Abstract
A blastocyst will implant only when the uterus becomes receptive. Following attachment, luminal epithelial cells undergo degeneration at the site of the blastocyst. Although many genes critical for uterine receptivity are primarily regulated by ovarian hormones, Kruppel-like factor 5 (KLF5), a zinc finger-containing transcription factor, is persistently expressed in epithelial cells independently of ovarian hormones. Loss of uterine Klf5 causes female infertility due to defective implantation. Cox2 is normally expressed in the luminal epithelium and stroma at the site of blastocyst attachment, but luminal epithelial COX2 expression is absent with loss of Klf5. This is associated with the retention of the epithelium around the implantation chamber with arrested embryonic growth. These results suggest that Klf5 is indispensable for normal implantation.
Keywords: decidualization, fertility, pregnancy
Ovarian estrogen and progesterone (P4) direct phases of uterine sensitivity to implantation. In mice, the uterus is prereceptive on days 1–3, assumes receptivity on day 4 of pregnancy or pseudopregnancy (day 1 = vaginal plug), and becomes refractory to implantation by day 5 (1). The implantation process, which is initiated by blastocyst attachment to the receptive uterine luminal epithelium, occurs in the evening of day 4 and becomes more prominent on the morning of day 5. This coincides with an increased uterine vascular permeability at the site of the blastocyst. The event can be visualized as discrete blue bands (sites of increased vascular permeability) by an i.v. injection of a blue dye solution before sacrifice (2, 3). By day 6, epithelial cells lining the implantation chamber disintegrate, presumably due to apoptosis in response to the invading blastocyst (4). The blastocyst is in direct contact with underlying stromal cells that are then undergoing proliferation and differentiation into decidual cells. A previous report indicated that trophoblasts do not directly partake in epithelial cell degeneration (5), but the epithelium is programmed to apoptosis at the site of the blastocyst following the attachment reaction (6). However, the epithelium away from the attachment site remains intact, suggesting that the blastocyst signals trigger epithelial cell death (5).
In mice, uterine receptivity is achieved when the uterus is exposed to estrogen after 24–48 h of P4 priming (7). However, the gene network that confers uterine receptivity is complex, making it difficult to manipulate the window of receptivity by changing the expression of several critical genes (8). Genetic studies in mice have shown that certain genes, including Lif (9), Ptgs2 (10), Ihh (11), Msx (12), and Fkbp52 (13), are critical to uterine receptivity. However, it is still unclear whether the signaling pathways work independently, in parallel, or converge onto a common pathway to confer uterine receptivity.
The luminal epithelium plays a critical role in implantation: it transmits embryonic signals to underlying stromal cells to initiate and progress the implantation process. In mice, the luminal epithelium around the embryo is still intact on the morning of day 5, when stromal cells have already initiated decidualization. This suggests that embryonic signals are transmitted to the stroma via epithelial cells. In fact, removal of the epithelium prevents decidualization (14). Although the exact nature of this signal has not been clearly identified, prostaglandins (PGs) are considered to play a role in triggering decidualization (10), and COX2-deficient females have decidualization failure (10). However, it is not clearly understood how uterine COX2 is regulated during implantation and decidualization.
The Kruppel-like factors (KLFs) belong to a family of zinc finger-containing transcription factors that regulate diverse cellular processes, including development, differentiation, proliferation, and apoptosis. Similar to the other 16 members in the family, KLF5 contains three zinc-finger domains that function in DNA binding (15). KLF5 is expressed in the human and mouse digestive tract, pancreas, placenta, testis, prostate, skeletal muscle, and lung (15). Studies in cell culture and animal models show that KLF5 has essential roles in cell proliferation, apoptosis, migration, differentiation, and stemness in a context-dependent manner. Klf5 expression is regulated by estrogen and P4 in breast cancer cells: although estrogen facilitates KLF5 degradation (16), P4 induces its expression, and KLF5 mediates P4's effects on breast epithelial cell proliferation and dedifferentiation (17). However, it is not known whether Klf5 is expressed in the uterus or whether it has a role in pregnancy events.
Klf5 null (Klf5−/−) mice show embryonic lethality due to defects in preimplantation embryo development (18). In its absence, trophectoderm development is defective, resulting in developmental arrest at the blastocyst stage. In addition, the expression of Oct4 and Nanog, two pluripotency markers, is down-regulated in the inner cell mass, whereas Sox17 expression is increased in the primitive endoderm. These results suggest that Klf5 is a key regulator of all three lineages in preimplantation embryos (19). The generation of a conditional deletion mouse model has helped to study Klf5's function in adulthood (20). To study KLF5's role in female fertility, we established a mouse line with uterine deletion of Klf5 (Klf5d/d) using floxed Klf5 mice (Klf5f/f) crossed with mice carrying a Cre gene driven by the progesterone receptor (Pgrcre/+). We found that Klf5d/d females are mostly infertile due to defective implantation and decidualization.
Results
KLF5 Is Expressed in a Spatiotemporal Manner in the Periimplantation Uterus.
Embryonic expression of Klf5 and developmental arrest of Klf5−/− blastocysts provide evidence that Klf5 has a critical role in stem cell differentiation and development of various organs. Conditional deletions in mice show that Klf5 is required for perinatal lung morphogenesis (20), formation and differentiation of the bladder urothelium (21), epithelial proliferation and differentiation of the gastrointestinal tract (22), and normal eyelid development (23). However, its role in female fertility was not known. To address this issue, we first examined KLF5 expression in the mouse ovary, oviduct, and uterus. Immunohistochemistry results show that KLF5-positive cells (if any) are very sparse in the ovary (Fig. 1). In the oviduct, epithelial cell nuclei show KLF5 staining.
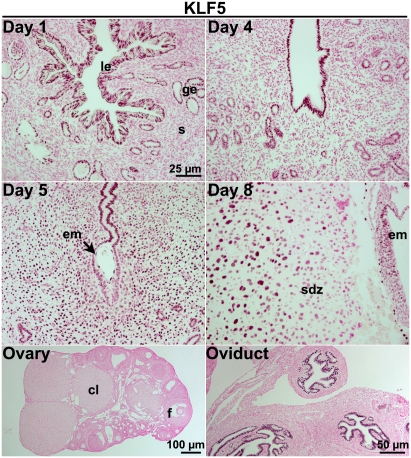
Fig. 1.
KLF5 is expressed in the oviduct and periimplantation mouse uterus in a spatiotemporal fashion. Immunohistochemistry of KLF5 in WT tissues. Nuclear KLF5 staining is shown as dark-brown deposits on tissue sections counterstained with eosin. KLF5 is present in the epithelia of day 1 and day 4 uteri and oviducts. On days 5 and 8, KLF5 is also expressed in decidual cells. Positive signals were not detected in the ovary. le, luminal epithelium; s, stroma; ge, glandular epithelium; em, embryo; sdz, secondary decidual zone; cl, corpus luteum; f, follicle.
In the uterus, KLF5 expression is spatiotemporal. On day 1 of pregnancy, KLF5 is present in the luminal and glandular epithelia when the uterus is primarily under the influence of preovulatory estrogen secretion. On day 4, its expression is also limited to the epithelium when the uterus is under the influence of rising P4 levels from newly formed corpora lutea superimposed with a small amount of estrogen secreted from the ovary. With blastocyst attachment in progress on the morning of day 5, stromal cells at the attachment site proliferate and differentiate into decidual cells. Later on day 5, stromal cells immediately surrounding the implantation chamber undergo differentiation to decidual cells, giving rise to the primary decidual zone (PDZ). At this time, proliferating stromal cells express KLF5, but the expression declines in stromal cells that had already differentiated into the PDZ (Fig. 1 and Fig. S1). In parallel, KLF5 expression is also down-regulated in the epithelium, especially the epithelium surrounding the blastocyst. On day 8, little epithelium is left at the implantation site, and stromal cells next to the PDZ have undergone decidualization, forming the secondary decidual zone. KLF5 expression persists in proliferating cells, but disappears once the cells complete terminal differentiation. The dynamic KLF5 expression in epithelial and decidual cells suggests that it contributes to uterine receptivity to implantation and decidualization.
Ovarian Hormones Modestly Influence KLF5 Expression.
To address whether estrogen and P4 regulate uterine KLF5 expression, WT females were ovariectomized and rested for 10 d. They were then given a s.c. injection of oil (0.1 mL/mouse), estradiol-17β (E2, 100 ng/mouse), or P4 (2 mg/mouse) and killed 2, 6, or 12 h later. The presence of KLF5 in the epithelium of oil-injected ovariectomized mice indicates that E2 and P4 are dispensable for KLF5 expression. However, E2 or P4 treatment increased the staining intensity in the epithelium at 2 h, and both hormones modestly decreased the staining at 12 h, specifically in the glandular epithelium (Fig. S2). These results suggest that uterine KLF5 expression is not greatly influenced by ovarian hormones.
Uterine Deletion of Klf5 Drastically Impairs Fertility.
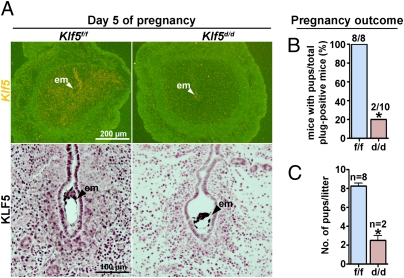
To examine the role of Klf5 in uterine biology and pregnancy, we conditionally deleted the floxed Klf5 gene via Cre expression driven by a progesterone receptor (Pgr) promoter (Pgr-Cre). Because KLF5 is expressed in neonatal uteri when Pgr-Cre is active (Fig. S3), it is possible that uterine loss of Klf5 from the neonatal stage to puberty would affect normal uterine development and maturation. We found that the uterus develops normally in Klf5d/d females, precluding its role in uterine growth and maturation after birth. To confirm efficient deletion of Klf5 in Klf5d/d females, Klf5d/d females were mated with fertile WT males. In situ hybridization and immunohistochemistry performed on sections of implantation sites on day 5 show that Klf5 is expressed in epithelial and stromal cells in Klf5f/f females, but is absent in Klf5d/d uteri except for Klf5flox/+ blastocysts that are positive for Klf5 (Fig. 2A). To test KLF5's role in female fertility, Klf5f/f and Klf5d/d females were mated with WT males. Females in both genotypes showed normal mating with the formation of vaginal plugs. However, only 2 of 10 Klf5d/d females gave birth to only two and three pups, whereas all Klf5f/f females delivered normal numbers of pups (approximately eight pups/mouse) (Fig. 2 B and C). The results show that Klf5d/d females are mostly infertile.
Fig. 2.
Uterine deletion of Klf5 impairs female fertility. (A) In situ hybridization of Klf5 and immunohistochemistry of KLF5 in day 5 implantation sites of Klf5f/f and Klf5d/d females. White and black arrowheads indicate the locations of blastocysts. (B) Pregnancy outcomes in Klf5f/f and Klf5d/d mice. (C) Litter sizes in Klf5f/f and Klf5d/d mice. Unpaired t test, *P < 0.05.
Klf5 Is Critical for Normal Implantation.
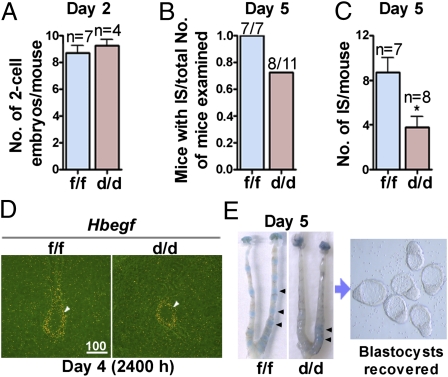
Appropriate ovulation, fertilization, preimplantation embryo development, oviductal embryo transport, implantation, and decidualization all contribute to a successful pregnancy. To ascertain the stage-specific failure of pregnancy in Klf5d/d females, Klf5f/f and Klf5d/d females were mated with WT males and pregnancy events were examined on different days. On day 2, the number of two-cell embryos retrieved from oviducts of both Klf5f/f and Klf5d/d females was comparable, suggesting normal ovulation and fertilization in Klf5d/d females (Fig. 3A). A comparable number of blastocysts was recovered from day 4 uteri from these two groups, suggesting that Klf5d/d oviducts and uteri support normal preimplantation embryo development (Fig. S4).
Fig. 3.
Klf5 is critical for normal implantation. (A) Ovulation and fertilization in Klf5f/f and Klf5d/d mice. (B and C) Implantation rate and number of implantation sites in Klf5f/f and Klf5d/d mice on day 5. (D) In situ hybridization of Hbegf at the implantation site at 2400 hours on day 4. Arrowheads indicate the position of embryos. (E) Representative uteri from Klf5f/f and Klf5d/d females on day 5 after blue dye injection and embryos recovered from Klf5d/d uteri. Arrowheads indicate the implantation sites. IS, implantation sites.
The expression of Hbegf at the site of blastocyst apposition heralds the onset of attachment reaction (24). Hbegf is normally expressed in the luminal epithelium surrounding the blastocyst before and at the time of attachment reaction. In situ hybridization results show that Hbegf expression is comparable between Klf5f/f and Klf5d/d mice (Fig. 3D), suggesting that initiation of the embryo–uterine interplay was not delayed in Klf5d/d females. We then examined the status of implantation in these mice on day 5. After an i.v. injection of a blue dye solution, the implantation sites are demarcated as discrete blue bands along the uterus (2). We found that the number of Klf5d/d mice with implantation sites was lower than that of Klf5f/f females (Fig. 3B), but the most dramatic effect seen in Klf5d/d females was the remarkably reduced number of implantation sites (Fig. 3C). In addition, blue bands in Klf5d/d uteri were not as distinct as those in the Klf5f/f group (Fig. 3E). When flushed with saline, Klf5d/d uteri with weak or no blue bands yielded many blastocysts (Fig. 3E). These results suggest that, although embryos develop to blastocysts in Klf5d/d uteri, most fail to implant or show defective implantation. We then sought to explore the reason for implantation failure in Klf5d/d females.
Luminal Epithelium Surrounding the Blastocyst in Klf5d/d Females Is Retained Past the Timing of Implantation.
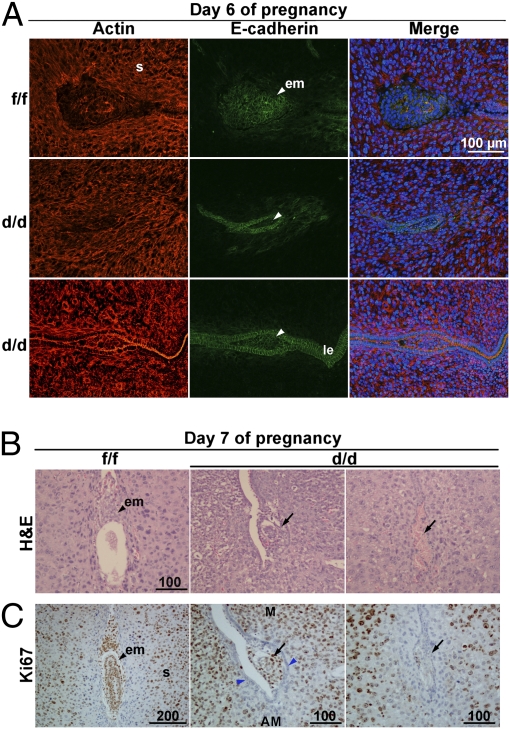
The above observations led us to examine the implantation status at later stages of pregnancy in Klf5d/d females. On day 6, the epithelium around the implantation chamber degenerates in Klf5f/f females, as is evident from the disappearance of E-cadherin, an established marker of epithelial cells. However, blastocysts remain entrapped within the lumen with the intact epithelium in Klf5d/d females, and the epithelium shows distinct expression of E-cadherin (Fig. 4A).
Fig. 4.
Blastocysts remain entrapped within the luminal epithelium past the window of implantation in Klf5d/d females. (A) Confocal images of E-cadherin and actin colocalization. Although the luminal epithelium (le) in Klf5d/d mice at the site of the blastocyst on day 6 of pregnancy is retained, it disintegrates in Klf5f/f mice with the loss of E-cadherin. Nuclei are shown in blue color. s, stroma; em, embryo. (B) H&E staining of Klf5f/f and Klf5d/d implantation sites on day 7. (C) Immunostaining of Ki67 in Klf5f/f and Klf5d/d implantation sites on day 7. M, mesometrial pole; AM, antimesometrial pole. Arrowheads indicate the location of blastocysts. Arrows indicate the degenerating embryos or residue of embryo debris, and the blue arrowheads indicate the luminal epithelium demarcation in which a blastocyst is retained.
Interestingly, the persistent epithelial barrier is associated with retarded embryonic growth in Klf5d/d females. This is further evident from H&E staining of sections of day 7 implantation sites; embryos in Klf5d/d females are either very small or resorbed (Fig. 4B). Ki67 immunostaining further confirmed our histological observations. Some embryos in Klf5d/d females were still surrounded by epithelial cells, but showed signs of degeneration (Fig. 4C and Fig. S5). Collectively, these data show that an intact epithelium surrounding the implantation chamber in Klf5d/d females past the normal window of implantation is not conducive to blastocyst implantation and growth.
Klf5d/d Females Show Compromised Decidualization.
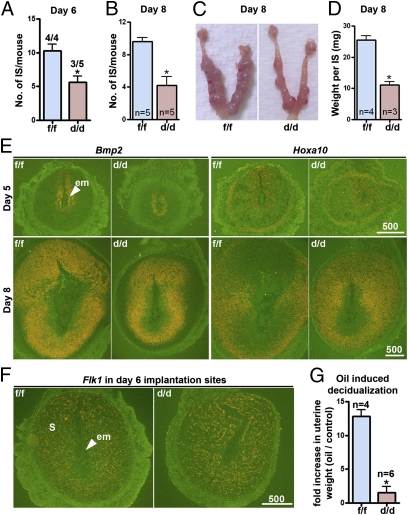
We found that the number of implantation sites on day 6 is similar to that seen on day 5 in Klf5d/d mice (Fig. 5A), suggesting that those implantation sites on day 5 with weak blue bands continued to grow in size. This increase in size is attributed to stromal growth (Fig. S6) because embryonic growth is arrested at the implantation sites in Klf5d/d females. Actin cytoskeleton reorganization is critical for normal decidualization (25). On day 4 before decidualization, actin is distributed around individual stromal cells without any obvious pattern (Fig. S7). In day 6 implantation sites, actin expression is up-regulated and becomes more organized, forming a network around the embryo (Fig. 4A). A similar pattern of cytoskeletal reorganization was observed in day 6 implantation sites in both Klf5f/f and Klf5d/d females (Fig. 4A), suggesting that Klf5d/d stromal cells were proliferating and differentiating, despite the presence of the intact luminal epithelium. Most Klf5d/d implantation sites showed continued growth until day 8, and the number of sites was similar on day 8 (Fig. 5B) compared with those on days 5 and 6 (Figs. 3C and 5A). However, decidual response in Klf5d/d implantation sties was dramatically compromised, and implantation sites were smaller compared with those in Klf5f/f mice (Fig. 5 C and D). Although KLF5 is expressed in decidual cells (Fig. 1), the results suggest that stromal cells are able to initiate decidualization in the absence of KLF5. However, the decidual response of Klf5d/d stromal cells is greatly attenuated in the absence of trophoblast penetration through the luminal epithelium.
Fig. 5.
Klf5d/d females show compromised decidualization. (A) Number of implantation sites on day 6 in Klf5f/f and Klf5d/d mice. Numerals above bars indicate numbers of dams with implantation sites over total number of dams examined. (B) Number of implantation sites on day 8 pregnancy in Klf5f/f and Klf5d/d mice. (C) Representative uteri from Klf5f/f and Klf5d/d females on day 8. (D) Weight of implantation sites on day 8 in Klf5f/f and Klf5d/d mice. (E) In situ hybridization of Bmp2 and Hoxa10 in implantation sites on days 5 and 8 in Klf5f/f and Klf5d/d females. (F) In situ hybridization of Flk1 in sections of day 6 implantation sites of Klf5f/f and Klf5d/d females. (G) Fold changes in uterine weights of oil-infused horns over noninfused horns indicate the extent of decidualization in Klf5f/f and Klf5d/d females on day 8 of pseudopregnancy. Unpaired t test, *P < 0.05.
To further delineate the decidualization process in these mice, we examined the expression of Bmp2 and Hoxa10, which are critical for decidualization (1). In situ hybridization results show that these genes are expressed in stromal cells at implantation sites on days 5 and 8 in Klf5d/d females, encompassing a smaller domain of the stromal bed correlating with smaller decidual sizes. Notably, viable embryos were not present in the implantation chambers of Klf5d/d females on day 8 (Fig. 5E). Increased vascular permeability and angiogenesis are also critical to successful decidualization (6). Because Klf5 was shown to play a role in angiogenesis, we asked whether angiogenesis is defective at implantation sites in Klf5d/d females. In situ hybridization of Flk1 (an endothelial cell marker) in sections of day 6 implantation sites showed that Klf5f/f and Klf5d/d females have comparable density of microvasculature in decidual beds (Fig. 5F). This suggests that changes in angiogenesis are apparently not a cause for smaller decidual size.
The implanting blastocyst is the normal stimulus for decidualization. However, it can also be experimentally induced by intraluminal oil infusion in pseudopregnant mice. Klf5d/d and Klf5f/f females were mated with vasectomized males to induce pseudopregnancy. On day 4, sesame oil (10 μL/horn) was intraluminally infused into one uterine horn and the noninfused contralateral horn was severed as a control. Mice were killed on day 8 (day of maximal decidualization) to assess the extent of decidualization by recording fold increases in uterine weights of infused-vs.-noninfused horns. Klf5d/d females showed a remarkably reduced decidual response (Fig. 5G). Collectively, these results suggest that signals emanating from the blastocyst entrapped within the Klf5d/d luminal epithelium contributed to decidual response in Klf5d/d stroma, albeit at a reduced scale.
Klf5d/d Mice Have Normal Levels of E2 and P4 and Uterine Responsiveness to These Hormones.
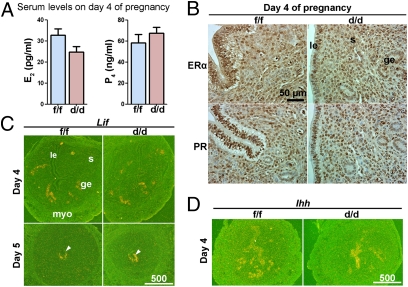
Uterine functions are primarily directed by estrogen and P4, and most of their actions are mediated by estrogen receptor-α (ERα) and progesterone receptor (PR), respectively (26, 27). Because KLF5 can interact with these hormones in other systems (17, 28), observed infertility in Klf5d/d females could be due to abnormal uterine responsiveness to estrogen and P4. We found that serum levels of E2 and P4 in Klf5d/d females on day 4 are comparable to those in Klf5f/f females (Fig. 6A). Immunohistochemistry results show that Klf5d/d uteri have a normal expression pattern of ERα and PR on day 4 (Fig. 6B and Fig. S8). These results suggest that infertility in Klf5d/d females is not due to altered steroid hormone levels or reduced uterine responsiveness to these hormones. Normal expression of Lif and Ihh is indispensable to implantation (9, 11, 29). Whereas Lif is regulated by E2, P4 regulates Ihh expression. In situ hybridization showed that Klf5d/d uteri have normal expression of Lif and Ihh on day 4 and day 5 (Fig. 6 C and D), suggesting that Klf5d/d uteri can appropriately respond to these genes.
Fig. 6.
Klf5f/f and Klf5d/d mice have comparable levels of ovarian steroid hormones and uterine responsiveness to these hormones. (A) Serum levels of E2 and P4 on day 4 of pregnancy. (B) Immunohistochemistry of ERα and PR on day 4 uteri. (C) In situ hybridization of Lif in day 4 and day 5 uteri. (D) In situ hybridization of Ihh in day 4 uteri. myo, myometrium. Arrowheads indicate the position of embryos.
Klf5 Regulates COX2 Expression in Luminal Epithelial Cells During Implantation.
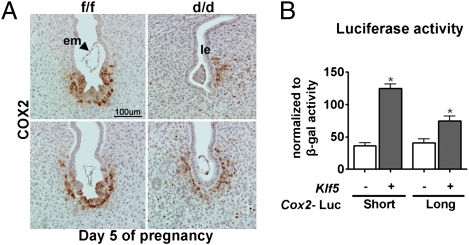
COX2 is expressed at the implantation site, and Ptgs2−/− females show defective implantation and decidualization (10). The luminal epithelial and stromal COX2 expression is differently regulated in some genetically altered mouse models. For example, COX2 is expressed in the luminal epithelium, but not in stromal cells, around the implantation chambers in Lif−/− females that are infertile (29). Thus, we examined COX2 expression at the implantation site on day 5. In Klf5f/f implantation sites, COX2 was localized in both the epithelium and the stroma (Fig. 7A). In contrast, COX2 was absent in the epithelium, but present in stromal cells at the blastocyst site in Klf5d/d females. These results suggest that poor attachment reaction in Klf5d/d females could be due to aberrant COX2 expression. To complement our in vivo observation, we examined whether KLF5 can regulate Ptgs2 expression in cells in vitro. We transfected a human endometrial epithelial cell line (Ishikawa cells) with Ptgs2 promoter constructs fused to a luciferase gene together with a full-length construct of the Klf5-coding region. We found that KLF5 up-regulated luciferase activity under the control of Ptgs2 promoters (Fig. 7B). These results suggest that COX2 expression in luminal epithelial cells requires KLF5 activity.
Fig. 7.
Klf5 differentially regulates COX2 expression in the uterus during implantation. (A) Immunohistochemistry of COX2 in day 5 implantation sites of Klf5f/f and Klf5d/d females. Immunoreactive COX2 is absent in Klf5d/d luminal epithelial cells. (B) Luciferase assay as evident from the luciferase gene activity driven by a short and long form of the Ptgs2 promoter. Ishikawa cells were transfected with a construct spanning the full-length coding region of Klf5 or control plasmid together with luciferase reporter constructs. A CMV–β-galactosidase plasmid was severed as a control for transfection efficiency.
Discussion
Successful implantation requires an intimate cross-talk between the blastocyst and the receptive uterus. However, the molecular basis for the initiation of uterine receptivity is not fully understood. The uterine luminal epithelium is the first cell type to interact with the blastocyst trophectoderm to initiate the attachment reaction. Thus, a receptive and functional epithelium is essential for implantation. In WT females, the demise of the luminal epithelium surrounding the blastocyst with the progression of the attachment reaction helps trophoblast invasion into the stroma for continued embryonic growth. We found that Klf5 is persistently expressed in the Klf5f/f uterine epithelium throughout the preimplantation period and that deletion of uterine Klf5 confers female infertility due to defective and/or failed implantation. The epithelium at the site of blastocysts fails to undergo degeneration in most Klf5d/d females, thus preventing further progression of the implantation process and embryonic growth.
The data suggest that Klf5 is critical to making the uterine luminal epithelium conducive to blastocyst implantation and growth. Klf5 is unique compared with other genes critical for implantation. For example, Ihh and Lif are transiently expressed before and during implantation and are regulated by P4 or E2 (9, 11, 29). However, Klf5 is present in the uterine epithelium even in the absence of ovaries (Fig. S1), suggesting that Klf5 signaling may be parallel to E2 and P4 signaling. This raises the possibility that there are other unidentified genes that are independent of E2 and/or P4 regulation, but critical for implantation.
Klf5 is expressed mainly in the epithelia of various tissues (15, 30), but is also expressed in cardiovascular smooth muscle cells (31), lymphoid cells (32), and neuronal cells (33). Our present study shows that Klf5 expression is not limited to the epithelium, but also occurs in stromal cells undergoing decidualization, suggesting that Klf5 has a role in stromal cell transformation. However, stromal decidualization, albeit at a reduced level, with the expression of decidualization markers in Klf5d/d uteri suggests that Klf5 has a limited role in decidualization. Because Klf5d/d epithelium seems to compromise decidualization, the definitive role of Klf5 in decidualization warrants further investigation using a mouse model with stromal cell-specific deletion of Klf5.
We speculate that Klf5 regulates uterine receptivity via luminal epithelial expression of COX2, which is an inducible proinflammatory gene. A recent study also shows that Klf5 expression in the renal collecting duct is essential for inflammatory responses to unilateral ureteral obstruction (34). The apoptotic cell fractions and glomerular sclerosis and tubular injury scores were all reduced in the absence of Klf5. Implantation is also considered a proinflammatory response, and COX2-derived PGs are important for implantation (10). Our study suggests that the absence of luminal epithelial COX2 in Klf5d/d females may contribute to implantation failure. Because KLF5 is known to exert an array of context-dependent functions, COX2 signaling may not be the only pathway that is dysregulated in the absence of Klf5. Furthermore, pregnancy phenotype in Ptgs2−/− mice is genetic-background-dependent, and COX1 can compensate for the loss of COX2, partially rescuing pregnancy failure in Ptgs2−/− females on certain backgrounds. These studies and COX2's conserved role in implantation in various species, including humans, have previously been discussed (35). Future studies will determine which PG ligands and corresponding receptors interact with KLF5 in regulating pregnancy events.
In Lif mutant females or mice with uterine deletion of Msx1/Msx2 , epithelial COX2 expression persists around the embryo on d 5 of pregnancy, but stromal COX2 is lost (12, 29). In the present study, only epithelial COX2 expression was affected, although Klf5 was deleted in both epithelial and stromal cells. Collectively, the findings suggest that COX2 in the epithelium and stroma is differently regulated and affects implantation in diverse ways.
It is interesting to note that Klf5d/d uteri show an initial response to decidualization in the presence of a blastocyst, but at a reduced rate. In contrast, oil-induced decidual response is very poor in Klf5d/d uteri. Similar phenotype is observed in several other knockout mouse models in which implantation is aberrant (12, 13). It is possible that the blastocyst stimulates stromal cell decidualization indirectly via its interaction with the epithelium in addition to sending paracrine signals to stromal cells. We speculate that stromal cells respond to a paracrine signaling from the blastocyst, although the stimulus arising from the Klf5-deficient epithelium is disturbed. Collectively, the results suggest that a circuitry connecting a viable blastocyst with the functional epithelium and stroma is essential for successful decidualization and establishment of pregnancy. Because KLF5 is expressed in both the epithelium and the stroma, it would be exciting to define the relative contribution of epithelial vs. stromal KLF5 in female fertility. By deleting floxed Klf5 by Cre driven by the Müllerian inhibiting substance type II receptor (36, 37) or Wnt7a promoter (38) in the stroma or the epithelium, future experiments may help in dissecting the roles of epithelial vs. stromal KLF5 in implantation and decidualization.
Nearly 1 in 12 US married couples under 45 y of age are infertile; 40% of such infertility results from female reproductive disorders. Despite significant improvement in in vitro fertilization technology, implantation failure is still high. There is a need to better understand the mechanism of uterine receptivity to develop the means to extend uterine receptivity. Uteri in Klf5d/d females are mostly unfavorable for normal implantation, providing evidence that Klf5 is a key player in uterine receptivity. Klf5 is unique in that it is crucial for implantation, but not influenced by ovarian hormones. It would be interesting to see whether KLF5 is expressed in the human endometrium similarly to that in mice. If so, this may have an implication in regulating the window of implantation in humans by manipulating Klf5 expression independently of ovarian hormones.
Materials and Methods
Animals and Treatments.
To generate mice with uterine deletion of Klf5, Klf5loxP/loxP mice (20) were crossed with PgrCre/+ mice (39). Klf5f/f and Klf5d/d mice were housed in the animal care facility of the Cincinnati Children's Hospital Medical Center according to National Institutes of Health and institutional guidelines for laboratory animals. All protocols of the present study were approved by the Cincinnati Children's Hospital Research Foundation Institutional Animal Care and Use Committee.
Immunostaining.
Immunohistochemistry was performed in paraffin-embedded sections using specific antibodies as indicated. Tissues fixed in 4% cold paraformaldehyde were used for KLF5 immunostaining; tissues fixed in 10% neutral buffered formalin were used for other stainings. Antigen retrieval using microwave heating is required for KLF5 staining. A Histostain-Plus kit (Invitrogen) was used to visualize specific antigens.
In Situ Hybridization.
In situ hybridization was performed as previously described (40) and is detailed in SI Text.
Cell Transfection and Luciferase Assay.
Ishikawa cells maintained in DMEM plus 10% FBS were transfected with specific plasmids (0.3 μg) and CMV–β-galactosidase (0.06 μg), as transfection efficiency control (20), using polyethylenimine (2.4 μg). KLF5 overexpressing and control plasmids have been described (19). Plasmids containing two regions of the Ptgs2 promoter fused with the luciferase gene were used to measure Ptgs2 transcriptional activity. The long and short forms of the promoter containing 3.2- and 1-kb fragments, respectively, upstream of the translation start site were used. The promoter activity was determined by luciferase activity normalized to β-galactosidase activity 48 h after transfection. All experiments were done in duplicates (n = 3).
Statistical Analysis.
Comparison of means was performed by using Student's t test. Data are shown as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Serenity Curtis for editing the manuscript and John Lydon and Francesco DeMayo (Baylor College of Medicine) for providing us with PgrCre/+ mice. This work was supported in part by National Institutes of Health Grants HD12304 and HD068524 (to S.K.D.) and HL090156 and HL095580 (to J.A.W.). X.S. is supported by a Lalor Foundation postdoctoral fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118411109/-/DCSupplemental.
References
- 1.Wang H, Dey SK. Roadmap to embryo implantation: Clues from mouse models. Nat Rev Genet. 2006;7(3):185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 2.Psychoyos A. Endocrine control of egg implantation. In: Greep RO, Astwood EG, Geiger SR, editors. Handbook of Physiology. Washington, DC: American Physiology Society; 1973. pp. 187–215. [Google Scholar]
- 3.Paria BC, Huet-Hudson YM, Dey SK. Blastocyst's state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci USA. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parr EL, Tung HN, Parr MB. Apoptosis as the mode of uterine epithelial cell death during embryo implantation in mice and rats. Biol Reprod. 1987;36:211–225. doi: 10.1095/biolreprod36.1.211. [DOI] [PubMed] [Google Scholar]
- 5.Welsh AO, Enders AC. Chorioallantoic placenta formation in the rat: I. Luminal epithelial cell death and extracellular matrix modifications in the mesometrial region of implantation chambers. Am J Anat. 1991;192:215–231. doi: 10.1002/aja.1001920302. [DOI] [PubMed] [Google Scholar]
- 6.Dey SK, Lim H. Implantation. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd Ed. Vol I. Amsterdam; London; New York: Elsevier; 2005. pp. 147–188. [Google Scholar]
- 7.Huet-Hudson YM, Dey SK. Requirement for progesterone priming and its long-term effects on implantation in the mouse. Proc Soc Exp Biol Med. 1990;193:259–263. doi: 10.3181/00379727-193-43032. [DOI] [PubMed] [Google Scholar]
- 8.Reese J, et al. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J Biol Chem. 2001;276:44137–44145. doi: 10.1074/jbc.M107563200. [DOI] [PubMed] [Google Scholar]
- 9.Stewart CL, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359(6390):76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 10.Lim H, et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 11.Lee K, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38:1204–1209. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- 12.Daikoku T, et al. Conditional deletion of MSX homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev Cell. 2011;21:1014–1025. doi: 10.1016/j.devcel.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tranguch S, et al. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci USA. 2005;102:14326–14331. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lejeune B, Van Hoeck J, Leroy F. Transmitter role of the luminal uterine epithelium in the induction of decidualization in rats. J Reprod Fertil. 1981;61:235–240. doi: 10.1530/jrf.0.0610235. [DOI] [PubMed] [Google Scholar]
- 15.Sogawa K, et al. cDNA cloning and transcriptional properties of a novel GC box-binding protein, BTEB2. Nucleic Acids Res. 1993;21:1527–1532. doi: 10.1093/nar/21.7.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao KW, et al. Oestrogen causes degradation of KLF5 by inducing the E3 ubiquitin ligase EFP in ER-positive breast cancer cells. Biochem J. 2011;437:323–333. doi: 10.1042/BJ20101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu R, Zhou Z, Zhao D, Chen C. The induction of KLF5 transcription factor by progesterone contributes to progesterone-induced breast cancer cell proliferation and dedifferentiation. Mol Endocrinol. 2011;25:1137–1144. doi: 10.1210/me.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ema M, et al. Krüppel-like factor 5 is essential for blastocyst development and the normal self-renewal of mouse ESCs. Cell Stem Cell. 2008;3:555–567. doi: 10.1016/j.stem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Lin SC, Wani MA, Whitsett JA, Wells JM. Klf5 regulates lineage formation in the pre-implantation mouse embryo. Development. 2010;137:3953–3963. doi: 10.1242/dev.054775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan H, et al. Kruppel-like factor 5 is required for perinatal lung morphogenesis and function. Development. 2008;135:2563–2572. doi: 10.1242/dev.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell SM, et al. Kruppel-like factor 5 is required for formation and differentiation of the bladder urothelium. Dev Biol. 2011;358(1):79–90. doi: 10.1016/j.ydbio.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McConnell BB, et al. Kruppel-like factor 5 is important for maintenance of crypt architecture and barrier function in mouse intestine. Gastroenterology. 2011;141(4):1302–1313. doi: 10.1053/j.gastro.2011.06.086. 1313.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenchegowda D, et al. Conditional disruption of mouse Klf5 results in defective eyelids with malformed meibomian glands, abnormal cornea and loss of conjunctival goblet cells. Dev Biol. 2011;356(1):5–18. doi: 10.1016/j.ydbio.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das SK, et al. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: A possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 25.Ihnatovych I, Livak M, Reed J, de Lanerolle P, Strakova Z. Manipulating actin dynamics affects human in vitro decidualization. Biol Reprod. 2009;81:222–230. doi: 10.1095/biolreprod.108.074666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couse JF, Korach KS. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 27.Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–355. doi: 10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima Y, et al. Estrogen regulates tumor growth through a nonclassical pathway that includes the transcription factors ERβ and KLF5. Sci Signal. 2011;4:ra22. doi: 10.1126/scisignal.2001551. [DOI] [PubMed] [Google Scholar]
- 29.Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol. 2000;14:1147–1161. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- 30.Conkright MD, Wani MA, Anderson KP, Lingrel JB. A gene encoding an intestinal-enriched member of the Krüppel-like factor family expressed in intestinal epithelial cells. Nucleic Acids Res. 1999;27:1263–1270. doi: 10.1093/nar/27.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe N, et al. BTEB2, a Krüppel-like transcription factor, regulates expression of the SMemb/Nonmuscle myosin heavy chain B (SMemb/NMHC-B) gene. Circ Res. 1999;85(2):182–191. doi: 10.1161/01.res.85.2.182. [DOI] [PubMed] [Google Scholar]
- 32.Yang XO, Doty RT, Hicks JS, Willerford DM. Regulation of T-cell receptor D beta 1 promoter by KLF5 through reiterated GC-rich motifs. Blood. 2003;101:4492–4499. doi: 10.1182/blood-2002-08-2579. [DOI] [PubMed] [Google Scholar]
- 33.Yanagi M, et al. Expression of Kruppel-like factor 5 gene in human brain and association of the gene with the susceptibility to schizophrenia. Schizophr Res. 2008;100:291–301. doi: 10.1016/j.schres.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 34.Fujiu K, Manabe I, Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest. 2011;121:3425–3441. doi: 10.1172/JCI57582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dey SK, et al. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 36.Arango NA, et al. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75:1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- 37.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 38.Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor α is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci USA. 2010;107:19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soyal SM, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- 40.Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–5321. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.