Abstract
Obligate intracellular pathogens such as Leishmania specifically target host phagocytes for survival and replication. Phosphoinositide 3-kinase γ (PI3Kγ), a member of the class I PI3Ks that is highly expressed by leukocytes, controls cell migration by initiating actin polymerization and cytoskeletal reorganization, which are processes also critical for phagocytosis. In this study, we demonstrate that class IB PI3K, PI3Kγ, plays a critical role in pathogenesis of chronic cutaneous leishmaniasis caused by L. mexicana. Using the isoform-selective PI3Kγ inhibitor, AS-605240 and PI3Kγ gene-deficient mice, we show that selective blockade or deficiency of PI3Kγ significantly enhances resistance against L. mexicana that is associated with a significant suppression of parasite entry into phagocytes and reduction in recruitment of host phagocytes as well as regulatory T cells to the site of infection. Furthermore, we demonstrate that AS-605240 is as effective as the standard antileishmanial drug sodium stibogluconate in treatment of cutaneous leishmaniasis caused by L. mexicana. These findings reveal a unique role for PI3Kγ in Leishmania invasion and establishment of chronic infection, and demonstrate that therapeutic targeting of host pathways involved in establishment of infection may be a viable strategy for treating infections caused by obligate intracellular pathogens such as Leishmania.
Entry into host cells is essential for the survival and replication of obligate intracellular pathogens. Leishmania pathogens are obligate intracellular protozoan parasites that infect macrophages and neutrophils (1). Although macrophages are the principle effector cells involved in eliminating Leishmania, they are also the target cells used by the parasites for their survival and replication within the host. Neutrophils have been suggested to act as a “Trojan horse” in establishing Leishmania infection (2) by internalizing parasites via mechanisms that fail to induce antileishmanial host responses (3, 4). Furthermore, a recent study by Peters et al. (5) has shown that neutrophils harbor viable parasites during early stages of infection with Leishmania major and facilitate establishment of chronic infection by protecting parasites from extracellular destruction. To this effect, therapeutic targeting of pathways that mediate parasite entry into host cells could be a viable strategy for treating infections caused by Leishmania and possibly other obligate intracellular pathogens that target phagocytes.
The PI3Ks are a large family of enzymes that phosphorylate phosphoinositol-containing lipids (6). Activation of PI3Ks results in the generation of phosphatidylinositol-3,4,5-triphosphate [PtdIns(3,4,5)P3], an important intermediate involved in intracellular signal transduction (6). PI3Kγ is a class IB PI3K predominantly expressed by immune cells and consists of a catalytic subunit (p110γ) and a regulatory subunit (p101 or p84). PI3Kγ mediates signaling initiated primarily through G-protein coupled receptors (6) and plays a critical role in chemoattractant- induced cell migration by controlling actin cytoskeletal rearrangement (6–9).
Activation of PI3Kγ results in the generation of PtdIns(3,4,5)P3 and the activation of Akt (6). PtdIns(3,4,5)P3 cooperates with Gβγ subunits to initiate actin polymerization and subsequent F-actin accumulation induced by PI3Kδ (6). Neutrophils from PI3Kγ−/− mice display impaired activation of Rac and reduced F-actin accumulation at the leading edge, which correlate with their reduced ability to migrate in response to chemotactic stimuli (10, 11). Studies using PI3K inhibitors, such as wortmannin or LY294002, show that type I PI3Ks are involved in phagocytosis (12–15) and mediate the entry of parasites, such as Trypanosoma cruzi, into host cells (16–18). The precise role of each isoform, however, remains unclear. A study by Gagnon et al. (19) has suggested that endoplasmic reticulum (ER)-mediated phagocytosis is a possible mechanism of entry of Leishmania into macrophages and that inhibition of PI3K activity using 3-methyladenine and wortmannin markedly suppresses ER-mediated uptake of latex beads into macrophages in vitro. Taken together, these findings led us to hypothesize that by initiating actin polymerization and cytoskeletal rearrangement, PI3Kγ may contribute to establishment of chronic Leishmania infection by recruiting macrophages and/or neutrophils to the site of infection and by facilitating uptake of parasites into these cells.
In this study, we examined the role of PI3Kγ in the development of chronic cutaneous leishmaniasis (CL) caused by Leishmania mexicana and determined whether this enzyme might be a potential therapeutic target for the treatment of this disease. Our results demonstrate that PI3Kγ-mediated pathways play a critical role in establishment of chronic L. mexicana infection by mediating the recruitment of phagocytes and regulatory T cells (Tregs) to the site of infection and by facilitating entry of parasites into phagocytes. Most importantly, we provide “proof-of-concept” that targeting the host pathway contributing to establishment of chronic infection could be a therapeutically viable option for treating infections caused by obligate intracellular pathogens like Leishmania.
Results
Blockade of PI3Kγ Activity Reduces Uptake of Leishmania Parasites by Phagocytes in Vitro.
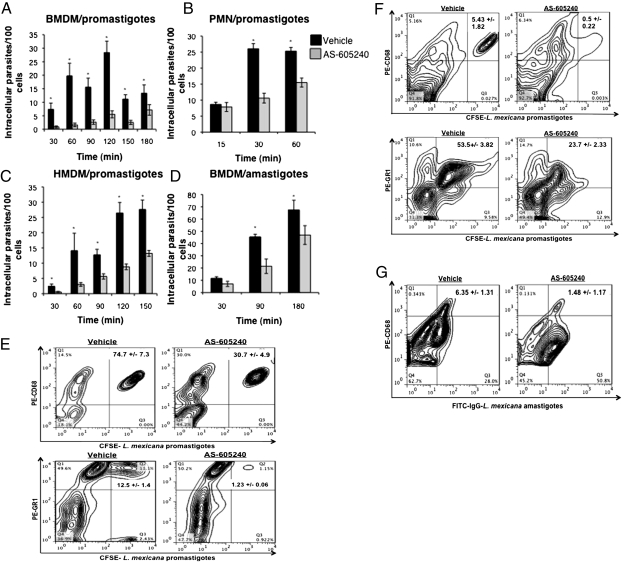
Because PI3Kγ has been implicated in cytoskeletal reorganization, we hypothesized that this enzyme may play a role in mediating entry of Leishmania into host leukocytes, and therefore establishment of chronic infection. To test this hypothesis, we examined the effect of PI3Kγ blockade on parasite uptake by mouse macrophages and neutrophils, as well as human macrophages, in vitro using AS-605240, a small-molecule isoform-selective inhibitor of PI3Kγ. AS-605240 effectively competes with ATP for its binding pocket on the enzyme, rendering the kinase inactive (20). We found that AS-605240 significantly reduced the uptake of L. mexicana promastigotes into mouse bone marrow-derived macrophages (BMDMs) (Fig. 1A) and neutrophils (PMNs) (Fig. 1B), as well as human monocyte-derived macrophages (HMDMs) (Fig. 1C). AS-605240 was also effective in inhibiting uptake of L. mexicana amastigotes into mouse BMDMs (Fig. 1D) The inhibitory effect of AS-605240 on parasite entry into BMDMs and neutrophils was further confirmed using flow cytometry (Fig. 1E).
Fig. 1.
Blockade of PI3Kγ activity significantly reduces the entry of L. mexicana parasites into macrophages and neutrophils in vitro and in vivo. Quantification of intracellular promastigotes in BMDMs (A), PMNs (B), and HMDMs (C) by fluorescence microscopy. (D) Quantification of intracellular axenic amastigotes in BMDMs by fluorescence microscopy. Cells were treated with AS-605240 (1.25 M) or saline before infection with CFSE-labeled parasites. At least 1,000 cells were enumerated for each time point. Data are expressed as the mean number of intracellular parasites per 100 cells ± SE from two independent experiments. *P < 0.05 as determined by an unpaired Student's t test. (E) Quantification of intracellular parasites in BMDMs and PMNs by flow cytometry. Mice were infected by inoculating 4 × 106 CFSE-labeled promastigotes (F) or IgG-coated axenic amastigotes (G) into air pouches in the presence or absence of AS-605240 (15 mg/kg, administered i.p.). Cells were obtained by lavage and stained for macrophage and neutrophils using antibodies against the cell surface markers CD68 and GR1, respectively. Data are representative of results from one of three independent experiments with similar results.
The inhibitory effect of AS-605240 on phagocytosis by macrophages was not specific for the uptake of Leishmania parasites alone, because C57BL/6 WT primary BMDMs (Fig. S1A), as well as macrophage cell line (ANA-1) (Fig. S1B), treated with AS-605240 and incubated with collagen-coated fluorobeads showed nearly a 50% reduction in the number of intracellular beads compared with saline-treated controls. Similarly, using macrophage cell lines generated from bone marrow of neonatal C57BL/6 WT and PI3Kγ−/− mice (Fig. S1C), as well as primary BMDMs from C57BL/6 WT and PI3Kγ−/− mice (Fig. S1 D and E), we further confirmed that PI3Kγ is involved in mediating parasite entry into macrophages in vitro, because L. mexicana-infected macrophages derived from PI3Kγ−/− mice contained significantly fewer intracellular parasites compared with PI3Kγ+/+ WT-derived macrophages.
AS-605240 Significantly Reduces Entry of L. mexicana into Neutrophils and Macrophages in Vivo.
To investigate the effect of PI3Kγ blockade on parasite uptake in vivo, dorsal s.c. air pouches were created on WT C57BL/6 mice as described previously (21, 22). These pouches were injected with LPS or thioglycollate to recruit neutrophils and macrophages, respectively, followed by subsequent injection of fluorescent L. mexicana promastigotes in the presence of AS-605240 or saline vehicle. C57BL/6 mice injected with AS-605240 contained fewer parasitized macrophages and neutrophils in their dorsal pouches compared with saline controls (Fig. 1F). Similar decreases in parasitized macrophages were also observed in macrophages infected with axenic L. mexicana amastigotes (Fig. S2), as well as IgG-opsonized axenic amastigotes, with the latter representing the physiologically relevant form of amastigotes present in the mammalian host (Fig. 1G). The effect of AS-605240 on amastigote uptake by neutrophils was not investigated, because neutrophils are known to interact primarily with Leishmania promastigotes in vivo only during the early phase of infection after sand fly bite (5).
The phagocytic receptors complement receptor (CR)3 and Fc gamma receptor (FcγR) have been implicated as the major receptors used by Leishmania parasites to gain entry into host macrophages. We therefore determined whether the reduced uptake of Leishmania parasites into phagocytes observed both in vitro and in vivo could be attributed to decreased expression of phagocytic receptors (CD11b, CD18, and CD16/32) on AS-605240–treated cells. We found that AS-605240 treatment did not alter the level of these receptors expressed by macrophages in vitro (Fig. S3). Taken together, these data demonstrate that suppression of parasite phagocytosis into AS-605240–treated macrophages was not attributable to reduction in levels of CR3 and FcγR.
Treatment with AS-605240 Significantly Reduces Lesion Growth and Parasite Loads in L. mexicana-Infected C57BL/6 Mice.
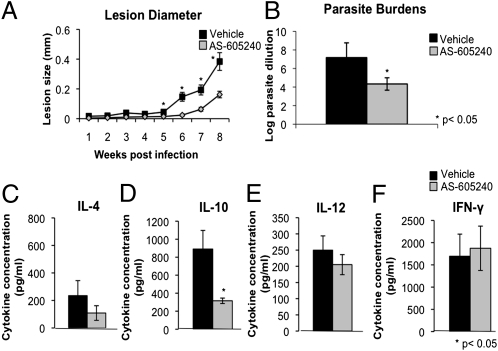
Because AS-605240 treatment displayed no cytotoxic effects against host cells in vitro (Fig. S4) and effectively reduced entry of Leishmania into macrophages and neutrophils both in vitro and in vivo, we investigated whether AS-605240 could be used as a therapeutic agent to treat CL. L. mexicana-infected mice treated with AS-605240 failed to develop lesions, or developed significantly smaller lesions containing significantly fewer parasites, compared with saline-treated controls (Fig. 2 A and B).
Fig. 2.
Effect of PI3Kγ inhibition on disease progression and pathogenesis of cutaneous L. mexicana infection. Disease progression was monitored by measuring the thickness of infected ears using a dial-gauge micrometer at weekly intervals. (A) Treatment with AS-605240 (15 mg⋅kg⋅d, administered i.p.) or saline began 2 wk postinfection and ended at week 6 postinfection. Data are expressed as the increase in the thickness of infected ears compared with uninfected ears. Data shown are from three independent experiments (n = 15 mice per group) and are presented as the mean lesion size ± SE. *P < 0.05 as determined by an unpaired Student's t test. (B) Parasite loads at week 8 postinfection as determined by limiting dilution analysis. Cell filtrates from infected ears were serially diluted across 96-well plates in duplicate and incubated at 26 °C for 7–14 d, when the greatest dilution yielding viable parasites was recorded for each ear. Data are presented as the mean log parasite dilution ± SE from three independent experiments (n = 15 mice per group). *P < 0.05 as determined by a Mann–Whitney U prime test. Quantification of Th1/Th2 cytokine production was done by draining lymph node cells from infected mice as determined by ELISA. Cells were restimulated ex vivo with LmAg (20 μg/mL) for 72 h and analyzed for production of IL-4 (C), IL-10 (D), IL-12 (E), and IFN-γ (F). Data are expressed as the mean cytokine level (pg/mL) ± SE from three independent experiments (n = 9–15 mice per group) with similar results. *P < 0.05 as determined by an unpaired Student's t test.
The ear lesions from AS-605240–treated mice contained fewer inflammatory cells and parasitized macrophages compared with the lesions of control mice, which showed ulceration and necrosis, as well as an increased inflammatory infiltrate composed of heavily parasitized macrophages, neutrophils, and eosinophils (Figs. S5 and S6). L. mexicana-infected mice treated with AS-605240 contained significantly fewer macrophages (1.09 ± 0.2 × 103 F4/80+ cells) within their lesions compared with control mice treated only with saline (5.83 ± 0.11 × 103 F4/80+ cells; P < 0.05). This is perhaps not surprising, because PI3Kγ is well known for mediating intracellular signaling involved in initiating actin polymerization and cytoskeletal reorganization in response to inflammatory stimuli. This impairment in recruitment of inflammatory cells to the site of infection likely contributes, in part, to the protective phenotype observed in mice treated with AS-605240 to inhibit PI3Kγ activity in vivo. These findings are promising and suggest that therapeutic use of PI3Kγ isoform-selective kinase inhibitors may be a viable strategy for treating Leishmania infection and possibly other intracellular pathogens of phagocytes.
Draining Lymph Node Cells from AS-605240–Treated Mice Show Impaired Production of T Helper 2- but Not T Helper 1-Associated Cytokines.
The T helper (Th) 2-associated cytokines IL-4 and IL-10 mediate susceptibility to L. mexicana infection by suppressing protective Th1 response (1, 23–25). We therefore examined the effect of AS-605240 treatment on Th1/Th2 cytokine responses. Eight weeks postinfection, lymph node cells from AS-605240–treated and control mice were stimulated with L. mexicana antigen (LmAg) in vitro, and IFN-γ, IL-12p70, IL-10, and IL-4 levels were determined by ELISA. LmAg-stimulated lymph node cells from AS-605240–treated mice produced less Th2- associated IL-4 and IL-10 than saline controls; however, the levels of Th1-associated IL-12 and IFN-γ were comparable between both groups (Fig. 2 C–F). We believe that suppression of Th2 cytokine production is an indirect effect of AS-605240 on T cells, because AS-605240–treated naive T cells from C57BL/6 WT mice, as well as T cells from PI3Kγ−/− mice, did not show reduced production of Th2 cytokines IL-4 and IL-10 after in vitro stimulation with anti- CD3/anti-CD28 antibodies (Fig. S7). Taken together, these findings demonstrate that AS-605240 treatment is associated with a reduction in Th2 cytokine production in L. mexicana-infected mice, which could contribute to control of parasite replication observed in these mice.
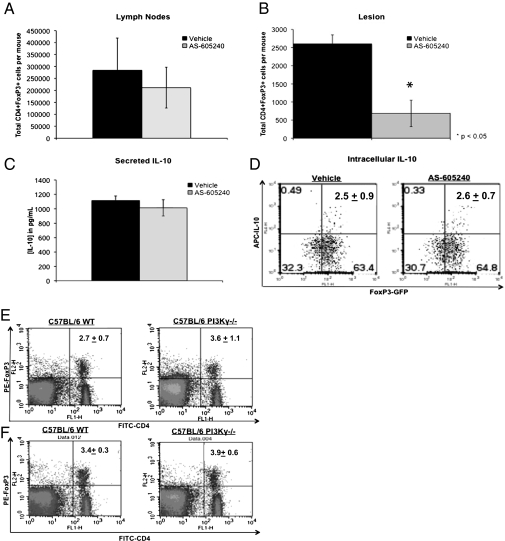
A previous study by Belkaid et al. (26) has shown that CD4+ CD25+ Tregs play a critical role in pathogenesis of chronic L. major infection by suppressing activity of CD4+ effector cells in an IL-10–dependent and –independent manner. Recently, Liu et al. (27) reported that PI3Kδ, a class IA PI3K that regulates actin polymerization together with PI3Kγ, controls susceptibility to L. major by regulating expansion and tissue homing of Tregs. We therefore examined the effect of PI3Kγ blockade on recruitment and IL-10 production by CD4+ FoxP3+ Tregs in L. mexicana-infected C57BL/6 FoxP3-EGFP knock-in mice (Fig. 3).
Fig. 3.
Blockade of PI3Kγ reduces recruitment of CD4+ FoxP3+ Tregs into the lesion but has no effect on Treg function or development. Quantification of CD4+ FoxP3+ Tregs within the lymph nodes (A) and the lesions (B) of vehicle- and AS-605240–treated mice. At 9 wk postinfection, infected mice were killed and CD4+ FoxP3+ cell populations within the lesions and draining lymph nodes were analyzed by flow cytometry. The data shown are the average numbers of CD4+ FoxP3+ Tregs per mouse from each treatment group and represent collective data from three independent experiments with similar results. *P < 0.05 as determined by an unpaired Student's t test. (C and D) Quantification of IL-10 production by GFP+ cells isolated from the spleens of naive FoxP3-EGFP knock-in C57BL/6 mice. Cells were stimulated in vitro with plate-bound anti-CD3/anti-CD28 antibodies in the presence of AS-605240 or vehicle, and IL-10 levels were determined by ELISA (C) and intracellular cytokine (ICC)-flow cytometry (D). ELISA data are expressed as the mean cytokine level (pg/mL) ± SE from three independent experiments with similar results. Data shown for ICC-flow cytometry are from one representative experiment of two with similar results. The effect of PI3Kγ deficiency on Treg populations in C57BL/6 mice is shown. Cells were obtained from the lymph nodes (E) and spleens (F) of naive C57BL/6 WT and PI3Kγ−/− mice and stained using anti-CD4 (FITC) and anti-FoxP3 phycoerythrin (PE) antibodies. C57BL/6 WT and PI3Kγ−/− mice contain comparable proportions of CD4+ FoxP3+ Tregs within their secondary lymphoid organs. Data are presented as the percentage of CD4+ FoxP3+ cell populations ± SE and represent results from one of three independent experiments (n = 3 mice) with similar results.
AS-605240 treatment significantly reduced recruitment of CD4+ FoxP3+ Tregs to the infected lesions but had no effect on Treg populations within the draining lymph nodes of these mice (Fig. 3 A and B). Furthermore, AS-605240 had no significant effect on IL-10 production by CD4+ FoxP3+ Treg cells in vitro (Fig. 3 C and D). Naive C57BL/6 WT and PI3Kγ−/− mice were found to contain comparable proportions of CD4+ FoxP3+ Tregs within their lymph nodes and spleens, suggesting that class IB PI3Kγ is not involved in the generation of these cells in vivo (Fig. 3 E and F). Collectively, these data suggest that PI3Kγ does not regulate development, expansion, or IL-10 production by Tregs but, instead, may contribute to the pathogenesis of L. mexicana infection by mediating trafficking of these cells to the site of infection. This is perhaps not surprising in light of a recent study by Anderson et al. (28) reporting that CD4+ CD25− Foxp3− Th1 cells are the source of IL-10–mediated immune suppression in chronic CL.
Treatment with AS-605240 Is as Effective as Sodium Stibogluconate in Reducing Parasite Growth in L. mexicana-Infected C57BL/6 Mice.
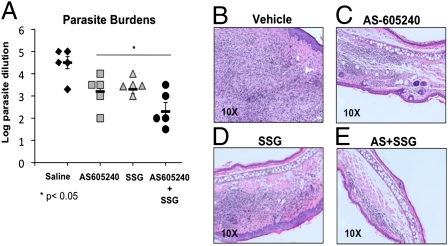
Because AS-605240 treatment was found to limit disease progression and reduce disease-associated pathology in mice, we compared the therapeutic efficacy of AS-605240 with that of the standard antileishmanial drug sodium stibogluconate (SSG). Over a 7-wk period, L. mexicana-infected C57BL/6 mice were monitored for the development of visible ulcerating cutaneous lesions at the site of infection. Beginning at week 7 postinfection, infected mice received twice-daily treatment with AS-605240 (15 mg/kg), SSG (20 mg/kg), or saline vehicle for a period of 2 wk. On cessation of treatment (9 wk postinfection), infected mice were euthanized and parasite burdens were determined by limiting dilution analysis. As was expected 9 wk postinfection, saline-treated control mice contained significant numbers of parasites within their lesions (Fig. 4A). In contrast, the infected ears of mice treated with AS-605240 or SSG contained significantly fewer parasites (nearly 2 logs less) than saline-treated controls (Fig. 4A). Most importantly, however, both AS-605240–treated and SSG-treated mice displayed comparable reductions in ear parasite burdens (Fig. 4A).
Fig. 4.
Efficacy of AS-605240 vs. SSG in treatment of L. mexicana infection. WT C57BL/6 mice were infected by inoculation of 1,000 L. mexicana stationary phase promastigotes into the ear dermis and allowed to develop visible lesions. Treatment with AS-605240 (15 mg/kg), SSG (20 mg/kg), or a combination of the two (AS-605240 + SSG) began 7 wk postinfection and continued for 14 d. (A) Parasite loads were determined at week 9 postinfection for each treatment group. Data are expressed as log parasite burdens ± SE compared with untreated (saline) controls. Data presented are collective of results from two independent experiments with similar results (n = 10 mice per group). Histopathological findings of infected lesions from vehicle- (B), AS-605240- (C), SSG- (D), and combination AS-605240– and SSG- (E) treated mice 10 wk postinfection; ear lesions from all groups were excised, fixed, cut, and stained by routine H&E staining. Data are representative of results from one of three independent experiments with similar results.
Histopathological analysis of ear lesions obtained from L. mexicana-infected C57BL/6 control mice revealed significant tissue inflammation and extensive inflammatory cell infiltration composed of heavily parasitized macrophages, neutrophils, and eosinophils (Fig. 4B). In contrast, AS-605240–treated mice exhibited a substantial reduction in tissue inflammation and harbored significantly fewer parasites within the infected lesions (Fig. 4C). The diminished disease-associated pathological findings observed in AS-605240–treated mice were comparable to those observed in mice receiving standard therapy with SSG (Fig. 4D). These results show that AS-605240 is as effective as SSG in reducing disease progression and limiting parasite growth in mice when administered late after disease onset.
Finally, we determined the efficacy of AS-605240 to limit disease progression when administered in conjunction with SSG. L. mexicana-infected mice receiving a combination therapy of AS-605240 and SSG developed smaller lesions and harbored fewer parasites within their lesions than mice treated with either AS-605240 or SSG alone (Fig. 4A). As might be expected, histopathological analysis of mice receiving a combination therapy of AS-605240 and SSG demonstrated a similar reduction in inflammatory cell infiltration and tissue inflammation at the site of infection compared with controls (Fig. 4E). Collectively, these findings suggest that a combination therapy using standard chemotherapy, in conjunction with PI3Kγ isoform-selective kinase inhibitors, could be more effective in the treatment of CL.
L. mexicana Parasites Fail to Establish Efficient Infection in p110γ Gene-Deficient C57BL/6 Mice.
To confirm the role of PI3Kγ in the pathogenesis of L. mexicana infection further, C57BL/6 mice lacking the p110γ catalytic subunit of the enzyme (PI3Kγ−/− mice) were infected by injecting 104 L. mexicana promastigotes into the ear dermis. Infection was monitored as described previously. L. mexicana-infected PI3Kγ−/− mice developed significantly smaller lesions than their WT counterparts (Fig. 5A) and harbored significantly lower parasite burdens (Fig. 5B). Lesion sizes and parasite burdens in PI3Kγ−/− mice were comparable to those observed in AS-605240–treated WT mice (Fig. 2 A and B). PI3Kγ−/− mice also recruited fewer cells to the site of infection and showed a 50–60% reduction in immune cells, including macrophages, neutrophils, and T cells. Furthermore, LmAg-stimulated lymph node cells from these mice also produced significantly less IL-10 compared with WT controls (1,416 ± 271 pg/mL and 416 ± 112 pg/mL in WT and PI3Kγ−/− mice, respectively; P < 0.05). No significant difference was observed in IL-4 production between the groups, although levels of IL-4 were slightly lower in culture supernatants from PI3Kγ−/− mice (125 ± 15 pg/mL and 82 ±17 pg/mL in WT and PI3Kγ−/− mice, respectively). These findings validate the role of PI3Kγ in the pathogenesis of cutaneous L. mexicana infection.
Fig. 5.
Effect of PI3Kγ deficiency on disease progression and pathogenesis of cutaneous L. mexicana infection. C57BL/6 mice genetically deficient of the p110γ subunit of PI3Kγ (PI3Kγ−/− mice) were infected by inoculating 104 metacyclic L. mexicana promastigotes into the left ear dermis. (A) Disease progression was monitored by measuring the thickness of infected ears using a dial-gauge micrometer at weekly intervals. Data are expressed as the increase in the thickness of infected ears compared with uninfected ears. Data shown are from three independent experiments (n = 15 mice per group) and are presented as mean lesion size ± SE. For lesion sizes, P < 0.05 by an unpaired Student's t test. (B) Parasite loads at weeks 3, 6, and 9 postinfection. Data are expressed as the mean log parasite dilution ± SE and are representative of three independent experiments with similar results. For determination of parasite burdens, *P < 0.05 by a Mann–Whitney U prime test.
Discussion
Our study reveals a unique role for PI3Kγ in mediating host cell invasion by Leishmania and provides “proof-of-principle” that targeting the intracellular kinase activity of PI3Kγ could be a therapeutically viable option for treating infections caused by obligate intracellular pathogens and for limiting infection-induced pathological changes by inhibiting the recruitment of disease-exacerbating immune cells to the site of infection.
Conventional approaches for treating infections caused by obligate intracellular pathogens such as Leishmania and Mycobacteria have focused on drugs that target unique molecules or pathways in the pathogen. Although such strategies have been effective, the emergence of drug-resistant pathogens to conventional therapeutics is becoming a significant problem in the treatment of leishmaniasis, as well as many other pathogens such as Mycobacterium tuberculosis and Salmonella (29, 30). Furthermore, the standard drugs that are used to treat leishmaniasis, such as SSG, are toxic and have poor patient compliance because of long treatment times ranging from 3 to 5 wk and the requirement for parenteral administration. Therefore, treating infectious diseases by targeting host pathways that are critical for pathogen invasion and survival has recently been considered as a viable option for developing new therapeutic agents against infectious diseases (31). Some studies have reported that targeting host molecules inhibits intracellular growth of bacteria and parasites such as Plasmodium in the host cell in vitro (32). However, the therapeutic efficacy of this approach in vivo has not been tested in the treatment of bacterial or parasitic diseases in vivo. Our findings in the present study demonstrate that in vivo targeting of host pathways essential for pathogen invasion could be effective in limiting infection and show that PI3Kγ signaling could be a potential drug target for the treatment of intracellular pathogens that require host hematopoietic cells for survival and replication.
A recent study by Liu et al. (27) has found that PI3Kδ also controls susceptibility to CL caused by L. major by regulating the expansion and tissue homing of the Tregs that are involved in pathogenesis (27). In the present study, we found that although PI3Kγ blockade/deficiency significantly reduced recruitment of Tregs to the lesion, it had no effect on development, expansion, or IL-10 production from these cells. Interestingly, Liu et al. (27) found that PI3Kδ was not involved in mediating uptake of parasites into host phagocytes. This is perhaps not surprising, because both PI3Kγ and PI3Kδ, which are primarily expressed in hematopoietic cells and endothelial cells, have been reported to have related yet nonredundant roles. Taken together, these findings indicate that both PI3Kγ and PI3Kδ mediate susceptibility to CL through multiple mechanisms, and could therefore be therapeutic targets for treatment of this infection. Dual PI3Kδ/PI3Kγ-deficient mice have been shown to have defects in T-cell development and thymocyte survival (33, 34); therefore, one concern for targeting of PI3Kγ and PI3Kδ, or both, in host cells could be that blockade of these enzymes could be toxic and lead to enhanced susceptibility of the host to other infections. Therefore, PI3Kδ/PI3Kγ inhibitors may need to be used in combination with conventional antimicrobial agents. Nonetheless, in the present study, we found that Leishmania antigen-stimulated lymph node cells from both control and AS-605240–treated mice produced comparable levels of IFN-γ, indicating that PI3Kγ blockade has no effect on induction of Th1-like responses. Furthermore, several PI3Kδ and PI3Kγ inhibitors, as well as dual-specificity PI3Kδ/PI3Kγ inhibitors, are currently in development for clinical use as antiinflammatory and antiproliferative agents (35), and have been found to be safe in phase I clinical trials.
In conclusion, our study shows that PI3Kγ mediates susceptibility CL caused by L. mexicana by mediating recruitment of Tregs and phagocytes into the lesions and by facilitating uptake of parasites in macrophages and neutrophils. In addition, our findings show that blockade of PI3Kγ activity in vivo using an isoform-selective small-molecule inhibitor is therapeutically as effective as the standard antileishmanial drug SSG in limiting disease progression and parasite growth. These findings suggest that dual-specificity PI3Kδ/PI3Kγ inhibitors or inhibitors with similar activity, such as AS-605240, could be therapeutic agents to treat infections caused by intracellular pathogens.
Materials and Methods
In Vitro Parasite Uptake Assay by Fluorescence Microscopy.
BMDMs, PMNs, or HMDMs (3.5 × 105 cells/mL) were cultured on 1.5-mm glass coverslips at 37 °C/5% (vol/vol) CO2 and allowed to adhere overnight. Adherent cells were treated with AS-605240 (1.25 μM) or 1× sterile saline (vehicle) 30 min before infection. Infected cells were incubated at 33 °C/5% (vol/vol) CO2. Cells were washed, fixed in 3% paraformaldehyde, stained, and mounted on glass coverslips (see above) for imaging. All uptake assays were performed at a ratio of 7:1 parasites/cells.
In Vivo Parasite Uptake Assay.
Dorsal s.c. air pouches were created on the shaven backs of C57BL/6 mice by injection with 3 mL of sterile air. These pouches subsequently received injections with either thioglycollate (2 mL) or LPS (20 μg/mL) to recruit macrophages or neutrophils, respectively. Ninety-six hours (for macrophage studies) or 3 h (for neutrophil studies) later, these pouches were infected with 2–5 × 106 carboxylfluorescein succinimidylester (CFSE)-labeled L. mexicana parasites in the presence of either AS-605240 (15 mg/kg) or vehicle for a period of 1–3 h. Cells were obtained from the pouches by lavage, washed, and stained for flow cytometric analysis of infected macrophage (CD68+/CFSE+) or neutrophil (GR1+/CFSE+) cell populations.
Supplementary Material
Acknowledgments
Research in the Satoskar laboratory is funded by grants from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110339109/-/DCSupplemental.
References
- 1.Alexander J, Satoskar AR, Russell DG. Leishmania species: Models of intracellular parasitism. J Cell Sci. 1999;112:2993–3002. doi: 10.1242/jcs.112.18.2993. [DOI] [PubMed] [Google Scholar]
- 2.Laskay T, van Zandbergen G, Solbach W. Neutrophil granulocytes—Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol. 2003;11:210–214. doi: 10.1016/s0966-842x(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 3.Laufs H, et al. Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infect Immun. 2002;70:826–835. doi: 10.1128/iai.70.2.826-835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Zandbergen G, et al. Cutting edge: Neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 5.Peters NC, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deane JA, Fruman DA. Phosphoinositide 3-kinase: Diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 7.Rückle T, Schwarz MK, Rommel C. PI3Kgamma inhibition: Towards an ‘aspirin of the 21st century’? Nat Rev Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- 8.Ferrandi C, et al. Phosphoinositide 3-kinase gamma inhibition plays a crucial role in early steps of inflammation by blocking neutrophil recruitment. J Pharmacol Exp Ther. 2007;322:923–930. doi: 10.1124/jpet.107.123026. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki T, Suzuki A, Sasaki J, Penninger JM. Phosphoinositide 3-kinases in immunity: Lessons from knockout mice. J Biochem. 2002;131:495–501. doi: 10.1093/oxfordjournals.jbchem.a003126. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, et al. Roles of PLC-β2 and -β3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 11.Hannigan M, et al. Neutrophils lacking phosphoinositide 3-kinase gamma show loss of directionality during N-formyl-Met-Leu-Phe-induced chemotaxis. Proc Natl Acad Sci USA. 2002;99:3603–3608. doi: 10.1073/pnas.052010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamen LA, Levinsohn J, Swanson JA. Differential association of phosphatidylinositol 3-kinase, SHIP-1, and PTEN with forming phagosomes. Mol Biol Cell. 2007;18:2463–2472. doi: 10.1091/mbc.E07-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leverrier Y, Ridley AJ. Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr Biol. 2001;11:195–199. doi: 10.1016/s0960-9822(01)00047-1. [DOI] [PubMed] [Google Scholar]
- 15.Pomel V, et al. Furan-2-ylmethylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3-kinase gamma. J Med Chem. 2006;49:3857–3871. doi: 10.1021/jm0601598. [DOI] [PubMed] [Google Scholar]
- 16.Wilkowsky SE, Barbieri MA, Stahl P, Isola EL. Trypanosoma cruzi: Phosphatidylinositol 3-kinase and protein kinase B activation is associated with parasite invasion. Exp Cell Res. 2001;264:211–218. doi: 10.1006/excr.2000.5123. [DOI] [PubMed] [Google Scholar]
- 17.Woolsey AM, et al. Novel PI 3-kinase-dependent mechanisms of trypanosome invasion and vacuole maturation. J Cell Sci. 2003;116:3611–3622. doi: 10.1242/jcs.00666. [DOI] [PubMed] [Google Scholar]
- 18.Todorov AG, Einicker-Lamas M, de Castro SL, Oliveira MM, Guilherme A. Activation of host cell phosphatidylinositol 3-kinases by Trypanosoma cruzi infection. J Biol Chem. 2000;275:32182–32186. doi: 10.1074/jbc.M909440199. [DOI] [PubMed] [Google Scholar]
- 19.Gagnon E, et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–131. doi: 10.1016/s0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- 20.Camps M, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 21.Edwards JC, Sedgwick AD, Willoughby DA. The formation of a structure with the features of synovial lining by subcutaneous injection of air: An in vivo tissue culture system. J Pathol. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- 22.Matte C, Olivier M. Leishmania-induced cellular recruitment during the early inflammatory response: Modulation of proinflammatory mediators. J Infect Dis. 2002;185:673–681. doi: 10.1086/339260. [DOI] [PubMed] [Google Scholar]
- 23.Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nat Immunol. 2002;3:1041–1047. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- 24.Sato N, et al. CC chemokine receptor (CCR)2 is required for langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, b cell outgrowth, and sustained neutrophilic inflammation. J Exp Med. 2000;192:205–218. doi: 10.1084/jem.192.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosas LE, et al. Genetic background influences immune responses and disease outcome of cutaneous L. mexicana infection in mice. Int Immunol. 2005;17:1347–1357. doi: 10.1093/intimm/dxh313. [DOI] [PubMed] [Google Scholar]
- 26.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 27.Liu D, et al. The p110delta isoform of phosphatidylinositol 3-kinase controls susceptibility to Leishmania major by regulating expansion and tissue homing of regulatory T cells. J Immunol. 2009;183:1921–1933. doi: 10.4049/jimmunol.0901099. [DOI] [PubMed] [Google Scholar]
- 28.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406:775–781. doi: 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
- 30.Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol. 2008;6:17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwegmann A, Brombacher F. Host-directed drug targeting of factors hijacked by pathogens. Sci Signal. 2008;1:re8. doi: 10.1126/scisignal.129re8. [DOI] [PubMed] [Google Scholar]
- 32.Lindenthal C, Weich N, Chia YS, Heussler V, Klinkert MQ. The proteasome inhibitor MLN-273 blocks exoerythrocytic and erythrocytic development of Plasmodium parasites. Parasitology. 2005;131:37–44. doi: 10.1017/s003118200500747x. [DOI] [PubMed] [Google Scholar]
- 33.Webb LM, Vigorito E, Wymann MP, Hirsch E, Turner M. Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol. 2005;175:2783–2787. doi: 10.4049/jimmunol.175.5.2783. [DOI] [PubMed] [Google Scholar]
- 34.Swat W, et al. Essential role of PI3Kdelta and PI3Kgamma in thymocyte survival. Blood. 2006;107:2415–2422. doi: 10.1182/blood-2005-08-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams O, et al. Discovery of dual inhibitors of the immune cell PI3Ks p110δ and p110γ: A prototype for new anti-inflammatory drugs. Chem Biol. 2010;17:123–134. doi: 10.1016/j.chembiol.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.