Abstract
For subunit vaccines, adjuvants play a key role in shaping immunological memory. Nanoparticle (NP) delivery systems for antigens and/or molecular danger signals are promising adjuvants capable of promoting both cellular and humoral immune responses, but in most cases the mechanisms of action of these materials are poorly understood. Here, we studied the immune response elicited by NPs composed of multilamellar “stapled” lipid vesicles carrying a recombinant Plasmodium vivax circumsporozoite antigen, VMP001, both entrapped in the aqueous core and anchored to the lipid bilayer surfaces. Immunization with these particles and monophosphoryl lipid A (MPLA), a US Food and Drug Administration–approved immunostimulatory agonist for Toll-like receptor-4, promoted high-titer, high-avidity antibody responses against VMP001, lasting more than 1 y in mice at 10-fold lower doses than conventional adjuvants. Compared to soluble VMP001 mixed with MPLA, VMP001-NPs promoted broader humoral responses, targeting multiple epitopes of the protein and a more balanced Th1/Th2 cytokine profile from antigen-specific T cells. To begin to understand the underlying mechanisms, we examined components of the B-cell response and found that NPs promoted robust germinal center (GC) formation at low doses of antigen where no GC induction occurred with soluble protein immunization, and that GCs nucleated near depots of NPs accumulating in the draining lymph nodes over time. In parallel, NP vaccination enhanced the expansion of antigen-specific follicular helper T cells (Tfh), compared to vaccinations with soluble VMP001 or alum. Thus, NP vaccines may be a promising strategy to enhance the durability, breadth, and potency of humoral immunity by enhancing key elements of the B-cell response.
Outside of sub-Saharan Africa, Plasmodium vivax is the most frequent cause of recurring malaria and infects 130–390 million people each year, representing approximately 50% of all malaria cases globally (1). Although not as virulent as Plasmodium falciparum, P. vivax has a long dormant liver stage, lasting months in some cases, and poses a significant threat to the global health (2). The circumsporozoite protein (CSP) is the most prevalent protein in Plasmodium sporozoites (3) and has been the target of clinical vaccine trials for P. falciparum (4). To date, however, there have been limited attempts to advance vaccines for P. vivax. We recently developed VMP001, a recombinant protein antigen composed of CSP core sequences derived from two widespread isolates of P. vivax (5, 6). Although VMP001 mixed with either alum or Montanide elicits antigen-specific antibody responses (5, 6), adjuvants capable of eliciting high-affinity antibodies against protective regions within CSP, which may lead to opsonization of sporozoites (7) or inhibition of their entry into hepatocytes (8), are of great interest.
Recently, antigens and adjuvants delivered by synthetic nanoparticles (NPs) have emerged as promising vaccine formulations. NPs may allow co-delivery of antigen and immunostimulatory molecules to the same intracellular compartment in antigen-presenting cells (APCs) (9) and promote cross-presentation of antigens, enhancing CD8+ T-cell expansion and functionality (10–12). NP delivery of antigens and adjuvant molecules such as Toll-like receptor (TLR) agonists also has been shown to promote humoral immune responses (13, 14). However, a greater understanding of the mechanisms by which synthetic particles enhance immunity will be critical to maximize the potential of NP vaccine strategies.
We recently reported the design of a unique class of lipid-based NPs, interbilayer-crosslinked multilamellar vesicles (ICMVs), possessing many favorable characteristics for vaccine delivery (10). ICMVs, synthesized by forming covalent cross-links across lipid layers within multilayered lipid vesicles, are stable in the extracellular milieu following injection and retain entrapped proteins until the particles are internalized into intracellular compartments, thereby increasing the delivery of antigen to APCs in draining lymph nodes (dLNs). Immunization with ICMVs generated strong humoral and cellular immune responses to the model antigen ovalbumin (OVA) (10). In these studies, OVA was entrapped in the aqueous core of ICMVs. However, because cross-linking of B-cell receptors (BCRs) and B-cell stimulation are facilitated by structurally repetitive antigens, such as in viral and bacterial membranes (15, 16), we hypothesized that multivalent display of antigen on the surfaces of ICMVs would enhance the humoral response.
Here, we tested the efficacy of ICMVs for delivery of the VMP001 malaria antigen, and exploited available terminal cysteine groups in the protein to both encapsulate VMP001 in the core of the NPs and anchor a fraction of the protein to the lipid membranes of the vesicle walls, creating VMP001-ICMVs. Vaccination with VMP001-ICMVs dramatically enhanced both the quantitative strength of the antibody response and qualitative breadth and isotype bias relative to the conventional adjuvants, such as MPLA, alum, or Montanide. ICMV vaccination also promoted robust GC formation with the majority of GCs nucleated adjacent to depots of ICMVs accumulating in dLNs. Furthermore, ICMVs induced antigen-specific Tfh cells, surpassing the levels induced by 10-fold greater doses of antigen adsorbed to alum. Thus, NP vaccines have the capacity to enhance the breadth, avidity, and durability of humoral responses by promoting multiple key stages of antibody development.
Results
Design of ICMV NPs Combining Membrane Display and Encapsulation of Soluble Antigen.
The synthesis of antigen-loaded ICMVs proceeds in three steps: formation of anionic maleimide (MAL)-functionalized vesicles by hydration of lipid films in the presence of antigen, fusion of vesicles via divalent cations, and finally “stapling” of the vesicles by addition of membrane-permeable dithiols that cross-link lipid headgroups bilayer-to-bilayer (Fig. S1) (10). We previously showed that immunization with the TLR4 agonist MPLA and ICMVs entrapping OVA in their aqueous core provided substantially enhanced antibody responses against the protein, compared to soluble protein/MPLA vaccinations. To determine whether the effectiveness of ICMVs could be further enhanced by combining aqueous encapsulation with anchoring of a fraction of the antigen to the membranes of the particles, we exploited the MAL groups in ICMV precursor vesicles as sites for both cross-linking of the bilayers and for conjugation to free cysteines of the malaria antigen VMP001 (Fig. S1 and Fig. 1A). We thus formed ICMVs in the presence of VMP001 containing cysteines at the N and C termini. In a typical synthesis, a total of 45 ± 8.2 μg protein was incorporated in ICMVs per mg of lipids with 50 ± 9.1% loading efficiency. PEGylated VMP001-loaded ICMVs (VMP001-ICMVs) had hydrodynamic diameters of 180 ± 14 nm and a relatively narrow size distribution with polydispersity index of 0.29 ± 0.021.
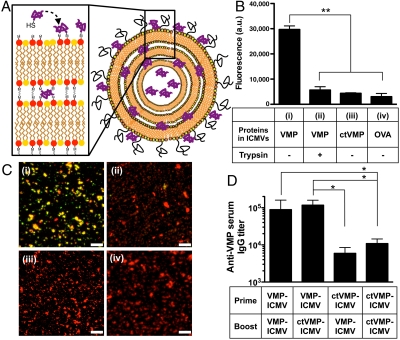
Fig. 1.
ICMVs with surface-conjugated VMP001 induce potent humoral immune responses. (A) Schematic illustration of VMP001-loaded ICMVs: VMP001 is surface-displayed on ICMVs via coupling of cysteine residues with MAL-functionalized lipids. (B and C) (i) VMP001 bound on membranes of ICMVs was detected by staining with anti-his-tag Ab (recognizing the C terminus of the antigen) followed by a labeled secondary Ab; bound Abs (green) on particles (lipids, red) was detected by (B) a fluorescence plate reader or (C) confocal microscopy (colocalization of Ab with lipids appears yellow). Controls included (ii) ICMVs treated with trypsin to digest surface-exposed antigens, or loaded with capped-thiol VMP001 [(iii) ctVMP001] or (iv) OVA. (D) C57Bl/6 mice were immunized with either VMP001-ICMVs or ctVMP001-ICMVs in different combinations (prime day 0, boost day 21; 1 μg VMP and 0.1 μg MPLA). Anti-VMP001 IgG sera titers were measured on day 50 by ELISA. Scale bars, 10 μm. Data represent mean ± SEM of two to three independent experiments conducted with n = 3.
To assess whether VMP001 was linked to the membranes of ICMV particles, we probed for surface-accessible VMP001 bound to the NPs using antibodies (Abs) against the C-terminal his-tag of the VMP001 protein. Particles incubated with fluorophore-conjugated anti-his-tag Abs showed Ab binding to VMP001-ICMVs, but not to OVA-ICMVs or VMP001-ICMVs treated with trypsin to digest surface-bound protein (Fig. 1B). Blockade of MAL-mediated coupling by capping the free thiols of VMP001 with ethyl maleimide before particle synthesis (ctVMP001-ICMVs) did not affect protein loading efficiency significantly (44 ± 8.5%, p = 0.45), but eliminated anti-his-tag Ab binding, suggesting that VMP001 display on the particle surfaces was due to conjugation with MAL lipid headgroups rather than by surface adsorption. In agreement with these bulk measurements, confocal micrographs of fluorescently tagged VMP001-ICMVs showed lipid colocalization with anti-his-tag Ab staining, which was absent for OVA-ICMVs, trypsin-treated VMP001-ICMVs, or ctVMP001-ICMVs (Fig. 1C).
To test the impact of membrane conjugation on the immunogenicity of ICMVs, mice were immunized with VMP001-ICMVs, where antigen was both membrane-conjugated and encapsulated, or with ctVMP001-ICMVs, where antigen was only encapsulated in a soluble state in the particle interior. Mice in each group were primed and then boosted on day 21 with a total of 1 μg VMP001 and 0.1 μg MPLA. Measurement of resulting serum VMP001-specific IgG titers showed that membrane display of the antigen clearly increased the potency of ICMV vaccination, as homologous VMP001-ICMV immunization elicited IgG titers approximately 9-fold greater than homologous ctVMP001-ICMV immunization (p < 0.05, Fig. 1D). Heterologous immunizations with VMP001-ICMVs and ctVMP001-ICMVs revealed that vaccination with antigen bound on particle membranes during the prime rather than boost was more critical to elicit high IgG titers (Fig. 1D).
VMP001-ICMV Vaccination Induces Durable Antibody Responses at 10-Fold Lower Doses of Antigen than Soluble Protein with Traditional Adjuvants.
Immunization of C57Bl/6 mice with VMP001-ICMVs mixed with MPLA as a molecular adjuvant elicited durable, high titers of serum anti-VMP001 IgG, sustained for more than 1 y following a prime and boost with as little as 100 ng of the malaria antigen (Fig. 2A). In contrast, vaccines composed of soluble VMP001 mixed with MPLA or adjuvanted with Montanide or alum required at least 10-fold more protein to elicit a response, and exhibited waning titers over time (Fig. 2A). VMP001-ICMV + MPLA vaccination induced antigen-specific IgG1 and IgG2c isotype responses, while soluble protein + MPLA elicited only Th2-skewed IgG1 antibodies (Fig. 2 B and C). Priming of a more balanced Th1/Th2 antibody response correlated with the enhanced production of IFN-γ and TNF-α by splenocytes from NP-immunized mice, compared to soluble protein vaccination (Fig. 2D). VMP001-ICMVs incubated with sera from naïve, VMP001-ICMV-, or OVA-ICMV-immunized mice had detectable binding only to VMP001-ICMV sera, indicating minimal cross-reactivity of elicited IgG to nonprotein components of ICMVs; i.e., maleimide, lipids, or PEG (Fig. S2). Notably, VMP001-ICMVs reconstituted in saline from lyophilized powders maintained their original particle diameter and size distribution (diameter of 200 nm ± 11, polydispersity index of 0.23 ± 0.05), and elicited similar humoral responses, suggesting that freeze-dried materials could be used to enhance the storage life of ICMV vaccines (Fig. S3).
Fig. 2.
VMP001-ICMV vaccines elicit robust, durable antibody titers with significantly reduced antigen/adjuvant doses. C57Bl/6 mice were immunized s.c. on day 0 and day 21 with the indicated doses of VMP001 in ICMVs mixed with 25 μg MPLA, or as soluble proteins mixed with either 25 μg MPLA, Montanide ISA-50, or alum. (A) Anti-VMP001 IgG sera titers were assessed over time by ELISA. Anti-VMP001 IgG sera were further characterized on day 90 for (B) IgG1 and (C) IgG2c titers (n.d., not detected). (D) Splenocytes isolated 7 d after priming and boosting with 1 μg VMP001 and 0.1 μg MPLA were stimulated with PBS (white bars) or 2 μg/mL VMP001-ICMVs (black bars) ex vivo, and the cell media were analyzed on day 2 for the concentrations of cytokines. (E) Mice were immunized with titrated amounts of MPLA mixed with 1 μg of VMP001 in either soluble or ICMV formulations. Shown are anti-VMP001 IgG sera titers measured by ELISA on d 50. Data represent the mean ± SEM of two independent experiments with n = 3–4 per group.
Enhanced Immunogenicity of NP-Formulated Antigen Enables Dose Sparing of MPLA.
Strategies to reduce the dose of potent immunostimulatory adjuvants such as MPLA or other TLR agonists are attractive, to lower the risk of reactogenicity or other side effects that could hamper the safety of vaccine candidates. To determine whether the enhanced immunogenicity of ICMVs would permit dose sparing of MPLA, we titrated down the dose of MPLA with a fixed dose of VMP001 antigen (1 μg) in soluble or ICMV form. Strikingly, the peak IgG response was comparable for 25 μg or 1 μg MPLA given with VMP001-ICMVs, and MPLA doses as low as 100 ng elicited IgG titers comparable to a 250-fold greater dose of MPLA given with soluble protein (Fig. 2E).
NP Delivery Increases the Breadth and Avidity of the Humoral Responses.
Several studies thus far have suggested that a humoral response against the Type I repeat in P. vivax CSP, specifically toward the AGDR motif within the VK210 sequence, may confer protective immunity against sporozoites (17–19). Thus, to evaluate the quality of the antibody response raised against VMP001, we assessed the avidity and epitope specificity of sera from immunized animals. ICMV vaccinations elicited IgG responses with up to 4.3-fold higher avidity than soluble protein immunization (VMP001-ICMV vs. VMP001 at 1 μg, p < 0.05) (Fig. 3A). Sera from mice immunized with VMP001-ICMVs bound the Type I repeat sequence with significantly higher titers, and explicitly recognized the AGDR motif, while sera from soluble protein immunizations did not recognize this fragment (Fig. 3B). ICMV vaccination also generated antibodies capable of recognizing the Region I domain, which may inhibit sporozoite invasion into hepatocytes by blocking receptor-ligand interactions during parasite entry (8). In contrast, the C-terminal fragment of VMP001 and the Region II domain, which share sequence homology to endogenous thrombospondin (20), were not recognized by antibodies elicited with either vaccine, suggesting lack of activation of self-reactive B cells. Taken together, these results suggest that NP vaccination generates antibody responses that are more durable and have higher avidity than those elicited by traditional adjuvants even using 10-fold less antigen, and elicit broader humoral responses with the capacity to recognize the sporozoite domains thought to be critical in protective immunity against infection.
Fig. 3.
VMP001-ICMV immunization elicits antibodies with high avidity and broader specificity than soluble protein vaccination. C57Bl/6 mice were immunized with VMP001-ICMVs or soluble VMP001 (25 μg MPLA with 0.1 or 1 μg protein) as in Fig. 2 and avidity and specificity of sera were analyzed on day 90. (A) Avidity index of sera from immunized mice binding to whole VMP001 protein. (B) Anti-VMP001 IgG antibodies elicited with VMP001-ICMVs + MPLA (blue) or VMP001 + MPLA (red) were further analyzed for binding to fragments of VMP001, including the Type I insert, AGDRx5, Region I, Region II, C terminus, or a scrambled peptide negative control. Data represent the mean ± SEM of two independent experiments conducted with n = 3.
Enhanced Antigen Delivery and Germinal Center Formation Triggered by ICMVs.
Durable and high-affinity humoral responses elicited by ICMVs suggest that NP vaccines may effectively stimulate formation of GCs, where activated B cells proliferate, undergo immunoglobulin isotype-switching and somatic hypermutation, and eventually form memory B cells that can rapidly differentiate into plasma cells on re-exposure to antigen (21, 22). To investigate GC induction and its anatomic relationship with NP delivery to dLNs, we immunized mice with fluorophore-conjugated OVA (soluble or encapsulated in fluorescent ICMVs) and MPLA, and carried out histological analyses of dLNs at serial time points. Soluble OVA was detected in the dLNs within 4 h, but was rapidly cleared within 24 h (Fig. 4A). In contrast, OVA-loaded ICMVs were detected at the subcapsular sinus (SCS) of dLNs by 24 h, with continued accumulation over the next 2 wk, depositing a large amount of antigen beneath the SCS (Fig. 4A). Flow cytometry and histological analysis of OVA+ cells on 1 or 4 d post NP injection showed that the major APC population acquiring particles were LN-resident macrophages, although OVA+ CD11b+CD11c+ DCs were also detected (Fig. S4), suggesting that both free draining and cell-mediated transport of particles to the dLN contributed at both time points.
Fig. 4.
ICMV immunization sustains antigen deposition in dLNs and induces germinal center (GC) formation. (A and B) C57Bl/6 mice were immunized with 100 μg fluorophore-conjugated OVA (shown in red) either in soluble or ICMV (shown in blue) formulations with 5 μg MPLA, and dLNs excised at indicated time points were examined by immunohistochemical analysis. (A) Representative confocal sections of dLNs; pink signals indicate colocalized OVA/ICMV lipids. Day 14 sections were stained with anti-GL-7 to identify GCs. (B) Enumeration of GCs located within 100 μm of ICMV-draining sites in dLNs. (C–G) C57Bl/6 mice were immunized with 1 μg VMP001 and 0.1 μg MPLA in either soluble or ICMV formulations, and dLNs were analyzed for GC formation on day 14. Shown are representative flow cytometry scatter plots of (C) isotype-switched GC B cells (GL-7+PNA+), gated on B220+IgDlow populations, and (D) their absolute numbers in dLNs. (E) Number of GCs observed in sections from whole dLNs (n = 4), cryosectioned and stained with anti-B220 and anti-GL-7. (F and G) Representative confocal micrographs of dLNs from immunized animals. Scale bars, 200 μm. Data represent the mean ± SEM of two to three independent experiments conducted with n = 2–3.
OVA-ICMV immunizations elicited prominent GC formation, and notably, in the majority of cases, GCs were nucleated within 100 μm of ICMV-draining sites in dLNs (Fig. 4 A and B). Immunization with VMP001-ICMVs also induced GCs; compared with soluble VMP001+MPLA immunizations, NPs induced a significantly enhanced frequency and 8-fold increase in the absolute number of isotype class-switched GC B cells (B220+IgDlowGL-7+PNA+) in dLNs by day 14 (p < 0.05, Fig. 4 C and D). Histological analysis revealed 2–5 GCs per dLN in mice immunized with 100 ng VMP001-ICMV+MPLA, in contrast to their complete absence in the VMP001+MPLA immunization groups (Fig. 4 E and F). Notably, as observed with OVA, VMP001-ICMVs promoted GCs in close proximity to the particle-draining sites, with approximately 75% of GCs observed directly adjacent to NP deposits (Fig. 4 B and G).
ICMV Vaccination Enhances the Generation of Antigen-Specific Tfh Cells.
Strong humoral immune responses, characterized by GC formation and long-lived plasma and memory B cells, are dependent on help provided by CD4+ Tfh cells (23, 24). To determine whether ICMVs amplify the humoral response in part by enhanced CD4+ T-cell differentiation, we turned to a model system to trace antigen-specific T helper responses: TCR-transgenic CD45.2+ OT-II CD4+ T cells recognizing OVA peptides were adoptively transferred into CD45.1+ recipient mice that were subsequently immunized with 1 μg MPLA mixed with 10 μg soluble OVA or OVA-ICMVs. As an additional comparison, mice were also immunized with the traditional adjuvant alum and a 10-fold greater dose of OVA (100 μg). Eight days post priming, soluble protein immunization induced 6.3-fold expansion of OT-II CD4+ T cells in spleens compared to mice treated with PBS after adoptive transfer; in contrast, OVA-ICMV vaccination induced a 21-fold expansion of the transferred cells, compared with PBS controls (p < 0.05, Fig. 5A). Alum also induced robust expansion of OVA-specific T cells (23-fold increase, compared to PBS, p < 0.05). Notably, OVA-ICMV vaccination promoted differentiation of OT-II CD4+ T cells toward Tfh phenotypes (CXCR5+PD-1+), leading to a substantially increased frequency of antigen-specific Tfh cells, compared to the other immunization regimens (Fig. 5 B and C). Taken together, these results suggest that the enhanced humoral responses elicited by ICMVs compared to soluble vaccines or other traditional adjuvants are a product of enhanced GC formation and increased expansion/differentiation of antigen-specific CD4+ T cells toward Tfh phenotypes.
Fig. 5.
ICMV immunization enhances follicular helper T-cell expansion. (A–C) One day after adoptive transfer of 105 CD45.2+CD4+OT-II T cells i.v. into naïve CD45.1+ C57Bl/6 mice, recipient mice were immunized s.c. with 100 μg OVA in alum or 10 μg OVA and 1 μg MPLA in either soluble or ICMV formulations. (A) On day 8 after immunization, CD45.2+CD4+OT-II T cells were enumerated from spleens and dLNs. (B and C) CD45.2+CD4+OT-II T cells from spleens were further analyzed for expression of Tfh markers (CXCR5+PD-1+). (B) Representative flow cytometry scatter plots, gated on CD45.2+CD4+OT-II T cells in spleens, are shown with the percentages of CXCR5+PD-1+ populations, and (C) the percentage of OT-II cells with Tfh phenotypes were enumerated. Data represent mean ± SEM of two independent experiments conducted with n = 3.
Discussion
Strategies to enhance the efficacy of recombinant protein subunit vaccines without sacrificing safety are of great interest, due to the weaker magnitude and durability of immune responses elicited by subunit vaccines relative to more potent live attenuated or recombinant vectors. This is particularly relevant in the context of malaria, where short-lived immunity has been observed in the field trials of candidate subunit vaccines (4, 25). Analogous to the development of recombinant live viruses or bacteria as vectors to deliver nucleic acid-encoded antigens, synthetic NPs are emerging as promising vectors for the delivery of protein subunit antigens. Like live vectors, NPs can serve multiple roles in vaccination, carrying antigen into target tissue sites, controlling the antigen uptake by immune cells, and co-delivering “danger” signals that provide critical cues for the development of early effectors and late memory (10–14). Here using ICMVs as a lipid-based NP vector, we assessed the impact of NP delivery on immune responses elicited by a candidate P. vivax subunit vaccine, and particularly focused on defining qualitative and quantitative aspects of the humoral response and biological pathways contributing to this response.
We first optimized antigen incorporation into ICMVs with the goal of promoting the antibody response. Our original NP design was based on the entrapment of soluble antigen in the core of these multilamellar “stapled” lipid vesicles (10). Although T-cell responses rely on recognition of antigen fragments following intracellular antigen processing by APCs, antibody responses are ultimately linked to B-cell recognition of intact antigen via cell surface BCRs. Therefore, we hypothesized that particles with antigens anchored to their surfaces, mimicking the multivalent display of epitopes on the surfaces of pathogens, may facilitate cross-linking of BCRs and enhance the activation of B cells for stronger humoral immune responses, compared to soluble bolus injection of antigens (15, 16). To achieve such antigen surface-display, we exploited available cysteine residues in VMP001 and utilized maleimide groups in ICMV precursor vesicles for the dual purpose of both “stapling” the vesicle walls together in the presence of cross-linker and anchoring a fraction of the antigen to the vesicle membranes. Notably, we found that ICMVs incorporating VMP001 by both entrapment in the core and anchoring to the membranes enhanced the antigen-specific antibody response by 9-fold relative to NPs that contained protein only in the particle core (Fig. 1D).
VMP001-ICMVs adjuvanted with MPLA elicited strong antibody titers, surpassing the response induced by soluble VMP001 injected with MPLA or the conventional adjuvants alum or Montanide, and maintaining high titers for more than 1 y in mice. ICMV vaccines required 10-fold and 250-fold less antigen and MPLA, respectively, to produce similar antibody titers as soluble formulations. In addition to these quantitative effects on the antibody response, VMP001-ICMV immunization also changed the qualitative nature of the antibody response, promoting a more balanced Th1/Th2 response (both in terms of T-cell cytokine production and antibody isotypes) and broadening the antibody targets within VMP001, including epitopes implicated in protection against Plasmodium (5). We previously reported that immunization with Freund’s adjuvant, a very strong but toxic adjuvant for small-animal experimental immunization, could elicit responses against multiple epitopes within VMP001 (5). Here we have made the important advance of achieving broad antibody responses using clinically relevant adjuvant materials. Previous studies using recombinant proteins or multipeptide constructs to target multiple vivax CSP regions showed evidence of immunodominance with some peptide combinations, with antibody responses focused on only a single epitope (19) or responses that failed to bind the critical protective AGDR sequence (26). Although the precise mechanism underlying NP-mediated broadening of the antibody response is the subject of further studies beyond the scope of this first report, we speculate that multivalent display of VMP001 on ICMVs may stimulate a more diverse set of naïve B cells, by allowing some lower-avidity B cells to compete for the antigen.
In order to gain a deeper mechanistic understanding of how NP-mediated delivery of antigen generates high-affinity, long-lasting antibody responses, we examined antigen distribution in dLNs following ICMV immunizations and subsequent B- and T-cell differentiation. Whereas soluble OVA injected s.c. drained to dLNs and was rapidly cleared within 24 h, OVA-loaded ICMVs drained to the SCS in dLNs after 24 h and accumulated over the next 2 wk (Fig. 4A). The ability of particles in the 200–300 nm size range to drain to LNs is consistent with recent work characterizing the transport of similarly sized synthetic or viral particles to LNs (27, 28), and other studies have reported that synthetic particles can continue to drain from s.c. injection sites to dLNs for at least 8 d (29). GC formation tended to occur directly adjacent to NP accumulation sites, suggesting that increased deposition/retention of antigen drives B-cell responses locally in the tissue. Indeed, we have shown previously that ICMV vaccines effectively increased antigen delivery to dLNs, enhancing antigen presentation by DCs, compared with soluble protein immunizations (10). Prolonged antigen presentation mediated by ICMV vaccines may have also contributed to enhanced development of CD4+ Tfh cells (Fig. 5 B and C) (24), which provide critical cytokines and signals required to initiate somatic hypermutation and affinity maturation for B cells (23).
In summary, ICMV lipid NP vectors enhanced a range of quantitative and qualitative features of the immune response to the VMP001 CSP antigen, suggesting their utility in recombinant protein subunit vaccination. Enhanced humoral responses correlated with enhanced GC formation and induction of antigen-specific Tfh cells. Here we focused on vaccination using a single TLR agonist, MPLA, and future studies will be needed to determine if other clinically relevant molecular adjuvants combined with the current NP vaccine may allow further amplification of humoral immune responses in a synergistic manner, thus enhancing the protective efficacy of the NP vaccine.
Materials and Methods
Synthesis of ICMVs Loaded with VMP001.
VMP001 was prepared as previously described (5, 6). Synthesis of ICMVs was performed as described previously with slight modifications (10). Briefly, dried films of 1.26 μmol of lipids [DOPC∶DOPG∶MPB (1,2-dioleoyl-sn-glycero-3-phosphoethanol amine-N-[4-(p- maleimidophenyl) butyramide) at 4∶1∶5 mol ratio (Avanti Polar Lipids)] were rehydrated in 20 mM bis-tris propane at pH 7.0 with 50 μg VMP001 or ovalbumin (Worthington) for 1 h with vortexing every 10 min, and sonicated in alternating power cycles of 6 W and 3 W in 30 s intervals for 5 min on ice (Misonix Microson XL probe tip sonicator). DTT and Ca2+ were then sequentially added at final concentrations of 3 mM and 40 mM, respectively, and incubated for 1 h at 37 °C to form ICMVs. The particles were recovered by centrifugation, washed twice, and incubated with 10 mg/mL 2 kDa PEG-thiol (Laysan Bio) for 30 min at 37 °C. For some experiments, capped-thiol VMP001 (ctVMP001) was prepared by incubating 0.5 mg VMP001 with 3.6 mM TCEP for 2 h at 25 °C, followed by incubation with 40 mM ethyl-maleimide (Pierce) at 37 °C for 2 h. The extent of thiol protection was measured to be > 98% by Ellman’s assay. Characterization of ICMVs is described in SI Materials and Methods.
Immunizations.
Animals were cared for following National Institutes of Health, state, and local guidelines. Groups of 6- to 10-wk-old female C57Bl/6 mice (Jackson Laboratories) were immunized s.c. at the tail base with VMP001-ICMVs and indicated doses of MPLA (Sigma-Aldrich) in 100 μL PBS on day 0 and 21. Control immunizations with soluble VMP001 were performed using MPLA, Montanide ISA-50 V2 (Seppic), or alum (Imject alum, Pierce) at an adjuvant∶protein solution volume ratio of 1∶1 following the manufacturer’s instructions. Characterization of humoral responses, germinal center formation, and follicular helper T-cell analysis is described in SI Materials and Methods.
Statistical Analysis.
Datasets were analyzed using one- or two-way analysis of variance (ANOVA), followed by Tukey’s honestly significant difference (HSD) test for multiple comparisons with Prism 5.0 (GraphPad Software). P values less than 0.05 were considered statistically significant, and marked with one asterisk. P values less than 0.01 were marked with two asterisks. All values are reported as mean ± SEM.
Supplementary Material
Acknowledgments.
This work was supported in part by the Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard University; the Gates Foundation; the Department of Defense (Contract W911NF-07-D-0004); and National Institutes of Health (AI095109 and 1U19AI091693). D.J.I. is an investigator of the Howard Hughes Medical Institute. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.A.S. is a guest editor invited by the Editorial Board.
See Commentary on page 999.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112648109/-/DCSupplemental.
References
- 1.Price RN, et al. Vivax malaria: Neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller I, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 3.Nussenzweig V, Nussenzweig RS. Circumsporozoite proteins of malaria parasites. Cell. 1985;42:401–403. doi: 10.1016/0092-8674(85)90093-5. [DOI] [PubMed] [Google Scholar]
- 4.Ballou WR. The development of the RTS,S malaria vaccine candidate: Challenges and lessons. Parasite Immunol. 2009;31:492–500. doi: 10.1111/j.1365-3024.2009.01143.x. [DOI] [PubMed] [Google Scholar]
- 5.Yadava A, et al. A novel chimeric Plasmodium vivax circumsporozoite protein induces biologically functional antibodies that recognize both VK210 and VK247 sporozoites. Infect Immun. 2007;75:1177–1185. doi: 10.1128/IAI.01667-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell BA, et al. Process development for the production of an E.coli produced clinical grade recombinant malaria vaccine for Plasmodium vivax. Vaccine. 2009;27:1448–1453. doi: 10.1016/j.vaccine.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Schwenk R, et al. Opsonization by antigen-specific antibodies as a mechanism of protective immunity induced by Plasmodium falciparum circumsporozoite protein-based vaccine. Parasite Immunol. 2003;25:17–25. doi: 10.1046/j.1365-3024.2003.00495.x. [DOI] [PubMed] [Google Scholar]
- 8.Ancsin JB, Kisilevsky R. A binding site for highly sulfated heparan sulfate is identified in the N terminus of the circumsporozoite protein: Significance for malarial sporozoite attachment to hepatocytes. J Biol Chem. 2004;279:21824–21832. doi: 10.1074/jbc.M401979200. [DOI] [PubMed] [Google Scholar]
- 9.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 10.Moon JJ, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy ST, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 12.Heit A, Schmitz F, Haas T, Busch DH, Wagner H. Antigen co-encapsulated with adjuvants efficiently drive protective T cell immunity. Eur J Immunol. 2007;37:2063–2074. doi: 10.1002/eji.200737169. [DOI] [PubMed] [Google Scholar]
- 13.Kasturi SP, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demento SL, et al. TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis. J Immunol. 2010;185:2989–2997. doi: 10.4049/jimmunol.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jegerlehner A, et al. Regulation of IgG antibody responses by epitope density and CD21-mediated costimulation. Eur J Immunol. 2002;32:3305–3314. doi: 10.1002/1521-4141(200211)32:11<3305::AID-IMMU3305>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Chen YH. High epitope density in a single protein molecule significantly enhances antigenicity as well as immunogenicity: A novel strategy for modern vaccine development and a preliminary investigation about B cell discrimination of monomeric proteins. Eur J Immunol. 2005;35:505–514. doi: 10.1002/eji.200425749. [DOI] [PubMed] [Google Scholar]
- 17.Jones TR, et al. Low immunogenicity of a Plasmodium vivax circumsporozoite protein epitope bound by a protective monoclonal antibody. Am J Trop Med Hyg. 1992;47:837–843. doi: 10.4269/ajtmh.1992.47.837. [DOI] [PubMed] [Google Scholar]
- 18.Wirtz RA, et al. Evaluation of monoclonal antibodies against Plasmodium vivax sporozoites for ELISA development. Med Vet Entomol. 1991;5:17–22. doi: 10.1111/j.1365-2915.1991.tb00515.x. [DOI] [PubMed] [Google Scholar]
- 19.Udhayakumar V, et al. Immunogenicity of Plasmodium falciparum and Plasmodium vivax circumsporozoite protein repeat multiple antigen constructs (MAC) Vaccine. 1998;16:982–988. doi: 10.1016/s0264-410x(97)00290-9. [DOI] [PubMed] [Google Scholar]
- 20.Bilsborough J, Baumgart K, Bathurst I, Barr P, Good MF. Fine epitope specificity of antibodies to region II of the Plasmodium vivax circumsporozoite protein correlates with ability to bind recombinant protein and sporozoites. Acta Trop. 1997;65:59–80. doi: 10.1016/s0001-706x(97)00648-7. [DOI] [PubMed] [Google Scholar]
- 21.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 22.Nutt SL, Tarlinton DM. Germinal center B and follicular helper T cells: Siblings, cousins or just good friends? Nat Immunol. 2011;131:472–477. doi: 10.1038/ni.2019. [DOI] [PubMed] [Google Scholar]
- 23.Breitfeld D, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deenick EK, et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bojang KA, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: A randomised trial. Lancet. 2001;358:1927–1934. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 26.Charoenvit Y, et al. Inability of malaria vaccine to induce antibodies to a protective epitope within its sequence. Science. 1991;251:668–671. doi: 10.1126/science.1704150. [DOI] [PubMed] [Google Scholar]
- 27.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Junt T, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 29.Manolova V, et al. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 30.Yue Y, Xu W, Hu L, Jiang Z, Xiong S. Enhanced resistance to coxsackievirus B3-induced myocarditis by intranasal co-immunization of lymphotactin gene encapsulated in chitosan particle. Virology. 2009;386:438–447. doi: 10.1016/j.virol.2009.01.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.