Abstract
Purpose of review
This review describes the evolution of the clinical criteria for Alzheimer’s disease over the past 25 years, with special emphasis on those recently published that have incorporated the use of biomarkers.
Recent findings
One of the most important advances in the knowledge of Alzheimer’s disease was the development of cerebrospinal fluid, PET and MRI biomarkers. These have shown that the Alzheimer’s disease is present in cognitively normal individuals, suggesting that there is a long incubation process that precedes the onset of the symptoms. Although there are diagnostic criteria for Alzheimer’s disease, the National Institute on Aging and the Alzheimer’s Association has proposed a set of diagnostic criteria oriented to provide a unified vision of the pathological process from preclinical, to mild cognitive impairment, and to full-blown dementia. These new criteria take advantage of different biomarkers to support the clinical diagnosis of the different stages of the disease.
Summary
The new guidelines provide a definition of the dementia syndrome and core diagnostic features to be used in research and clinical practice, although they caution about the use of biomarkers, since they still require validation, and the longitudinal interaction and dynamics of these biomarkers in relationship to the manifestation of the symptoms are not fully understood.
Keywords: Alzheimer’s disease, dementia, diagnostic criteria, mild cognitive impairment, preclinical Alzheimer’s disease
Introduction
The improvement in life expectancy in practically every population around the world has led the prevalence of Alzheimer’s disease to rise to epidemic proportions. It is estimated that 6–7% of the individuals aged above 65 are affected, and Alzheimer’s disease represents 80% of the dementia in this age group [1]; approximately 45% of the population aged above 85 have dementia [2]. Therefore, there are continuing efforts to better understand the physiopathology of the disease and to develop therapies, for which it is essential to have accurate and reliable clinical diagnoses.
As the search for useful diagnostic criteria moves towards the earliest manifestations of the disease, it is increasingly important to explicitly distinguish between the neuropathological disease – in this case Alzheimer’s disease –and its clinical manifestation (i.e. mild cognitive impairment, dementia). Thus, the goal of the clinical criteria has been to maximize the likelihood of identifying the pathological disease. This is critical to bear in mind, and to recall that it has only been in the past 10–15 years that increasingly valid biomarkers for Alzheimer’s disease have been developed.
During the past 30 years specific diagnostic criteria for Alzheimer’s disease have allowed researchers to conduct pathological and clinical studies, and to compare incidence and prevalence of dementia across multiple populations. Here we will discuss the evolution of the clinical diagnosis of Alzheimer’s disease, especially the newly published criteria that incorporate the use of biomarkers [3•].
Dementia
The first step in the diagnostic process of Alzheimer’s disease is to determine whether a patient is actually demented. Although the syndrome of dementia has been described in medical writings for centuries [4], at the time that Alzheimer described his case, the concept of dementia involved behavioral and cognitive symptoms [5], and there was an emphasis on nondegenerative causes (e.g. neurosyphilis). Over time, the central component of the syndrome shifted towards cognitive symptoms, and the presumed cause evolved primarily from nondegenerative diseases to atherosclerosis to neurodegeneration, and to a better appreciation of the variety of pathologies that result in dementia [6].
Over the past 30 years, the bases of the cognitive syndrome of dementia have been driven by the criteria proposed by the Diagnostic and Statistical Manual of Mental Disorders (DSM) [7–10] (see Table 1); for the diagnosis of dementia there must be impairments in memory and one additional cognitive domain. These cognitive deficits can cause significant impairment in social or occupational functioning, and represent a significant decline from previous levels of functioning. These DSM criteria are still being used in clinical practice and research, although it is expected that there will be major modifications in the fifth edition of the manual in 2013.
Table 1.
Cognitive features of different dementia criteria used for clinical practice and research
| Impairments | DSM-III (1980) to DSM-IV-TR (2001) | ICD-10 (1994) | NIA-AA (2010) | ADRC of Pittsburgh (1984) |
|---|---|---|---|---|
| Memory + one cognitive domain | Yes | |||
| Memory + two domainsa | Yesa,b | |||
| Two domains or more, not necessarily memory impaired | Yesc | Yes |
ADRC, Alzheimer’s Disease Research Center.
Memory (verbal + nonverbal) + abstract thinking, judgment and problem solving + other cognitive domain.
Behavioral symptoms strengthen the diagnosis.
One of the domains could be behavioral abnormalities.
The International Classification of Disease (ICD-10) has a more strict definition for dementia, requiring that memory and abstract thinking, judgement and problem solving all are impaired, as well as one additional cognitive domain. Although these criteria have a high specificity for dementia, they have low sensitivity because cases with mild disease are missed. For example, in one study, the prevalence of dementia by DSM-IV was 13.7%, whereas using ICD-10 it was only 3.1% [11].
Because not all dementia patients experience memory deficits as the initial symptom, it seems reasonable that the definition of dementia should be less restrictive [12]. For example, since 1984, the Alzheimer’s Disease Research Center at the University of Pittsburgh has required impairments in two cognitive domains for the diagnosis of dementia, but these do not have to include memory functions [13]. Using these criteria, we have a sensitivity of 98% and specificity of 88% relative to autopsy [13]. The recently developed National Institute on Aging – Alzheimer’s Association (NIA-AA) criteria for all-cause dementia takes a similar approach requiring deficits in any two of the following domains: memory, judgement/problem-solving, visuospatial, language, and behavior (e.g. agitation) [3•].
Dementia and Alzheimer’s disease
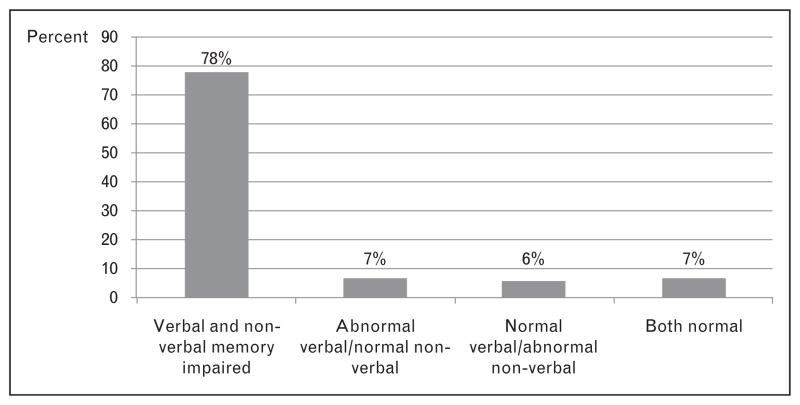
Although the memory-centric DSM criteria seemed the perfect fit for the diagnosis of Alzheimer’s disease, they lack sensitivity for Alzheimer’s disease syndromes [14–16] and other dementias (e.g. frontotemporal) that do not present with memory deficits [17]. Even Alzheimer’s disease cases without focal cognitive deficits can have a relative preservation of their memory functions [18]; 7% of the Alzheimer’s disease cases can have normal verbal and nonverbal memory, and 6% can have normal verbal memory with abnormal nonverbal memory (see Fig. 1). This point is particularly important for clinicians and epidemiologists as it points out the diagnostic problems when only verbal memory measures are used for diagnosis.
Figure 1.
Material-specific memory loss in 194 patients with the diagnosis of probable Alzheimer’s disease
Evolution of the diagnostic criteria for Alzheimer’s disease
The concept of the chronic organic brain syndrome codified in the DSM in 1952 [19] and DSM-II in 1968 [20] evolved to current criteria for dementia and Alzheimer’s disease in the third [7] and subsequent editions (DSM-III-R, DSM-IV and DSM-IV-TR) [8–10], and to the 1984 NINCDS-ADRDA clinical criteria [21]. What was critical about these latter criteria, and is often forgotten, is that they ranked the diagnosis in terms of the certainty that the dementia actually was caused by Alzheimer’s disease pathology. The grade of certainty ‘Definite Alzheimer’s disease’ was reserved for cases with neuropathological confirmation (usually at autopsy). ‘Probable Alzheimer’s disease’ was the term used to describe the clinical syndrome most likely expected in the context of Alzheimer’s disease, whereas ‘Possible Alzheimer’s disease’ was used when the patient had the core clinical symptoms for Alzheimer’s disease but there was evidence of other disease processes that in and of themselves could account for the cognitive deficits.
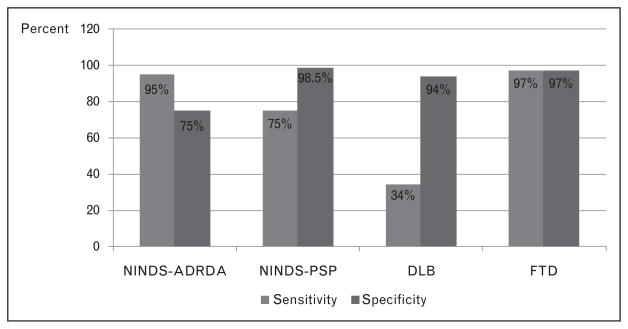
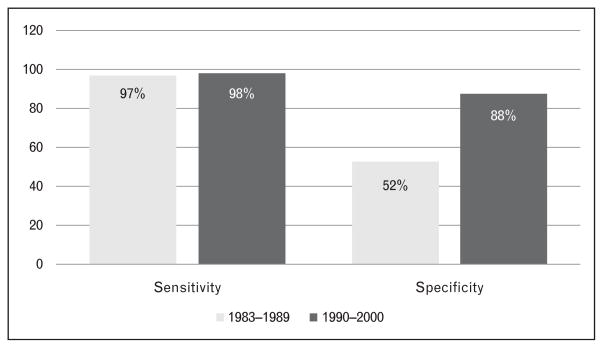
As the clinical criteria for Alzheimer’s disease become more specific, investigators began to test the validity of these multiple research diagnostic criteria. The sensitivity of the clinical diagnosis of Alzheimer’s disease tended to be higher when more detailed clinical criteria were used, and when the study was conducted in referral clinics [13,22–38] compared to population [39–41] or hospital-based studies [42–44] (see Table 2). However, the increased knowledge of the Alzheimer’s disease phenotype and the introduction of clinical criteria for other dementia syndromes in the 1990s [45–50] resulted in an increase in the accuracy of the diagnosis of Alzheimer’s disease. Although the sensitivity of the clinical diagnosis for Alzheimer’s disease improved after 1990, the specificity improved dramatically (Table 2). One study simultaneously tested the diagnostic criteria for neurodegenerative dementias [Alzheimer’s disease, progressive supranuclear palsy, Lewy body dementia (LBD), frontotemporal dementia (FTD)], and found good sensitivity and specificity for all except LBD (low sensitivity and high specificity) (see Fig. 2) [51]. This was also reflected in large longitudinal series; for example, the specificity for Alzheimer’s disease at the ADRC of Pittsburgh rose to 88% in the 1990s [13] (Fig. 3).
Table 2.
Sensitivity and specificity for Alzheimer’s disease before and after 1990a
| Authors | Clinical criteria | Number of cases | Sensitivity | Specificity |
|---|---|---|---|---|
| Before 1990 | ||||
| Todorov et al. (1975) [43] | Clinical diagnosis | 726 | 43% | 19% |
| Mölsa et al. (1985) [44] | Clinical diagnosis | 58 | 71% | 73% |
| Alafuzoff et al. (1987) [42] | DSM-III | 55 | 63% | N/A |
| Wade et al. (1987) [30] | Standardized criteria | 65 | 87% | 78% |
| Joachim et al. (1988) [27] | Standardized criteria | 150 | 87% | N/A |
| Boller et al. (1989) [23] | NINCDS-ADRDA | 54 | 95% | 33% |
| After 1990 | ||||
| Kukul et al. (1990) [28] | NINCDS-ADRDA | 62 | 92% | 65% |
| DSM-III | 76% | 80% | ||
| Jellinger et al. (1990) [25] | NINCDS-ADRDA | 675 | 85% | N/A |
| Burns et al. (1990) [22] | NINCDS-ADRDA | 50 | 84% | N/A |
| Mendez et al. (1991) [29] | Clinical diagnosis | 394 | 88% | N/A |
| Kazee et al. (1993) [33] | NINCDS-ADRDA | 123 | 98% | 69% |
| Galasko et al. (1994) [26] | NINCDS-ADRDA | 151 | 86% | N/A |
| Blacker et al. (1994) [24] | NINCDS-ADRDA | 60 | 81% | 73% |
| Gearing et al. (1995) [34] | NINCDS-ADRDA | 106 | 87% | N/A |
| Kosunen et al. (1996) [31] | NINCDS-ADRDA | 56 | 96% | N/A |
| Jobst et al. (1998) [35] | NINCDS-ADRA | 151 | 96% | 61% |
| DSM-III-R | 51% | 97% | ||
| Nagy et al. (1998) [36] | NINCDS-ADRA | 73 | 98% | 61% |
| Holmes et al. (1999) [39] | NINCDS-ADRDA | 80b | 66% | 75% |
| Lim et al. (1999) [40] | NINCDS-ADRDA | 134b | 75% | 54% |
| Massoud et al. (1999) [37] | NINCDS-ADRDA | 63 | 98% | 84% |
| Lopez et al. (2000) [13] | NINCDS-ADRDA | 295 | 98% | 88% |
| Petrovich et al. (2001) [41] | NINCDS-ADRDA | 79b | 87% | N/A |
| Hogervorst et al. (2003) [38] | NINCDS-ADRDA | 204 | 92% | 69% |
N/A, not available.
Clinicopathological studies with 50 or more patients.
Population-based study.
Figure 2.
Accuracy of four clinical diagnostic criteria for the diagnosis of neurodegenerative dementias
Figure 3.
Sensitivity and specificity for Alzheimer’s disease using standardized clinical criteria at the Alzheimer’s Disease Research Center of Pittsburgh
Revised NINCDS-ADRDA criteria for probable Alzheimer’s disease
The increased knowledge of the natural history of Alzheimer’s disease, the development of positive bio-markers, and the need to better characterize Alzheimer’s disease patients in the earliest, even predementia stage led to a proposed modification of the criteria for probable Alzheimer’s disease [52]. The proposed criteria require the presence of gradual and progressive change in memory function, characterized by deficits in memory storage [53]. These criteria elevated the importance of biomarkers and genotypes to support the diagnosis of Alzheimer’s disease by requiring that the Alzheimer’s disease phenoptype should include one of the following: atrophy in the medial temporal lobe structures in the MRI, an abnormal cerebrospinal fluid (CSF) study, an ‘Alzheimer’s disease pattern’ on positron emission tomography (PET) studies [i.e. 2-[18F]-fluoro-2-deoxy-D-glucose (FDG) or with amyloid ligands], or the presence of an autosomal dominant mutation in the immediate family.
These revised criteria also addressed the issue of prodromal or preclinical Alzheimer’s disease; these mildly affected patients can be classified as probable Alzheimer’s disease when they have an isolated memory deficit and at least one of the biomarkers described above. The term ‘definite’ Alzheimer’s disease was again used for patients with pathologically confirmed Alzheimer’s disease, and those living patients with Alzheimer’s disease clinical symptoms and genetic evidence of Alzheimer’s disease (mutations in chromosome 1, 14, or 21).
NIA-AA criteria for Alzheimer’s disease
Following the publication of the revised criteria for probable Alzheimer’s disease, the NIA and the Alzheimer’s Association organized a workgroup to revise the entire NINCDS-ADRDA criteria by integrating advances in the knowledge of the disease into the decision tree, and to provide general practitioners with the tools to make the diagnosis at the bedside. These NIA-AA criteria retained the degrees of certainty that the clinical syndrome represented the neurodegenerative condition, that is probable and possible Alzheimer’s disease (see Table 3).
Table 3.
Relevant aspects of the NINCDS-ADRDA and NIA-AA diagnostic criteria for Alzheimer’s disease
| NINCDS-ADRDA (1984) | NIA-AA (2011) | |
|---|---|---|
| Dementia criteria | Not provided | Provides criteria for all-cause dementia |
| Probable AD | ||
| Dementia diagnosis | Impairments in two cognitive domains based on clinical exam and documented by cognitive testing [1] | Impairments in two cognitive domains, and expands the definition on the nonmemory forms of AD (language, visuospatial, executive) [1] |
| Onset and progression | Progressive worsening of memory symptoms and other cognitive functions [1] | Insidious onset and clear-cut history of worsening of cognition by report or observation [1] |
| Comorbid systemic or neurological disorders | Absence of systemic or neurological disorders that in and of themselves could account for the cognitive deficits [1] | Absence of cerebrovascular disease or other neurological, nonneurological comorbidities or use of medication that could have substantial effect on cognition [1] |
| Age | Between ages 40 and 90 [1] | No age limitation |
| Behavioral and neurological symptoms | Altered pattern of behavior and mood-related disorders (e.g. depression), increased muscle tone, myoclonus, gait disorders | Mood-related and behavioral symptoms are considered a ‘domain’ in the definition of dementia |
| Level of consciousness | The diagnosis of AD cannot be made in patients with delirium, drowsiness, stupor/coma, or other abnormality that prevent adequate evaluation. | Symptoms cannot be explained by delirium or other major psychiatric disorder |
| Laboratory test | Normal lumbar puncture and blood tests. CT scan normal or with atrophy | Not stated |
| Biomarkers | Not available in 1984 | MRI, PET, and CSF studies. Any biomarker positive increases the certainty of AD in patients with probable AD. Recommended only for research purposes or clinical trials |
| Familial forms | Familial history of similar disorders, particularly if confirmed by autopsy supports the diagnosis of AD | Evidence of a causative gene (APP, PSEN1, and PSEN2) increases the likelihood of AD pathology. The APOE-4 allele is not sufficiently specific to be considered in this category |
| Possible AD | ||
| Comorbid conditions | Presence of systemic or neurological disorders that in and of themselves could account for the cognitive deficits, which is not considered to be the cause of the dementia | Meets clinical criteria for AD but there is cerebrovascular disease, or other neurological or non-neurological comorbidities, or use of medication that could have substantial effect on cognition |
| Atypical presentations | Presence of variations in the presentation, onset, or clinical course | Presence of atypical course, sudden onset, or there is insufficient historical detail or documentation of progressive decline |
| Single cognitive domain | Presence of a single cognitive deficit in the absence of other identifiable cause | Replaced by MCI |
| Non-AD phenotype | Not addressed | At least two biomarker categories positive (Aβ CSF, tau CSF, PET, or MRI) to support the presence of underlying AD pathology |
AD, Alzheimer’s disease; CSF, cerebrospinal fluid; MCI, mild cognitive impairment.
The NIA-AA criteria for dementia require deficits in two cognitive domains, not necessarily memory, and they also included a description of each domain for use in clinical practice. This is an improvement over the NINCDS-ADRDA criteria that stated that the dementia syndrome was present only when patients had deficits in two cognitive domains, but they were less clear whether memory had to be affected. This type of broader definition of dementia provides higher sensitivity and specificity for the Alzheimer’s disease diagnosis [13].
The inclusion of biomarkers in the criteria brings to the classification of Alzheimer’s disease aspects of its pathophysiology (see also [52]). Low amyloid (A)β-42 and high tau protein levels in CSF [54,55], decreased cerebral metabolism [56], decreased mesial temporal and parietal lobe volumes [57,58], and increased amyloid deposition in the brain with PET amyloid ligands [59] have been replicated in multiple studies. It is believed that these biomarkers measure two aspects of the disease: amyloid deposition (i.e. low CSF Aβ-42 levels and positive PET amyloid imaging), and neuronal damage (i.e. high tau protein levels in CSF, decreased cerebral glucose metabolism, and disproportionate temporal/parietal atrophy). However, there is still a lack of standardization of the technologies and limited access to them by the medical community. For example, there is a large standard deviation in CSF tau and Aβ-42 levels in cases with definite Alzheimer’s disease [60], and whereas volumetric MRI studies have consistently showed that specific areas of the brain are affected in Alzheimer’s disease and mild cognitive impairment (MCI) [61], visual MRI ratings have shown poor accuracy and inter-rater reliability [62] (cf. [63]). Therefore, biomarkers are, at present, most useful for research or clinical trials, or in limited clinical circumstances.
The original NINCDS-ADRDA criteria assumed that Alzheimer’s disease occurred in the 40–90 years age range and that patients with age of onset below 65 should be considered a separate subgroup – both of these were rejected by the new criteria. Considering age as a part of the diagnostic criteria had been a holdover from the 1960s–1970s when Alzheimer’s disease was a presenile syndrome and dementia after age 65 was referred to as senile dementia of Alzheimer’s type, and there was little knowledge about the prevalence of dementia in the old-old (age >85). It is now clear that the neuropathology and clinical presentation of Alzheimer’s disease is unrelated to age [64,65], and that the central underlying pathology is similar in familial cases with early (age <40 years) and late-onset Alzheimer’s disease [66,67].
Possible Alzheimer’s disease
The term ‘possible Alzheimer’s disease’ was used for patients with atypical presentation or clinical course, when another disease process that could cause cognitive disorders was present, or when there was present a progressive deficit in a single cognitive domain [21]. The NIA-AA criteria have redefined this classification, and considered possible Alzheimer’s disease when the patient has sudden onset of symptoms; insufficient historical detail to document progression of symptoms; evidence of concomitant disorders; or, medication use that can affect cognition. It should be noted that possible Alzheimer’s disease can evolve to probable Alzheimer’s disease if the concomitant condition resolves in the context of progressive dementia.
The NIA-AA criteria also recognize that two conditions that can cause dementia may coexist. Patients with core Alzheimer’s disease features may have signs and symptoms of other neurodegenerative processes (e.g. LBD), and patients with dementia syndomes (e.g. FTD) can have the biomarkers for Alzheimer’s disease. This approach is used successfully in research clinics where patients were classified primarily based on the NINCDS-ADRDA clinical criteria for Alzheimer’s disease, and secondarily according to the specific diagnostic criteria for other disease processes (e.g. DLB) [68].
The original NINDCS-ADRDA criteria classified patients with deficits in a single cognitive domain as possible Alzheimer’s disease; this has been revised by the NIA-AA criteria, and these patients are now classified as MCI. However, these new criteria recommend that when there is significant interference in the ability to function, clinicians should use their own judgment to distinguish MCI from a dementia syndrome when only a single cognitive domain affected. This change in the use of possible Alzheimer’s disease has important conceptual implications, perhaps the most important of which is that it assumes that MCI is preclinical Alzheimer’s disease.
Mild cognitive impairment
As patients progress to dementia they go through a state of mild cognitive deficit, and multiple diagnostic criteria have been proposed to characterize this transitional state [69–74]. The MCI with memory loss is the most similar to Alzheimer’s disease, and these patients are the most likely to develop Alzheimer’s disease [75]. However, epidemiological studies have shown that the ‘pure’ MCI-amnestic-type (i.e. idiopathic amnesia) has a low prevalence in the general population compared to those patients with a much wider range of cognitive impairments [76,77], and MCI patients without memory deficits can also progress to Alzheimer’s disease [78]. The initial memory-based criteria for MCI [75] were expanded in 2004 to include all the possible cognitive manifestations of the syndrome (see Table 4) [79,80].
Table 4.
Evolution of the construct of the MCI criteria
| MCI: Petersen et al. (1999) [75] | MCI: Petersen (2004) [79] | MCI: Winblad et al. (2004) [80] | NIA-AA: MCI due to Alzheimer’s disease (2011) [81•] | |
|---|---|---|---|---|
| Cognitive complaints | By patient or informant | By patient or informant | By patient or informant | By patient or informant |
| Memory deficits detected on testing | Abnormal memory for age | Abnormal memory for age | Abnormal memory for age | Abnormal memory for age. Memory tests 1–1.5 SD below the norms |
| Other cognitive domains affected | Normal | Four subtypes:
|
Three subtypes:
|
Impairments in other domains could be present |
| Activities of daily living | Normal | Normal or they could be minimally affected | Normal or they could be minimally affected | Normal; complex tasks could be minimally affected |
| Clinical impression | Not demented | Not demented | Not demented | Not demented |
MCI, mild cognitive impairment.
With or without memory impairments.
The NIA-AA criteria for MCI were developed primarily to identify a syndrome that had a high likelihood of being caused by Alzheimer’s disease pathology [81•] and are reminiscent of what had been referred to as ‘very mild Alzheimer’s disease’ [82,83]. These criteria state that there must be evidence of impairment in one or more cognitive domains, typically including memory, and there are groupings only for patients with memory impaired or memory + other cognitive domains. The criteria maximize the likelihood that the syndrome is associated with Alzheimer’s disease by explicitly ruling out vascular, traumatic, or other causes of mild central nevous system dysfunction. However, given that all Alzheimer’s disease patients do not have memory loss at initial presentation, and that memory loss may not be the most severe deficit, this is a limiting aspect of the criteria.
The foundation of the recent NIA-AA MCI guidelines is the core clinical criteria (see Table 4). The general structure retains that established over the past 10 years but clarifies a few points to reflect the evolution of the MCI paradigm. Included in this are: the emphasis that a subjective cognitive complaint or change can come from the patient, an informant or the physician observing the patient; independence in functioning remains necessary but examples of mild changes in more ‘complex functional tasks’ that may be affected are provided; impairment in one or more cognitive domains, although episodic memory is emphasized as being the most commonly associated with the development of Alzheimer’s disease. Additional support for the diagnosis of MCI is provided when there is evidence of a decline on serial testing.
Whereas the identification of the ‘pure Alzheimer’s disease’ form of MCI is critical for research studies and clinical trials, the reality is that MCI patients with multiple comorbidities are the most common in clinical practice and they also progress to Alzheimer’s disease at the same rate as those without comorbidities [78]. Thus, even in the presence of a disease process that could explain the MCI syndrome, there is frequently an underlying neurodegenerative process that will eventually lead to the dementia symptoms. In this case the biomarkers have a critical role in identifying Alzheimer’s disease patients in the context of other pathologies, and thus improving treatment and management.
The use of biomarkers in the classification of MCI not only supports the presence of the Alzheimer’s disease pathology, but also increases the likelihood that the progression to dementia will occur within a relatively short period of time [84–87]. The NIA-AA criteria graded the certainty that the MCI is due to Alzheimer’s disease as follows: high likelihood: when both beta amyloid and neuronal damage biomarkers are present; intermediate likelihood: when the core clinical symptoms are present and there is a single positive biomarker, either amyloid deposition or neuronal damage; and unlikely due to Alzheimer’s disease: neither types of biomarkers are positive. However, the authors cautioned against the use of combination biomarkers until more experience is gained in this respect. Indeed, the Alzheimer’s Disease Neuroimaging Initiative found that over a short term, single marker models were as effective as multiple marker models, and their accuracy was only 64% [88].
Preclinical Alzheimer’s disease
The pathology of Alzheimer’s disease likely starts decades before the onset of the clinical syndrome, and cognitively normal elderly individuals who later develop Alzheimer’s disease have a different cognitive performance than those who do not [89,90]. Pathological [91,92] and amyloid imaging studies [93,94] find cognitively normal individuals with Alzheimer’s disease neuropathology. The NIA-AA work group position is that Alzheimer’s disease is a pathological–clinical continuum that starts with the amyloid deposition in cognitively normal individuals and gradually progresses to clinical dementia [95•]. Therefore, three disease stages were proposed: stage 1: normal cognition with positive cerebral amyloidosis by CSF or amyloid ligand studies, and with normal markers of neuronal damage; stage 2: normal cognition with cerebral amyloidosis and markers of downstream neurodegeneration; and stage 3: subtle cognitive change with cerebral amyloidosis and markers of neurodegeneration. The latter stage includes individuals who are in the borderzone between normal and MCI; ‘not normal’ and ‘not MCI’.
There are limitations in the preclinical staging because the cause of Alzheimer’s disease is complex, and the majority of the existing data are from cross-sectional or short-term follow-up studies. It is still not clear whether there is a single sequence of events in the pathological cascade during the preclinical phase. The central pathological event appears to be amyloid deposition, and this should precede damage to neurons. However, cognitively normal individuals aged 50–65, APOE-4+, have decreased cerebral metabolism in the brain regions usually affected by Alzheimer’s disease [96]. Thus, amyloid deposition and neuronal damage can progress in parallel, although the critical multimodal longitudinal data are lacking.
Conclusion
There have been significant advances in the understanding of the Alzheimer’s disease phenotype. The incorporation of biomarkers to clinical diagnostic criteria is a major step forward in research and clinical practice. However, studies to validate the new criteria will be difficult to perform due to the need for large numbers of individuals with biomarkers, and waiting for autopsy data. Nevertheless, small studies have been conducted, and a recent radiological–pathological study conducted in 26 institutionalized dementia patients showed that an amyloid ligand had 96% accuracy for Alzheimer’s disease [97].
It is not clear whether there is a single or multiple underlying causes of Alzheimer’s disease, and consequently a single specific biomarker may not be possible. However, the clinical observations conducted over the past 30 years have led to the development of clinical criteria that can detect the underlying disease with high accuracy. The inclusion of biomarkers and the characterization of a prodromal Alzheimer’s disease phenotype represent an important step forward in the new conceptualization of the diagnosis of the disease. The strict clinical definition makes these criteria suitable for research purposes in which homogeneous groups are needed. By including these biomarkers, we can constrain the patients who are included in clinical trials, and maximize the likelihood that we are studying only Alzheimer’s disease pathology. However, with the increased reliance on such biological tools, it is critical to remember the admonition of German Berrios [6]:
So, paradoxically, the more that scientific detail has accumulated on Alzheimer’s disease, the more elusive the ‘illness’ has become. In an effort to make it a separate entity, the creators of the disease may have narrowed its clinical boundaries unduly, so that current research workers are trapped in the vicious circle of only finding what they themselves put there in the first place’ (p. 7).
Key points.
The evolution of the clinical criteria for Alzheimer’s disease has improved significantly the sensitivity and specificity for the disease.
The use of biomarkers improves detection of the Alzheimer’s disease pathology before the development of the symptoms of dementia.
The use of biomarkers is limited to research studies and clinical trials at this time.
Acknowledgments
Preparation of this manuscript was supported, in part, by funds from the National Institute on Aging (AG20098 and AG05133). M.R. is supported by a grant from Fundación Caja Madrid (Spain).
Footnotes
Conflicts of interest
O.L.L. has served as a consultant for Lundbeck and Johnson & Johnson.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 653–654).
- 1.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzpatrick AL, Kuller LH, Ives D, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 3•.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. New clinical criteria for all-cause dementia and for Alzheimer’s disease. These criteria incorporate CSF and radiological biomarkers to support the diagnosis of Alzheimer’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berrios GE. The history of mental symptoms. Descriptive psychopathology since the nineteenth century. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- 5.Alzheimer A. Uber eine eigenartige Erkrankung der Hirnrinde. Allg Zeits f Psychiat. 1907;64:146–148. [Google Scholar]
- 6.Berrios GE, Freeman HL. Alzheimer and the dementias (Eponymists in Medicine) London: Royal Society of Medicine Services Ltd; 1991. [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. New York: APA; 1980. (DSM-III) [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and statistical manual on mental disorders. 3. Washington, DC: American Psychiatric Press; 1987. Revised (DSM-III-R) [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and statistic manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. (DSM-IV) [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistic Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- 11.Erkinjuntti T, Ostbye T, Steenhuis R, Hachinski V. The effect of different diagnostic criteria on the prevalence of dementia. N Engl J Med. 1997;337:1667–1674. doi: 10.1056/NEJM199712043372306. [DOI] [PubMed] [Google Scholar]
- 12.Becker JT, Boller F, Lopez OL, et al. The natural history of Alzheimer’s disease: description of study cohort and accuracy of diagnosis. Arch Neurol. 1994;51:585–594. doi: 10.1001/archneur.1994.00540180063015. [DOI] [PubMed] [Google Scholar]
- 13.Lopez OL, Becker JT, Klunk W, et al. Research evaluation and diagnosis of probable Alzheimer’s disease over the last two decades. I Neurology. 2000;55:1854–1862. doi: 10.1212/wnl.55.12.1854. [DOI] [PubMed] [Google Scholar]
- 14.Hof PR, Archin N, Osmand AP, et al. Posterior cortical atrophy in Alzheimer’s disease: analysis of a new case and re-evaluation of a historical report. Acta Neuropathol (Berl) 1993;86:215–223. doi: 10.1007/BF00304135. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JK, Head E, Kim R, et al. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- 16.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130 (Pt 10):2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 17.Neary D, Snowden J. Fronto-temporal dementia: nosology, neuropsychology, and neuropathology. Brain Cogn. 1996;31:176–187. doi: 10.1006/brcg.1996.0041. [DOI] [PubMed] [Google Scholar]
- 18.Becker JT, Lopez OL, Wess J. Material specific memory loss in probable Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1992;55:1177–1181. doi: 10.1136/jnnp.55.12.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and statistic manual of mental disorders. Washington, DC: American Psychiatric Association; 1952. [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistic manual of mental disorders. 2. Washington, DC: American Psychiatric Association; 1968. (DSM-II) [Google Scholar]
- 21.McKhann G, Drachman DA, Folstein MF, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Burns A, Luthert P, Levy R, et al. Accuracy of clinical diagnosis of Alzheimer’s disease. Br Med J. 1990;301:1026. doi: 10.1136/bmj.301.6759.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boller F, Lopez OL, Moossy J. Diagnosis of dementia: clinicopathological correlations. Neurology. 1989;39:76–79. doi: 10.1212/wnl.39.1.76. [DOI] [PubMed] [Google Scholar]
- 24.Blacker D, Albert MS, Bassett SS, et al. Reliability and validity of NINCDS-ADRDA criteria for Alzheimer’s disease. Arch Neurol. 1994;51:1198–1204. doi: 10.1001/archneur.1994.00540240042014. [DOI] [PubMed] [Google Scholar]
- 25.Jellinger K, Danielczyk W, Fischer P, Gabriel E. Clinicopathological analysis of dementia disorders in the elderly. J Neurol Sci. 1990;95:239–258. doi: 10.1016/0022-510x(90)90072-u. [DOI] [PubMed] [Google Scholar]
- 26.Galasko D, Hansen LA, Katzman R, et al. Clinical-neuropathological correlations in Alzheimer’s disease and related dementias. Arch Neurol. 1994;51:888–895. doi: 10.1001/archneur.1994.00540210060013. [DOI] [PubMed] [Google Scholar]
- 27.Joachim CL, Morris JH, Selkoe DJ. Clinically diagnosed Alzheimer’s disease: autopsy neuropathological results in 150 cases. Ann Neurol. 1988;24:50–56. doi: 10.1002/ana.410240110. [DOI] [PubMed] [Google Scholar]
- 28.Kukull WA, Larson EB, Reifler BV, et al. The validity of 3 clinical diagnostic criteria for Alzheimer’s disease. Neurology. 1990;40:1364–1369. doi: 10.1212/wnl.40.9.1364. [DOI] [PubMed] [Google Scholar]
- 29.Mendez MF, Mastri AR, Sung JH, et al. Neuropathologically confirmed Alzheimer’s disease: clinical diagnoses in 394 cases. J Geriatr Psychiatry Neurol. 1991;4:26–29. doi: 10.1177/089198879100400105. [DOI] [PubMed] [Google Scholar]
- 30.Wade JP, Mirsen TR, Hachinski VC, et al. The clinical diagnosis of Alzheimer’s disease. Arch Neurol. 1987;44:24–29. doi: 10.1001/archneur.1987.00520130016010. [DOI] [PubMed] [Google Scholar]
- 31.Kosunen O, Soininen H, Paljarvi L, et al. Diagnostic accuracy of Alzheimer’s disease: a neuropathological study. Acta Neuropathol (Berl) 1996;91:185–193. doi: 10.1007/s004010050412. [DOI] [PubMed] [Google Scholar]
- 32.Victoroff J, Mack WJ, Lyness SA, Chui HC. Multicenter clinicopathological correlation in dementia. Am J Psychiatry. 1995;152:1476–1484. doi: 10.1176/ajp.152.10.1476. [DOI] [PubMed] [Google Scholar]
- 33.Kazee AM, Eskin TA, Lapham LW, et al. Clinicopathologic correlates in Alzheimer disease: assessment of clinical and pathologic diagnostic criteria. Alzheimer Dis Assoc Disord. 1993;7:152–164. doi: 10.1097/00002093-199307030-00004. [DOI] [PubMed] [Google Scholar]
- 34.Gearing M, Mirra SS, Hedreen JC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part X. Neuropathology confirmation of the clinical diagnosis of Alzheimer’s disease. Neurology. 1995;45:461–466. doi: 10.1212/wnl.45.3.461. [DOI] [PubMed] [Google Scholar]
- 35.Jobst KA, Lin PD, Barnetson PD, Shepstone BJ. Accurate prediction of histologically confirmed Alzheimer’s disease and the differential diagnosis of dementia: the use on NINCDS-ADRDA and DSM-III-R criteria, SPECT, X-ray CT, and Apo E4 in medial temporal lobe dementias. Int Psychogeriatrics. 1998;10:271–302. doi: 10.1017/s1041610298005389. [DOI] [PubMed] [Google Scholar]
- 36.Nagy Z, Esiri MM, Hindley NJ, et al. Accuracy of clinical operational diagnostic criteria for Alzheimer’s disease in relation to different pathological diagnostic protocols. Dement Geriatr Cogn Disord. 1998;9:219–226. doi: 10.1159/000017050. [DOI] [PubMed] [Google Scholar]
- 37.Massoud F, Devi G, Stern Y, et al. A clinicopathological comparison of community-based and clinic-based cohorts of patients with dementia. Arch Neurol. 1999;56:1368–1373. doi: 10.1001/archneur.56.11.1368. [DOI] [PubMed] [Google Scholar]
- 38.Hogervorst E, Bandelow S, Combrinck M, et al. The validity and reliability of 6 sets of clinical criteria to classify Alzheimer’s disease and vascular dementia in cases confirmed postmortem: added value of a decision tree approach. Dement Geriatr Cogn Disord. 2003;16:170–180. doi: 10.1159/000071006. [DOI] [PubMed] [Google Scholar]
- 39.Holmes C, Cairns N, Lantos P, Mann A. Validity of current clinical criteria for Alzheimer’s disease, vascular dementia, and dementia with Lewy bodies. Br J Psychiatry. 1999;174:45–50. doi: 10.1192/bjp.174.1.45. [DOI] [PubMed] [Google Scholar]
- 40.Lim A, Tsuang D, Kukull W, et al. Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J Am Geriatr Soc. 1999;47:564–569. doi: 10.1111/j.1532-5415.1999.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 41.Petrovich H, White LR, Ross GW, et al. Accuracy of clinical criteria for AD in the Honolulu-Asia Aging Study, a population-based study. Neurology. 2001;57:226–234. doi: 10.1212/wnl.57.2.226. [DOI] [PubMed] [Google Scholar]
- 42.Alafuzoff I, Igbal K, Friden H, et al. Histopathological criteria for progressive dementia disorders: clinical-pathological correlation and classification by multivariate data analysis. Acta Neuropathol (Berl) 1987;74:209–225. doi: 10.1007/BF00688184. [DOI] [PubMed] [Google Scholar]
- 43.Todorov A, Go R, Constantinidis J, Elston R. Specificity of the clinical diagnosis of dementia. J Neurol Sci. 1975;26:81–98. doi: 10.1016/0022-510x(75)90116-1. [DOI] [PubMed] [Google Scholar]
- 44.Molsa P, Paljarvi L, Rinne J, et al. Validity of clinical diagnosis in dementia. J Neurol Neurosurg Psychiatry. 1985;48:1085–1090. doi: 10.1136/jnnp.48.11.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKeith IG, Fairbairn AF, Perry RH. Clinical diagnostic criteria for Lewy body dementia. Dementia. 1992;3:251–252. [Google Scholar]
- 47.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP International Workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Chui HC, Victoroff JI, Margolin D, et al. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 49.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 50.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2000;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 51.Lopez OL, Litvan I, Catt KE, et al. Accuracy of four clinical diagnostic criteria for the diagnosis of neurodegenerative dementias. Neurology. 1999;53:1292–1299. doi: 10.1212/wnl.53.6.1292. [DOI] [PubMed] [Google Scholar]
- 52.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 53.Delis DC, Wetter SR, Jacobson MW, et al. Recall discriminability: utility of a new CVLT-II measure in the differential diagnosis of dementia. J Int Neuropsychol Soc. 2005;11:708–715. doi: 10.1017/S1355617705050812. [DOI] [PubMed] [Google Scholar]
- 54.Andreasen N, Vanmechelen E, Van de Voorde A, et al. Cerebrospinal fluid tau protein as a biochemical marker for Alzheimer’s disease: a community based follow up study. J Neurol Neurosurg Psychiatry. 1998;64:298–305. doi: 10.1136/jnnp.64.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galasko D, Chang L, Motter R, et al. High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol. 1998;55:937–945. doi: 10.1001/archneur.55.7.937. [DOI] [PubMed] [Google Scholar]
- 56.Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. J Am Med Assoc. 2001;286:2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 57.Thompson PM, Moussai J, Zohoori S, et al. Cortical variability and asymmetry in normal aging and Alzheimer’s disease. Cereb Cortex. 1998;8:492–509. doi: 10.1093/cercor/8.6.492. [DOI] [PubMed] [Google Scholar]
- 58.Jack CR, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compond-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 60.Clark CM, Xie S, Chittams J, et al. Cerebrospinal fluid tau and beta-amyloid. How well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol. 2003;60:1696–1702. doi: 10.1001/archneur.60.12.1696. [DOI] [PubMed] [Google Scholar]
- 61.Thompson PM, Hayashi KM, Dutton RA, et al. Tracking Alzheimer’s disease. In: De Leon MJ, Federoff H, Hirsch J, et al., editors. Proceedings of the New York Academy of Sciences. 2006. Special Issue on Imaging and the Aging Brain. [Google Scholar]
- 62.Raji CA, Lee C, Lopez OL, et al. Initial experience in using continuous arterial spin-labeled MR imaging for early detection of Alzheimer disease. AJNR Am J Neuroradiol. 2010;31:847–855. doi: 10.3174/ajnr.A1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duara R, Loewenstein DA, Potter E, et al. Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer disease. Neurology. 2008;71:1986–1992. doi: 10.1212/01.wnl.0000336925.79704.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terry RD, Davies P. Dementia of the Alzheimer type. Annu Rev Neurosci. 1980;3:77–95. doi: 10.1146/annurev.ne.03.030180.000453. [DOI] [PubMed] [Google Scholar]
- 65.Sulkava R. Alzheimer’s disease and senile dementia of Alzheimer type. Acta Neurol Scand. 1982;26:81–98. doi: 10.1111/j.1600-0404.1982.tb03117.x. [DOI] [PubMed] [Google Scholar]
- 66.Shepherd C, McCann H, Halliday GM. Variations in the neuropathology of familial Alzheimer’s disease. Acta Neuropathol. 2009;118:37–52. doi: 10.1007/s00401-009-0521-4. [DOI] [PubMed] [Google Scholar]
- 67.Klunk W, Price JC, Mathis CA, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopez OL, Becker JT, Kaufer DI, et al. Research evaluation and prospective diagnosis of dementia with Lewy bodies. Arch Neurol. 2002;59:43–46. doi: 10.1001/archneur.59.1.43. [DOI] [PubMed] [Google Scholar]
- 69.Kral VA. Senescent forgetfullness: benign and malignant. Br Med J. 1962;304:5–6. [PMC free article] [PubMed] [Google Scholar]
- 70.Crook TH, Bartus RT, Ferris SH, et al. Age-associated memory impairment: proposed diagnostic criteria and measures of clinical change. Report of a National Institute of Mental Health Work Group. Dev Neuropsychol. 1986;2:261–276. [Google Scholar]
- 71.Blackford RC, LaRue A. Criteria for diagnosing age associated memory impairment: proposed improvements from the field. Dev Neuropsychol. 1989;5:295–306. [Google Scholar]
- 72.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 73.Graham JE, Rockwood K, Beattie EL. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 74.Levy R. Aging-associated cognitive decline. From the Aging-Associated Cognitive Decline Working Party. Int Psychogeriatr. 1994;6:63–68. [PubMed] [Google Scholar]
- 75.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 76.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognitive Study Part 1. Arch Neurology. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 77.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 78.Lopez OL, Kuller LH, Becker JT, et al. Incidence of dementia in mild cognitive impairment in the Cardiovascular Health Study. Arch Neurol. 2007;64:416–420. doi: 10.1001/archneur.64.3.416. [DOI] [PubMed] [Google Scholar]
- 79.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 80.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment: beyond controversies, towards a consensus – report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 81•.Albert MS, Dekosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging and Alzheimer’s Association workgroup. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. These are the new clinical criteria for the mild cognitive impairment due to Alzheimer’s disease. These criteria included memory and memory and other cognitive domain forms of mild cognitive impairment and are supported by the presence of biomarkers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.desRosiers G, Hodges JR, Berrios G. The neuropsychological differentiation of patients with very mild Alzheimer’s disease and/or major depression. J Am Geriatr Soc. 1995;43:1256–1263. doi: 10.1111/j.1532-5415.1995.tb07402.x. [DOI] [PubMed] [Google Scholar]
- 83.Perry RJ, Hodges JR. Fate of patients with questionable (very mild) Alzheimer’s disease: longitudinal profiles of individual subjects’ decline. Dement Geriatr Cogn Disord. 2000;11:342–349. doi: 10.1159/000017264. [DOI] [PubMed] [Google Scholar]
- 84.Hansson O, Zetterberg H, Buchhave P, et al. Prediction of Alzheimer’s disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:316–320. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- 85.Jack CR, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chetelat G, Desgranges B, de la Sayette V, et al. Mild cognitive impairment. Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 87.Wolk DA, Price JC, Saxton J, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ewers M, Walsh C, Trojanowski JQ, et al. Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.10.019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawas CH, Corrada MM, Brookmeyer R, et al. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology. 2003;60:1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- 90.Saxton J, Lopez OL, Ratcliff GR, et al. Preclinical Alzheimer disease. Neuropsychological test performance 1. 5 to 8 years prior to dementia onset. Neurology. 2004;63:2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- 91.Morris JC, Stornadt M, McKeel DW, et al. Cerebral amyloid deposition and diffuse plaques in ‘normal’ aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 92.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 93.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 95•.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. These are recommendations oriented to describe Alzheimer’s disease in its earliest stages, when the clinical symptoms are not evident but the disease is present. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 97.Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. J Am Med Assoc. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]