Myelin is the membranous structure that wraps around axonal segments and supports the fast saltatory conduction of action potentials along axons (1, 2). Disorders that disrupt myelin during development or in adulthood, such as multiple sclerosis, lead to severe pathologies, illustrating myelin's crucial role in normal neural functioning. In the central nervous system, oligodendrocytes (OLs) are the cell type responsible for producing myelin. An individual OL is multiprocessed and can myelinate up to 60 nearby axon segments, generating an incredibly complex cell morphology (3, 4). To achieve complete, yet nonredundant, myelination of neural circuits, there must be very accurate matching of myelin segments to axonal surfaces. How these intricate intercellular interactions are so precisely coordinated both temporally and spatially has been a long-standing and elusive question. A study reported in PNAS by Chong et al. (4) provides evidence for a competition-based mechanism of OL myelin refinement that signals via OL-derived Nogo-A during both development and remyelination after injury.
In vivo, only OLs that make appropriate axonal contacts survive and initiate myelination (5). Therefore, it has long been recognized that competition for limited quantities of survival and inductive axonal cues is one mechanism driving myelin spatial refinement. Are there also repellant signals between neighboring OLs that further enhance this precise segregation? It has been previously demonstrated that the differentiation of OL-lineage cells can be modulated by factors such as oligodendrocyte precursor cell (OPC) density and mature myelin. Notably, the presence of a myelin substrate strongly inhibits the maturation of OPCs in vitro, suggesting the existence of contact-mediated signals between OL-lineage cells (6). Little is known, however, about the signals involved.
To address this question, Chong et al. (4) use in vitro myelinating cocultures. OPC density affects OL differentiation in these systems (7), which introduces a potentially confounding variable when trying to study a contact-based mechanism. Therefore, to normalize basal levels of myelination, Chong et al. (4) use polystyrene beads to achieve robust myelination at low OL densities. As predicted by a competitive model, decreasing OL density increased the average number of myelin segments formed by a given OL. Interestingly, this increase was completely reversed when the surrounding polystyrene beads were coated with membranes isolated from either OPCs or OLs, but not from non–OL-lineage cells. Taken together, these data support the existence of a membrane-bound OL-lineage–derived signal that restricts the myelinogenic potential of neighboring cells.
What is the molecular identity of this restrictive signal? A candidate-based screen revealed that coating the polystyrene beads with the amino terminal of Nogo-A was sufficient to reduce the myelinogenic potential of low-density OLs. Furthermore, depletion of Nogo-A from OPCs before membrane isolation for bead coating impaired the ability of those membranes to impact myelination. This was true both for siRNA knockdown of Nogo-A and OPCs isolated from Nogo knockout mice. Removing Nogo from surrounding OL membranes, however, only partially restored the myelinogenic potential of low-density OLs, indicating that there are likely other molecules involved.
Significantly, Chong et al. (4) demonstrate that Nogo is also important for myelin refinement in vivo. Nogo knockout mice exhibited increased myelination in the upper layers of the cortex coincident with a minor decrease in the total number of OLs. Thus, there was an increase in the number of myelin segments made by individual OLs, which was independent of OL differentiation or survival. It seems that Nogo-A signaling can restrict the myelinogenic potential of OLs during normal developmental myelination as well (Fig. 1).
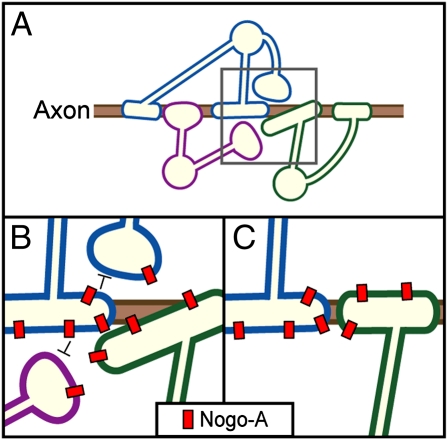
Fig. 1.
Mechanisms regulating myelinogenic potential of OLs. (A) Myelinating OLs compete for axon space. (B) Enlargement of gray square in A; Nogo-A molecules (in red) on OLs mediate repulsive interactions between OLs (black negative arrows). (C) In the final state, OL competition has helped achieve a precise nonredundant matching between the axon and myelin segments.
Might Nogo therefore similarly restrict remyelination ability? Promoting myelin repair is an attractive treatment goal for demyelinating diseases, including multiple sclerosis. Understanding the normal developmental mechanisms regulating myelination has yielded many potential targets, but only some have been effective at promoting remyelination. Using an in vivo model of demyelination, Chong et al. (4) demonstrate that, in addition to exuberant developmental myelination, there is also enhanced remyelination in the Nogo knockout mice. Two weeks after lysolecithin injury, 31% of axons in the wild-type spinal cords remained unmyelinated, whereas only 6% remained unmyelinated in the Nogo knockout animals. Interestingly, the acceleration of remyelination was not accompanied by an increase in the total number of mature OLs populating the lesions. Therefore, blockade of Nogo-A signaling was able accelerate the amount of myelin produced by each individual remyelinating OL.
A barrier to understanding the mechanisms that control OL morphology has been the challenge of visualizing individual OLs. Extensive OL morphological diversity is generally acknowledged on the basis of innumerable qualitative observations and some regionally focused quantitative studies (8). Comprehensive analyses, however, have been lacking owing to a dearth of techniques to efficiently probe the complexity of OLs in vivo. Chong et al. (4) generated a unique transgenic mouse that expresses membrane-associated EGFP in less than 1% of myelinating OLs. Using this mouse, the authors were able to provide the most broad and quantitative examination of OL diversity to date. The results confirm that a vast degree of variability in OL morphology exists both throughout the brain and within axonal tracts. It will be interesting to use this tool to address other basic questions about myelin biology, such as regional thickness, segment length along different axonal tracts, variety in axon diameter wrapped by each OL, or changes in OL morphology in aging and disease.
Chong et al. (4) provide mechanistic insight into how contact-dependent signaling between neighboring OLs contributes to the elegant coordination of axons and myelin segments. A clear future direction would be to further elucidate the mechanism by determining the relevant Nogo-A receptor on OLs. Additionally, the intracellular program that drives the alteration in OL morphology and myelin production downstream of Nogo-A is unknown. Recent research has begun to describe the transcriptional networks regulating OL differentiation and myelination
Chong et al. demonstrate that Nogo is also important for myelin refinement in vivo.
(9). It would be of interest to test the regulation of these major pathways by Nogo-A or OL membranes.
The authors demonstrate that reducing Nogo-A signaling enhances the myelinogenic potential of individual remyelinating OLs, which makes this an intriguing prospective drug target. If such a mechanism could be harnessed therapeutically, fewer healthy OLs would potentially be needed to repair damaged areas, resulting in a reduced need for stimulating OPC recruitment and differentiation, which are themselves complex therapeutic challenges. It will thus be of great interest to test the potential therapeutic effects of Nogo-A inhibition in other models of demyelinating disease. We still have much to learn about the molecular mechanisms regulating the intricate architecture of myelinated axons, but Chong et al. (4) have added some noteworthy pieces to the puzzle.
Footnotes
The authors declare no conflict of interest.
See companion article on page 1299.
References
- 1.Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 2.Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- 3.Simons M, Trotter J. Wrapping it up: The cell biology of myelination. Curr Opin Neurobiol. 2007;17:533–540. doi: 10.1016/j.conb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Chong SY, et al. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci USA. 2012;109:1299–1304. doi: 10.1073/pnas.1113540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barres BA, Jacobson MD, Schmid R, Sendtner M, Raff MC. Does oligodendrocyte survival depend on axons? Curr Biol. 1993;3:489–497. doi: 10.1016/0960-9822(93)90039-q. [DOI] [PubMed] [Google Scholar]
- 6.Miller RH. Contact with central nervous system myelin inhibits oligodendrocyte progenitor maturation. Dev Biol. 1999;216:359–368. doi: 10.1006/dbio.1999.9466. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci USA. 2008;105:14662–14667. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murtie JC, Macklin WB, Corfas G. Morphometric analysis of oligodendrocytes in the adult mouse frontal cortex. J Neurosci Res. 2007;85:2080–2086. doi: 10.1002/jnr.21339. [DOI] [PubMed] [Google Scholar]
- 9.Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]