Abstract
Neural stem cells (NSCs) reside in specialized niches in the adult mammalian brain, including the subventricular zone and the dentate gyrus, which act to control NSC behavior. Among other cell types within these niches, NSCs are found in close proximity to blood vessels. We carried out an analysis of the interaction between endothelial cells and NSCs, and show that betacellulin (BTC), a member of the EGF family and one of several signaling molecules made by the former, induces NSC proliferation and prevents spontaneous differentiation in culture. When infused into the lateral ventricle, BTC induces expansion of NSCs and neuroblasts, and promotes neurogenesis in the olfactory bulb and dentate gyrus, whereas specific blocking antibodies reduce the number of stem/progenitor cells. BTC-null mice are less able to regenerate neuroblast numbers compared with WT littermates following depletion of proliferating cells using cytosine-β-d-arabinofuranoside. BTC acts via both the EGF receptor, located on NSCs, and ErbB4, located on neuroblasts, with the latter explaining why its effects are distinct from those of EGF itself. Our results suggest that BTC could be a good candidate to aid regenerative therapies.
Keywords: regeneration, vasculature, choroid plexus, cerebral spinal fluid, neurospheres
Neural stem cells (NSCs) reside in a specialized microenvironment, known as a stem cell niche, that shapes their fate (1, 2). Two main NSC niches have been described in the adult mouse brain: the subventricular zone (SVZ) and the dentate gyrus (DG) in the hippocampus (1, 2). Located on the lateral wall of the lateral ventricles, the SVZ is composed of different cell populations, including a monolayer of ependymal cells that lines the ventricle, NSCs, transit-amplifying cells, neural progenitors (neuroblasts), astrocytes, and a dense network of blood vessels. NSCs extend a process to the ventricle through the ependymal layer and also to endothelial cells (ECs) in the niche, and therefore maintain contact with both the ventricular space and the blood vessels (3).
In the SVZ, which is densely vascularized, neurogenesis is associated with the vasculature and most proliferating cells are found adjacent or very close to blood vessels (4, 5). GFAP+ astrocytic stem cells and transit-amplifying progenitor cells are located in the proximity of the capillaries and chains of neuroblasts line the vessels as they migrate along the rostral migratory stream (RMS), although without direct contact (4, 5). Progenitor cells are recruited to the niche vasculature by SDF-1, and their continuing interaction is dependent on α6β1 integrin (5, 6). The functional effect that ECs exert on NSCs can be reproduced in vitro. ECs, but not smooth muscle cells, induce NSC proliferation in coculture experiments, prevent their differentiation, and enhance their neurogenic capacity (7). Both ECs and ependymal cells support NSC self-renewal, at least in part, by secreting pigment epithelium-derived factor (8). However, although these data strongly suggest that blood vessels have an impact on NSCs, niche homeostasis, and neurogenesis, the underlying molecular mechanisms are far from being understood and are likely to involve additional components. Interestingly, stem/progenitor cells localized around capillaries in the SVZ show strong expression of EGF receptor (EGFR), which is further up-regulated by SDF-1, suggesting a role for the EGF family of growth factors in the regulation of NSC function (4, 6).
Here, we investigate communication between ECs and NSCs and unveil that betacellulin (BTC), an EGF-like protein initially described to mediate the generation of β cells in the pancreas (9), induces NSC proliferation and enhances neurogenesis.
Results
Global Analysis of the Effect of ECs on NSCs.
In agreement with others, we found that NSCs are located in close proximity (<15 μm) to ECs of the microvasculature (Fig. S1 and SI Results). To determine the molecular processes that mediate the effect of ECs on NSCs, we cocultured both cell types in a Transwell device (Costar) for 7 d, allowing exchange of soluble factors between ECs and NSCs but not direct cell-cell contact (Fig. S2A). NSCs grown in the absence of ECs were used as a negative control. To take full advantage of the resources available, we made use of the human NSC line CTX0E03. Using an antibody array, we observed that a number of tyrosine kinase receptors presented differential phosphorylation in the presence of ECs, including the EGF receptors ErbB1 and ErbB4; the FGF receptors 3 and 4; the receptors for ephrins A3, A4, and B6; the VEGF receptors 1 and 2; and the glial-derived neurotrophic factor receptor c-Ret (Fig. S2B and Dataset S1).
Antibody array analysis of soluble factors revealed a number of chemokines and cytokines increased in the coculture supernatant compared with either NSCs or ECs alone, as well as growth factors of the hepatocyte growth factor [HGF and macrophage stimulating protein alpha (MSPa)] and EGF (BTC) families (Fig. S2C and Dataset S2). To investigate the effect that EC-released factors have on NSCs, we extracted RNA from NSCs, either grown alone or in coculture with ECs, and analyzed gene expression profiles using oligonucleotide DNA microarrays. NSCs grown in the presence of ECs showed an increase >1.4-fold in 679 genes, compared with NSCs grown alone, and a decrease in 1,898 genes (Datasets S3 and S4). Gene ontology analysis (Methods) suggested that coculture with ECs stimulates ribosomal RNA and protein synthesis in NSCs, whereas it represses the regulation of gene transcription and cell signaling, neurogenesis, cell growth, the Wnt pathway, and the regulation of the cell cycle (Datasets S5–S10). Validation of these results by quantitative reverse-transcribed PCR (qRT-PCR) confirmed reduced expression of the negative regulators of the cell cycle CDKN1A/p21 and CDKN1C/p57; their regulator FOXO3A; and the differentiation markers GFAP, MAP2, and OLIG2 in the presence of ECs (Fig. S2D), suggesting that EC-derived factors promote NSC proliferation while inhibiting spontaneous differentiation.
To determine whether some of the proteins identified in the analysis of the coculture supernatant were sufficient to stimulate NSC growth, we studied the ability of NSCs to form neurospheres in the presence of HGF, MSP, BTC, Gro, Serpin, or soluble tumor necrosis factor-receptor II. As shown in Fig. S2E, both HGF and BTC increased the number of neurospheres in culture. To test the functional relevance of the increased phosphorylation of VEGF receptors 1 and 2 in the presence of ECs, we cultured neurospheres with VEGF-A in combination with FGF and/or EGF. VEGF-A synergized with FGF to increase the number of neurospheres, although it was not able to increase the number of neurospheres further in the presence of FGF + EGF as shown by both BTC and HGF (Fig. S2F). Moreover, VEGF-A addition produced smaller neurospheres compared with FGF and EGF (Fig. S2G). VEGF-C acting via VEGF receptor 3 (both absent from the antibody arrays used here) has recently been shown to stimulate NSC self-renewal and thereby promote (nonspecifically) both neurogenesis and gliogenesis (10).
BTC Stimulates Neurosphere Growth.
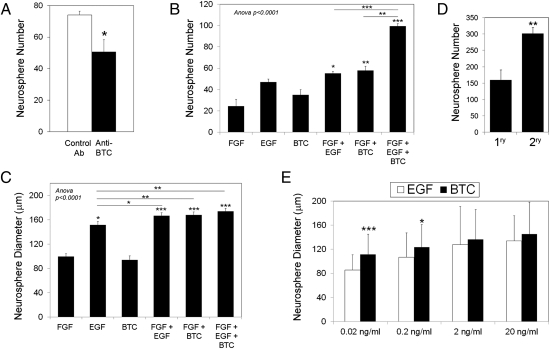
Because it has already been described that HGF can stimulate NSC proliferation (11), and given the relatively weak effects of VEGF-A, we focused on the potential of BTC as an NSC growth factor. We investigated whether BTC was necessary for the action of ECs on NSCs by adding a neutralizing anti-BTC or an isotype control antibody to the coculture. As shown in Fig. 1A, blockade of BTC decreased the number of neurospheres induced by EC supernatant. We also compared the efficiency of BTC with that of EGF by growing mouse NSCs in the presence of these factors, either combined or separately. We found that BTC is sufficient to induce neurosphere growth (Fig. 1 B and C) and to derive and grow secondary neurospheres (Fig. 1D), indicating that BTC induces NSC self-renewal. In combination with the prosurvival factor FGF, which is generally used for the growth of neurospheres with EGF, BTC showed similar efficiency to EGF at 20 ng/mL and higher efficiency at lower concentrations (Fig. 1E). In addition, whereas a small percentage of spontaneously differentiating neurospheres could be detected in the presence of EGF (20 ng/mL), BTC reproducibly blocked spontaneous differentiation (Fig. S3A). Exposure of NSCs to BTC plus FGF also mimicked the effect of ECs by reducing the expression of differentiation markers and cell cycle regulators (Fig. S3B), compared with FGF plus EGF, reinforcing the idea that BTC was more efficient than EGF. Immunofluorescence analysis of NSCs grown in the presence of BTC (20 ng/mL) for 10 d and then allowed to differentiate in the absence of BTC showed that BTC-stimulated NSCs were able to differentiate into the three different lineages: neurons (Tuj1), oligodendroglia [2',3'-cyclic nucleotide 3'-phosphodiesterase (CNPase)], and astrocytes (GFAP), maintaining their full differentiation potential (Fig. S3C).
Fig. 1.
BTC stimulates neurosphere growth. (A) Adult mouse NSCs were grown in Transwell devices in the presence of ECs and were treated with 10 μg/mL anti-BTC blocking antibody or an isotype control. Neurosphere number is expressed as the average per well ± SE. (B and C) NSCs were stimulated as indicated with 20 ng/mL FGF, EGF, and/or BTC. Neurosphere number (B) and diameter (C) were determined after 10 d and expressed as the average ± SE. *P < 0.05; **P < 0.005; and ***P < 0.0005 vs. FGF or as indicated (one-way ANOVA plus Bonferroni posttest). (D) Primary neurospheres were generated in the presence of BTC (20 ng/mL) for 10 d, after which they were disgregated, seeded at the initial concentration, and grown with BTC to generate secondary neurospheres. 1ry, primary; 2ry, secondary. (E) NSCs were grown as in B in the presence of FGF (20 ng/mL) plus the indicated concentrations of EGF or BTC, and the diameter ± SE was quantified.
BTC Is Expressed by ECs and the Choroid Plexus.
To identify the cell type producing BTC in the coculture, we analyzed RNA from NSCs and ECs by qRT-PCR. We found that ECs were expressing BTC mRNA, whereas it was barely detectable in NSCs, regardless of whether they were cocultured or grown alone (Fig. S4A). In addition, we observed that the EC lines ccec and b.End.3 expressed BTC protein at levels comparable to the pancreatic cell line PARC1 (Fig. S4B). Analysis of BTC expression in the adult brain showed the highest BTC expression in the cerebellum and lower amounts in the cortex and the SVZ and DG NSC niche regions (Fig. S4D). Immunofluorescence analysis of BTC distribution in the brain revealed BTC staining within ECs (Fig. S4F), with a similar pattern to that observed for other soluble factors (6, 8). Interestingly, BTC mRNA expression and protein staining were also observed in the choroid plexus of the lateral ventricle (Fig. S4 D and E), which was stronger than that observed in blood vessels. In agreement with these results, we detected BTC in mouse cerebrospinal fluid (CSF) by Western blot analysis (Fig. S4C), suggesting that both blood vessels and the choroid plexus produce and secrete BTC in the brain.
BTC Stimulates Growth of NSCs and Precursor Cells in the Adult Mouse Brain.
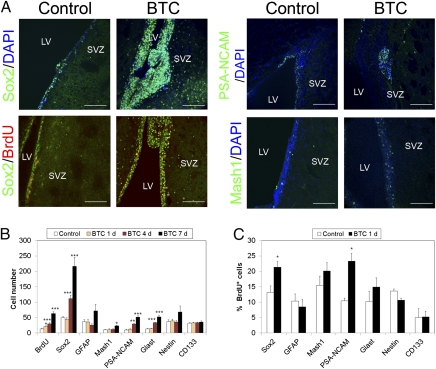
We performed a series of experiments infusing BTC directly into the brain lateral ventricle using an osmotic minipump that allows continuous delivery of the protein. Brains were analyzed after 1, 4, or 7 d by immunofluorescence using antibodies directed against different SVZ cell populations. We found that BTC progressively induced proliferation of SOX2+ cells, which more than quadrupled their number after 1 wk (Fig. 2 A and B). Accordingly, we found a similar increase in the number of cells positive for BrdU, which labels proliferating cells, and a proportional increase in the SVZ thickness. Whereas cells positive for Glast, a nonexclusive marker of NSCs and some astrocytes, were also found to be increased in response to BTC, only a modest increase in the number of GFAP+, Nestin+, and Mash1+ cells was observed, and the number of CD133+ ependymal cells remained unaltered (Fig. 2 A and B and Fig. S5A). A similar response was detected in the DG, where BTC induced proliferation of SOX2+ cells (Fig. S6C). A progressive increase in the number of neuroblasts was also observed, as determined with an anti–polysialic acid neural cell adhesion molecule (PSA-NCAM) antibody, suggesting that BTC could induce neurogenesis. These effects were likely attributable to increased proliferation rather than reduced apoptosis, because we detected very few apoptotic cells in the SVZ by TUNEL staining (Fig. S5B).
Fig. 2.
BTC stimulates growth of NSCs and precursor cells in the SVZ of the adult mouse brain in vivo. (A and B) Vehicle (Control) or BTC (400 ng/d) was infused into the mouse lateral ventricle (LV), and mice were killed 1, 4, or 7 d later. For BrdU experiments, BrdU (10 μg/g) was administered daily by i.p. injection for 7 d. Brains were fixed, sectioned, and analyzed by immunofluorescence using antibodies directed against Sox2, BrdU, PSA-NCAM, Glast, GFAP, CD133/Prominin, and Nestin (additional examples are provided in Fig. S1). Nuclei were counterstained using DAPI. (Scale bar, 100 μm.) (B) Average number of cells ± SE in each population were quantified in the SVZ. (C) BTC or vehicle (Control) was infused into the lateral ventricle for 24 h, and a single dose of BrdU (10 μg/g) was administered i.p. at the time of cannula implantation. The percentage of proliferating cells was quantified as 100 × the number of cells positive for each cell population marker that was also positive for BrdU. *P < 0.05; **P < 0.005; ***P < 0.0005.
To determine whether endogenous BTC was necessary for maintenance of the stem/progenitor population in the SVZ, we infused blocking anti-BTC antibody or an isotype control antibody into the lateral ventricle and analyzed the SVZ 7 d later. As shown in Fig. 2C and Fig. S6A, blockade of endogenous BTC activity resulted in a decrease in the number of SOX2+ and Glast+ stem/precursor cells in the SVZ, together with fewer PSA-NCAM+ neuroblasts, suggesting that BTC is both necessary and sufficient to regulate proliferation of NSCs and neuroblasts. Preincubation with recombinant BTC neutralized effects of the blocking antibody, demonstrating the specificity of the result (Fig. S6B). A decrease in proliferating (BrdU+) SOX2+ cells was also observed in the DG after infusion of anti-BTC (Fig. S6E).
To find out which cell subpopulation in the SVZ first reacted to BTC, we injected BrdU i.p. and infused BTC or vehicle into the lateral ventricle for 24 h. We quantified the percentage of cells in each cell population that had duplicated their DNA (BrdU+). As shown in Fig. 2C, BTC significantly increased the percentage of SOX2+ cells and neuroblasts that were also BrdU+. No increase in total SOX2+ or PSA-NCAM+ cells was detected within the first 24 h (Fig. 2B), suggesting that these cell subpopulations enter the cell cycle within the first 24 h but need longer to complete cell division.
Together, these results demonstrate that BTC induces expansion of progenitor populations in the mouse brain, notably NSCs and neuroblasts. We found that this effect is mediated by both EGFR and ErbB4, which, in turn, activate the MEK/Erk and Akt signaling pathways (Fig. 3C, Fig. S7, and SI Results). In contrast, EGF mainly activates EGFR and signals through the MEK/Erk pathway.
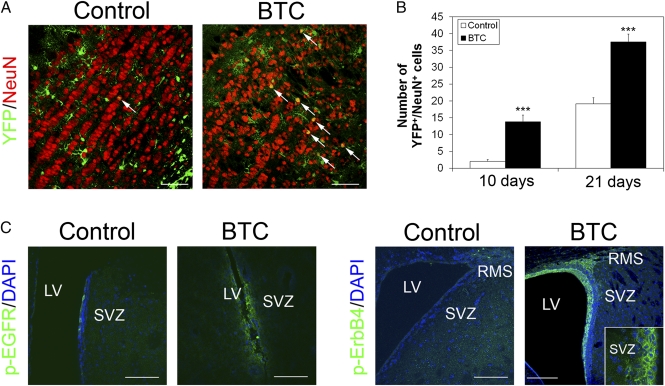
Fig. 3.
BTC induces neurogenesis in the olfactory bulb. (A and B) R26REYFP mice were crossed to Glast::Cre-ERT2 mice, and YFP expression was induced in Glast+ adult progenitors by injecting tamoxifen for 5 d. BTC or vehicle was infused for 7 d, and the presence of Glast+ cell-derived neurons in the olfactory bulb was analyzed 10 d (B) and 21 d (A and B) later. (Scale bar, 50 μm.) The percentage of YFP+ cells that were also NeuN+ was scored in three random fields on view. White arrows indicate YFP/NeuN double positive cells. (C) BTC activates EGFR and ErbB4 in vivo. BTC (400 ng/d) or vehicle was infused into the lateral ventricle (LV), and activation of EGFR and ErbB4 was determined by immunofluorescence using phospho-specific antibodies. (Scale bar, 100 μm.) (Inset) p-ErbB4 staining in the SVZ. (Scale bar, 50 μm.)
BTC Increases Neurogenesis.
To investigate whether BTC induces neurogenesis in vivo, we bred R26REYFP into mice carrying Glast::CreERT2. YFP expression was activated in adult Glast+ progenitor cells by tamoxifen injection for 5 d. BTC or vehicle was then infused into the lateral ventricle for 7 d. Ten days after removing the minipump, a clear increase in Glast+ cell-derived YFP+ neurons was detected in the olfactory bulb of mice infused with BTC compared with vehicle (Fig. 3 A and B and Fig. S8). Similar data were obtained with a method (12) whereby NSCs are labeled after a single injection of an adenovirus Cre into the lateral ventricle of R26REYFP mice (Fig. S8). These results suggest that BTC induces expansion of NSCs and neuroblasts in the SVZ and that these cells subsequently migrate to the olfactory bulb to contribute to the neuron population. Similarly, we observed increased neurogenesis in the DG using a complementary approach. We infused BTC into the lateral ventricle for 7 d, injected BrdU i.p. daily during the same period, and processed the brains after 10 d. Immunofluorescence analysis of BrdU and the neuron markers NeuN and Tuj1 showed an increase in the number of BrdU+ neurons in the DG 10 d after implantation of the minipump (Fig. S9), confirming that BTC enhances neurogenesis.
BTC Mediates Regeneration of the NSC Niche.
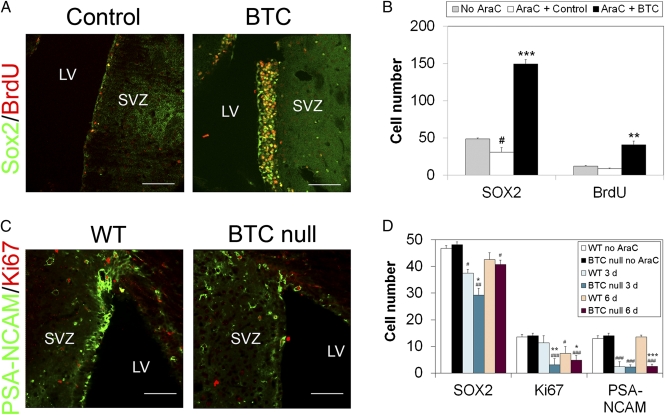
We next analyzed the capacity of BTC to induce regeneration of the SVZ. We administered the antimitotic drug cytosine-β-d-arabinofuranoside (AraC) directly into the lateral ventricle using osmotic minipumps. Six days later, we implanted minipumps delivering BTC or vehicle. After 3 d, immunofluorescence analysis showed a fourfold increase in the number of SOX2+ cells after BTC treatment (Fig. 4 A and B), together with an increase in the number of BrdU+ cells, all of which were also positive for SOX2. Because only quiescent NSCs remain after AraC treatment, this also confirms that BTC can act on these cells. To determine whether BTC was necessary for replenishment of the SVZ niche, we treated WT and BTC-null mice with AraC for 6 d and allowed regeneration to proceed for a further 3 or 6 d. Compared with WT, BTC-null animals showed reduced levels of SOX2+ NSCs and proliferating cells after 3 d (Fig. 4D) and far fewer PSA-NCAM+ neuroblasts after 6 d (Fig. 4 C and D). These results suggest that BTC plays a key role in the timely regeneration of the NSC niche.
Fig. 4.
BTC contributes to the regeneration of the NSC niche. (A and B) AraC [2% (wt/vol) in saline] was infused into the lateral ventricle (LV) for 6 d, and either vehicle (AraC + Control) or BTC (AraC + BTC; 400 ng/d) was infused for 3 d. Vehicle alone was used as a negative control for AraC (No AraC). BrdU (10 μg/g) was administered daily by i.p. injection for the last 7 d. Brains were fixed, sectioned, and analyzed by immunofluorescence using the antibodies indicated on the left. (Scale bar, 100 μm.) (B) Number of cells was quantified only in the SVZ and expressed as the average ± SE. WT and BTC-null mice were treated with AraC for 6 d as in A and analyzed 3 d (D) or 6 d (C and D) later. Cells were quantified as in B. (Scale bar, 100 μm.) *P < 0.05; **P < 0.005; and ***P < 0.0005 BTC vs. control (B) or BTC-null vs. WT at each time point (D) as determined by the Student t test. #P < 0.05; ##P < 0.005; and ###P < 0.0005 for AraC vs. no AraC for each time point and condition.
Discussion
The molecular mechanisms underlying the effects of ECs on NSCs are largely unknown. Here, we have identified BTC as an EC-derived factor capable of inducing NSC proliferation and neurogenesis. BTC belongs to the EGF family of growth factors, which bind receptors of the ErbB family and induce their dimerization (13). Interestingly, stem and progenitor cells localized around capillaries in the SVZ niche show strong expression of EGFR (4). The effect of different ErbB ligands on the SVZ appears to be unique. Infusion of EGF into the lateral ventricle induces disorganized proliferation and invasion of progenitors (transit-amplifying cells), as well as formation of oligodendroglial cells, although decreasing neuroblast differentiation and neurogenesis (14–18). This is in clear contrast to the effects of BTC, which induces organized expansion of NSCs, some transit-amplifying cells, and particularly neuroblasts in the SVZ and DG, without leading to an invasion of the subjacent tissue. Some of the differences between BTC and EGF are likely to be attributable to the more promiscuous activity of BTC, which can function via EGFR and ErbB4, whereas EGF does not bind ErbB4. Thus, BTC can affect neuroblast proliferation directly, because such cells express ErbB4, in addition to promoting expansion of NSCs via EGFR and/or ErbB4. The finding that many neuroblasts persist as clusters, particularly in the SVZ, might suggest that BTC action also leads to their increased aggregation in a manner similar to that shown for NRG1 (19). However, this may simply reflect the limited size of the pathway normally taken by neuroblasts within the RMS (i.e., with insufficient substrate, only a subset of the cells can form migrating chains, whereas the remainder adhere only to each other and remain behind in the SVZ). Importantly, additional BTC increases the number of neurons derived from SVZ cells in the olfactory bulb and new (BrdU+) early neurons in the DG, indicating that BTC induces both NSC proliferation and neurogenesis. This is a critical difference between EGF and BTC. Further differences are outlined in vitro, where low concentrations of BTC stimulate neurosphere growth and prevent spontaneous differentiation more efficiently than EGF. This could be partially explained by activation of both the Erk and Akt pathways by BTC, whereas EGF activates only the former (Fig. S7 and SI Results).
Two main routes are responsible for the delivery of small molecules to the brain: the vascular network and the ventricles, where CSF in the latter is made largely by the choroid plexus (4). We found high levels of endogenous BTC in both. Our blocking antibody experiments imply that ventricular activity of BTC helps to maintain the stem/progenitor population in the SVZ; indeed, choroid plexus-derived BTC may be one of the factors contributing to the existence of an NSC niche in the SVZ. However, the anti-BTC neutralizing antibody also decreased the number of proliferating SOX2+ cells in the DG, suggesting that endogenous BTC may be part of the vascular NSC niche. In this regard, we found BTC expression in primary ECs and EC lines in culture, both mRNA and protein, and we observed BTC immunostaining in brain blood vessels, notably in microcapillaries in the SVZ. BTC expression in the latter was strongest toward the surface of the ECs away from the lumen, suggesting that it is secreted into the niche rather than the bloodstream. Interestingly, the metalloproteinase responsible for BTC shedding and release to the extracellular milieu, ADAM10, shows a similar distribution to that of BTC, expressed in the choroid plexus and in the blood vessels of the developing and adult brain (20, 21).
Clearly, several factors found in NSC niches, and made by various cell types within the niche or present in CSF, have overlapping roles in the behavior of NSCs and their progeny. In contrast to the blocking antibody experiments, where the effect on BTC will be acute, Btc-null mutant mice appear grossly normal. However, when challenged with AraC, the recovery of the niche was severely compromised. This suggests that BTC may be especially relevant in situations where repair is needed. In summary, our results show that BTC is produced endogenously in the brain and that it is sufficient to induce NSC proliferation and neurogenesis. Our findings suggest that BTC has therapeutic potential for the treatment of neurodegenerative diseases.
Methods
An extended version of the experimental procedures is provided in SI Methods.
Cell Culture.
NSCs were isolated from the mouse SVZ as previously described (22). The human NSC line CTX0E03 (Reneuron) (23) was grown in NSC growth medium in the presence of EGF (20 ng/mL) and FGF-2 (20 ng/mL). Mouse NSCs were grown in NSC growth medium, and the different growth factors were added at the indicated concentrations. Human umbilical cord endothelial cells (HUVECs) were grown in M199 supplemented with 10% (vol/vol) FCS and Endothelial Growth Factor (Upstate). For coculture experiments, HUVECs were grown in the upper chamber of a Transwell device with a 0.4-μm pore (Costar) until confluence was reached. Neurospheres were disaggregated and seeded in the bottom chamber in NSC medium containing no EGF or FGF-2.
Immunostaining.
Adult MF1 mice were perfused intracardially with 4% (vol/vol) paraformaldehyde (PFA) for fixation. Brains were excised and mounted in agarose, and 70-μm sections were sliced in the vibratome. Each section was blocked using 10% (vol/vol) donkey serum in PBS/0.1% Triton X-100 for 30 min at room temperature and incubated with primary antibodies overnight at 4 °C. BrdU immunostaining was performed as described (12). Sections were washed and incubated with secondary antibodies, and DAPI was added before mounting. For ICAM2/BTC coimmunostaining, sections were fixed in 8% (wt/vol) PFA and staining was performed without Triton X-100.
BTC Infusion.
Infusions were carried out as previously described (15, 19). Briefly, osmotic minipumps (Alzet 1007D; flow rate of 0.5 μL/h) were loaded with BTC (3.3 μg in 100 μL PBS, 0.1% BSA) or vehicle. Cannulas were implanted at 0.0 mm relative to bregma, 1.2 mm lateral and 3.5 mm deep, and the attached minipumps were implanted s.c. in the intercapsular region. Mice were killed 1 wk after minipump implantation and analyzed as described above. For regeneration experiments, Ara-C [2% (wt/vol)] was infused 1 wk before BTC infusion. For fate mapping, conditional R26REYFP adult male mice, which express the YFP reporter after Cre-mediated recombination, were crossed with Glast::Cre-ERT2 mice, which express a tamoxifen-inducible form of Cre in Glast-expressing cells. Recombination was induced by injecting tamoxifen for 5 d, and BTC or vehicle was infused. Cells were quantified as described in SI Methods.
Supplementary Material
Acknowledgments
We are grateful to C. Wise, R. Subramaniam, and C. Parras for technical support. Glast::CreERT2 mice were generously supplied by Magdalena Goetz. CTX0E03 cells were a gift from Dr. J. Sinden, and mouse CSF was supplied by Dr. E. Bides, Dr. C. Guaza, and Dr. P. Bovolenta. This work was supported by UK Medical Research Council Grant U117512772 and by a Quantum grant from the US National Institutes of Health (National Institute of Biomedical Imaging and Bioengineering) (to R.L.-B.). M.V.G.-G. was supported by a Marie Curie Intra-European Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016199109/-/DCSupplemental.
References
- 1.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 2.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokovay E, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 8.Ramírez-Castillejo C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 9.Dunbar AJ, Goddard C. Structure-function and biological role of betacellulin. Int J Biochem Cell Biol. 2000;32:805–815. doi: 10.1016/s1357-2725(00)00028-5. [DOI] [PubMed] [Google Scholar]
- 10.Calvo C-F, et al. Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev. 2011;25:831–844. doi: 10.1101/gad.615311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokuzawa J, et al. Hepatocyte growth factor promotes proliferation and neuronal differentiation of neural stem cells from mouse embryos. Mol Cell Neurosci. 2003;24(1):190–197. doi: 10.1016/s1044-7431(03)00160-x. [DOI] [PubMed] [Google Scholar]
- 12.Scott CE, et al. SOX9 induces and maintains neural stem cells. Nat Neurosci. 2010;13:1181–1189. doi: 10.1038/nn.2646. [DOI] [PubMed] [Google Scholar]
- 13.Linggi B, Carpenter G. ErbB receptors: New insights on mechanisms and biology. Trends Cell Biol. 2006;16:649–656. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 16.Jin K, et al. Heparin-binding epidermal growth factor-like growth factor: Hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci. 2002;22:5365–5373. doi: 10.1523/JNEUROSCI.22-13-05365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Perez O, Romero-Rodriguez R, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells. 2009;27:2032–2043. doi: 10.1002/stem.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gampe K, Brill MS, Momma S, Götz M, Zimmermann H. EGF induces CREB and ERK activation at the wall of the mouse lateral ventricles. Brain Res. 2011;1376:31–41. doi: 10.1016/j.brainres.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 19.Ghashghaei HT, et al. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc Natl Acad Sci USA. 2006;103:1930–1935. doi: 10.1073/pnas.0510410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahin U, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J, Luo J, Redies C. Differential expression of five members of the ADAM family in the developing chicken brain. Neuroscience. 2008;157:360–375. doi: 10.1016/j.neuroscience.2008.08.053. [DOI] [PubMed] [Google Scholar]
- 22.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 23.Pollock K, et al. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006;199(1):143–155. doi: 10.1016/j.expneurol.2005.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.