Abstract
At birth, synaptic sites in developing rodent muscles are innervated by numerous motor axons. During subsequent weeks, this multiple innervation disappears as one terminal strengthens and all the others are eliminated. Experimental perturbations that alter neuromuscular activity affect the rate of synaptic refinement with more activity accelerating the time to single innervation and neuromuscular blockade retarding it. But it remains unclear whether patterns of muscle use (driven by endogenous neuronal activity) contribute to the rate of synapse elimination. For this reason we examined the timing of supernumerary nerve terminal elimination at synapses in extraocular muscles (EOMs), a specialized set of muscles that control eye movements. On the basis of their exceptionally high patterns of activity, we hypothesized that synaptic refinement would be greatly accelerated at these synapses. We found, however, that rates of synaptic refinement were only modestly accelerated in rectus and oblique EOMs compared with synapses in somite-derived skeletal muscle. In contrast to these results, we observed a dramatic delay in the elimination of supernumerary nerve terminals from synapses in the levator palpebrae superioris (LPS) muscle, a specialized EOM that initiates and maintains eye-lid elevation. In mice, natural eye-opening occurs at the end of the second postnatal week of development. Thus, while synapse elimination is occurring in most EOMs and somite-derived skeletal muscles it appears dramatically delayed in a set of specialized eyelid muscles that remain immobile during early postnatal development.
Keywords: Neuromuscular junction, synapse elimination, extraocular muscle, muscle fiber, postsynaptic maturation
Introduction
Following their initial formation, vertebrate synapses undergo a period of refinement in which some synapses are strengthened and maintained compensating for the elimination of others. The mechanisms controlling this process have been most thoroughly investigated at the mammalian neuromuscular junction (NMJ) – a large, accessible synapse between motor neurons and muscle fibers. At birth, postsynaptic specializations on rodent muscle fibers are contacted by multiple motor axons. Within several weeks however, all but one axon terminal are eliminated at each NMJ (reviewed in Sanes and Lichtman 1999; Fox 2009). The loss of axonal branches is a protracted process in which initially intermingled branches segregate to occupy non-overlapping domains on the postsynaptic site (Gan and Lichtman, 1998). Subsequently, all motor axons – save one – undergo atrophy and disconnect from the synapse while the remaining nerve terminal expands to occupy postsynaptic sites vacated by the retracting nerves (Walsh and Lichtman 2003). In mice, few multiply innervated NMJs persist in somite-derived skeletal muscles beyond postnatal day 14 (Keller-Peck et al. 2001; Personius and Balice-Gordon 2001).
While the initial formation of the NMJ may be genetically driven (reviewed in Sanes and Lichtman 1999, Fox and Umemori 2006; Wu et al. 2010), neuromuscular use appears to significantly contribute to synapse elimination (Thompson 1985; Wyatt and Balice-Gordon 2003; Bosetto et al. 2003). In general, experiments that increase motor neuron activity accelerate synapse elimination at the NMJ, whereas those that reduce activity delay it (Thompson et al. 1979; Thompson 1983; Benoit and Changeux 1975). Additionally, differences in intra-axonal activity, local postsynaptic activity, and even neuronal identity contribute to the mechanisms driving synaptic refinement at the vertebrate NMJ (Callaway et al. 1987; Balice-Gordon and Lichtman 1994; Buffelli et al. 2003; Kasthuri and Lichtman 2003). Moreover, a classic study by W. Thompson (1983) revealed that the pattern of muscle use strongly influenced synaptic refinement; chronic bursts of high frequency stimulation accelerated the rate at which excess motor nerve terminals were eliminated from synapses in rat soleus muscle (Thompson 1983). Despite such studies, it remains largely unclear whether endogenous patterns of muscle use alter the rate of supernumerary motor nerve terminal elimination in different classes of muscles. A few differences have been noted in the timing of synapse elimination in various muscles, however in this previous work there did not appear to be a correlation with the muscle’s predominant mature fiber type or activity (Bixby and Van Essen, 1979). Whether endogenous patterns of activity in more specialized classes of muscles result in altered rates of synaptic refinement remains unclear. Here, we examined the rate of synapse elimination in extraocular muscles (EOMs) – a specialized class of muscles that control the complex and diverse movements of the eye and eye-lid.
In vertebrates, 6 EOMs control eye movements – the superior, inferior, medial and lateral rectus muscles (SR, IR, MR and LR, respectively) and the superior and inferior oblique muscles (SO and IO, respectively). Mammals contain a seventh EOM that is responsible for initiating and maintaining eye-lid elevation – the levator palpebrae superioris (LPS) muscle. EOMs serve diverse functions, which include the generation of fine reflexive eye movements to stabilize images on the retina, slow and smooth eye movements associated with visual pursuit and vestibulo-ocular reflexes, and rapid eye movements associated with saccades (reviewed in Porter 2002; Spencer and Porter 2006). To accomplish such functions extraocular motor neurons fire at very high rates, exceeding 300 hertz (hz) during visual fixation and up to 600 hz during saccadic eye movement (Robinson 1970). Physiological specialization of extraocular motor neurons occurs early in rodent development: these motor neurons fire at high rates shortly after birth and they reach their mature electrophysiological state by natural eye opening (Tsuzuki et al 1995).
In addition to these physiological specializations, EOMs have unique embryonic origins, genetic profiles, types of myosin heavy chains (MHCs), and muscle fiber classifications (Couly et al. 1992, Khanna et al. 2003a; Rubinstein and Hoh 2000; reviewed in Porter 2002; Spencer and Porter 2006). Differences also exist between the patterns of innervation of EOMs and typical skeletal muscles. While most somite-derived mammalian skeletal muscle fibers have only a single synaptic site, EOMs contain both single- and multi-synaptic site muscle fibers. Like synapses on typical skeletal muscle fibers, NMJs on single-site fibers are clustered in a central end-plate band and are morphologically defined as ‘en plaque’-type synapses based upon the typical shield-shape branch patterns of the motor nerve terminals and adjacent postsynaptic muscle membrane. In contrast, multi-site fibers have neuromuscular junctions that are simple, small, and arranged at regular intervals along the length of the fiber (Khanna et al. 2002,Khanna et al. 2003b; Porter 2002; Spencer and Porter 2006). In other vertebrates, multi-site fibers are known to be slowly contracting and do not fire action potentials, suggesting they play a role in tonic maintenance of tension (Wilkinson and Lichtman 1985; Lichtman and Wilkinson 1987). In addition to having multiple neuromuscular junctions (sometimes referred to as ‘en grappe’ or ‘grape-like’ NMJs) each synaptic site on a multi-site fiber retains multiple axons throughout life and does not appear to undergo synaptic refinement (Lichtman et al., 1985). This is in sharp contrast to the single axonal innervation that becomes the norm at ‘en plaque’ neuromuscular junctions after a period of multiple innervation during development. Based upon the high firing frequency of EOM single site ‘en plaque’ junctions (Robinson 1970; Fuchs et al. 1992), we hypothesized that the rate of synapse elimination would be significantly accelerated at ‘en plaque’ junctions in EOM. Here we used transgenic mice in which motor axons express fluorescent proteins to assess NMJ development and the number of motor nerve terminals at each junction. While we observed only a modest acceleration in attaining single innervation of synaptic sites in rectus and oblique EOMs (compared to other somite-derived muscles), we were surprised to discover a dramatic delay in the elimination of supernumerary inputs at synapses in LPS muscles – a delay that led to persistent multiple innervation many months into adulthood at some synaptic sites.
Methods
Reagents
All chemicals and reagents were obtained from either Sigma (St Louis, MO) or Fisher (Fairlawn, NJ), unless otherwise noted.
Animals
The generation of thy1:yfp line H, thy1:yfp line 16, thy1:gfp line S, and thy1:cfp line 23 mice were described previously (Feng et al. 2000). All analyses conformed to NIH guidelines and were carried out under protocols approved by the Harvard University Standing Committee on the Use of Animals in Research and Teaching and the VCU Institutional Animal Care and Use Committees.
Antibodies
A monoclonal antibody directed against synaptotagmin 2 (Syt2) was used in these studies (see Table 1). This antibody, called znp1, was identified in a screen for tissue specific antibodies in zebrafish (Trevarrow et al. 1990). This antibody was recently shown to detect mouse Syt2 by both western blot and IHC (Fox and Sanes 2007). In western blots of mouse cerebellum, hippocampi and synaptosome fractions this antibody detects a single band at 60 kDa (Fox and Sanes 2007; Su et al. 2010). Protein immunoprecipitated with this antibody was identified as Syt2 by mass spectrometry and western blots demonstrated that this antibody specifically detected recombinant Syt2 but not Syt1, a closely related family member (Fox and Sanes 2007). Syt2-immunolabeled nerve terminals were detected with AlexaFluor647-conjugated secondary antibodies, obtained from Molecular Probes/Invitrogen (Eugene, OR) and applied at a 1:1000 dilution.
Table 1.
Antibody used in this study.
| Antigen | Isotype | Description of immunogen | Source/Catalogue number | Dilution For IHC | Reference |
|---|---|---|---|---|---|
| Synaptotagmin 2 | Mouse IgG2a | 1–5 day zebrafish embryo | Zebrafish International Resource Center (cat# znp-1) | 1:200 | Fox and Sanes 2007 |
Tissue preparation
Mice were given a lethal dose of sodium pentobarbital, then transcardially perfused with phosphate-buffered saline (PBS)(pH 7.4) followed by 4% paraformaldehyde (PFA) in PBS. Heads were removed and post-fixed in 4% PFA for at least twelve hours at 4°C. After washing in PBS, heads were bisected and EOM were dissected by removing the superior portion of the bony orbit. Dissected EOMs were incubated in PBS containing a 1:1000 dilution of 1μg/μl tetramethylrhodamine conjugated α-bugarotoxin (Invitrogen/Molecular Probes) for 4 hours at room temperature. Following 12 hours of washing in PBS, whole muscles were mounted in VectaShield (Vector Laboratories; Burlingame, CA). Once cover-slipped muscles were flattened using alnico button magnets (Eclipse Magnets; Sheffield, England). Muscles were viewed on a Zeiss AxioImager A1 fluorescent microscope (Carl Zeiss, Inc., Thornwood, NY) and high-resolution images were acquired on either an Olympus FV1000 (Olympus America Inc., Melville, NY) or Leica SP2 (Leica Microsystems, Bannockburn, IL) confocal microscopes. Image stacks were exported to Metamorph where maximal projection images were generated. Adobe Photoshop CS3 was used to adjust gamma levels, montage adjacent images, and merge color images into single images.
Immunohistochemistry (IHC)
After images of GFP expression and BTX-labeling were acquired on an Olympus FV1000, cover-slips were removed and whole-mount muscles were washed thoroughly in PBS to remove the VectaShield mounting reagent (Vector Laboratories; Burlingame, CA). Muscles were immunostained as described previously (Fox et al. 2007; Fox and Sanes 2007). Briefly, muscles were incubated in blocking buffer (5% normal goat serum, 2.5% bovine serum albumin, and 0.1%TritonX100 in PBS) for 1 hour, in primary antibodies (diluted 1:200 in blocking buffer) for 12 hours at 4°C, and in AlexaFluor647-conjugated anti-mouse IgG2A secondary antibodies for 12 hours at 4°C. After washing in PBS, muscles were remounted in VectaShield mounting reagent (Vector Laboratories; Burlingame, CA) and were re-imaged on an Olympus FV1000 confocal microscope.
Results
Synapse elimination at EOM NMJs
To characterize the development of ‘en plaque’-type EOM synapses, we examined NMJ morphology on EOM fibers that have a single junctional site during the first three weeks of postnatal mouse development. Postsynaptic sites in SR muscles (Figure 1A) were labeled with alpha-bungarotoxin (BTX), which selectively binds to acetylcholine receptors (AChRs). Postnatal development of AChR cluster morphology at single site EOM muscle fibers appeared similar to that described in typical skeletal muscle (reviewed in Sanes and Lichtman 1999; Sanes and Lichtman 2001, Fox 2009): shortly after birth (~P5) AChR clusters appeared simple and plaque-like, but by P7 perforations formed in these simple clusters, and from P11 to P21 these perforations expanded to form complex, branched AChR clusters that resembled adult postsynaptic sites (Figure 1B′-E′)(Sanes and Lichtman 2001; Khanna et al. 2003b).
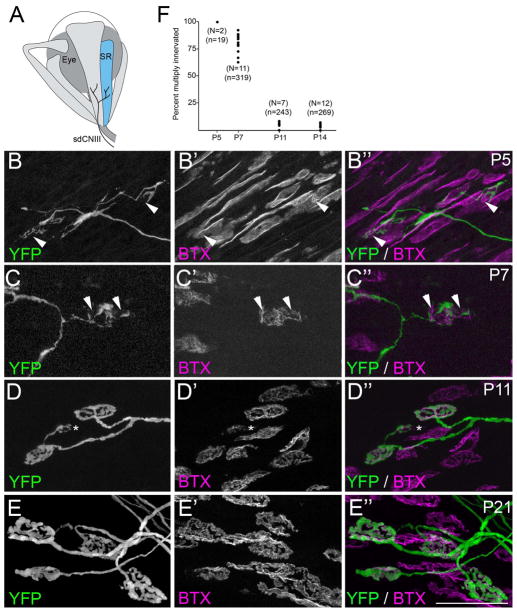
Fig. 1. Development of motor nerve terminals and AChR clusters at ‘en plaque’-type synapses in SR muscle.
A. Schematic representation of SR muscle (in blue) and its innervation by the superior division of the oculomotor nerve (sdCNIII). B–E. YFP-expressing motor nerve terminals (green) were imaged in P5 (B), P7 (C), P11 (D) and P21 (E) SR muscles isolated from thy1:yfp line H mice. AChR clusters were simultaneously visualized by labeling with fluorescently-conjugated bungarotoxin (BTX; magenta). At early ages (B,C) YFP-containing motor nerve terminals failed to innervate the entire postsynaptic membrane, suggesting that synaptic sites remained multiply innervated at these ages. Arrowheads highlight AChR-rich regions of the postsynaptic membrane that were unoccupied by YFP-labeled nerve terminals. By P11 (D), few synaptic sites contacted by YFP-labeled axons appeared multiply innervated. Asterisk in D, highlights an ‘en grappe’-type AChR cluster contacted by the same motor axon that contacted 2 ‘en plaque’-type NMJs in this field of view. Scale bar = 50 μm. F. Numbers of multiply innervated NMJs were quantified at P5, P7, P11, and P14 from thy1:yfp line H SR muscles. Each black dot represents the percentage of YFP-labeled NMJs that were multiply innervated from a single muscle. N = number of muscles analyzed per age. n = total of number of NMJs analyzed per age.
We next investigated the rate of synapse elimination of ‘en plaque’-type synapses. The small size of EOM motor units (a single motor axon innervates ~5 single site EOM muscle fibers) resulted in a much higher density of motor axons within the endplate band of EOMs than in typical skeletal muscles. Moreover the close association of different axons projecting to a junction made counting the number of axons entering a junction difficult. For this reason, we employed an alternative approach to assess the extent of multiple innervation: we counted the number of sites that were singly or multiply innervated in sparsely labeled thy1:yfp-lineH transgenic mice (Keller-Peck et al., 2001). While a single motor axon was labeled with yellow fluorescent protein (YFP) in most skeletal muscles of thy1:yfp-lineH mice (Feng et al. 2000; Keller-Peck et al 2001), several axons were labeled in EOMs. Multiply innervated synaptic sites in thy1:yfp-lineH EOM muscles were identified by observing unoccupied AChR-rich regions of postsynaptic membranes at synapses contacted by YFP-labeled nerve terminals (see arrowheads in Figure 1B,C)(see Keller-Peck et al. 2001 for details). Comparative analysis with wild-type mice has previously shown that synapse elimination proceeds normally in thy1:yfp-lineH mice despite the expression of YFP (Keller-Peck et al. 2001).
YFP expression in thy1:yfp-lineH mice appeared low in EOMs at early postnatal ages, however, labeled motor axons were observed in 2 out of 12 P5 SR muscles. All synaptic sites innervated by YFP-labeled axons in these muscles contained unoccupied regions of AChR-rich postsynaptic membrane suggesting that they were multiply innervated (Figure 1B,F and Table 2). By P7, substantially more axons were labeled with YFP in the SR muscles of thy1:yfp-lineH mice. Nearly 80% of synaptic sites contacted by a single labeled axon in P7 thy1:yfp-lineH SR muscles contained regions of unoccupied AChR-rich postsynaptic muscle membrane (Figure 1C,F and Table 2). These results strongly suggest that a high proportion of synapses on SR single junctional site muscle fibers contain supernumerary motor nerve terminals at the end of the first postnatal week of mouse development. However, by P11 – and at all ages thereafter – AChR-rich postsynaptic membranes appeared fully covered by single nerve terminals (Figure 1D-F and Table 2). Thus, at single junctional site muscle fibers, supernumerary nerve terminals appeared largely eliminated by P11, which is a few days earlier than the P14 endpoint previously found in neck and limb muscles of wild-type and thy1:yfp-lineH mice (Balice-Gordon and Lichtman 1993; Keller-Peck et al. 2001; Personius and Balice-Gordon 2001).
Table 2. Percent multiple innervation in thy1-YFP-lineH EOM muscles during postnatal development.
Numbers of multiply innervated ‘en plaque’-type NMJs were counted in SR, SO, LR, and LPS muscles throughout development. Each percentage represents the percent of multiply innervated synapses (±SD). N = the number of muscles analyzed per age; n = the total number of NMJs analyzed per age; ND – not determined. Tukey-Kramer tests were performed on data obtained from all age-matched muscles. For P21 LPS muscles, data was compared against all EOMs at P14. All statistically significant data sets are highlighted with asterisks and p values. For comparison with rates of synapse elimination in somite-derived muscles in thy1:yfp-lineH mice see Keller-Peck et al. 2001.
| EOM muscle | % Multiple innervation at various postnatal ages | |||||
|---|---|---|---|---|---|---|
| P5 | P7 | P11 | P14 | P21 | P56 | |
| Superior Rectus (SR) muscle | 100% (N=2, n=19) | 79.5 ± 9.2% (N=11, n=319) | 4.8 ± 3.4% (N=7, n=243) | 2.2 ± 2.8% (N=12, n=269) | ND | 1.0± 1.0% (N=9, n=121) |
| Superior Oblique (SO) muscle | 100% (N=1, n=8) | 77.6 ± 6.7% (N=7, n=175) | 6.9 ± 1.4% (N=7, n=243) | 7.5 ± 3.7% (N=12, n=269) | ND | ND |
| Lateral Rectus (LR) muscle | ND | 84.8 ± 10.5% (N=5, n=75) | 7.7 ± 4.4% (N=7, n=243) | 3.8 ± 3.6% (N=12, n=269) | ND | ND |
| Levator Palpebrae Superioris (LPS) muscle | 100% (N=1, n=5) Not statistically different than P5 SR or SO muscles |
87.0 ± 7.7% (N=7, n=120) Not statistically different than P7 SR, SO, or LR muscles |
45.1 ± 4.1% (N=8, n=151) *p<0.0001 vs P11 SR, SO and LR muscles |
42.7 ± 6.6% (N=6, n=106) *p<0.0002 vs P14 SR, SO and LR muscles |
22.1 ± 9.1% (N=5, n=103) *p<0.004 vs P14 SR, SO, LR, and LPS muscles |
7.3± 2.5% (N=8, n=80) *p<0.05 vs P56 SR muscle |
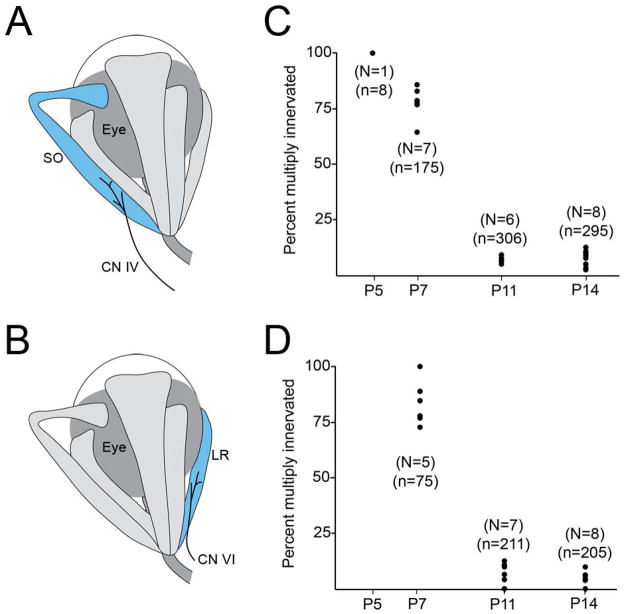
EOMs are innervated by 3 cranial nerves (CNs). Since SR muscles are innervated by the superior division of the oculomotor nerve (CN III)(Figure 1A), we also investigated the timing of synapse elimination in SO and LR muscles, which are innervated by the trochlear (CN IV) and abducens (CN VI) nerves, respectively (Figure 2A,B). The percent of multiply innervated ‘en plaque’-type NMJs in SO and LR muscles from thy1:yfp-lineH mice were statistically similar to those observed in age-matched SR muscles (Figure 1F, 2C,D and Table 2).
Fig 2. Percent of multiply innervated ‘en plaque’-type NMJs in developing SO and LR muscles.
A,B. Schematic representation of SO (A) and LR (B) muscles (in blue) and their innervation by CN IV and CN VI, respectively. Numbers of multiply innervated NMJs at P5, P7, P11, and P14 were quantified from SO (C), and LR (D) muscles isolated from thy1:yfp line H mice. Each black dot represents the percentage of YFP-labeled NMJs that were multiply innervated from a single muscle. N = number of muscles analyzed per age. n = total of number of NMJs analyzed per age.
In addition to being able to infer the extent of multiple innervation at synaptic sites on single-site muscle fibers, labeling of sparse motor axons in thy1:yfp-lineH mice proved useful in assessing patterns of innervation to ‘en grappe’-type NMJs, especially those within EOM central end-plate bands. Single motor axons were observed innervating chains of ‘en grappe’ synapses in thy1:yfp-lineH mice (Figure 3). As ‘en grappe’ end-plates are scattered longitudinally along a single muscle fiber, this suggests that many ‘en grappe’-type junctions along a single muscle fiber are innervated by the same motor neuron. Moreover, unlike ‘en grappe’-type junctions in other vertebrate muscle (Wilkinson and Lichtman 1985; Lichtman et al 1985), a lack of unoccupied AChR-rich regions of the muscle membrane suggested that EOM ‘en grappe’-type junctional sites are innervated by a single nerve terminal (Bach-y-rita and Lennerstrand 1975; but see Dimitrova et al. 2009). Unexpectedly, analysis in thy1:yfp-lineH mice also demonstrated that some ‘en grappe’ and ‘en plaque’ NMJs were contacted by the same motor axon (Figure 1D and 3). As ‘en plaque’ and ‘en grappe’ NMJs are associated with twitch and non-twitch fiber types, respectively (Khanna et al. 2003b; Porter 2002; Spencer and Porter 2006), these observations suggest that EOM motor units may contain heterogeneous populations of muscle fibers. In other mouse muscles motor neurons have been observed to innervate both intra and extrafusal fibers (so called “beta” motor neurons; see Keller-Peck et al. 2001), however to our knowledge this is the first example of a motor axon innervating both single- and multi-synaptic site type extrafusal muscle fibers. It is important to note that only ‘en grappe’ AChR clusters within proximity of ‘en plaque’ junctions in the central end-plate band were contacted by the axons that also innervated ‘en plaque’ endings. None of the ‘en grappe’ NMJs residing proximal or distal to the end-plate band appeared to be contacted by motor axons that innervated synaptic sites within the central end-plate band.
Fig 3. “En plaque’- and ‘en grappe’-type AChR clusters were contacted by the same motor axons.
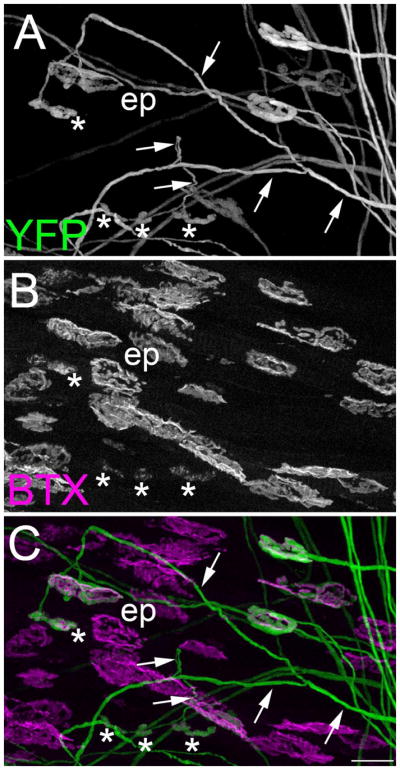
YFP-expressing motor nerve terminals (A)(green) were imaged in P21 SR muscles isolated from thy1:yfp line H mice. AChR clusters were labeled with fluorescently-conjugated bungarotoxin (B)(BTX; magenta). A merged image is shown in C. Note that the YFP-labeled axon highlighted with arrows contacts both ‘en plaque’- (ep) and ‘en grappe’-type (asterisks) synaptic sites. Scale bars = 25 μm.
Delayed synapse elimination in the LPS
We next assessed the development of NMJs in LPS muscle, which is not only adjacent to the SR muscle but is similarly innervated by the superior division of CN III (Figure 4A). AChR cluster morphology analysis revealed a similar temporal pattern of postsynaptic maturation in the LPS compared with other EOMs: at P5 AChR clusters appeared simple and plaque-like, by P7 perforations began to appear in AChR clusters, and at the beginning of the second week these perforations began to expand to form the complex, branched morphology of adult-like AChR clusters (Figure 4B′–E′). Labeling of AChR clusters in the developing LPS also confirmed that, as opposed to other EOMs, LPS muscles contained only single site ‘en plaque’ synapses and lacked evidence of muscle fibers innervated by multiple ‘en grappe’-type junctions.
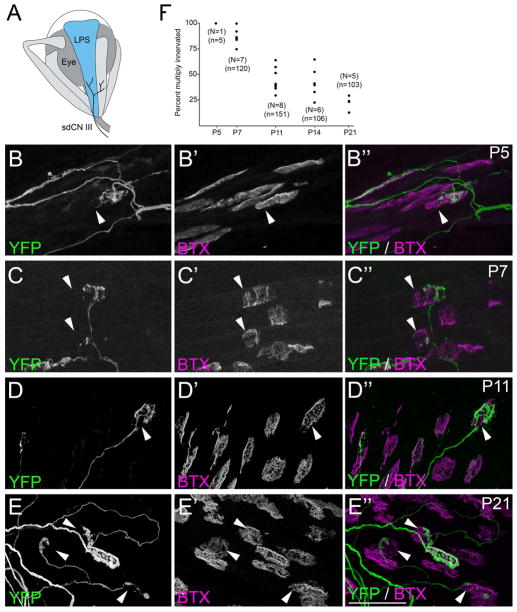
Fig 4. Development of nerve terminals and AChR clusters in LPS muscle.
A. Schematic representation of LPS muscle (in blue) and its innervation by the superior division of the oculomotor nerve (sdCNIII). B–E. YFP-expressing motor nerve terminals (green) were imaged in P5 (B), P7 (C), P11 (D) and P21 (E) LPS muscles isolated from thy1:yfp line H mice. AChR clusters were simultaneously visualized by labeling with fluorescently conjugated bungarotoxin (BTX; magenta). At all ages, YFP-containing motor nerve terminals were observed that failed to innervate entire postsynaptic sites, indicating these synapses were multiply innervated. Arrowheads highlight AChR-rich regions of the postsynaptic membrane unoccupied by YFP-labeled nerve terminals. Scale bar = 50μm. F. Numbers of NMJs multiple innervated synapses in P5, P7, P11, P14 and P21 LPS muscles isolated from thy1:yfp line H mice. Each black dot represents the percentage of YFP-labeled NMJs that were multiply innervated from a single LPS muscle. N = number of LPS muscles analyzed per age. n = total of number of NMJs analyzed per age.
As described above, thy1:yfp-lineH mice were used to infer the amount of multiple innervation at NMJs in the LPS at various developmental stages. At early postnatal ages (i.e. P5 and P7) the percent of multiply innervated synaptic sites were similar to other EOMs (Figure 4B,C,F and Table 2). At P11, ~45% of YFP-labeled nerve terminals failed to fully occupy synaptic sites in LPS (Figure 4D,F and Table 2). As described above, unoccupied regions of the postsynaptic membrane were rare at this age in other EOMs (Figure 1F and 2C,D). At P14, more than a third of LPS synapses appeared multiply innervated (Figure 4F and Table 2). The percent of multiply innervated synaptic sites in P11 and P14 LPS muscles were statistically significant compared to other EOMs (Table 2). Multiply innervated NMJs remained common in the LPS at P21 and were also observed in young adult (P56) LPS muscles (Figure 4E,F and Table 2). Previous studies have demonstrated that synapse elimination stalls at a small proportion of NMJs in bullfrog (Rana catesbeiana) muscle leaving supernumerary motor nerve terminals at adult synapses (Letinsky and Morrison-Graham 1980; Morrison-Graham 1983). To assess whether some synapses remained multiply innervated forever or whether synapse elimination occurred over an extremely protracted period in LPS muscle, we examined the occupancy of YFP-labeled nerve terminals in 1 year old thy1:yfp-lineH mice. At one year of age, we observed no multiply innervated NMJs in LPS muscles. Together, these data reveal an unexpected and significant delay in the elimination of supernumerary inputs in mouse LPS muscle.
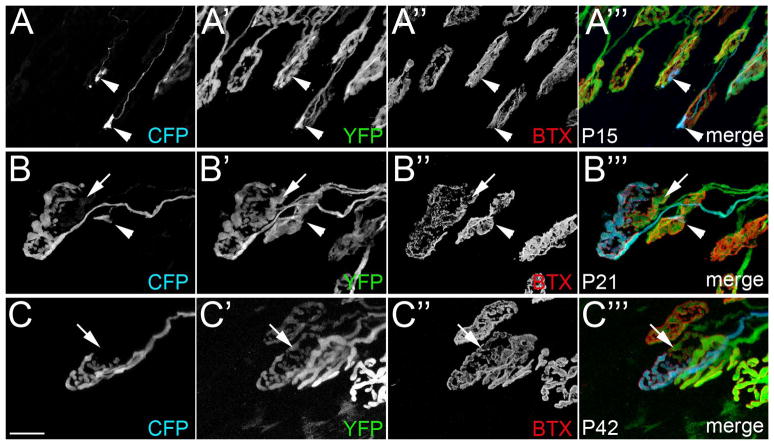
As detailed above, we interpreted unoccupied receptors at a synaptic site innervated by a YFP-labeled motor nerve terminal to indicate that additional, unlabeled motor axons were present at these sites. To directly demonstrate that synaptic sites were contacted by multiple axons we analyzed LPS muscles in transgenic mice in which motor axons were labeled by different fluorescent proteins (see Walsh and Lichtman 2003; Lichtman and Sanes 2003; Kasthuri and Lichtman 2003). A transgenic mouse line in which all motor axons were labeled with YFP (thy1:yfp-line16 mice; see Figure S2) was crossed with a transgenic line in which only some motor axons innervating the LPS contained cyan fluorescent protein (CFP)(thy1:cfp-line23 mice)(Feng et al. 2000). The resulting mice (thy1:yfp-line16; thy1:cfp-line23) had a small cohort of motor axons labeled with both CFP and YFP, whereas most motor axons contained only YFP (Figure 5A). Focusing on synaptic sites contacted by CFP-labeled axons, we asked whether YFP-positive, CFP-negative axons co-innervated these sites. At P10, an age in which synapse elimination appeared largely complete in other EOMs (Figure 1F and 2C,D), only a small number of synaptic sites contacted by CFP-labeled axons appeared singly innervated (Figure 5B). In contrast, the majority of synaptic sites were contacted by both axons containing CFP and axons that lacked CFP (Figure 5C). Similar results were obtained at synapses in P15, P21 and P42 LPS muscles (Figure 6), although two distinctions were noted at these older ages. First, as expected from analysis in thy1:yfp-lineH mice, fewer multiply innervated synapses were observed at older ages. Second, and more importantly, nerve terminals sharing space at single synaptic sites in older muscles appeared segregated from each other, with each occupying a distinct domain of the postsynaptic membrane (Figure 6C).
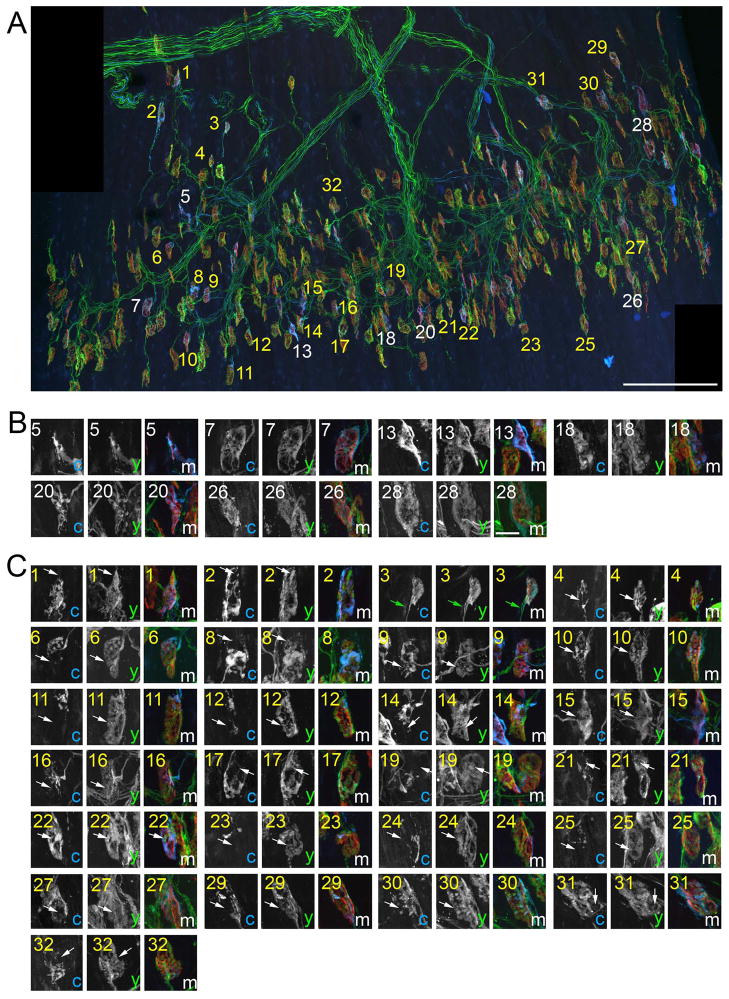
Fig 5. Multiple nerve terminals were observed at most synaptic sites in P10 LPS muscles.
To conclusively determine synaptic sites in P10 LPS muscle were contacted by multiple axons, NMJs were imaged in thy1:yfp line 16; thy1:cfp line23 mice in which all motor axons were labeled with YFP and only a few were also labeled with CFP. A. A montage of high-resolution images encompassing the entire central end-plate band. AChR clusters were labeled with BTX (red). 32 NMJs contacted by a CFP-labeled axon were labeled with numbers: white numbers depict singly innervated NMJs; yellow numbers depict synapses contacted by both CFP-labeled axons and YFP-labeled (CFP-non-labeled) axons. B. High magnification images of singly innervated NMJs from (A). C. High magnification images of NMJs that are contacted by both a CFP-expressing axon and supernumerary YFP-expressing axons. Arrows in C highlight domains of these NMJs that are innervated by YFP-labeled (CFP-non-labeled) nerve terminals. In some cases although CFP-labeled terminals appeared to fully occupy a postsynaptic site, YFP-labeled (CFP-non-labeled) axons were still observed co-innervating these site (see green arrows in image number 3 of C). c = CFP; y = YFP; m = merge. Scale bar in A = 200 μm, in B = 10 μm for B,C.
Fig 6. Multiple nerve terminals were observed at synaptic sites in P15, P21 and P42 LPS muscles.
NMJs were imaged from P15 (A), P21 (B) and P42 (C) LPS muscles from thy1:yfp line 16; thy1:cfp line23 transgenic mice in which all motor axons were labeled with YFP and only a few were also labeled with CFP. Note the segregation of axonal terminals into distinct domains of the postsynaptic membrane in P21 and P42 LPS muscles (B,C). Arrows highlight AChR-rich regions unoccupied by CFP-labeled motor nerve terminals. Arrowhead in B highlights a small portion of this synaptic site contacted by a CFP-labeled axon. Scale bar = 10 μm.
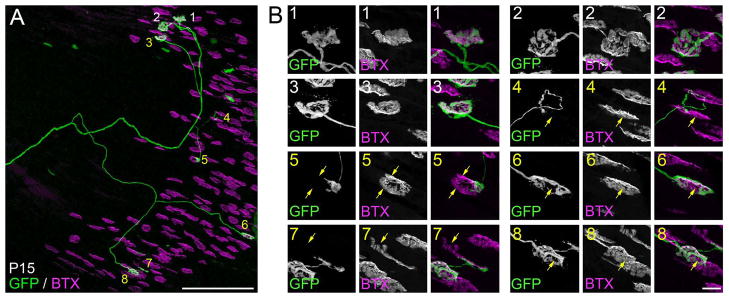
By using either thy1:yfp lineH or thy1:yfp-line16; thy1:cfp-line23 mice numerous axons in the LPS muscle were analyzed simultaneously. Therefore, we could not determine whether all motor axons were undergoing branch withdrawal and synapse elimination or whether just a select subset were undergoing branch trimming at many sites. To answer this question we analyzed axonal arbors and NMJs in thy1:gfp-lineS mice – a transgenic line in which only one or two motor axons were labeled in LPS muscles. We analyzed single motor axons in 9 P15 LPS muscles. In all but one case (8/9 axons), at least one synaptic site contacted by the GFP-labeled axon appeared multiply innervated (Figure 7). These results lead us to 2 important conclusions. First, synapse elimination occurs asynchronously at synaptic sites in a single motor unit in this unusual muscle as has been previously described in more typical muscles (Keller-Peck et al., 2001). Second, nearly all motor axons innervating LPS muscle are still undergoing branch withdrawal at some synaptic sites suggesting the entire population of motor neurons in this muscle is developmentally delayed compared to the innervation of all other muscles in the body.
Fig 7. Reconstruction of a single LPS motor unit revealed a high degree of multiple innervation at P15.
A. Confocal reconstruction of a single motor axon in a P15 thy1:gfp line S LPS muscle. Synaptic sites contacted by this axon are labeled with numbers. White numbers depict singly innervated synapses. Yellow numbers depict synapses in which GFP-labeled axons do not fully occupy all of the AChR-rich postsynaptic sites, indicating they are multiple innervated. B. High magnification images of synaptic sites contacted by the labeled motor axon shown in A. Yellow arrows highlight regions of AChR-rich postsynaptic membranes not contacted by the GFP-labeled axon. Scale bar in A = 250 μm and in B = 10 μm.
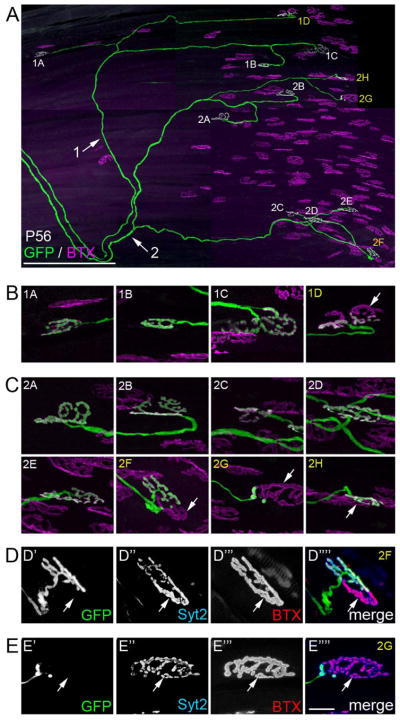
As synapse elimination proceeds fewer motor axons contain branches that contact multiply innervated synapses. However, young adult (P56) LPS motor axons still undergoing synaptic refinement do so asynchronously – with some terminal branches projecting to multiply innervated junctions and others contacting singularly innervated junctions. Figure 8 shows 2 GFP-labeled axons in a P56 thy1:gfp-lineS LPS muscle which both show signs of sharing postsynaptic sites with other axons at some of the NMJs they contact (see arrows in Figure 8B,C). To demonstrate that these adult sites were multiply innervated, we confirmed the presence of non-GFP-labeled terminals by immunostaining for synaptotagmin 2 (Syt2), a hallmark component of all motor nerve terminals (Fox and Sanes 2007) (Figure 8D,E). Thus, in this muscle some fibers maintain multiple innervation for far longer than any other known mammalian muscle.
Fig 8. Reconstruction of two LPS motor units revealed multiply innervated synaptic sites persisted at P56.
A. Confocal reconstruction of two motor axons innervating the same P56 LPS muscle from a thy1:gfp line S mouse. Each axon is labeled (with arrows and either a 1 or 2) as they course toward the end-plate band. Synaptic sites contacted by each axon are labeled with numbers that correspond to the axon innervating them and a letter. Sites labeled in white appear singly innervated; sites labeled in yellow synapses appear multiply innervated. B. High magnification images of synaptic sites in motor unit 1. Arrow indicates AChR-rich regions of a synaptic site not fully innervated by the GFP-labeled axon. C. High magnification images of synaptic sites in motor unit 2. Arrows indicates AChR-rich regions of synaptic sites not fully innervated by the GFP-labeled axon. D,E. After imaging, the LPS muscle was immunostained for synaptotagmin 2 (Syt2), a component of the presynaptic machinery within all motor nerve terminals. Immuno-labeling for Syt2 (blue) revealed that portions of the synaptic sites not occupied by the GFP-labeled were occupied by other axons (see arrows). Scale bar in A = 250 μm and in E = 10 μm for B–E.
Discussion
We examined the timing of postnatal elimination of multiple innervation at neuromuscular synapses in mouse eye muscles. Our initial hypothesis was that the elimination of excess motor nerve terminals would be accelerated in EOMs (compared with typical, somite-derived skeletal muscles) based in part upon their extraordinarily high frequency of extraocular motor neuron firing which develops at early perinatal ages before natural eye-opening (Tsuzuki et al. 1995; Carrascal et al. 2006, 2010). Synapse elimination appeared modestly accelerated in these muscles – which were singly innervated by P11 compared to a number of other neck and limb muscles that appear to reach the mature, singly innervated state by P14 in the same transgenic mouse line (Keller-Peck et al. 2001). A comparable acceleration in the rate of synapse elimination has been shown to be elicited experimentally by 100Hz bursts of action potentials in the rat soleus muscle (Thompson, 1983). The present results corroborate those findings and others (Personius and Balice-Gordon 2001) and support the notion that endogenous activity rates affect the time at which neuromuscular junctions become singly innervated.
Our studies also provide several results that were less expected. First, we found a dramatic general delay in the elimination of multiple innervation in the muscle that raises the eyelid (LPS muscle). Remarkably, some NMJs remained multiply innervated even 8 weeks after birth when animals were in all other respects considered fully adult. This multiple innervation was caused by a profound developmental delay in synapse elimination, rather than a permanent impairment, because by one year of age all signs of multiple innervation in LPS muscles had disappeared. A second unexpected result was that this delay was not due to a special subset of axons that were unable to undergo synapse elimination because analysis of single motor units showed that some terminal branches of these delayed axons had undergone synapse elimination early while other branches had not. This asynchronous synapse elimination suggests that these axons are not fundamentally different from axons in more typical mouse muscles, which also show asynchronous synapse elimination (Keller-Peck et al., 2001; Kasthuri and Lichtman 2003). Third, the extraordinary delay in nerve terminal withdrawal was not matched by a comparable delay in the developmental rate of postsynaptic differentiation. In typical mouse muscles, synaptic refinement coincides with postsynaptic maturation, such that in the same developing muscle singly innervated synapses have mature postsynaptic specializations whereas multiply innervated synapses have immature postsynaptic specializations (Balice-Gordon and Lichtman 1993; Lichtman and Colman 2000). For this reason, it was surprising that despite the delay in synapse loss, the time course of postsynaptic maturation appeared to occur similar in LPS muscles compared to other EOMs whose postsynaptic maturation is largely complete by the end of the second postnatal week. This presynaptic vs. postsynaptic mismatch is also surprising because prior work has suggested that the postsynaptic cell may be the intermediary in the synaptic interactions between different axons vying for the same postsynaptic cell (e.g., Balice-Gordon and Lichtman 1994). Thus, although the target may be regulating synapse loss, the delay in refinement does not seem to be explained by immaturity or delayed development of the muscle fibers in LPS. Lastly, that these motor axons innervated both fast twitch style ‘en plaque’- and slower contracting type ‘en grappe’-type synapses, which were on two separate classes of muscle fibers, suggests that EOM motor units drive a heterogeneous population of muscle fibers.
Delayed elimination of supernumerary inputs at LPS synapses
What could be the reason for the delay in synapse elimination in LPS muscles? It appears to be unlike the sustained partial multiple innervation of frog muscle fibers (Morrison-Graham 1983), because amphibian fibers maintain multiple innervation throughout life (Letinsky and Morrison-Graham 1980; Morrison-Graham 1983). It also seems different from than the persistent multiple innervation known to occur in reptile muscle fibers (Lichtman et al., 1985; Wilkinson and Lichtman, 1985) as those tonic fibers do not fire action potentials. One possibility is that the initial delay in synapse elimination within LPS muscles may be caused by the eyelids remaining closed in mice until P12–P14. Thus, during the time period when synapse elimination is occurring in most skeletal muscles the LPS muscle is entirely immobile. Even though eyelids are not open during perinatal development, rodent EOM motor neurons are highly active shortly after birth, generate phasic activity in perinatal EOMs as early as P3, and generate adult-like eye movements (such as Rapid Eye Movements [REMs]) as early as P6 (Seelke et al. 2005; Tsuzuki et al. 1995; Carrascal et al. 2006, 2010; Kablar 2003; Jouvet-Mounier et al. 1970).
It is unlikely, however, that the absence of perinatal LPS muscle activity during the first two weeks accounts for the sustained delay in synapse elimination in 8 week old mice. An alternative possibility, is that the activity of LPS muscles is unlike the activity patterns observed in other muscles. For example, approximately 10% of their muscle fibers are a unique classification of fibers absent in other EOMs and other skeletal muscles (Porter et al. 1989). Based upon their internal membrane system development and mitochondrial content, these LPS-specific fibers were initially designated as slow-twitch fibers with extreme fatigue resistance (Porter et al. 1989; Frueh et al 1994). Indeed the role of LPS muscles in sustained eyelid elevation (Porter et al. 1989; Frueh et al. 1994) may mean that in normal use all these muscle fibers are active chronically in awake animals. If synapse elimination is, as supposed, promoted by activity differences between innervating axons (reviewed in Sanes and Lichtman, 1999) then a muscle in which all axons are active all the time may not provide the kind of ordered recruitment (Henneman 1985) necessary for postsynaptic cells to disambiguate their inputs and foment synapse elimination. Consistent with this view is the experimental evidence that shows if motor axons are synchronized by exogenous stimulation then synapse elimination is delayed (Busetto et al. 2000, 2003). These LPS-specific fibers then may be more like tonic fibers in reptiles that sustain multiple innervation despite, in this case, an ability to fire action potentials. While these specialized fiber types may contribute to the long lasting delay of synaptic refinement at some synaptic sites in LPS muscles, they most likely do not contribute to the initial widespread delay in refinement since they account for only a small fraction of LPS muscle fibers.
In addition to containing a unique class of muscle fibers, LPS muscles have both a unique genetic profile and susceptibility to disease (Porter et al. 2001). For this reason, it also remains possible that the delay in synaptic refinement is due to activity-independent mechanisms in this specialized muscle.
Heterogeneous fiber-types in EOM motor units
In typical skeletal muscles, alpha-motor neurons contact homogenous populations of muscle fibers (Burke et al. 1971; Burke 1999). While retrograde labeling studies have suggested this holds true in eye muscles (Büttner-Ennever et al. 2001; Eberhorn et al. 2005, 2006) we observed branches of the same YFP-labeled axon innervating both ‘en plaque’ and ‘en grappe’ NMJs in SR muscle (Figure 4). While our results seem to contradict those of Büttner-Ennever et al. (2001) and Eberhorn et al. (2006), their studies assessed whether motor neurons innervating the central end plate band also innervated distally located ‘en grappe’ synapses, whereas we assessed whether motor axons in the end plate band could innervate ‘en plaque’ and ‘en grappe’ type synapses within only this region. Based upon sites of delivery, these previous retrograde tracer studies could not address whether a single population of motor neurons could innervate both ‘en plaque’ and ‘en grappe’ synapses within the central end-plate band (Büttner-Ennever et al. 2001; Eberhorn et al. 2006).
The finding that both fast and slow contracting muscle fibers are innervated by the same motor axon raises interesting questions about the functional properties of these fibers since motor units are subject to use-dependent plasticity and motor neuron activity influences the contractile properties of muscle (Buller et al. 1960). This raises the question of whether a single axon innervating both ‘twitch’ and ‘tonic’-type EOM fibers alters the contractile properties of these fibers. Perhaps it does. Physiological recordings have demonstrated that multi-synaptic site EOM muscle fibers with ‘en grappe’-type synapses exhibit mixed contractile properties in EOMs: in distal regions these fibers behave as ‘tonic’ fibers, however, in the central end plate region they behave as ‘twitch-like’ fibers (Jacoby et al. 1989).
Our studies also raise an interesting question regarding the identity of these axons that innervate both fast and slow contracting EOM fibers. While alpha-motor neurons innervate homogenous populations of muscle fibers, another class of motor neurons – termed beta-motor neurons (or skeleto-fusimotor neurons) – innervate both fast contracting extrafusal skeletal muscle fibers and slow-contracting intrafusal fibers associated with muscle spindles (Emonet-Denand et al 1975; Burke and Tsairis 1977; Keller-Peck et al. 2001). This begs the question of whether motor neurons studied here are abnormal alpha-motor neurons or a novel class of beta-motor neurons.
Acknowledgments
We thank Dr. J.R. Sanes (Harvard University) for generously providing mice and reagents and Dr. J. Su, S. Haddad and D. Pelusi for animal care and genotyping. Microscopy was performed, in part, at the VCU Department of Anatomy and Neurobiology Microscopy Facility supported by funding from NIH-NINDS Center Core grant (5P30NS047463-02). This work was supported in part by an AD Williams grant (M.A.F.), the Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust (M.A.F.) and the National Institute of Health (NIH; NS020364) (J.W.L.).
References
- Bach-y-rita P, Lennerstrand G. Absence of polyneuronal innervation in cat extraocular muscles. J Physiol. 1975;244(3):613–624. doi: 10.1113/jphysiol.1975.sp010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Lichtman JW. In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions. J Neurosci. 1993;13(2):834–855. doi: 10.1523/JNEUROSCI.13-02-00834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Chua CK, Nelson CC, Lichtman JW. Gradual loss of synaptic cartels precedes axon withdrawal at developing neuromuscular junctions. Neuron. 1993;11(5):801–815. doi: 10.1016/0896-6273(93)90110-d. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372(6506):519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- Benoit P, Changeux JP. Consequences of tenotomy on the evolution of multiinnervation in developing rat soleus muscle. Brain Res. 1975;99(2):354–358. doi: 10.1016/0006-8993(75)90036-0. [DOI] [PubMed] [Google Scholar]
- Bixby JL, van Essen DC. Regional differences in the timing of synapse elimination in skeletal muscles of the neonatal rabbit. Brain Res. 1979;169(2):275–286. doi: 10.1016/0006-8993(79)91030-8. [DOI] [PubMed] [Google Scholar]
- Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424(6947):430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Zajac FE., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971;174(10):709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Burke RE, Tsairis P. Histochemical and physiological profile of a skeletofusimotor (beta) unit in cat soleus muscle. Brain Res. 1977;129(2):341–345. doi: 10.1016/0006-8993(77)90013-0. [DOI] [PubMed] [Google Scholar]
- Burke RE. Revisiting the notion of ‘motor unit types’. Prog Brain Res. 1999;123:167–175. [PubMed] [Google Scholar]
- Busetto G, Buffelli M, Tognana E, Bellico F, Cangiano A. Hebbian mechanisms revealed by electrical stimulation at developing rat neuromuscular junctions. J Neurosci. 2000;20(2):685–695. doi: 10.1523/JNEUROSCI.20-02-00685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busetto G, Buffelli M, Cangiano L, Cangiano A. Effects of evoked and spontaneous motoneuronal firing on synapse competition and elimination in skeletal muscle. J Neurocytol. 2003;32(5–8):795–802. doi: 10.1023/B:NEUR.0000020624.48032.ed. [DOI] [PubMed] [Google Scholar]
- Buttner-Ennever JA, Horn AK, Scherberger H, D’Ascanio P. Motoneurons of twitch and nontwitch extraocular muscle fibers in the abducens, trochlear, and oculomotor nuclei of monkeys. J Comp Neurol. 2001;438(3):318–335. doi: 10.1002/cne.1318. [DOI] [PubMed] [Google Scholar]
- Callaway EM, Soha JM, Van Essen DC. Competition favouring inactive over active motor neurons during synapse elimination. Nature. 1987;328(6129):422–426. doi: 10.1038/328422a0. [DOI] [PubMed] [Google Scholar]
- Carrascal L, Luque MA, Sobrino V, Torres B, Nunez-Abades P. Postnatal development enhances the effects of cholinergic inputs on recruitment threshold and firing rate of rat oculomotor nucleus motoneurons. Neuroscience. 171(2):613–621. doi: 10.1016/j.neuroscience.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Carrascal L, Nieto-Gonzalez JL, Nunez-Abades P, Torres B. Temporal sequence of changes in electrophysiological properties of oculomotor motoneurons during postnatal development. Neuroscience. 2006;140(4):1223–1237. doi: 10.1016/j.neuroscience.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992;114(1):1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- Dimitrova DM, Allman BL, Shall MS, Goldberg SJ. Polyneuronal innervation of single muscle fibers in cat eye muscle: inferior oblique. J Neurophysiol. 2009;101(6):2815–2821. doi: 10.1152/jn.90828.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhorn AC, Ardeleanu P, Buttner-Ennever JA, Horn AK. Histochemical differences between motoneurons supplying multiply and singly innervated extraocular muscle fibers. J Comp Neurol. 2005;491(4):352–366. doi: 10.1002/cne.20715. [DOI] [PubMed] [Google Scholar]
- Eberhorn AC, Buttner-Ennever JA, Horn AK. Identification of motoneurons supplying multiply- or singly-innervated extraocular muscle fibers in the rat. Neuroscience. 2006;137(3):891–903. doi: 10.1016/j.neuroscience.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Emonet-Denand F, Jami L, Laporte Y. Skeleto-fusimotor axons in the hind-limb muscles of the cat. J Physiol. 1975;249(1):153–166. doi: 10.1113/jphysiol.1975.sp011008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28(1):41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Fox MA, Umemori H. Seeking long-term relationship: axon and target communicate to organize synaptic differentiation. J Neurochem. 2006;97(5):1215–1231. doi: 10.1111/j.1471-4159.2006.03834.x. [DOI] [PubMed] [Google Scholar]
- Fox MA, Sanes JR. Synaptotagmin I and II are present in distinct subsets of central synapses. J Comp Neurol. 2007;503(2):280–296. doi: 10.1002/cne.21381. [DOI] [PubMed] [Google Scholar]
- Fox MA. Development of the Vertebrate Neuromuscular Junction. In: Hortsch M, Umemori H, editors. The Sticky Synapse. New York: Springer; 2009. pp. 39–84. [Google Scholar]
- Frueh BR, Hayes A, Lynch GS, Williams DA. Contractile properties and temperature sensitivity of the extraocular muscles, the levator and superior rectus, of the rabbit. J Physiol. 1994;475(2):327–336. doi: 10.1113/jphysiol.1994.sp020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF, Becker W, Ling L, Langer TP, Kaneko CR. Discharge patterns of levator palpebrae superioris motoneurons during vertical lid and eye movements in the monkey. J Neurophysiol. 1992;68(1):233–243. doi: 10.1152/jn.1992.68.1.233. [DOI] [PubMed] [Google Scholar]
- Gan WB, Lichtman JW. Synaptic segregation at the developing neuromuscular junction. Science. 1998;282(5393):1508–1511. doi: 10.1126/science.282.5393.1508. [DOI] [PubMed] [Google Scholar]
- Henneman E. The size-principle: a deterministic output emerges from a set of probabilistic connections. J Exp Biol. 1985;115:105–112. doi: 10.1242/jeb.115.1.105. [DOI] [PubMed] [Google Scholar]
- Jacoby J, Chiarandini DJ, Stefani E. Electrical properties and innervation of fibers in the orbital layer of rat extraocular muscles. J Neurophysiol. 1989;61(1):116–125. doi: 10.1152/jn.1989.61.1.116. [DOI] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2(4):216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Kablar B. Determination of retinal cell fates is affected in the absence of extraocular striated muscles. Dev Dyn. 2003;226(3):478–490. doi: 10.1002/dvdy.10256. [DOI] [PubMed] [Google Scholar]
- Kasthuri N, Lichtman JW. The role of neuronal identity in synaptic competition. Nature. 2003;424(6947):426–430. doi: 10.1038/nature01836. [DOI] [PubMed] [Google Scholar]
- Keller-Peck CR, Walsh MK, Gan WB, Feng G, Sanes JR, Lichtman JW. Asynchronous synapse elimination in neonatal motor units: studies using GFP transgenic mice. Neuron. 2001;31(3):381–394. doi: 10.1016/s0896-6273(01)00383-x. [DOI] [PubMed] [Google Scholar]
- Khanna S, Porter JD. Conservation of synapse-signaling pathways at the extraocular muscle neuromuscular junction. Ann N Y Acad Sci. 2002;956:394–396. doi: 10.1111/j.1749-6632.2002.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Khanna S, Richmonds CR, Kaminski HJ, Porter JD. Molecular organization of the extraocular muscle neuromuscular junction: partial conservation of and divergence from the skeletal muscle prototype. Invest Ophthalmol Vis Sci. 2003;44(5):1918–1926. doi: 10.1167/iovs.02-0890. [DOI] [PubMed] [Google Scholar]
- Khanna S, Merriam AP, Gong B, Leahy P, Porter JD. Comprehensive expression profiling by muscle tissue class and identification of the molecular niche of extraocular muscle. Faseb J. 2003;17(10):1370–1372. doi: 10.1096/fj.02-1108fje. [DOI] [PubMed] [Google Scholar]
- Letinsky MS, Morrison-Graham K. Structure of developing frog neuromuscular junctions. J Neurocytol. 1980;9(3):321–342. doi: 10.1007/BF01181540. [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Wilkinson RS, Rich MM. Multiple innervation of tonic endplates revealed by activity-dependent uptake of fluorescent probes. Nature. 1985;314(6009):357–359. doi: 10.1038/314357a0. [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Wilkinson RS. Properties of motor units in the transversus abdominis muscle of the garter snake. J Physiol. 1987;393:355–374. doi: 10.1113/jphysiol.1987.sp016827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25(2):269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Sanes JR. Watching the neuromuscular junction. J Neurocytol. 2003;32(5–8):767–775. doi: 10.1023/B:NEUR.0000020622.58471.37. [DOI] [PubMed] [Google Scholar]
- Morrison-Graham K. An anatomical and electrophysiological study of synapse elimination at the developing frog neuromuscular junction. Dev Biol. 1983;99(2):298–311. doi: 10.1016/0012-1606(83)90279-8. [DOI] [PubMed] [Google Scholar]
- Personius KE, Balice-Gordon RJ. Loss of correlated motor neuron activity during synaptic competition at developing neuromuscular synapses. Neuron. 2001;31(3):395–408. doi: 10.1016/s0896-6273(01)00369-5. [DOI] [PubMed] [Google Scholar]
- Porter JD, Burns LA, May PJ. Morphological substrate for eyelid movements: innervation and structure of primate levator palpebrae superioris and orbicularis oculi muscles. J Comp Neurol. 1989;287(1):64–81. doi: 10.1002/cne.902870106. [DOI] [PubMed] [Google Scholar]
- Porter JD, Merriam AP, Hack AA, Andrade FH, McNally EM. Extraocular muscle is spared despite the absence of an intact sarcoglycan complex in gamma- or delta-sarcoglycan-deficient mice. Neuromuscul Disord. 2001;11(2):197–207. doi: 10.1016/s0960-8966(00)00171-1. [DOI] [PubMed] [Google Scholar]
- Porter JD. Extraocular muscle: cellular adaptations for a diverse functional repertoire. Ann N Y Acad Sci. 2002;956:7–16. doi: 10.1111/j.1749-6632.2002.tb02804.x. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Oculomotor unit behavior in the monkey. J Neurophysiol. 1970;33(3):393–403. doi: 10.1152/jn.1970.33.3.393. [DOI] [PubMed] [Google Scholar]
- Rubinstein NA, Hoh JF. The distribution of myosin heavy chain isoforms among rat extraocular muscle fiber types. Invest Ophthalmol Vis Sci. 2000;41(11):3391–3398. [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2(11):791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Seelke AM, Karlsson KA, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements and the development of active and quiet sleep. Eur J Neurosci. 2005;22(4):911–920. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RF, Porter JD. Biological organization of the extraocular muscles. Prog Brain Res. 2006;151:43–80. doi: 10.1016/S0079-6123(05)51002-1. [DOI] [PubMed] [Google Scholar]
- Su J, Gorse K, Ramirez F, Fox MA. Collagen XIX is expressed by interneurons and contributes to the formation of hippocampal synapses. J Comp Neurol. 518(2):229–253. doi: 10.1002/cne.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W, Kuffler DP, Jansen JK. The effect of prolonged, reversible block of nerve impulses on the elimination of polyneuronal innervation of new-born rat skeletal muscle fibers. Neuroscience. 1979;4(2):271–281. doi: 10.1016/0306-4522(79)90088-5. [DOI] [PubMed] [Google Scholar]
- Thompson W. Synapse elimination in neonatal rat muscle is sensitive to pattern of muscle use. Nature. 1983;302(5909):614–616. doi: 10.1038/302614a0. [DOI] [PubMed] [Google Scholar]
- Thompson WJ. Activity and synapse elimination at the neuromuscular junction. Cell Mol Neurobiol. 1985;5(1–2):167–182. doi: 10.1007/BF00711091. [DOI] [PubMed] [Google Scholar]
- Trevarrow B, Marks DL, Kimmel CB. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990;4(5):669–679. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- Tsuzuki S, Yoshida S, Yamamoto T, Oka H. Developmental changes in the electrophysiological properties of neonatal rat oculomotor neurons studied in vitro. Neurosci Res. 1995;23(4):389–397. doi: 10.1016/0168-0102(95)00966-W. [DOI] [PubMed] [Google Scholar]
- Walsh MK, Lichtman JW. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 2003;37(1):67–73. doi: 10.1016/s0896-6273(02)01142-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson RS, Lichtman JW. Regular alternation of fiber types in the transversus abdominis muscle of the garter snake. J Neurosci. 1985;5(11):2979–2988. doi: 10.1523/JNEUROSCI.05-11-02979.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 137(7):1017–1033. doi: 10.1242/dev.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt RM, Balice-Gordon RJ. Activity-dependent elimination of neuromuscular synapses. J Neurocytol. 2003;32(5–8):777–794. doi: 10.1023/B:NEUR.0000020623.62043.33. [DOI] [PubMed] [Google Scholar]