Abstract
The aryl hydrocarbon receptor (AhR) knockout mice raised in the laboratory of Fujii-Kuriyama have been under investigation for several years because of the presence in their urinary bladder of large, yellowish stones. The stones are composed of uric acid and become apparent in the bladders as tiny stones when mice are 10 wk of age. By the time the mice are 6 mo of age, there are usually two or three stones with diameters of 3–4 mm. The urate concentration in the serum was normal but in the urine the concentration was 40–50 mg/dL, which is 10 times higher than that in the WT littermates. There were no apparent histological pathologies in the kidney or joints and the levels of enzymes involved in elimination of purines were normal. The source of the uric acid was therefore judged to be from degradation of nucleic acids due to a high turnover of cells in the bladder itself. The bladder was fibrotic and the luminal side of the bladder epithelium was filled with eosinophilic granules. There was loss of E-cadherin between some epithelial cells, with an enlarged submucosal area filled with immune cells and sometimes invading epithelial cells. We hypothesize that in the absence of AhR there is loss of detoxifying enzymes, which leads to accumulation of unconjugated cytotoxins and carcinogens in the bladder. The presence of bladder toxins may have led to the increased apoptosis and inflammation as well as invasion of epithelial cells in the bladders of older mice.
Keywords: gout; 2,3,7,8-tetrachlorodibenzoparadioxin; xenobiotic; uricase; bladder toxicity
The aryl hydrocarbon receptor (AhR) has been extensively studied for its role in regulating xenobiotic-metabolizing enzymes and the curious observation that one of its most potent ligands is the environmental contaminant, 2,3,7,8-tetrachlorodibenzoparadioxin (TCDD) (1). Important enzymes that are regulated by AhR are members of the CYP1 family (1), glucuronyl transferase, and a number of enzymes involved in purine metabolism: xanthine oxidase (2), ADP ribose polymerase (PARP) (3), and adenosine deaminase (ADA) (4). Adenosine deaminase is the enzyme that regulates how much adenosine is metabolized to urate (5). TCDD is a potent immunosuppressant in several animal species (6) and via AhR it is a suppressor of adenosine deaminase, which is essential for the proper functioning of the immune system (4).
Three strains of aryl hydrocarbon receptor knockout (AhR−/−) mice have been produced in three different laboratories, those of Gonzales, Bradfield, and Fujii-Kuriyama (7–9). These three mouse strains exhibit different phenotypes. Common features of the AhR−/− mice are decreased liver size, hepatic portal fibrosis, and decreased constitutive expression of drug metabolizing enzymes such a P4501A1 and 1B1 (10). In the AhR mice produced in the Bradfield laboratory, seminal vesicle weight was higher than that of WT mice at postnatal day 35 (11), whereas in the AhR−/− mice produced in the Fujii-Kuriyama laboratory, seminal vesicles were reported to regress in an age-dependent manner (12). To date no abnormalities in the metabolism of purines or excretion of urate have been reported in AhR−/− mice and variable effects on the immune system have been reported in the three AhR−/− strains. One of the knockouts had decreased lymphocyte numbers in the spleen and lymphocyte infiltration of lung, intestine, and urinary tract (13). One strain was more susceptible to infection (14) and one had immune impairment and hepatic fibrosis (15).
We examined the mice generated in the laboratory of Fujii-Kuriyama and found that the most outstanding phenotype was the presence of urate stones in the urinary bladder. We investigated the source of these stones and found that they were due to abnormal cellular turnover in the urinary bladder itself. There was extensive inflammation and development of bladder cancer in older mice.
Results
Uric Acid Stones and Elevated Urinary Uric Acid in the AhR−/− Mice.
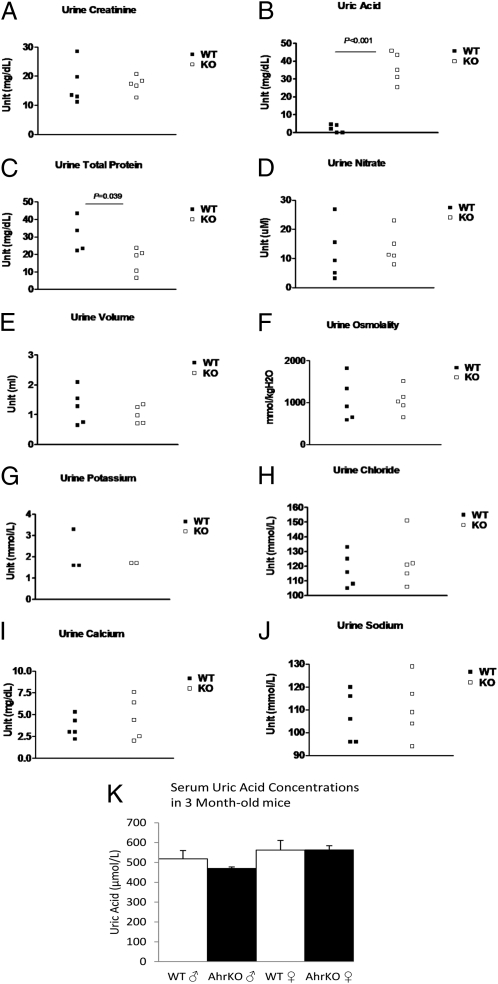
All AhR−/− mice at 8 mo of age have urinary bladder stones ∼3–4 mm in diameter and their composition is almost 100% urate. These stones first appeared in some of the mice at 10 wk of age and by the age of 6 mo, all of the mice have a stone occupying most of the bladder (Fig. 1). At 3 mo of age, uric acid levels in the urine of these mice are ∼10-fold higher than those of wild-type littermates (Fig. 2).
Fig. 1.
Urate stones in the urinary bladder of AhR knockout mice. Urinary bladder of a 10-wk-old AhR knockout mouse (A) with a urate stone indicated by the arrow. These stones are rough and pitted in appearance (B). Urate stones at 6 mo of age (C) with diameters of 3–4 mm. (Scale bar in A, 500 μm; B, 200 μm; and C, 1 mm.)
Fig. 2.
Urine solutes and volume and serum uric acid measurements of wild-type and AhR knockout mice at 3 mo of age. Urine creatinine (A), nitrate (D), volume (E), osmolality (F), potassium (G), chloride (H), calcium (I), and sodium (J) were not significantly different in the knockout mice compared with their wild-type littermates. Uric acid (B) was ∼10-fold higher in the urine of knockout mice (P < 0.001). There was a small decrease in the total protein (C) in the AhR knockout mice (P = 0.039). Uric acid levels in the serum were not significantly changed at 3 mo of age (K). Error bars in K represent SD.
Interestingly, the serum levels of uric acid in the knockouts were not significantly different from the wild-type mice at 3 mo (Fig. 2). There were also no urate stones or histological abnormalities in the kidneys or the joints of the AhR−/− mice, which are characteristics of elevated uric acid in humans.
Uric acid is the end point of purine metabolism in humans but in mice, unlike humans, there is an enzyme called uricase, which catalyzes the conversion of uric acid to allantoin. We examined several enzymes in the purine degradation pathway and found no significant difference in the RNA or protein levels in the liver for adenosine deaminase, uricase, hypoxanthine-guanine phosphoribosyltransferase, or xanthine oxidase (Fig. S1). This result is compatible with the lack of high urate in the circulation and suggested an abnormality in the bladder itself.
Aquaporins Are Not Involved in Uric Acid Stone Formation.
Changes in water transporters (aquaporins) in the kidney is known to cause the urine to become concentrated and may lead to the formation of stones. However, in the AhR−/− mice the urine osmolality was not different from their control littermates at 3 mo (Fig. 2) or at 4, 5, and 6 mo of age. There were also no changes in the total urine volume, nitrate, potassium, sodium, calcium, creatinine, or chloride in these mice (Fig. 2). The pH was 6.5 in all knockout and wild-type mice. There was a small decrease in the total protein concentration in the urine (Fig. 2). The mRNA levels of several urate transporters were also unchanged in the kidney (Fig. S2).
Fibrosis in the AhR−/− Mouse Submucosal Layer.
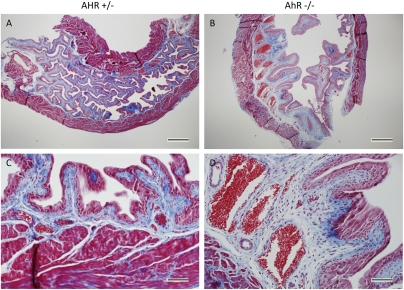
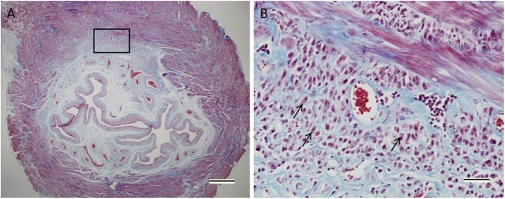
In view of the lack of changes in other pathways that may have led to elevated uric acid in the urine, we examined the histology of the urinary bladders of mice at 10 wk of age. There was increased fibrosis of the submucosal layer in AhR−/− mice compared with their heterozygous littermates (Fig. 3). There was a thicker collagen layer in these bladders as well as very large, dilated blood vessels. This phenotype is even more severe at 6 mo of age and in some of the mice, epithelial cells had invaded the muscle and submucosal layers of the bladder (Fig. 4). It appeared possible that a high turnover of cells in the enlarged bladders may have caused an increase in nucleic acid degradation into uric acid. This is a situation similar to tumor lysis syndrome in leukemia when tumor cells are killed too rapidly.
Fig. 3.
Fibrosis in the submuscosal layer of the 10-wk-old AhR knockout bladders. Masson's trichrome stain of AhR+/− (A and C) and AhR−/− (B and D) bladders, demonstrating fibrosis of the knockout bladder with an increased amount of collagen (blue) and enlarged blood vessels. (Scale bars in A and B, 500 μm; C and D, 20 μm.)
Fig. 4.
Invading epithelial cells are observed in the submucosal and muscle layers of the 6-mo-old AhR−/− bladder. Some epithelial cells are marked with arrows (B). Masson's trichrome stain was used to stain collagen blue, cytoplasm red, and nuclei purple. (Scale bar in A, 500 μm; B, 20 μm.)
Numerous Round Structures in the Cytoplasm and Death of Urothelium.
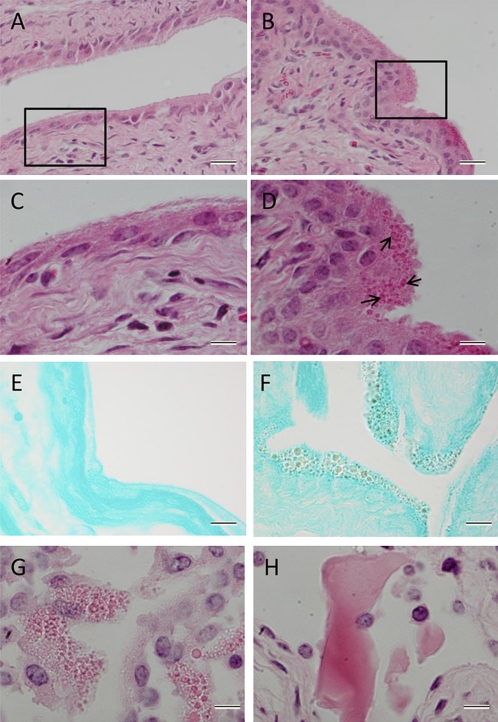
In the urothelial cells in the bladder of AhR knockout mice, there were numerous round structures concentrated toward the luminal surface, which are not present in wild-type or heterozygous mice (Fig. 5). These structures stain positive for the histological stain for acidic substances, eosin and for a stain commonly used to detect uric acid, methenamine silver, whereas the control mice remain negative (Fig. 5). We speculated that these structures are vesicles filled with uric acid. Therefore, we treated some fresh 4-mo-old AhR−/− bladders with uricase (an enzyme that converts uric acid to allantoin) to see whether these structures would dissolve. Even though the hydrogen peroxide used in this reaction caused much of the epithelium to fall off, the remaining epithelium after the uricase treatment did not contain any round granules, whereas the control did (Fig. 5). Because the uricase appeared to eliminate the presence of the epithelial inclusions, we conclude they contain uric acid.
Fig. 5.
Numerous round particles were visible in the luminal side of the urothelium of a 10-wk-old AhR knockout mouse (B and D), whereas no particles exist in the heterozygous control (A and C). Cytoplasm and particles are stained pink with eosin and nuclei are stained purple with Mayer's hematoxylin. These structures stain positive (brown-black color) for Grocott's methenamine silver stain, which can be a marker for uric acid (F), whereas the control bladder remains negative (E). After a 1-h treatment with uricase and hydrogen peroxide, these granules are not present in the epithelium of the 4-mo-old AhR−/− bladder (H). The granules are still present in the control treatment of only hydrogen peroxide in PBS (G). (Scale bars in A, B, E, and F, 20 μm; C, D, G, and H, 10 μm.)
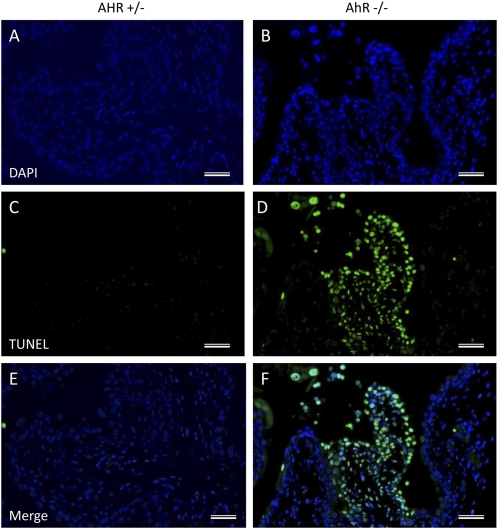
Because increased cell death is a possible explanation for the elevated uric acid, we measured apoptosis in the AhR−/− bladders with the TUNEL assay. There were more TUNEL+ cells in the 4-mo-old knockout bladders than in heterozygous littermates and there were several areas in the knockouts where cells had lost their cytoplasm and the nuclei were condensed (Fig. 6).
Fig. 6.
Apoptotic cells are present in some areas of the 4-mo-old AhR−/− bladder (B, D, and F), whereas there are very few in the AhR+/− mouse (A, C, and E). DAPI is used to stain the nuclei blue (A and B) and FITC stains TUNEL+ cells green (C and D). (Scale bars, 20 μm.)
Immunohistochemical Characteristics of the AhR−/− Bladder.
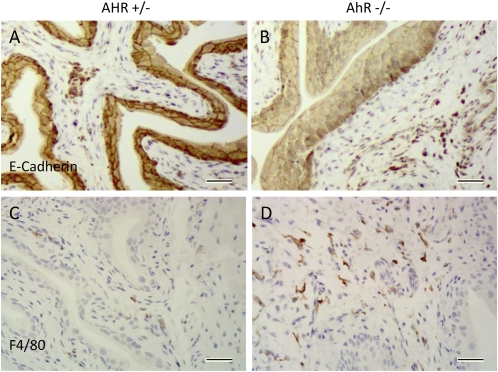
E-cadherin, an important constituent of the epithelial adherens junction, was lower in the AhR−/− bladders with almost complete absence in some disorganized areas of epithelium (Fig. 7). There was a marked increase in the number of macrophages (positive staining for the macrophage marker F4/80) in the stroma of the AhR−/− mouse bladders (Fig. 7). Because macrophages can degrade purines and secrete uric acid, these cells may be the source of uric acid in the urine of the knockout mice.
Fig. 7.
E-cadherin and F4/80 staining in 10-wk-old AhR−/− and AhR+/− mice. There is loss of E-cadherin in some areas of the AhR−/− urothelium (B), whereas the heterozygous mice appear to have normal E-cadherin expression throughout the bladder (A). There were many more cells that stained positive for the macrophage marker, F4/80, in the stroma of the knockout bladders (D) compared with the heterozygous controls (C). (Scale bars, 20 μm.)
Discussion
In this study we have described a unique phenotype of the aryl hydrocarbon receptor knockout mouse, i.e., uric acid stones in the urinary bladder. In most human diseases that involve elevated urinary uric acid, such as gout and Lesch-Nyhan disease, the serum uric acid is also elevated (16, 17). The AhR knockout mice in our study had elevated uric acid in the urine but normal levels of serum uric acid, setting this model apart from any human diseases. Unlike humans, mice have an enzyme, urate oxidase (uricase), which catalyzes the conversion of uric acid to allantoin. Because of the presence of uricase in the liver, mice excrete very low levels of uric acid in their urine and do not suffer from uric acid-related diseases. Uricase knockout mice have elevated serum uric acid, stones in the kidney, and a very high mortality rate (18), none of which occurs in the AhR knockout mice. Because there is no uricase expressed in humans, loss of AhR in humans would be expected to lead to a much more severe disease with elevated levels of uric acid in the circulation and in joints. For this reason, defective AhR signaling should be considered as a risk factor for development of gout.
In the AhR knockout mice, there were no detectable abnormalities in the purine degradation pathway during which uric acid is formed. There were normal protein and RNA levels of uricase and other members of this pathway in the knockout mice (Fig. S1). There were also no significant changes in the urine volume, osmolality, or concentrations of any other solutes measured (Fig. 2), which rules out the involvement of water transporters concentrating the urine. No significant changes in the mRNA levels of several urate transporters in the kidney were found (Fig. S2). These results led us to the conclusion that there was a problem with the urinary bladder itself in these mice.
AhR is known to regulate several enzymes involved in the metabolism of toxic substances including Cyp1A1, Cyp1B1, and GST (19). The cytochrome P450 1A1 and 1B1 enzymes are capable of converting polycyclic aromatic hydrocarbons into their most carcinogenic forms and AhR knockout mice are less susceptible to the cancer-causing effects of some of these substances (20). Some of the GST enzymes, however, are known to detoxify many harmful substances, which can end up in the bladder, and a characteristic of the GSTM1-null genotype is bladder carcinogenesis (21). Because levels of GST are much reduced in the AhR knockout mice, it is possible that more unmetabolized toxins are entering the bladder, leading to bladder cancer. The urothelial layer of the AhR−/− bladders contains more cells, have more disorganized areas with decreased expression of E-cadherin, and in some cases, there is invasion of the muscle layers by epithelial cells (Fig. 4).
Tumor lysis syndrome in humans is a situation when a large amount of cellular components from the dying cells in a tumor are released into the bloodstream, causing hyperuricemia (22). There have been cases of tumor lysis, which led to large amounts of uric acid in the bladder, causing stone formation (23). In the AhR−/− mice, the urate seemed to accumulate as granules on the luminal side of the urothelial cells, many of which appeared to be undergoing necrosis or apoptosis. Because the granules present in the urothelium stained positive for a uric acid marker and were degraded with uricase (Fig. 5), we believe uric acid is being secreted from the bladder into the urine by these structures. There is a possibility that the process ongoing in the AhR−/− bladder is similar to tumor lysis syndrome in that the large number of cells in the stroma and urothelium may be releasing their components into the bladder. If uric acid produced in the bladder entered the bloodstream, it would be detoxified by uricase in the liver. This would explain why urate levels in the serum are not elevated.
In human beings there is a rare disorder, keratinizing squamous metaplasia, in which the epithelial cell layer of the bladder grows and the stromal layer becomes thicker (24). This disorder can be caused by irritation of urinary tract by objects such as catheters, stones or infections but may also occur due to genetic factors (24). There have also been studies showing that foreign bodies introduced in animal bladders can cause cancer-like phenotypes of the urothelium (25). At present, we are unsure of the exact cause of the elevated uric acid in AhR knockout mouse bladders. We do not know to what extent the pathological changes are due to uric acid itself and what the contribution is of irritation caused by the presence of stones. The macrophages in the stromal and muscle layers of the AhR−/− bladders may have been recruited to the bladder to clear away cells damaged by toxins in the urine. Macrophages engulf cell debris and can degrade DNA to produce and secrete uric acid (26). It is possible that the large numbers of macrophages in these bladders are secreting uric acid, which ends up in the urine. The presence of large stones in the bladder is a further irritant leading to more inflammation and a worsening of pathological changes. If cells in the urinary bladder are dying because of exposure to toxins, and the elevated uric acid is due to degradation of these cells, the process is ongoing in very young mice because bladder stones were detectable when mice were 10 wk of age.
In conclusion, we have demonstrated that the AhR knockout mice developed in the Fujii-Kuriyama laboratory have markedly elevated uric acid in the urine and develop urate stones in their bladders. The bladders of the AhR knockout mice had an enlarged submucosal area, numerous round particles in the urothelium, and increased macrophage infiltration. In some older mice, there was invasion of the epithelium into the stromal and muscle compartment, indicating the development of malignant changes. Thus, the absence of AhR confers a predisposition for bladder toxicity without exposure of mice to any known carcinogen. Other AhR−/− mouse strains remain to be studied with regard to this bladder phenotype and it would be valuable to see whether the phenotype is consistent across all strains. Although we are not certain about the mechanism for this phenomenon, we have described a unique connection between AhR and uric acid production with possible associations to human diseases such as gout.
Materials and Methods
Animals.
AhR-deficient mice were generated in the laboratory of Fujii-Kuriyama by using a homologous recombination as described in Shimizu (2000) (27). Male AhR−/− mice were backcrossed to C57BL/6J AhR+/+ females to give rise to heterozygotes. The AhR+/− mice were interbred to yield AhR+/+, AhR+/−, and AhR−/− mice. Among 100 offspring obtained from heterozygous matings, the relative frequencies of AhR+/+, AhR+/−, and AhR−/− mice were ∼1:2:1, as expected from Mendelian law. Mice were shipped to the Huddinge animal facility at the Karolinska Institute and to the animal facility at the University of Houston and cleansed into the system. Heterozygous littermates were used as a control when wild-type mice were not available (AhR+/− mice are phenotypically similar to wild-type mice). Urine chemistry measurements were performed at Taconic and stone composition measurements were performed at the Urolithiasis Laboratory (Methodist Hospital, Houston, TX).
Examination of Mice for Gross Morphological Changes.
Mice between the ages of 3 mo and 1.5 y of age were asphyxiated with carbon dioxide. Internal organs were examined. Urinary bladders were removed, placed in 4% buffered paraformaldehyde overnight, and thereafter switched to 70% ethanol, dehydrated, paraffin embedded, and 5-μm sections were cut for histological examination. Kidneys and livers were removed and frozen in liquid nitrogen before storage at −80° C.
Immunohistochemistry.
Paraffin-embedded sections were dewaxed in xylene followed by rehydration in graded concentrations of ethanol. Antigen retrieval was achieved by placing slides in 97° C citrate buffer (pH 6.0) for 5–10 min. Endogenous peroxidase was quenched by a 30-min incubation in 1% H2O2 in 50% methanol followed by blocking of unspecific protein binding with 3% BSA for 10 min. Sections were incubated with rabbit anti–E-cadherin (1:200; Santa Cruz Biotechnology) and rat F4/80 (1:50; BD Pharmingen) in 3% BSA with PBS, 0.1% Nonidet P-40 at room temperature overnight. Corresponding HRP polymer solution (Biocare Medical) was added for 30 min at room temperature. The slides were developed using the DAB method (Dako) and counterstained with hematoxylin. TUNEL staining was done using the in situ cell death detection kit, Fluorescein (Roche). Masson's trichrome staining was performed according to the Electron Microscopy Sciences manufacturer's protocol. Hematoxylin and eosin staining was performed by incubating sections for 5 s in eosin solution and 1 min in Mayer's hematoxylin solution (Sigma-Aldrich). Grocott's methenamine silver stain was conducted according to the manufacturer's protocol (Richard-Allan Scientific). Microscopic sections were analyzed using Cell Sense Dimension software. Representative histological pictures were taken and the sample size for each age group was: 10-wk-old mice, n = 3; 4-mo-old mice, n = 4; and 6-mo-old mice, n = 6.
Uricase Treatment.
A fresh 4-mo-old AhR−/− bladder was cut in half and one part was placed in 1% hydrogen peroxide in PBS and the other part was placed in 1% hydrogen peroxide with 25.6 mmol/L of uricase in PBS (Sigma-Aldrich) for 1 h. The tissues were then fixed, processed, and stained for hematoxylin/eosin as previously described.
Real-Time PCR.
RNA was extracted from homogenized kidney and liver tissues from six, 6-mo-old wild-type and AhR−/− mice (n = 6) using the Qiagen RNeasy mini kit followed by cDNA synthesis using Invitrogen's SuperScript II RT method. Real-time qPCR was performed using Sybr Green for detection with an Applied Biosystems 7500 fast qPCR machine and each sample was plated in triplicate. Primers used in this study are given in Table S1.
Supplementary Material
Acknowledgments
This study was supported by grants from the Swedish Cancer Society, Robert A. Welch Foundation, and the Emerging Technology Fund of Texas.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120581109/-/DCSupplemental.
References
- 1.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 2.Sugihara K, et al. Aryl hydrocarbon receptor (AhR)-mediated induction of xanthine oxidase/xanthine dehydrogenase activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem Biophys Res Commun. 2001;281:1093–1099. doi: 10.1006/bbrc.2001.4464. [DOI] [PubMed] [Google Scholar]
- 3.Lin PH, Lin CH, Huang CC, Chuang MC, Lin P. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces oxidative stress, DNA strand breaks, and poly(ADP-ribose) polymerase-1 activation in human breast carcinoma cell lines. Toxicol Lett. 2007;172:146–158. doi: 10.1016/j.toxlet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Muralidhara Matsumura F, Blankenship A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-induced reduction of adenosine deaminase activity in vivo and in vitro. J Biochem Toxicol. 1994;9:249–259. doi: 10.1002/jbt.2570090505. [DOI] [PubMed] [Google Scholar]
- 5.Moriwaki Y, Yamamoto T, Higashino K. Enzymes involved in purine metabolism—a review of histochemical localization and functional implications. Histol Histopathol. 1999;14:1321–1340. doi: 10.14670/HH-14.1321. [DOI] [PubMed] [Google Scholar]
- 6.Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta. 2003;1619:263–268. doi: 10.1016/s0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140:173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- 8.Lahvis GP, et al. The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol Pharmacol. 2005;67:714–720. doi: 10.1124/mol.104.008888. [DOI] [PubMed] [Google Scholar]
- 9.Nakatsuru Y, et al. Dibenzo[A,L]pyrene-induced genotoxic and carcinogenic responses are dramatically suppressed in aryl hydrocarbon receptor-deficient mice. Int J Cancer. 2004;112:179–183. doi: 10.1002/ijc.20365. [DOI] [PubMed] [Google Scholar]
- 10.Lahvis GP, Bradfield CA. Ahr null alleles: Distinctive or different? Biochem Pharmacol. 1998;56:781–787. doi: 10.1016/s0006-2952(98)00134-8. [DOI] [PubMed] [Google Scholar]
- 11.Lin TM, et al. Effects of aryl hydrocarbon receptor null mutation and in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on prostate and seminal vesicle development in C57BL/6 mice. Toxicol Sci. 2002;68:479–487. doi: 10.1093/toxsci/68.2.479. [DOI] [PubMed] [Google Scholar]
- 12.Baba T, et al. Disruption of aryl hydrocarbon receptor (AhR) induces regression of the seminal vesicle in aged male mice. Sex Dev. 2008;2:1–11. doi: 10.1159/000117714. [DOI] [PubMed] [Google Scholar]
- 13.Esser C. The immune phenotype of AhR null mouse mutants: Not a simple mirror of xenobiotic receptor over-activation. Biochem Pharmacol. 2009;77:597–607. doi: 10.1016/j.bcp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol. 1997;34:605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Salguero P, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 16.Jinnah HA, et al. Lesch-Nyhan Disease International Study Group Attenuated variants of Lesch-Nyhan disease. Brain. 2010;133:671–689. doi: 10.1093/brain/awq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stark K, et al. Common polymorphisms influencing serum uric acid levels contribute to susceptibility to gout, but not to coronary artery disease. PLoS ONE. 2009;4:e7729. doi: 10.1371/journal.pone.0007729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X, et al. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci USA. 1994;91:742–746. doi: 10.1073/pnas.91.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowlands JC, Gustafsson J-A. Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol. 1997;27:109–134. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto Y, et al. Aryl hydrocarbon receptor plays a significant role in mediating airborne particulate-induced carcinogenesis in mice. Environ Sci Technol. 2007;41:3775–3780. doi: 10.1021/es062793g. [DOI] [PubMed] [Google Scholar]
- 21.Chico DE, Listowsky I. Diverse expression profiles of glutathione-S-transferase subunits in mammalian urinary bladders. Arch Biochem Biophys. 2005;435:56–64. doi: 10.1016/j.abb.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy LD, Ajiboye VO. Rasburicase for the prevention and treatment of hyperuricemia in tumor lysis syndrome. J Oncol Pharm Pract. 2010;16:205–213. doi: 10.1177/1078155209348719. [DOI] [PubMed] [Google Scholar]
- 23.Chubb EA, Maloney D, Farley-Hills E. Tumour lysis syndrome: An unusual presentation. Anaesthesia. 2010;65:1031–1033. doi: 10.1111/j.1365-2044.2010.06414.x. [DOI] [PubMed] [Google Scholar]
- 24.Chan T-S. Purine excretion by mouse peritoneal macrophages lacking adenosine deaminase activity. Proc Natl Acad Sci USA. 1979;76:925–929. doi: 10.1073/pnas.76.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmad I, Barnetson RJ, Krishna NS. Keratinizing squamous metaplasia of the bladder: A review. Urol Int. 2008;81:247–251. doi: 10.1159/000151398. [DOI] [PubMed] [Google Scholar]
- 26.Shirai T, Ikawa E, Fukushima S, Masui T, Ito N. Uracil-induced urolithiasis and the development of reversible papillomatosis in the urinary bladder of F344 rats. Cancer Res. 1986;46:2062–2067. [PubMed] [Google Scholar]
- 27.Shimizu Y, et al. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.