Abstract
BACKGROUND
Early invasive electrical stimulation studies suggested that enhancement of cerebellar vermal activity might prove valuable in symptomatic treatment of refractory neuropsychiatric diseases via modulation of emotion and affect. This proof of principle study aimed to test this hypothesis using noninvasive brain stimulation, and to explore the safety of this protocol in schizophrenia.
METHODS
Eight treatment-refractory patients with schizophrenia underwent ten sessions of intermittent theta burst stimulation (TBS) to the cerebellar vermis using MRI-guided transcranial magnetic stimulation (TMS). Assessments included side effect questionnaires, cardiovascular monitoring, psychiatric evaluations and comprehensive neuropsychological testing before and after TBS and at one-week follow-up.
RESULTS
Overall, TBS was tolerated well with mild side effects primarily comprising neck pain and headache. No serious adverse events occurred. Diastolic blood pressure (BP) showed mild decreases for five minutes post-TBS; no significant changes were detected for systolic BP or pulse. PANSS negative subscale showed significant improvements following TBS and during the follow-up. Calgary Depression Scale and self-report visual analog scales for Happiness and Sadness pointed to significant mood elevation. Neuropsychological testing revealed significantly fewer omissions in working memory and interference conditions of a Continuous Performance Test, a longer spatial span and better delay organization on the Rey-Osterrieth Complex Figure during follow-up. No significant worsening in psychiatric or neuropsychological measures was detected.
CONCLUSIONS
Theta burst stimulation of the cerebellar vermis is safe and well-tolerated, while offering the potential to modulate affect, emotion and cognition in schizophrenia. Future randomized, sham-stimulation controlled studies are warranted to support the clinical efficacy of this technique.
Keywords: Transcranial Magnetic Stimulation (TMS), Intermittent Theta Burst Stimulation (TBS), Vermis, Cerebellum, Schizophrenia, Safety
1. INTRODUCTION
The possibility of a pathophysiogical role for cerebellum in schizophrenia has become increasingly likely (Weinberger et al., 1979; Snider, 1982; Schmahmann, 1991; Andreasen et al., 1996). Clinical reports of patients with cerebellar pathology provide evidence for a cerebellar role in cognition, affect and psychosis (Keschner et al., 1937; Rubinstein and Freeman, 1940; Courville and Friedman, 1940; Keddie, 1969; Smith, 1975; Hamilton et al., 1983; Schmahmann, 1991, 1997, 2000; Sandyk, 1993; Jurjus et al., 1994; Schmahmann and Sherman, 1998; Spranger et al., 1999; Tashiro et al., 1999; Turner and Schiavetto, 2004; Duggal, 2005; Schmahmann et al., 2007; Tavano et al., 2007). Neuroimaging and postmortem studies in schizophrenia report cerebellar dysmorphology, and altered gray-white matter proportions in the vermis (Nopoulos et al., 1999; Loeber et al., 2001; Ichimiya et al., 2001; Okugawa et al., 2002, 2003; Joyal et al., 2004; Levitt et al., 1999; Lee et al., 2007; Lawyer et al., 2009), decreased Purkinje cell (PC) input to the deep nuclei and reduction in size and linear density of the PC layer (Eastwood et al., 2001; Tran et al., 1998; Reyes and Gordon, 1981). Further, accumulating evidence indicates prominent cerebellar dysfunction within the cerebello-thalamo-cortical networks in schizophrenia and points to lower N-acetylaspartate levels and volumetric reductions in cerebello-thalamo-cortical networks (Rusch et al., 2007; Deicken et al., 2001; Ende et al., 2005), disrupted connectivity in the middle and superior cerebellar peduncles (Okugawa et al., 2004, 2005, 2006; Kyriakopoulos et al., 2008; Magnotta et al. 2008), and disruption of the interactions between cerebellum and cerebral cortex by way of the thalamus as shown by transcranial magnetic stimulation (TMS) and positron emission tomography (PET) (Daskalakis et al., 2005; Andreasen et al., 1996, 1998).
It now appears that cerebellum is a critical neuromodulator not only of motor control but also of intellect and mood, optimizing these functions that are represented with topographic precision in distinct regions of the cerebellum (Stoodley and Schmahmann, 2010). The cerebellar vermis and fastigial nucleus (the limbic cerebellum [Snider 1976; Heath 1977; Schmahmann, 1991]) seem to be particularly involved in the regulation of emotion and affect (Stoodley and Schmahmann, 2009). They are connected with limbic/paralimbic regions in the frontal and temporal lobes, amygdale hippocampus, septal region, hypothalamus, periaqueductal gray matter and monoamine brainstem nuclei (Anand et al., 1959; Riklan et al., 1974; Cooper et al., 1976; Tolbert et al., 1976; Batini et al., 1978; Heath et al., 1978; Snider et al., 1982; Schmahmann, 2001), and manipulation of the vermis in animals and humans produces alterations in complex behaviors and mood (Reis et al., 1973; Heath, 1977; Berman et al., 1978).
In the 1970_s Cooper (Riklan et al., 1974; Cooper et al., 1976) implanted stimulators on the cerebellar surface to treat epilepsy, hypothesizing that cerebellar cortical stimulation would induce upstream inhibition of cerebral cortex via inhibition of thalamus. In addition to improvements in epilepsy, patients demonstrated improved attention and amelioration of aggression. Heath and colleagues (1977) followed up their observations of abnormal electrical activity within the cerebellar vermis and limbic sites in emotional disorders, by high frequency electrical stimulation of cerebellar vermal-paravermal regions for the treatment of schizophrenia, severe neurosis and uncontrollable aggression; and reported clinical improvements. These invasive manipulations suggested a direct relationship between cerebellum, mood and psychosis, but they have not been replicated.
In the current era, repetitive TMS (rTMS) is a promising therapeutic tool for refractory neuropsychiatric diseases on the basis of neural network modulations, and is a noninvasive analogue to electrical stimulation (Pascual-Leone et al., 1996; George et al., 2000; Kobayashi and Pascual-Leone, 2003). Theta burst stimulation (TBS) is a relatively new rTMS protocol that modulates activity in the underlying region in a shorter period of time, enabling more potent and longer-lasting post stimulation effects compared with standard rTMS (Huang et al., 2005; Stefan et al., 2008). Consecutive sessions of TBS have been employed safely and with promising clinical efficacy in schizophrenia (Bor et al., 2009; Sidhoumi et al., 2010), levodopa induced dyskinesias (Koch et al., 2009), spasticity (Mori et al., 2010) and depression (Chistyakov et al., 2010). Notably, ten sessions of standard TBS to lateral cerebellar hemispheres have led to improvement in levodopa-induced dyskinesias, highlighting the capability of TBS to augment long lasting plasticity via modulation of the cerebello-thalamo-cortical circuits (Koch et al., 2009).
Here, we conducted the first clinical trial to test whether enhancing cerebellar vermal activity using intermittent TBS (iTBS) may be a safe noninvasive method for augmenting the cerebellar modulation of the putatively dysfunctional neural networks in schizophrenia. The primary objective of this study was to determine the safety and tolerability of repeated sessions of iTBS over the cerebellar vermis. As a secondary aim, we explored the potential therapeutic efficacy in an effort to provide an early proof of principle for this novel approach.
2. METHODS
2.1. PATIENTS
Eligible subjects were ≥18 years of age, with a DSM-IV diagnosis of schizophrenia diagnosed by a board-certified psychiatrist using the clinician-administered Structured Clinical Interview for DSM-IV Axis I Disorders. For the month before enrollment they received outpatient care, with no visits to emergency psychiatry departments, and on stable doses of psychotropic medications. Exclusion criteria were alcohol or drug abuse in the prior six months, a history of seizures, head injury or prior neurosurgical procedures, DSM-IV diagnosis of other Axis I disorders, contraindications to TMS or MRI, gross organic pathology on neuroimaging, and pregnancy in females.
Eight patients were included in the study (Table 1). All had treatment refractory schizophrenia, having failed at least three trials of therapeutic doses of antipsychotic use during their lifetime. All had a baseline PANSS score of ≥58, indicating moderate to severe illness at the time of study (Leucht et al., 2005). With one exception, all had been hospitalized several times in the past. Two were medication-free at time of study as a result of self-reported noncompliance. Medications remained unchanged for the duration of the study. Five were smokers; two used alcohol occasionally; all denied substance abuse. All participants were right-handed as confirmed by the Annett_s Handedness Inventory (Annett, 1970).
Table 1.
Demographic and Clinical Characteristics of Patients with Schizophrenia
| Patients (n=8) | |
|---|---|
| Gender | |
| Male | 7 |
| Female | 1 |
| Age at Study Entry | |
| Mean | 41±9.2 |
| Min-Max | 29–54 |
| Education | 13±2.1 |
| Age at Disease Onset | 22.5±2.14 |
| Duration of Disease | 18.9±8.3 |
| Premorbid IQ estimate (WTAR) | 103.0±11.6 |
| Current IQ (WASI, Full Scale IQ) | 91.5±14.2 |
| Handedness | |
| Right | 8 |
| Race | |
| Caucasian | 8 |
| Schizophrenia subtypes | |
| Paranoid | 4 |
| Undifferentiated | 3 |
| Disorganized | 1 |
| Medications | |
| Atypical antipsychotics | 8 |
| Antianxiety (benzodiazepines, buspiron) | 4 |
| Antidepressant (SSRI) | 3 |
| Mood stabilizer (lamotrigine, sodium valproate) | 2 |
| Unmedicated | 2 |
Abbreviations: WTAR: Wechsler Test of Adult Reading (Wechsler, 2001); WASI: Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Full Scale IQ derived from Two-Subtest Form (Vocabulary and Matrix Reasoning).
The study was approved by the Institutional Review Board and Scientific Advisory Committee of the Harvard-Thorndike Clinical Research Center (CRC) at Beth Israel Deaconess Medical Center (BIDMC). The approval of the Massachusetts Department of Mental Health was granted by the Central Office Research Review Committee. Two study investigators explained the study protocol, assessed competency to sign informed consent and discussed potential risks of the study with patients before enrollment. All participants provided written informed consent.
2.2. TBS APPLICATIONS
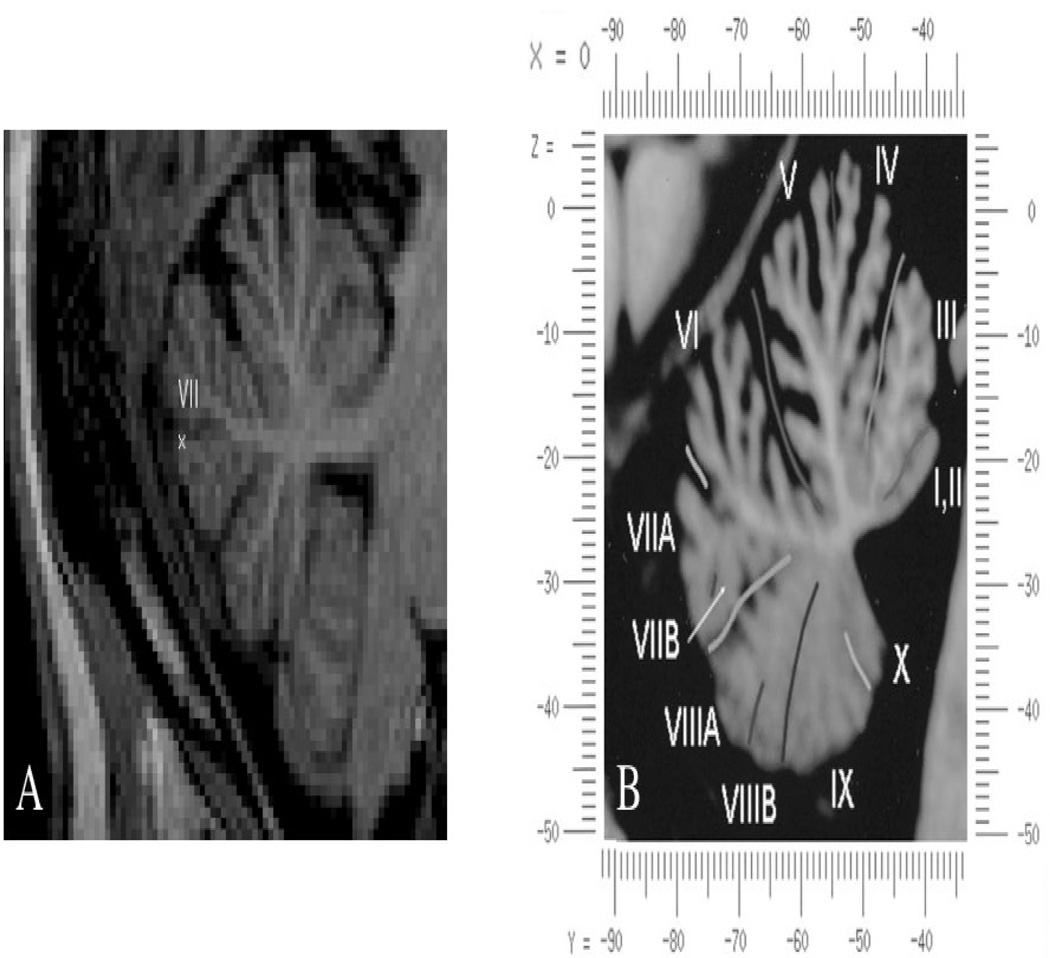
Anatomical brain MRI (Philips 3T-scanner, 0.5mm resolution) was performed prior to baseline assessment to enable use of the Brainsight frameless stereotaxic system (Rogue Research, Montreal, Canada) that effectively localizes the area of stimulation throughout the TBS application and ensures consistency of coil placement across days. The precise location of stimulation is illustrated in Figure 1.
FIGURE 1.
(A) Location of stimulation in a patient (Talairach coordinates x=0, y=−82, z=−30). Stimulation was performed using frameless stereotaxic system enabling precise targeting in all patients. (B) Vermis warped into the proportional stereotaxic space of Talairach, midsagittal plane (Adapted from Schmahmann et al., 1999).
Participants received 10 sessions of TBS to the cerebellar vermis. TBS sessions were administered twice daily (8.30 am and 1.30 pm) on five consecutive days. TBS was applied via a MagPro X100 stimulator and a figure-of-eight cool coil (Tonica, Farum, Denmark) held tangentially to the scalp with the handle pointing upwards. TBS was applied at 100% of active motor threshold (AMT) intensity (A/µs) with the standard iTBS burst pattern described by Huang et al. (2005) (3 pulses at 50-Hz repeated at a rate of 5-Hz; 20 trains of 10 bursts given with 8-s intervals; 600 pulses).
2.3. SAFETY
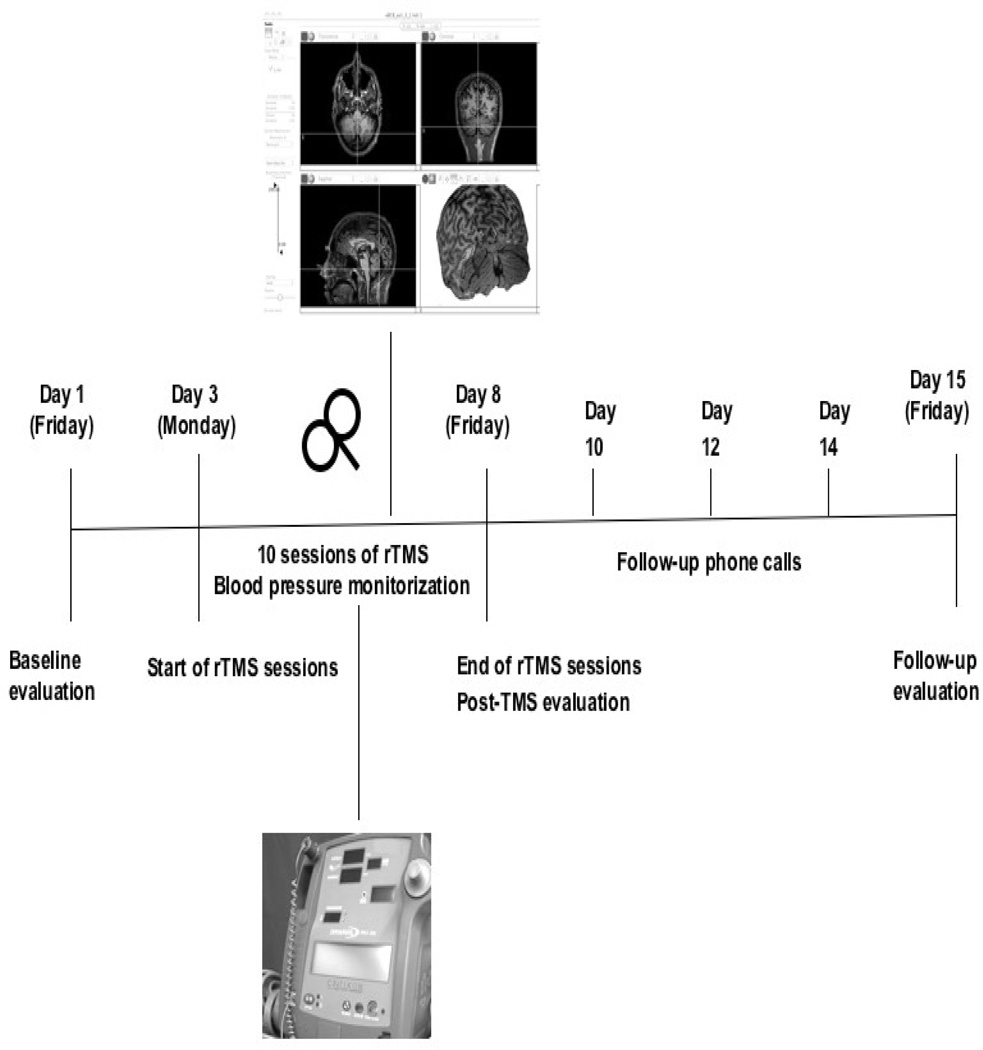
All patients were admitted to the inpatient CRC of BIDMC during the week of TBS to maximize observation and subject protection (Figure 2). Animal studies suggest a role for posterior vermis in cardiovascular control (Bradley et al., 1991), therefore all participants underwent cardiovascular monitoring before and after every TBS application with systemic blood pressure (BP) and heart rate measurements every five minutes for a total of nine times during one session. To avoid possible BP surges potentially influenced by diet, participants were not permitted caffeine, and they were given a low-sodium diet.
FIGURE 2.
Schematic representation of the experimental design. Clinical safety evaluations comprised psychiatric and neuropsychological assessments at three time points: prior to and following the application of 10 sessions of TBS, and one week later for follow-up purposes. Blood pressure was monitored throughout each TBS session.
Adverse events were recorded using standard adverse event forms, from the start of TBS treatment through the end of study participation. Patients reported pain/discomfort level on a visual analog scale (VAS) before and after each TBS session. Neurological examination focused on cerebellar signs and was repeated after every session. Patients were followed by telephone every other day after discharge to their follow-up visit.
2.4. ASSESSMENTS
Serial clinical assessments included psychiatric evaluations and neuropsychological testing performed on three consecutive weeks. Psychiatric evaluations were carried out by an experienced board-certified psychiatrist, a certified rater for Positive and Negative Symptom Scale(PANSS)(LS). Outcome measures for clinical efficacy comprised standardized ratings using the PANSS and Clinical Global Impression (CGI) for rating the symptoms of Schizophrenia, and Calgary Depression Scale for Schizophrenics (CDSS) to index effects on mood. Subjects rated themselves on self-administered scales, including the Profile of Mood States (POMS) test and VAS for dimensions of mood (Happiness, Sadness, Calmness, Anxiety, Wellbeing, Anger, Self-confidence, Fear, Alertness, and Energy).
Table 2 summarizes the neuropsychological battery with emphasis on attention, working memory, long-term memory, speed of processing, executive functions, visuospatial skills, and motor functions. For all time points, tests were administered in the same session by a post-doctoral fellow in clinical neuropsychology (JRC) supervised by neuropsychologists experienced with clinical assessment of individuals with schizophrenia (LJS, WSS). To minimize practice effects, alternative versions were used where possible. Raw or standardized scores were used, depending upon the particular measure.
Table 2.
Neuropsychological battery according to cognitive domains.
| COGNITIVE DOMAIN | NEUROPSYCHOLOGICAL TEST |
|---|---|
| Attention/Vigilance | Auditory Continuous Performance Test (CPT; Seidman, vigilance condition; Seidman et al., 1998) |
| Working Memory | Auditory CPT (Seidman, memory & interference conditions; Seidman et al., 1998) |
| Letter-Number Span (Gold et al., 1997) | |
| Wechsler Memory Scale, 3rd Edition (WMS-III)- Spatial Span (Wechsler, 1997) | |
| Speed of Processing | Phonemic/Letter Fluency (FAS; Benton, 1978) |
| Category Fluency (Animals; Spreen and Strauss, 1991) | |
| Brief Assessment of Cognition in Schizophrenia (BACS)-Symbol Coding (Keefe, 1999) | |
| Trail Making Test: Parts A&B (Reiten and Wolfson, 1985) | |
| Executive Functions/ | Wisconsin Card Sorting Test (WCST; Heaton, 1981) |
| Abstract Thinking | Delis-Kaplan Executive Function System (D-KEFS) - Proverbs Test (Delis et al., 2001) |
| Verbal Learning | California Verbal Learning Test, 2nd Edition (CVLT-II; Delis et al., 2000) |
| Visual Learning | Rey Osterrieth Complex Figure Test (Denman, 1984) |
| Motor Functions | Grooved Pegboard (Matthews and Klove, 1964) |
2.5. STATISTICAL ANALYSIS
Nonparametric statistical analyses were performed due to the small cohort. Baseline, post-TBS and follow-up evaluation scores were compared using non-parametric Friedman_s ANOVA. When appropriate, post-hoc comparisons were performed using Wilcoxon signed-rank test. Spearman_s rho was employed for nonparametric correlational analyses to explore possible associations. Given the exploratory character of the study, the alpha level was not lowered using Bonferroni_s correction. Resulting p-values <0.05 were considered statistically significant while p-values of <0.1 were reported to show a trend. Cohen_s d effect sizes were computed to estimate effects that might be overlooked due to the small sample size and to compare magnitude of change in a standardized way across disparate measures (Cohen, 1994). Analyses were carried out using STATA statistical software (version 9.1).
3. RESULTS
3.1. SAFETY
All patients completed the protocol without complications. Side effects were mild including neck pain and headache (both responded to acetaminophen), discomfort at the location of stimulation, and light-headedness. The pre- and post-TBS VAS for pain/discomfort did not detect a significant change (p>0.05). Inpatient stay with low-sodium and caffeine-free diet was tolerated by all patients but one; our last patient received eight of ten sessions of TBS because he refused to stay more days at the CRC unit. No seizures occurred. Neurological examination did not change. Patients reported no new symptoms or worsening of existing symptoms.
3.1.1. CARDIOVASCULAR MONITORING
There were no cardiovascular adverse events. Friedman tests yielded a significant effect of TBS on diastolic BP (X2=26.19, p<0.001) but not for systolic BP or pulse rate. Diastolic BP measured immediately post-TBS and five minutes later showed mild decreases (p<0.01), with a tendency to return to the baseline thereafter.
Highest and lowest BP values for each day were identified to investigate possible trends or fluctuations throughout the study. Changes varied and revealed elevation (N=3), no change (N=2), or a decrement (N=3). Elevation of BP was remarkable in only one patient with a history of hypertension. Overall, the group showed a 6±19 mmHg mean increase in systolic and 4.85±13 mmHg increase in diastolic BP (p>0.05).
3.2. CLINICAL PSYCHIATRIC DATA
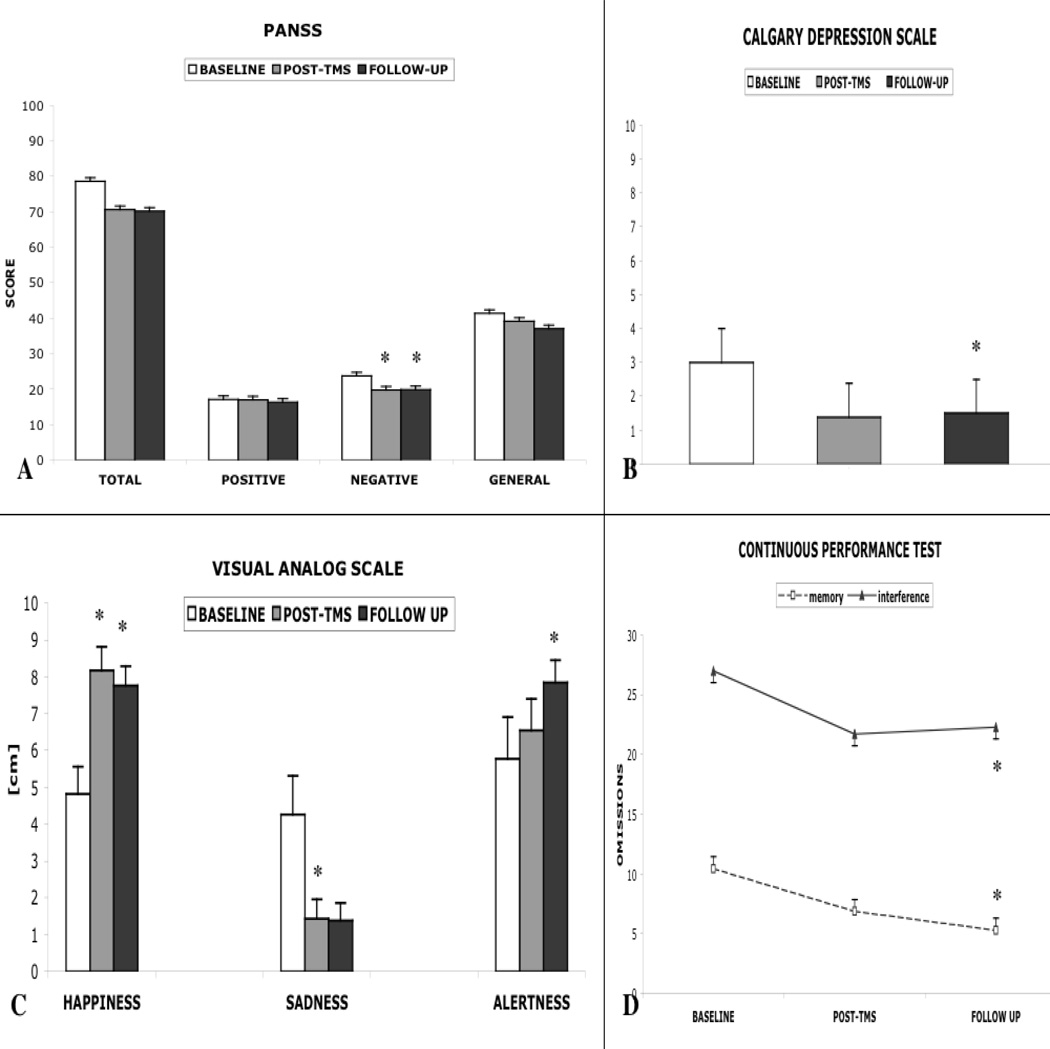
Friedman test revealed no significant changes between baseline, post-TBS and follow-up PANSS total scores (X2=3.56,p=0.16). However, when analyzed separately in positive, negative, and general subscales, a significant condition effect of TBS on PANSS negative subscale was detected (X2= 9.2, p<0.01). Post-hoc comparisons showed significant differences between baseline vs. post-TBS (p< 0.05,d=0.69), and baseline vs. follow-up (p<0.01,d=0.60). The mean peak reduction of the baseline score for negative symptoms was 19.3±12.3% (7.1%–43.8%) and was ≥15% in six patients. Improvement of symptoms showed no correlation with age, age at disease onset, duration of disease or IQ. There was no significant effect of TBS on either positive or general subscales of PANSS, or CGI scale (p>0.05).
CDSS revealed significant increases in mood following TBS (X2= 6.8, p<0.05). Post-hoc tests showed a significant difference only for baseline vs. follow-up (p<0.01,d=0.72) while a trend was present between baseline vs. post-TBS (p=0.077,d=0.64). Parallel results were noted in the POMS; however, comparisons did not reach statistical significance. A Friedman test for Happiness showed a significant overall effect (X2= 9.43, p<0.01). Post-hoc tests revealed significant paired comparisons for baseline vs. post-TBS (p<0.01,d=1.39) and baseline vs. follow-up (p<0.05,d=1.2), with ratings increasing following TBS. There was a significant decrease in Sadness (X2=7, p<0.05); the sole statistically significant difference was observed between baseline and post-TBS conditions (p<0.05,d=1.15) while a trend was present between baseline and follow-up (p=0.06,d=1.05). Friedman test for Alertness showed a significant difference (X2=6.2, p<0.05) and post-hoc comparisons pointed to an increase at follow-up only (p<0.05,d=0.8). Other VAS ratings showed no significant effects; however, the Anxiety (X2= 4.7,p=0.093, baseline vs. follow-up d=0.84) and Fear (X2=4.68,p=0.096, baseline vs. post-TBS d=0.96) categories showed a trend toward a reduction.
3.3. NEUROPSYCHOLOGICAL DATA
Neuropsychological data indicated no significant negative effects of cerebellar TBS on the tested domains (Table 3). Four tests revealed improved performance during post-TBS and follow-up assessments compared to baseline. Continuous performance test (CPT) results revealed significantly fewer omissions during the memory and interference conditions (X2=7.1,p<0.05). Post-hoc comparisons showed a significant difference only between baseline vs. follow-up time points for both conditions (p<0.05;d=0.78 and d=1.04, respectively). Spatial span forward performance improved significantly between baseline (X2= 6.06,p<0.05) and both post-TBS and follow up assessments (p<0.05,d=0.69). The delay organization score of the Rey-Osterrieth Complex figure also showed improvement (X2=7.56,p<0.05) and this was significant for the follow-up assessment (p<0.05,d= 0.68).
Table 3.
Neuropsychological Functioning in Patients with Schizophrenia During Baseline, Post-TBS and Follow Up
| N | Baseline (B) |
Post-TBS (P) |
Follow up (F) |
d B=P |
d B=F |
X2 |
p value |
Pairwise Comparisons |
|
|---|---|---|---|---|---|---|---|---|---|
| Attention - Vigilance | |||||||||
| Seidman Auditory Continuous | |||||||||
| Performance Test (Omission errors) | |||||||||
| Vigilance | 7 | 7.57±12.2 | 5.71±6.6 | 3.29±2.8 | 0.19 | 0.49 | 2 | 0.36 | ns |
| Working Memory | |||||||||
| Seidman Auditory Continuous | |||||||||
| Performance Test (Omission errors) | |||||||||
| Memory | 7 | 10.5±8.2 | 6.9±5.4 | 5.3±4.5 | 0.52 | 0.78 | 7.14 | 0.028 | F<B |
| Interference | 7 | 27.0±5.6 | 21.7±7.6 | 22.3±3.2 | 0.79 | 1.04 | 6.5 | 0.039 | F<B |
| Wechsler Memory Scale, 3rd | |||||||||
| Edition (WMS-III) | |||||||||
| Spatial Span Forward | 8 | 7.4±1.5 | 8.6±1.9 | 8.4±1.4 | 0.74 | 0.69 | 6.06 | 0.048 | P>B F>B |
| Spatial Span Backward | 8 | 6.1±3.0 | 6.3±3.0 | 6.1±3.0 | 0.04 | 0.00 | 0.19 | 0.91 | Ns |
| University of Maryland | 7 | 13.0±3.2 | 13.6±3.1 | 13.0±2.9 | 0.18 | 0.00 | 0.5 | 0.77 | ns |
| Letter-Number Span | |||||||||
| Speed of Processing | |||||||||
| Phonemic Fluency (FAS) | 8 | 33.1±10.8 | 35.3±12.5 | 35.6±13.0 | 0.18 | 0.21 | 1.75 | 0.42 | ns |
| Category Fluency (Animals) | 8 | 18.1±5.0 | 17.6±5.8 | 19.8±6.2 | −0.09 | 0.29 | 2.44 | 0.3 | ns |
| Brief Assessment of Cognition in Schizophrenia | |||||||||
| Symbol Coding | 8 | 36.0±7.9 | 36.3±11.0 | 36.3±10.8 | 0.03 | 0.03 | 0.25 | 0.88 | ns |
| Trail Making Test (seconds) | |||||||||
| Time Part A | 8 | 31.5±11.1 | 34.1±18.6 | 35.3±18.8 | −0.17 | −0.24 | 0.25 | 0.88 | ns |
| Time Part B | 8 | 94.6±33.8 | 96.3±83.7 | 105.9±81.9 | −0.03 | −0.18 | 1 | 0.6 | ns |
| Executive Functioning | |||||||||
| Wisconsin Card Sorting Test | |||||||||
| Categories Completed | 7 | 3.9±2.3 | 2.9±3.0 | 2.4±1.8 | −0.37 | −0.68 | 1.5 | 0.47 | ns |
| Perseverative Errors | 7 | 17.6±13.5 | 19.3±9.7 | 21.3±9.5 | −0.15 | −0.32 | 1.79 | 0.4 | ns |
| Delis-Kaplan Executive | |||||||||
| Function System (D-KEFS), Proverbs Test | |||||||||
| Accuracy | 7 | 7.7±5.4 | 7.4±5.3 | 8.0±4.7 | −0.05 | 0.06 | 0.29 | 0.87 | ns |
| Abstraction | 7 | 9.4±5.6 | 10.3±3.9 | 9.7±4.7 | 0.18 | 0.06 | 1.36 | 0.5 | ns |
| Achievement | 7 | 14.9±8.8 | 17.7±8.8 | 17.7±9.0 | 0.33 | 0.32 | 3.7 | 0.16 | ns |
| Verbal Learning | |||||||||
| California Verbal Learning | |||||||||
| Test, 2nd Edition (CVLT-2) | |||||||||
| (Number of words recalled) | |||||||||
| Total 1–5 standard score | 8 | 35.3±9.7 | 34.4±11.9 | 40.0±12.6 | −0.08 | 0.42 | 3.06 | 0.21 | ns |
| Short delay free recall | 8 | 6.0±3.6 | 6.6±3.1 | 7.9±4.1 | 0.19 | 0.49 | 4.19 | 0.12 | ns |
| Long delay free recall | 8 | 6.3±3.5 | 7.3±4.3 | 8.3±4.6 | 0.29 | 0.49 | 6.06 | 0.048 | ns |
| Short delay cued recall | 8 | 6.8±3.6 | 7.8±3.8 | 8.0±4.6 | 0.27 | 0.30 | 3.56 | 0.17 | ns |
| Long delay cued recall | 8 | 6.8±3.5 | 7.7±3.6 | 8.3±3.7 | 0.24 | 0.45 | 2.69 | 0.26 | ns |
| Visual Learning | |||||||||
| Rey-Osterrieth Complex | |||||||||
| Figure | |||||||||
| Copy Organization | 8 | 63.1±12.9 | 62.0±15.3 | 63.0±13.5 | −0.08 | −0.01 | 0.06 | 0.96 | ns |
| Delay Organization | 8 | 21.9±13.7 | 27.9±16.9 | 32.9±18.5 | 0.39 | 0.68 | 7.56 | 0.02 | F>B |
| Motor Functions (seconds) | |||||||||
| Grooved Pegboard Right | 7 | 85.4±16.1 | 82.4±6.8 | 80.6±8.5 | 0.01 | 0.02 | 0.92 | 0.63 | ns |
| Grooved Pegboard Left | 7 | 98.0±19.7 | 94.0±14.1 | 95.6±16.4 | 0.02 | 0.01 | 0.64 | 0.72 | ns |
Abbreviations B: baseline; P: post-TBS; F: follow up; d Cohen_s d effect size measurement; X2: Friedman_s ANOVA; ns: nonsignificant, p>0.05. Higher scores indicate improvement except for the following variables: Auditory CPT omission errors, Trail Making Test seconds, Wisconsin Card Sorting Test perseverative errors, Grooved Pegboard seconds.
No test showed significant decline during the study, although Wisconsin Card Sort categories score showed a nonsignificant decrement compared to baseline (X2=1.5,p>0.05,d=0.68). Overall, we note that 70% of the neuropsychological variables tested were in the direction of improvement (mean d=+0.33), 4% showed zero effect sizes, and 26% showed negative effects (mean d=−0.18). Thus, this pattern, combined with four significant results indicating improvement, suggests a trend toward improvement in neuropsychological function.
4. DISCUSSION
We have investigated the safety of repeated applications of iTBS over the cerebellar vermis in patients with schizophrenia, and have observed no cognitive decline, psychiatric worsening, or serious systemic adverse events. No seizures occurred; indeed, previous reports indicate reduced seizure activity following electrical stimulation of the cerebellar cortex (Cooper et al., 1976; Heath, 1977; Brighina et al., 2006). Side effects of mild occipital headache were similar to those reported following single cerebellar TBS sessions (Koch et al., 2008). Fluctuations in BP were noted and were generally mild except in one patient with hypertension. The posterior vermis (lobules VI–VII) and fastigial nucleus constitute a cardiovascular module in cerebellum (Bradley et al., 1991), and we consider it likely that the BP changes were related to the cerebellar stimulation.
Serial psychiatric assessments and neuropsychological testing revealed no safety concerns. On the contrary, evidence of efficacy was detected for negative symptoms, mood, and cognition, in agreement with earlier reports following invasive electrical stimulation (Cooper et al., 1976; Heath, 1977). Increased cerebellar activity in PET studies has been considered a compensatory mechanism for dysfunctional cerebrocerebellar circuitry in patients with hypofrontal/negative symptoms (Andreasen et al., 1997; Kim et al., 2000; Potkin et al., 2002) and, given the changes in frontal gamma spectrum following excitatory stimulation of the vermis (Schutter et al., 2003), it is theoretically plausible that potentiation of cerebellar inhibitory output via excitatory iTBS may modulate impairments in frontal gamma activity (Farzan et al., 2010; Cho et al., 2006). While the mechanism of improvement remains to be shown, our findings provide empirical support for the dysmetria of thought theory (Schmahmann 1991, 1996, 1998, 2000), that cerebellum acts to correct errors in the realms of thought and emotion maintaining behavior around a homeostatic baseline, and loss of the universal cerebellar transform in schizophrenia.
In this study, stimulation intensity was set at 100% AMT, with a slight modification from the original protocol described by Huang et al. (2005), because the estimated vermis-coil distance is approximately 2.5cm (Schmahmann et al., 1999). However, the applicability of motor threshold intensities to cerebellum is still under debate (Del Olmo et al., 2007), and future studies employing cerebellar TBS should specifically address this issue. We chose a twice-daily regimen of iTBS to minimize length of inpatient stay and maximize patient compliance, but other combinations of TBS protocols may also prove effective. It is now well established that TBS can be safely performed using a range of stimulation parameters. Twice daily iTBS each comprising 1800 stimuli, performed at 100% AMT over the dorsolateral prefrontal cortex (DLPFC) in patients with depression was reported to be safe without significant adverse effects (Chistyakov et al., 2010), and twice daily iTBS over the DLPFC at 80% MT resulted in improvement of negative symptoms in a patient with schizophrenia (Bor et al., 2009). EEG recordings of standard TBS protocols over the DLPFC proved safe with no epileptiform activity in normal subjects (Grossheinrich et al., 2009). Standard TBS protocols targeting the motor cortex in patients with multiple sclerosis and amyotrophic lateral sclerosis did not produce serious adverse events (Mori et al., 2010; Di Lazzaro et a., 2009), and weekly use of TBS for up to 12 consecutive months has provided evidence in favor of its long-term safety (Di Lazzaro et al., 2009). Finally, ten consecutive sessions of TBS to lateral cerebellar hemispheres in levodopa-induced dyskinesias reported no adverse events (Koch et al., 2009).
It is not possible to know whether cerebellar vermal iTBS could be more effective in schizophrenia than invasive electrical stimulation; early data on electrical stimulation are limited. Invasive stimulation offers the advantage of being able to stimulate any desired location while TBS is mostly limited to more superficial structures. The major advantage of TBS, however, lies in its noninvasive, morbidity-free application and its safe use within a range of stimulation parameters. Future modifications of TBS may result in clinically significant changes in efficacy, but the safety of such protocols could differ (Rossi et al., 2009).
The strengths of this study include the novel hypothesis-driven approach of stimulating the cerebellum and particularly the vermis in this disorder, the precise targeting of the vermis and minimized interindividual anatomical variability achieved via the use of neuronavigation, and continuous monitoring of the patients in the CRC unit to ensure their safety. There are notable limitations in this exploratory study. The principal limitation is the open-label nature and the absence of a control intervention; this was not included because of our primary focus on safety. Whereas our results demonstrate the safety and tolerability of repeated sessions of rTMS over the vermis in schizophrenia and provide early proof of principle to proceed further, future placebo controlled trials will need to define clinical efficacy. Electroencephalography and functional neuroimaging may help characterize changes in neural circuitry induced by cerebellar stimulation, and shed light on the neurobiology of this disorder. A second limitation is the number of patients studied which can lead to type II errors. Despite the small n, psychiatric and cognitive results actually showed improvement in some functions. Lastly, smoking habits, caffeine restriction and medications may have influenced our results. The refractoriness of our patients precluded withdrawal or changing their existing medications in favor of one antipsychotic, although it would be desirable to study medication-free patients because psychotropics may affect the response to TBS.
In sum, this study demonstrates that repeated sessions of iTBS to the cerebellar vermis in patients with refractory schizophrenia are safe and well-tolerated. Improvement in negative symptoms, mood and cognition represents an encouraging initial step towards treatment of refractory schizophrenia through noninvasive neuromodulation of the cerebellum. These findings are potentially important because available treatments for negative symptoms of schizophrenia remain only partially effective (Alphs et al., 2006). Further studies of clinical efficacy and mechanisms of cerebellar TBS are warranted. By demonstrating the safety of cerebellar vermal iTBS in schizophrenia, it may be possible to perform future studies in the outpatient setting, although caution is warranted in patients with hypertension.
FIGURE 3.
The graph demonstrates the changes in (A) PANSS and subscales, (B) Calgary Depression Scale, (C) Visual Analogue Scales (Happiness, Sadness and Alertness) and (D) Continuous Performance Test (omissions during memory and interference conditions) for all time points. Significant changes are marked *.
ACKNOWLEDGEMENTS
ROLE OF THE FUNDING SOURCE: Funding for this study was provided in part by Harvard Clinical and Translational Science Center, from the National Center for Research Resources (UL1 RR025758) and National Institutes of Health grant (K24 RR018875) to APL, and by the Birmingham Foundation, the Executive Committee on Research of the Massachusetts General Hospital and the MINDlink foundation to JDS. CF was supported by the Foundation for Science and Technology, Portugal (SFRH/BPD/44126/2008).
We gratefully acknowledge the contributions of Daniel Z. Press, Donald C. Goff, MD, Benjamin Brent, MD and Chester Pearlman, MD. Special thanks to the staff at the Harvard-Thorndike Clinical Research Center, this study would not have been accomplished without their dedicated help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: All authors declare that they have no conflicts of interest.
CONTRIBUTORS: JDS conceptualized the initial idea. ADT, JDS, LJS, WSS and APL designed the study. ADT, JDS and APL wrote the protocol. DO was involved in patient screening and recruitment. ADT, CF, JRC and LS collected the data. ADT and JRC participated in statistical analyses and LJS, WSS and DO contributed to interpretation of the data. ADT wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
REFERENCES
- Alphs L. An industry perspective on the NIMH consensus statement on negative symptoms. Schizophr Bull. 2006;32:225–230. doi: 10.1093/schbul/sbj056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand BK, Malhotra CL, Singh B, Dua S. Cerebellar Projections to the Limbic System. J Neurophysiol. 1959;22:451–458. doi: 10.1152/jn.1959.22.4.451. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, et al. Schizophrenia and Cognitive Dysmetria: A Positron Emission Tomography Study of Dysfunctional Prefrontal-Thalamic-Cerebelar Circuitry. Proc Natl Acad Sci USA. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Flaum M, et al. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O'Leary DS. Cognitive dysmetria as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Batini C, Buisseret-Delmas C, Corvisier J, Hardy O, Jassik-Gerschenfeld Brain stem nuclei giving fibers to lobules VI and VII of the cerebellar vermis. Brain Res. 1978;153:241–261. doi: 10.1016/0006-8993(78)90405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. Multilingual Aphasia Examination Manual Revised. Iowa City: University of Iowa; 1978. [Google Scholar]
- Berman AF, Berman D, Prescott JW. The effect of cerebellar lesions on emotional behavior in the rhesus monkey. In: Cooper IS, Riklan M, Snider RS, editors. The Cerebellum, Epilepsy and Behavior. New York: Plenum Press; 1978. pp. 277–284. [Google Scholar]
- Bor J, Brunelin J, Rivet A, et al. Effects of theta burst stimulation on glutamate levels in a patient with negative symptoms of schizophrenia. Schizophr Res. 2009;111:196–197. doi: 10.1016/j.schres.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Ghelarducci B, Spyer KM. The role of the posterior cerebellar vermis in cardiovascular control. Neurosci Res. 1991;12:45–56. doi: 10.1016/0168-0102(91)90099-k. [DOI] [PubMed] [Google Scholar]
- Brighina F, Daniele O, Piazza A, Giglia G, Fierro B. Hemispheric cerebellar rTMS to treat drug-resistant epilepsy: case reports. Neurosci Lett. 2006;397:229–233. doi: 10.1016/j.neulet.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Chistyakov AV, Rubicsek O, Kaplan B, Zaaroor M, Klein E. Safety, tolerability and preliminary evidence for antidepressant efficacy of theta-burst transcranial magnetic stimulation in patients with major depression. Int J Neuropsychopharmacol. 2010;13:387–393. doi: 10.1017/S1461145710000027. [DOI] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. The earth is round. Am Psychol. 1994;49:997–1003. [Google Scholar]
- Cooper IS, Amin I, Riklan M, Waltz JM, Poon TP. Chronic Cerebellar Stimulation in Epilepsy. Arch Neurol. 1976;33:559–570. doi: 10.1001/archneur.1976.00500080037006. [DOI] [PubMed] [Google Scholar]
- Courville C, Friedman A. Chronic progressive degeneration of superior cerebellar cortex (parenchymatous conical cerebellar atrophy) with particular reference to its pathogenesis and pathology. Bull Los Angeles Neurol Soc. 1940;5:171–183. [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Fountain SI, Chen R. Reduced cerebellar inhibition in schizophrenia: a preliminary study. Am J Psychiatry. 2005;162:1203–1205. doi: 10.1176/appi.ajp.162.6.1203. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Feiwell R, Schuff N, Soher B. Evidence for altered cerebellar vermis neuronal integrity in schizophrenia. Psychiatry Res. 2001;107:125–134. doi: 10.1016/s0925-4927(01)00103-2. [DOI] [PubMed] [Google Scholar]
- Del Olmo MF, Cheeran B, Koch G, Rothwell JC. Role of the cerebellum in externally paced rhythmic finger movements. J Neurophysiol. 2007;98:145–152. doi: 10.1152/jn.01088.2006. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test, 2nd Edition (CVLT-II) San Antonio, Texas: Pearson Assessment; 2000. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS: Proverbs Test) San Antonio, Texas: Pearson Assessment; 2001. [Google Scholar]
- Denman SB. Manual for the Denman Neuropsychology Memory Scale. Charleston, South Carolina: Private publisher; 1984. [Google Scholar]
- Di Lazzaro V, Pilato F, Profice P, et al. Motor cortex stimulation for ALS: a double blind placebo-controlled study. Neurosci Lett. 2009;464:18–21. doi: 10.1016/j.neulet.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Duggal HS. Cognitive Affective Psychosis Syndrome in a Patient with Sporadic Olivopontocerebellar Atrophy. J Neuropsychiatry Clin Neurosci. 2005;17:260–262. doi: 10.1176/jnp.17.2.260. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Cotter D, Harrison PJ. Cerebellar synaptic protein expression in schizophrenia. Neuroscience. 2001;105:219–229. doi: 10.1016/s0306-4522(01)00141-5. [DOI] [PubMed] [Google Scholar]
- Ende G, Hubrich P, et al. Further evidence for altered cerebellar neuronal integrity in schizophrenia. Am J Psychiatry. 2005;162:790–792. doi: 10.1176/appi.ajp.162.4.790. [DOI] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Levinson AJ, Chen R, Wong W, Fitzgerald PB, Daskalakis ZJ. Evidence for gamma inhibition deficits in the dorsolateral prefrontal cortex of patients with schizophrenia. Brain. 2010;133:1505–1514. doi: 10.1093/brain/awq046. [DOI] [PubMed] [Google Scholar]
- George MS, Nahas Z, Molloy M, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry. 2000;48:962–970. doi: 10.1016/s0006-3223(00)01048-9. [DOI] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Grossheinrich N, Rau A, Pogarell O, et al. Theta burst stimulation of the prefrontal cortex: safety and impact on cognition, mood, and resting electroencephalogram. Biol Psychiatry. 2009;65:778–784. doi: 10.1016/j.biopsych.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Hamilton NG, Frick RB, Takahashi T, Hopping MW. Psychiatric symptoms and cerebellar pathology. Am J Psychiatry. 1983;140:1322–1326. doi: 10.1176/ajp.140.10.1322. [DOI] [PubMed] [Google Scholar]
- Heath RG. Modulation of Emotion with a Brain Pacemaker. J Nerv Ment Dis. 1977;165:300–317. [PubMed] [Google Scholar]
- Heath RG, Dempesy CW, Fontana CJ, Myers WA. Cerebellar Stimulation: Effects on Septal Region, Hippocampus, and Amygdala of Cats and Rats. Biol Psychiatry. 1978;13:501–529. [PubMed] [Google Scholar]
- Heaton RK. A Manual for the Wisconsin Card Sorting Test. Odessa, Florida: Psychological Assessment Resources; 1981. [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Ichimiya T, Okubo Y, Suhara T, Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biol Psychiatry. 2001;49:20–27. doi: 10.1016/s0006-3223(00)01081-7. [DOI] [PubMed] [Google Scholar]
- Jurjus GJ, Weiss KM, Jaskiw GE. Schizophrenia-like psychosis and cerebellar degeneration. Schizophr Res. 1994;12:183–184. doi: 10.1016/0920-9964(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Joyal CC, Pennanen C, Tiihonen E, Laakso MP, Tiihonen J, Aronen HJ. An MRI study of cerebellar vermis morphology in patients with schizophrenia: evidence in support of the cognitive dysmetria concept. Psychiatry Res. 2004;131:115–124. [Google Scholar]
- Keddie KM. Hereditary ataxia, presumed to be of the Menzel type, complicated by paranoid psychosis, in a mother and two sons. J Neurol Neurosurg Psychiatry. 1969;32:82–87. doi: 10.1136/jnnp.32.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RSE. Brief Assessment of Cognition in Schizophrenia (BACS) Manual A: Version 2.1. Durham, North Carolina: Duke University Medical Center; 1999. [Google Scholar]
- Keschner M, Bender M, Strauss I. Mental symptoms in cases of subtentorial tumor. Arch Neurol Psychiatry. 1937;37:1–18. [Google Scholar]
- Kim JJ, Mohamed S, Andreasen NC, et al. Regional neural dysfunctions in chronic schizophrenia studied with positron emission tomography. Am J Psychiatry. 2000;157:542–548. doi: 10.1176/appi.ajp.157.4.542. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Koch G, Mori F, Marconi B, et al. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin Neurophysiol. 2008;119:2559–2569. doi: 10.1016/j.clinph.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Koch G, Brusa L, Carrillo F, et al. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology. 2009;73:113–119. doi: 10.1212/WNL.0b013e3181ad5387. 2009. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S. A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biol Psychiatry. 2008;63:519–523. doi: 10.1016/j.biopsych.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Lawyer G, Nesvåg R, Varnäs K, Okugawa G, Agartz I. Grey and white matter proportional relationships in the cerebellar vermis altered in schizophrenia. Cerebellum. 2009;8:52–60. doi: 10.1007/s12311-008-0071-7. [DOI] [PubMed] [Google Scholar]
- Lee KH, Farrow TF, Parks RW, et al. Increased cerebellar vermis white-matter volume in men with schizophrenia. J Psychiatr Res. 2007;41:645–651. doi: 10.1016/j.jpsychires.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res. 2005;79:231–238. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, McCarley RW, Nestor PG, et al. Quantitative volumetric MRI study of the cerebellum and vermis in schizophrenia: clinical and cognitive correlates. Am J Psychiatry. 1999;156:1105–1107. doi: 10.1176/ajp.156.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber RT, Cintron CMB, Yurgelun-Todd DA. Morphometry of Individual Cerebellar Lobules in Schizophrenia. Am J Psychiatry. 2001;158:952–954. doi: 10.1176/appi.ajp.158.6.952. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Adix ML, Caprahan A, Lim K, Gollub R, Andreasen NC. Investigating connectivity between the cerebellum and thalamus in schizophrenia using diffusion tensor tractography: a pilot study. Psychiatry Res. 2008;163:193–200. doi: 10.1016/j.pscychresns.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews CG, Klove H. Instruction manual for the Adult Neuropsychology Test Battery. Madison, Wisconsin: Lafayette Instrument; 1964. Grooved Pegboard. [Google Scholar]
- Mori F, Codecà C, Kusayanagi H, Monteleone F, Boffa L, Rimano A, Bernardi G, Koch G, Centonze D. Effects of intermittent theta burst stimulation on spasticity in patients with multiple sclerosis. Eur J Neurol. 2010;17:295–300. doi: 10.1111/j.1468-1331.2009.02806.x. [DOI] [PubMed] [Google Scholar]
- Nopoulos PC, Ceilley JW, Gailis EA, Andreasen NC. MRI volumetry of the vermis and the cerebellar hemispheres in men with schizophrenia. Biol Psychiatry. 1999;46:703–711. doi: 10.1016/s0006-3223(99)00093-1. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Sedvall G, Nordström M, et al. Selective reduction of the posterior superior vermis in men with chronic schizophrenia. Schizophr Res. 2002;55:61–67. doi: 10.1016/s0920-9964(01)00248-1. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Sedvall GC, Agartz I. Smaller cerebellar vermis but not hemisphere volumes in patients with chronic schizophrenia. Am J Psychiatry. 2003;160:1614–1617. doi: 10.1176/appi.ajp.160.9.1614. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Nobuhara K, Minami T, et al. Subtle disruption of the middle cerebellar peduncles in patients with schizophrenia. Neuropsychobiology. 2004;50:119–123. doi: 10.1159/000079101. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Nobuhara K, Sugimoto T, Kinoshita T. Diffusion tensor imaging study of the middle cerebellar peduncles in patients with schizophrenia. Cerebellum. 2005;4:123–127. doi: 10.1080/14734220510007879. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Nobuhara K, Minami T, et al. Neural disorganization in the superior cerebellar peduncle and cognitive abnormality in patients with schizophrenia: A diffusion tensor imaging study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1408–1412. doi: 10.1016/j.pnpbp.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Alva G, Fleming K, et al. A PET Study of the Pathophysiology of Negative Symptoms in Schizophrenia. American Journal of Psychiatry. 2002;159:227–237. doi: 10.1176/appi.ajp.159.2.227. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson, Arizona: Neuropsychology Press; 1985. [Google Scholar]
- Reis DJ, Doba N, Nathan MA. Predatory attack, grooming, and consummatory behaviors evoked by electrical stimulation of cat cerebellar nuclei. Science. 1973;182:845–847. doi: 10.1126/science.182.4114.845. [DOI] [PubMed] [Google Scholar]
- Reyes MG, Gordon A. Cerebellar vermis in schizophrenia. Lancet. 1981;2:700–701. doi: 10.1016/s0140-6736(81)91039-4. [DOI] [PubMed] [Google Scholar]
- Riklan M, Marisak I, Cooper IS. Psychological studies of chronic cerebellar stimulation in man. In: Cooper IS, Riklan M, Snider RS, editors. The cerebellum epilepsy and behavior. New York: Plenum Press; 1974. pp. 285–342. [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A The Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein H, Freeman W. Cerebellar agenesis. J Nerv Ment Dis. 1940;92:485–502. [Google Scholar]
- Rusch N, Spoletini I, et al. Prefrontal-thalamic-cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr Res. 2007;93:79–89. doi: 10.1016/j.schres.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Sandyk R. Psychotic behavior associated with cerebellar pathology. Int J Neurosci. 1993;71:1–7. doi: 10.3109/00207459309000586. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. An Emerging Concept: The Cerebellar Contribution to Higher Function. Arch Neurol. 1991;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: Anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Rediscovery of an early concept. Int Rev Neurobiol. 1997;41:3–27. doi: 10.1016/s0074-7742(08)60345-1. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The Cerebellar Cognitive Affective Syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Dysmetria of thought. Clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci. 1998;2:362–370. doi: 10.1016/s1364-6613(98)01218-2. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The Role of Cerebellum in Affect and Psychosis. J Neurolinguistics. 2000;13:189–214. [Google Scholar]
- Schmahmann JD. The cerebrocerebellar system: Anatomic substrates of the cerebellar contribution to cognition and emotion. Int Rev Psychiatry. 2001;13:247–260. [Google Scholar]
- Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of cerebellum-insights from the clinic. Cerebellum. 2007;6:254–267. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J, d'Alfonso AA, Peper JS, Panksepp J. High frequency repetitive transcranial magnetic over the medial cerebellum induces a shift in the prefrontal electroencephalography gamma spectrum: a pilot study in humans. Neurosci Lett. 2003;336:73–76. doi: 10.1016/s0304-3940(02)01077-7. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Breiter H, Goodman JM, et al. A functional magnetic resonance imaging study of auditory vigilance with low and high information processing demands. Neuropsychology. 1998;12:505–518. doi: 10.1037//0894-4105.12.4.505. [DOI] [PubMed] [Google Scholar]
- Sidhoumi D, Braha S, Bouaziz N, Brunelin J, Benadhira R, Januel D. Evaluation of the therapeutic effect of theta burst stimulation on drug-resistant auditory hallucinations in a schizophrenic patient and its impact on cognitive function and neuronal excitability: a case study. Clin Neurophysiol. 2010;121:802. doi: 10.1016/j.clinph.2009.12.033. [DOI] [PubMed] [Google Scholar]
- Smith MC. Histological findings after hemicerebellectomy in man: Anterograde, retrograde, and transneuronal degeneration. Brain Res. 1975;95:423–442. doi: 10.1016/0006-8993(75)90119-5. [DOI] [PubMed] [Google Scholar]
- Snider RS. Cerebellar contributions to the Papez circuit. J Neurosci Res. 1976;2:133–146. doi: 10.1002/jnr.490020204. [DOI] [PubMed] [Google Scholar]
- Snider SR. Cerebellar pathology in schizophrenia--cause or consequence? Neurosci Biobehav Rev. 1982;6:47–53. doi: 10.1016/0149-7634(82)90006-9. [DOI] [PubMed] [Google Scholar]
- Spranger M, Spranger S, Schwab S, Benninger C, Dichgans M. Familial hemiplegic migraine with cerebellar ataxia and paroxysmal psychosis. Eur Neurol. 1999;41:150–152. doi: 10.1159/000008039. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 1991. [Google Scholar]
- Stefan K, Gentner R, Zeller D, Dang S, Classen J. Theta-burst stimulation: remote physiological and local behavioral after-effects. Neuroimage. 2008;40:265–274. doi: 10.1016/j.neuroimage.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro H, Suzuki SO, Hitotsumatsu T, Iwaki T. An autopsy case of spinocerebellar ataxia type 6 with mental symptoms of schizophrenia and dementia. Clin Neuropathol. 1999;18:198–204. [PubMed] [Google Scholar]
- Tavano A, Grasso R, Gagliardi C, et al. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130:2646–2660. doi: 10.1093/brain/awm201. [DOI] [PubMed] [Google Scholar]
- Tolbert DL, Bantli H, Bloedel JR. Anatomical and physiological evidence for a cerebellar nucleo-cortical projection in the cat. Neuroscience. 1976;1:205–217. doi: 10.1016/0306-4522(76)90078-6. [DOI] [PubMed] [Google Scholar]
- Tran KD, Smutzer GS, Doty RL, Arnold SE. Reduced Purkinje cell size in the cerebellar vermis of elderly patients with schizophrenia. Am J Psychiatry. 1998;155:1288–1290. doi: 10.1176/ajp.155.9.1288. [DOI] [PubMed] [Google Scholar]
- Turner R, Schiavetto A. The cerebellum in schizophrenia: a case of intermittent ataxia and psychosis--clinical, cognitive, and neuroanatomical correlates. J Neuropsychiatry Clin Neurosci. 2004;16:400–408. doi: 10.1176/jnp.16.4.400. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading (WTAR) San Antonio, Texas: Pearson Assessment; 2001. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, Texas: Pearson Assessment; 1999. [Google Scholar]
- Wechsler D. The Wechsler Memory Scale, 3rd ed. San Antonio, Texas: Pearson Assessment; 1997. [Google Scholar]
- Weinberger DR, Torrey EF, Wyatt RJ. Cerebellar atrophy in chronic schizophrenia. Lancet. 1979;1:718–719. doi: 10.1016/s0140-6736(79)91164-4. [DOI] [PubMed] [Google Scholar]