding of the function of the whole organ. However, relatively little work has been performed to link information obtained at the protein structure/interaction and cellular levels to the operation of the whole lung.
The international Physiome Project (www.physiome.org.nz) aims to construct an integrative computational modelling framework, including databases and tools, to define and represent multi-scale structure–function relationships within an organism, spanning from genes through to organ and to whole organism. Development towards a Lung Physiome (www.physiome.org.nz/lung) includes compiling a digital atlas of the human lung for male and female subjects spanning several decades of life (Hoffman et al. 2004), deriving computation-ready models of geometric structure to partner imaged subjects in the digital atlas (Tawhai et al. 2004; Burrowes et al. 2005; Lin et al. 2007), and implementation of models for various pulmonary functions to study structure–function relationships and system interactions (Burrowes & Tawhai 2006; Tawhai et al. 2006; Lin et al. 2007). Here, we review some of the efforts to develop integrative models of lung structure and function at the organ and tissue levels, and discuss the challenges and benefits of coupling with models at the cellular level, and gas exchange.
2. Organ-level models of structure for integrated simulation of function
(a) Anatomically based models of the lung, airway and vascular trees
Because the lung tissue is extremely deformable, this negates the possibility of deriving models of lung geometry from an excised specimen as is possible for muscular organs or bone (e.g. LeGrice et al. 1997); however, high-resolution anatomical data can be obtained from in vivo imaging of the lung. Currently, the imaging modality of choice for the lung tissue is spiral multi-row detector computed tomography (MDCT). From the standard clinical MDCT, the lungs, the lobes and the major airways and blood vessels can be segmented, and computation-ready models for the geometry of the lung and its lobes can be created using geometry fitting methods (Fernandez et al. 2004). This approach fits a high-order finite-element (FE) mesh to the surface shape of the structure of interest. This method can be used to derive models for the geometry of human or animal lungs, in a form that is ready for computational analysis of tissue stress and strain (Tawhai et al. 2006). Figure 1 illustrates a volumetric FE mesh of the left lung derived from MDCT data, including a surface mesh of the central airways also fitted to MDCT data.
Figure 1.
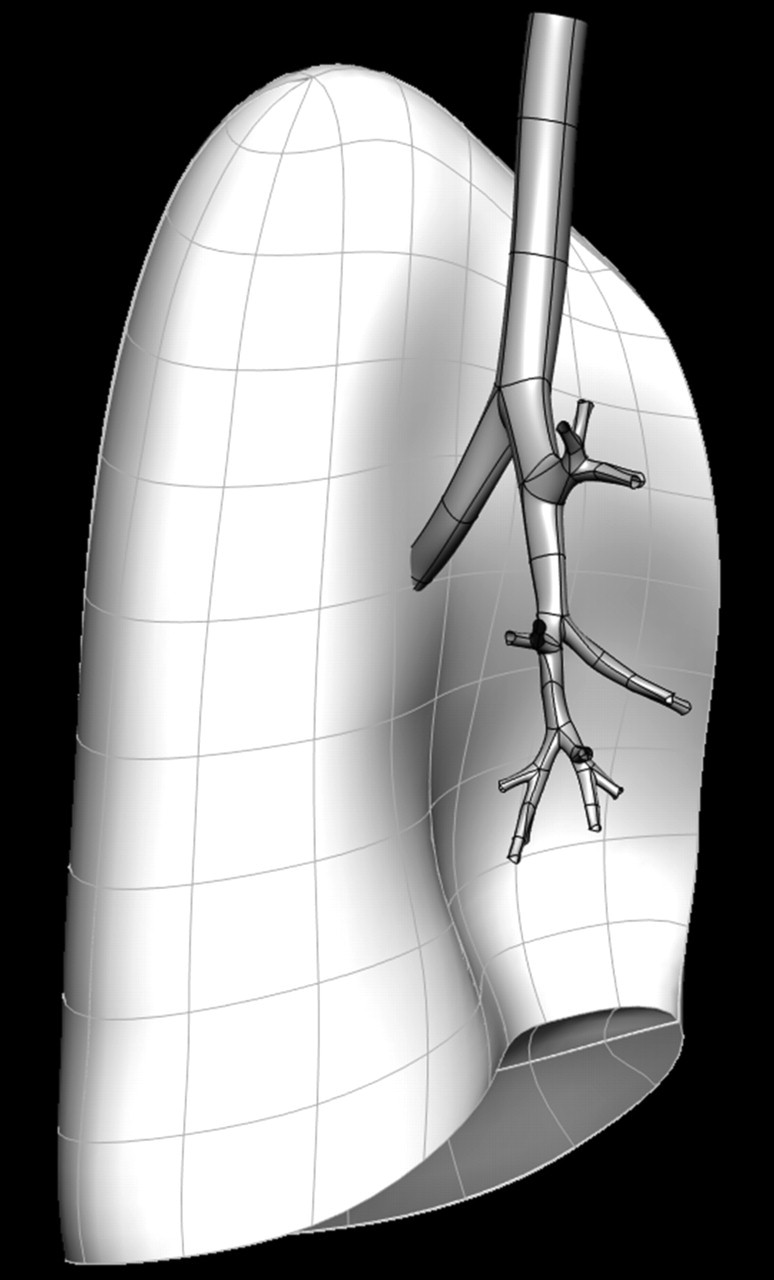
Volumetric FE mesh of the left lung derived from geometry fitting to imaging data. The surfaces of the geometry-fitted CT-defined airways are in dark grey. Note that models of the lung and airway are fitted to imaging data from a single individual. The models can be used in independent studies, or in studies that consider interaction between tissue and airway.
Models for the geometry of the conducting airways can be derived as one-dimensional (centreline), two-dimensional (surface) or three-dimensional (volume) structures. The dimension of a model depends on the functional problem for which it will be used. For example, one-dimensional models are suitable for simulating airway heat and humidity transport (Tawhai & Hunter 2004), whereas three-dimensional models are necessary for detailed computational fluid dynamics (CFD) simulation (van Ertbruggen et al. 2005; Lin et al. 2007, in press). Traditional one-dimensional airway models used for functional simulation are Weibel's symmetric model A (Weibel 1963) and Horsfield's model with regular asymmetry of branching (Horsfield et al. 1971). Both of these models provide a convenient simplification of the complex branching airway tree, and have been used widely for one-dimensional simulation studies or extrapolated to three-dimensional studies (van Ertbruggen et al. 2005). But neither model is specific to an individual, nor does it include spatially accurate distributions of the airway branches.
Spatially distributed models for the airway tree can be derived by tracing the bifurcating airway paths using imaging of the in vivo lung (Tawhai et al. 2004) or imaging of airway casts from an excised lung. Tawhai et al. (2004) supplemented in vivo imaging-based airway geometry with a volume-filling branching (VFB) method to fill an imaging-based lung volume with a morphometrically consistent tree. Using an airway cast to define the branching geometry has the advantage of defining connectivity through an extensive airway tree, but casting introduces geometric distortion of the tissue, and it does not extend through the entire conducting airway system. It is also not applicable to deriving individualized models for living subjects. Combining in vivo imaging with supplemental airways has the advantage of producing a model that is specific to an imaged subject and morphometrically consistent. The method can also be used for humans or animals. Figure 2 illustrates a subject-specific model of the human conducting airway tree, including central branches derived from MDCT imaging data and additional branches generated using the VFB method of Tawhai et al. (2004).
Figure 2.

Subject-specific models generated to represent the human conducting airway tree for an imaged subject. (a) Left upper (yellow) and left lower (orange) lobe-generated airways, viewed from the r.h.s. (b) Airways generated in all five lobes using a VFB method, with CT-defined airway surfaces (grey). (c) Right upper (green), right middle (red) and right lower (blue) lobe-generated airways.
The model from Tawhai et al. (2004) is a centreline (one-dimensional) tree with associated branch diameters. It is therefore equivalent in basic structure to the Weibel and Horsfield models, but with subject-specific geometry and spatial positioning of the airways. This becomes important when considering interacting function, such as the effect of changing tissue properties on airway tethering and airway collapse or for comparison of simulated results with spatially distributed experimental results (Burrowes & Tawhai 2006). The one-dimensional tree can also be converted to a three-dimensional mesh for CFD simulation (Lin et al. in press). The consistent relationship between the one- and three-dimensional models from Lin et al. (in press) means that the accuracy of simplified (one-dimensional) models of airway function can be evaluated by comparing directly with their more detailed three-dimensional counterpart.
Similar methods have been used to derive models for the pulmonary arteries and veins (Burrowes et al. 2005). The addition of supernumerary blood vessels is a unique feature of the vascular tree model (Burrowes et al. 2005). Each airway is known to be accompanied by an artery and vein, but there is also a vast system of supernumerary blood vessels, which far outnumber the conventional accompanying vessels. The exact functional significance of these vessels is still unclear, but they are likely to provide alternative perfusion pathways when a main vessel is occluded. Burrowes et al. (2005) developed a model generation algorithm based on the limited available structural information on these vessels: that they are known to branch off at approximately 90° from the accompanying blood vessels and branch rapidly towards the closest parenchymal tissue.
(b) Perfusion of the arterial and venous networks
Early low-resolution studies of pulmonary perfusion concluded that hydrostatic pressure differences were the main determinant of flow distribution (West et al. 1964). Since then, several studies of pulmonary perfusion, both experimental and theoretical, have highlighted the intimate relationship between structure and function in the pulmonary circulation. For example, experimental studies using high-resolution radionuclide-labelled microsphere techniques (reviewed by Robertson & Hlastala 2007) and functional imaging measurements (e.g. Jones et al. 2001) have suggested a prominent role of the asymmetric vascular branching structure in determining the distribution of perfusion, and a less significant contribution from gravity. Several modelling studies have also investigated structure–function relationships in this system: most of these have reduced the complexity of the pulmonary vascular tree geometry by representing the arteries and veins as a symmetric tree (Parker et al. 1997), as a self-similar fractal tree (e.g Glenny & Robertson 1991), or an average flow path via summary morphometric parameters (Dawson et al. 1999). These early models were implemented to investigate the effects of large-scale alterations of branching geometry on pulmonary perfusion, and therefore only represent the average geometry of the branching structure. The models have not been created to accurately reflect the geometry of the vascular structure; they have instead been designed for use in a particular functional investigation. However, these studies all suggested a strong dependence of flow distribution, in the isolated perfusion system, on vascular geometry.
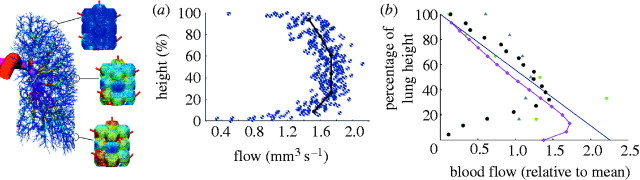
Burrowes et al. (2005) used pulmonary vascular models with anatomical detail to investigate the relative roles of the vascular branching structure and gravity on perfusion by comparing flow in symmetric and anatomical models, and in models with and without gravity. Solution of a one-dimensional form of the Navier–Stokes equations including gravity (Burrowes & Tawhai 2006) gave predictions of spatially distributed blood pressure, vessel radius and blood flow. Figure 3a shows a plot of blood flow exiting the terminal vessels against height in an arterial model exposed to normal gravity. The model predicted a large amount of flow heterogeneity emerging as a result of the asymmetric branching structure; the relative position of the main vessel inlet/outlet to the system also played a relatively large role in flow distribution, and this is most apparent when changing between the prone and supine postures. The influence of branching geometry dominated regardless of the magnitude and direction of gravity (Burrowes & Tawhai 2006).
Figure 3.
Illustration of previous perfusion predictions across multi-scale circulatory models from the arteries (Burrowes & Tawhai 2006) to the capillary network (Burrowes et al. 2004). The colour spectrum ranges from red (highest flow) to dark blue (lowest flow). (a) Flow predictions at terminal vessel locations in the arterial model plotted with respect to vertical height. Results are averaged within 1 mm (blue circles) and 50 mm (black line) slices. (b) Flow solutions (relative to mean) in the isolated capillary model plotted with respect to gravitationally dependent height. Experimental flow data are included from supine dogs (green down triangles and grey up triangles; Hogg et al. 1985), upright baboons (blue line; Glenny et al. 1999) and supine pigs (black circles; Glenny et al. 2000). Pink diamonds, model results.
3. Tissue-level models
(a) Modelling the structure of the pulmonary parenchyma
The pulmonary alveoli form a volume-filling honeycomb-like structure such that the division of the alveolar surface, rather than the lung volume, provides a sufficiently large surface area for gas exchange. Several models that seek to mimic the anatomical structure of alveolar tissue have been developed, with varying levels of geometric complexity. The level of detail in these models has been dictated somewhat by the functional investigations in which they have been used.
Alveolar model geometry has progressed from simple spherical approximations to more complex geometric representations. From the detailed measurement of the anatomy of the lung, Weibel (1963) developed early geometric models of the airways and alveolar capillary units. Alveolar shape varies considerably; therefore, several geometric simplifications were evaluated in an attempt to best represent the measured surface-to-volume ratio of 4.87. A 5/6 sphere was chosen to represent an average alveolus with the inclusion of a corrugated surface due to capillary bulging.
Fung (1988) started with the assumption that alveoli are all initially equal in size and shape and are volume filling. The geometry of all possible space-filling polyhedra was analysed, and a 14-hedron was selected to represent the average alveolus because it has the minimum surface-to-volume ratio of all space-filling polyhedra of the same volume and the geometry compared well with the measured alveolar structure. A volume-filling structure was created from several 14-hedrons. Geometric properties of the model were analysed by removing various combinations of alveolar walls and central faces to allow aeration. The model was later used to derive theoretical incremental elastic constants (Fung 1989).
In a series of investigations into the micromechanical properties of alveolar tissue, Denny & Schroter (Dale et al. 1980; Kowe et al. 1986; Denny & Schroter 1995, 1996, 2006) developed several FE models of the alveolar geometry. Their model consisted of a network of adjacent truncated octahedral alveoli surrounding a longitudinal air duct (Denny & Schroter 1995). This was extended to a three-dimensional space-filling model of an acinar ventilatory unit within a cuboidal block (Denny & Schroter 1996). Truncated octahedra were again used to represent individual alveoli with specified faces removed to create ducts and allow ventilation. All alveoli were assumed to be uniform in shape and size. The model has most recently been used to study the effect of large non-uniform distortions of the lung tissue, and to derive elastic constants that define the material behaviour of the parenchyma (Denny & Schroter 2006).
The structure of the polyhedral models is termed ‘volume filling’ because the polyhedra can pack together without leaving any gaps. This is a reasonable representation of alveoli that are deep within the lung tissue, but not for alveoli that have their shape constrained by a lung or lobe surface. That is, the polyhedral models have not been used to represent a shape-constrained alveolated structure that may have a smooth surface.
To derive a volume-filling alveolated structure that can conform to any host shape, Burrowes et al. (2004) developed a Voronoi–Delaunay meshing method, which effectively partitions the space around a collection of points that represent either the centre of an alveolus or the central axis of an alveolar duct. Advantages of this method are that it is both volume filling and surface constrained, and that by varying the density of seeding points the alveoli can be generated with varying size. To date, an alveolar sac isolated from the model has been used as a base structure for creating an alveolar capillary network (Burrowes et al. 2004), and for simulating mixing in the alveolar region using CFD analysis (Kumar et al. 2007).
In each of the methods described above, polyhedral faces are removed to create a duct–alveolus structure. Primary septa are defined by their connectivity with alveoli in adjacent ducts or sacs, whereas secondary septa form the boundary between adjacent alveoli. In each study, the geometric structure has compared well with anatomical measurements, and the structures have been used to simulate various functions.
(b) Lung tissue mechanics
An important outcome from Denny & Schroter (2006) is the demonstration of how mechanical behaviour at the level of the lung tissue microstructure can be used to derive material laws for a lung tissue continuum. The elastic behaviour of the lung tissue is a combination of tissue elasticity and its interaction with surface forces. The surface forces are moderated by the dynamic behaviour of lung surfactant, which acts to decrease surface tension. Early attempts to derive mechanical properties for the lung tissue either estimated linear moduli for the intact lung (e.g. Lai-Fook & Hyatt 2000) or derived material laws for the tissue independent of surface forces, using measurements from the excised tissue (e.g. Fung 1990). The derivation of material laws from experimental measurements on the lung tissue is confounded by the distortion of the tissue when it is excised. The approach demonstrated by Denny & Schroter (2006) holds promise for linking microstructural mechanics to mechanics at the organ level, without relying directly on axial stretch experiments.
Tawhai et al. (2006) developed a continuum model for the ovine lung coupled to ventilation through a one-dimensional system of airways. Limitations of the model include the assumed material law, which was based on Fung (1990), and that the effect of gravity was not included. The study did however demonstrate a method for coupling function in the airways (organ level) to the function of the tissue. Burrowes & Tawhai (2007) demonstrated a similar method in the human lung, for coupling perfusion of the model arterial tree to the volume change and elastic recoil calculated from a soft tissue mechanics model. Importantly, these coupled modelling approaches allow inclusion of regionally varying transmural and transpulmonary pressures in simulations of air and blood transport.
(c) Microcirculatory flow in the pulmonary capillaries
An example at the tissue level that illustrates the importance of anatomically detailed model geometry is the study of microcirculatory flow in the alveolar capillary network (Burrowes et al. 2004). Owing to the high density of pulmonary capillaries, early ‘sheet flow’ models for the pulmonary microcirculation had approximated blood to flow as a continuous sheet bounded on either side by a compliant endothelium, and flowing between ‘posts’ of connective tissue (Fung & Sobin 1969) rather than through discrete vessels. Distributions determined in the sheet flow model are averaged over the entire capillary network; segment-to-segment variability in individual capillaries cannot be predicted. While this type of model can give a good representation of the average properties and function at this level, it does not allow some physiological predictions to be made, such as the effect of capillary blockage on perfusion redistribution or distributions of cellular transit times within discrete pathways. Dhadwal et al. (1997) and Huang et al. (2001) modelled blood flow through discrete tubules to represent the segmented structure of the capillary network; however, these early models used a simplified geometric structure consisting of a relatively small number of capillaries to represent the complex capillary network.
Burrowes et al. (2004) extended the functional model of Huang et al. (2001) by implementing their model equations in a more realistic representation of the capillary network geometry. A two-dimensional Voronoi meshing method was developed to generate a continuous network of capillaries over adjacent model alveoli in a single alveolar sac. The capillary model wraps over the surface of adjacent alveoli, making multiple connection points between capillaries of surrounding alveoli and a single capillary sheet between the alveolar faces. This study was the first attempt to accurately model the relationship between the segmented geometry of the pulmonary microcirculation and the space-filling alveoli over which it lies. Gravity-dependent transpulmonary and transmural pressure boundary conditions were specified to simulate cell and plasma transit (figure 3b). More realistic cellular transit times were predicted in this anatomically based geometry than in previous more simple geometries. Owing to the large amount of reserve capacity in the model, the blockage of capillaries by white blood cells (as found physiologically) was shown to have only a minor effect due to the large number of alternative perfusion pathways.
4. Cellular-level models in pulmonary physiology
Only limited attention has been paid to modelling at the cellular or subcellular level in the lung, with the exception of erythrocyte gas kinetics. To date, only a handful of electrophysiological models of any pulmonary cell type have been published. Of these, models of airway myocytes are the most developed. With very few models for pulmonary cellular function, no computational studies have been published that link cellular function to whole lung function. It is unfortunate and somewhat surprising that this coupling between scales has been neglected in the lung, considering that drug or gene therapies are designed to act at the subcellular level with the intention of amending whole organ function. This is in contrast to modelling efforts in the cardiac and musculoskeletal systems, where the approach of coupling detailed models of cellular operation to spatially dependent representations of the whole organ has been in development for some time (e.g. Fernandez et al. 2005).
Of the recent publications of airway myocyte models, Mijailovich et al. (2000) focused on myosin-binding kinetics and the effect of length fluctuations on the dynamically evolving cross-bridge distributions, which occur during breathing, whereas the models of Marhl et al. (2006) and Roux et al. (2006) paid particular attention to signal transduction, modelling changes in cytosolic calcium levels after cholinergic stimulation. These models were used to explain the small influence of extracellular calcium on myocyte contraction under cholinergic stimulation, and the influence of different inositol triphosphate receptor isoforms on calcium oscillations. Mbikou et al. (2006) investigated the calcium contraction coupling in airway myocytes, with their model correctly predicting the time course of myosin light chain phosphorylation and force in a short-time response. A more recent model by Wang et al. (2008), where intracellular calcium oscillations were linked to contraction of airway myocytes and changes in airway calibre, showed how models of cellular function can be linked with macroscopic measures.
Airway epithelial cell dysfunction is clearly a feature of diseases such as cystic fibrosis, yet models for the ciliated airway epithelial cells are few in number and have had varying levels of success. The first of these were based on the models of tight epithelia (e.g. Lew et al. 1979; Latta et al. 1984); however, airway epithelium is leaky, and thus involves paracellular as well as transcellular ion transport. Of the few published airway epithelial cell models known to the authors, those of Hartmann & Verkman (1990), Duszyk & French (1991) and Horisberger (2003) concentrated only on ion transport rates, ignoring water transport. Miller (1992) proposed a model that took into account water movement and cell swelling, but details of the model were not published. An attempt was made to explain ASL depth regulation through opening and closing of paracellular pathways when the cell swells/shrinks after changes in osmolarity of the ASL. The model was not able to explain how cells can reach homeostasis after an isotonic challenge. Novotny & Jakobsson (1996) produced a model that accounted for lumen, intracellular and serosal compartments and described osmotic water transport more accurately. It is currently the only published model that includes a term to account for evaporation of water from the ASL. This model also attempted to propose a regulating mechanism for the ASL depth through volume-sensing Cl− channels. By introducing an equation for a volume-sensing Cl− channel into the model, a feedback is introduced, which ensures that the system will always reach equilibrium at a prescribed depth. Despite the arbitrary nature of the equation used for the Cl− channel, the model allows a useful conclusion to be drawn: ASL regulation is, at least in part, capable of being controlled by modifying apical membrane Cl− channel conductance. The later model by Horisberger (2003) was used to investigate the electrical coupling between the Na+ and Cl− channels. It has been suggested that epithelial cells control ion movement through a blended operation of the Na+ and Cl− channels (Tarran et al. 2006) with regulation of the epithelial sodium channel (ENaC) operating through the cAMP-dependent cystic fibrosis transmembrane conductance regulator (CFTR; e.g. Reddy & Quinton 2003). It was the absence of Na+ channel inhibition by CFTR chloride channel that was postulated to be responsible for the abnormal electrolyte transport in cystic fibrosis airway epithelia. The Horisberger (2003) model demonstrated that the Cl− conductance has a significant effect on the rate of the Na+ transport through parallel and apical pathways. This result suggested that there was little coupling between the two channels other than through electrical potential. This was later confirmed experimentally by Nagel et al. (2005) who found no evidence that activated CFTR channels regulate Na+ transport through the ENaC.
Of these published airway epithelial cell models, only one provides any rigorous attempt to consider transcellular water movement and evaporation (Novotny & Jakobsson 1996). Furthermore, none of the models made any significant attempt at including second messengers or autocrine signalling. It is this cellular signalling that is well known to be an important part of regional tissue-level response. For example, waves of calcium moving through epithelial cells (Evans & Sanderson 1999) are known to control a variety of functions such as ciliary beat frequency (Gertsberg et al. 2004) and transepithelial water movement (Lee et al. 2007). Autocrine signalling through ATP and UTP stimulates mucin secretion from adjoining goblet cells (Kreda et al. 2007) while feeding back onto epithelial cells to activate release of calcium from internal stores.
It is possible that the minimal number of pulmonary cell models has been due to the past lack of ‘exciting’ dynamic signals in the lung, antonymic with propagation of electrical waves seen in cardiac physiology. However, with the development of new measurement techniques, or improvement of old ones, many dynamic signalling phenomena are now capable of being measured. This has resulted in many signalling pathways being elucidated and increasingly well characterized.
5. Towards integration of models for gas exchange into anatomically based models of geometry
Exchange of respiratory gases has been a major focus of mathematical and computational modelling of the lung over the past century. Considering the extensive amount of research in this area, we will give only a brief outline of several modelling approaches and consider their merits in terms of developing an integrative model for whole lung gas exchange.
(a) Chemical interactions
Modelling gas exchange requires a mathematical description of the diffusion of gases through the air–blood barrier coupled with the complex chemical interactions between oxygen (O2), carbon dioxide (CO2) and blood. Detailed models for these interactions have been developed by many authors (e.g. Staub et al. 1962; Huang & Hellums 1994). Such models incorporate reaction velocities, which have been shown to be slow enough to limit O2 uptake, and take into account the two-phase nature of the blood, considering the reactions within both the plasma and intracellular phases. Bohr and Haldane effects are often included (Hill et al. 1973), and become important when considering abnormal gas exchange where the O2 and CO2 dissociation curves are shifted. The mathematical descriptions generally present a system of ordinary differential equations, or occasionally partial differential equations, that include the various chemical species involved in the reactions: oxyhaemoglobin; carbaminohaemoglobin; bicarbonate; dissolved O2 and CO2; and hydrogen ions.
(b) Detail at the level of the red blood cell and capillary
Efforts to understand gas exchange at the scale of the red blood cell (RBC) have led to models that incorporate small-scale structure. While it is often difficult to extrapolate small-scale models to whole lung function directly, they are useful for focusing on a particular function in detail, and for setting up a foundation from which to build multi-scale models.
A number of authors have implemented FE models to investigate the effect of anatomical geometry at the scale of the RBC on diffusing capacity. Nabors et al. (2003) noted that the degree of lung inflation and/or microvascular pressure affects capillary cross sections and hence the orientation of erythrocytes within the capillaries. This was investigated using a two-dimensional FE model. Further studies have used this modelling technique to investigate the effect of uniform, random and clustered distributions of RBCs in a single capillary (Hsia et al. 1999), and the effect of RBC shape (Wang & Popel 1993). These studies demonstrate the importance of modelling small-scale structure; however, they do not easily extend to larger scales. In an effort to overcome this limitation, Chakraborty et al. (2004) derived an analytic expression for the diffusing capacity of the RBC for any reactive gas in terms of size and shape of the RBC, thickness of the unstirred plasma layer surrounding the RBC, diffusivities and solubilities of the gas in the RBC, and boundary layer, haematocrit and the slope of the dissociation curve. This is a particularly interesting and novel model, as it incorporates the size and the shape of the RBC within the analytic expression.
(c) Whole lung gas exchange
To understand gas exchange function of the whole lung, many models represent the lung as a number of lumped gas exchange units. For example, early attempts to describe changes in partial pressures as blood traverses the pulmonary capillaries used a single lumped alveolar air compartment coupled to a single lumped capillary blood compartment (Flumerfelt & Crandall 1968; Milhorn & Pulley 1968). This type of model assumes homogeneous function throughout the lung. Although their assumptions are simplistic, such models have proved to be useful in understanding mechanisms of pulmonary control. For example, Trueb et al. (1971) predicted changes in arterial PO2 and PCO2 in response to changes in ventilation using a simple lumped gas exchange model. Simple descriptions of gas exchange are also necessary for coupling with more complex models that describe the interaction of the cardiovascular and respiratory systems, and that incorporate airway mechanics, gas exchange and blood flow (Liu et al. 1998).
Multiple lumped compartment models were introduced to incorporate the effects of shunt (i.e. perfusion, no ventilation) and functional dead space (i.e. ventilation, no perfusion), and have subsequently been used to investigate the effects of heterogeneous ventilation perfusion ratio (V/Q) distributions. An example is the 50-compartment model developed by West (1969). This model allows V/Q to vary between compartments, but it does not include spatial or structural information. The approach could conceivably be extended to study regional variation in V/Q by including anatomic detail such as the lobar and vertical location of the compartment; this would provide insight into the gas exchange functionality of different regions in the lung.
(d) Towards multi-scale models for an integrative model of gas exchange
Multi-scale models link together different spatial and temporal scales. Some models extrapolate whole lung gas exchange measures by scaling up results from small-scale models (Hill et al. 1973); however, to our knowledge, only one study exists that includes structural and functional details from multiple scales to simulate gas exchange (Chakraborty et al. 2007). In this study, the investigators presented a multi-scale model of oxygen uptake in the lung by simultaneously solving equations for the micro (RBC), meso (capillary) and macro (lung) scales. Spatial averaging techniques were applied so that different spatial and time scales could be solved simultaneously. This model incorporates details at the level of the RBC with large-scale information, such as the V/Q distribution and shunt. At the macro scale, the lung is divided into six compartments (five ventilated and one shunt). One disadvantage is that the V/Q distribution and perfusion are assumed to depend solely on gravity: the effects of meso and macro structures are ignored.
An area that is not yet well modelled is the spatial variation in whole lung gas exchange. Pulmonary disorders generally manifest in a specific region of the lung (at least in the initial stages of the disease) resulting in a heterogeneous deterioration of structure and function. To date, models for gas exchange have typically incorporated considerable detail at the level of chemical reactions, with less focus on the influence of anatomical structures or coupling physical processes that occur over different spatial or temporal scales. Gas exchange occurs at the confluence of air and blood transport and it is this ‘V/Q’ matching that governs the efficiency of gas exchange. Blood and airflow distributions are influenced by the asymmetric branching geometry through which they flow, and by the pressures to which the conduits are exposed (tissue elastic recoil pressure, air and blood pressures). Hence, the anatomical geometries of the airway and vascular systems will have an important bearing on regional distributions of air and blood, and therefore on whole lung gas exchange. While the effect of anatomical structure is undoubtedly important, this has not yet been addressed.
6. Conclusion
To understand the integrated function of the pulmonary system, we need to understand the downstream and upstream effects of changes at each functional scale. Multi-scale modelling provides a powerful approach to investigate interdependent processes that span many orders of magnitude. Multi-scale approaches include deriving systems of equations with greater or lesser complexity to represent the same function (e.g. one- and three-dimensional models), or linking models at different spatial scales (e.g. cell and tissue) or over different temporal scales. As with models of the heart, where a description of a dysfunctional ion channel or pump has been used to explore the flow-on effects to the whole organ (Noble 2002), the same approach should be encouraged in developing models for the lung. This is particularly appealing for the study of the lung due to limited options for experimental measurement in the in vivo system. Multi-scale modelling will allow a bridging per se between the well-studied cellular systems and whole organ operation.
Acknowledgments
The authors thank collaborators at the University of Iowa (Profs Eric A. Hoffman and Ching-Long Lin) and colleagues at the Auckland Bioengineering Institute for contributing data, discussion and advice. This work was supported by the National Institutes of Health grant NIH 2 RO1 HL064368-06 A1, a LSI EPSRC Postdoctoral Fellowship and a Maurice Paykel Postdoctoral Fellowship.
Footnotes
One contribution of 11 to a Theme Issue ‘The virtual physiological human: building a framework for computational biomedicine II’.
References
- Burrowes K.S, Tawhai M.H.2006Computational predictions of pulmonary blood flow gradients: gravity versus structure. Respir. Physiol. Neurobiol. 154, 515–523. 10.1016/j.resp.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Burrowes, K. S. & Tawhai, M. H. 2007 The effect of lung orientation on functional imaging of blood flow. In Proc. SPIE Medical Imaging: Physiology, Structure, and Function from Medical Images, vol. 6511.
- Burrowes K.S, Tawhai M.H, Hunter P.J.2004Modeling RBC and neutrophil distribution through an anatomically based pulmonary capillary network. Ann. Biomed. Eng. 32, 585–595. 10.1023/B:ABME.0000019178.95185.ad. [DOI] [PubMed] [Google Scholar]
- Burrowes K.S, Hunter P.J, Tawhai M.H.2005Anatomically-based finite element models of the human pulmonary arterial and venous trees including supernumerary vessels. J. Appl. Physiol. 99, 731–738. 10.1152/japplphysiol.01033.2004. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Balakotaiah V, Bidani A.2004Diffusing capacity reexamined: relative roles of diffusion and chemical reaction in red cell uptake of O2, CO, CO2, and NO. J. Appl. Physiol. 97, 2284–2302. 10.1152/japplphysiol.00469.2004. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Balakotaiah V, Bidani A.2007Multiscale model for pulmonary oxygen uptake and its application to quantify hypoxemia in hepatopulmonary syndrome. J. Theor. Biol. 244, 190–207. 10.1016/j.jtbi.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Dale P.J, Matthews F.L, Schroter R.C.1980Finite element analysis of lung alveolus. J. Biomech. 13, 865–873. 10.1016/0021-9290(80)90174-8. [DOI] [PubMed] [Google Scholar]
- Dawson C.A, Krenz G.S, Karau K.L, Haworth S.T, Hanger C.C, Linehan J.H.1999Structure–function relationships in the pulmonary arterial tree. J. Appl. Physiol. 86, 569–583. [DOI] [PubMed] [Google Scholar]
- Denny E, Schroter R.C.1995The mechanical behavior of a mammalian lung alveolar duct model. J. Biomech. Eng. Trans. ASME. 117, 254–261. 10.1115/1.2794178. [DOI] [PubMed] [Google Scholar]
- Denny E, Schroter R.C.1996A mathematical model for the morphology of the pulmonary acinus. J. Biomech. Eng. Trans. ASME. 118, 210–215. 10.1115/1.2795961. [DOI] [PubMed] [Google Scholar]
- Denny E, Schroter R.C.2006A model of non-uniform lung parenchyma distortion. J. Biomech. 39, 652–663. 10.1016/j.jbiomech.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Dhadwal A, Wiggs B, Doerschuk C, Kamm R.1997Effects of anatomic variability on blood flow and pressure gradients in the pulmonary circulation. J. Appl. Physiol. 83, 1711–1720. [DOI] [PubMed] [Google Scholar]
- Duszyk M, French A.S.1991An analytical model of ionic movements in airway epithelial cells. J. Theor. Biol. 151, 231–247. 10.1016/S0022-5193(05)80362-5. [DOI] [PubMed] [Google Scholar]
- Evans J.H, Sanderson M.J.1999Intracellular calcium oscillations induced by ATP in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 277, L30–L41. [DOI] [PubMed] [Google Scholar]
- Fernandez J.W, Mithraratne P, Thrupp S, Tawhai M.H, Hunter P.J.2004Anatomically based geometric modelling of the musculo-skeletal system and other organs. Biomech. Model. Mechanobiol. 2, 139–155. 10.1007/s10237-003-0036-1. [DOI] [PubMed] [Google Scholar]
- Fernandez J.W, Buist M.L, Nickerson D.P, Hunter P.J.2005Modelling the passive and nerve activated response of the rectus femoris muscle to a flexion loading: a finite element framework. Med. Eng. Phys. 27, 862–870. 10.1016/j.medengphy.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Flumerfelt R.W, Crandall E.D.1968An analysis of external respiration in man. Math. Biosci. 3, 205–230. 10.1016/0025-5564(68)90081-3. [DOI] [Google Scholar]
- Fung Y.1988A model of the lung structure and its validation. J. Appl. Physiol. 64, 2132–2141. [DOI] [PubMed] [Google Scholar]
- Fung Y.1989Connecting incremental shear modulus and Poisson's ratio of lung tissue with morphology and rheology of microstructure. Biorheology. 26, 279–289. [DOI] [PubMed] [Google Scholar]
- Fung Y.C.Biomechanics: motion, flow, stress, and growth. In Springer 1990New York, NY:Springer [Google Scholar]
- Fung Y, Sobin S.1969Theory of sheet flow in lung alveoli. J. Appl. Physiol. 26, 472–488. [DOI] [PubMed] [Google Scholar]
- Gertsberg I, Hellman V, Fainshtein M, Weil S, Silberberg S.D, Danilenko M, Priel Z.2004Intracellular Ca2+ regulates the phosphorylation and the dephosphorylation of ciliary proteins via the NO pathway. J. Gen. Physiol. 124, 527–540. 10.1085/jgp.200409153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenny R.W, Robertson H.T.1991Fractal modeling of pulmonary blood flow heterogeneity. J. Appl. Physiol. 70, 1024–1030. [DOI] [PubMed] [Google Scholar]
- Glenny R.W, Bernard S, Robertson H.T, Hlastala M.P.1999Gravity is an important but secondary determinant of regional pulmonary blood flow in upright primates. J. Appl. Physiol. 86, 623–632. [DOI] [PubMed] [Google Scholar]
- Glenny R, Lamm W, Bernard S, An D, Chornuk M, Pool S, Wagner W, Jr, Hlastala M.P, Rovertson H.2000Physiology of a microgravity environment, selected contribution: redistribution of pulmonary perfusion during weightlessness and increased gravity. J. Appl. Physiol. 89, 1239–1248. [DOI] [PubMed] [Google Scholar]
- Hartmann T, Verkman A.S.1990Model of ion transport regulation in chloride-secreting airway epithelial cells. Integrated description of electrical, chemical, and fluorescence measurements. Biophys. J. 58, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E.P, Power G.G, Longo L.D.1973Mathematical simulation of pulmonary O2 and CO2 exchange. Am. J. Physiol. 224, 904–917. [DOI] [PubMed] [Google Scholar]
- Hoffman E.A, et al. 2004The comprehensive imaging-based analysis of the lung: a forum for team science (1). Acad. Radiol. 11, 1370–1380. 10.1016/j.acra.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Hogg J, Martin B, Lee S, McLean T.1985Regional differences in erythrocyte transit in normal lungs. J. Appl. Physiol. 59, 1266–1271. [DOI] [PubMed] [Google Scholar]
- Horisberger J.-D.2003ENaC–CFTR interactions: the role of electrical coupling of ion fluxes explored in an epithelial cell model. Eur. J. Physiol. 445, 522–528. [Google Scholar]
- Horsfield K, Dart G, Olson D.E, Filley G.F, Cumming G.1971Models of the human bronchial tree. J. Appl. Physiol. 31, 207–217. [DOI] [PubMed] [Google Scholar]
- Hsia C.C, Johnson R.L, Jr, Shah D.1999Red cell distribution and the recruitment of pulmonary diffusing capacity. J. Appl. Physiol. 86, 1460–1467. 10.1063/1.370965. [DOI] [PubMed] [Google Scholar]
- Huang N.S, Hellums J.D.1994A theoretical model for gas transport and acid/base regulation by blood flowing in microvessels. Microvasc. Res. 48, 364–388. 10.1006/mvre.1994.1062. [DOI] [PubMed] [Google Scholar]
- Huang Y, Doerschuk C.M, Kamm R.D.2001Computational modeling of RBC and neutrophil transit through the pulmonary capillaries. J. Appl. Physiol. 90, 545–564. 10.1063/1.1379354. [DOI] [PubMed] [Google Scholar]
- Jones A.T, Hansell D.M, Evans T.W.2001Pulmonary perfusion in supine and prone positions: an electron-beam computed tomography study. J. Appl. Physiol. 90, 1342–1348. 10.1063/1.1376404. [DOI] [PubMed] [Google Scholar]
- Kowe R, Schroter R, Matthers F, Hitchings D.1986Analysis of elastic and surface tension effects in the lung alveolus using finite element methods. J. Biomech. 19, 541–549. 10.1016/0021-9290(86)90127-2. [DOI] [PubMed] [Google Scholar]
- Kreda S.M, Okada S.F, van Heusden C.A, O'Neal W, Gabriel S, Abdullah L, Davis C.W, Boucher R.C, Lazarowski E.R.2007Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J. Physiol. 584, 245–259. 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, H., Lin, C.-L., Tawhai, M. H., McLennan, G. & Hoffman, E. A. 2007 Patterns of mixing in the alveolar region of the human lungs. In Proc. 60th Annual Meeting of the Division of Fluid Dynamics.
- Lai-Fook S.J, Hyatt R.E.2000Effects of age on elastic moduli of human lungs. J. Appl. Physiol. 89, 163–168. [DOI] [PubMed] [Google Scholar]
- Latta R, Clausen C, Moore L.C.1984General method for the derivation and numerical simulation of epithelial transport models. J. Membr. Biol. 82, 67–82. 10.1007/BF01870733. [DOI] [PubMed] [Google Scholar]
- Lee R.J, Limberis M.P, Hennessy M.F, Wilson J.M, Foskett J.K.2007Optical imaging of Ca2+-evoked fluid secretion by murine nasal submucosal gland serous acinar cells. J. Physiol. 582, 1099–1124. 10.1113/jphysiol.2007.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGrice I, Hunter P.J, Smaill B.H.1997Laminar structure of the heart: a mathematical model. Am. J. Physiol. Heart Circ. Physiol. 272, H2466–H2476. [DOI] [PubMed] [Google Scholar]
- Lew V.L, Ferreira H.G, Moura T.1979The behaviour of transporting epithelial cells. I. Computer analysis of a basic model. Proc. R. Soc. B. 206, 53–83. 10.1098/rspb.1979.0091. [DOI] [PubMed] [Google Scholar]
- Lin C.-L, Tawhai M.H, McLennan G, Hoffman E.A.2007Characteristics of the turbulent laryngeal jet and its effect on airflow in the human intra-thoracic airways. Respir. Physiol. Neurobiol. 157, 295–309. 10.1016/j.resp.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.-L., Tawhai, M. H., McLennan, G. & Hoffman, E. A. In press. Multiscale simulation of gas flow in subject-specific models of the human lung. IEEE Eng. Med. Biol.
- Liu C.H, Niranjan S.C, Clark J.W, Jr, San K.Y, Zwischenberger J.B, Bidani A.1998Airway mechanics, gas exchange, and blood flow in a nonlinear model of the normal human lung. J. Appl. Physiol. 84, 1447–1469. [DOI] [PubMed] [Google Scholar]
- Marhl M, Noble D, Roux E.2006Modeling of molecular and cellular mechanisms involved in Ca2+ signal encoding in airway myocytes. Cell. Biochem. Biophys. 46, 285–302. 10.1385/CBB:46:3:285. [DOI] [PubMed] [Google Scholar]
- Mbikou P, Fajmut A, Brumen M, Roux E.2006Theoretical and experimental investigation of calcium-contraction coupling in airway smooth muscle. Cell. Biochem. Biophys. 46, 233–252. 10.1385/CBB:46:3:233. [DOI] [PubMed] [Google Scholar]
- Mijailovich S.M, Butler J.P, Fredberg J.J.2000Perturbed equilibria of myosin binding in airway smooth muscle: bond-length distributions, mechanics, and ATP metabolism. Biophys. J. 79, 2667–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhorn H.T, Jr, Pulley P.E., Jr1968A theoretical study of pulmonary capillary gas exchange and venous admixture. Biophys. J. 8, 337–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller I.F.1992Mechanisms for ion and water transport across tracheal epithelium. Ind. Eng. Chem. Res. 31, 721–726. 10.1021/ie00003a011. [DOI] [Google Scholar]
- Nabors L.K, Baumgartner W.A, Jr, Janke S.J, Rose J.R, Wagner W.W, Jr, Capen R.L.2003Red blood cell orientation in pulmonary capillaries and its effect on gas diffusion. J. Appl. Physiol. 94, 1634–1640. [DOI] [PubMed] [Google Scholar]
- Nagel G, Barbry P, Chabot H, Brochiero E, Hartung K, Grygorczyk R.2005CFTR fails to inhibit the epithelial sodium channel ENaC expressed in Xenopus laevis oocytes. J. Physiol. 564, 671–682. 10.1113/jphysiol.2004.079046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D.2002The rise of computational biology. Nat. Rev. Mol. Cell Biol. 3, 459–463. 10.1038/nrm810. [DOI] [PubMed] [Google Scholar]
- Novotny J.A, Jakobsson E.1996Computational studies of ion–water flux coupling in the airway epithelium. I. Construction of model. Am. J. Physiol. Cell Physiol. 270, C1751–C1763. [DOI] [PubMed] [Google Scholar]
- Parker J.C, Cave C.B, Ardell J.L, Hamm C.R, Williams S.G.1997Vascular tree structure affects lung blood flow heterogeneity simulated in three dimensions. J. Appl. Physiol. 83, 1370–1382. [DOI] [PubMed] [Google Scholar]
- Reddy M, Quinton P.2003Functional interaction of CFTR and ENaC in sweat glands. Pflugers Arch. 445, 499–503. 10.1007/s00424-002-0959-x. [DOI] [PubMed] [Google Scholar]
- Robertson H.T, Hlastala M.P.2007Invited review: microsphere maps of regional blood flow and regional ventilation. J. Appl. Physiol. 102, 1265–1272. 10.1152/japplphysiol.00756.2006. [DOI] [PubMed] [Google Scholar]
- Roux E, Noble P.J, Noble D, Marhl M.2006Modelling of calcium handling in airway myocytes. Prog. Biophys. Mol. Biol. 90, 64–87. 10.1016/j.pbiomolbio.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Staub N.C, Bishop J.M, Forster R.E.1962Importance of diffusion and chemical reaction rates in O2 uptake in the lung. J. Appl. Physiol. 17, 21–27. [DOI] [PubMed] [Google Scholar]
- Tarran R, Button B, Boucher R.C.2006Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu. Rev. Physiol. 68, 543–561. 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- Tawhai M.H, Hunter P.J.2004Modeling water vapor and heat transfer in the normal and the intubated airway. Ann. Biomed. Eng. 32, 609–622. 10.1023/B:ABME.0000019180.03565.7e. [DOI] [PubMed] [Google Scholar]
- Tawhai M.H, Hunter P.J, Tschirren J, Reinhardt J.M, McLennan G, Hoffman E.A.2004CT-based geometry analysis and finite element models of the human and ovine bronchial tree. J. Appl. Physiol. 97, 2310–2321. 10.1152/japplphysiol.00520.2004. [DOI] [PubMed] [Google Scholar]
- Tawhai M.H, Nash M.P, Hoffman E.A.2006An imaging-based computational approach to model ventilation distribution and soft tissue deformation in the ovine lung. Acad. Radiol. 13, 113–120. 10.1016/j.acra.2005.09.088. [DOI] [PubMed] [Google Scholar]
- Trueb T.J, Cherniack N.S, D'Souza A.F, Fishman A.P.1971A mathematical model of the controlled plant of the respiratory system. Biophys. J. 11, 810–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ertbruggen C, Hirsch C, Paiva M.2005Anatomically based three-dimensional model of airways to simulate flow and particle transport using computational fluid dynamics. J. Appl. Physiol. 98, 970–980. 10.1152/japplphysiol.00795.2004. [DOI] [PubMed] [Google Scholar]
- Wang C.H, Popel A.S.1993Effect of red blood cell shape on oxygen transport in capillaries. Math. Biosci. 116, 89–110. 10.1016/0025-5564(93)90062-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang I, Politi A.Z, Tania N, Bai Y, Sanderson M.J, Sneyd J.2008A mathematical model of airway and pulmonary arteriole smooth muscle. Biophys. J. 94, 2053–2064. 10.1529/biophysj.107.113977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E.R.Morphometry of the human lung. In Springer 1963Berlin, Germany:Springer [Google Scholar]
- West J.B.1969Ventilation–perfusion inequality and overall gas exchange in computer models of the lung. Respir. Physiol. 7, 88–110. 10.1016/0034-5687(69)90071-1. [DOI] [PubMed] [Google Scholar]
- West J.B, Dollery C.T, Naimark A.1964Distribution of blood flow in isolated lung; relation to vascular and alveolar pressures. J. Appl. Physiol. 19, 713–724. [DOI] [PubMed] [Google Scholar]