Abstract
Generation of antiviral IgM is usually considered as a marker of a short-lived initial antibody response that is replaced by hypermutated and more-efficient IgG. However, once viruses have established a particular niche for their persistence (e.g., within the CNS), the immune system has to specifically mobilize a broad range of antimicrobial effectors to contain the pathogen in the long term. Infection of the CNS with the mouse hepatitis virus (MHV) provides a unique model situation in which the extent of inflammatory CNS disease is determined by the balance between antiviral immune control, viral replication, and immune-mediated damage. We show here that whereas antibody- or B cell-deficient mice failed to contain MHV CNS infection and developed progressive demyelinating disease, germline IgM produced in activation-induced cytidine deaminase-deficient mice (aicda−/−) provided long-term protection against the chronic multiple sclerosis-like disease. Furthermore, we found that appropriate B-cell activation within the CNS-draining lymph node and subsequent CXCR3-mediated migration of antiviral IgM-secreting cells to the infected CNS was dependent on CD40-mediated interaction of B cells with T helper cells. These data indicate that the CD40-mediated collaboration of T and B cells is critical to secure neuroprotective IgM responses during viral CNS infection.
Keywords: coronavirus, encephalomyelitis, neutralizing antibodies
Multiple sclerosis (MS) is one of the most common neurological disorders in young adults. However, the complex etiology of this CNS inflammatory demyelination has not yet been fully resolved. Several lines of research support the view that MS is an autoimmune disease (reviewed in ref. 1) that is influenced by a broad range of host factors (2). However, a recent genome analysis of monozygotic twins with discordant MS onset revealed that genetic, epigenetic, or transcriptome differences do not suffice to explain the discordant disease course (3). Furthermore, migration studies have shown that environmental factors are important determinants for disease susceptibility (4). It has been suggested that different neurotropic infectious insults predispose to the stereotyped pathological tissue responses in MS (5). Indeed, several viruses, including Epstein–Barr virus (6), human herpes virus-6 (7), or human coronaviruses (8), can be found in the CNS of MS patients, and both cellular and humoral immune responses against these viruses can be detected in affected individuals (5, 9). The importance of the interplay between viral replication and antiviral immunity in the CNS is illustrated by the fact that although JC virus infection is highly prevalent in the general population, infection-associated demyelinating disease occurs only when the host immune system is compromised (10). Hence, it is most likely that the balance between viral persistence and efficient immune surveillance determines the development of virus-induced demyelinating CNS disease.
One of the histopathological characteristics of MS brains is the presence of tertiary lymphoid tissue in the cerebral perivascular space (11). It has been recently shown that the presence of B-cell follicle-like structures correlates with an earlier onset and more severe disease. Such lymphoid tissue-like structures are thought to support the activation of B cells producing intrathecal oligoclonal immunoglobulins (12). The presence of oligoclonal IgG in the cerebrospinal fluid is a hallmark of early-onset MS, whereas the predictive value of oligoclonal IgM in MS patients is still a controversial issue (13, 14). Nevertheless, intrathecal IgG production against several neurotropic viruses has been shown in both pediatric and adult-onset MS (15). The fact that intrathecal immunoglobulins directed against neurotropic pathogens persist for a long period (16) suggests that B cells are critical for the containment of infectious agents in this compartment. Indeed, the long-term control of coronavirus CNS infection is dependent on B cells secreting neutralizing antibodies (17, 18). However, previous studies on the importance of antiviral B cells in virus-induced demyelinating diseases have mainly focused on the role of neutralizing IgG (19, 20), whereas the potential contribution of neutralizing IgM in this context has been neglected.
Antiviral IgM functions either as a natural, polyvalent antibody that fixes pathogens for better retention in the splenic marginal zone, or as induced IgM that specifically neutralizes the virus (21). The importance of antiviral neutralizing IgM has been demonstrated, for example, for infection with influenza virus (22) or West Nile virus (23). In the latter infection, antiviral IgM was shown to prevent access of the virus to the CNS (23). To address the importance of neutralizing IgM during virus-induced demyelinating disease and to clarify whether and how antiviral IgM is induced and maintained, we used the infection with the molecularly defined mouse hepatitis virus (MHV) A59 strain (24). MHV is a natural mouse pathogen that infects all cell types within the CNS (25). Particular strains of MHV, such as John Howard Mueller (JHM), display a distinct CNS tropism leading to severe acute encephalitis. Strains with a less pronounced neurotropism, such as the gliatropic MHV A59 strain, generally establish a persistent infection within the CNS, leading to chronic demyelination associated with axonal death (25). We show here that coronavirus-induced demyelinating CNS disease can be efficiently prevented by a long-lasting germline-encoded IgM response. Furthermore, we found that CD40-mediated T- and B-cell collaboration was essential to induce and maintain the neuroprotective IgM.
Results and Discussion
Germline IgM Prevents Virus-Mediated Chronic Demyelination.
To assess the importance of antibodies in the control of CNS infection with the gliatropic MHV strain A59, we used B cell-deficient JHT mice and antibody-deficient but B cell-competent IgMi mice (26) and applied the virus via the intranasal route (i.n.). This route of infection grants the virus access to the CNS (27) and leads to a transient weight loss during the acute phase (i.e., between days 8 and 12 after infection) (Fig. S1A). Whereas C57BL/6 (B6) control mice recovered, both JHT and IgMi mice failed to gain weight, whereby IgMi mice showed a rapidly deteriorating health status with persisting weight loss (Fig. S1A). Both antibody-deficient strains developed a progressive neurological disease with ascending paralysis, a pronounced demyelination in the spinal cord (Fig. S1 B and C) that was not only associated with failure to clear the virus from the CNS but also with significant mortality (Fig. S1 D and E). Furthermore, the absence of B cells did not affect the induction of antiviral effector T cells and their recruitment to the CNS (Fig. S1F).
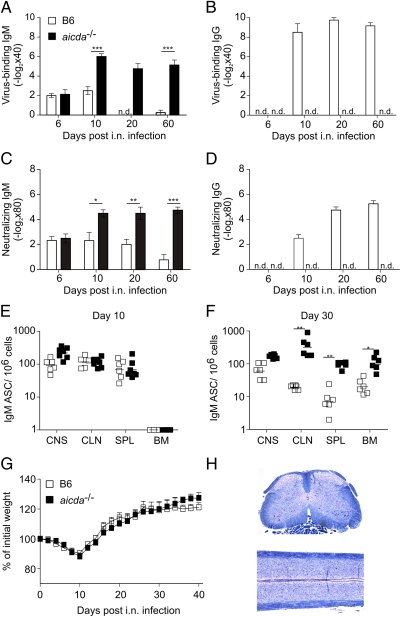
To assess the role of antiviral IgM in this viral CNS disease, we first determined both virus-binding (Fig. 1 A and B) and -neutralizing (Fig. 1 C and D) antibodies in serum. As expected, the early serum IgM response declined after day 10, and high IgG serum titers were reached in B6 mice after the virus had been eliminated (Fig. 1 A–D). Furthermore, the induction of serum IgG was associated with the appearance of IgG-producing antibody-secreting cells (ASCs) in the CNS (Fig. S2). Interestingly, B cells secreting virus-specific IgM were not only found in the CNS early after infection, but these cells also persisted in the CNS for at least 30 d (Fig. 1 E and F). To test whether IgM alone would be sufficient to mediate protection from MHV-induced demyelinating disease, we infected activation-induced cytidine deaminase (AID)-deficient mice (aicda−/−) i.n. with MHV. aicda−/− mice cannot perform class switch recombination and somatic hypermutation and hence possess only IgM antibodies with germline-encoded sequences (22). As shown in Fig. 1 A–D, aicda−/− mice mounted exclusively IgM antibody responses against the virus (Fig. 1 A–D) and exhibited a robust expansion of antiviral B cells during the acute and chronic phase (Fig. 1 E and F) that compensated efficiently for the lack of IgG (Fig. S2B). Importantly, aicda−/− mice stayed healthy and were fully protected from MHV-induced demyelinating disease [i.e., they showed normal weight gain (Fig. 1G) and a lack of histopathological signs of demyelination (Fig. 1H)]. These data indicated that germline-encoded antiviral IgM was sufficient to provide long-term protection from virus-induced demyelinating CNS disease.
Fig. 1.
IgM controls MHV CNS infection and prevents demyelinating disease. B6 or aicda−/− mice were i.n. infected with 5 × 104 pfu of MHV A59. (A–D) Serum samples were obtained at days 6, 10, 20, and 60 after infection, and virus-binding IgM (A) and IgG (B) and neutralizing IgM (C) and IgG (D) were determined. Values indicate mean ± SEM (n = 5 mice per group and time point). (E and F) IgM-specific antibody-secreting cells were enumerated in CNS, CLN, spleen (SPL), and bone marrow (BM) at days 10 (E) and 30 (F) after infection. (G) Weight loss was recorded during the indicated time period after infection. Values indicate mean percentage of the initial weight ± SEM (n = 8 mice per group). (H) Spinal cord sections from i.n. infected aicda−/− mice were analyzed on day 40 using Luxol fast blue staining. The statistical analyses in E and F were performed using Student's t test. *P < 0.05; **P < 0.01; ***P < 0.001.
CD40-Mediated T Helper- and B-Cell Collaboration Is Critical for CNS-Protective IgM.
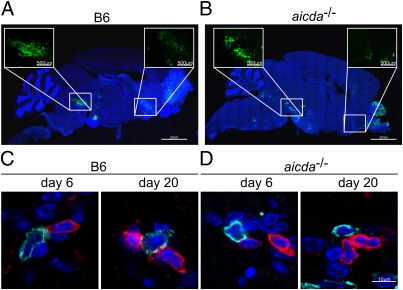
IgM is a pentameric antibody whose capacity to cross the blood–brain barrier is limited. We therefore reasoned that the access and maintenance of antiviral IgM-secreting B cells within the CNS should be a critical step for the protection against MHV-induced demyelination. As shown in Fig. 2 A and B for day 6 after i.n. infection, the virus replicated both in B6 (Fig. 2A) and aicda−/− mice (Fig. 2B) preferentially in the olfactory bulb, the hypothalamic area, and in the striatum/pons region. These areas of viral replication at day 6 after infection showed extensive lymphocyte infiltration, with both T and B cells being present in B6 (Fig. 2C) and aicda−/− mice (Fig. 2D). After viral clearance, the lymphocytic infiltrates persisted (Fig. 2 C and D, Right), and T and B cells were frequently found in close juxtaposition, suggesting that the collaboration of T and B cells might be important to secure the protective IgM response.
Fig. 2.
Viral antigen localization and composition of inflammatory infiltrates in aicda−/− mice. Virus distribution was assessed in whole-brain confocal laser scanning analysis. Mice were infected with 5 × 104 pfu MHV encoding for eGFP. Viral antigen distribution was visualized using anti-GFP antibody on day 6 after infection in B6 (A) and aicda−/− mice (B). Boxed and enlarged areas in A and B show viral antigen deposition in striatum (Right) and hypothalamus/pons (Left) areas. (C and D) Juxtaposition of T (red) and B cells (blue) in virus-infected hypothalamus/pons areas in B6 (C) and aicda−/− mice (D) on days 6 (Left) and 20 (Right) after infection.
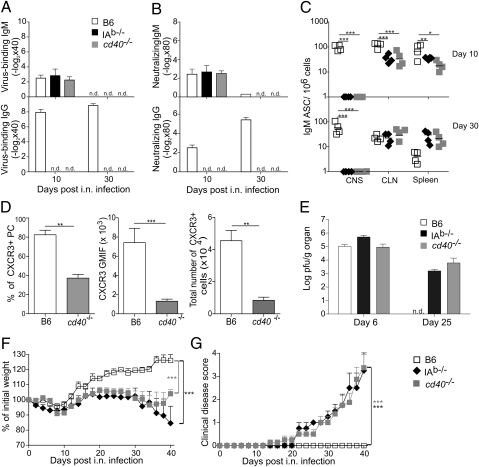
To dissect the T–B collaboration in MHV CNS infection in more detail, we infected mice lacking the MHC class II molecule I-Ab (IAb−/−) and mice lacking the CD40 molecule (cd40−/−) with MHV. In the absence of CD4+ T cells or one of the central regulatory molecules of T–B collaboration, MHV-infected mice mounted a T-independent neutralizing IgM response during the early phase of the infection and failed to produce antiviral IgG (Fig. 3 A and B). Serum IgM levels dropped below the level of detection in the late infection (i.e., on day 30; Fig. 3 A and B), whereas antiviral IgM-secreting B cells could still be detected in cervical lymph node (CLN) and spleen (Fig. 3C). Importantly, in the absence of CD4+ T cells or CD40, antiviral B cells could not be found in the CNS on either day 10 or day 30 after infection, indicating that CD40-mediated T-help was crucial to grant IgM-producing B cells access to the virus-infected CNS. Because the chemokine receptor CXCR3 is important to guide MHV-specific B cells to the CNS (20), we assessed here CXCR3 expression during the acute infection and found that the lack CD40-mediated T-help significantly reduced CXCR3 expression on plasma cells in the CLN (Fig. 3D). The lack of antiviral IgM-secreting B cells in the CNS and the absence of serum IgM in the late phase of the infection was associated with the inability of CD4+ T cell- and CD40-deficient mice to clear the virus (Fig. 3E). The consequence of the persisting viral infection was a progressive weight loss (Fig. 3F), development of neurological disease with ascending paralysis (Fig. 3G), and increased mortality in the later phase of the disease (Fig. S3), suggesting that the selective lack of antiviral IgM due to impaired T–B collaboration precipitated the virus-induced demyelinating disease.
Fig. 3.
CD4+ T cells and CD40 signals are critical for long-term control of MHV and for prevention of demyelinating disease. Assessment of virus-binding (A) and neutralizing (B) antibody responses in B6, MHC class II-deficient (IAb−/−), and CD40-deficient (cd40−/−) mice at the indicated time points after i.n. MHV infection. (C) IgM-specific antibody secreting cells were enumerated in CNS, CLN, and spleen (SPL) at days 10 and 30 after infection. (D) CXCR3 expression on CD45+B220+CD138+ plasma cells was determined at 10 d after infection in B6 and cd40−/− CLNs by flow cytometry; shown is percentage of CXCR3+ plasma cells (Left), geometric mean fluorescence intensity of CXCR3 staining on CD45+B220+CD138+ plasma cells (Center), and total numbers of CXCR3+ plasma cells (Right). Data are from two separate experiments (n = 4 mice). (E) Viral titers in CNS determined at days 6 and 25 after infection. Data indicate means of log-transformed titers ± SEM (n = 5 mice per group). n.d., not detectable. (F) Weight loss and (G) development of clinical symptoms were recorded during the indicated time points after infection. Values in F indicate mean percentage of the initial weight ± SEM (n = 8–10 mice per group). Data in G indicate clinical scores (n = 8–10 mice per group). Statistical analyses in C and D were performed using Student's t test, and in F and G using one-way ANOVA with Tukey's postanalysis. *P < 0.05; **P < 0.01; ***P < 0.001.
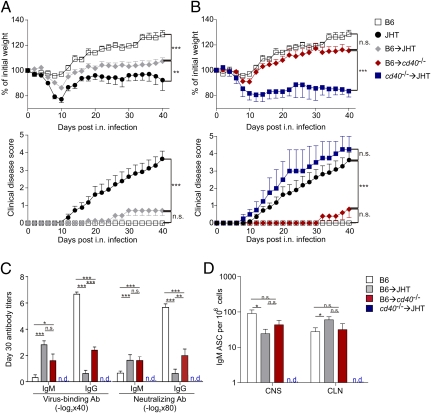
In several systemic viral infections, the lack of CD4+ T cells or the CD40 molecule is not critical to mount protective CD8+ T-cell or IgM responses (28). Likewise, systemic infection of B cell-deficient mice with MHV results in viral clearance (17). It seems therefore that the immune system requires a broad armament of antiviral effectors once the viral replication niche in the CNS has been established. To further substantiate our conclusion that B cells secreting antiviral IgM are of particular importance to prevent MHV-induced CNS disease and to exclude that the interaction of other CD40+ antigen-presenting cells such as dendritic cells could be responsible for the observed phenotype, we performed a series of adoptive B-cell transfers. To this end, we used a protocol of B-cell transfer into JHT mice that reconstitutes 5–10% of the B-cell compartment (29). Here, adoptive transfer of wild-type (B6) B cells into JHT mice significantly improved weight gain and completely prevented clinical disease (Fig. 4A and Fig. S4A). Likewise, JHT mice reconstituted with AID-deficient B cells were protected from the disease (Fig. S5 A and B), and germline IgM-producing B cells from aicda−/− mice were present in the CNS in MHV-infected JHT mice on day 10 after infection (Fig. S5C). Efficient protection against virus-induced demyelinating disease was also achieved in CD40-deficient recipients that had received B6 B cells, whereas adoptive transfer of CD40-deficient B cells into JHT mice did not have a significant effect on the disease in JHT mice (Fig. 4B and Fig. S4B). The adoptive transfer of B6 B cells into JHT and CD40-deficient recipients fully restored the serum IgM response and led to a partial recovery of the serum IgG response, whereas cd40−/− B cells in JHT hosts failed to produce antiviral antibodies (Fig. 4C). Furthermore, the absence of CD40 on B cells prevented their access to the CNS, whereas antiviral IgM-secreting, CD40-competent B cells in JHT or cd40−/− recipients were not only found in high numbers in the CLN but also in the CNS (Fig. 4D and Fig. S5C). The importance of CD40-mediated B-cell activation with full up-regulation of CXCR3 on plasma cells (Fig. 3D) is shown by the lack of protection from virus-induced CNS disease when CXCR3-deficient cells were adoptively transferred into JHT recipients (Fig. S2 A and B). Taken together, these data demonstrate that the CD40-dependent T–B collaboration facilitated the generation of neuroprotective IgM during MHV CNS infection.
Fig. 4.
CD40 expression on B cells is required to protect against demyelinating disease. Sorted B cells (1.5 × 107) from B6 or CD40-deficient donors were transferred into JHT or CD40-deficient recipient mice on days 3 and 1 before MHV infection. B6 and JHT mice served as controls. (A and B) Weight loss and development of clinical symptoms were recorded during the indicated time period after infection in the indicated strains or B cell-reconstituted recipients (n = 8 mice per group). (C) Serum antibody titers on day 30 after infection in the indicated B-cell recipients. Values indicate mean titers ± SEM from eight mice per group. (D) IgM-specific antibody-secreting cells were enumerated in CNS and CLN on day 30 after infection. Values indicate mean number of IgM-secreting cells per 106 cells ± SEM from eight mice per group. n.d., not detectable. Statistical analyses in A and B were performed using one-way ANOVA with Tukey's postanalysis, and Student's t test in C and D. *P < 0.05; **P < 0.01, ***P < 0.001. n.s., not significant.
The present study has revealed that IgM can provide efficient protection against virus-mediated demyelinating disease. Importantly, generation of neuroprotective IgM (i.e., sufficient B-cell activation and migration of specific B cells to the infected CNS) was dependent on the CD40-mediated interaction of B cells with T helper cells within the CNS-draining lymph node. Thus, CD4+ T cells provide critical “help” not only for B-cell differentiation for Ig class switch but also for the instruction of IgM-producing B cells for CXCR3-mediated migration into the CNS. As shown for MHV-specific IgG-secreting B cells (20), also B cells producing IgM persisted in the CNS for a prolonged period after they had received appropriate T help. It seems, therefore, that once either IgG or IgM antibody-secreting cells have reached the CNS, the particular environment rich in B cell-stimulatory factors (30) provides a long-term survival niche. At this point the question arises of why a particular CD40-mediated, T helper-dependent activation- and CNS migration-control for IgM-producing cells should be beneficial for the host. We believe that persisting antiviral IgM in the CNS provides an important additional layer of protection because the immunopathological “costs” of the attempt to completely eradicate the virus from the CNS are high and that therefore IgM-secreting cells in the CNS are well-suited to block recrudescence of the persisting virus. Persisting viruses such as MHV can escape from T cell-mediated control, and this evasion can be efficiently prevented by neutralizing antibodies (31). Given the importance of B cells in the protection against demyelinating disease in persisting coronavirus infection, it is likely that the virus not only evades from CD8+ T-cell recognition but also from the neutralizing B-cell responses. IgM, and in particular germline IgM, usually exhibits a low affinity to its antigen but can bind antigens owing to the high valency with a wide range of avidities (21). Therefore, CNS-resident IgM-secreting B cells most likely provide critical protection by neutralizing antibody escape variants.
Concluding Remarks.
Production of IgM in the course of an infection is usually considered as a marker of the early adaptive immune response that is short-lived and replaced by hypermutated and more-efficient IgG. Our study and other work on IgM in the maintenance of long-term immunity during a chronic intracellular bacterial infection (32) shows that IgM can serve as an important immune effector in the long-term protection against particular pathogens. Therefore, the induction of long-lived IgM at locations of pathogen persistence such as the CNS should be considered in future vaccine development strategies.
B cells are an important pathogenic component of MS, as shown by recent clinical trials that demonstrated efficacy of B-cell depletion. On the other hand, different biological therapies, including B-cell depletion, can foster the development of progressive multifocal leukoencephalopathy, the demyelinating CNS disease caused by the JC virus (33). Thus, improved mechanistic insight into the biological processes involved in the generation of neuroprotective antibodies is needed to guide the development of MS diagnostics and therapies.
Materials and Methods
Mice.
Experiments were performed in accordance with federal and cantonal guidelines under permission numbers SG08/79, SG09/83, and SG09/87 after review and approval by the Cantonal Veterinary Office (St. Gallen, Switzerland). B6 mice were purchased from Charles River Laboratories. CXCR3−/−, CD40−/−, IAb−/−, JHT, AID-deficient mice B6 aicdatm1Hon (22), and IgMi (26) mice were maintained locally and at the Institute for Laboratory Animal Sciences at the University of Zürich. All mice were on the C57BL/6 genetic background and were maintained in individually ventilated cages and were used between 6 and 9 wk of age.
Virus Infections and Determination of Virus Titers.
Mice were infected i.n. with 5 × 104 pfu of MHV A59 or MHV-eGFP as previously described (24, 27). Clinical disease symptoms were scored as follows: 0, no signs of disease; 1, limb tail and wobbly gait; 2, paralysis of one hind leg; 3, paralysis of two hind legs; 4, paralysis of hind legs and at least one front leg. MHV titers were determined by standard plaque assay using L929 cells.
Histology.
For histological analysis, mice were killed, immediately perfused with PBS (pH 7.5), and organs were fixed in 4% formaldehyde. Sections were stained with Luxol Fast Blue to visualize spinal cord demyelination. Images were acquired using a Leica DMRA microscope and processed using Adobe Photoshop (Adobe Systems). For immunofluorescence analysis brains were fixed in PBS/4% paraformaldehyde. Twenty-micrometer sections were cut using a vibratom (Leica VT 1200S). Images were acquired using Zeiss LSM710 microscope and processed using ZEN software (Zeiss) and Adobe Photoshop. Further information on histological procedures is available in SI Materials and Methods.
Cell Isolation, Adoptive Transfer, and Flow Cytometry.
Mice were killed and immediately perfused with PBS. Single-cell suspensions from brain, spleen, and cervical LNs were prepared by mechanical disruption of the organs. For adoptive transfer, B cells were sorted from spleen cell suspensions using anti-B220 MACS beads (Miltenyi Biotec) to a purity of >95% as confirmed by flow cytometry. On days 1 and 3 before infection, 1.5 × 107 purified B cells were adoptively transferred into the indicated recipients, resulting in a reconstitution of 5–10% of the B-cell compartment (29). Further information on cell isolation and flow cytometry is available in SI Materials and Methods.
Antibody Detection.
MHV neutralizing titers were determined using a well-established protocol for the vesicular stomatitis virus (34). For IgG titers, sera were incubated with equal volumes of 0.1 M 2-ME in PBS for 1 h at room temperature before dilution. Neutralizing IgM titers were calculated by subtracting the IgG from the total Ig titers. Neutralizing antibody titers are given as −log2 dilution × 80. For measurement of virus-binding antibodies, high-binding 96-well polystyrene plates (Corning) were coated with 5 × 105 pfu of A59 MHV/mL, and the assay was performed as previously described (35). Antibody titers are given as −log2 dilution × 40. Positive titers were defined as 3 SD above the mean values of the negative controls.
Antibody-Producing Cell Assay.
Enzyme-linked immunosorbent spot (ELISPOT) assays were performed according to the manufacturer's instructions (Mabtech). Plates with 5 × 105 pfu of MHV A59 per well were incubated with 105 CNS-infiltrating, CLN, spleen, or bone marrow cells for 24 h at 37°C. Plates were counted with an ELISPOT reader and analyzed with the software ELISPOT 3.1SR (Autoimmun Diagnostika). ELISPOT responses of individual mice are expressed as mean number of specific antibody-forming cells (experimental sample − negative control) per 106 cells from triplicate measurements.
Statistical Analysis.
Statistical analyses were performed with Graphpad Prism 5.0 using nonpaired, two-tailed Student t test. Longitudinal comparison between different groups was done with one-way ANOVA with Tukey's posttest. Statistical significance was defined as P < 0.05.
Supplementary Material
Acknowledgments
This study received financial support from the Swiss National Science Foundation and Promedica Foundation (Chur). C.G.-C. and C.P.-S. received a postdoctoral and a Ph.D. fellowship, respectively, from the Mexican National Science and Technology Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. L.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115154109/-/DCSupplemental.
References
- 1.Bhat R, Steinman L. Innate and adaptive autoimmunity directed to the central nervous system. Neuron. 2009;64:123–132. doi: 10.1016/j.neuron.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Hafler DA, et al. International Multiple Sclerosis Genetics Consortium Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 3.Baranzini SE, et al. Genome, epigenome and RNA sequences of monozygotic twins discordant for multiple sclerosis. Nature. 2010;464:1351–1356. doi: 10.1038/nature08990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pugliatti M, et al. Evidence of early childhood as the susceptibility period in multiple sclerosis: Space-time cluster analysis in a Sardinian population. Am J Epidemiol. 2006;164:326–333. doi: 10.1093/aje/kwj190. [DOI] [PubMed] [Google Scholar]
- 5.Münz C, Lünemann JD, Getts MT, Miller SD. Antiviral immune responses: Triggers of or triggered by autoimmunity? Nat Rev Immunol. 2009;9:246–258. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin LI, Munger KL, O'Reilly EJ, Falk KI, Ascherio A. Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann Neurol. 2010;67:824–830. doi: 10.1002/ana.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soldan SS, et al. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: Increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med. 1997;3:1394–1397. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- 8.Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74:8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boucher A, Desforges M, Duquette P, Talbot PJ. Long-term human coronavirus-myelin cross-reactive T-cell clones derived from multiple sclerosis patients. Clin Immunol. 2007;123:258–267. doi: 10.1016/j.clim.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brew BJ, Davies NW, Cinque P, Clifford DB, Nath A. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol. 2010;6:667–679. doi: 10.1038/nrneurol.2010.164. [DOI] [PubMed] [Google Scholar]
- 11.Prineas JW. Multiple sclerosis: presence of lymphatic capillaries and lymphoid tissue in the brain and spinal cord. Science. 1979;203:1123–1125. doi: 10.1126/science.424741. [DOI] [PubMed] [Google Scholar]
- 12.Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F. B cells and multiple sclerosis. Lancet Neurol. 2008;7:852–858. doi: 10.1016/S1474-4422(08)70192-3. [DOI] [PubMed] [Google Scholar]
- 13.Sola P, et al. Primary progressive versus relapsing-onset multiple sclerosis: Presence and prognostic value of cerebrospinal fluid oligoclonal IgM. Mult Scler. 2011;17:303–311. doi: 10.1177/1352458510386996. [DOI] [PubMed] [Google Scholar]
- 14.Stauch C, et al. Intrathecal IgM synthesis in pediatric MS is not a negative prognostic marker of disease progression: Quantitative versus qualitative IgM analysis. Mult Scler. 2011;17:327–334. doi: 10.1177/1352458510388543. [DOI] [PubMed] [Google Scholar]
- 15.Pohl D, et al. Intrathecal antibody production against Epstein-Barr and other neurotropic viruses in pediatric and adult onset multiple sclerosis. J Neurol. 2010;257:212–216. doi: 10.1007/s00415-009-5296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobi C, Lange P, Reiber H. Quantitation of intrathecal antibodies in cerebrospinal fluid of subacute sclerosing panencephalitis, herpes simplex encephalitis and multiple sclerosis: Discrimination between microorganism-driven and polyspecific immune response. J Neuroimmunol. 2007;187:139–146. doi: 10.1016/j.jneuroim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Matthews AE, et al. Antibody is required for clearance of infectious murine hepatitis virus A59 from the central nervous system, but not the liver. J Immunol. 2001;167:5254–5263. doi: 10.4049/jimmunol.167.9.5254. [DOI] [PubMed] [Google Scholar]
- 18.Ramakrishna C, Stohlman SA, Atkinson RD, Shlomchik MJ, Bergmann CC. Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J Immunol. 2002;168:1204–1211. doi: 10.4049/jimmunol.168.3.1204. [DOI] [PubMed] [Google Scholar]
- 19.Ramakrishna C, Bergmann CC, Atkinson R, Stohlman SA. Control of central nervous system viral persistence by neutralizing antibody. J Virol. 2003;77:4670–4678. doi: 10.1128/JVI.77.8.4670-4678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marques CP, et al. CXCR3-dependent plasma blast migration to the central nervous system during viral encephalomyelitis. J Virol. 2011;85:6136–6147. doi: 10.1128/JVI.00202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: The two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- 22.Harada Y, Muramatsu M, Shibata T, Honjo T, Kuroda K. Unmutated immunoglobulin M can protect mice from death by influenza virus infection. J Exp Med. 2003;197:1779–1785. doi: 10.1084/jem.20021457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond MS, et al. A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med. 2003;198:1853–1862. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Züst R, et al. Identification of coronavirus non-structural protein 1 as a major pathogenicity factor—implications for the rational design of live attenuated coronavirus vaccines. PLoS Pathog. 2007;3:e109. doi: 10.1371/journal.ppat.0030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: Host-virus stand-off. Nat Rev Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waisman A, Croxford AL, Demircik F. New tools to study the role of B cells in cytomegalovirus infections. Med Microbiol Immunol (Berl) 2008;197:145–149. doi: 10.1007/s00430-008-0088-z. [DOI] [PubMed] [Google Scholar]
- 27.Cervantes-Barragán L, et al. Type I IFN-mediated protection of macrophages and dendritic cells secures control of murine coronavirus infection. J Immunol. 2009;182:1099–1106. doi: 10.4049/jimmunol.182.2.1099. [DOI] [PubMed] [Google Scholar]
- 28.Oxenius A, et al. CD40-CD40 ligand interactions are critical in T-B cooperation but not for other anti-viral CD4+ T cell functions. J Exp Med. 1996;183:2209–2218. doi: 10.1084/jem.183.5.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar V, et al. Global lymphoid tissue remodeling during a viral infection is orchestrated by a B cell-lymphotoxin-dependent pathway. Blood. 2010;115:4725–4733. doi: 10.1182/blood-2009-10-250118. [DOI] [PubMed] [Google Scholar]
- 30.Phares TW, Marques CP, Stohlman SA, Hinton DR, Bergmann CC. Factors supporting intrathecal humoral responses following viral encephalomyelitis. J Virol. 2011;85:2589–2598. doi: 10.1128/JVI.02260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dandekar AA, Jacobsen G, Waldschmidt TJ, Perlman S. Antibody-mediated protection against cytotoxic T-cell escape in coronavirus-induced demyelination. J Virol. 2003;77:11867–11874. doi: 10.1128/JVI.77.22.11867-11874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Racine R, et al. IgM production by bone marrow plasmablasts contributes to long-term protection against intracellular bacterial infection. J Immunol. 2011;186:1011–1021. doi: 10.4049/jimmunol.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosch X, Saiz A, Ramos-Casals M, BIOGEAS Study Group Monoclonal antibody therapy-associated neurological disorders. Nat Rev Neurol. 2011;7:165–172. doi: 10.1038/nrneurol.2011.1. [DOI] [PubMed] [Google Scholar]
- 34.Ludewig B, et al. Induction of optimal anti-viral neutralizing B cell responses by dendritic cells requires transport and release of virus particles in secondary lymphoid organs. Eur J Immunol. 2000;30:185–196. doi: 10.1002/1521-4141(200001)30:1<185::AID-IMMU185>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Cervantes-Barragan L, et al. TLR2 and TLR4 signaling shapes specific antibody responses to Salmonella typhi antigens. Eur J Immunol. 2009;39:126–135. doi: 10.1002/eji.200838185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.