Abstract
A requisite component of nervous system development is the achievement of cellular recognition and spatial segregation through competition-based refinement mechanisms. Competition for available axon space by myelinating oligodendrocytes ensures that all relevant CNS axons are myelinated properly. To ascertain the nature of this competition, we generated a transgenic mouse with sparsely labeled oligodendrocytes and establish that individual oligodendrocytes occupying similar axon tracts can greatly vary the number and lengths of their myelin internodes. Here we show that intercellular interactions between competing oligodendroglia influence the number and length of myelin internodes, referred to as myelinogenic potential, and identify the amino-terminal region of Nogo-A, expressed by oligodendroglia, as necessary and sufficient to inhibit this process. Exuberant and expansive myelination/remyelination is detected in the absence of Nogo during development and after demyelination, suggesting that spatial segregation and myelin extent is limited by microenvironmental inhibition. We demonstrate a unique physiological role for Nogo-A in the precise myelination of the developing CNS. Maximizing the myelinogenic potential of oligodendrocytes may offer an effective strategy for repair in future therapies for demyelination.
The spatial alignment of myelin internodes along axons facilitates the process of saltatory conduction, maximizing the speed and efficacy of action potential propagation throughout the nervous system. In the CNS, myelin is formed by oligodendrocytes, cells with the capacity to form multiple myelin internodes (1). What developmental mechanisms control the generation and coordination of the precise number and length of myelin internodes? Though recent studies have revealed extrinsic cues and transcriptional and epigenetic determinants that regulate oligodendrocyte differentiation (2–7), achievement of the precise spatial organization of myelin internodes necessitates a mechanism whereby neighboring oligodendroglial cells coordinate the appropriate number of myelin internodes generated. This coordination could be achieved through oligodendroglial competition for inductive cues and/or available axonal space (8). Variations in the number and length of myelin internodes formed by individual oligodendrocytes support the likelihood of environmental regulation of myelination. However, providing evidence of this variation requires a strategy that facilitates the resolution and analysis of individual myelinating oligodendrocytes in vivo.
Results
Individual Oligodendrocytes Exhibit Great Variability in the Number and Lengths of Myelin Internodes Throughout the CNS.
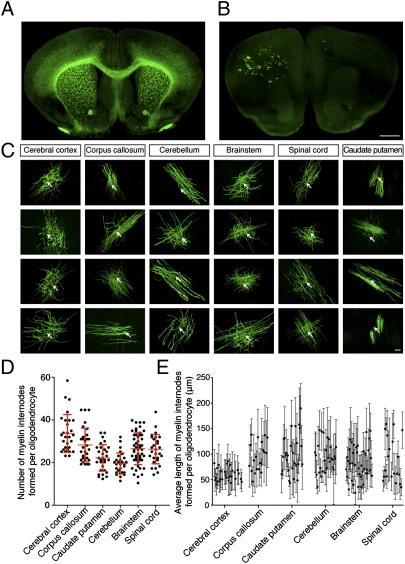
Though the heterogeneity of oligodendrocyte morphology has been recognized for the past century (9, 10), efforts to accurately examine oligodendrocytes have been hindered by the high density of myelinating cells, limiting the ability to confidently attribute specific myelin internodes to any particular oligodendrocyte. Existing methods approximate the number and length of myelin internodes produced by individual oligodendrocytes and are limited by sample size and brain region (11–13). To explore the likelihood of competitive oligodendroglial interactions, we developed a unique transgenic mouse line permitting resolution and analysis of individual oligodendrocytes in vivo. Based on position-effect variegation (14), we used a construct expressing membrane-associated EGFP (maEGFP) under the control of the myelin basic protein (MBP) enhancer to successfully generate a transgenic mouse line displaying maEGFP expression in <1% of myelinating cells (Fig. 1B). In contrast to knockin (Fig. 1A) and transgenic mouse lines in which the majority of oligodendrocytes are labeled, this sparsely labeled mouse line (Fig. 1B) allows for the identification and characterization of individual oligodendrocytes and their associated myelin internodes (Fig. 1C), providing a unique opportunity to characterize the factors that regulate myelinogenic potential. We systematically analyzed single oligodendrocytes throughout the CNS, and demonstrate the remarkable heterogeneity that exists in the orientation, number, and length of myelin internodes formed by individual oligodendrocytes in vivo. By compressing 100-μm z-stack images, we were able to resolve individual myelin internodes associated with each oligodendrocyte (Fig. S1A), and verify the quantification by 3D rendering. This analysis reveals a sixfold range in the number of myelin internodes formed per cell, as well as a variation in the average length of internodes ranging from ∼20 to 200 μm (Fig. 1 D and E). Importantly, the variability in number and length of internodes is not region specific, and can be detected within the same local region and along similar axonal tracts (Fig. 1 C–E). Though the orientation of myelin internodes is dependent on axon orientation, the observed variability in myelin internode number and length suggests that the myelinogenic potential of individual oligodendrocytes is not determined solely by axonal density, size, or molecular signals. Instead, the variation within brain regions suggests that localized microenvironmental cues may regulate myelinogenic potential.
Fig. 1.
Oligodendrocytes exhibit striking diversity in the number and length of myelin internodes in vivo and in vitro. Brain section from (A) 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP)-GFP transgenic mouse where all oligodendrocytes are fluorescently labeled. (B) Transgenic mouse expressing maGFP in <1% of oligodendrocytes. (Scale bar: 1 mm.) (C) Individual oligodendrocytes from different CNS regions of the sparsely labeled mouse. Arrows indicate cell bodies. (Scale bar: 20 μm.) (D and E) Comparison of myelinogenic potential between CNS regions. (D) Individual oligodendrocytes from indicated regions form a varied number of myelin internodes. Error bars represent SD, and the middle line represents the average number of myelin internodes formed per oligodendrocyte for each indicated region. (E) Average length of myelin internodes formed by individual oligodendrocytes. Error bars represent SD of myelin internode lengths for each individual oligodendrocyte, and demonstrate the variation of length from single cells.
The in vitro myelinating coculture represents a reduced system that is ideal for manipulating the microenvironment (15). Similar to oligodendroglial development in vivo, oligodendrocyte precursor cells (OPCs) cocultured with purified sensory neurons undergo an initial period of proliferation in response to axonal cues (15, 16) before differentiating into myelinating oligodendrocytes. The cocultures create an environment characterized by minimal variability in density and heterogeneity in the type of cultured neurons. Despite this relatively homogeneous environment, individual oligodendrocytes exhibit variability in morphology and myelinogenic potential, comparable to the variation found in vivo (Fig. S1B). This observation suggests that the neuronal diversity present in vivo may not be necessary for establishing the heterogeneous morphology of myelinating oligodendrocytes, and underscores the likelihood that competitive interactions between oligodendroglial cells, which take place both in vivo and in vitro, are responsible for shaping the myelinogenic potential of individual oligodendrocytes.
Oligodendroglial Density Controls the Myelinogenic Potential: A Competition-Based Model for Myelination.
A competition-based model for regulating myelination suggests a direct relationship between oligodendroglial numbers and myelinogenic potential. We hypothesize that an increase in the density of oligodendroglial cells should result in a corresponding decrease in the number of myelin internodes formed per oligodendrocyte (Fig. S2A). Efforts to manipulate the density of oligodendroglial cells are complicated in light of previous findings demonstrating that a critical density of OPCs along axons is required for the initiation of oligodendrocyte differentiation (17). However, differentiation can be induced in the absence of a critical density by replacing the majority of OPCs with comparably sized polystyrene beads (Fig. S2B). Conjugation of these beads to the surface of cultured axons creates spatial constraints that are sufficient to induce differentiation (17). The addition of polystyrene beads to cocultures allows for a 10-fold reduction in the density of oligodendroglial cells without inhibiting robust myelination (Fig. 2 A and B). Using this approach we tested the impact of oligodendroglial cell density on the average myelinogenic potential of oligodendrocytes. We find that a 10-fold decrease in OPC density leads to a significant increase in the average number of myelin internodes formed per oligodendrocyte (Fig. 2 C, D, and F). This finding is consistent with the possibility that repulsive interactions between oligodendroglial cells mediate myelinogenic potential.
Fig. 2.
Membrane-bound inhibitory cues expressed by oligodendroglia decrease the number of myelin internodes formed per oligodendrocyte. (A and B) Robust myelination is achieved as indicated by MBP in red (Right) in cocultures seeded with a high density of OPCs (A Left) and a low density of OPCs (B Left) where the majority of OPCs are replaced with polystyrene beads. OPCs are visualized by immunostaining for PDGF receptor-α (PDGFRα) axons with neurofilament (NF). Nuclei are labeled with DAPI. (Scale bar: 50 μm.) (C–E) High-magnification images of an individual oligodendrocyte from high-density coculture (C), low-density coculture with polystyrene beads (D), and low-density coculture with OPC membrane-coated beads (E). (Scale bar: 20 μm.) (F) Only beads coated with OPC/oligodendrocyte membranes significantly decrease the number of internodes formed per cell in a manner comparable to high-density cocultures (P < 0.001, Tukey post hoc comparison after one-way ANOVA). All error bars represent SD unless otherwise stated.
Oligodendroglia Express Membrane-Bound Cue(s) to Inhibit Myelinogenic Potential.
To confirm that a membrane-bound factor is sufficient to modulate myelinogenic potential, and to mimic the localized contact that may be required, we coated custom-designed polystyrene beads with OPC and/or oligodendrocyte membranes (18, 19) (Fig. S2B). This approach essentially creates artificial cells to test whether membrane-bound molecules are responsible for the decrease in myelinogenic potential that occurs at a high density of oligodendroglial cells. We find that compared with controls, beads coated with oligodendroglial membranes induce a twofold decrease in the number of myelin internodes formed per cell (Fig. 2 E and F). In contrast, beads coated with control membranes from other cell types, including astrocytes, do not alter the number of myelin internodes (Fig. 2F). Additionally, oligodendroglial membranes also decrease the length of myelin internodes (Fig. S3), suggesting that a membrane-bound cue(s) expressed specifically by oligodendroglia is sufficient to regulate myelinogenic potential.
Nogo-A Expressed by Oligodendroglia Is both Necessary and Sufficient for Regulating Myelinogenic Potential.
Expression of inhibitory cues by developing oligodendroglia could regulate the extension and/or retraction of neighboring glial projections through inhibitory mechanisms reminiscent of growth cone collapse. To identify the membrane-bound oligodendroglial signal(s), we performed a candidate screen testing factors previously identified as myelin-based inhibitors of axon regeneration. Because the number of myelin internodes is robustly inhibited by oligodendroglia (twofold), we initially focused our assay on the number of myelin internodes formed per oligodendrocyte. Our candidate screen included myelin-associated glycoprotein (MAG), Nogo-A, Nogo-66, p75NTR, Ephrin-B3, and oligodendrocyte-myelin glycoprotein (OMgp) (20). After verifying the expression of these inhibitory cues on cultured OPCs and oligodendrocytes by Western blot analysis (Fig. 3A), we conjugated soluble Fc-linked fusion constructs containing extracellular domains of the candidates to Protein A-coated polystyrene beads (Fig. S2C). This strategy allows for the local presentation of the candidate molecules to myelinating cells, and the examination of molecular sufficiency in a contact-mediated fashion. The majority of factors screened did not modulate myelinogenic potential (Fig. 3B). However, a 181-aa fragment located on the amino terminal of Nogo-A (also known as Nogo-Δ20) significantly inhibited the number of myelin internodes formed by individual oligodendrocytes (Fig. 3 B and C) (21, 22). We have performed a homology search using the National Center for Biotechnology Information (NCBI) BLASTP (23) and Conserved Domain Database (24), and we did not find similar peptide sequences or domains, suggesting the unique role of this portion of Nogo-A in inhibiting myelin internode generation.
Fig. 3.
Nogo-A is necessary and sufficient for reducing the number of myelin internodes formed per oligodendrocyte in vitro. (A) Expression of myelin-based inhibitors of axon regeneration are analyzed by Western blot in oligodendroglia, astrocytes, COS, and 3T3 cells. PDGFRα is a marker for OPCs, and MBP and MAG are markers of mature oligodendrocytes. β-actin serves as a loading control. (B) Fc-fusion candidates conjugated to Protein A-coated polystyrene beads are presented locally to oligodendroglia. (Scale bar: 50 μm.) (C) Only the amino terminal of Nogo-A (Nogo-A/Fc) significantly decreases the number of myelin internodes formed per oligodendrocyte across all conditions (P < 0.001, Tukey post hoc comparison after one-way ANOVA). (D) Knockdown of Nogo-A expression in OPC membranes before adsorption onto polystyrene beads leads to an increase in the number of myelin internodes formed per cell. Polystyrene beads are coated with membranes extracted from OPCs nucleofected with control siRNA or siRNA targeting Nogo-A. (Scale bar: 20 μm.) (E) OPC membranes treated with siRNA targeting Nogo-A or extracted from Nogo KO mice (with all isoforms of Nogo deleted) significantly rescue the number of myelin internodes formed compared with their respective controls (P < 0.0001, Student t test).
We next sought to examine whether Nogo-A is necessary to inhibit myelin internode formation through contact-mediated interactions. siRNA was used to knock down Nogo-A expression in cultured OPCs before membrane extraction (Fig. S4 A and B). Membranes were then coated onto beads and presented to oligodendrocytes. Reduction in Nogo-A expression significantly increased the myelinogenic potential of individual oligodendrocytes, suggesting that Nogo-A is an essential component responsible for the inhibitory effect of oligodendroglial membranes (Fig. 3 D and E). It is important to note that manipulating the expression of Nogo-A does not restore internode formation to that of the controls. To ensure that this observation is not due to incomplete knockdown or off-target effects of the siRNA, we conducted similar experiments with oligodendroglial membranes isolated from Nogo KO mice lacking all forms of Nogo (25), and confirm that additional cues that regulate the myelinogenic potential remain to be identified (Fig. 3E). Additionally, control oligodendroglial membranes exhibited an unusual influence in decreasing the percentage of myelinating oligodendrocytes, resulting in a corresponding increase in the nonmyelinating population (Table S1). To determine whether oligodendroglial membranes and Nogo expression could influence OPC differentiation, we analyzed the extent of differentiation across all experimental conditions and found that differentiation was unaffected (Table S1). Additionally, the axonal contribution of Nogo-A was examined in the dorsal root ganglion (DRG) neurons via siRNA knockdown. Reduction in Nogo-A expression in DRG neurons did not result in any changes in myelin internode numbers (Fig. S4C). Together, these findings support a model in which myelinogenic potential is directly influenced by contact-mediated competitive interactions between oligodendroglia.
Nogo-A Mediates Competitive Interactions Between Oligodendroglia.
Demonstrating competition necessitates a mosaic environment with select populations of cells possessing a competitive advantage. By combining different proportions of WT and Nogo KO OPCs in our cocultures, we demonstrate competition between oligodendroglia (Fig. S5A). Consistent with our hypothesis, the number of myelin internodes formed by WT oligodendrocytes is dependent on the expression of Nogo by neighboring oligodendroglia (Fig. S5C). Together, these findings demonstrate that myelinogenic potential is dependent on the expression of Nogo by neighboring oligodendroglia, supporting the role for Nogo-mediated competitive interactions in regulating myelinogenic potential.
Oligodendrocytes in Nogo KO Mice Exhibit a Significant Increase in Myelinogenic Potential and Display Exuberant and Expansive Myelination.
To examine the role of Nogo in the formation of myelin internodes in vivo, we crossed Nogo KO mice (25) with our sparsely labeled mouse line. We find that the average myelinogenic potential of oligodendrocytes within the cerebral cortex is significantly increased in the absence of Nogo, with individual cells forming an average of 67.0 ± 15.0 internodes per cell, whereas the WT littermate controls formed 58.8 ± 15.5 internodes per cell (Fig. 4 A and B). This increase in myelinogenic potential is significant when considering the high density of oligodendrocytes present throughout the CNS. Upon quantification of the average length of myelin internodes formed in vitro, we find that Nogo-A does not inhibit myelin internode length compared with high-density cocultures or in the presence of oligodendroglial membranes (Fig. S3), suggesting that other cues contribute to the modulation of length.
Fig. 4.
Deletion of Nogo in vivo results in enhanced myelinogenic potential and a spatial expansion of myelin in the cerebral cortex. (A and B) Deletion of Nogo in vivo results in an increase in the number of myelin internodes formed per oligodendrocyte. (A) Representative oligodendrocytes from the cerebral cortex of WT and Nogo KO. (Scale bar: 50 μm.) (B) There is a significant increase in the average number of myelin internodes made by cortical oligodendrocytes from Nogo KO compared with WT (P < 0.03, Student t test). (C Upper) Coronal views of WT and Nogo KO brains at P30. Boxes indicate locations for magnified views shown in Lower panels. (Scale bar: 1 mm.) (Lower) Exuberant myelin is seen in the upper cortical layers for the Nogo KO compared with WT. (Scale bar: 200 μm.) (D) Nogo KO mice have significantly more myelin internodes per mm2 in cortical layers 1–3 compared with WT (P < 0.0001, Student t test). (E) Magnified coronal views of the cerebral cortex at P30. OPCs are identified with PDGFRα in red and oligodendrocyte cell bodies with an antibody against Adenomatosis polyposis coli (CC1) in white. (Scale bar: 50 μm.) (F) There are significantly fewer CC1+ oligodendrocytes (P < 0.002, Student t test) and more OPCs (P < 0.007, Student t test) in the Nogo KO.
Does the increase in the number of myelin internodes formed by oligodendrocytes in the absence of Nogo result in an expansion of myelination leading to a disruption in spatial segregation? This question is challenging to address because the CNS is characterized by marked heterogeneity in the spatiotemporal regulation of myelination (1, 26, 27) and the density of myelin limits the feasibility and accuracy of precise quantification. To circumvent these complications, we chose to perform a temporally restricted regional evaluation of myelination in the sparsely myelinated upper layers of the cerebral cortex. At postnatal day (P) 30, layers 1–3 of the somatomotor region of the cortex are actively undergoing myelination, making this an ideal region to evaluate changes in the extent of myelination in the absence of Nogo. Our analysis demonstrates that the elimination of Nogo does indeed result in exuberant myelination in the upper layers of the somatomotor cortex compared with WT littermates, as shown by the marked increase in the number of myelin internodes per millimeter squared (Fig. 4 C and D). The extent of myelination in Nogo KO mice is expanded along axonal projections in both the medial-lateral (Fig. 4C) and rostral-caudal axes of orientation (Fig. S6A). Upon quantification, we find that myelination is expanded by ∼600 μm rostrally across the somatomotor cortex in the absence of Nogo (Fig. S6B). This finding is consistent with the increase of ∼10 myelin internodes formed per cell in the Nogo KO, spanning an average internode length of 70 μm in the cerebral cortex (Figs. 1E and 4B and Fig. S6C).
Oligodendroglial Numbers and Precocious Differentiation in Nogo KO Mice.
Could the exuberant myelination detected in the Nogo KO mice be attributed to (i) a shift in the temporal onset of OPC differentiation, (ii) an increase in the number of myelinating oligodendrocytes, or (iii) ectopic myelination of axons that would normally remain unmyelinated? Because differentiation of OPCs into oligodendrocytes plays an important role in regulating the timing of myelination (4, 28), we analyzed the somatomotor cortex at P20, before the onset of active myelination in this region (Fig. S7A). At P20, we observe similar numbers of differentiated oligodendrocytes in both Nogo KO mice and WT littermate controls (Fig. S7B), suggesting that a shift in the onset of oligodendrocyte differentiation is not responsible for the accelerated progression of myelination in the absence of Nogo. We next quantified the number of oligodendroglial cells present in the cortex at P30 to assess whether an increase in oligodendrocyte numbers may be responsible for the formation of exuberant myelin (Fig. 4E). Rather than detecting an increase in oligodendrocytes, we observe a significant decrease in the number of cortical oligodendrocytes present in the Nogo KO mice compared with WT littermate controls (Fig. 4F). This decrease in cortical oligodendrocytes is accompanied by a significant increase in OPC numbers, consistent with a reduction in differentiation.
Aberrant Myelination in Nogo KO Mice.
Does the presence of exuberant myelin in the absence of Nogo represent aberrant myelination of axons that normally remain unmyelinated? Analysis of the somatomotor cortex at P150 reveals a similar extent of myelination in both Nogo KO mice and WT littermate controls (Fig. S7C), demonstrating that changes in the extent of myelination in the absence of Nogo do not represent aberrant myelination. Instead, the elimination of Nogo disrupts spatial segregation, prematurely expanding myelination without altering the global pattern of myelination in the adult animals. Additionally, at P150 we still observe a decrease in the number of oligodendrocytes and a corresponding increase in the number of OPCs, suggesting that the increase in the myelinogenic potential of Nogo KO mice is maintained (Fig. S7D).
Maximizing the Myelinogenic Potential of Oligodendrocytes Enhances Remyelination.
To determine whether maximizing the myelinogenic potential of oligodendrocytes represents an effective means for remyelination, injection of lysolecithin into the dorsal funiculus and ventrolateral white matter of adult mouse spinal cords was performed in the Nogo KO and WT controls (4). Because oligodendrocytes in the white matter of the spinal cord of Nogo KO mice display an approximate twofold increase in myelinogenic potential (Fig. 5 A and B), the spinal cord represents a promising region that may allow for the detection of enhancement in repair. Spinal cords were harvested for analyses at 10 and 14 d postlesion (dpl), corresponding to the initiation of differentiation and myelination, respectively. At 10 dpl, Nogo KO samples were indistinguishable from control, because the onset of differentiation was just beginning to occur. Consistent with our hypothesis, at 14 dpl, remyelination was significantly more extensive in the Nogo KO spinal cords, with only 6% of the axons in the lesion that remained unmyelinated compared with 31% of the axons in the WT controls (Fig. 5 C–G). The enhancement of remyelination was apparent when G-ratios were analyzed because the proportion of axons with a ratio of 1 (demyelinated axons) were significantly fewer in the Nogo KO compared with the WT (Fig. 5 E–G). The accelerated remyelination is very likely attributed to the increase in myelinogenic potential, because the number of oligodendrocytes found within the lesion sites of Nogo KO and WT controls were unchanged (Fig. 5 H–J). Together, these findings suggest that Nogo may limit the efficacy of remyelination by restricting the myelinogenic potential of oligodendrocytes in the demyelinated lesion.
Fig. 5.
Myelinogenic potential and remyelination after lysolecithin-induced demyelination are significantly enhanced in the spinal cord of Nogo KO. (A) Representative oligodendrocytes from the white and gray matter of the spinal cord in WT and Nogo KO. (Scale bar: 50 μm.) (B) Quantification of the average number of myelin internodes made by individual oligodendrocytes from the gray and white matter of Nogo KO and WT (P < 0.0001, Student t test). (C and D Left) Light micrographs showing the dorsal funiculus of the spinal cord for WT (C) and Nogo KO (D) at 14 dpl. The lysolecithin-induced lesion sites are outlined in red. (Right) Electron micrographs of the lesion sites. Unmyelinated axons are marked with red asterisks. (Scale bar: 5 μm.) (E–G) Quantification of myelin sheath thickness and myelinated axons in WT (E) and Nogo KO (F) mice by G-ratio analysis. Scatterplots display G ratios of individual axons as a function of axonal diameter. (G) Extensive remyelination is detected in lesions induced in Nogo KO (black diamonds and line) compared with WT (gray circles and line), as evident by more axons with lower G-ratio values. A G-ratio value of 1.0 represents unmyelinated axons. (H–J) Quantification of proteolipid protein (PLP) mRNA+ oligodendrocytes in lesions sites at 14 dpl for Nogo KO (H) compared with WT (I). (Scale bar: 20 μm.) (J) There is no statistically significant difference between Nogo KO and WT.
Discussion
Our findings begin to address one of the long-standing questions in the myelination field: What mechanisms control the generation and coordination of the precise number of myelin internodes made by oligodendrocytes? Insight into this question is essential for improving our understanding of developmental myelination and for developing novel strategies to promote repair. Recent efforts have provided valuable insight into the extrinsic cues and transcriptional and epigenetic determinants that regulate oligodendrocyte differentiation (2–7). These studies originate under the general premise that upon differentiation, oligodendrocyte myelination is an all-or-none event solely dependent on the factors responsible for the temporal maturation of the oligodendrocyte under a permissive environment. However, it is clearly evident that the CNS is composed of heterogeneous microenvironments that shape the unique architecture and relationship between neurons and glia, which leads to the question of how the nervous system ensures the precise spatial and temporal pattern of myelination of all relevant axons. Underlying this developmental mechanism is the capacity of oligodendrocytes to form numerous myelin internodes. Therefore, we set out to investigate the potential environmental cues that control the generation and coordination of the number and length of myelin internodes, referred to as the myelinogenic potential. The periodic and nonoverlapping placement of myelin internodes along axons necessitates the presence of precise spatial cues that allow for coordination between myelinating oligodendrocytes. These cues should control the generation of the appropriate number, length, and the proper placement of myelin internodes along axonal tracts. To achieve this precise spatial alignment, it is essential that extraneous overlapping internodes are not deposited. Therefore, as an alternative approach, myelination can be viewed as a graded process, whereby oligodendrocytes integrate environmental signals to help achieve the precise spatial organization of myelin internodes. It is clear that during development, competition-based refinement mechanisms provide numerous instructive cues to aid in the precise patterning and establishment of neuronal connections, and oligodendrocyte myelination is no exception. We demonstrate that myelination in the CNS necessitates a mechanism whereby neighboring oligodendroglial cells coordinate the appropriate number of myelin internodes through oligodendroglial competition for available axonal space. In addition, we demonstrate a unique physiological role for Nogo during development in regulating the myelinogenic potential of oligodendrocytes, and suggest a degree of plasticity that allows for variations in myelinogenic potential and differentiation, while still ensuring the precise myelination of the CNS. Previous work indicates that the relative expression level of Nogo-A in the CNS is highly variable, with measurable differences both between brain regions and between different cell types and layers in a given region (29). This variable expression pattern is consistent with the heterogeneity seen in the number and length of myelin internodes throughout the CNS—both within and between brain regions. Together, these findings support a scenario in which localized expression of Nogo-A may act to regulate the formation of myelin internodes.
However, it must be noted that manipulation of Nogo expression does not affect the length of myelin internodes or the number of internodes with the same potency as oligodendroglial membranes, suggesting that other membrane-bound cues are also likely to be involved in the regulation of myelinogenic potential. In addition, oligodendroglial membranes exhibit a significant and specific activity in reducing the percentage of myelinating oligodendrocytes with a subsequent increase in the percentage of nonmyelinating oligodendrocytes that was not exhibited by any of the other candidates screened, including Nogo-A (Table S1). These findings suggest that additional potent cues still remain unidentified on oligodendroglia, which likely contribute to the precise patterning and spatial organization of myelin internodes. Further exploration of both intrinsic and extrinsic mechanisms that directly regulate myelination will be important to expand our understanding of developmental myelination and to enhance efforts aimed at promoting remyelination.
Many studies have focused on the inhibition of differentiation by the demyelinated environment as a major limitation to successful remyelination (30, 31). Our findings suggest that endogenous inhibitors present as a result of the myelin debris and in resident oligodendroglial cells surrounding the demyelinated environment may also inhibit remyelination by severely limiting the number and length of internodes that oligodendrocytes can form (32). Therefore, maximizing myelination at the level of internode numbers and lengths represents a promising alternative strategy for promoting repair and remyelination after injury or disease.
Methods
For a detailed description of the materials and methods used, see SI Methods.
Generation of Transgenic Mice with Sparsely Labeled Oligodendrocytes.
maEGFP (a gift from Bruce Appel, University of Colorado School of Medicine, Aurora, CO) was inserted after a 1.9-kb Mbp enhancer previously shown to direct transgene expression specifically in oligodendrocytes (33). Founder mice carrying mosaic Mbp-maGFP expression based on position-effect variegation were bred and characterized based on the sparseness of labeling of individual oligodendrocytes. Further details can be found in SI Methods.
Preparation of Membrane-Coated Polystyrene Beads.
Using an adapted protocol (18), membrane extracts were subjected to freeze/thaw cycles before ultracentrifugation. The membrane pellets were subjected to sonication and normalized to protein concentration before incubation with NH2-coated 20-μm polystyrene beads (G. Kisker). Detailed methods can be found in SI Methods.
Supplementary Material
Acknowledgments
We thank Dr. Marc Tessier-Lavigne for reviewing our manuscript and for his contribution with the Nogo KO mice. Support for this work was provided by US National Multiple Sclerosis Society Career Transition Award TA 3008A2/T, Harry Weaver Neuroscience Scholar Award TA 3008A/T, JF 2142-A-2, and National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant NS062796-02 (to J.R.C.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113540109/-/DCSupplemental.
See Commentary on page 1003.
References
- 1.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 2.Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- 3.Li H, He Y, Richardson WD, Casaccia P. Two-tier transcriptional control of oligodendrocyte differentiation. Curr Opin Neurobiol. 2009;19:479–485. doi: 10.1016/j.conb.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fancy SP, et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci. 2009;12:1398–1406. doi: 10.1038/nn.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emery B, et al. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138(1):172–185. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nave KA. Oligodendrocytes and the “micro brake” of progenitor cell proliferation. Neuron. 2010;65:577–579. doi: 10.1016/j.neuron.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Barres BA, Jacobson MD, Schmid R, Sendtner M, Raff MC. Does oligodendrocyte survival depend on axons? Curr Biol. 1993;3:489–497. doi: 10.1016/0960-9822(93)90039-q. [DOI] [PubMed] [Google Scholar]
- 9.Del Rio-Hortega P. Tercera aportacion al conocimiento morfologica e interpretacion funcional de la oligodendroglia [Third contribution to the morphological knowledge and functional interpretation of the oligodendroglia] Mem Real Soc Espan Hist Nat. 1928;14:5–122. [Google Scholar]
- 10.Butt AM, Ransom BR. Morphology of astrocytes and oligodendrocytes during development in the intact rat optic nerve. J Comp Neurol. 1993;338(1):141–158. doi: 10.1002/cne.903380110. [DOI] [PubMed] [Google Scholar]
- 11.Murray JA, Blakemore WF. The relationship between internodal length and fibre diameter in the spinal cord of the cat. J Neurol Sci. 1980;45(1):29–41. doi: 10.1016/s0022-510x(80)80004-9. [DOI] [PubMed] [Google Scholar]
- 12.Murtie JC, Macklin WB, Corfas G. Morphometric analysis of oligodendrocytes in the adult mouse frontal cortex. J Neurosci Res. 2007;85:2080–2086. doi: 10.1002/jnr.21339. [DOI] [PubMed] [Google Scholar]
- 13.Vinet J, et al. Subclasses of oligodendrocytes populate the mouse hippocampus. Eur J Neurosci. 2010;31:425–438. doi: 10.1111/j.1460-9568.2010.07082.x. [DOI] [PubMed] [Google Scholar]
- 14.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28(1):41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 15.Chan JR, et al. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004;43(2):183–191. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci USA. 2008;105:14662–14667. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimes ML, et al. Endocytosis of activated TrkA: Evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamauchi J, Chan JR, Shooter EM. Neurotrophins regulate Schwann cell migration by activating divergent signaling pathways dependent on Rho GTPases. Proc Natl Acad Sci USA. 2004;101:8774–8779. doi: 10.1073/pnas.0402795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oertle T, et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci. 2003;23:5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwab ME. Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci. 2010;11:799–811. doi: 10.1038/nrn2936. [DOI] [PubMed] [Google Scholar]
- 23.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchler-Bauer A, et al. CDD: A Conserved Domain Database for protein classification. Nucleic Acids Res. 2005;33(Database issue):D192–D196. doi: 10.1093/nar/gki069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JK, et al. Reassessment of corticospinal tract regeneration in Nogo-deficient mice. J Neurosci. 2009;29:8649–8654. doi: 10.1523/JNEUROSCI.1864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foran DR, Peterson AC. Myelin acquisition in the central nervous system of the mouse revealed by an MBP-Lac Z transgene. J Neurosci. 1992;12:4890–4897. doi: 10.1523/JNEUROSCI.12-12-04890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg SS, Chan JR. Modulating myelination: Knowing when to say Wnt. Genes Dev. 2009;23:1487–1493. doi: 10.1101/gad.1824009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber AB, Weinmann O, Brösamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- 31.Franklin RJM, Ffrench-Constant C. Remyelination in the CNS: From biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 32.Kotter MR, Li WW, Zhao C, Franklin RJM. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26:328–332. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gow A, Friedrich VL, Jr, Lazzarini RA. Myelin basic protein gene contains separate enhancers for oligodendrocyte and Schwann cell expression. J Cell Biol. 1992;119:605–616. doi: 10.1083/jcb.119.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.