Abstract
The sudden infant death syndrome (SIDS) is the sudden death of an infant under one year of age that is typically associated with sleep and that remains unexplained after a complete autopsy and death scene investigation. A leading hypothesis about its pathogenesis is that many cases result from defects in brainstem-mediated protective responses to homeostatic stressors occurring during sleep in a critical developmental period. Here we review the evidence for the brainstem hypothesis in SIDS with a focus upon abnormalities related to the neurotransmitter serotonin in the medulla oblongata, as these are the most robust pathologic findings to date. In this context, we synthesize the human autopsy data with genetic, whole-animal, and cellular data concerning the function and development of the medullary serotonergic system. These emerging data suggest an important underlying mechanism in SIDS that may help lead to identification of infants at risk and specific interventions to prevent death.
Keywords: arousal, chemosensitivity, gasping, intermittent hypoxia, serotonin, raphé
INTRODUCTION
The sudden infant death syndrome (SIDS) is the sudden and unexpected death of an infant under 12 months of age that is usually associated with a sleep period and remains unexplained after a complete autopsy, death scene investigation, and review of the clinical history (1). Typically, without warning an apparently healthy infant is found dead sometime after being placed to sleep for the night or for an afternoon nap. In the United States today, SIDS is the leading cause of death in infants between 1 and 12 months, and it is the third leading cause of all infant deaths between birth and 12 months (after congenital malformations and low birth weight) (2, 3). The overall incidence of SIDS is currently 0.57 per 1000 live births in the United States (3)—or approximately six infant deaths per day. The most significant advance in SIDS research in the past two decades has been the recognition that the prone (belly-down) sleep position more than triples the risk for SIDS (4). This seminal observation has led to vigorous national public health campaigns advocating the supine (belly-up) sleep position for infants, to a decline in the placement of infants in the prone position to sleep to fewer than 20% of parents putting infants in this position (5, 6), and to a reduction in the national SIDS rate of almost 50% (3).
Despite the dramatic public health achievement in the reduction of SIDS, this disorder remains a major problem that must not be underestimated. It is still the leading cause of post-neonatal infant mortality in the United States [with an overall rate higher than those of several European countries and Japan (2, 3, 7)]. Moreover, the decline in rate may be due in part to diagnostic shift, in which terms such as positional asphyxia, accidental suffocation, and undetermined are used on death certificates (from which national statistics are derived) in cases previously identified as SIDS (8, 9). In addition, SIDS occurs in infants found in the supine position and in infants who were inadvertently placed in the prone position (even for the first time) or who had rolled from the supine to prone position (10). SIDS is also associated with several significant risk factors that are less modifiable than infant sleep position, e.g., maternal smoking and alcohol use during pregnancy, prematurity, and poverty (7, 11–15), as well as with genetic (immutable) factors that appear to interact with environmental factors to compound SIDS risk (16–19). Of major concern today is the substantial discrepancy in SIDS rates between white and nonwhite populations, with rates two or more times higher in certain racial and ethnic groups whose health care is commonly marginalized, e.g., African Americans (20), American Indians in the Northern Plains (102), Maori in New Zealand (15, 21), aboriginal Australians (14), and mixed-ancestry populations in Cape Town (22). In order to establish the means to eradicate all SIDS deaths—the ultimate goal of SIDS research—as well as to give biologic plausibility to the current public health messages, the underlying cause and pathogenesis of SIDS must be established.
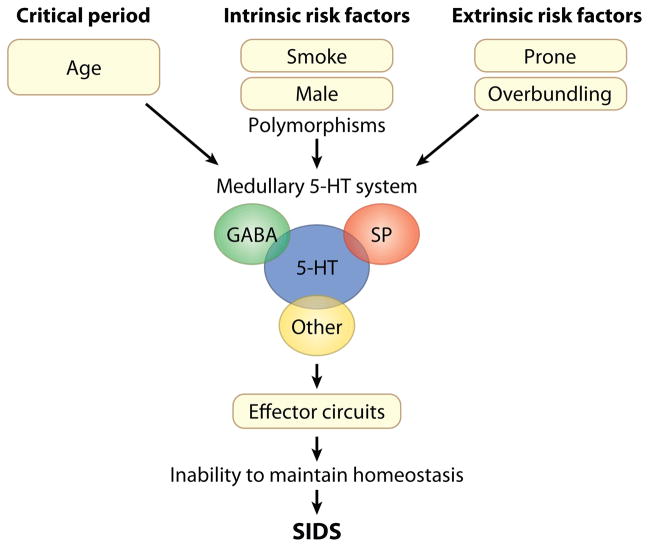
This review summarizes two decades of brainstem research in SIDS in large part under the auspices of a National Institute of Child Health and Human Development–funded program project led by the authors, combined with a synthesis of relevant analysis reported by others. We focus primarily (but not exclusively) upon pathways in the medulla oblongata that involve the neurotransmitter serotonin (known as 5-hydroxytryptamine or 5-HT) because medullary 5-HT abnormalities are the most robust and reproducible findings in SIDS brainstem research to date (23–26). Based upon the collective work of our group and others, we offer the hypothesis that an important subset of SIDS is due to a developmental disorder of the medullary 5-HT and related neurotransmitter systems and that it is incurred prenatally but exerts its effects postnatally (Figure 1). This brainstem disorder leads in turn, we believe, to impaired protective responses to life-threatening (but common) stressors during infant sleep and to subsequent asphyxia and/or other homeostatic derangements—each stressor is potentially nonlethal in itself, but in combination they can precipitate death (Figure 1). Given that approximately 90% of SIDS cases occur in the first six months of life, we postulate that the transition period from fetal to extrauterine life represents a critical time frame in which the brainstem circuitry for the maintenance of homeostasis undergoes rapid maturation and therefore is at greatest risk (Figure 1). In essence, we envision SIDS as a complex and heterogeneous process that involves multiple neurotransmitters and neuromodulators, multiple stressors acting simultaneously, and multiple genetic and environmental factors augmenting the intrinsic brainstem defects (Figure 1). Our objective here is to provide an up-to-date review of the rationale for brainstem research in SIDS, as well as an integrative overview of human, whole-animal, cellular, and developmental studies involving the medullary 5-HT and related neurotransmitter systems in an effort to elucidate potential mechanisms underlying sudden death in early life.
Figure 1.
Schematic representation of our concept of sudden infant death syndrome (SIDS) as a disorder of homeostasis due to abnormalities in the medullary serotonin (5-HT) system. This disorder involves multiple neurotransmitters and neuromodulators that interact with the defective medullary 5-HT system; multiple intrinsic and extrinsic (exogenous) stressors acting simultaneously; and the critical developmental period, i.e., the first six months of postnatal life when 90% of SIDS cases occur. Abbreviations: GABA, γ-aminobutyric acid; SP, substance P.
THE BRAINSTEM HYPOTHESIS
In 1987, just prior to the international recognition of the importance of the prone sleep position in SIDS, Hunt & Brouillette (27) articulated the consensus of the scientific community: “Although numerous general theories of cause are probably worthy of further clinical study, the most compelling hypothesis continues to be that SIDS is related to a brainstem abnormality in the neuroregulation of cardiorespiratory control.” This statement is, in our opinion, even more valid today than it was 20 years ago due to independent reports of subtle brainstem pathology in SIDS cases since 1987 (Table 1). The rationale for the brainstem hypothesis is based upon the following: (a) established evidence that the brainstem plays a critical role in respiratory and autonomic regulation, sleep, and arousal (28), i.e., the physiological processes considered abnormal in SIDS infants; (b) analogy to instances of sleep-related sudden death in children or adults in whom isolated or primary pathology is found by neuroimaging and/or at autopsy in the brainstem (29–31); and (c) reports of subclinical defects in cardiorespiratory control and/or arousal that are consistent with brainstem dysfunction in infants who are studied prospectively and who subsequently die of SIDS. These defects include impaired autoresuscitation (gasping), abnormal respiratory patterning, episodic obstructive apnea during sleep, autonomic dysfunction (episodic tachycardia/bradycardia, abnormal heart rate variability), and arousal deficits (32–42).
Table 1.
Neurotransmitter abnormalities in the brainstem in sudden infant death syndrome infants
| Substance | Our laboratory | Other investigators |
|---|---|---|
| 5-HT | Widespread decreased 5-HT1A binding, increased 5-HT cells, relative decrease in 5-HTT binding (25) | Increase 5-HT in raphé obscurus (HPLC) (189); reduced immunostaining for 5-HT1A and 5-HT2A receptors in NTS, DMX, VLM, locus coeruleus; increased immunostaining in periaqueductal gray (26) |
| ChAT | No studies performed | Decreased ChAT immunostaining in HG and DMX (62) |
| Muscarinic receptor | Decreased binding in arcuate nucleus (69) | Decreased number of neurons immunopositive for muscarinic receptors in arcuate nucleus (190), no decrease in muscarinic receptor immunostaining in arcuate nucleus (62) |
| Nicotine receptor | No abnormal binding, except in selected mesopontine nuclei upon stratification by history of exposures (64, 65) | No studies performed |
| CA | No abnormal α2-adrenergic receptor binding (191) | Increased dendritic spines in CA neurons in VLM (68): absent PNMT immunoreactivity in NTS (66), decreased TH immunoreactivity in VLM, DMX (192), TH immunostaining not correlated with density of TH-immunostained neurons in DMX, NTS, NA, VLM (193) |
| α2-adrenergic receptor | No abnormal binding (191) | Reduced α-2A (but not -2B and -2C) receptors by ICC in NTS, VLM (194) |
| NMDA receptor | No studies performed | Increased mRNA to NR1 subunit in six nuclei in medulla, increased NR1 protein in DMX, and decreased NR1 protein in spinal trigeminal nucleus (70) |
| Kainate receptor | Reduced binding in arcuate nucleus (69) | No studies performed |
| Opioids | No abnormal binding to 3H-naloxone (92) | No changes in homogenates of medulla (195) |
| GABA | No studies performed | No studies performed |
| Substance P | No studies performed | Increased in homogenates of medulla (195), increased immunostaining in trigeminal fibers (196), increased immunostaining in NTS and spinal trigeminal nucleus (197), no difference in binding in medulla (198) |
Abbreviations: 5-HT, 5-hydroxytryptamine; CA, catecholamines; ChAT, acetylcholine; DMX, dorsal motor nucleus of the vagus; GABA, γ-aminobutyric acid; HG, hypoglossal nucleus; HPLC, high-pressure liquid chromatography; ICC, immunocytochemistry; NA, nucleus ambiguous; NMDA, N-methyl-D-aspartic acid; NTS, nucleus of the solitary tract; PNMT, phenylethanolamine N-methyltransferase; TH, tyrosine hydroxylase; VLM, ventrolateral medulla.
In the decade following the review by Hunt & Brouillette, several major risk factors for SIDS in addition to prone sleep position were discovered, including face-down position, covered face in the supine position, soft bedding, bed sharing, overbundling, elevated room temperature, and minor infection at the time of death (4, 7, 10, 14, 20, 21, 42–44). The association of SIDS with these risk factors underscores the importance of the brainstem hypothesis, as these factors can be considered collectively as exogenous stressors that lead to asphyxia, hypoxia, hypercapnia, or thermal imbalance requiring intact brainstem defense systems to protect against lethal consequences. These defense systems involve central chemosensitivity to carbon dioxide (CO2) and peripheral and central chemosensitivity to oxygen (O2), autoresuscitation, and arousal with head lifting and turning to escape asphyxiating microenvironments by gaining fresh air. There is now evidence, for example, that the prone position can lead to rebreathing exhaled gases, particularly in soft bedding, which in turn leads to asphyxia (combined hypercapnia and hypoxia) (43). A defect in brainstem-mediated chemosensitivity could lead to the failure of arousal to protect against lethal asphyxia in this situation. Alternatively, a defect in brainstem-mediated autoresuscitation could result in death due to an inability to respond to hypoxic gasping. The possibility of defective autoresuscitation is suggested by cardiorespiratory tracings of SIDS infants monitored at the time of death, in whom abnormal and ineffectual gasping patterns have been observed (32, 33). Hyperthermia may result from a lack of (facial) heat loss in the face-down position, leading to heat-induced respiratory depression that is unchecked due to defective brainstem thermoregulatory mechanisms.
THE TRIPLE RISK MODEL
In 1994, as epidemiological reports emerged describing the benefit of supine sleep position in reducing SIDS rates worldwide, we proposed the Triple Risk Model as a framework for integrating the evolving epidemiological, physiological, and neuropathological data on SIDS (45). Like other models, the Triple Risk Model emphasizes the interaction of multiple factors in the pathogenesis of sudden death (46–48), but it reduces the concept to a simple Venn diagram with three interlocking circles. According to this model, SIDS results when three factors simultaneously influence the infant: (a) an underlying vulnerability in the infant, (b) a critical developmental period, and (c) an exogenous stressor, e.g., prone sleep position (45). In this model, such exogenous stressors are postulated to induce asphyxia, hypercapnia, and hypoxia. The critical period for SIDS (i.e., the first six months of life) coincides with dramatic and rapid changes in cardiorespiratory control and the sleep-wake cycle (45, 49–52). According to the Triple Risk Model, only infants with an underlying brainstem disease process die of SIDS, which explains why all infants who are placed prone to sleep or who bed share do not die of SIDS: They do not all have the underlying vulnerability. The model also explains why SIDS rates are reduced by the change to supine sleep position: The exogenous stressor is removed, allowing the vulnerable infant to pass through the critical period unharmed. In the context of the Triple Risk Model, the risk factors for SIDS can be divided into extrinsic and intrinsic categories. Extrinsic risk factors are acquired physical stressors related to the circumstances of death, e.g., prone sleep position. Intrinsic risk factors, however, are associated with the underlying vulnerability/abnormality in the infant and increase the likelihood of SIDS by exacerbating this abnormality. The intrinsic risk factors include prematurity, male gender, African American race, poverty, adverse prenatal factors such as maternal smoking or alcohol use during pregnancy, and genetic polymorphisms (7, 11–20, 53–56).
In a recent study of sudden infant death, 85% of 209 deaths were associated with circumstances consistent with asphyxia, including prone or face-down position when found, covered head, wedging, bed sharing, and sleeping on a couch, suggesting a major role for asphyxia in the pathogenesis of sudden infant death (57). Unquestionably, normal infants can become accidentally trapped in lethal, asphyxiating situations, such as wedging and overlaying (58, 59). We postulate, however, that SIDS infants (i.e., infants with underlying brainstem vulnerability/pathology) die in asphyxia-producing circumstances from which normal infants escape. In essence, SIDS is a disorder of homeostasis in which the infant with an underlying brainstem abnormality cannot adjust to or defend against asphyxia or other metabolic challenges that result from life-threatening events during sleep. Baseline brainstem function is likely adequate (albeit with probable subclinical compromise), given that those infants who subsequently die of SIDS look normal to their caretakers when awake: A homeostatic stress during sleep, in some critical way, unmasks the brainstem defect and triggers lethal decompensation. Indeed, very small perturbations with minor external stimuli can cause abrupt transitions in respiratory and other neural control systems in human infants (60).
BRAINSTEM PATHOLOGY IN SUDDEN INFANT DEATH SYNDROME
The fundamental paradox of the brainstem hypothesis in SIDS is that the brainstem looks normal upon review of conventionally stained sections under the light microscope. Thus, if the SIDS brainstem is abnormal, it is clear that quantitative methods at the cellular, subcellular, neurochemical, and/or molecular levels are needed to uncover the putative underlying pathology. The modern era of brainstem research in SIDS began with the seminal 1976 report describing subtle brainstem gliosis, which was detected and quantified with a specific astroglial fiber stain by the pediatric pathologist Dr. Richard Naeye (61). Naeye attributed this gliosis to chronic hypoxia due to sleep apnea and alveolar hypoventilation prior to the lethal event. Subsequent research focused upon the hypothesis that SIDS is due to a neurotransmitter defect in brainstem cardiorespiratory–related pathways, and investigators worldwide, including our group, reported various neurotransmitter and morphological abnormalities in SIDS cases in brainstem nuclei related (and unrelated) to cardiorespiratory control using immunocytochemistry, tissue autoradiography, and cellular quantitation (Table 1) (23–26, 62–72).
The Arcuate Nucleus
The evidence for rebreathing in the prone position set forth in the early 1990s led our group to focus initially upon the protective brainstem circuits involved in chemosensitivity to CO2 (63, 69, 73–75). A role for abnormal chemosensitivity in SIDS was also supported by the report of impaired responses to CO2 and central hypoventilation during sleep in 11 near-miss SIDS infants, 2 of whom subsequently died of SIDS, compared to normal infants (76). We proposed that the arcuate nucleus at the ventral medullary surface is a candidate region for central chemosensitivity in humans, based upon cytological and positional homologies between arcuate neurons and neurons within the respiratory chemosensitive fields on the ventral medullary surface in cats (73, 75). Structural underdevelopment of the arcuate nucleus was subsequently observed in SIDS cases (74, 77). We also reported reduced binding to the muscarinic cholinergic and glutamate-related (kainate) receptors, as these receptors contribute to the mediation of chemosensitivity by ventral neuronal populations in experimental animals (63, 69, 75). This arcuate anomaly was similar to that reported in an infant with clinical insensitivity to CO2 and sleep-related sudden death (31). Serotonergic neurons at the medullary ventral surface and in the midline (raphé) are now known to be preferentially chemosensitive to CO2, and although they are not the only central chemosensitive neurons (78, 79), they appear to play a critical, potentially modulatory role (78, 80–83). A small but important population of 5-HT neurons is embedded within the human arcuate nucleus (84), suggesting that the putative dysfunction in chemosensitivity related to the arcuate anomaly specifically involves these embedded 5-HT neurons. This defect likely reflects developmental disturbance in neuron proliferation, migration, and/or differentiation because, in the majority of cases, it is characterized by an underpopulation of neurons without gliosis. Yet the report (85) of acute neuronal necrosis, apoptosis, and gliosis in the arcuate nucleus in stillborn fetuses as early as midgestation and associated with hypoxic-ischemic injury elsewhere in the brain suggests the possibility that secondary injury occurs at this site as well.
The Medullary 5-HT System
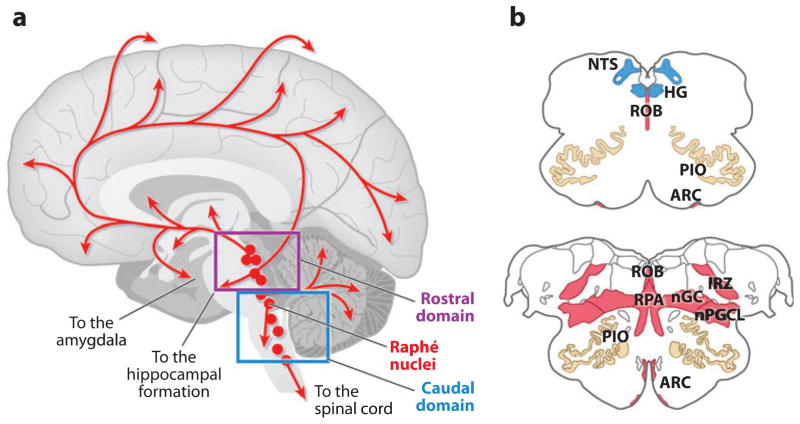
Our analysis of the medulla in SIDS led to our recognition that the ventral (arcuate) 5-HT neurons are part of a much larger 5-HT system in the caudal brainstem that, based upon extrapolations from animal data, is critical for the modulation and integration of diverse homeostatic functions (see below). This so-called medullary 5-HT system comprises the caudal domain of the entire brainstem 5-HT system (Figure 2). Notably, all of the 5-HT cell bodies in the brain are located in either rostral or caudal domains in the brainstem that differentially project to virtually all regions of the central nervous system (Figure 2) (86). These rostral and caudal domains are distinct not only in their anatomic location, but also in their developmental origins, functions, and connectivity. The rostral domain, located in the upper brainstem, projects diffusely to the cerebral cortex, thalamus, hypothalamus, basal ganglia, hippocampus, and amygdala (Figure 2). It helps mediate arousal, cognition, mood, motor activity, and cerebral blood flow (86) and has been implicated in cognitive problems and/or derangements in autism (87), depression (88), and fetal alcohol syndrome (89). The medullary 5-HT system projects diffusely to other brainstem sites, the cerebellum, and the spinal cord (Figure 2) and is critical for respiratory and autonomic output (Figure 3). This 5-HT caudal system may be involved in other disorders of respiratory and autonomic function than SIDS, e.g., Rett syndrome (90).
Figure 2.
(a) Sagittal view of whole human brain upon which the site of the 5-hydroxytryptamine (5-HT) neuronal cell bodies (red circles) and their projections (red lines) are superimposed. Serotonergic neurons are located in the brainstem in either the rostral or the caudal domain, each of which has different projections and functions. In the rostral domain, 5-HT neurons are present in the rostral pons and midbrain and project rostrally to the cerebral cortex, thalamus, hypothalamus, hippocampus, and basal ganglia to help modulate cognition and mood, among other functions. In the caudal domain, 5-HT neurons are located primarily in the medulla and project caudally to other brainstem regions, cerebellum, and spinal cord; this domain, known as the medullary 5-HT system, is postulated to integrate and modulate homeostatic function relative to the individual’s state. Interconnections exist between 5-HT neurons in the rostral and caudal domains but are poorly characterized. (b) Cross-sectional diagrams of the medullary 5-HT system at representative rostral-and midlevels. Panel b based upon the human brainstem atlas of Olszewski & Baxter (188). The nuclei shown in red are the site of 5-HT cell bodies, positioned in the midline raphé, extra-raphé, and ventral surface. The nucleus of the solitary tract (NTS) and the hypoglossal nucleus (HG) (blue), along with the principal inferior olive (PIO) (tan), are a major target site of 5-HT projections within the medulla. Abbreviations: ARC, arcuate nucleus; IRZ, intermediate reticular zone; nGC, nucleus gigantocellularis; nPGCL, nucleus paragigantocellularis; ROB, raphé obscurus; RPA, raphé pallidus.
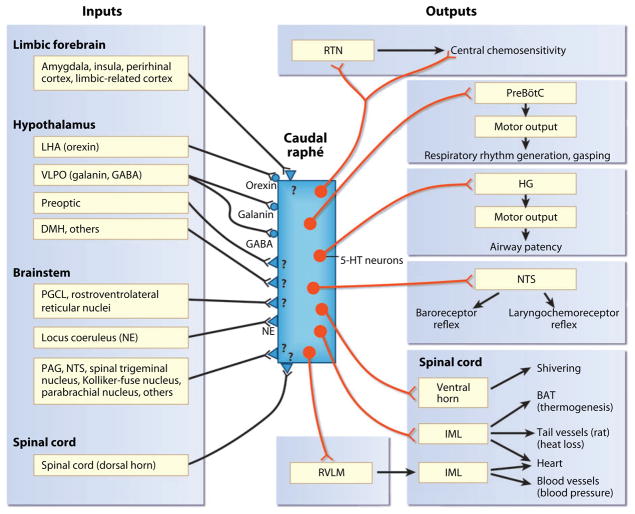
Figure 3.
Schematic diagram of the inputs and outputs of the caudal raphé in the medulla relevant to multiple homeostatic functions. The 5-hydroxytryptamine (5-HT) neurons (red dots) innervate the specific effector systems (right). Inputs into the caudal raphé (left) include known transmitter and receptor phenotypes that are specifically on 5-HT neurons (circles) and unknown transmitter/modulator phenotypes (triangles and question marks). The caudal raphé innervates the major effector nuclei of respiration, chemosensitivity, upper airway control, and autonomic regulation, including temperature. It receives inputs from the limbic system, the hypothalamus, other brainstem regions, and the spinal cord. Abbreviations: BAT, brown adipose tissue; GABA, γ-aminobutyric acid; HG, hypoglossal nucleus; IML, intermediolateral column of the spinal cord; LHA, lateral hypothalamic area; NE, norepinephrine; NTS, nucleus of the solitary tract; PAG, periaqueductal gray; PGCL, paragigantocellularis lateralis; PreBötC, pre-Bötzinger complex; RTN, retrotrapezoid nucleus; RVLM, rostral ventrolateral medulla; VLPO, ventrolateral preoptic area.
Anatomically, the medullary 5-HT system comprises the 5-HT neuronal cell bodies in the medulla that are located in the raphé, extra-raphé (medial and lateral reticular formation), and ventral surface (arcuate nucleus) (Figure 2). The inhibitory or excitatory effect of 5-HT is determined largely by the pre-and postsynaptic receptor subtypes. At least 17 5-HT receptor subtypes are currently recognized and are organized into 7 groups (5-HT1–5-HT7) based upon genetic and pharmacologic features, and all but one (5-HT3) act as neuromodulators through G proteins and second-messenger systems (91). Serotonergic receptors are located both synaptically and extrasynaptically with 5-HT1A receptors in the highest abundance as autoreceptors on the soma and dendrites of 5-HT neurons; 5-HT released from axodendritic processes of the same neuron, or from adjacent neurons, may activate these autoreceptors to inhibit firing.
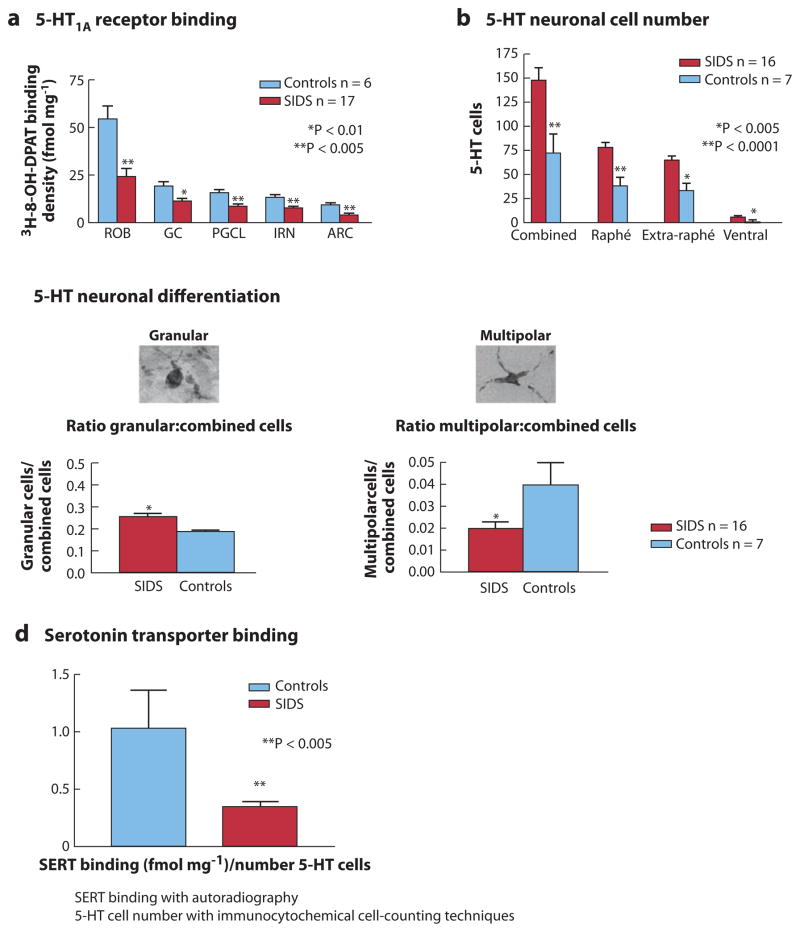
To date, the most robust neurochemical abnormality in SIDS involves the medullary 5-HT system (23–26), which is involved in approximately 70% of SIDS deaths (23–25) (Figure 4). For the neurochemical analysis of SIDS brainstems we use tissue receptor autoradiography, as it allows for precise neurochemical measurements in virtually all anatomic sites—even those as small as the arcuate nucleus—and it is capable of screening every nucleus for neurochemical abnormalities; these are important features for the initial analysis of brainstem neurochemistry in SIDS, in which abnormalities are postulated but for which there may be no specific histopathologic clues. We have now published 5-HT-related brainstem abnormalities in SIDS cases in three independent data sets: (a) cases from Children’s Hospital Boston and the San Diego Medical Examiner’s Office, 1985–1995 (period of case accrual, prior to the onset of the national supine sleep campaign) (23, 63, 69, 92, 93); (b) American Indian infants from the Northern Plains (a major high-risk population for SIDS), 1992–1994 (35, 65); and (c) cases from the San Diego Medical Examiner’s Office, 1996–2005 (in the era of safe sleep campaigns) (25).
Figure 4.
Multiple abnormalities in 5-hydroxytryptamine (5-HT) markers in sudden infant death syndrome (SIDS) infants compared to controls adjusted for postconceptional age in cases from the San Diego Medical Examiner’s Office, 1996–2005 (modified from Reference 25). (a) Binding to 5-HT1A receptors is significantly reduced in nuclei that contain 5-HT neurons and comprise the source neurons of the medullary 5-HT system. (b) The number of 5-HT neurons is increased in the same regions in SIDS cases compared to controls in the same data set. (c) The ratio of granular (immature) 5-HT neurons to total 5-HT neurons is significantly increased in the SIDS cases compared to controls, whereas the ratio of multipolar (mature or well-differentiated) 5-HT neurons to total 5-HT neurons is decreased, suggesting a developmental failure in 5-HT neuronal differentiation in the SIDS cases. (d ) The relative binding of the 5-HT transporter (SERT) to total number of 5-HT neurons is proportionately reduced in SIDS cases compared to controls, suggesting a relative failure in 5-HT transporter regulation relative to 5-HT cell number in SIDS. Abbreviations: ARC, arcuate nucleus; GC, ganglion cells; IRN, intermediate reticular nucleus; PGCL, paragigantocellularis lateralis; ROB, raphé obscurus.
In the first data set, we examined binding in multiple nuclei in the same brainstems (midbrain, pons, and medulla) for six neurotransmitter receptors (5-HT, muscarinic, nicotinic, opioid, α2-adrenergic, and kainate) with intentionally broad radioligands. We first observed the 5-HT receptor binding abnormality characterized by a reduction in binding or a decrease in binding with age compared to an increase in controls (age versus diagnosis interaction) with the radioligand 3H-lysergic acid diethylamide (3H-LSD), which binds to multiple 5-HT receptor subtypes, including 5-HT1A and 5-HT2A (23). This 5-HT receptor binding defect was localized almost exclusively to nuclei containing the 5-HT cell bodies in the medulla (raphé, extra-raphé, and ventral components of the medullary 5-HT system) (Figure 2) (23). In this first data set, we found no differences between SIDS and controls in binding to opioid and α2-adrenergic receptors in the same cases that had 5-HT receptor binding abnormalities (92, 93), indicating that the 5-HT binding deficits in SIDS cases are not part of a generalized receptor deficit, but rather represent a specific pattern whose cause has yet to be determined. The same pattern of 5-HT receptor binding abnormalities demonstrated by 3H-LSD in this data set was reproducible in the second independent data set of a high-risk population for SIDS, i.e., the American Indians of the Northern Plains.
In the third data set, we focused exclusively upon the medullary 5-HT system and analyzed several 5-HT-related tissue markers in SIDS and control cases. Using a radioligand specific for the 5-HT1A receptor, we found reduced binding not only in medullary nuclei containing 5-HT cell bodies, but also in nuclei receiving 5-HT projections, e.g., nucleus of the solitary tract and hypoglossal nucleus (Figure 4) (25). In addition, we found that this reduced 5-HT1A binding was associated with increased 5-HT cell number and density, morphological immaturity of 5-HT cell types, and a relative reduction in binding to the 5-HT transporter (5-HTT) compared to 5-HT cell number in the same SIDS cases (25). Notably, the 5-HTT is the key regulator of 5-HT levels at the synapse, and its upregulation can result from genetic factors, e.g., polymorphisms, and/or environmental factors, e.g., cytokines, alcohol, nicotine, and cocaine (94–96). Importantly, other groups have reported 5-HT abnormalities in the brainstem or cerebrospinal fluid of SIDS cases, including reduced 5-HT1A and 5-HT2A receptors detected by immunocytochemistry in the medulla (26), as well as genetic polymorphisms in the promoter region of the 5-HTT (56). Parenthetically, the LL (as opposed to the SS) genotype of the 5-HTT polymorphism associated with increased SIDS risk is thought to produce a mild 5-HT deficiency due to increased 5-HT uptake (97); this gene variation could potentiate an underlying medullary 5-HT abnormality when affected SIDS infants are stressed and optimal 5-HT modulation of homeostatic responses is essential.
Risk Factors and the Medullary 5-HT System
In the third data set we analyzed, all the SIDS cases (n = 31) had at least one intrinsic or extrinsic risk factor, 74% had at least three risk factors, and 64% were bed sharing or were found prone at the time of death (25). Medullary 5-HT abnormalities were found in SIDS infants who were (a) found dead in either the prone or the supine position, (b) found either face down or not face down, (c) either bed sharing or not bed sharing, and (d ) born either prematurely or at term (25). Thus, although 5-HT abnormalities were common among SIDS infants irrespective of particular risk factors, they were also associated with potentially asphyxiating environments such as prone sleep position and bed sharing. The small sample of SIDS cases in this study did not uncover a significant association between the LL genotype for the 5-HTT polymorphism, 5-HTT binding levels, and SIDS (25). The possibility that defects in autonomic and respiratory regulation are linked specifically to medullary 5-HT abnormalities is substantiated by our case report of a two-week-old SIDS infant who had subclinical autonomic and respiratory dysfunction at two days of age and whose autopsy revealed medullary 5-HT abnormalities (35). In the American Indians, 5-HT receptor binding in the arcuate nucleus was significantly reduced in infants of mothers who smoked during pregnancy compared with those infants whose mothers did not smoke (p = 0.010), irrespective of the cause of death (24). This association between a prenatal exposure and reduced brainstem 5-HT receptor binding in the postnatal period provides biologic plausibility as to how environmental risk factors potentiate an underlying brainstem abnormality and thereby increase the likelihood of sudden death associated with medullary 5-HT defects.
The Relationship of Brainstem Pathology to Hypoxia-Ischemia
Is the neurochemical brainstem pathology in SIDS infants secondary to hypoxia-ischemia, given the evidence that infants who subsequently died of SIDS had episodes of bradycardia and/or apnea days or even weeks prior to death? The possibility of chronic intermittent hypoxia (CIH) occurring prior to death is supported by reports in SIDS cases of hypoxic tissue markers, i.e., hyperplasia of pulmonary neuroendocrine cells (98), reduced hypoxanthine oxidase in the vitreous humor (99), and elevated vascular endothelial growth factor in the cerebrospinal fluid (100). The reports in SIDS of subtle, albeit nonspecific, gliosis in the brainstem (61, 67, 101, 102) are of particular interest, as the brainstem is a recognized region of vulnerability to hypoxia-ischemia in early life. Interestingly, gliosis develops within days of a brain insult, underscoring the possibility of subacute or chronic (nonagonal) injury in at least some cases. In addition, apoptosis has been reported in brainstem nuclei of SIDS cases, consistent with hypoxia-induced programmed cell death (103, 104).
Histopathologic abnormalities consistent with hypoxia-ischemia have also been reported in regions rostral to the brainstem, including reactive microglia in the hippocampus (105), cerebral white matter gliosis (106), and periventricular leukomalacia (106, 107), as well as in an increased number of copper/zinc superoxide dismutase– and glutathione peroxidase–expressing neurons in the hippocampus (108). Importantly, chronically ill infants who die from prolonged oxygenation disorders such as cyanotic congenital heart disease do not have medullary 5-HT abnormalities (or muscarinic/kainate receptor deficits) that mimic those found in SIDS (23, 69, 74); this finding counters the possibility that these neurotransmitter abnormalities are secondary to hypoxia-ischemia. Indeed, SIDS infants with medullary 5-HT abnormalities may be unable to respond to hypoxic challenges because impairment in adaptive responses to CIH is known to be regulated in part by the caudal 5-HT raphé (109). Intermittent hypoxia triggers an increase in output of the phrenic nerve (which drives the diaphragm) to activate postsynaptic 5-HT2A receptors and enhance glutamatergic-mediated respiratory drive (110).
Brainstem Abnormalities Associated with Maternal Cigarette Smoking during Pregnancy
Although prenatal exposure to nicotine (a major toxic component of cigarette smoke) reduces utero-placental blood flow and can cause fetal brain injury secondary to hypoxia-ischemia, nicotine itself—via interactions with the brain’s endogenous nicotinic receptors—can adversely affect neural development and function (111). In the first and second data sets described above, we found no alterations in nicotinic receptor binding between SIDS and control brainstems except when the cases were stratified by a history of maternal smoking during pregnancy (64, 65). Upon stratification, we found impaired regulation of nicotinic receptor binding in exposed SIDS cases compared to non-exposed SIDS cases, as well as to exposed and nonexposed controls in the same data sets with 5-HT receptor binding abnormalities (64, 65). The affected regions were in the rostral pons and/or midbrain in the exposed SIDS cases and involved nuclei critical for arousal (e.g., locus coeruleus, raphé dorsalis, and oral pontine reticular formation) and for the coordination of defense responses (e.g., periaqueductal gray) (64, 65).
Altered nicotinic receptor regulation upon exposure to cigarette smoke in SIDS infants is of interest in light of reports of arousal deficits in living infants exposed to prenatal cigarette smoke; these deficits include reduced frequency of spontaneous arousals (112) and reduced arousal responses to auditory stimuli and mild hypoxia (113, 114). The autopsy observations in SIDS brainstems, although demonstrated in two independent studies, involve small sample sizes and thus require confirmation in larger populations. Additional brainstem findings of gliosis, apoptosis, and arcuate nucleus hypoplasia in SIDS infants associated with histories of maternal smoking during pregnancy support the possibility of harmful effects of cigarette smoke/nicotine upon brainstem development in SIDS (104, 115). The association of altered nicotinic receptor binding in SIDS infants whose mothers smoked during pregnancy with 5-HT receptor binding abnormalities suggests that certain risk factors can adversely influence non-5-HT systems, which in turn compound the primary 5-HT abnormality with further dysfunction in cardiorespiratory patterning and/or arousal. In effect, a systematic analysis of multiple transmitters/modulators that interface with the medullary 5-HT system is needed to establish the precise neurochemical pathology in all or subsets of SIDS cases. Without such information, the pathogenesis of the disorder cannot be established, and optimal interventions cannot be developed.
The Cause and Pathogenesis of Medullary 5-HT Abnormalities
Although the cause and pathogenesis of medullary 5-HT abnormalities in SIDS are unknown, the finding of an increased number of 5-HT neurons in SIDS cases suggests a primary developmental defect in the regulation of 5-HT cell number during gestation, when 5-HT number is mainly (but not completely) determined (84). The increased number of 5-HT neurons of simple morphology (i.e., granular) and a decreased number of neurons of complex morphology (i.e., multipolar) in SIDS cases compared to controls supports a maturational delay in 5-HT neuron differentiation (25). However, we do not know whether the extracellular 5-HT levels in the medulla in SIDS cases are higher or lower compared to controls, nor do we know the nature of the mechanistic sequence of events leading to the changes in receptors and transporter. Nevertheless, the increased number of 5-HT neurons, coupled with the reduction in 5-HT1A receptor binding and the relative reduction in 5-HTT binding in medullary sites in SIDS cases, suggests that the synthesis and availability of 5-HT (and by extrapolation neuronal firing) are altered within 5-HT pathways.
The 5-HTT is expressed predominantly in perisynaptic sites on 5-HT neuron terminals, and is therefore a marker of 5-HT innervation in a given region. A relative reduction in 5-HTT expression may be due to reduced expression of 5-HTT protein at 5-HT neuron terminals and/or to a reduced number of 5-HT terminals and synapses. Mutations/polymorphisms in the genes coding for transcription factors and/or neurotrophins that regulate 5-HT development, along with exposure to harmful prenatal toxins, may adversely affect the development of the medullary 5-HT system. Maternal drinking and smoking during pregnancy, which are major risk factors for SIDS, impair 5-HT development in experimental animals (89, 116–118). An increased number of 5-HT neurons may lead to an excess of extracellular 5-HT and a compensatory downregulation of 5-HT1A receptors; alternatively, 5-HT synthesis and/or release may be dysfunctional in the 5-HT neurons (which become overabundant in compensation), resulting in a deficiency of extracellular 5-HT. Clearly, further research is needed to establish the cause and consequences of the medullary 5-HT defects in SIDS.
THE FUNCTION OF THE MEDULLARY 5-HT SYSTEM AND ITS RELATIONSHIP TO SUDDEN INFANT DEATH SYNDROME
How the abnormalities in the medullary 5-HT system translate into a mechanism for sudden and unexpected death in apparently healthy infants likely relates to the importance of this and related brainstem systems in multiple homeostatic processes that, if dysfunctional, could in sum promote death. These processes involve central chemoreception, modulation of inhibitory reflexes (e.g., laryngeal chemoreflex [LCR]), sleep, arousal, temperature regulation, and autoresuscitation. SIDS research depends upon the use of animal models of homeostatic dysfunction because there are no available biomarkers for identification and study of living infants at risk. Also, given that there are no known spontaneous models of SIDS in animals, we have focused upon the analysis of homeostatic function in young rats, mice, and piglets, in which abnormalities of the human medullary 5-HT and related pathology are produced by various pharmacological and genetic methods, as well as in reduced brainstem preparations and cell culture systems.
Breathing
Synaptic projections from 5-HT neurons are present in all of the major respiratory nuclei, including (a) the phrenic nucleus in the cervical spinal cord (drive to the diaphragm), (b) the hypoglossal nucleus (upper airway patency), (c) the nucleus of the solitary tract (visceral sensory reception for baroreceptor reflex, LCR, and peripheral chemosensitivity), (d ) the retrotrapezoid nucleus (additional site of chemoreceptors), and (e) the pre-Bötzinger complex (preBötC; the putative site of central rhythm generation for breathing) (81, 119, 120) (Figure 3). These terminals, many of which also contain the neuropeptides substance P (SP) and thyrotrophin-releasing hormone (TRH), arise from the raphé pallidus and the raphé obscurus (81, 121, 122). In addition, a subset of individual 5-HT axons arising in the raphé branch and send collaterals to both the preBötC and the hypoglossal nucleus (G.B. Richerson, unpublished observations; 123), suggesting a means by which raphé neurons coordinate and integrate functions between two different homeostatic-related sites. Receptors for 5-HT, SP, and TRH are located on neurons within each of the major respiratory nuclei (81); their activation in vitro induces modulatory effects on respiratory neurons that enhance excitability of respiratory neurons and respiratory network activity. For example, serotonin stimulates neurons of the preBötC via activation of 5-HT2A receptors by enhancing persistent sodium currents (124). Such 5-HT2A receptor activation also converts some preBötC neurons into intrinsic “bursters” by modulation of leak channels (G.B. Richerson, unpublished observations; 123); SP also helps induce bursting activity in these neurons, and TRH induces bursting pacemaker activity in neurons within the portion of the nucleus of the solitary tract involved in respiratory control (125). Serotonin, SP, and TRH also stimulate neurons in the retrotrapezoid nucleus (83) and in the hypoglossal nucleus (126). Thus, several neurotransmitters/neuropeptides released by 5-HT neurons directly enhance the excitability of multiple subsets of neurons within the respiratory network.
Using transverse, 350–600-μm-thick slice preparations of the brainstem, the rostral portion of the respiratory network containing the preBötC can be isolated (127). Similarly, the respiratory network can be isolated in more intact preparations that contain the whole brainstem and spinal cord (128). In all of these reduced brainstem preparations, rhythmic respiratory activity continues to be generated, and motor output can be recorded in the phrenic or hypoglossal nerves. This motor output is responsive to manipulations of the 5-HT system: 5-HT2A receptor blockers, for example, reduce the frequency and amplitude of respiratory activity generated by the preBötC in rostral medullary slice preparations (G.B. Richerson, unpublished observations; 123; 124), indicating that the generation of respiratory output depends upon the release of endogenous 5-HT from 5-HT neurons in the rostral medulla. Genetic mouse models that lead to either a decrease or an increase in 5-HT levels result in unstable respiratory output in vitro (129, 130). The Pet-1 knockout mouse with decreased 5-HT neuronal number and brain 5-HT levels, for example, generates an abnormal respiratory output with increased irregularity in rostral medullary slices (129). In contrast, mice with genetic deletion of necdin (130) and of monoamine oxidase A (an enzyme that degrades synaptic 5-HT) (131) demonstrate both increased brain 5-HT levels and substantial respiratory irregularity in the neonatal brainstem/spinal cord preparation.
Chemoreception: Response to Hypercapnia
An abnormal ventilatory response to increased CO2 has long been postulated as a determinant of SIDS (63, 69, 74). In addition, recent observations indicate a key role for asphyxia (i.e., the combination of hypercapnia and hypoxia) in the pathogenesis of SIDS (42, 57). Certainly, reduced chemosensitivity would be detrimental in asphyxia. Serotonergic neurons are well suited to a role as central respiratory chemoreceptors, as they are closely associated with the basilar artery and its largest branches near the ventral surface of the medulla; i.e., they are in a position to directly monitor arterial PCO2 (80). In brain slices and dissociated cell cultures in which 5-HT neurons are analyzed after isolation from synaptic inputs, 5-HT neurons respond intrinsically to increased PCO2 with a large increase in firing rate (132, 133) (Figure 5); this response is due to a decrease in intracellular pH induced by hypercapnia (133). On average, these neurons increase their firing rate threefold in response to a decrease in pH from 7.4 to 7.2. Chemosensitivity increases during postnatal development, with a blunted response to pH before postnatal day 12 in rats (134). This physiological delay in chemosensitivity is potentially relevant to SIDS because it indicates that 5-HT neurons may be immature during the critical developmental period, throughout which all infants are susceptible to hypercapnia.
Figure 5.
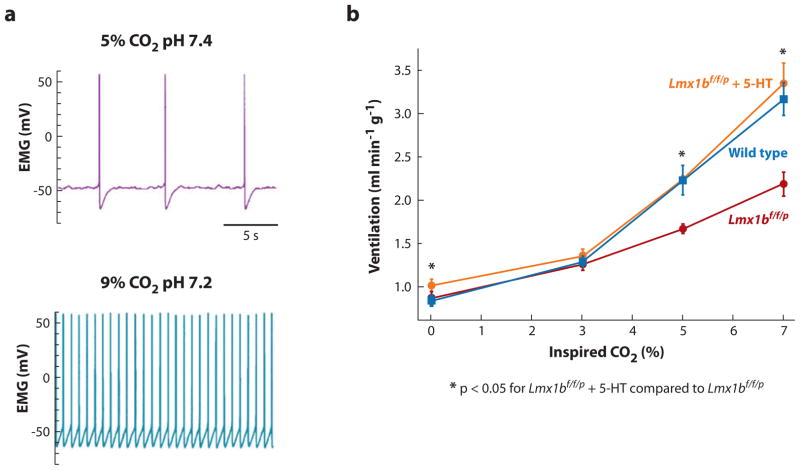
(a) Serotonergic neuronal firing in response to changes in carbon dioxide (CO2) and pH. Serotonergic neurons respond to CO2/acidosis with an increase in firing rate. Shown is the membrane potential of a 5-hydroxytryptamine (5-HT) neuron from a rat in primary cell culture recorded using a patch clamp electrode. Hypercapnic acidosis (high CO2 plus low pH) causes an increase in firing rate. This response is due to intrinsic chemosensitivity. Panel a modified from Reference 133 with permission. (b) Pharmacological “rescue” of abnormal CO2 chemosensitivity by intraventricular infusion of 5-HT in Lmx1b f/f/p mice. Central CO2 chemosensitivity is severely depressed in the absence of 5-HT neurons in these mice but can be rescued by therapeutic replacement of 5-HT. Ventilation in response to an increase in inhaled CO2 from 0% to 7% is shown for wild-type mice (blue curve), mice in which development of all 5-HT neurons is prevented by selective deletion of the gene for Lmx1b in all 5-HT neuron precursor cells (Lmx1b f/f/p) (red curve), and Lmx1b f/f/p mice (orange curve), whose lateral cerebral ventricles are infused with 5-HT via a catheter. Baseline ventilation is normal in the Lmx 1b mice, but the hypercapnic ventilatory response is decreased by 50%. Exogenous 5-HT stimulates baseline ventilation, and also restores the CO2 response to normal. Panel b adapted from Reference 135 with permission.
In whole animals, the role of 5-HT neurons in the response to hypercapnia is complex and depends upon the species, the anatomic locus in the medullary 5-HT system, age, gender, and the experimental paradigm. In adult rats, reduction of approximately 28% of the medullary raphé 5-HT neurons by injection of the cell-specific toxin anti-5-HT-saporin decreases the CO2 response by 18–20% both in waking and in non–rapid eye movement (NREM) sleep (136). Inhibition of these neurons by focal dialysis of the 5-HT1A receptor agonist 8-hydroxy-2-di-n-propylaminotetralin (DPAT), which presumably leads to inhibition of 5-T neuronal activity via autoreceptor activation, lowers the CO2 response by approximately 20% in these states. However, daily focal treatment of the medullary raphé by microdialysis of the specific 5-HT reuptake inhibitor fluoxetine increases the CO2 response (137).
Other neurotransmitters and neuromodulators influence chemosensitivity. For example, lesioning neurons that express the neurokinin-1 receptor (NK1R), which binds to SP, are associated with a decrease in the CO2 response, an effect that is exaggerated if 5-HT and the NK1R-expressing neurons are lesioned in combination (136). Our group studied unique conditional knockout adult mice in which the transcription factor Lmx1b was genetically deleted in Pet-1-expressing cells (Lmx1b f/f/p), which resulted in a near-complete absence of brainstem 5-HT neurons. In these mice the hypercapnic ventilatory response was decreased by 50% compared to wild-type mice, whereas baseline ventilation and the hypoxic ventilatory response remained normal (82).
Of major relevance to potential pharmacological treatment of 5-HT-related deficits and defective chemosensitivity in SIDS infants is our finding that infusion of 5-HT into the ventricle of these mice stimulated the baseline ventilation and “rescued” the blunted hypercapnic ventilatory response (82) (Figure 5). In this model, 5-HT appears to enhance the response of the remaining respiratory network to CO2; this effect could occur, for example, by enhancing the chemosensitivity of the neurons within the retrotrapezoid nucleus (83). Taken together, these adult mouse studies show that medullary raphé 5-HT cells participate in the CO2 response, that their effect depends on an interaction with NK1R-expressing neurons, and that there is an interaction with cells having GABA receptors, suggesting that endogenous GABA release normally modulates the CO2 response. Moreover, microdialysis of DPAT into the medullary raphé in newborn conscious piglets inhibits the activity of 5-HT neurons and decreases the CO2 response in an age-dependent manner, i.e., piglets younger than eight postnatal days show an increased response, whereas piglets older than eight postnatal days show a decreased response (138). When studied in early postnatal life, Pet-1 knockout mice also demonstrate irregular breathing and posthypoxic respiratory depression (129).
Of interest is the mounting evidence for sexually dimorphic features in the brainstem 5-HT system (139), given (a) that SIDS occurs twice as often in males compared to females (140), (b) our report of a greater reduction of 5-HT1A binding in the medullary raphé in males compared to females dying of SIDS (75), and (c) the report that plasma levels of testosterone, but not estradiol, are significantly higher in both male and female SIDS infants compared to age-matched control infants (141). In this regard, a substantial ablation (approximately 60%) of medullary raphé neurons with a selective toxin for 5-HT decreases the CO2 responses in NREM sleep in males only (142). In 5-HTT knockout mice, the reduction in ventilatory response is greater in males (68% reduction) compared to females (22% reduction) (142a). Notably, adult 5-HTT knockout mice demonstrate increased extracellular 5-HT (143), decreased tissue 5-HT, increased 5-HT synthesis, reduced 5-HT neuronal activity, reduced 5-HT1A receptor binding (144), and varying changes in postsynaptic 5-HT receptor function (145). Thus, this neurochemical phenotype mimics two of the 5-HT-related abnormalities described in the brainstem of the SIDS cases, i.e., reduced 5-HT1A and relative reduction of 5-HTT binding. In sum, these data underscore the gender differences in brainstem-mediated 5-HT function, with females’ brains apparently relying less on 5-HT neurons in chemoreception and adapting more readily to the loss of 5-HT function.
Chemoreception: Response to Hypoxia
Although multiple neurotransmitters influence protective brainstem responses to hypoxia (146), there is evidence for a role of the medullary 5-HT neurons in such responses (147). In newborn piglets with a 60% reduction in medullary 5-HT neuronal number due to specific neurotoxic injections into the cisterna magna, breathing frequency is significantly increased, for example, in response to hypoxia but only during NREM sleep (142). Thus, 5-HT neuronal activity in the caudal raphé may normally suppress the ventilatory response to hypoxia in a state-dependent manner, a finding that requires verification.
The Laryngeal Chemoreflex
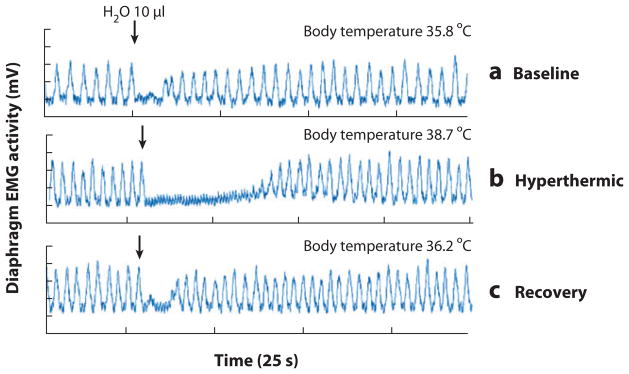
The LCR is elicited by water or other foreign liquids in the lumen of the larynx that stimulate mucosal receptors, which in turn project to respiratory-related medullary nuclei in the ventrolateral medulla via vagal projections to the nucleus of the solitary tract, resulting in apnea and swallowing. The LCR is developmentally regulated and occurs more commonly in newborn human infants and animals compared to adults (42, 148, 149). The LCR has long been suspected to be a cause of or a contributing factor in some SIDS cases; investigators hypothesize that infants during the susceptible age range aspirate gastric contents during sleep and thereby trigger this reflex, which, if prolonged or unchecked, leads to lethal apnea (148–151). In anaesthetized piglets, the nonspecific inhibition of all neurons in the rostral medullary raphé (by focal dialysis of the GABAA receptor agonist muscimol) causes a prolonged duration of apnea in the LCR (152). The LCR is also exaggerated in neonatal piglets and rats, whose body temperature rises 1–3°C above normal (150) (Figure 6). In piglets, the hyperthermic exaggeration of the LCR is most striking under postnatal day 5 and disappears by postnatal day 20 (151). Intravenous infusion of GABAA receptor blockers reverses the enhancement of laryngeal apnea by whole-body hyperthermia, suggesting that the effect of hyperthermia on the LCR is mediated by GABAergic inhibition of respiration (151a). The specific role of medullary 5-HT neurons in modulation of the LCR in early life is under investigation.
Figure 6.
The laryngeal chemoreflex (LCR) induced by intralaryngeal water in a 14-day-old male rat anesthetized with chloralose and urethane. The tracings represent (a) baseline, (b) hyperthermic, and (c) recovery conditions. Apnea is prolonged during hyperthermia. Modified from Reference 151. Abbreviation: EMG, electromyography.
Autoresuscitation
Asphyxia is strongly implicated in the sequence of events leading to SIDS (42), and is postulated to complicate rebreathing or upper airway compression in the face-down position or, alternatively, an exaggerated LCR triggered by gastric regurgitation during sleep. This asphyxia leads to death if effective life-saving mechanisms (e.g., autoresuscitation and/or arousal) do not intervene. In normal infants, autoresuscitation can terminate hypoxic gasping and thereby restore eupnea (normal breathing) (124, 153, 154); however, in vulnerable infants, autoresuscitation may fail and cause death. Tracings of infants who subsequently die of SIDS show effectual gasping with large-amplitude breaths, abnormally complex breaths, and failure to increase heart rate (33). Gasping appears to involve neurons within the rostral medulla that have a bursting discharge due to pacemaker: The initiation of gasping requires severe hypoxia, a loss of inhibition, potentiation by elevated potassium levels (which often accompany hypoxia), and activation of persistent sodium channels (153). Gasping (and more specifically, the activity of persistent sodium channels) is modulated by a variety of neurotransmitters, including 5-HT and norepinephrine via 5-HT2A and α1-adrenergic receptors, respectively (124, 154–156). Notably, neither 5-HT nor norepinephrine is required for hypoxic gasping in the isolated, perfused brainstem preparation in rats (154, 156). Yet when 5-HT1, 5-HT2, and α1 noradrenergic receptors are all blocked simultaneously, the number of gasps is significantly reduced and the restoration of eupnea is markedly impaired (154). Therefore, multiple neurotransmitter systems are likely involved in the regulation of gasping and autoresuscitation and no single neurotransmitter system appears essential.
Arousal
This protective mechanism allows for escape from asphyxiating microenvironments during sleep (42) and is postulated to be abnormal in at least some SIDS infants (39–41). Arousal comprises cortical and subcortical components and involves a progressive activation of specific subcortical and cortical brain structures. Cortical arousal results from ascending systems that originate in serotonergic, noradrenergic, dopaminergic, cholinergic, and histaminergic neurons in the rostral brainstem, basal fore-brain, and hypothalamus; these systems excite neurons in the cerebral cortex, increase their firing rate, and cause electroencephalography (EEG) activation (157). Descending (subcortical) arousal, however, is mediated mainly by brainstem pathways that increase heart rate, blood pressure, respiration, and postural tone (158). Subcortical arousal involves autonomic and respiratory brainstem-mediated changes without changes in cortical (EEG) activity; in contrast, complete arousals involve cortical and autonomic activation. Positive feedback loops involving the ascending and descending arousal pathways produce brain and somatic activation (159). In light of 5-HT neurons’ preferential chemosensitivity to CO2, their diffuse and widespread ascending and descending projections, and their close association with large arteries (160), we suggest that 5-HT neurons in the rostral and caudal domain mediate in part the arousal response to hypercapnia during sleep.
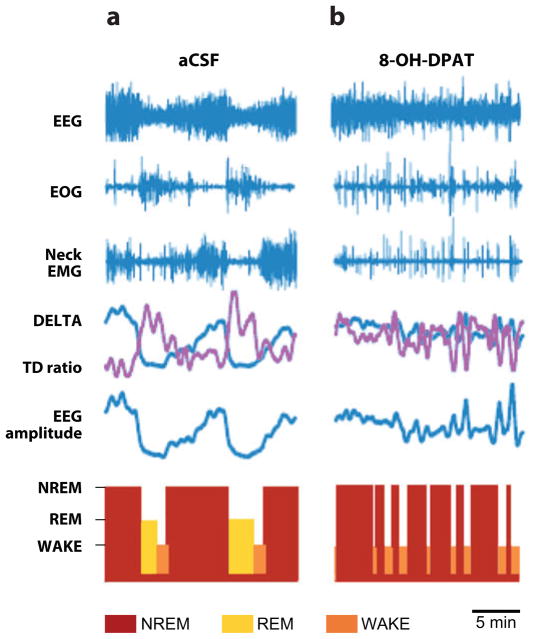
A distinct feature of 5-HT neurons in both the rostral and caudal domains is that they fire differentially according to the individual’s level of arousal, with increased firing during waking, decreased firing during NREM, and almost-complete absence of firing during REM (161, 162). Based upon the relationship between arousal level and neuronal excitability, different global roles for the caudal 5-HT domain have been proposed (161, 163, 164); for example, the overall function of the caudal raphé is to act as a “gain-setter” that modulates different autonomic and somatomotor control systems at levels appropriate to arousal (164) (Figure 3). We conceptualize the medullary 5-HT system as a modulatory circuit comprising 5-HT neurons in the raphé, extra-raphé, and ventral surface of the medulla that almost completely encircle and/or intermingle with neurons within nuclei that form the final common pathways that directly produce (i.e., mediate) the specific homeostatic function (Figures 2, 3). Inputs from the limbic system, hypothalamus, spinal cord, and other brainstem sites also modulate the medullary 5-HT system (Figure 3). Although it is widely recognized that 5-HT neurons in the rostral domain are important for arousal, researchers have devoted little attention to the role of 5-HT neurons in the caudal domain in this function. Recently, however, we reported that 5-HT neurons in the medullary extra-raphé [paragigantocellularis lateralis (PGCL)] of the piglet play an important role in the sleep/wake cycle such that their inhibition results in sleep fragmentation, alternating periods of NREM and waking, and almost complete loss of REM (165) (Figure 7). Similarly, activation of 5-HT1A receptors in the piglet raphé obscurus and raphé pallidus disrupt sleep and abolish REM (166). Thus, these findings implicate the medullary (raphé and extra-raphé) in the sleep/wake cycle.
Figure 7.
A typical experiment showing the effects of 8-hydroxy-2-di-n-propylaminotetralin (8-OH-DPAT, a 5-HT1A receptor agonist) dialyzed into the paragigantocellularis lateralis (PGCL) of the piglet (165). Data include electroencephalography (EEG), electrooculogram (EOG), neck electromyography (EMG), delta power (DELTA), the ratio of theta to delta power (TD ratio), and a hypnogram showing near–rapid eye movement (NREM) sleep (red bars), REM sleep ( yellow bars), and wakefulness (WAKE) (orange bars). Periods of NREM sleep are characterized by increases in EEG amplitude and DELTA power, no rapid eye movements on the EOG, and relatively increased neck EMG activity. REM is indicated by low EEG amplitude and DELTA power, an increased TD ratio, rapid eye movements, and low neck EMG activity. (a) A recording during a control period where artificial cerebrospinal fluid (aCSF) is dialyzed into the PGCL. (b) A recording after 30 min of dialyzing DPAT into the PGCL. After 5-HT1A receptor activation, there is sleep fragmentation characterized by alternating periods of NREM and WAKE and a complete absence of REM sleep.
Thermoregulation
Abnormal thermoregulation in SIDS is suggested by increased risk from heavy wrapping with blankets and/or clothing (overbundling), elevated ambient temperatures at the time of death (44), and increased periadrenal brown adipose tissue (BAT) at autopsy (61). Significantly, BAT, regulated by the sympathetic nervous system, is the major source of nonshivering thermogenesis in newborn humans and animals and plays an essential role in arousal during hibernation (167). In rats and rabbits, neurons in the medullary raphé modulate the sympathetic control of BAT thermogenesis and thermoregulatory skin vasoconstriction (168, 169). Specifically, 5-HT neurons increase their firing rates during cooling in parallel with increased BAT temperature (170, 171). Moreover, local application of 5-HT into the intermediolateral column of the spinal cord (preganglionic sympathetic outflow) increases sympathetic drive to BAT (172). In newborn piglets that are chronically instrumented and conscious, activation of 5-HT1A receptors by microdialysis of DPAT directly into the raphé or PGCL reduces shivering and peripheral vasoconstriction in response to acute and chronic cold stress (166). In addition, Lmx1bf/f/p mice with a profound deficiency in 5-HT neurons rapidly become hypothermic when exposed to cold ambient temperatures (4°C); this thermoregulatory failure is due to impaired shivering and nonshivering thermogenesis, while thermosensation and heat conservation remain intact (82). These studies implicate 5-HT in modulation of temperature.
Cardiovascular Control
Animal studies have implicated serotonergic modulation in the control of heart rate, heart rate variability, and the baroreceptor reflex (173, 174). In the rat nucleus of the solitary tract, for example, 5-HT2A receptors facilitate modulation of the cardiovagal component in the baroreceptor reflex; this component is triggered by 5-HT released from the nodose ganglion and/or the caudal raphé (173). In rats, 5-HT2A receptors are also expressed upon the dendrites of catecholaminergic neurons, suggesting 5-HT-catecholaminergic interactions in the function of the nucleus of the solitary tract (174).
THE DEVELOPMENT OF BRAINSTEM 5-HT NEURONS
Serotonergic abnormalities in SIDS brainstems are likely to originate during prenatal development. Evidence supporting this hypothesis includes the increased risk for SIDS associated with adverse exposures during pregnancy (7, 11, 13), features of abnormal 5-HT maturation (i.e., aberrant regulation of 5-HT cell number and morphology) in the SIDS medulla (25), and altered 5-HT and nicotinic receptor binding the medulla in the postnatal period associated with adverse influences of prenatal alcohol and smoking exposure (24, 175). Given the important role of 5-HT as a growth factor in early cell division, migration, and differentiation in the brain (176, 177), 5-HT may be especially critical in the pathogenesis of the 5-HT-related abnormalities in medullary development in SIDS as well. Thus, a requisite step toward identifying underlying mechanisms in SIDS is the delineation of where, when, and how 5-HT neurons develop during embryonic and fetal life. Substantial progress has recently been made in this area of developmental neurobiology through the use of various model organisms (e.g., mouse, rat, chicken) and cutting-edge, molecular genetic approaches (e.g., gene gain- or loss-of-function studies and genetic fate mapping) (178–181). Several of these advances, which are indeed shaping contemporary SIDS research, are summarized below.
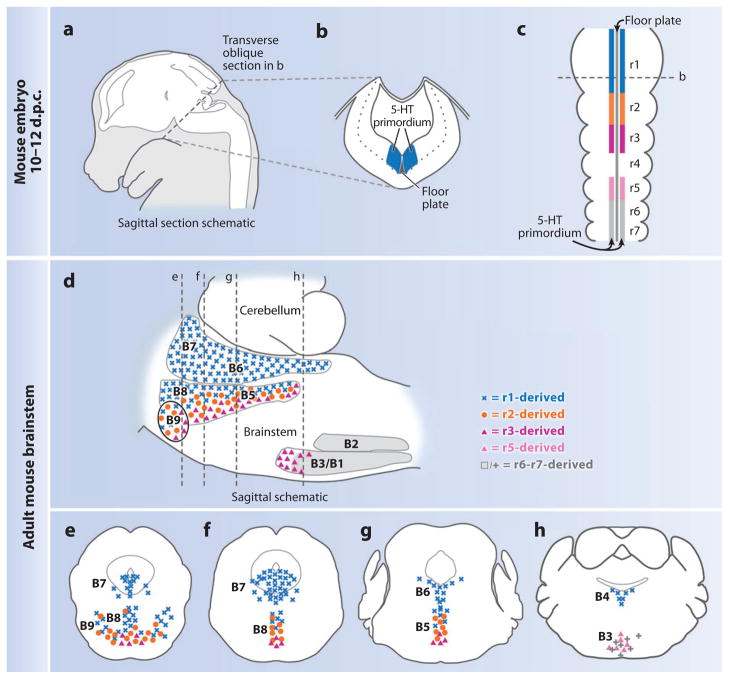
Serotonergic progenitor cells reside in the embryonic hindbrain in territories that bilaterally flank the floor plate and span much of the rostrocaudal extent of the hindbrain (182) (Figure 8). Acquiring generic 5-HT neuron identity from these progenitor cells involves turning on the expression of the transcription factor–encoding genes, which include Nkx2.2, Mash1/Ascl1, and Foxa2 (178–180, 183). This is then followed by the activation of expression of the transcription factors Pet1, Lmx1b, and Gata2/3, which take descendant postmitotic precursor cells to a state of 5-HT production, the hallmark of the mature 5-HT neuron. Sonic hedgehog, a signaling molecule secreted by nearby floor plate cells, initiates this cascade of cellular and molecular events (178–180, 184). Understanding the genetic programs and signaling pathways critical to 5-HT neuron development is important because they identify avenues for exploration with respect to SIDS pathogenesis, such as alterations in various transcription factors either through genetic mutation or upon prenatal exposure to known risk factors for SIDS, such as alcohol and cigarette smoke (discussed above).
Figure 8.
The organization of 5-hydroxytryptamine (5-HT) neurons as defined by embryonic origin and developmental gene expression profile. This organization differs from that based historically on anatomical architecture. (a–c) Schematics of mouse embryo at 10–12 days postcoitum (d.p.c.) illustrate the sagittal section (with the brain shown in white) (a), the transverse section (b), and the dorsal view of the hindbrain (c). The dashed lines in panels a and c represent the level of section in panel b. The 5-HT primordium is situated on either side of the floor plate and spans almost the entire length of the hindbrain. (d ) Sagittal schematic of adult brainstem compressed along the mediolateral axis. Blue represents the 5-HT progenitor cells in r1 (c) or r1-derived mature 5-HT neurons (d ); orange, r2 5-HT progenitors (c) or descendants (d ); magenta, r3; pink, presumed r5; gray, r6–r7. B1–B9 refer to the names given to nuclei defined anatomically. The dashed lines represent coronal sections (e–h) presented rostral to caudal, left to right. r1-derived 5-HT neurons (blue) populate the B7, B6, and B4 groups in their entirety, as well as aspects of the B9, B8, and B5 nuclei. r2-derived 5-HT neurons (orange) populate the B9, B8, and B5 nuclei intermingled with both the r1-derived (blue) and the r3-derived (magenta) 5-HT neurons. Presumed r5-derived 5-HT neurons ( pink) populate the B3 nucleus intermingled with more caudal (r6–r7) 5-HT neurons ( gray). Figure modified from Reference 187.
Although 5-HT production generally de-fines a neuron as serotonergic, there are in fact many different subtypes of 5-HT neurons that are distinguishable by their different and widespread positions in the brainstem (as constituents of the nine different types of 5-HT nuclei historically referred to as B1–B9) (185), by differences in their synaptic targets (86), and by their coexpression of different neuropeptides and neurotransmitters (e.g., GABA, SP, and/or TRH) (186), as well as by their different cellular, electrophysiological, and functional properties (e.g., some 5-HT neurons are chemosensitive and others are not; some 5-HT neurons are involved in pain, others in respiration) (183). Understanding which of these parameters come together in a single cell to define a specific functional subtype of 5-HT neuron is an area of active investigation for our group, given that different 5-HT neuronal subtypes are likely associated with different disease vulnerabilities. Of interest here is the identification of those 5-HT subtypes most relevant to SIDS; achieving this goal requires knowledge of the molecular markers of these different subtypes. In working toward this goal, we recently developed an approach that takes advantage of molecular differences among 5-HT progenitor cells, rather than relying on the identification of molecular differences within mature 5-HT neurons (187). We are investigating the possibility that each of the molecularly distinct 5-HT progenitor cell pools gives rise to a functionally distinct subtype of 5-HT neurons. Our rationale is based upon the concept that developmental programs that define the fate and function of neurons are often set in motion by the action of factors that are differentially expressed among their antecedent progenitors.
The 5-HT progenitor territory can be subdivided along the rostrocaudal axis into groups on the basis of the broader partitioning of the hindbrain into segments (rhombomeres) with distinguishing gene expression profiles (Figure 8). Thus, aspects of mature 5-HT neuron subtype identity may be determined through the actions of rhombomere (r)-specific genetic programs on resident 5-HT progenitor and precursor cell subsets. Using the method of genetic fate mapping, we deconstructed the 5-HT neural system based on rhombomere-defined 5-HT sublineages, distinguishing those 5-HT neurons arising from the primordium as r1, r2, r3, r5, or r6–r8 (187) (Figure 8). [5-HT neurons are thought not to arise from r4 (183).] Interestingly, we found that the generated developmental and molecular map of the 5-HT neural system differs from the classical anatomically defined groupings (B1–B9) of mature 5-HT neurons (187) (Figure 8). Some anatomically defined B1–B9 nuclei receive contributions from multiple (molecularly distinct) progenitor cell pools, whereas others receive contributions from only one such pool. Are these newly identified genetic sublineages of physiological relevance? That is, do genetically defined subtypes of 5-HT neurons serve different functions, despite coresiding within a single anatomical nucleus? If so, which particular subtypes serve functions that, if defective, might render an infant vulnerable to SIDS? Answering these questions has become possible because a set of tools similar to that used to identify these genetic sublineages of 5-HT neurons can be used to selectively manipulate properties of individual sublineages, such as neurotransmission, in otherwise normal mice.
CONCLUSIONS
We postulate that SIDS results from the convergence of multiple factors primarily (but not exclusively) involving 5-HT-mediated mechanisms in the medulla (Figure 1). Given the wide array of homeostatic functions modulated by the medullary 5-HT system, we propose that an important subset of SIDS is due to a convergence of abnormal protective responses that are modulated by 5-HT and potentially by other neuromodulators and neurotransmitters (Figures 1, 3). Findings from laboratories worldwide, including ours, indicate that medullary 5-HT neurons have multiple roles in the control of breathing, blood pressure, temperature, and arousal and that their function differs by gender, age, and level of arousal. Moreover, these 5-HT neurons respond directly to CO2 as CO2 detectors, modulate the function of other CO2 receptor neurons, and affect hypercapnic and hypoxic responses in a gender-dependent manner. Neurons in the medullary raphé modulate the LCR, although we do not know whether 5-HT neurons specifically participate in this important inhibitory reflex. We speculate that primary medullary 5-HT defects cause subclinical homeostatic dysfunction days or weeks prior to the final lethal event and that they also cause repetitive hypoxia, leading in turn to secondary subtle brain injury and systemic hypoxic injury prior to death. It is also likely that particular sites of origin within the embryonic neural tube, with their unique profiles of gene expression, set in motion developmental programs that subsequently define functionally different subsets of raphé and extra-raphé 5-HT neurons (Figure 8), such that differences in origin likely determine postnatal functional differences within the 5-HT system.
Based upon the combined human and animal data presented above, we propose that SIDS results from the convergence of exogenous stressors (e.g., face-down sleep position, overbundling), a critical developmental period, sleep, and dysfunctional and/or immature cardiorespiratory and/or arousal systems (i.e., underlying vulnerability) that often involve abnormal 5-HT mechanisms (Figure 1). A lethal sequence, for example, might begin with intermittent hypoventilation, apnea, and periods of tachycardia and/or bradycardia—not uncommon events in early life. Uninterrupted by arousal, this process leads to a downward spiral of progressive asphyxia, bradycardia, metabolic acidosis, and ultimately hypoxic gasping that is ineffectual, potentially related to an intrinsic 5-HT defect in the neural network underlying gasping. A similar sequence could begin with the LCR, a developmental reflex that is occasionally stimulated by regurgitated gastric contents in virtually all babies, resulting in a few seconds of apnea without serious consequences. If an infant’s LCR is potentiated by a mildly elevated temperature, if the medullary 5-HT and/or GABAergic systems are at a particular developmental stage, and/or if the infant happens to be in a particular sleep state at the time of the LCR, the result may be prolonged apnea (Figure 9). Further, if an infant’s ventilatory response to the progressive hypoxia and hypercapnia during the apnea is depressed, and if the hypoxic gasping and/or arousal mechanism is abnormal, the lack of oxygen from uninterrupted apnea may lead to death (Figure 9).
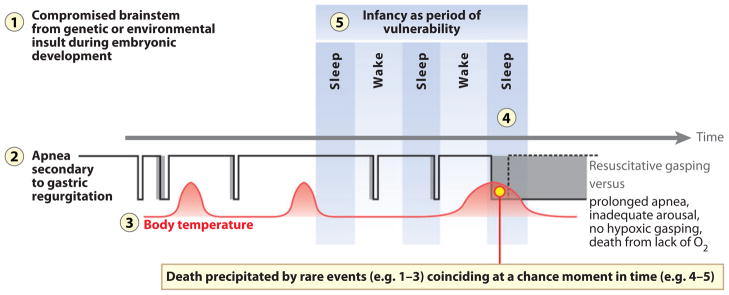
Figure 9.
Schematic diagram of the concept of sudden infant death syndrome (SIDS) as the biologic version of the perfect storm, in which the chance combination of multiple events results in sleep-related sudden death during a critical developmental period and in which the combination of events is far more powerful than each individual event alone. Depicted here is one possible lethal scenario, in which the vulnerable infant with an underlying abnormality in the medullary 5-HT system (influenced by adverse prenatal exposures and/or genetic susceptibilities) (1) passes through the critical postnatal period in homeostatic development (5) and experiences regurgitation of gastric contents and triggering of the laryngeal chemoreflex (LCR), i.e., a life-threatening challenge (2). The infant may be slightly febrile due to an otherwise trivial upper respiratory tract infection (3); as a consequence, the apnea component of the LCR is inordinately prolonged by mild hyperthermia (4). Further, if the infant’s ventilatory response to the progressive hypoxia and hypercapnia during the apnea is depressed, and if the hypoxic gasping and/or arousal mechanism is abnormal, oxygen lack from uninterrupted apnea results (red circle). Ultimately death occurs within minutes to hours.
The concept of a combination of events coming together to result in death extends to the cellular level as well, as exemplified by the neurotransmitter modulation of gasping. In this context, blocking 5-HT receptors (primarily 5-HT2A) alone does not alter gasping in the isolated perfused rat brainstem preparation; however, when much smaller doses of 5-HT blockers are combined with equally small doses of noradrenergic blocker, gasping is markedly inhibited (156). Thus, gasping does not appear to depend upon a single neurotransmitter system, but it is quite vulnerable to disruption by much smaller defects in multiple neurotransmitter systems. We now conceptualize SIDS as the biologic version of the perfect storm, in which the simultaneous and chance combination of multiple events is far more powerful than any individual event alone (Figure 9). The emerging data about the role of the medullary 5-HT system in many SIDS infants may pave the way for future identification of infants at risk and specific interventions to prevent death.
SUMMARY POINTS.
The brainstem hypothesis in SIDS states that a majority of SIDS cases are due to a defect in brainstem-mediated protective responses to homeostatic stressors during sleep in a critical developmental period.
The brainstem hypothesis in SIDS is supported by several lines of evidence, including independent reports of subtle brainstem findings in SIDS cases at autopsy and reports of cardiorespiratory and arousal (subclinical) deficits in infants who subsequently die of SIDS, which are consistent with brainstem dysfunction.
The most reproducible and robust neurochemical findings in the SIDS brainstems studied to date involve abnormalities in the medullary 5-HT system, as detected by immunocytochemical, quantitative cellular, and tissue autoradiographic methods in brainstem tissues specially prepared at the time of autopsy.
Extensive experimental analysis indicates that the medullary 5-HT system is critical for the modulation and integration of a variety of homeostatic functions (e.g., respiration, chemosensitivity, autoresuscitation, blood pressure, temperature) that are state and age dependent, and underscores the possibility that deficits in this system could lead to sleep-related sudden death under stress in a critical developmental period.
State-of-the-art genetic mapping studies indicate that different subpopulations of 5-HT neurons originate from different progenitor pools in the embryonic brainstem, underscoring the possibility that these different populations may mediate different homeostatic functions.
A prenatal origin of medullary 5-HT abnormalities in SIDS is suggested by (a) the finding of deficits in the regulation of 5-HT cell number and differentiation in the medullae of affected cases, (b) epidemiologic data indicating that prenatal adverse exposures (e.g., alcohol and cigarette smoke/nicotine) are major risk factors for SIDS, and (c) autopsy data showing that prenatal exposure to cigarette smoke is associated with reduced 5-HT receptor binding in the arcuate nucleus in the postnatal period, irrespective of the cause of death.
FUTURE ISSUES.
Centralized sites for the collection and disposition of SIDS and control tissues specifically handled for research analysis need to be developed.
Future studies are needed to delineate the number and types of neurotransmitters and neuromodulators that may be part of the neurochemical brainstem pathology in SIDS in association with serotonin.
Ongoing basic science research is needed to determine the precise mechanisms whereby multiple homeostatic stressors interact with multiple brainstem neurotransmitter systems, including 5-HT, as well as with complex genetic and environmental risk factors, to cause sudden death in early life.
Fundamental research is needed to understand where, how, and when specific subpopulations of 5-HT neurons develop during fetal life, and if and how they are programmed to regulate specific homeostatic functions after birth.
Translational research is needed to develop methods to identify living infants at risk for SIDS with 5-HT-related brainstem pathology and to establish preventative strategies against sudden death during the critical period.
Acknowledgments
We would like to thank our many colleagues, medical examiners, students, and technicians for the dedicated work in SIDS research (presented above) from our laboratories. We are also grateful to the many SIDS families and organizations that have supported this research throughout the years. We appreciate the ongoing support of Dr. Holcombe E. Grier. Our work is supported by grants from the National Institutes of Health [R37-HD20991, R37-HL28066, PO1-HD36379, and Children’s Hospital Boston Developmental Disabilities Research Center (P30-HD18655)], the C.J. Murphy Foundation, the First Candle/SIDS Alliance, a Deborah Evelyn Barrett Fellowship for SIDS Research, the C.J. Foundation for SIDS, and the Scottish Crib Death Foundation.
Glossary
- Asphyxia
severe hypoxia and hypercapnia
- 5-hydroxytryptamine (5-HT)
also known as serotonin; a major monoamine neurotransmitter produced by neurons located in the rostral and caudal brainstem (raphé, extra-raphé, and medullary ventral surface regions) that plays a role in virtually all functions of the central nervous system via diffuse and collateralized projections throughout the neuroaxis
- Arousal
transition from sleep to wakefulness
- Autoresuscitation
process by which a newborn can recover from severe asphyxia
- Gasping
abrupt large breaths that occur in severe asphyxia or hypoxia
- Hypoxia
below-normal oxygen levels in lung, blood, or other tissues (measured at sea level)
- Hypercapnia
arterial carbon dioxide levels above 40 mm Hg
- Chemosensitivity
refers to the ventilatory response to a change in carbon dioxide/pH as sensed by tissue chemoreceptors, which are composed of neurons and/or astrocytes
- Chronic intermittent hypoxia (CIH)
episodic periods of low oxygen interspersed between variable periods of normoxia; typically associated with sleep apnea
- Laryngeal chemoreflex (LCR)
developmentally regulated reflex in which apnea and swallowing are triggered by the stimulation of receptors in the laryngeal lumen by a foreign liquid
- Pre-Bötzinger complex (preBötC)
collection of bilateral neurons in the rostral ventrolateral medulla of experimental animals; the putative site of central rhythm generation critical for breathing
- GABA
γ-aminobutyric acid
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Krous HF, Beckwith JB, Byard RW, Rognum TO, Bajanowski T, et al. Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114:234–38. doi: 10.1542/peds.114.1.234. [DOI] [PubMed] [Google Scholar]
- 2.Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B. Annual summary of vital statistics: 2004. Pediatrics. 2006;117:168–83. doi: 10.1542/peds.2005-2587. [DOI] [PubMed] [Google Scholar]
- 3.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2003 period linked birth/infant death data sets. Natl Vital Stat Rep. 2006;54:1–29. [PubMed] [Google Scholar]
- 4.Willinger M, Hoffman HJ, Hartford RB. Infant sleep position and risk for sudden infant death syndrome: report of meeting held January 13 and 14, 1994, National Institutes of Health, Bethesda, MD. Pediatrics. 1994;93:814–19. [PubMed] [Google Scholar]
- 5.Corwin MJ, Lesko SM, Heeren T, Vezina RM, Hunt CE, et al. Secular changes in sleep position during infancy: 1995–1998. Pediatrics. 2003;111:52–60. doi: 10.1542/peds.111.1.52. [DOI] [PubMed] [Google Scholar]
- 6.Willinger M, Ko CW, Hoffman HJ, Kessler RC, Corwin MJ. Factors associated with caregivers’ choice of infant sleep position, 1994–1998: the National Infant Sleep Position Study. JAMA. 2000;283:2135–42. doi: 10.1001/jama.283.16.2135. [DOI] [PubMed] [Google Scholar]
- 7.Moon RY, Horne RS, Hauck FR. Sudden infant death syndrome. Lancet. 2007;370:1578–87. doi: 10.1016/S0140-6736(07)61662-6. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro-Mendoza CK, Tomashek KM, Anderson RN, Wingo J. Recent national trends in sudden, unexpected infant deaths: more evidence supporting a change in classification or reporting. Am J Epidemiol. 2006;163:762–69. doi: 10.1093/aje/kwj117. [DOI] [PubMed] [Google Scholar]
- 9.Malloy MH, MacDorman M. Changes in the classification of sudden unexpected infant deaths: United States, 1992–2001. Pediatrics. 2005;115:1247–53. doi: 10.1542/peds.2004-2188. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell E, Thach B, Thompson J, Williams S. Changing infants’ sleep position increases risk of sudden infant death syndrome. Arch Pediatr Adolesc Med. 1999;154:1136–41. doi: 10.1001/archpedi.153.11.1136. [DOI] [PubMed] [Google Scholar]
- 11.Iyasu S, Randall LL, Welty TK, Hsia J, Kinney HC, et al. Risk factors for sudden infant death syndrome among Northern Plains Indians. JAMA. 2002;288:2717–23. doi: 10.1001/jama.288.21.2717. [DOI] [PubMed] [Google Scholar]
- 12.Horne RS. Effects of prematurity on heart rate control: implications for sudden infant death syndrome. Exp Rev Cardiovasc Ther. 2006;4:335–43. doi: 10.1586/14779072.4.3.335. [DOI] [PubMed] [Google Scholar]
- 13.Blair PS, Sidebotham P, Berry PJ, Evans M, Fleming PJ. Major epidemiological changes in sudden infant death syndrome: a 20-year population-based study in the UK. Lancet. 2006;367:314–19. doi: 10.1016/S0140-6736(06)67968-3. [DOI] [PubMed] [Google Scholar]
- 14.Beal SM. Sudden infant death syndrome in South Australia 1968–97. Part I: Changes over time. J Paediatr Child Health. 2000;36:540–47. doi: 10.1046/j.1440-1754.2000.00563.x. [DOI] [PubMed] [Google Scholar]
- 15.Ford RP, Nelson KP. Higher rates of SIDS persist in low-income groups. J Paediatr Child Health. 1995;31:408–11. doi: 10.1111/j.1440-1754.1995.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 16.Hunt CE. Sudden infant death syndrome and other causes of infant mortality: diagnosis, mechanisms, and risk for recurrence in siblings. Am J Respir Crit Care Med. 2001;164:346–57. doi: 10.1164/ajrccm.164.3.9910045. [DOI] [PubMed] [Google Scholar]
- 17.Weese-Mayer DE, Ackerman MJ, Marazita ML, Berry-Kravis EM. Sudden infant death syndrome: review of implicated genetic factors. Am J Med Genet A. 2007;143:771–88. doi: 10.1002/ajmg.a.31722. [DOI] [PubMed] [Google Scholar]
- 18.Hunt CE. Gene-environment interactions: implications for sudden unexpected deaths in infancy. Arch Dis Child. 2005;90:48–53. doi: 10.1136/adc.2004.051458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Opdal SH, Rognum TO. The sudden infant death syndrome gene: Does it exist? Pediatrics. 2004;114:e506–12. doi: 10.1542/peds.2004-0683. [DOI] [PubMed] [Google Scholar]
- 20.Hauck FR, Herman SM, Donovan M, Iyasu S, Merrick Moore C, et al. Sleep environment and the risk of sudden infant death syndrome in an urban population: the Chicago Infant Mortality Study. Pediatrics. 2003;111:1207–14. [PubMed] [Google Scholar]
- 21.Mitchell EA, Brunt JM, Everard C. Reduction in mortality from sudden infant death syndrome in New Zealand: 1986–92. Arch Dis Child. 1994;70:291–94. doi: 10.1136/adc.70.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molteno CD, Ress E, Kibel MA. Early childhood mortality in Cape Town. S Afr Med J. 1989;75:570–74. [PubMed] [Google Scholar]
- 23.Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, et al. Decreased serotonergic receptor binding in rhombic lip–derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2000;59:377–84. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- 24.Kinney HC, Randall LL, Sleeper LA, Willinger M, Belliveau RA, et al. Serotonergic brainstem abnormalities in Northern Plains Indians with the sudden infant death syndrome. J Neuropathol Exp Neurol. 2003;62:1178–91. doi: 10.1093/jnen/62.11.1178. [DOI] [PubMed] [Google Scholar]
- 25.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, et al. Multiple sero-tonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–32. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 26.Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002;33:142–49. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- 27.Hunt CE, Brouillette RT. Sudden infant death syndrome: 1987 perspective. J Pediatr. 1987;110:669–78. doi: 10.1016/s0022-3476(87)80001-x. [DOI] [PubMed] [Google Scholar]
- 28.Saper CB, Richerson GB, Lumsden A. The brainstem and cranial nerves. In: Kandal ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 5. New York: McGraw-Hill; 2008. In press. [Google Scholar]
- 29.Parenti A, Macchi V, Snenghi R, Porzionato A, Scaravilli T, et al. Selective stroke of the solitary tract nuclei in two cases of central sleep apnoea. Clin Neuropathol. 2005;24:239–46. [PubMed] [Google Scholar]
- 30.Morrell MJ, Heywood P, Moosavi SH, Guz A, Stevens J. Unilateral focal lesions in the rostrolateral medulla influence chemosensitivity and breathing measured during wakefulness, sleep, and exercise. J Neurol Neurosurg Psychiatry. 1999;67:637–45. doi: 10.1136/jnnp.67.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folgering H, Kuyper F, Kille JF. Primary alveolar hypoventilation (Ondine’s curse syndrome) in an infant without external arcuate nucleus. Case report. Bull Eur Physiopathol Respir. 1979;15:659–65. [PubMed] [Google Scholar]
- 32.Poets CF. Apparent life-threatening events and sudden infant death on a monitor. Paediatr Respir Rev. 2004;5(Suppl A):S383–86. doi: 10.1016/s1526-0542(04)90068-1. [DOI] [PubMed] [Google Scholar]
- 33.Sridhar R, Thach BT, Kelly DH, Henslee JA. Characterization of successful and failed autoresuscitation in human infants, including those dying of SIDS. Pediatr Pulmonol. 2003;36:113–22. doi: 10.1002/ppul.10287. [DOI] [PubMed] [Google Scholar]
- 34.Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics. 1994;93:44–49. [PubMed] [Google Scholar]
- 35.Kinney HC, Myers MM, Belliveau RA, Randall LL, Trachtenberg FL, et al. Subtle autonomic and respiratory dysfunction in sudden infant death syndrome associated with serotonergic brainstem abnormalities: a case report. J Neuropathol Exp Neurol. 2005;64:689–94. doi: 10.1097/01.jnen.0000174334.27708.43. [DOI] [PubMed] [Google Scholar]
- 36.Franco P, Szliwowski H, Dramaix M, Kahn A. Decreased autonomic responses to obstructive sleep events in future victims of sudden infant death syndrome. Pediatr Res. 1999;46:33–39. doi: 10.1203/00006450-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Schechtman VL, Lee MY, Wilson AJ, Harper RM. Dynamics of respiratory patterning in normal infants and infants who subsequently died of the sudden infant death syndrome. Pediatr Res. 1996;40:571–77. doi: 10.1203/00006450-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Southall DP, Stevens V, Franks CI, Newcombe RG, Shinebourne EA, Wilson AJ. Sinus tachycardia in term infants preceding sudden infant death. Eur J Pediatr. 1988;147:74–78. doi: 10.1007/BF00442617. [DOI] [PubMed] [Google Scholar]
- 39.Kato I, Franco P, Groswasser J, Scaillet S, Kelmanson I, et al. Incomplete arousal processes in infants who were victims of sudden death. Am J Respir Crit Care Med. 2003;168:1298–303. doi: 10.1164/rccm.200301-134OC. [DOI] [PubMed] [Google Scholar]
- 40.Schechtman VL, Harper RM, Wilson AJ, Southall DP. Sleep state organization in normal infants and victims of the sudden infant death syndrome. Pediatrics. 1992;89:865–70. [PubMed] [Google Scholar]
- 41.Kahn A, Groswasser J, Rebuffat E, Sottiaux M, Blum D, et al. Sleep and cardiorespiratory characteristics of infant victim of sudden death: a prospective case-control study. Sleep. 1992;15:287–92. doi: 10.1093/sleep/15.4.287. [DOI] [PubMed] [Google Scholar]
- 42.Thach B. Tragic and sudden death: potential and proven mechanisms causing sudden infant death syndrome. EMBO Rep. 2008;9:114–18. doi: 10.1038/sj.embor.7401163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kemp JS, Kowalski RM, Burch PM, Graham MA, Thach BT. Unintentional suffocation by rebreathing: a death scene and physiologic investigation of a possible cause of sudden infant death. J Pediatr. 1993;122:874–80. doi: 10.1016/s0022-3476(09)90010-5. [DOI] [PubMed] [Google Scholar]
- 44.Fleming PJ, Levine MR, Azaz Y, Wigfield R, Stewart AJ. Interactions between thermoregulation and the control of respiration in infants: possible relationship to sudden infant death. Acta Paediatr Suppl. 1993;82(Suppl 389):57–59. doi: 10.1111/j.1651-2227.1993.tb12878.x. [DOI] [PubMed] [Google Scholar]
- 45.Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonat. 1994;65:194–97. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- 46.Guntheroth WG, Spiers PS. The triple risk hypothesis in sudden infant death syndrome. Pediatrics. 2002;110:e64. doi: 10.1542/peds.110.5.e64. [DOI] [PubMed] [Google Scholar]
- 47.Vege A, Ole Rognum T. Sudden infant death syndrome, infection and inflammatory responses. FEMS Immunol Med Microbiol. 2004;42:3–10. doi: 10.1016/j.femsim.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 48.Raza MW, Blackwell CC. Sudden infant death syndrome, virus infections, and cytokines. FEMS Immunol Med Microbiol. 1999;25:85–96. doi: 10.1111/j.1574-695X.1999.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 49.Ramanathan R, Corwin MJ, Hunt CE, Lister G, Tinsley LR, et al. Cardiorespiratory events recorded on home monitors: comparison of healthy infants with those at increased risk for SIDS. JAMA. 2001;285:2199–207. doi: 10.1001/jama.285.17.2199. [DOI] [PubMed] [Google Scholar]
- 50.Scher MS, Steppe DA, Dokianakis SG, Sun M, Guthrie RD, Sclabassi RJ. Cardiorespiratory behavior during sleep in full-term and preterm neonates at comparable postconceptional term ages. Pediatr Res. 1994;36:738–44. doi: 10.1203/00006450-199412000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Fifer WP, Myers MM, Sahni R, Ohira-Kist K, Kashyap S, et al. Interactions between sleeping position and feeding on cardiorespiratory activity in preterm infants. Dev Psychobiol. 2005;47:288–96. doi: 10.1002/dev.20096. [DOI] [PubMed] [Google Scholar]
- 52.Myers MM, Gomez-Gribben E, Smith KS, Tseng A, Fifer WP. Developmental changes in infant heart rate responses to head-up tilting. Acta Paediatr. 2006;95:77–81. doi: 10.1080/08035250500325074. [DOI] [PubMed] [Google Scholar]
- 53.Malloy MH, Hoffman HJ. Prematurity, sudden infant death syndrome, and age of death. Pediatrics. 1995;96(3 Pt 1):464–71. [PubMed] [Google Scholar]
- 54.Blair PS, Fleming PJ, Bensley D, Smith I, Bacon C, et al. Smoking and the sudden infant death syndrome: results from 1993–5 case-control study for confidential inquiry into stillbirths and deaths in infancy. Confidential enquiry into stillbirths and deaths regional coordinators and researchers. BMJ. 1996;313:195–98. doi: 10.1136/bmj.313.7051.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oyen N, Skjaerven R, Irgens LM. Population-based recurrence risk of sudden infant death syndrome compared with other infant and fetal deaths. Am J Epidemiol. 1996;144:300–5. doi: 10.1093/oxfordjournals.aje.a008925. [DOI] [PubMed] [Google Scholar]
- 56.Narita N, Narita M, Takashima S, Nakayama M, Nagai T, Okado N. Serotonin transporter gene variation is a risk factor for sudden infant death syndrome in the Japanese population. Pediatrics. 2001;107:690–92. doi: 10.1542/peds.107.4.690. [DOI] [PubMed] [Google Scholar]
- 57.Pasquale-Styles MA, Tackitt PL, Schmidt CJ. Infant death scene investigation and the assessment of potential risk factors for asphyxia: a review of 209 sudden unexpected infant deaths. J Forensic Sci. 2007;52:924–29. doi: 10.1111/j.1556-4029.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 58.Collins KA. Death by overlaying and wedging: a 15-year retrospective study. Am J Forensic Med Pathol. 2001;22:155–59. doi: 10.1097/00000433-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 59.Byard RW. Hazardous infant and early childhood sleeping environments and death scene examination. J Clin Forensic Med. 1996;3:115–22. doi: 10.1016/s1353-1131(96)90000-0. [DOI] [PubMed] [Google Scholar]
- 60.Paydarfar D, Buerkel DM. Sporadic apnea: paradoxical transformation to eupnea by perturbations that inhibit inspiration. Med Hypotheses. 1997;49:19–26. doi: 10.1016/s0306-9877(97)90246-2. [DOI] [PubMed] [Google Scholar]
- 61.Naeye RL. Brain-stem and adrenal abnormalities in the sudden-infant-death syndrome. Am J Clin Pathol. 1976;66:526–30. doi: 10.1093/ajcp/66.3.526. [DOI] [PubMed] [Google Scholar]
- 62.Mallard C, Tolcos M, Leditschke J, Campbell P, Rees S. Reduction in choline acetyltransferase immunoreactivity but not muscarinic-m2 receptor immunoreactivity in the brainstem of SIDS infants. J Neuropathol Exp Neurol. 1999;58:255–64. doi: 10.1097/00005072-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF. Decreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science. 1995;269:1446–50. doi: 10.1126/science.7660131. [DOI] [PubMed] [Google Scholar]
- 64.Nachmanoff DB, Panigrahy A, Filiano JJ, Mandell F, Sleeper LA, et al. Brainstem 3H-nicotine receptor binding in the sudden infant death syndrome. J Neuropathol Exp Neurol. 1998;57:1018–25. doi: 10.1097/00005072-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Duncan JR, Randall LL, Belliveau RA, Trachtenberg FL, Randall B, et al. The effect of maternal smoking and drinking during pregnancy upon (3)H-nicotine receptor brainstem binding in infants dying of the sudden infant death syndrome: initial observations in a high-risk population. Brain Pathol. 2007 doi: 10.1111/j.1750-3639.2007.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kopp N, Chigr F, Denoroy L, Gilly R, Jordan D. Absence of adrenergic neurons in nucleus tractus solitarius in sudden infant death syndrome. Neuropediatrics. 1993;24:25–29. doi: 10.1055/s-2008-1071508. [DOI] [PubMed] [Google Scholar]
- 67.Kinney HC, McHugh T, Miller K, Belliveau RA, Assmann SF. Subtle developmental abnormalities in the inferior olive: an indicator of prenatal brainstem injury in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2002;61:427–41. doi: 10.1093/jnen/61.5.427. [DOI] [PubMed] [Google Scholar]
- 68.Takashima S, Becker LE. Delayed dendritic development of catecholaminergic neurons in the ventrolateral medulla of children who died of sudden infant death syndrome. Neuropediatrics. 1991;22:97–99. doi: 10.1055/s-2008-1071424. [DOI] [PubMed] [Google Scholar]
- 69.Panigrahy A, Filiano J, Sleeper L, Mandell F, Valdes-Dapena M, et al. Decreased kainate receptor binding in the arcuate nucleus of the sudden infant death syndrome. J Neuropathol Exp Neurol. 1997;56:1253–61. doi: 10.1097/00005072-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 70.Machaalani R, Waters KA. NMDA receptor 1 expression in the brainstem of human infants and its relevance to the sudden infant death syndrome (SIDS) J Neuropathol Exp Neurol. 2003;62:1076–85. doi: 10.1093/jnen/62.10.1076. [DOI] [PubMed] [Google Scholar]
- 71.Quattrochi JJ, McBride PT, Yates AJ. Brainstem immaturity in sudden infant death syndrome: a quantitative rapid Golgi study of dendritic spines in 95 infants. Brain Res. 1985;325:39–48. doi: 10.1016/0006-8993(85)90300-2. [DOI] [PubMed] [Google Scholar]
- 72.Takashima S, Becker LE. Developmental abnormalities of medullary “respiratory centers” in sudden infant death syndrome. Exp Neurol. 1985;90:580–87. doi: 10.1016/0014-4886(85)90155-4. [DOI] [PubMed] [Google Scholar]
- 73.Filiano JJ, Choi JC, Kinney HC. Candidate cell populations for respiratory chemosensitive fields in the human infant medulla. J Comp Neurol. 1990;293:448–65. doi: 10.1002/cne.902930308. [DOI] [PubMed] [Google Scholar]
- 74.Filiano JJ, Kinney HC. Arcuate nucleus hypoplasia in the sudden infant death syndrome. J Neuropathol Exp Neurol. 1992;51:394–403. doi: 10.1097/00005072-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Paterson DS, Thompson EG, Kinney HC. Serotonergic and glutamatergic neurons at the ventral medullary surface of the human infant: observations relevant to central chemosensitivity in early human life. Auton Neurosci. 2006;124:112–24. doi: 10.1016/j.autneu.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 76.Shannon DC, Kelly DH, O’Connell K. Abnormal regulation of ventilation in infants at risk for sudden-infant-death syndrome. N Eng J Med. 1977;297:747–50. doi: 10.1056/NEJM197710062971403. [DOI] [PubMed] [Google Scholar]
- 77.Matturri L, Biondo B, Mercurio P, Rossi L. Severe hypoplasia of medullary arcuate nucleus: quantitative analysis in sudden infant death syndrome. Acta Neuropathol (Berlin) 2000;99:371–75. doi: 10.1007/s004010051138. [DOI] [PubMed] [Google Scholar]
- 78.Nattie E, Li A. Central chemoreception 2005: a brief review. Auton Neurosci. 2006;126–27:332–38. doi: 10.1016/j.autneu.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 79.Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol. 2005;90:247–53. doi: 10.1113/expphysiol.2004.029637. discussion 53–57. [DOI] [PubMed] [Google Scholar]
- 80.Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci. 2002;5:401–2. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- 81.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–61. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 82.Hodges P. Defects in breathing and thermoregulation in mice with near complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, et al. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–38. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kinney HC, Belliveau RA, Trachtenberg FL, Rava LA, Paterson DS. The development of the medullary serotonergic system in early human life. Auton Neurosci. 2007;132:81–102. doi: 10.1016/j.autneu.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 85.Folkerth RD, Zanoni S, Andiman SE, Billiards SS. Neuronal cell death in the arcuate nucleus of the medulla oblongata in stillbirth. Int J Dev Neurosci. 2008;26:133–40. doi: 10.1016/j.ijdevneu.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 86.Tork I, Hornung JP. Raphé nuclei and the 5-HT system. In: Paxinos G, editor. The Human Nervous System. San Diego: Academic; 1990. pp. 1001–22. [Google Scholar]
- 87.Cook EH, Leventhal BL. The serotonin system in autism. Curr Opin Pediatr. 1996;8:348–54. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 88.Kapornai K, Vetro A. Depression in children. Curr Opin Psychiatry. 2008;21:1–7. doi: 10.1097/YCO.0b013e3282f25b01. [DOI] [PubMed] [Google Scholar]
- 89.Zhou FC, Sari Y, Powrozek TA. Fetal alcohol exposure reduces serotonin innervation and compromises development of the forebrain along the serotonergic pathway. Alcohol Clin Exp Res. 2005;29:141–49. doi: 10.1097/01.alc.0000150636.19677.6f. [DOI] [PubMed] [Google Scholar]
- 90.Paterson DS, Thompson EG, Belliveau RA, Antalffy BA, Trachtenberg FL, et al. Serotonin transporter abnormality in the dorsal motor nucleus of the vagus in Rett syndrome: potential implications for clinical autonomic dysfunction. J Neuropathol Exp Neurol. 2005;64:1018–27. doi: 10.1097/01.jnen.0000187054.59018.f2. [DOI] [PubMed] [Google Scholar]
- 91.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 92.Kinney HC, Filiano JJ, Assmann SF, Mandell F, Valdes-Dapena M, et al. Tritiated-naloxone binding to brainstem opioid receptors in the sudden infant death syndrome. J Auton Nerv Syst. 1998;69:156–63. doi: 10.1016/s0165-1838(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 93.Mansouri J, Panigrahy A, Assmann SF, Kinney HC. Distribution of α2-adrenergic receptor binding in the developing human brain stem. Pediatr Dev Pathol. 2001;4:222–36. doi: 10.1007/s100240010138. [DOI] [PubMed] [Google Scholar]
- 94.Blakely RD, Bauman AL. Biogenic amine transporters: regulation in flux. Curr Opin Neurobiol. 2000;10:328–36. doi: 10.1016/s0959-4388(00)00088-x. [DOI] [PubMed] [Google Scholar]
- 95.Kelai S, Aissi F, Lesch KP, Cohen-Salmon C, Hamon M, Lanfumey L. Alcohol intake after serotonin transporter inactivation in mice. Alcohol Alcohol. 2003;38:386–89. doi: 10.1093/alcalc/agg095. [DOI] [PubMed] [Google Scholar]
- 96.Awtry TL, Werling LL. Acute and chronic effects of nicotine on serotonin uptake in prefrontal cortex and hippocampus of rats. Synapse. 2003;50:206–11. doi: 10.1002/syn.10259. [DOI] [PubMed] [Google Scholar]
- 97.Heils A, Mossner R, Lesch KP. The human serotonin transporter gene polymorphism: basic research and clinical implications. J Neural Transm. 1997;104:1005–14. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- 98.Cutz E, Perrin DG, Pan J, Haas EA, Krous HF. Pulmonary neuroendocrine cells and neuroepithelial bodies in sudden infant death syndrome: potential markers of airway chemoreceptor dysfunction. Pediatr Dev Pathol. 2007;10:106–16. doi: 10.2350/06-06-0113.1. [DOI] [PubMed] [Google Scholar]
- 99.Vege A, Chen Y, Opdal SH, Saugstad OD, Rognum TO. Vitreous humor hypoxanthine levels in SIDS and infectious death. Acta Paediatr. 1994;83:634–39. doi: 10.1111/j.1651-2227.1994.tb13096.x. [DOI] [PubMed] [Google Scholar]
- 100.Jones KL, Krous HF, Nadeau J, Blackbourne B, Zielke HR, Gozal D. Vascular endothelial growth factor in the cerebrospinal fluid of infants who died of sudden infant death syndrome: evidence for antecedent hypoxia. Pediatrics. 2003;111:358–63. doi: 10.1542/peds.111.2.358. [DOI] [PubMed] [Google Scholar]
- 101.Kinney HC, Burger PC, Harrell FE, Jr, Hudson RP., Jr “Reactive gliosis” in the medulla oblongata of victims of the sudden infant death syndrome. Pediatrics. 1983;72:181–87. [PubMed] [Google Scholar]
- 102.Takashima S, Armstrong D, Becker L, Bryan C. Cerebral hypoperfusion in the sudden infant death syndrome? Brainstem gliosis and vasculature. Ann Neurol. 1978;4:257–62. doi: 10.1002/ana.410040312. [DOI] [PubMed] [Google Scholar]
- 103.Machaalani R, Rodriguez M, Waters KA. Active caspase 3 in the sudden infant death syndrome (SIDS) brainstem. Acta Neuropathol (Berlin) 2007;113:577–84. doi: 10.1007/s00401-007-0216-7. [DOI] [PubMed] [Google Scholar]
- 104.Waters KA, Meehan B, Huang JQ, Gravel RA, Michaud J, Cote A. Neuronal apoptosis in sudden infant death syndrome. Pediatr Res. 1999;45:166–72. doi: 10.1203/00006450-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 105.Del Bigio MR, Becker LE. Microglial aggregation in the dentate gyrus: a marker of mild hypoxic-ischaemic brain insult in human infants. Neuropathol Appl Neurobiol. 1994;20:144–51. doi: 10.1111/j.1365-2990.1994.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 106.Kinney HC, Brody BA, Finkelstein DM, Vawter GF, Mandell F, Gilles FH. Delayed central nervous system myelination in the sudden infant death syndrome. J Neuropathol Exp Neurol. 1991;50:29–48. doi: 10.1097/00005072-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 107.Takashima S, Armstrong D, Becker LE, Huber J. Cerebral white matter lesions in sudden infant death syndrome. Pediatrics. 1978;62:155–59. [PubMed] [Google Scholar]
- 108.Huggle S, Hunsaker JC, 3rd, Coyne CM, Sparks DL. Oxidative stress in sudden infant death syndrome. J Child Neurol. 1996;11:433–38. doi: 10.1177/088307389601100603. [DOI] [PubMed] [Google Scholar]
- 109.Bocchiaro CM, Feldman JL. Synaptic activity–independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci USA. 2004;101:4292–95. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, et al. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- 111.Slikker W, Jr, Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction–developmental neurotoxicity of nicotine. Crit Rev Toxicol. 2005;35:703–11. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- 112.Horne RS, Ferens D, Watts AM, Vitkovic J, Lacey B, et al. Effects of maternal tobacco smoking, sleeping position, and sleep state on arousal in healthy term infants. Arch Dis Child Fetal Neonatal Ed. 2002;87:F100–5. doi: 10.1136/fn.87.2.F100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lewis KW, Bosque EM. Deficient hypoxia awakening response in infants of smoking mothers: possible relationship to sudden infant death syndrome. J Pediatr. 1995;127:691–99. doi: 10.1016/s0022-3476(95)70155-9. [DOI] [PubMed] [Google Scholar]
- 114.Franco P, Groswasser J, Hassid S, Lanquart JP, Scaillet S, Kahn A. Prenatal exposure to cigarette smoking is associated with a decrease in arousal in infants. J Pediatr. 1999;135:34–38. doi: 10.1016/s0022-3476(99)70324-0. [DOI] [PubMed] [Google Scholar]
- 115.Matturri L, Ottaviani G, Lavezzi AM. Maternal smoking and sudden infant death syndrome: epidemiological study related to pathology. Virchows Arch. 2006;449:697–706. doi: 10.1007/s00428-006-0308-0. [DOI] [PubMed] [Google Scholar]
- 116.Kim EK, Lee MH, Kim H, Sim YJ, Shin MS, et al. Maternal ethanol administration inhibits 5-hydroxytryptamine synthesis and tryptophan hydroxylase expression in the dorsal raphé of rat offspring. Brain Dev. 2005;27:472–76. doi: 10.1016/j.braindev.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 117.Sari Y, Zhou FC. Prenatal alcohol exposure causes long-term serotonin neuron deficit in mice. Alcohol Clin Exp Res. 2004;28:941–48. doi: 10.1097/01.alc.0000128228.08472.39. [DOI] [PubMed] [Google Scholar]
- 118.Xu Z, Seidler FJ, Ali SF, Slikker W, Jr, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–78. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- 119.Voss MD, De Castro D, Lipski J, Pilowsky PM, Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol. 1990;295:208–18. doi: 10.1002/cne.902950205. [DOI] [PubMed] [Google Scholar]
- 120.Pilowsky PM, de Castro D, Llewellyn-Smith I, Lipski J, Voss MD. Serotonin immunoreactive boutons make synapses with feline phrenic motoneurons. J Neurosci. 1990;10:1091–98. doi: 10.1523/JNEUROSCI.10-04-01091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holtman JR, Jr, Norman WP, Skirboll L, Dretchen KL, Cuello C, et al. Evidence for 5-hydroxytryptamine, substance P, and thyrotropin-releasing hormone in neurons innervating the phrenic motor nucleus. J Neurosci. 1984;4:1064–71. doi: 10.1523/JNEUROSCI.04-04-01064.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Manaker S, Tischler LJ. Origin of serotoninergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334:466–76. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- 123.Ptak K, Zhang R, Milescu LS, Richerson GB, Smith JC. Modulation of neuronal activity in the pre-Bötzinger complex by medullary raphé neurons. Soc Neurosci Abstr. 2006:32. [Google Scholar]
- 124.Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–64. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dekin MS, Richerson GB, Getting PA. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science. 1985;229:67–69. doi: 10.1126/science.3925552. [DOI] [PubMed] [Google Scholar]
- 126.Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 127.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–29. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suzue T. Respiratory rhythm generation in the in vitro brain stem–spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–83. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol. 2007;159:85–101. doi: 10.1016/j.resp.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zanella S, Watrin F, Mebarek S, et al. Necdin plays a role in the serotonergic modulation of the mouse respiratory network: implication for Prader-Willi syndrome. J Neurosci. 2008;28:1745–55. doi: 10.1523/JNEUROSCI.4334-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bou-Flores C, Lajard AM, Monteau R, De Maeyer E, Seif I, et al. Abnormal phrenic motoneuron activity and morphology in neonatal monoamine oxidase A–deficient transgenic mice: possible role of a serotonin excess. J Neurosci. 2000;20:4646–56. doi: 10.1523/JNEUROSCI.20-12-04646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor–immunoreactive neurons of the ventral respiratory group in the rat. J Comp Neurol. 2001;434:128–46. doi: 10.1002/cne.1169. [DOI] [PubMed] [Google Scholar]
- 133.Wang W, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphé neurons to independent changes in pH(o) and P(CO2) J Physiol. 2002;540(Pt. 3):951–70. doi: 10.1113/jphysiol.2001.013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphé neurons. Neuroscience. 1999;90:1001–11. doi: 10.1016/s0306-4522(98)00505-3. [DOI] [PubMed] [Google Scholar]
- 135.Hodges MR, Tattersall MB, Harris SD, McEvoy DN, Richerson ES, et al. Defects in breathing and thermoregulation after genetic deletion of central serotonin neurons. J Neurosci. 2008;28:2495–505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nattie EE, Li A, Richerson G, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–53. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taylor NC, Li A, Green A, Kinney HC, Nattie EE. Chronic fluoxetine microdialysis into the medullary raphé nuclei of the rat, but not systemic administration, increases the ventilatory response to CO2. J Appl Physiol. 2004;97:1763–73. doi: 10.1152/japplphysiol.00496.2004. [DOI] [PubMed] [Google Scholar]
- 138.Messier ML, Li A, Nattie EE. Inhibition of medullary raphé serotonergic neurons has age-dependent effects on the CO2 response in newborn piglets. J Appl Physiol. 2004;96:1909–19. doi: 10.1152/japplphysiol.00805.2003. [DOI] [PubMed] [Google Scholar]
- 139.Greaves JM, Russo SS, Azmitia EC. Gender-specific 5-HT1A receptor changes in BrdU nuclear labeling patterns in neonatal dentate gyrus. Brain Res Dev Brain Res. 2005;157:65–73. doi: 10.1016/j.devbrainres.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 140.Task Force Sudd. Infant Death Syndr. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116:1245–55. doi: 10.1542/peds.2005-1499. [DOI] [PubMed] [Google Scholar]
- 141.Emery MJ, Krous HF, Nadeau-Manning JM, Marck BT, Matsumoto AM. Serum testosterone and estradiol in sudden infant death. J Pediatr. 2005;147:586–91. doi: 10.1016/j.jpeds.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 142.Penatti EM, Berniker AV, Kereshi B, Cafaro C, Kelly ML, et al. Ventilatory response to hypercapnia and hypoxia after extensive lesion of medullary serotonergic neurons in newborn conscious piglets. J Appl Physiol. 2006;101:1177–88. doi: 10.1152/japplphysiol.00376.2006. [DOI] [PubMed] [Google Scholar]
- 142a.Li A, Nattie E. Serotonin transporter knockout mice have a reduced ventilatory response to hypercapnia (predominantly in males) but not to hypoxia. J Physiol. 2008;586:2321–29. doi: 10.1113/jphysiol.2008.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, et al. Altered serotonin synthesis, turnover, and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49:798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 144.Bouali S, Evrard A, Chastanet M, Lesch KP, Hamon M, Adrien J. Sex hormone-dependent desensitization of 5-HT1A autoreceptors in knockout mice deficient in the 5-HT transporter. Eur J Neurosci. 2003;18:2203–12. doi: 10.1046/j.1460-9568.2003.02960.x. [DOI] [PubMed] [Google Scholar]
- 145.Qu Y, Villacreses N, Murphy DL, Rapoport SI. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berlin) 2005;180:12–20. doi: 10.1007/s00213-005-2231-5. [DOI] [PubMed] [Google Scholar]
- 146.Hehre DA, Devia CJ, Bancalari E, Suguihara C. Brainstem amino acid neurotransmitters and ventilatory response to hypoxia in piglets. Pediatr Res. 2008;63:46–50. doi: 10.1203/PDR.0b013e31815b4421. [DOI] [PubMed] [Google Scholar]
- 147.Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–82. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- 148.Downing SE, Lee JC. Laryngeal chemosensitivity: a possible mechanism for sudden infant death. Pediatrics. 1975;55:640–49. [PubMed] [Google Scholar]
- 149.Thach BT. The role of respiratory control disorders in SIDS. Respir Physiol Neurobiol. 2005;149:343–53. doi: 10.1016/j.resp.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 150.Xia L, Damon TA, Leiter JC, Bartlett D., Jr Focal warming in the nucleus of the solitary tract prolongs the laryngeal chemoreflex in decerebrate piglets. J Appl Physiol. 2007;102:54–62. doi: 10.1152/japplphysiol.00720.2006. [DOI] [PubMed] [Google Scholar]
- 151.Xia L, Damon T, Leiter JC, Bartlett D., Jr Elevated body temperature exaggerates laryngeal chemoreflex apnea in decerebrate piglets. Adv Exp Med Biol. 2008;605:249–54. doi: 10.1007/978-0-387-73693-8_44. [DOI] [PubMed] [Google Scholar]
- 151a.Xia L, Damon T, Niblock MM, Bartlett D, Jr, Leiter JC. Unilateral microdialysis of gabazine in the dorsal medulla reverses thermal prolongation of laryngeal chemoreflex in decerebrate piglets. J Appl Physiol. 2007;103:1864–72. doi: 10.1152/japplphysiol.00524.2007. [DOI] [PubMed] [Google Scholar]
- 152.Van Der Velde L, Curran AK, Filiano JJ, Darnall RA, Bartlett D, Jr, Leiter JC. Prolongation of the laryngeal chemoreflex after inhibition of the rostral ventral medulla in piglets: a role in SIDS? J Appl Physiol. 2003;94:1883–95. doi: 10.1152/japplphysiol.01103.2002. [DOI] [PubMed] [Google Scholar]
- 153.Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci. 2006;9:311–13. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- 154.St-John WM, Leiter JC. Maintenance of gasping and restoration of eupnea after hypoxia is impaired following blockers of α-1 adrenergic receptors and serotonin 5HT2 receptors. J Appl Physiol. 2007;104:665–73. doi: 10.1152/japplphysiol.00599.2007. [DOI] [PubMed] [Google Scholar]
- 155.Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–34. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Toppin VA, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol. 2007;103:220–27. doi: 10.1152/japplphysiol.00071.2007. [DOI] [PubMed] [Google Scholar]
- 157.Sinton CM, McCarley RW. Neurophysiological mechanisms of sleep and wakefulness: a question of balance. Semin Neurol. 2004;24:211–23. doi: 10.1055/s-2004-835067. [DOI] [PubMed] [Google Scholar]
- 158.McNamara F, Lijowska AS, Thach BT. Spontaneous arousal activity in infants during NREM and REM sleep. J Physiol. 2002;538:263–69. doi: 10.1113/jphysiol.2001.012507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Siegel J. The Neural Control of Sleep and Waking. New York: Springer-Velag; 2002. [Google Scholar]
- 160.Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–40. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- 161.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 162.McGinty DJ, Harper RM. Dorsal raphé neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–75. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 163.Mason P. Contributions of the medullary raphé and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–77. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- 164.Lovick TA. The medullary raphé nuclei: a system for integration and gain control in autonomic and somatomotor responsiveness? Exp Physiol. 1997;82:31–41. doi: 10.1113/expphysiol.1997.sp004013. [DOI] [PubMed] [Google Scholar]
- 165.Darnall RA, Harris MB, Gill WH, Hoffman JM, Brown JW, Niblock MM. Inhibition of serotonergic neurons in the nucleus paragigantocellularis lateralis fragments sleep and decreases rapid eye movement sleep in the piglet: implications for sudden infant death syndrome. J Neurosci. 2005;25:8322–32. doi: 10.1523/JNEUROSCI.1770-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brown JW, Sirlin EA, Benoit AM, Hoffman JM, Darnall RA. Activation of 5-HT1A receptors in medullary raphé disrupts sleep and decreases shivering during cooling in the conscious piglet. Am J Physiol Regul Integr Comp Physiol. 2007;294:R884–94. doi: 10.1152/ajpregu.00655.2007. [DOI] [PubMed] [Google Scholar]
- 167.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 168.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling–evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292:R127–36. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ootsuka Y, Blessing WW. Activation of 5-HT1A receptors in rostral medullary raphé inhibits cutaneous vasoconstriction elicited by cold exposure in rabbits. Brain Res. 2006;1073–1074:252–61. doi: 10.1016/j.brainres.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 170.Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphé neurons to environmental cooling in freely moving cats. Neuroscience. 2000;98:301–9. doi: 10.1016/s0306-4522(00)00133-0. [DOI] [PubMed] [Google Scholar]
- 171.Nason MW, Jr, Mason P. Medullary raphé neurons facilitate brown adipose tissue activation. J Neurosci. 2006;26:1190–8. doi: 10.1523/JNEUROSCI.4707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol. 2006;577(Pt. 2):525–37. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Raul L. Serotonin-2 receptors in the nucleus tractus solitarius: characterization and role in the baroreceptor reflex arc. Cell Mol Neurobiol. 2003;23:709–26. doi: 10.1023/A:1025096718559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nosjean A, Hamon M, Darmon M. 5-HT2A receptors are expressed by catecholaminergic neurons in the rat nucleus tractus solitarii. Neuroreport. 2002;13:2365–69. doi: 10.1097/00001756-200212030-00039. [DOI] [PubMed] [Google Scholar]
- 175.Duncan JR, Randall LL, Belliveau RA, Trachtenberg FL, Randall M, et al. The effect of maternal smoking and drinking during pregnancy upon (3)H-nicotine receptor brainstem binding in infants dying of the sudden infant death syndrome: initial observations in a high-risk population. Brain Pathol. 2008;18:21–31. doi: 10.1111/j.1750-3639.2007.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. [DOI] [PubMed] [Google Scholar]
- 177.Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:413–24. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 178.Hynes M, Rosenthal A. Specification of dopaminergic and serotonergic neurons in the vertebrate CNS. Curr Opin Neurobiol. 1999;9:26–36. doi: 10.1016/s0959-4388(99)80004-x. [DOI] [PubMed] [Google Scholar]
- 179.Goridis C, Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci. 2002;3:531–41. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- 180.Jacob J, Ferri AL, Milton C, Prin F, Pla P, et al. Transcriptional repression coordinates the temporal switch from motor to serotonergic neurogenesis. Nat Neurosci. 2007;10:1433–39. doi: 10.1038/nn1985. [DOI] [PubMed] [Google Scholar]
- 181.Joyner AL, Zervas M. Genetic inducible fate mapping in mouse: establishing genetic lineages and defining genetic neuroanatomy in the nervous system. Dev Dyn. 2006;235:2376–85. doi: 10.1002/dvdy.20884. [DOI] [PubMed] [Google Scholar]
- 182.Buse E. Development of serotoninergic neurons from ventricular cells of the mouse neural plate in vitro. Int J Dev Neurosci. 1987;5:107–15. doi: 10.1016/0736-5748(87)90056-6. [DOI] [PubMed] [Google Scholar]
- 183.Cordes SP. Molecular genetics of the early development of hindbrain serotonergic neurons. Clin Genet. 2005;68:487–94. doi: 10.1111/j.1399-0004.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- 184.Alenina N, Bashammakh S, Bader M. Specification and differentiation of serotonergic neurons. Stem Cell Rev. 2006;2:5–10. doi: 10.1007/s12015-006-0002-2. [DOI] [PubMed] [Google Scholar]
- 185.Dahlstroem A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand. 1964;62(Suppl 232):1–55. [PubMed] [Google Scholar]
- 186.Hokfelt T, Arvidsson U, Cullheim S, Millhorn D, Nicholas AP, et al. Multiple messengers in descending serotonin neurons: localization and functional implications. J Chem Neuroanat. 2000;18:75–86. doi: 10.1016/s0891-0618(99)00037-x. [DOI] [PubMed] [Google Scholar]
- 187.Jensen P, Farago A, Awatramani R, Scott MM, Deneris E, Dymecki S. Redefining the central serotonergic system based on genetic lineage. Nat Neurosci. 2008;11:417–19. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Olszewski J, Baxter D. Cytoarchitecture of the Human Brainstem. 2 Basel: Karger; 1982. [Google Scholar]
- 189.Kopp N, Denoroy L, Eymin C, Gay N, Richard F, et al. Studies of neuroregulators in the brain stem of SIDS. Biol Neonate. 1994;65:189–93. doi: 10.1159/000244051. [DOI] [PubMed] [Google Scholar]
- 190.Kubo S, Orihara Y, Gotohda T, Tokunada I, Tsuda R, et al. Immunohistochemical studies on neuronal changes in brain stem nucleus of forensic autopsied cases. II Sudden infant death syndrome. Nihon Hoigaku Zasshi ( Jpn J Leg Med) 1998;52:350–54. [PubMed] [Google Scholar]
- 191.Mansouri J, Panigrahy A, Filiano JJ, Sleeper LA, St-John WM, Kinney HC. α2 receptor binding in the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2001;60:141–46. doi: 10.1093/jnen/60.2.141. [DOI] [PubMed] [Google Scholar]
- 192.Obonai T, Yasuhara M, Nakamura T, Takashima S. Catecholamine neurons alteration in the brainstem of sudden infant death syndrome victims. Pediatrics. 1998;101:285–88. doi: 10.1542/peds.101.2.285. [DOI] [PubMed] [Google Scholar]
- 193.Sawaguchi T, Patricia F, Kadhim H, Groswasser J, Sottiaux M, et al. The correlation between serotonergic neurons in the brainstem and sleep apnea in SIDS victims. Early Hum Dev. 2003;75:S31–40. doi: 10.1016/j.earlhumdev.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 194.Sawaguchi T, Ozawa Y, Patricia F, Kadhim H, Groswasser J, et al. Catecholaminergic neurons in the brain-stem and sleep apnea in SIDS victims. Early Hum Dev. 2003;75:S41–50. doi: 10.1016/j.earlhumdev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 195.Bergstrom L, Lagercrantz H, Terenius L. Post-mortem analyses of neuropeptides in brains from sudden infant death victims. Brain Res. 1984;323:279–85. doi: 10.1016/0006-8993(84)90298-1. [DOI] [PubMed] [Google Scholar]
- 196.Yamanouchi H, Takashima S, Becker LE. Correlation of astrogliosis and substance P immunoreactivity in the brainstem of victims of sudden infant death syndrome. Neuropediatrics. 1993;24:200–3. doi: 10.1055/s-2008-1071539. [DOI] [PubMed] [Google Scholar]
- 197.Obonai T, Takashima S, Becker LE, Asanuma M, Mizuta R, et al. Relationship of substance P and gliosis in medulla oblongata in neonatal sudden infant death syndrome. Pediatr Neurol. 1996;15:189–92. doi: 10.1016/s0887-8994(96)00217-2. [DOI] [PubMed] [Google Scholar]
- 198.Jordan D, Kermadi I, Rambaud C, Bouvier R, Dijoud F, et al. Autoradiographic distribution of brainstem substance P binding sites in humans: ontogenic study and relation to sudden infant death syndrome (SIDS) J Neural Transm. 1997;104:1101–5. doi: 10.1007/BF01273322. [DOI] [PubMed] [Google Scholar]