Abstract
The canonical Wnt signaling pathway is critical for development of the mammalian central nervous system and regulates diverse processes throughout adulthood, including adult neurogenesis. Glycogen synthase kinase-3 (GSK-3) antagonizes the canonical Wnt pathway and therefore also plays a central role in neural development and adult neurogenesis. Lithium, the first line of therapy for bipolar disorder, inhibits GSK-3, activates Wnt signaling and stimulates adult neurogenesis, which may be important for its therapeutic effects. GSK-3 also regulates other critical signaling pathways which may contribute to the therapeutic effects of lithium, including growth factor/neurotrophin signaling downstream of Akt. Here we will review the roles of GSK-3 in CNS development and adult neurogenesis, with a focus on the canonical Wnt pathway. We will also discuss the validation of GSK-3 as the relevant target of lithium and the mechanisms downstream of GSK-3 that influence mammalian behavior.
Keywords: Wnt, GSK-3, neurogenesis, lithium, bipolar disorder, behavior, development, psychiatric
Introduction
Wnt signaling is essential for patterning, cell fate specification, and stem cell regulation during the development of many tissues and organs, including the mammalian brain (reviewed in Ciani and Salinas, 2005; Petersen and Reddien, 2009; Kim and Snider, 2011). In adults, Wnt signals continue to influence the maintenance and regeneration of many tissues by regulating stem cell homeostasis and cell proliferation (reviewed in Gu et al., 2010; Wend et al., 2010; Yeung et al., 2011). In the adult brain, Wnt signaling also regulates critical processes such as neurite outgrowth, axon remodeling, synapse formation and plasticity, and neurogenesis (reviewed in Ciani and Salinas, 2005; Budnik and Salinas, 2011). In the canonical Wnt pathway, Wnts inhibit glycogen synthase kinase-3 (GSK-3) to activate downstream signaling. Lithium, which has been used to treat bipolar disorder (BPD) for decades, inhibits GSK-3 both directly and indirectly, raising the possibility that the therapeutic effects of lithium may involve activation of downstream Wnt signaling (reviewed in MacDonald et al., 2009; O’Brien and Klein, 2009). However, GSK-3 also regulates other pathways – distinct from the canonical Wnt pathway – that are involved in neuronal development and function, and these may also play important roles in the response to lithium. As these pathways are discussed in detail elsewhere in this volume (reviewed in Kaidanovich-Beilin and Woodgett, 2011; Kim and Snider, 2011), this review will focus on GSK-3 and canonical Wnt signaling in CNS development, adult neurogenesis, and BPD.
GSK-3 is a Critical Regulator of Diverse Signaling Pathways
Glycogen synthase kinase-3 constitutively antagonizes the canonical Wnt signaling pathway and must be inhibited for the pathway to function (Figure 1). In the absence of Wnt ligands, GSK-3, the transcriptional co-activator β-catenin, and the tumor suppressor adenomatous polyposis coli (APC) bind directly to the scaffolding protein Axin in a complex that facilitates phosphorylation of β-catenin by GSK-3, which targets β-catenin for proteasome-dependent degradation. Wnt ligands bind to the receptor Frizzled, inducing phosphorylation of the essential co-receptors low density lipoprotein receptor-related protein 5 (LRP5) and LRP6 which results in GSK-3 inhibition and β-catenin stabilization. Stabilized β-catenin enters the nucleus and interacts with the lymphocyte enhancer factor/T-cell factor (LEF/TCF) family of transcription factors to activate transcription (reviewed in Clevers, 2006; MacDonald et al., 2009; Wu and Pan, 2010).
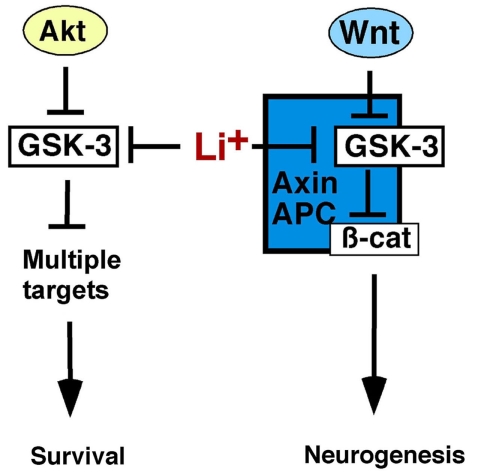
Figure 1.
Glycogen synthase kinase-3 regulates Wnt and Akt-dependent signaling. Growth factors, including neurotrophins, activate Akt, which phosphorylates, and inhibits GSK-3 allowing activation of downstream effectors to promote cell survival. Both Akt and GSK-3 have multiple targets not shown here for the sake of clarity. Wnts inhibit GSK-3 within the Axin complex stabilizing β-catenin, which activates Wnt target genes, and promotes neurogenesis. Axin-associated GSK-3 is not regulated by N-terminal phosphorylation and is thus distinct from Akt-regulated GSK-3, and both pools are inhibited by lithium. Thus lithium may stimulate neurogenesis by activating Wnt/β-catenin signaling and enhancing cell survival.
In addition to its critical role in regulating Wnt signaling, GSK-3 phosphorylates over 100 substrates and regulates multiple signaling pathways, such as Sonic Hedgehog, Notch, and growth factor signaling through Akt which influences cell survival decisions in the brain (reviewed in Kockeritz et al., 2006; Kaidanovich-Beilin and Woodgett, 2011; Kim and Snider, 2011). Insulin, neurotrophins, and other growth factors activate phosphatidylinositol-3-kinase (PI3K) and Akt which phosphorylates GSK-3 at an N-terminal serine residue (Ser21 on the GSK-3α isoform, Ser9 on GSK-3β; Cross et al., 1995; McManus et al., 2005). This creates a pseudosubstrate motif that inhibits GSK-3 allowing activation of downstream effectors such as Glycogen Synthase and the mammalian target of rapamycin (mTOR; Dajani et al., 2001; reviewed in Proud, 2006; Kim et al., 2011). Importantly, GSK-3 in the Wnt-responsive Axin complex is not regulated by N-terminal serine phosphorylation, as neither insulin/Akt nor Wnt ligands induce Ser9/21 phosphorylation of Axin-associated GSK-3 (Ding et al., 2000; Ng et al., 2009). Additionally, Wnt signaling is normal in double knockin mice in which the Gsk3aser21 and Gsk3bser9 phosphorylation sites have been mutated to alanine, further demonstrating that Wnt ligands inhibit GSK-3 by a mechanism other than Ser21/9 phosphorylation (Ding et al., 2000; McManus et al., 2005). Therefore Wnt-responsive GSK-3 and growth factor/Akt responsive GSK-3 represent distinct subcellular pools regulated by distinct mechanisms.
Canonical Wnt Signaling during Central Nervous System Development
Wnt signaling regulates numerous critical processes throughout the development of the vertebrate central nervous system. These include patterning and cell fate specification, proliferation, and neuronal morphology (Chenn and Walsh, 2002; Woodhead et al., 2006; reviewed in Ciani and Salinas, 2005; Budnik and Salinas, 2011).
Patterning
In the early vertebrate embryo, Wnt signaling promotes posterior development and suppresses anterior development of the neural tube. Thus inhibition of Wnt signaling reduces posterior development and expands anterior regions, whereas aberrant Wnt pathway activation enhances posterior and reduces anterior development (reviewed in Ciani and Salinas, 2005). Consistent with this framework, anterior localization of Wnt antagonists such as DKK1 is required for anterior neural tube development (reviewed in Glinka et al., 1998; Mukhopadhyay et al., 2001; Mudher et al., 2004; Ciani and Salinas, 2005). At later stages, Wnt signaling further patterns the neural tube by establishing signaling centers such as the midbrain–hindbrain boundary, and restricting rhombomere boundaries in the developing hindbrain (reviewed in McMahon and Bradley, 1990; Thomas and Capecchi, 1990; Kim et al., 2000; Lekven et al., 2001; Kapsimali et al., 2004; Ciani and Salinas, 2005).
Wnt signaling is also essential for dorsal/ventral patterning of the neural tube. Several Wnts including Wnt1 and Wnt3a are expressed in the dorsal neural tube and combined deletion of Wnt1 and Wnt3a results in expansion of ventral cell fates at the expense of dorsal fates (Megason and McMahon, 2002; Muroyama et al., 2002). Overexpression of Wnt1 or Wnt3a causes expansion of dorsal cell fates (Dickinson et al., 1994; Muroyama et al., 2002). Wnts also promote dorsal and suppress ventral cell fates in the telencephalon and are essential for the specification of neural crest (reviewed in Saint-Jeannet et al., 1997; Wu et al., 2003; Ciani and Salinas, 2005).
Proliferation
Wnt signaling also regulates proliferation of neural precursor cells throughout CNS development. In the developing chick neural tube, overexpression of Wnt1, Wnt3a, or stabilized β-catenin increases neural precursor proliferation while expression of dominant negative TCF4 (dnTCF4) reduces cell proliferation (Megason and McMahon, 2002). In mice, Wnt1 overexpression increases proliferation and neuronal cell size in the caudal midbrain leading to significant midbrain overgrowth (Panhuysen et al., 2004). Furthermore, β-catenin loss of function in the diencephalon, mesencephalon, and hindbrain diminishes progenitor cell domains and decreases midbrain size, while β-catenin gain of function expands progenitor cell domains and increases midbrain size (Zechner et al., 2003). Wnts also regulate proliferation in the developing hippocampus. Wnt3a loss of function reduces hippocampal neural progenitor proliferation and disrupts hippocampal development (Lee et al., 2000). Similar defects are observed when β-catenin is deleted from the dorsal telencephalon (Machon et al., 2003). These data suggest Wnt/β-catenin signaling promotes progenitor proliferation in the developing neural tube as well as the midbrain and hippocampus.
Deletion of both Gsk3a and Gsk3b from mouse neural progenitors activates Wnt signaling and causes dramatic hyperproliferation of Sox2-positive early neural progenitors (known as radial progenitors) and increases proliferation as measured by the number of phospho-Histone H3, BrdU, and Ki67 positive cells, without affecting apoptosis. Gsk3 deletion also reduces differentiation into intermediate neural progenitors and postmitotic neurons. Deletion of Gsk3a or Gsk3b alone does not significantly affect brain development demonstrating mostly redundant functions for the two isoforms. Expression of a dominant negative TCF to block downstream Wnt signaling in neural precursors cultured from Gsk3a/b double mutant mice partially reduces cell proliferation, suggesting that Gsk3 loss of function increases neural precursor proliferation in part by activating Wnt signaling (Kim et al., 2009). In support of this conclusion, expression of stabilized β-catenin in neural precursors also increases precursor proliferation (Chenn and Walsh, 2002), and in utero electroporation of dnTCF reduces cell proliferation (Woodhead et al., 2006). Taken together, these data suggest a role for canonical Wnt signaling in promoting neural precursor proliferation in the embryo.
Neural morphology
In addition to regulating patterning and cell proliferation, Wnts promote neurite outgrowth and influence axon size and branching, as well as growth cone size, complexity, and remodeling (Purro et al., 2008). Wnt7a increases neurite, axon, and growth cone size in cultured cerebellar granular cells and the Wnt antagonist secreted frizzled related protein (sFRP)-1 reduces growth cone size and prevents axon remodeling (Lucas and Salinas, 1997; Hall et al., 2000). GSK-3 inhibitors also increase neurite size, promote neurite outgrowth and axon formation, and increase axon size and branching in many cell types, including cerebellar granular cells, dorsal root ganglion neurons, and hippocampal neurons (Lucas and Salinas, 1997; Hall et al., 2000; Gartner et al., 2006; Dill et al., 2008). Wnts can also act as attractants to regulate commissural axon guidance after crossing the midline in the developing spinal cord; however, this activity is dependent on PI3K and atypical protein kinase C (aPKC) but not on LRP6, suggesting it is not through the canonical Wnt/β-catenin pathway (Lyuksyutova et al., 2003; Wolf et al., 2008). Wnts and β-catenin also promote dendrite growth and branching and promote synapse formation and plasticity (reviewed in Yu and Malenka, 2003; Rosso et al., 2005; Budnik and Salinas, 2011). Wnt7a increases the number of excitatory synapses in the hippocampus and promotes synapse formation in the cerebellum (Hall et al., 2000; Ciani et al., 2011).
Neurodevelopmental phenotypes in mice containing Wnt pathway mutations are summarized in Table 1.
Table 1.
Wnt pathway components are essential for neural development.
| Mutated genes | Phenotype | Reference |
|---|---|---|
| Wnt1 | Absence of midbrain, cerebellum | McMahon and Bradley (1990), Thomas and Capecchi (1990) |
| Wnt3 | Reduced hippocampal neurogenesis | Lie et al. (2005) |
| Wnt3a | Absence of hippocampus | Lee et al. (2000) |
| Wnt1/Wnt3a double mutant | Loss of dorsal interneurons in the neural tube and expansion of ventral interneuron fates | Muroyama et al. (2002) |
| Wnt7a | Reduced hippocampal neurogenesis, delayed cerebellar synapse maturation | Hall et al. (2000) |
| Fzd3/Fzd6 double mutant | Midbrain morphogenesis defects | Stuebner et al. (2010) |
| Fzd4 | Progressive loss of cerebellar granule and Purkinje cells | Wang et al. (2001) |
| Fzd5 | Decreased number of neurons in the parafascicular nucleus | Liu et al. (2008) |
| Axin | Axis duplication | Zeng et al. (1997) |
| Apc | Embryonic lethality prior to gastrulation | Moser et al. (1995) |
| *Gsk3a/b double mutant | Hyperproliferation of neural progenitors | Kim et al. (2009) |
| Lrp6 | Midbrain and cerebellar malformations | Pinson et al. (2000) |
| Dkk1 | Absence of anterior head structures | Mukhopadhyay et al. (2001) |
| b-catenin | Defects in anterior/posterior axis formation | Huelsken et al. (2000) |
| Lef1 | Loss of granule cells of dentate gyrus | Galceran et al. (2000) |
Neurodevelopmental phenotypes are summarized in mice carrying loss of function mutations in the indicated genes. *Gsk3a/b indicates conditional deletion from neural progenitor cells. For more information please see Kim and Snider (2011) in this volume and also visit The Wnt Homepage at http://www.stanford.edu/group/nusselab/cgi-bin/wnt/
Canonical Wnt Signaling in Adult Neurogenesis
Neurogenesis, the generation of new neurons, is a dynamic process that involves proliferation and differentiation of neural progenitors to produce new neurons which can then migrate, mature, and integrate into neuronal circuitry. Neurogenesis was once thought to occur during development only, but has since been observed throughout the lifespan of adult mammals including humans (Eriksson et al., 1998; reviewed in Suh et al., 2009; Ming and Song, 2011). In adult mammals, neurogenesis occurs in the subgranular zone (SGZ) of the dentate gyrus within the hippocampus and in the subventricular (SVG) zone adjacent to the lateral ventricles in the forebrain. Neurons generated in the SGZ migrate into the granular layer of the dentate gyrus where they mature and integrate into the existing neuronal circuitry and eventually behave similar to their older neighbors. Neurons arising in the SVZ migrate anteriorly through the rostral migratory stream and differentiate into interneurons in the olfactory bulb (reviewed in Gage, 2000; Lledo et al., 2006; Ming and Song, 2011; Mongiat and Schinder, 2011).
Although definitive evidence of a functional role for adult neurogenesis is limited, work from many groups suggests adult neurogenesis may be important for learning, memory, and a subset of behavioral responses to antidepressant medications in adult mammals. Spatial and object recognition memory, stress response, and contextual fear conditioning are among the behaviors that neurogenesis has been proposed to influence. Hippocampal irradiation inhibits neurogenesis and impairs spatial learning, contextual fear conditioning, and behavioral and hormonal responses to stress (Snyder et al., 2005, 2011). Importantly, similar results have been observed using alternative methods to block hippocampal neurogenesis (Saxe et al., 2006; Jessberger et al., 2009). Adult neurogenesis in both the SGZ and SVZ has also been proposed to play roles in pattern separation and/or memory resolution (Clelland et al., 2009; reviewed in Aimone et al., 2011; Sahay et al., 2011). SVG neurogenesis may also contribute to olfaction (reviewed in Lazarini and Lledo, 2011). Interestingly new neurons in adult mammals exhibit unique properties at various stages of their maturation, including hyperexcitability, enhanced synaptic plasticity, and unique response to GABA, suggesting they may be able to carry out unique functions in the adult brain (reviewed in Ge et al., 2008; Deng et al., 2010). Thus, adult neurogenesis appears to be an important regulator of learning, memory, and other behaviors, although behavioral data from some groups challenges these conclusions, perhaps due to differing experimental conditions (reviewed in Deng et al., 2010). A better understanding of the regulation of neural stem and progenitor cell homeostasis will also likely contribute to the treatment of neurodegenerative disorders. Thus, considerable recent attention has focused on the signaling pathways and local factors that control adult neurogenesis.
Much of this attention has focused on the critical role for Wnt signaling in adult neurogenesis. Several Wnt ligands including Wnt3 and Wnt7a are expressed in adult hippocampal progenitors (AHPs) and astrocytes near the SGZ (Lie et al., 2005; Wexler et al., 2009). Adult mice lacking Wnt7a show reduced BrdU labeling in the dentate gyrus and expression of dominant negative Wnt in the dentate gyrus also decreases BrdU incorporation and decreases the number of cells expressing Doublecortin (DCX), which marks neuroblasts and newborn neurons (Lie et al., 2005; Qu et al., 2010). Similarly, deletion and shRNA mediated knockdown of β-catenin reduces the number of cells expressing neuronal markers in the adult dentate gyrus (Kuwabara et al., 2009). Conversely, overexpression of Wnt3 in the dentate gyrus increases the number of BrdU, DCX double positive cells (Lie et al., 2005). These data suggest Wnt signaling promotes proliferation of neural progenitor cells and hippocampal neurogenesis in vivo.
Additional support for the role of the Wnt pathway in neurogenesis comes from experiments with cultured AHPs. The Wnt pathway is active under basal conditions in cultured AHPs and is further activated by co-culture with adult hippocampal astrocytes (Lie et al., 2005; Wexler et al., 2009). Treatment with Wnt antagonists, including the Frizzled extracellular domain and sFRPs, reduces AHP proliferation and the number of cells expressing neuronal markers. Similarly, overexpression of Axin, GSK-3, dominant negative TCF (dnTCF), or a truncated N-cadherin that binds and sequesters β-catenin blocks Wnt signaling and results in long term depletion of multipotent AHPs and reduction of neuronal marker expression (Lie et al., 2005; Wexler et al., 2008, 2009). Furthermore, activating Wnt signaling in cultured AHPs by expressing Wnt3, stabilized β-catenin, a kinase dead GSK-3 mutant, or by adding the GSK-3 inhibitor lithium, increases AHP proliferation, BrdU incorporation, and neuronal marker expression (Lie et al., 2005; Wexler et al., 2008). Lithium-induced BrdU uptake is blocked by β-catenin knockdown, further suggesting lithium induces AHP proliferation by activating Wnt signaling (Wexler et al., 2008). Taken together, these data suggest Wnt/β-catenin signaling promotes progenitor proliferation and neurogenesis both in culture and in vivo.
The disrupted in schizophrenia 1 (DISC1) gene is mutated in affected family members in a Scottish pedigree with a high incidence of schizophrenia and depression (Millar et al., 2000). Although this familial syndrome and corresponding mutation are extremely rare, investigation of DISC1 function has been highly informative, as DISC1 protein was recently shown to bind and inhibit GSK-3 (Mao et al., 2009). DISC1 knockdown increases phosphorylation of β-catenin at the GSK-3 phosphorylation sites, reduces total β-catenin levels, and reduces Wnt reporter activation and Wnt target gene expression, consistent with the proposed role for DISC1 as an endogenous GSK-3 inhibitor. DISC1 knockdown also reduces proliferation of AHPs in culture and in vivo, and this effect is rescued by treatment with a GSK-3 inhibitor (Mao et al., 2009). These data suggest DISC1 promotes proliferation in the adult hippocampus by inhibiting GSK-3.
Several studies suggest Wnt signaling promotes progenitor proliferation and neurogenesis in the adult SVZ as well as in hippocampus. Expression of the Wnt antagonist DKK1 in the adult mouse SVZ reduces neural progenitor cell proliferation, and inhibition of GSK-3 or expression of stabilized β-catenin increases progenitor cell proliferation. Inhibition of GSK-3 also increases the number of new neurons in the olfactory bulb (Adachi et al., 2007). Adult mice lacking Wnt7a show reduced BrdU labeling in the subventricular zone, and overexpression of Axin in the SVZ of wild type mice also reduces BrdU incorporation (Qu et al., 2010). In cultured neural progenitor cells isolated from the SVZ, exposure to Wnt3a, Wnt7a, or expression of stabilized β-catenin promotes proliferation and neurogenesis (Yu et al., 2006; Qu et al., 2010).
Evidence that Wnt signaling is essential for adult neurogenesis has led to investigation of upstream factors that may promote neurogenesis by activating Wnt signaling and downstream effectors that may mediate the effects of Wnt signaling on neurogenesis. Recent evidence suggests the orphan nuclear receptor TLX and hypoxia inducible factor-1 (HIF-1) regulate neurogenesis upstream of Wnt/β-catenin signaling. TLX is expressed in adult neural stem cells in the dentate gyrus and the SVZ (Shi et al., 2004). TLX binds to the Wnt7a promoter in adult neural stem cells and promotes Wnt7a transcription, β-catenin stabilization, and activation of Wnt target genes. Furthermore, TLX knockdown reduces NSC proliferation, and this effect is blocked by Wnt7a treatment or expression of stabilized β-catenin. In vivo, TLX null mice show reduced BrdU labeling in the SVZ, and this is rescued by expression of stabilized β-catenin (Qu et al., 2010). These data suggest TLX promotes adult neurogenesis by activating Wnt/β-catenin signaling.
Similarly, HIF-1 promotes Wnt signaling and neurogenesis (Mazumdar et al., 2010). HIF-1 is heterodimer of HIF-1α and HIF-1β/ARNT that regulates the response to hypoxia. Knockout of Hif1a, which encodes the HIF-1α subunit, reduces Wnt target gene expression in the adult hippocampus. Hif1a knockout also reduces BrdU incorporation and the number of newborn DCX positive neurons in the hippocampus. These effects are rescued by inhibition of GSK-3 and by expression of stabilized β-catenin, suggesting that HIF-1 functions upstream of the Wnt pathway to promote neurogenesis (Mazumdar et al., 2010). Interestingly, AHPs occupy a hypoxic niche in vivo, and hypoxia dramatically increases TLX protein levels in cultured AHPs (Mazumdar et al., 2010; Chavali et al., 2011). Furthermore, TLX knockdown under hypoxic conditions reduces AHP proliferation, suggesting TLX promotes neural stem or progenitor cell proliferation under hypoxic conditions (Chavali et al., 2011). These data suggest a potential link between HIF-1 and TLX functions upstream of Wnt signaling.
Downstream of Wnt signaling, NeuroD1 and the prospero-related homeodomain transcription factor Prox1 represent two intriguing candidates for Wnt target genes that may mediate the effects of Wnt signaling on neurogenesis. The NeuroD1 promoter contains several LEF/TCF binding sites and activating Wnt signaling by addition of either Wnt3a or the GSK-3 inhibitor TDZD8 increases NeuroD1 mRNA expression in cultured adult rat hippocampal NSCs, while blocking Wnt signaling with DKK1, dominant negative Wnt or β-catenin shRNA reduces NeuroD1 mRNA. Wnt3a treatment increases the number of cells expressing neuronal markers in cultured adult hippocampal NSCs, and in neurospheres cultured from adult mice, and this effect is blocked by NeuroD1 loss of function (Kuwabara et al., 2009). Overexpression of NeuroD in cultured adult hippocampal NSCs increases neuronal marker expression, mimicking the effect of Wnt3a (Hsieh et al., 2004). In the adult SGZ, NeuroD1 is expressed in neural progenitors and neuroblasts, including Ki67, and BrdU positive cells (Gao et al., 2009; Kuwabara et al., 2009). NeuroD1 deletion mimics Wnt loss of function by reducing the number of newborn neurons in the adult dentate gyrus as well as in the olfactory bulb, while NeuroD1 overexpression in the forebrain induces neurogenesis in the SVZ and rostral migratory stream (Gao et al., 2009; Boutin et al., 2010). These data suggest Wnt signaling may promote neurogenesis by inducing NeuroD1 expression.
Prox1 may also mediate the effects of Wnts on neurogenesis. β-Catenin associates with LEF/TCF binding sites in the Prox1 enhancer and promotes Prox1 expression in adult hippocampal NSCs. Prox1 is expressed in neural progenitors and both immature and mature neurons in the adult dentate gyrus. Expression of dominant negative LEF (dnLEF) in the dentate gyrus reduces Prox1 expression and expression of stabilized β-catenin induces Prox1 expression. Prox1 knockdown in the dentate gyrus reduces the number of newborn neurons and Prox1 overexpression increases the number of newborn neurons (Karalay et al., 2011). These data suggest Prox1 is a direct Wnt target that promotes neurogenesis. Importantly, further investigation is necessary to determine in vivo whether NeuroD1 or Prox1 expression can rescue neurogenesis defects resulting from Wnt pathway inhibition or whether Wnt-induced neurogenesis can be blocked by NeuroD1 or Prox1 loss of function.
Lithium Action in Bipolar Disorder
Bipolar disorder is a common neuropsychiatric disorder characterized by episodes of mania and depression, affecting an estimated 50–100 million people world-wide, and is associated with significant morbidity and mortality (reviewed in Belmaker, 2004; Goodwin and Jamison, 2007; Chen et al., 2010). Lithium has been widely used to treat BPD for over half a century, but its mechanism of action is not fully understood. Lithium also has a narrow therapeutic index and therapy is associated with multiple side effects, including thyroid dysfunction, nephrogenic diabetes insipidus, weight gain, arrhythmias, leukocytosis, tremor, and a variety of CNS and neuromuscular side effects (reviewed in Gilman, 1996). Thus, defining the molecular and cellular mechanisms of lithium action may provide insights into the pathogenesis of BPD and also lead to the development of better treatments for this common and devastating disorder.
An animal model of BPD would be extremely valuable in testing pathogenic and therapeutic mechanisms, but accurately assessing affect or affective disorders, especially with the added complexity of cycling of mood, in a model organism amendable to experimental manipulation is inherently challenging. Thus we and others have turned to animal behaviors that are influenced by mood stabilizers, including lithium, and can be measured in an objective and reproducible manner. Until recently, surprisingly few behaviors had been described that are sensitive to chronic lithium, most notably the amphetamine-induced hyperactivity behavior (reviewed in Murphy, 1977; Crawley, 2000). We have found that the forced swim test, classically used to assess antidepressant effects in rodents, is robustly sensitive to chronic lithium, with marked decrease in immobility in lithium treated mice (O’Brien et al., 2004). In addition, chronic lithium affects behavior in the elevated zero maze, a test often used to assess anxiolytic efficacy, and exploratory behavior (O’Brien et al., 2004; reviewed in O’Brien and Klein, 2009). Beaulieu et al. (2008) have shown that lithium also affects the tail suspension test and light–dark emergence, and Thakker-Varia et al. (2010) showed that lithium affects the novelty suppressed feeding paradigm.
Lithium regulates mammalian behaviors by inhibiting GSK-3
Numerous hypotheses have been put forth to explain the mechanisms of lithium action in mammalian behavior and in BPD (reviewed in Gurvich and Klein, 2002), but the number of direct lithium targets is quite limited. These include GSK-3 (Klein and Melton, 1996), inositol monophosphatase (IMPase) and structurally related enzymes (reviewed in Berridge et al., 1989; York et al., 2001; Gurvich and Klein, 2002), phosphoglucomutase (reviewed in Gurvich and Klein, 2002), and a β-arrestin-2 complex that regulates response to dopamine in the striatum (Beaulieu et al., 2008). Given the multiple plausible targets of lithium, we have suggested that validation of a proposed target of lithium should include evidence that (1) lithium inhibits the target at therapeutically relevant concentrations in vitro and in vivo, (2) structurally distinct inhibitors of the target should mimic lithium, (3) genetic loss of function of the target should also mimic lithium, and (4) the effects of lithium should be reversed by restoring function of the target (reviewed in Phiel and Klein, 2001; O’Brien and Klein, 2009).
Evidence from invertebrates suggests lithium can regulate behavior by inhibiting inositol phosphatases. In the nematode Caenorhabditis elegans, mutation of the inositol monophosphatase TTX-7 disrupts thermotaxis, and this behavioral phenotype is mimicked by lithium treatment; lithium also causes synaptic defects that phenocopy the ttx-7 mutant and both behavior and synaptic defects are prevented by inositol treatment or by TTX-7 overexpression (Tanizawa et al., 2006). These findings strongly support a role for inositol depletion in the effect of lithium on thermotaxis in C. elegans. Mutation of inositol polyphosphate 1-phosphatase (IPP) in Drosophila causes defects in synaptic vesicle release that are phenocopied by lithium (Acharya et al., 1998), although it is not known whether lithium or loss of IPP affects myo-inositol levels in this setting. However, inositol depletion in mammals does not mimic lithium effects on behavior; heterozygous deletion of the sodium dependent myo-inositol cotransporter-1 (SMIT1) in mice reduces brain myo-inositol levels to a similar or greater degree than lithium, but has no effect on behavioral phenotypes that are robustly affected by lithium (Shaldubina et al., 2006). These data suggest that global inositol depletion is not responsible for the behavioral effects of lithium in mammals.
We have proposed that the behavioral effects of lithium in mammals are mediated through inhibition of GSK-3 (O’Brien et al., 2004). GSK-3 fulfills each of the validation criteria described above: Lithium inhibits GSK-3 both in vitro and in vivo at therapeutically relevant concentrations (Klein and Melton, 1996; Stambolic et al., 1996; Hedgepeth et al., 1997; Hong et al., 1997; Munoz-Montano et al., 1997; Mudher et al., 2004; O’Brien et al., 2004), and structurally distinct GSK-3 inhibitors mimic the effects of lithium on mouse behaviors such as the forced swim test, exploratory behavior (Kaidanovich-Beilin et al., 2004; Pan et al., 2011; reviewed in O’Brien and Klein, 2009), light/dark emergence, and amphetamine-induced hyperactivity (Gould et al., 2004; Beaulieu et al., 2008). Furthermore, heterozygous loss of Gsk3b mimics lithium action in multiple behaviors (Beaulieu et al., 2004; O’Brien et al., 2004) and homozygous deletion of Gsk3a was also recently shown to mimic the behavioral effects of lithium and other GSK-3 inhibitors (Table 2; Kaidanovich-Beilin et al., 2009). Importantly, Gsk3b overexpression blocks the behavioral effects of LiCl (O’Brien et al., 2011). Taken together, these data strongly support GSK-3 as the relevant target of lithium in mammalian behavior.
Table 2.
Parallels between GSK-3 inhibitors and antidepressants in adult neurogenesis and behavior.
| Phenotype | Lithium | Alternative GSK-3 inhibitors | GSK-3 deletion | Antidepressants |
|---|---|---|---|---|
| Adult neurogenesis | ↑ | ↑ (SB216763) | ↑ (Fluoxetine, tranylcypromine, reboxetine) | |
| Forced swim test (immobility) | ↓ | ↓ (CHIR99021, AR-A014418, L803mts) | ↓ (Gsk3b+/− or Gsk3a−/−) | ↓ (Fluoxetine, desipramine) |
| Exploratory behavior (hole pokes) | ↓ | ↓ (Gsk3b+/−) | ||
| Elevated zero/plus maze (time in open area) | ↑ | ↑ (Gsk3b+/−) | ↑ (Citalopram) | |
| Tail suspension test (immobility) | ↓ | ↓ (Gsk3a−/−) | ↓ (Citalopram) | |
| Novelty suppressed feeding | ↓ | ↓ (Fluoxetine, imipramine, desipramine) | ||
| Novelty induced locomotor activity | ↓ | ↓ (TDZD-8) | ↓ (Gsk3a−/−) | |
| Amphetamine induced hyperlocomotion | ↓ | ↓ (CHIR99021, TDZD-8, 6-BIO, AR-A014418, SB216763, alsterpaullone) | ↓ (Gsk3b+/−) |
Lithium stimulates adult neurogenesis and affects mammalian behaviors. Structurally diverse GSK-3 inhibitors as well as genetic loss of GSK-3 function mimic the effects of lithium, strongly implicating GSK-3 as the relevant target mediating lithium effects on behavior. Interestingly, distinct classes of antidepressants have similar effects as lithium on neurogenesis and many behaviors.
In an elegant series of experiments, Beaulieu, Caron, and colleagues showed that, in the striatum, β-arrestin-2 forms a scaffold that binds Akt and protein phosphatase-2 (PP2A). Within this scaffold, PP2A dephosphorylates and deactivates Akt, preventing Akt-mediated phosphorylation and inhibition of GSK-3. They also showed that lithium disrupts this complex and proposed that this leads to Akt-mediated inhibition of GSK-3 (Beaulieu et al., 2008). Their findings therefore suggested that lithium inhibits GSK-3 indirectly.
However, these data are also compatible with direct inhibition of GSK-3 by lithium. We recently showed that GSK-3, which also binds to β-arrestin-2, maintains the stability of the β-arrestin-2 complex and lithium disrupts the complex by directly inhibiting GSK-3 (Figure 2; Beaulieu et al., 2005; O’Brien et al., 2011). In support of this mechanism, structurally distinct GSK-3 inhibitors as well as heterozygous loss of Gsk3b disrupt the complex and overexpression of Gsk3b restores basal levels of complex in the presence of lithium (O’Brien et al., 2011). These data suggest that GSK-3 stabilizes the β-arrestin-2 complex and that lithium disrupts the complex by inhibiting GSK-3.
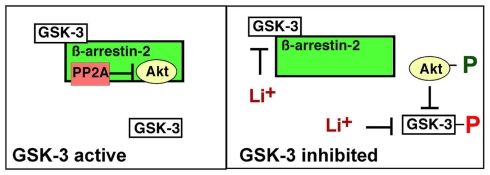
Figure 2.
Glycogen synthase kinase-3 regulation of the β-arrestin-2 scaffold. GSK-3 binds to and stabilizes the β-arrestin-2/PP2A/Akt complex, promoting PP2A-mediated dephosphorylation, and inactivation of Akt, which in turn maintains GSK-3 in an active state. Direct inhibition of GSK-3 disrupts the β-arrestin-2/PP2A/Akt interaction allowing activation of Akt, which then phosphorylates, and inhibits GSK-3.
Thus, the hypothesis that lithium modulates behaviors through direct inhibition of GSK-3 is strongly supported by each of the validation criteria described above, and while the data do not rule out contributions from other proposed targets, they provide a compelling rationale for pursuing GSK-3 substrates and downstream signaling pathways that mediate the behavioral and therapeutic effects of lithium.
Positive feedback regulation of GSK-3
These observations demonstrate both direct and indirect mechanisms of GSK-3 inhibition by lithium and imply a positive feedback system in which GSK-3 maintains its own activity by stabilizing the β-arrestin-2 complex, leading to dephosphorylation and inhibition of Akt (Figure 2). As Akt inhibits GSK-3, stabilization of the β-arrestin-2 complex by GSK-3 antagonizes this inhibition. In this model, a direct GSK-3 inhibitor is predicted to lead to indirect inhibition through enhanced N-terminal phosphorylation of GSK-3, as has been observed in many in vivo and cell culture contexts. This model can also explain how lithium activates Akt, as observed previously (Chalecka-Franaszek and Chuang, 1999). Through a parallel positive feedback circuit, GSK-3 regulates protein phosphatase 1 (PP1) and the PP1 inhibitor I-2 (Zhang et al., 2003). PP1 dephosphorylates and activates GSK-3; I-2 inhibits this reaction but I-2 in turn is deactivated by GSK-3; hence GSK-3 enhances its own activity by activating PP1. Taken together, these positive feedback systems provide discrete mechanisms to explain tissue-specific enhancement of GSK-3 inhibition by lithium (Figure 3), and may also help to explain why lithium is effective, both clinically and in mouse behaviors, at 1 mM, close to its in vitro IC50 (O’Brien et al., 2004).
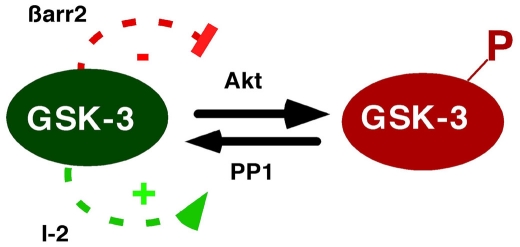
Figure 3.
Positive feedback regulation of GSK-3 through β-arrestin-2 and PP1. GSK-3 regulates its own activity by modulating its N-terminal phosphorylation state. As shown in Figure 2, GSK-3 enhances stability of the β-arrestin-2 scaffold, leading to inactivation of Akt, and thereby reducing GSK-3 phosphorylation. In addition, PP1 dephosphorylates and activates GSK-3, but is inhibited by the PP1-specific inhibitor-2 (I-2). GSK-3 inactivates I-2, preventing PP1 inhibition, and thereby maintaining dephosphorylation of GSK-3. Thus GSK-3 activity is regulated by positive feedback loops involving β-arrestin-2 and PP1.
Potential mechanisms mediating behavioral effects of GSK-3 inhibition
Since GSK-3 has emerged as the likely target of lithium in mammalian behavior, much attention has focused on mechanisms downstream of GSK-3 that may contribute to lithium effects. GSK-3 regulates dozens of substrates directly, regulates multiple signaling pathways including Wnt and Akt-dependent signaling, and controls cellular processes such as neurogenesis and survival (Doble et al., 2007). Lithium promotes adult hippocampal neurogenesis and several groups have proposed that this is an essential function of mood stabilizers (Chen et al., 1999; Son et al., 2003; Silva et al., 2008; Fiorentini et al., 2010; Hanson et al., 2011; reviewed in Samuels and Hen, 2011). In this regard, there are interesting parallels between the effects of mood stabilizing drugs and antidepressants in rodents: Structurally diverse antidepressants also stimulate neurogenesis (Malberg et al., 2000; Santarelli et al., 2003), have similar effects as chronic lithium in multiple behaviors (Table 2), and stimulate N-terminal inhibitory phosphorylation of GSK-3 (Li et al., 2004; reviewed in Polter and Li, 2010), suggesting that they may act through similar mechanisms. Hippocampal irradiation blocks the effects of antidepressants in the chronic unpredictable stress and novelty suppressed feeding paradigms suggesting hippocampal neurogenesis is required for the behavioral effects of antidepressants (Santarelli et al., 2003). By analogy, enhancing neurogenesis may be critical for the mood stabilizing effects of lithium. Although the requirement for neurogenesis in the behavioral response to lithium has not been tested, it would be intriguing and somewhat surprising to find that antimanic agents and antidepressants act through similar mechanisms.
Lithium inhibits GSK-3 to activate canonical Wnt signaling which promotes adult neurogenesis; thus chronic lithium may influence behavior by stimulating Wnt-dependent neurogenesis. In support of this proposed mechanism, chronic lithium activates Wnt signaling in the dentate gyrus in mice and expression of an activated form of β-catenin reduces immobility in the forced swim test (Gould et al., 2007). However, whether Wnt signaling is required for behavioral responses to lithium has not been explicitly tested. In addition, the requirement for Wnt-induced neurogenesis in behavior has not been studied. Lithium also activates Wnt signaling in the amygdala and hypothalamus, areas not known to support adult neurogenesis (O’Brien et al., 2004). Thus, Wnt target genes could function independently of neurogenesis to regulate lithium-sensitive behaviors. Importantly, lithium effects on non-neuronal cell types may also be important for its effects on behavior.
Glycogen synthase kinase-3 negatively regulates cell survival pathways suggesting additional mechanisms by which lithium could promote neurogenesis. Growth factors such as insulin, EGF, and neurotrophins activate Akt, which phosphorylates and inhibits GSK-3 to promote cell survival and proliferation (reviewed in Wada, 2009). In addition to inhibiting GSK-3 directly, lithium increases brain-derived neurotrophic factor (BDNF) levels (Fukumoto et al., 2001), and stimulates Akt and Akt-mediated inhibitory phosphorylation of GSK-3 (at Ser9/21) in the striatum (Beaulieu et al., 2008; Pan et al., 2011). Inhibitory GSK-3 phosphorylation may be important for the behavioral effects of lithium as Akt inhibition blocks the effects of lithium on amphetamine-induced hyperlocomotion (Pan et al., 2011), and Ser9/21 to alanine mutations have the opposite effects as lithium in some behaviors including increased exploratory behavior and increased amphetamine-induced hyperactivity (Ackermann et al., 2010; Polter et al., 2010). Ser9/21 to alanine mutations also reduce BrdU incorporation in the adult dentate gyrus and block stimulation of neurogenesis by co-treatment with lithium and fluoxetine (Eom and Jope, 2009a), suggesting GSK-3 phosphorylation promotes adult hippocampal neurogenesis and may be required for the effects of lithium and antidepressants on neurogenesis and behavior.
Interestingly, Akt may also enhance canonical Wnt pathway activation by lithium. As discussed above, lithium can activate Akt indirectly. Furthermore, lithium-induced Wnt reporter activation is attenuated by inhibition of Akt or PI3K, as well as by overexpression of the Akt inhibitor phosphatase and tensin homolog (PTEN; Pan et al., 2011). As N-terminal phosphorylation of GSK-3 does not promote Wnt signaling (Ding et al., 2000; McManus et al., 2005), Akt may either phosphorylate GSK-3 at other sites or regulate other components of the Wnt pathway. So far, alternative Akt phosphorylation sites in GSK-3 have not been described. However, Akt can phosphorylate β-catenin directly at a C-terminal site (Ser552), distinct from the GSK-3 phosphorylation sites, promoting β-catenin cytoplasmic and nuclear localization while reducing membrane localization (Fang et al., 2007). While speculative, these data raise the interesting possibility that Akt could contribute to β-catenin signaling in the response to lithium or other GSK-3 inhibitors.
Lithium may also affect behavior by inhibiting GSK-3 in the β-arrestin-2 complex in the striatum. β-Arrestin-2 prevents Akt-mediated inhibition of GSK-3, as β-arrestin-2 knockout activates Akt and increases inhibitory phosphorylation of GSK-3. Furthermore, β-arrestin-2 is required for effects of lithium on light/dark emergence and time immobile in the tail suspension test, and mimics the effect of lithium on novelty induced locomotor activity (Beaulieu et al., 2008).
Akt-mediated GSK-3 phosphorylation promotes cell growth, proliferation, and survival by activating downstream effectors such as mTOR, a key signaling complex that promotes protein translation and cell growth, regulates cell metabolism, and also regulates stem cell fate decisions (Huang et al., 2009; Duvel et al., 2010; Sato et al., 2010). GSK-3 negatively regulates mTOR by phosphorylating tuberous sclerosis complex-2 (TSC2), a core component of TSC (Inoki et al., 2006). The TSC is a GTPase activating factor that antagonizes the function of the small GTPase rheB, which is required to activate mTOR in response to nutrients (Inoki et al., 2003). Thus, inhibition of GSK-3 enhances rheB function and leads to activation of mTOR. Activation of mTOR by lithium or Gsk3 knockdown was demonstrated in cell culture and confirmed in vivo (Inoki et al., 2006; Huang et al., 2009). Interestingly, mTOR is also regulated by the Axin–GSK-3 complex, and canonical Wnt ligands activate mTOR in a β-catenin independent manner (Inoki et al., 2006). These data suggest mTOR may contribute to effects of Wnts, Akt, and lithium (Figure 4).
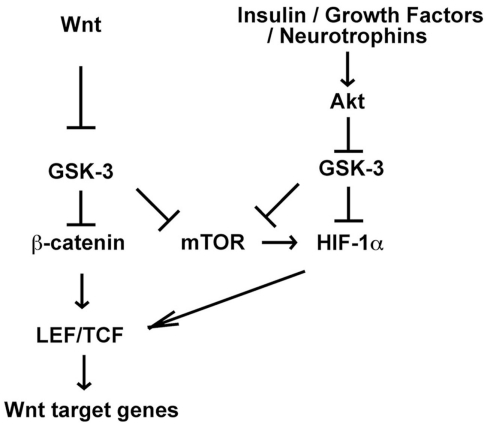
Figure 4.
Potential for crosstalk between Wnt and Akt-dependent signaling pathways through mTOR and HIF-1α. Wnt and Akt-dependent signaling pathways activate mTOR and mTOR promotes HIF-1α translation. Thus Wnt-induced mTOR activation may enhance HIF-1α function downstream of Akt. Furthermore HIF-1α promotes Wnt target gene expression by enhancing LEF/TCF transcription in embryonic and neural stem cells, suggesting that HIF-1α activation downstream of Akt may promote Wnt signaling. Interestingly, GSK-3 has also been shown to contribute to HIF-1α degradation, and therefore inhibition of GSK-3 by either small molecules or Akt-dependent pathways may also stabilize HIF-1α and enhance HIF-1 signaling (Mottet et al., 2003; Schnitzer et al., 2005; Flugel et al., 2007).
Hypoxia inducible factor-1 is another compelling candidate to mediate the effects of lithium as it promotes hippocampal neurogenesis and is functionally integrated with Insulin/PI3K signaling, Wnt signaling, GSK-3, and mTOR (Figure 4). GSK-3 phosphorylates HIF-1α resulting in proteasome-dependent HIF-1α degradation, and inhibition of GSK-3 by lithium, Gsk3 knockdown, or exposure to insulin increases HIF-1α protein levels (Flugel et al., 2007). HIF-1α stabilization in response to hypoxia can also be blocked by PI3K inhibition, which prevents inhibitory phosphorylation of GSK-3 (Mottet et al., 2003; Schnitzer et al., 2005).
Alternatively, GSK-3 may regulate HIF-1α abundance through regulation of mTOR (Figure 4). As discussed above, inhibition of GSK-3 activates mTOR, and mTOR activation increases HIF-1α translation and activates HIF-1α target genes including vascular endothelial growth factor (VEGF; Duvel et al., 2010). Fluoxetine and desipramine increase VEGF levels in the hippocampus, and inhibition of the VEGF receptor blocks the increase in neurogenesis and the behavioral effects caused by these two antidepressants (Warner-Schmidt and Duman, 2007; Greene et al., 2009). Increasing VEGF levels in the hippocampus is sufficient to stimulate neurogenesis, and has similar effects as lithium and antidepressants on behavior (Warner-Schmidt and Duman, 2007; Segi-Nishida et al., 2008; Udo et al., 2008). These data suggest HIF-1 and its target VEGF may be important for the effects of lithium on neurogenesis and behavior. Interestingly, HIF-1α also promotes canonical Wnt signaling (Mazumdar et al., 2010). HIF-1α promotes LEF-1 and TCF-1 transcription and antagonizes expression of APC, a negative regulator of Wnt signaling (Mazumdar et al., 2010; Newton et al., 2010). Thus HIF-1 may regulate neurogenesis and behavior through multiple downstream effectors.
Much evidence suggests GSK-3 also promotes apoptosis in many cell types, including neurons. Lithium blocks Bax and caspase-3 activation in response to trophic factor withdrawal and genotoxic stress in cultured neural precursor cells and protects against apoptosis resulting from glutamate induced excitotoxicity in multiple cell types (Nonaka et al., 1998; Eom et al., 2007). Lithium also increases levels of anti-apoptotic B-cell lymphoma protein-2 (bcl-2) in the hippocampus (Chen and Chuang, 1999; Chen et al., 1999). Neuronal apoptosis induced by trophic factor withdrawal or PI3K inhibition can be blocked by structurally diverse GSK-3 inhibitors, by an inhibitory GSK-3 binding protein (GBP), or by dominant negative GSK-3 (Hetman et al., 2000; Cross et al., 2001). Lithium and other GSK-3 inhibitors also block neuronal apoptosis induced by constitutively active c-Jun (Hongisto et al., 2003). In addition, GSK-3 enhances p53 function through direct interaction with p53 and through phosphorylation and activation of Tip60, an acetyltransferase required for p53-dependent apoptosis (Watcharasit et al., 2003; Eom and Jope, 2009b; Charvet et al., 2011). Thus, lithium and other GSK-3 inhibitors promote survival in part by attenuating p53 levels and/or activity. Gsk3b overexpression also induces apoptosis in cultured cortical neurons (Pap and Cooper, 1998; Hetman et al., 2000). Taken together, these data suggest GSK-3 promotes apoptosis and inhibiting GSK-3 blocks apoptosis in response to diverse challenges. Thus reduced apoptosis resulting from GSK-3 inhibition by lithium may contribute to increased neurogenesis and effects on behavior.
Conclusion
Wnt signaling regulates many aspects of mammalian CNS development and continues to play critical roles in the adult CNS, including adult neurogenesis. Canonical Wnt signaling may also play a key role in the therapeutic response to lithium in BPD, as lithium activates the pathway by inhibiting GSK-3. In animal models, lithium regulates multiple mammalian behaviors by inhibiting GSK-3. However, GSK-3 regulates multiple pathways in addition to Wnt signaling, most notably Akt-dependent signaling, and each of these pathways may contribute to neurogenesis, cell proliferation, cell survival, and neural morphology. Thus, how distinct GSK-3 regulated pathways interact to mediate the effects of lithium on neurogenesis and behavior has not yet been delineated. Furthermore, the evidence that GSK-3 inhibition mediates the therapeutic effects of lithium in patients with BPD is still limited. Exciting recent work has shown that inhibitory GSK-3 phosphorylation is reduced in peripheral blood mononuclear cells (PBMCs) of patients with BPD compared to healthy controls suggesting GSK-3 is aberrantly active in BPD patients (Polter et al., 2010). In addition, lithium and other mood stabilizers increase GSK-3 phosphorylation (reviewed in Li and Jope, 2010) in PBMCs of BPD patients. Thus, while the number of patients studied is still small, the therapeutic effects of lithium in BPD may involve both direct and indirect inhibition of GSK-3. Finally, an outstanding question has been whether lithium is a sufficiently potent GSK-3 inhibitor to explain its therapeutic action. Recent findings support several positive feedback circuits that confer GSK-3 autoregulation and these may enhance the sensitivity to lithium in a tissue-specific manner. Better understanding of these intriguing complexities will aid in the development of new therapeutics for patients with BPD.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Greg Bryman for his help in constructing Table 2 and W. Timothy O’Brien and Irwin Lucki for helpful discussions. Peter S. Klein is supported by grants from the NIH (1RO1MH58324 and 1R01HL110806). Alexander J. Valvezan was supported by the Training Program in Hematopoiesis (T32 DK07780).
References
- Acharya J. K., Labarca P., Delgado R., Jalink K., Zuker C. S. (1998). Synaptic defects and compensatory regulation of inositol metabolism in inositol polyphosphate 1-phosphatase mutants. Neuron 20, 1219–1229 10.1016/S0896-6273(00)80502-4 [DOI] [PubMed] [Google Scholar]
- Ackermann T. F., Kempe D. S., Lang F., Lang U. E. (2010). Hyperactivity and enhanced curiosity of mice expressing PKB/SGK-resistant glycogen synthase kinase-3 (GSK-3). Cell. Physiol. Biochem. 25, 775–786 10.1159/000315097 [DOI] [PubMed] [Google Scholar]
- Adachi K., Mirzadeh Z., Sakaguchi M., Yamashita T., Nikolcheva T., Gotoh Y., Peltz G., Gong L., Kawase T., Alavarez-Buylla A., Okano H., Sawamoto K. (2007). Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells 25, 2827–2836 10.1634/stemcells.2007-0177 [DOI] [PubMed] [Google Scholar]
- Aimone J. B., Deng W., Gage F. H. (2011). Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 70, 589–596 10.1016/j.neuron.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J. M., Marion S., Rodriguiz R. M., Medvedev I. O., Sotnikova T. D., Ghisi V., Wetsel W. C., Lefkowitz R. J., Gainetdinov R. R., Caron M. G. (2008). A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell 132, 125–136 10.1016/j.cell.2007.11.041 [DOI] [PubMed] [Google Scholar]
- Beaulieu J. M., Sotnikova T. D., Marion S., Lefkowitz R. J., Gainetdinov R. R., Caron M. G. (2005). An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122, 261–273 10.1016/j.cell.2005.05.012 [DOI] [PubMed] [Google Scholar]
- Beaulieu J. M., Sotnikova T. D., Yao W. D., Kockeritz L., Woodgett J. R., Gainetdinov R. R., Caron M. G. (2004). Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc. Natl. Acad. Sci. U.S.A. 101, 5099–5104 10.1073/pnas.0307921101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker R. H. (2004). Bipolar disorder. N. Engl. J. Med. 351, 476–486 10.1056/NEJMra035354 [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. (1989). Neural and developmental actions of lithium: a unifying hypothesis. Cell 59, 411–419 10.1016/0092-8674(89)90026-3 [DOI] [PubMed] [Google Scholar]
- Boutin C., Hardt O., de Chevigny A., Core N., Goebbels S., Seidenfaden R., Bosio A., Cremer H. (2010). NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 1201–1206 10.1073/pnas.0909015107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V., Salinas P. C. (2011). Wnt signaling during synaptic development and plasticity. Curr. Opin. Neurobiol. 21, 151–159 10.1016/j.conb.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalecka-Franaszek E., Chuang D. M. (1999). Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc. Natl. Acad. Sci. U.S.A. 96, 8745–8750 10.1073/pnas.96.15.8745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet C., Wissler M., Brauns-Schubert P., Wang S. J., Tang Y., Sigloch F. C., Mellert H., Brandenburg M., Lindner S. E., Breit B., Green D. R., McMahon S. B., Borner C., Gu W., Maurer U. (2011). Phosphorylation of Tip60 by GSK-3 determines the induction of PUMA and apoptosis by p53. Mol. Cell 42, 584–596 10.1016/j.molcel.2011.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavali P. L., Saini R. K., Matsumoto Y., Agren H., Funa K. (2011). Nuclear orphan receptor TLX induces Oct-3/4 for the survival and maintenance of adult hippocampal progenitors upon hypoxia. J. Biol. Chem. 286, 9393–9404 10.1074/jbc.M110.167445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Rajkowska G., Du F., Seraji-Bozorgzad N., Manji H. K. (2000). Enhancement of hippocampal neurogenesis by lithium. J. Neurochem. 75, 1729–1734 10.1046/j.1471-4159.2000.0751729.x [DOI] [PubMed] [Google Scholar]
- Chen G., Henter I. D., Manji H. K. (2010). Translational research in bipolar disorder: emerging insights from genetically based models. Mol. Psychiatry 15, 883–895 10.1038/mp.2010.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. W., Chuang D. M. (1999). Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. J. Biol. Chem. 274, 6039–6042 10.1074/jbc.274.53.37750 [DOI] [PubMed] [Google Scholar]
- Chenn A., Walsh C. A. (2002). Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297, 365–369 10.1126/science.1074192 [DOI] [PubMed] [Google Scholar]
- Chen G., Zeng W. Z., Yuan P. X., Huang L. D., Jiang Y. M., Zhao Z. H., Manji H. K. (1999). The mood stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J. Neurochem. 72, 879–882 10.1046/j.1471-4159.1999.720879.x [DOI] [PubMed] [Google Scholar]
- Ciani L., Boyle K. A., Dickins E., Sahores M., Anane D., Lopes D. M., Gibb A. J., Salinas P. C. (2011). Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca2+/Calmodulin-dependent protein kinase II. Proc. Natl. Acad. Sci. U.S.A. 108, 10732–10737 10.1073/pnas.1018132108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L., Salinas P. C. (2005). WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 6, 351–362 10.1038/nrg1601 [DOI] [PubMed] [Google Scholar]
- Clelland C. D., Choi M., Romberg C., Clemenson G. D. J., Fragniere A., Tyers P., Jessberger S., Saksida L. M., Barker R. A., Gage F. H., Bussey T. J. (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213 10.1126/science.1173215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. (2006). Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480 10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- Crawley J. N. (2000). What’s Wrong with my Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. New York, NY: Wiley-Liss [Google Scholar]
- Cross D., Alessi D., Cohen P., Andjelkovich M., Hemmings B. (1995). Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 10.1038/378785a0 [DOI] [PubMed] [Google Scholar]
- Cross D. A., Culbert A. A., Chalmers K. A., Facci L., Skaper S. D., Reith A. D. (2001). Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J. Neurochem. 77, 94–102 10.1046/j.1471-4159.2001.00251.x [DOI] [PubMed] [Google Scholar]
- Dajani R., Fraser E., Roe S. M., Young N., Good V., Dale T. C., Pearl L. H. (2001). Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 105, 721–732 10.1016/S0092-8674(01)00374-9 [DOI] [PubMed] [Google Scholar]
- Deng W., Aimone J. B., Gage F. H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350 10.1038/nrn2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson M. E., Krumlauf R., McMahon A. P. (1994). Evidence for a mitogenic effect of Wnt-1 in the developing mammalian central nervous system. Development 120, 1453–1471 [DOI] [PubMed] [Google Scholar]
- Dill J., Wang H., Zhou F., Li S. (2008). Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J. Neurosci. 28, 8914–8928 10.1523/JNEUROSCI.1178-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding V. W., Chen R. H., McCormick F. (2000). Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J. Biol. Chem. 275, 32475–32481 10.1074/jbc.M005342200 [DOI] [PubMed] [Google Scholar]
- Doble B. W., Patel S., Wood G. A., Kockeritz L. K., Woodgett J. R. (2007). Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev. Cell 12, 957–971 10.1016/j.devcel.2007.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvel K., Yecies J. L., Menon S., Raman P., Lipovsky A. I., Souza A. L., Triantafellow E., Ma Q., Gorski R., Cleaver S., Vander Heiden M. G., MacKeigan J. P., Finan P. M., Clish C. B., Murphy L. O., Manning B. D. (2010). Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183 10.1016/j.molcel.2010.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom T. Y., Jope R. S. (2009a). Blocked inhibitory serine-phosphorylation of glycogen synthase kinase-3alpha/beta impairs in vivo neural precursor cell proliferation. Biol. Psychiatry 66, 494–502 10.1016/j.biopsych.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom T. Y., Jope R. S. (2009b). GSK3 beta N-terminus binding to p53 promotes its acetylation. Mol. Cancer 8, 14. 10.1186/1476-4598-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom T. Y., Roth K. A., Jope R. S. (2007). Neural precursor cells are protected from apoptosis induced by trophic factor withdrawal or genotoxic stress by inhibitors of glycogen synthase kinase 3. J. Biol. Chem. 282, 22856–22864 10.1074/jbc.M702973200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P. S., Perfilieva E., Bjork-Eriksson T., Alborn A. M., Nordborg C., Peterson D. A., Gage F. H. (1998). Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317 10.1038/3305 [DOI] [PubMed] [Google Scholar]
- Fang D., Hawke D., Zheng Y., Xia Y., Meisenhelder J., Nika H., Mills G. B., Kobayashi R., Hunter T., Lu Z. (2007). Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J. Biol. Chem. 282, 11221–11229 10.1074/jbc.M611871200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini A., Rosi M. C., Grossi C., Luccarini I., Casamenti f. (2010). Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PLoS ONE 5, e14382. 10.1371/journal.pone.0014382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flugel D., Gorlach A., Michiels C., Kietzmann T. (2007). Glycogen synthase kinase 3 phosphorylates hypoxia-inducible factor 1alpha and mediates its destabilization in a VHL-independent manner. Mol. Cell. Biol. 27, 3253–3265 10.1128/MCB.00015-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto T., Morinobu S., Okamoto Y., Kagaya A., Yamawaki S. (2001). Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology (Berl.) 158, 100–106 10.1007/s002130100871 [DOI] [PubMed] [Google Scholar]
- Gage F. H. (2000). Mammalian neural stem cells. Science 287, 1433–1438 10.1126/science.287.5457.1433 [DOI] [PubMed] [Google Scholar]
- Galceran J., Miyashita-Lin E. M., Devaney E., Rubenstein J. L., Grosschedl R. (2000). Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development 127, 469–482 [DOI] [PubMed] [Google Scholar]
- Gao Z., Ure K., Ables J. L., Lagace D. C., Nave K. A., Goebbels S., Eisch A. J., Hsieh J. (2009). Neurod1 is essential for the survival and maturation of adult-born neurons. Nat. Neurosci. 12, 1090–1092 10.1038/nn.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner A., Huang X., Hall A. C. (2006). Neuronal polarity is regulated by glycogen synthase kinase-3 (GSK-3{beta}) independently of Akt/PKB serine phosphorylation. J. Cell. Sci. 119(Pt 19), 3927–3934 10.1242/jcs.03159 [DOI] [PubMed] [Google Scholar]
- Ge S., Sailor K. A., Ming G. L., Song H. (2008). Synaptic integration and plasticity of new neurons in the adult hippocampus. J. Physiol. (Lond.) 586, 3759–3765 10.1113/jphysiol.2008.155655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. (1996). The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill [Google Scholar]
- Glinka A., Wu W., Delius H., Monaghan A. P., Blumenstock C., Niehrs C. (1998). Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357–362 10.1038/34848 [DOI] [PubMed] [Google Scholar]
- Goodwin F. K., Jamison K. R. (2007). Manic-Depressive Illness: Bipolar and Recurrent Unipolar Disorders. New York: Oxford University Press [Google Scholar]
- Gould T. D., Einat H., Bhat R., Manji H. K. (2004). AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int. J. Neuropsychopharmacol. 7, 387–390 10.1017/S1461145704004535 [DOI] [PubMed] [Google Scholar]
- Gould T. D., Einat H., O’Donnell K. C., Picchini A. M., Schloesser R. J., Manji H. K. (2007). Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology 32, 2173–2183 10.1038/sj.npp.1301254 [DOI] [PubMed] [Google Scholar]
- Greene J., Banasr M., Lee B., Warner-Schmidt J., Duman R. S. (2009). Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology 34, 2459–2468 10.1038/npp.2009.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B., Watanabe K., Dai X. (2010). Epithelial stem cells: an epigenetic and Wnt-centric perspective. J. Cell. Biochem. 110, 1279–1287 10.1002/jcb.22650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvich N., Klein P. S. (2002). Lithium and valproic acid: parallels and contrasts in diverse signaling contexts. Pharmacol. Ther. 96, 45–66 10.1016/S0163-7258(02)00299-1 [DOI] [PubMed] [Google Scholar]
- Hall A. C., Lucas F. R., Salinas P. C. (2000). Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100, 525–535 10.1016/S0092-8674(00)80689-3 [DOI] [PubMed] [Google Scholar]
- Hanson N. D., Nemeroff C. B., Owens M. J. (2011). Lithium, but not fluoxetine or the corticotropin-releasing factor receptor 1 receptor antagonist R121919, increases cell proliferation in the adult dentate gyrus. J. Pharmacol. Exp. Ther. 337, 180–186 10.1124/jpet.110.175372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgepeth C., Conrad L., Zhang Z., Huang H., Lee V., Klein P. (1997). Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev. Biol. 185, 82–91 10.1006/dbio.1997.8552 [DOI] [PubMed] [Google Scholar]
- Hetman M., Cavanaugh J. E., Kimelman D., Xia Z. (2000). Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J. Neurosci. 20, 2567–2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M., Chen D. C., Klein P. S., Lee V. M.-Y. (1997). Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J. Biol. Chem. 272, 25326–25332 10.1074/jbc.272.31.19547 [DOI] [PubMed] [Google Scholar]
- Hongisto V., Smeds N., Brecht S., Herdegen T., Courtney M. J., Coffey E. T. (2003). Lithium blocks the c-Jun stress response and protects neurons via its action on glycogen synthase kinase 3. Mol. Cell. Biol. 23, 6027–6036 10.1128/MCB.23.17.6027-6036.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J., Nakashima K., Kuwabara T., Mejia E., Gage F. H. (2004). Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. U.S.A. 101, 16659–16664 10.1073/pnas.0405736101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhang Y., Bersenev A., O’Brien W. T., Tong W., Emerson S. G., Klein P. S. (2009). Pivotal role for glycogen synthase kinase–3 in hematopoietic stem cell homeostasis in mice. J. Clin. Invest. 119, 3519–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J., Vogel R., Brinkmann V., Erdmann B., Birchmeier C., Birchmeier W. (2000). Requirement for beta-catenin in anterior-posterior axis formation in mice. J. Cell Biol. 148, 567–578 10.1083/jcb.148.3.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K., Li Y., Xu T., Guan K. L. (2003). Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 17, 1829–1834 10.1101/gad.1110003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K., Ouyang H., Zhu T., Lindvall C., Wang Y., Zhang X., Yang Q., Bennett C., Harada Y., Stankunas K., Wang C. Y., He X., MacDougald O. A., You M., Williams B. O., Guan K. L. (2006). TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126, 955–968 10.1016/j.cell.2006.06.055 [DOI] [PubMed] [Google Scholar]
- Jessberger S., Clarke R. E., Broadbent N. J., Clemenson G. D. J., Consiglio A., Squire L. R., Gage F. H. (2009). Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 16, 147–154 10.1101/lm.1172609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O., Lipina T. V., Takao K., van Eede M., Hattori S., Laliberté C., Khan M., Okamoto K., Chambers J. W., Fletcher P. J., Macaulay K., Doble B. W., Henkelman M., Miyakawa T., Roder J., Woodgett J. R. (2009). Abnormalities in brain structure and behavior in GSK-3alpha mutant mice. Mol. Brain 2, 35. 10.1186/1756-6606-2-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O., Milman A., Weizman A., Pick C. G., Eldar-Finkelman H. (2004). Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol. Psychiatry 55, 781–784 10.1016/j.biopsych.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O., Woodgett J. R. (2011). GSK-3: functional insights from cell biology and animal models. Front. Mol. Neurosci. 4:40. 10.3389/fnmol.2011.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsimali M., Caneparo L., Houart C., Wilson S. W. (2004). Inhibition of Wnt/Axin/beta-catenin pathway activity promotes ventral CNS midline tissue to adopt hypothalamic rather than floorplate identity. Development 131, 5923–5933 10.1242/dev.01453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalay O., Doberauer K., Vadodaria K. C., Knobloch M., Berti L., Miquelajauregui A., Schwark M., Jagasia R., Taketo M. M., Tarabykin V., Lie D. C., Jessberger S. (2011). Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. U.S.A. 108, 5807–5812 10.1073/pnas.1013456108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. H., Oda T., Itoh M., Jiang D., Artinger K. B., Chandrasekharappa S. C., Driever W., Chitnis A. B. (2000). Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature 407, 913–916 10.1038/35038097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. Y., Snider W. D. (2011). Functions of GSK-3 signaling in development of the nervous system. Front. Mol. Neurosci. 4:44. 10.3389/fnmol.2011.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. Y., Wang X., Wu Y. H., Doble B. W., Patel S., Woodgett J. R., Snider W. D. (2009). GSK-3 is a master regulator of neural progenitor homeostasis. Nat. Neurosci. 12, 1390–1397 10.1038/nn.2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. T., Hur E. M., Snider W. D., Zhou F. Q. (2011). Role of GSK3 signaling in neuronal morphogenesis. Front. Mol. Neurosci. 4:48. 10.3389/fnmol.2011.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. S., Melton D. A. (1996). A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. U.S.A. 93, 8455–8459 10.1073/pnas.93.14.7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockeritz L., Doble B., Patel S., Woodgett J. R. (2006). Glycogen synthase kinase-3 – an overview of an over-achieving protein kinase. Curr. Drug Targets 7, 1377–1388 [DOI] [PubMed] [Google Scholar]
- Kuwabara T., Hsieh J., Muotri A., Yeo G., Warashina M., Lie D. C., Moore L., Nakashima K., Asashima M., Gage F. H. (2009). Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci. 12, 1097–1105 10.1038/nn.2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F., Lledo P. M. (2011). Is adult neurogenesis essential for olfaction? Trends Neurosci. 34, 20–30 10.1016/j.tins.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Lee S. M., Tole S., Grove E., McMahon A. P. (2000). A local Wnt-3a signal is required for development of the mammalian hippocampus. Development 127, 457–467 [DOI] [PubMed] [Google Scholar]
- Lekven A. C., Thorpe C. J., Waxman J. S., Moon R. T. (2001). Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev. Cell 1, 103–114 10.1016/S1534-5807(01)00007-7 [DOI] [PubMed] [Google Scholar]
- Li X., Jope R. S. (2010). Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology 35, 2143–2154 10.1038/npp.2010.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhu W., Roh M. S., Friedman A. B., Rosborough K., Jope R. S. (2004). In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology 29, 1426–1431 10.1038/sj.npp.1300439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie D. C., Colamarino S. A., Song H. J., Desire L., Mira H., Consiglio A., Lein E. S., Jessberger S., Lansford H., Dearie A. R., Gage F. H. (2005). Wnt signalling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375 10.1038/nature04108 [DOI] [PubMed] [Google Scholar]
- Liu C., Wang Y., Smallwood P. M., Nathans J. (2008). An essential role for Frizzled5 in neuronal survival in the parafascicular nucleus of the thalamus. J. Neurosci. 28, 5641–5653 10.1523/JNEUROSCI.3769-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo P. M., Alonso M., Grubb M. S. (2006). Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 7, 179–193 10.1038/nrn1867 [DOI] [PubMed] [Google Scholar]
- Lucas F. R., Salinas P. C. (1997). WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev. Biol. 192, 31–44 10.1006/dbio.1997.8734 [DOI] [PubMed] [Google Scholar]
- Lyuksyutova A. I., Lu C. C., Milanesio N., King L. A., Guo N., Wang Y., Nathans J., Tessier-Lavigne M., Zou Y. (2003). Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302, 1984–1988 10.1126/science.1089610 [DOI] [PubMed] [Google Scholar]
- MacDonald B. T., Tamai K., He X. (2009). Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 10.1016/j.devcel.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machon O., van den Bout C. J., Backman M., Kemler R., Krauss S. (2003). Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience 122, 129–143 10.1016/S0306-4522(03)00519-0 [DOI] [PubMed] [Google Scholar]
- Malberg J. E., Eisch A. J., Nestler E. J., Duman R. S. (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–9110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y., Ge X., Frank C. L., Madison J. M., Koehler A. N., Doud M. K., Tassa C., Berry E. M., Soda T., Singh K. K., Biechele T., Petryshen T. L., Moon R. T., Haggarty S. J., Tsai L. H. (2009). Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell 136, 1017–1031 10.1016/j.cell.2008.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J., O’Brien W. T., Johnson R. S., LaManna J. C., Chavez J. C., Klein P. S., Simon M. C. (2010). O2 regulates stem cells through Wnt/beta-catenin signalling. Nat. Cell Biol. 12, 1007–1013 10.1038/ncb2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. (1990). The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell 62, 1073–1085 10.1016/0092-8674(90)90385-R [DOI] [PubMed] [Google Scholar]
- McManus E. J., Sakamoto K., Armit L. J., Ronaldson L., Shpiro N., Marquez R., Alessi D. R. (2005). Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 24, 1571–1583 10.1038/sj.emboj.7600633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megason S. G., McMahon A. P. (2002). A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development 129, 2087–2098 [DOI] [PubMed] [Google Scholar]
- Millar J. K., Wilson-Annon J. C., Anderson S., Christie S., Taylor M. S., Semple C. A., Devon R. S., St Clair D. M., Muir W. J., Blackwood D. H., Porteous D. J. (2000). Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum. Mol. Genet. 9, 1415–1423 10.1093/hmg/9.9.1415 [DOI] [PubMed] [Google Scholar]
- Ming G. L., Song H. (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiat L. A., Schinder A. F. (2011). Adult neurogenesis and the plasticity of the dentate gyrus network. Eur. J. Neurosci. 33, 1055–1061 10.1111/j.1460-9568.2011.07603.x [DOI] [PubMed] [Google Scholar]
- Moser A. R., Shoemaker A. R., Connelly C. S., Clipson L., Gould K. A., Luongo C., Dove W. F., Siggers P. H., Gardner R. L. (1995). Homozygosity for the Min allele of Apc results in disruption of mouse development prior to gastrulation. Dev. Dyn. 203, 422–433 10.1002/aja.1002030405 [DOI] [PubMed] [Google Scholar]
- Mottet D., Dumont V., Deccache Y., Demazy C., Ninane N., Raes M., Michiels C. (2003). Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J. Biol. Chem. 278, 31277–31285 10.1074/jbc.M300763200 [DOI] [PubMed] [Google Scholar]
- Mudher A., Shepherd D., Newman T. A., Mildren P., Jukes J. P., Squire A., Mears A., Drummond J. A., Berg S., MacKay D., Asuni A. A., Bhat R., Lovestone S. (2004). GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol. Psychiatry 9, 522–530 10.1038/sj.mp.4001540 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M., Shtrom S., Rodriguez-Esteban C., Chen L., Tsukui T., Gomer L., Dorward D. W., Glinka A., Grinberg A., Huang S. P., Niehrs C., Belmonte J. C., Westphal H. (2001). Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev. Cell 1, 423–434 10.1016/S1534-5807(01)00041-7 [DOI] [PubMed] [Google Scholar]
- Munoz-Montano J. R., Moreno F. J., Avila J., Diaz-Nido J. (1997). Lithium inhibits Alzheimer’s disease-like tau protein phosphorylation in neurons. FEBS Lett. 411, 183–188 10.1016/S0014-5793(97)00688-1 [DOI] [PubMed] [Google Scholar]
- Muroyama Y., Fujihara M., Ikeya M., Kondoh H., Takada S. (2002). Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 16, 548–553 10.1101/gad.937102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. L. (1977). “Animal models for mania,” in Animal Models in Psychiatry and Neurology, eds Hanin I., Usdin E. (Oxford: Pergamon Press; ), 211–223 [Google Scholar]
- Newton I. P., Kenneth N. S., Appleton P. L., Nathke I., Rocha S. (2010). Adenomatous polyposis coli and hypoxia-inducible factor-1{alpha} have an antagonistic connection. Mol. Biol. Cell 21, 3630–3638 10.1091/mbc.E10-04-0312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. S., Mahmoudi T., Danenberg E., Bejaoui I., de Lau K., Korswagen H. C., Schuttle M., Clevers H. (2009). Phosphatidylinositol 3-kinase signaling does not activate the wnt cascade. J. Biol. Chem. 284, 35308–35313 10.1074/jbc.M109.078261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S., Hough C. J., Chuang D. M. (1998). Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc. Natl. Acad. Sci. U.S.A. 95, 2642–2647 10.1073/pnas.95.5.2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien W. T., Harper A. D., Jove F., Woodgett J. R., Maretto S., Piccolo S., Klein P. S. (2004). Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J. Neurosci. 24, 6791–6798 10.1523/JNEUROSCI.4753-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien W. T., Huang J., Buccafusca R., Garskof J., Valvezan A. J., Berry G. T., Klein P. S. (2011). Glycogen synthase kinase-3 is essential for beta-arrestin-2 complex formation and lithium-sensitive behaviors in mice. J. Clin. Invest. 121, 3756–3762 10.1172/JCI45194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien W. T., Klein P. S. (2009). Validating GSK3 as an in vivo target of lithium action. Biochem. Soc. Trans. 37, 1133–1138 10.1042/BST0371133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J. Q., Lewis M. C., Ketterman J. K., Clore E. L., Riley M., Richards K. R., Berry-Scott E., Liu X., Wagner F. F., Holson E. B., Neve R. L., Biechele T. L., Moon R. T., Scolnick E. M., Petryshen T. L., Haggarty S. J. (2011). AKT kinase activity is required for lithium to modulate mood-related behaviors in mice. Neuropsychopharmacology 36, 1397–1411 10.1038/npp.2011.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panhuysen M., Vogt Weisenhorn D. M., Blanquet V., Brodski C., Heinzmann U., Beisker W., Wurst W. (2004). Effects of Wnt1 signaling on proliferation in the developing mid-/hindbrain region. Mol. Cell. Neurosci. 26, 101–111 10.1016/j.mcn.2004.01.011 [DOI] [PubMed] [Google Scholar]
- Pap M., Cooper G. M. (1998). Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 273, 19929–19932 10.1074/jbc.273.32.19929 [DOI] [PubMed] [Google Scholar]
- Petersen C. P., Reddien P. W. (2009). Wnt signaling and the polarity of the primary body axis. Cell 139, 1056–1068 10.1016/j.cell.2009.11.035 [DOI] [PubMed] [Google Scholar]
- Phiel C. J., Klein P. S. (2001). Molecular targets of lithium action. Annu. Rev. Pharmacol. Toxicol. 41, 789–813 10.1146/annurev.pharmtox.41.1.789 [DOI] [PubMed] [Google Scholar]
- Pinson K. I., Brennan J., Monkley S., Avery B. J., Skarnes W. C. (2000). An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407, 535–538 10.1038/35035124 [DOI] [PubMed] [Google Scholar]
- Polter A., Beurel E., Yang S., Garner R., Song L., Miller C. A., Sweatt J. D., McMahon L., Bartolucci A. A., Li X., Jope R. S. (2010). Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology 35, 1761–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter A. M., Li X. (2010). 5-HT1A receptor-regulated signal transduction pathways in brain. Cell. Signal. 22, 1406–1412 10.1016/j.cellsig.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud C. G. (2006). Regulation of protein synthesis by insulin. Biochem. Soc. Trans. 34, 213–216 10.1042/BST20060213 [DOI] [PubMed] [Google Scholar]
- Purro S. A., Ciani L., Hoyos-Flight M., Stamatakou E., Siomou E., Salinas P. C. (2008). Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli. J. Neurosci. 28, 8644–8654 10.1523/JNEUROSCI.2320-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Q., Sun G., Li W., Yang S., Ye P., Zhao C., Yu R. T., Gage F. H., Evans R. M., Shi Y. (2010). Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat. Cell Biol. 12, 31–40 10.1038/ncb2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso S. B., Sussman D., Wynshaw-Boris A., Salinas P. C. (2005). Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat. Neurosci. 8, 34–42 10.1038/nn1374 [DOI] [PubMed] [Google Scholar]
- Sahay A., Wilson D. A., Hen R. (2011). Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 70, 582–588 10.1016/j.neuron.2011.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Jeannet J. P., He X., Varmus H. E., Dawid I. B. (1997). Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc. Natl. Acad. Sci. U.S.A. 94, 13713–13718 10.1073/pnas.94.25.13713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels B. A., Hen R. (2011). Neurogenesis and affective disorders. Eur. J. Neurosci. 33, 1152–1159 10.1111/j.1460-9568.2011.07614.x [DOI] [PubMed] [Google Scholar]
- Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 10.1126/science.1083328 [DOI] [PubMed] [Google Scholar]
- Sato A., Sunayama J., Matsuda K., Tachibana K., Sakurada K., Tomiyama A., Kayama T., Kitanaka C. (2010). Regulation of neural stem/progenitor cell maintenance by PI3K and mTOR. Neurosci. Lett. 470, 115–120 10.1016/j.neulet.2009.12.067 [DOI] [PubMed] [Google Scholar]
- Saxe M. D., Battaglia F., Wang J. W., Malleret G., David D. J., Monckton J. E., Garcia A. D., Sofroniew M. V., Kandel E. R., Santarelli L., Hen R., Drew M. R. (2006). Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 103, 17501–17506 10.1073/pnas.0607207103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer S. E., Schmid T., Zhou J., Eisenbrand G., Brune B. (2005). Inhibition of GSK3beta by indirubins restores HIF-1alpha accumulation under prolonged periods of hypoxia/anoxia. FEBS Lett. 579, 529–533 10.1016/j.febslet.2004.12.023 [DOI] [PubMed] [Google Scholar]
- Segi-Nishida E., Warner-Schmidt J. L., Duman R. S. (2008). Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc. Natl. Acad. Sci. U.S.A. 105, 11352–11357 10.1073/pnas.0710858105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaldubina A., Johanson R. A., O’Brien W. T., Buccafusca R., Agam G., Belmaker R. H., Klein P. S., Bersudsky Y., Berry G. T. (2006). SMIT1 haploinsufficiency causes brain inositol deficiency without affecting lithium-sensitive behavior. Mol. Genet. Metab. 88, 384–388 10.1016/j.ymgme.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Shi Y., Chichung Lie D., Taupin P., Nakashima K., Ray J., Yu R. T., Gage F. H., Evans R. M. (2004). Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature 427, 78–83 10.1038/nature02211 [DOI] [PubMed] [Google Scholar]
- Silva R., Mesquita A. R., Bessa J., Sousa J. C., Sotiropoulos I., Leao P., Almeida O. F., Sousa N. (2008). Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3beta. Neuroscience 152, 656–669 10.1016/j.neuroscience.2007.12.026 [DOI] [PubMed] [Google Scholar]
- Snyder J. S., Hong N. S., McDonald R. J., Wojtowicz J. M. (2005). A role for adult neurogenesis in spatial long-term memory. Neuroscience 130, 843–852 10.1016/j.neuroscience.2004.10.009 [DOI] [PubMed] [Google Scholar]
- Snyder J. S., Soumier A., Brewer M., Pickel J., Cameron H. A. (2011). Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461 10.1038/nature10287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H., Yu I. T., Hwang S. J., Kim J. S., Lee S. H., Lee Y. S., Kaang B. K. (2003). Lithium enhances long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus. J. Neurochem. 85, 872–881 10.1046/j.1471-4159.2003.01869.x [DOI] [PubMed] [Google Scholar]
- Stambolic V., Ruel L., Woodgett J. (1996). Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 6, 1664–1668 10.1016/S0960-9822(02)70790-2 [DOI] [PubMed] [Google Scholar]
- Stuebner S., Faus-Kessler T., Fischer T., Wurst W., Prakash N. (2010). Fzd3 and Fzd6 deficiency results in a severe midbrain morphogenesis defect. Dev. Dyn. 239, 246–260 [DOI] [PubMed] [Google Scholar]
- Suh H., Deng W., Gage F. H. (2009). Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 25, 253–275 10.1146/annurev.cellbio.042308.113256 [DOI] [PubMed] [Google Scholar]
- Tanizawa Y., Kuhara A., Inada H., Kodama E., Mizuno T., Mori I. (2006). Inositol monophosphatase regulates localization of synaptic components and behavior in the mature nervous system of C. elegans. Genes Dev. 20, 3296–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker-Varia S., Jean Y. Y., Parikh P., Sizer C. F., Jernstedt Ayer J., Parikh A., Hyde T. M., Buyske S., Alder J. (2010). The neuropeptide VGF is reduced in human bipolar postmortem brain and contributes to some of the behavioral and molecular effects of lithium. J. Neurosci. 30, 9368–9380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. (1990). Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature 346, 847–850 10.1038/346847a0 [DOI] [PubMed] [Google Scholar]
- Udo H., Yoshida Y., Kino T., Ohnuki K., Mizunoya W., Mukuda T., Sugiyama H. (2008). Enhanced adult neurogenesis and angiogenesis and altered affective behaviors in mice overexpressing vascular endothelial growth factor 120. J. Neurosci. 28, 14522–14536 10.1523/JNEUROSCI.3673-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A. (2009). Lithium and neuropsychiatric therapeutics: neuroplasticity via glycogen synthase kinase-3beta, beta-catenin, and neurotrophin cascades. J. Pharmacol. Sci. 110, 14–28 10.1254/jphs.09R02CR [DOI] [PubMed] [Google Scholar]
- Wang Y., Huso D., Cahill H., Ryugo D., Nathans J. (2001). Progressive cerebellar, auditory, and esophageal dysfunction caused by targeted disruption of the frizzled-4 gene. J. Neurosci. 21, 4761–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt J. L., Duman R. S. (2007). VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc. Natl. Acad. Sci. U.S.A. 104, 4647–4652 10.1073/pnas.0610282104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watcharasit P., Bijur G. N., Song L., Zhu J., Chen X., Jope R. S. (2003). Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J. Biol. Chem. 278, 48872–48879 10.1074/jbc.M305870200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wend P., Holland J. D., Ziebold U., Birchmeier W. (2010). Wnt signaling in stem and cancer stem cells. Semin. Cell Dev. Biol. 21, 855–863 10.1016/j.semcdb.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Wexler E. M., Geschwind D. H., Palmer T. D. (2008). Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol. Psychiatry 13, 285–292 10.1038/sj.mp.4002093 [DOI] [PubMed] [Google Scholar]
- Wexler E. M., Paucer A., Kornblum H. I., Palmer T. D., Geschwind D. H. (2009). Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells 27, 1130–1141 10.1002/stem.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A. M., Lyuksyutova A. I., Fenstermaker A. G., Shafer B., Lo C. G., Zou Y. (2008). Phosphatidy linositol-3-kinase-atypical protein kinase C signaling is required for Wnt attraction and anterior-posterior axon guidance. J. Neurosci. 28, 3456–3467 10.1523/JNEUROSCI.0029-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhead G. J., Mutch C. A., Olson E. C., Chenn A. (2006). Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J. Neurosci. 26, 12620–12630 10.1523/JNEUROSCI.3180-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Pan W. (2010). GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 35, 161–168 10.1016/j.tibs.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Saint-Jeannet J. P., Klein P. S. (2003). Wnt-frizzled signaling in neural crest formation. Trends Neurosci. 26, 40–45 10.1016/S0166-2236(02)00011-5 [DOI] [PubMed] [Google Scholar]
- Yeung T. M., Chia L. A., Kosinski C. M., Kuo C. J. (2011). Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell. Mol. Life Sci. 68, 2513–2523 10.1007/s00018-011-0687-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York J. D., Guo S., Odom A. R., Spiegelberg B. D., Stolz L. E. (2001). An expanded view of inositol signaling. Adv. Enzyme Regul. 41, 57–71 10.1016/S0065-2571(00)00025-X [DOI] [PubMed] [Google Scholar]
- Yu J. M., Kim J. H., Song G. S., Jung J. S. (2006). Increase in proliferation and differentiation of neural progenitor cells isolated from postnatal and adult mice brain by Wnt-3a and Wnt-5a. Mol. Cell. Biochem. 288, 17–28 10.1007/s11010-005-9113-3 [DOI] [PubMed] [Google Scholar]
- Yu X., Malenka R. C. (2003). Beta-catenin is critical for dendritic morphogenesis. Nat. Neurosci. 6, 1169–1177 10.1038/nn1132 [DOI] [PubMed] [Google Scholar]
- Zechner D., Fujita Y., Hulsken J., Muller T., Walther I., Taketo M. M., Crenshaw E. B., III, Birchmeier W., Birchmeier C. (2003). beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev. Biol. 258, 406–418 10.1016/S0012-1606(03)00123-4 [DOI] [PubMed] [Google Scholar]
- Zeng L., Fagotto F., Zhang T., Hsu W., Vasicek T. J., Perry W. L., Lee J. J., Tilghman S. M., Gumbiner B. M., Costantini F. (1997). The mouse fused locus encodes axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90, 181–192 10.1016/S0092-8674(00)80324-4 [DOI] [PubMed] [Google Scholar]
- Zhang F., Phiel C. J., Spece L., Gurvich N., Klein P. S. (2003). Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J. Biol. Chem. 278, 33067–33077 10.1074/jbc.M212635200 [DOI] [PubMed] [Google Scholar]