Abstract
Chronic hypoxia is an inciting factor for the development of pulmonary arterial hypertension. The mechanisms involved in the development of hypoxic pulmonary hypertension (HPH) include hypoxia-inducible factor 1 (HIF-1)–dependent transactivation of genes controlling pulmonary arterial smooth muscle cell (PASMC) intracellular calcium concentration ([Ca2+]i) and pH. Recently, digoxin was shown to inhibit HIF-1 transcriptional activity. In this study, we tested the hypothesis that digoxin could prevent and reverse the development of HPH. Mice were injected daily with saline or digoxin and exposed to room air or ambient hypoxia for 3 wk. Treatment with digoxin attenuated the development of right ventricle (RV) hypertrophy and prevented the pulmonary vascular remodeling and increases in PASMC [Ca2+]i, pH, and RV pressure that occur in mice exposed to chronic hypoxia. When started after pulmonary hypertension was established, digoxin attenuated the hypoxia-induced increases in RV pressure and PASMC pH and [Ca2+]i. These preclinical data support a role for HIF-1 inhibitors in the treatment of HPH.
Keywords: acriflavine, cardiac glycosides, pulmonary circulation
Prolonged exposure to hypoxia occurs in physiological and pathological contexts, such as during a high-altitude sojourn or as a result of chronic obstructive pulmonary disease, respectively. Chronic hypoxia (CH) triggers maladaptive responses in the lung vasculature, leading to the development of hypoxic pulmonary hypertension (HPH) (1). Typically, HPH results from arteriolar constriction followed by vascular wall remodeling, which includes both thickening of the wall due to smooth muscle cell (SMC) and fibroblast proliferation, as well as extension of SMCs into previously nonmuscular precapillary arterioles. Over time, HPH causes right ventricle hypertrophy (RVH), which can lead to right ventricular (RV) failure and death.
Although incompletely understood, some of the alterations in pulmonary arterial SMCs (PASMCs) that underlie the development of HPH have been delineated (2, 3). For example, in HPH, alterations in Ca2+ and pH homeostasis contribute to growth and contraction of PASMCs. Our previous work demonstrated that HPH is characterized by increased PASMC intracellular pH (pHi) due to increased activity and expression of Na+/H+ exchanger isoform 1 (NHE1) (4, 5), and elevated intracellular Ca2+ concentration ([Ca2+]i) (6) due to increased expression of canonical transient receptor potential (TRPC) proteins and enhanced Ca2+ entry through nonselective cation channels (7). Critical aspects of the pathogenesis of HPH, including both the increase in basal [Ca2+]i and alkalinization of PASMCs, are mediated by hypoxia-inducible factor 1 (HIF-1) (5, 7-9).
HIF-1 is a heterodimeric transcription factor composed of HIF-1α and HIF-1β subunits that regulates the expression of hundreds of genes in response to hypoxia, including many genes associated with HPH (10). HIF-1β is ubiquitously expressed, whereas HIF-1α expression is O2-regulated (11, 12). Under normoxic conditions, HIF-1α is hydroxylated on two proline residues by prolyl hydroxylase domain proteins, which use O2 as a substrate, marking the protein for ubiquitination and proteasomal degradation (13, 14). Under hypoxic conditions, HIF-1α accumulates and dimerizes with HIF-1β, allowing for the transcription of target genes. The ability to target HIF-1α would allow control of the expression of genes associated with the pathology of many diseases, including HPH.
Screening of the Johns Hopkins Drug Library, a collection of 3,120 clinically used compounds, revealed that all 11 cardiac glycosides tested inhibit HIF-1α, indicating a class effect (15). One member of this class, digoxin, inhibited HIF-1α protein translation and blocked HIF-1 activity in vivo (15, 16). Digoxin has been used for decades to treat heart failure on the basis of its inotropic potential, an effect that is most likely due to inhibition of the Na+/K+ ATPase. Given the central role of HIF-1 in the development of HPH, we hypothesized that digoxin would attenuate the pulmonary vascular effects of CH in a murine model of HPH.
Results
Effect of Digoxin on Body Weight (BW).
In the prevention protocol, mice were exposed to room air (21% O2; normoxia) or to 10% O2 (CH) for 3 wk to induce HPH while receiving daily injections of saline or digoxin at a dose of 1.0 mg/kg. Plasma digoxin levels measured 24 h after the final injection (Fig. S1A) were found to be at or below the therapeutic range for humans (0.5–2 ng/mL). BW increased in normoxic animals over 3 wk (Fig. S1B). All hypoxic mice experienced a sharp initial decrease in BW, but by day 5 mice receiving digoxin began to exhibit weight gain, eventually reaching 93.2% ± 2.4% of initial BW. Mice receiving saline began to gain weight by day 9–10, reaching 86.7% ± 1.3% of initial BW after 3 wk.
Effect of Digoxin on Hematocrit (Hct).
Prolonged exposure to hypoxia results in polycythemia via HIF-dependent induction of erythropoietin expression. In normoxic animals, Hct was similar in both saline- and digoxin-treated mice, with all values below 40% (Fig. S1C). In contrast, all animals exposed to CH experienced increased Hct levels, although values were significantly lower in hypoxic mice receiving digoxin.
Effect of Digoxin on RV Pressure and Heart Weight.
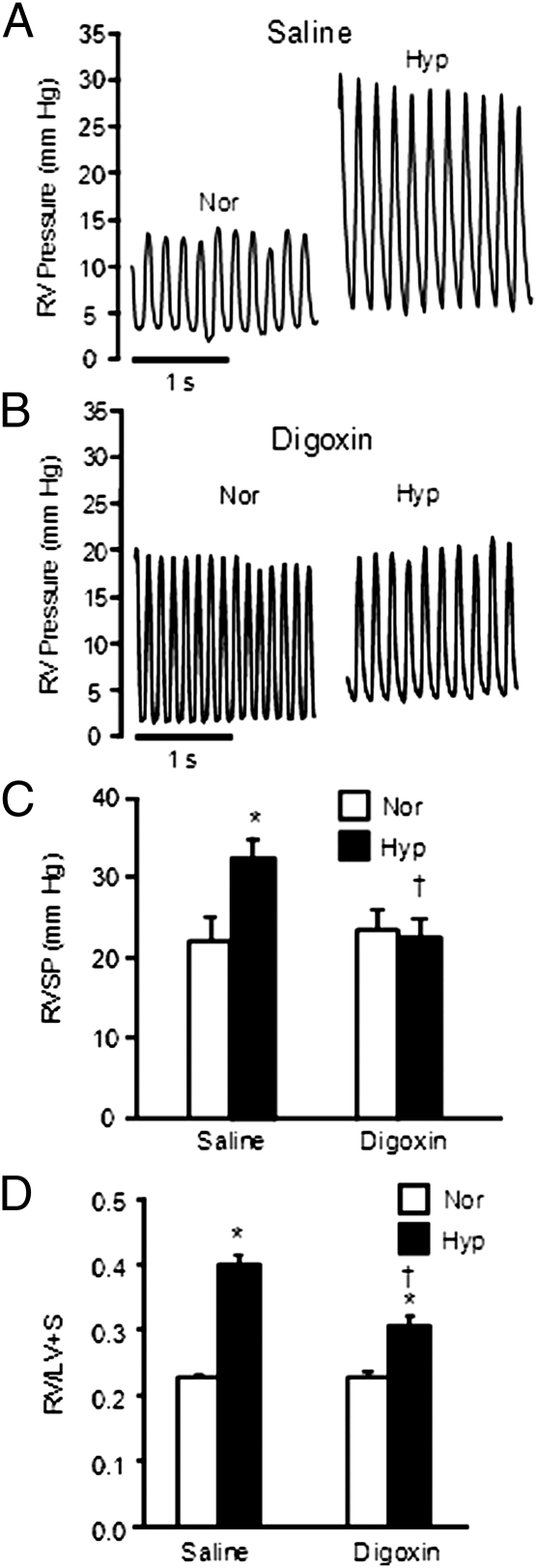
RV pressure readings (Fig. 1A) and heart weights were used to evaluate the effects of digoxin on the development of HPH. In normoxic mice, average RV systolic pressure (RVSP) was ≈20 mm Hg (Fig. 1B). RVSP in saline-treated chronically hypoxic mice was elevated. In hypoxic mice receiving digoxin, RVSP was significantly reduced compared with mice that received saline and was not statistically different from normoxic levels (Fig. 1C). Digoxin had no significant effect on heart rate (Table S1).
Fig. 1.
Effect of digoxin treatment on RV parameters. (A and B) Representative tracings of RV pressures in normoxic (Nor) and chronically hypoxic (Hyp) mice treated with saline (A) or 1 mg/kg digoxin (B). (C) Bar graph (mean ± SEM) shows effect of digoxin on RVSP. Mice were injected with saline or 1 mg/kg digoxin per day (n = 6 for Nor-saline, n = 7 for Hyp-saline, n = 8 for Nor-digoxin, and n = 7 for Hyp-digoxin). (D) Effect of digoxin treatment on RVH. Bar graphs (mean ± SEM) show RV/LV+S weight ratio in mice exposed to normoxia or hypoxia in the absence or presence of digoxin (n = 8 for Nor-saline and Hyp-saline; n = 9 for Nor-digoxin and Hyp-digoxin). *Significant difference compared to normoxia value of the same treatment; †significant difference compared to Hyp-saline.
In normoxic mice, digoxin had no effect on RV weight when normalized to BW (Table S1) or to combined weight of the left ventricle and septum (LV+S) (Fig. 1D). After exposure to CH, RV weight increased in mice receiving saline, whereas a much smaller increase in RV weight was observed in chronically hypoxic mice treated with digoxin (Table S1). Similar results were observed when RV weight was normalized to LV+S weight (Fig. 1D).
Effect of Digoxin on PASMC [Ca2+]i and pHi.
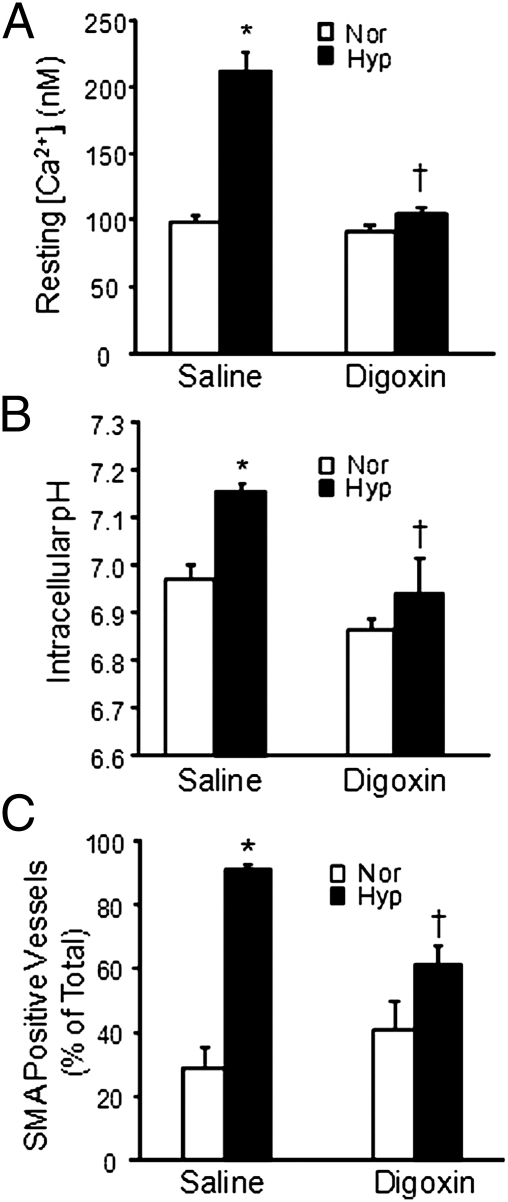
As anticipated, exposure to CH increased both basal [Ca2+]i and pHi in PASMCs isolated from saline-treated mice (Fig. 2 A and B). Treatment with digoxin caused a small but not statistically significant reduction in both basal [Ca2+]i and pHi in cells from normoxic mice and eliminated the hypoxia-induced increase in basal [Ca2+]i and pHi. To determine whether these results were due to acute effects of digoxin on Na+/K+ ATPase activity, we measured [Ca2+]i and pHi after acute exposure (10 min) to 5 nM digoxin. Acute administration of digoxin to PASMCs from normoxic mice caused a slight but significant increase in basal [Ca2+]i, whereas digoxin had no effect on pHi (Table S2). Acute exposure of PASMCs from hypoxic mice to digoxin had no effect on either [Ca2+]i or pHi.
Fig. 2.
Effect of digoxin on resting [Ca2+]i and pHi in PASMCs and on pulmonary vascular remodeling. (A) Basal [Ca2+]i and (B) basal pHi in PASMCs from normoxic (Nor) and chronically hypoxic (Hyp) mice treated with saline or 1 mg/kg per day digoxin (mean ± SEM). For [Ca2+]i, n = 67 cells from four mice for Nor-saline, n = 94 cells from five mice for Hyp-saline, n = 63 cells from four mice for Nor-digoxin, and n = 112 cells from seven mice for Hyp-digoxin. For pHi, n = 88 cells from four mice for Nor-saline, n = 110 cells from five mice for Hyp-saline, n = 54 cells from three mice for Nor-digoxin, and n = 104 cells from five mice for Hyp-digoxin. (C) Bar graph shows mean ± SEM data for the percentage of vessels identified as SMA positive in lung sections from normoxic and hypoxic mice treated with saline or digoxin. For each group, n = 5 mice. *Significant difference compared to normoxia value within treatment; †significant difference compared to Hyp-saline.
Effect of Digoxin on Pulmonary Vascular Remodeling.
The increase in pulmonary arterial pressure in response to CH occurs, in part, because of remodeling of the pulmonary vasculature. Extension of smooth muscle into previously nonmuscular vessels can be observed as an increase in small diameter vessels (<100 μm outer diameter) that are positive for smooth muscle-specific α-actin (SMA). In lungs from normoxic mice receiving saline or digoxin, the percentage of SMA-positive vessels was ≈30–40% (Fig. 2C). In saline-treated hypoxic mice, the number of small vessels exhibiting a complete muscle layer increased to nearly 90%. Significantly fewer SMA-positive vessels were observed in the lungs of digoxin-treated hypoxic mice.
Effect of Digoxin on HIF-1 Target Genes.
We previously reported that the increase in PASMC [Ca2+]i and pHi in response to hypoxia is due to HIF-1–dependent transcriptional activation of the Trpc1 and Nhe1 genes, respectively (5, 7). We found that levels of mRNA encoding TRPC1 and NHE1, as well as the classic HIF-1 target glucose transporter 1 (GLUT1), were increased in lung tissue from chronically hypoxic mice (Fig. 3A). The hypoxia-induced increase in mRNA levels for all HIF-1 target genes was not observed in lungs from chronically hypoxic mice treated with digoxin. To further confirm that digoxin could prevent HIF-1–dependent alterations in PASMCs, cells from normoxic animals were cultured under hypoxic conditions (4% O2; 60 h) in the presence of digoxin (100 nM) or vehicle. Digoxin treatment completely prevented the hypoxia-induced increase in GLUT1, TRPC1, and NHE1 mRNA levels (Fig. 3B).
Fig. 3.
Effect of digoxin on the expression of genes regulated by HIF-1. (A) Quantitative real-time RT-PCR analysis of GLUT1, TRPC1, and NHE1 mRNA levels in lung tissue from chronically hypoxic mice treated with saline or digoxin (1 mg/kg per day). (B) Analysis of GLUT1, TRPC1, and NHE1 mRNA levels in PASMCs exposed to hypoxia ex vivo (4% O2; 60 h). Levels of target gene mRNAs were normalized to cyclophilin mRNA levels within samples, and data are expressed as fold change relative to levels measured in normoxia under the same treatment conditions (n = 3–4 per group). *Significant difference compared to saline (in panel A) or vehicle (in panel B).
Effect of Digoxin on Established HPH.
To evaluate the ability of digoxin to reverse established HPH, mice were exposed to 10% O2 for 3 wk. After development of HPH, mice received daily injections of saline or digoxin (0.2 or 1.0 mg/kg) and were exposed to hypoxia for an additional 2 wk. At the end of the hypoxic exposure (5 wk total), there were no significant differences in Hct, BW, or LV weight among the three groups (Table S1). Both RV weight and RV weight/BW ratio were significantly reduced in mice treated with digoxin (Table S1). Digoxin treatment also resulted in significantly lower RVSP (Fig. 4A) compared with mice receiving saline. The RV/LV+S weight ratio was lower in digoxin-treated mice (0.29 ± 0.01 for 0.2 mg/kg and 0.308 ± 0.01 for 1 mg/kg; n = 7 each) compared with those receiving saline (0.327 ± 0.01; n = 8), but the difference did not reach statistical significance (P = 0.096). In a subset of mice, lung histology was examined for evidence of vascular remodeling. Similar to mice exposed to saline in the prevention protocol, mice in the reversal protocol exhibited an increase in the percentage of SMA-positive small-diameter vessels. Administration of digoxin had no significant effect on vascular remodeling, with all hypoxic groups exhibiting >80% SMA-positive vessels. As expected, both pHi and [Ca2+]i were elevated in PASMCs isolated from mice receiving saline (Fig. 4 B and C). Digoxin treatment markedly reduced both pHi and [Ca2+]i in PASMCs from mice exposed to 5 wk of hypoxia.
Fig. 4.
Effect of digoxin treatment on established HPH. Mice were injected with 0 (saline), 0.2 mg/kg, or 1.0 mg/kg digoxin per day for the final 2 wk of a 5-wk hypoxic exposure. (A) Effect of digoxin on RVSP (mean ± SEM; n = 5 for saline treated and n = 7 for 0.2-digoxin and 1.0-digoxin). (B and C) Mean basal [Ca2+]i and pHi in PASMCs isolated from chronically hypoxic mice. For [Ca2+]i, n = 49 cells from four mice for saline, n = 34 cells from three mice for 0.2-digoxin, and n = 32 cells from three mice for 1.0-digoxin. For pHi, n = 42 cells from three mice for saline, n = 38 cells from three mice for 0.2-digoxin, and n = 47 cells from three mice for 1.0-digoxin. †Significant difference compared to saline.
Effect of Acriflavine on HPH.
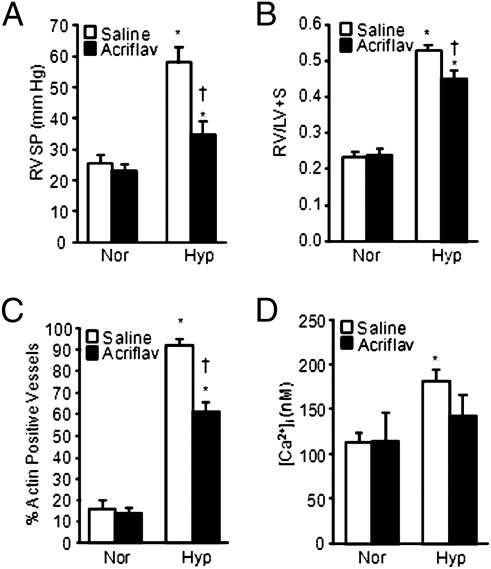
To further evaluate the effect of pharmacologic inhibition of HIF activity on HPH and to confirm that the effects of digoxin were not due to HIF-independent actions, prevention experiments were also performed with a different HIF inhibitor, acriflavine, which does not affect HIF-1α synthesis but inhibits the dimerization of HIF-1α with HIF-1β (17). Furthermore, the experiments were performed with rats exposed to CH, which represents a more robust model of HPH. Daily administration of acriflavine had no effect on normoxic rats but significantly reduced RVSP (Fig. 5A), RVH (Fig. 5B), the percentage of SMA-positive small-diameter vessels (Fig. 5C), and PASMC resting [Ca2+]i (Fig. 5D) in rats subjected to CH.
Fig. 5.
Effect of acriflavine treatment on HPH in rats. (A) Effect of acriflavine (acriflav) on RVSP (mean ± SEM) in normoxic (Nor) and chronically hypoxic (Hyp) rats. Rats were injected with saline or 2.0 mg/kg acriflavine per day. (B) RV/LV+S ratio (mean ± SEM) in rats exposed to normoxia or hypoxia in the absence or presence of acriflavine. (C) Percentage of total vessels (mean ± SEM) that were identified as SMA positive in lung sections from normoxic and hypoxic rats treated with saline or acriflavine. In all experiments, n = 5 rats per group. *Significant difference compared to normoxia value within treatment; †significant difference compared to Hyp-saline. (D) Basal [Ca2+]i (mean ± SEM) in PASMCs from normoxic and hypoxic rats treated with saline (n = 94 cells from five rats for normoxia and 92 cells from five rats for hypoxia) or acriflavine (n = 100 cells from five rats for normoxia and 110 cells from five rats for hypoxia).
Discussion
In the present study, we show that administration of digoxin, which inhibits HIF-1α synthesis and HIF-1 transcriptional activity (15, 16), prevents the development and slows the progression of HPH in a murine model. Although the doses of digoxin administered in our study are higher than those administered to humans, comparison of dosages between species is complicated by a number of factors. For example, on the basis of BW vs. surface area measurements, it has been suggested that a given dosage in humans requires a 12-fold higher dose in mice (18). Drug metabolism can also vary considerably owing to differential mechanisms of uptake, clearance, and/or degradation (19). With these caveats in mind, plasma digoxin levels measured in this study were at or below the therapeutic range for humans.
Weight loss associated with exposure to CH was reduced in mice treated with digoxin, suggesting that digoxin was well tolerated. This finding was surprising because HIF-1α heterozygous-null mice, which were protected from HPH, lost more weight during CH than wild-type mice (9). The reason for the difference in weight gain between these studies is unclear, but it could be due to several factors, including variable drug penetration among tissues or the use of different strains of mice.
In the prevention protocol, digoxin normalized RVSP. Intriguingly, digoxin reduced but did not normalize RVH. It is doubtful that the discrepancy between RVSP and RVH was due to a component of RVSP that rapidly reversed upon reoxygenation, because we previously demonstrated that RV pressures were minimally higher in chronically hypoxic mice ventilated with 10% O2 compared with reoxygenated animals (9). Although cardiac glycosides can cause cardiac hypertrophy (20–22), it is unlikely that digoxin had a direct hypertrophic effect, given that heart weights were unchanged in the normoxic mice. A plausible explanation is that there is a direct effect of hypoxia on the RV that is independent of HIF-1 or the pulmonary vascular effects of hypoxia (i.e., PH), a hypothesis that is supported by a recent study in which RVH was decoupled from PH in heme-oxygenase-1–deficient mice that received supplemental carbon monoxide or biliverdin (23). Further testing will be required to determine the mechanism by which RVH occurs during CH and to determine whether treatment with digoxin can alter long-term survival.
The effect of digoxin on Hct was modest but still significantly reduced compared with animals receiving saline. The small effect on Hct could be due to lower drug penetration in the tissues where erythropoietin is produced (i.e., kidney and liver). Alternatively, it has been proposed that in vivo, erythropoietin expression is driven predominately by HIF-2α (24, 25), which exhibits reduced sensitivity to digoxin compared with HIF-1α (15). Consistent with this possibility, the reduction in CH-induced polycythemia in mice with partial deficiency for HIF-1α was modest, albeit statistically significant (9). In either case, the fact that therapeutic concentrations of digoxin normalized RVSP and reduced RVH while maintaining slightly elevated Hct might prove beneficial in improving O2 transport while reducing HPH.
Digoxin had profound effects on PASMC [Ca2+]i and pHi during CH. We previously demonstrated that HPH was associated with increased [Ca2+]i, which is HIF-1–dependent, mediated by increased TRPC expression, and likely contributes to increased PASMC contraction and proliferation (6, 7). In the presence of digoxin, the reduction in [Ca2+]i in PASMCs from chronically hypoxic mice was correlated with an absence of hypoxia-induced induction of TRPC1 mRNA expression. Similarly, digoxin prevented the hypoxia-induced elevation in pHi, which is due to HIF-1–dependent induction of NHE1 mRNA and protein.
Initiating digoxin treatment after HPH and changes in ion homeostasis were established still resulted in a near normalization of pHi and [Ca2+]i, indicating significant plasticity in these responses. Despite the dramatic reduction in both pHi and [Ca2+]i with digoxin treatment and the fact that RVSP was reduced, vascular remodeling was unaltered, suggesting that once established, 2 wk may be insufficient time to reverse changes in muscularity of the small vessels or that HIF-1–dependent vascular remodeling may be an early response to hypoxic exposure and once initiated is maintained via HIF-independent mechanisms. Nonetheless, the reductions in RVSP, PASMC [Ca2+]i, and pHi observed in digoxin-treated mice, coupled with the lack of effect of digoxin on remodeling, suggests that at these later time points a reduction in vascular tone may have played a more prominent role in decreasing pulmonary arterial pressure.
Digoxin inhibits HIF-1 transcriptional activity by preventing HIF-1α protein translation (15, 16), consistent with the lack of hypoxic induction of HIF-1 target genes in lungs from chronically hypoxic animals and in PASMCs exposed to hypoxia ex vivo. Even so, it is also possible that the observed results in digoxin-treated animals may be due, in part, to modulation of HIF-1–independent pathways, such as inhibition of Na+/K+ ATPase, which could result in elevated intracellular [Na+] and altered PASMC pHi or [Ca2+]i via modulation of Na+/H+ exchange or Na+/Ca2+ exchange, respectively (26). However, acute administration of digoxin had no effect on [Ca2+]i or pHi in PASMCs from hypoxic animals. In contrast, Na+/K+ ATPase activity typically serves to reduce vascular tone (27–29), and digoxin slightly increased [Ca2+]i in PASMCs from normoxic animals, an expected consequence of Na+/K+ ATPase inhibition and subsequent reversal of Na+/Ca2+ exchange. Consistent with these results, inhibition of Na+/K+ ATPase increased pulmonary vascular tone (29–31) and cardiac output (32) and had no effect on (32, 33) or accentuated (34) hypoxic pulmonary vasoconstriction. These findings would argue against a role for blockade of Na+/K+ ATPase as the mechanism by which digoxin inhibited HPH. Moreover, the effects of digoxin on HPH were replicated by treatment with acriflavine, which inhibits HIF activity by a distinct molecular mechanism (17). Thus, although we cannot completely rule out HIF-1–independent effects of digoxin, our data suggest that the ability of digoxin to lower [Ca2+]i and pHi and prevent vascular remodeling was most likely due to inhibition of HIF-1.
In summary, we found that treatment with digoxin prevented the changes in pulmonary vascular [Ca2+]i, pHi, remodeling, and pressure that occur in mice exposed to CH, offering further evidence that HIF-1 plays a critical role in the development of HPH. Furthermore, administration of digoxin after HPH was established reduced RVSP and corrected Ca2+ and pH homeostasis, demonstrating the ability of this agent to slow the progression of HPH. Although the present study was restricted to HPH, other forms of PH are also associated with elevated levels of HIF-1α in the lung (35, 36) and similar defects in PASMC function (2, 3), suggesting the possibility that digoxin might reduce pulmonary vascular pressure and remodeling in non–hypoxia-associated PH. Digoxin has been proposed for use in the treatment of PH with associated RV failure as a means to increase cardiac contractility and output (37, 38). However, given the small therapeutic window in humans, possible issues of toxicity in the chronic obstructive pulmonary disease patient population (39, 40), and a lack of data supporting a positive effect of digoxin on survival, the use of this drug in patients with PH and RV failure remains controversial, and to date no clinical trials have been performed to evaluate its ability to lower pulmonary arterial pressure. In a disease for which treatment options are limited, this study provides “proof of concept” that HIF-1 inhibitors could be beneficial and suggests that further investigation is warranted.
Materials and Methods
In Vivo Exposure to CH.
All protocols were approved by The Johns Hopkins University Animal Care and Use Committee. Adult male C57BL/6J mice (8–10 wk; Jackson Laboratory) were placed in a chamber maintained at 10% O2 for 3 wk, as previously described (5, 7). The chamber was constantly flushed with room air to maintain low (<0.5%) CO2 concentrations. A servo-control system (PRO-OX; Hudson RCI) monitored O2 levels and injected 100% N2 as needed to maintain 10% ± 0.5% O2. Cages were cleaned and food and water replenished twice per week. Normoxic animals were kept in room air on a wire rack adjacent to the chamber. Animals were allowed free access to food and water. In the prevention protocol, beginning the day before the hypoxic exposure, mice were weighed and injected daily with digoxin (1.0 mg/kg i.p.), which was diluted in four times the volume of sterile saline, or injected with an equal volume of sterile saline. In the reversal protocol, mice were exposed to CH for 3 wk to establish HPH before being weighed and injected daily with saline or digoxin (0.2 or 1 mg/kg) during an additional 2 wk of hypoxic exposure. Injectable digoxin (Sandoz; 0.25 mg/mL) was obtained from The Johns Hopkins Hospital Research Pharmacy and was diluted in sterile saline before injection. For acriflavine experiments, adult male Wistar rats (Harlan; starting weight 250 g) were weighed daily, and saline or acriflavine (2 mg/kg in a total volume of 600 mL) was administered i.p. (17) starting the day before hypoxic exposure. Acriflavine (Sigma Aldrich) was obtained in crystalline form and dissolved in sterile PBS. Unless otherwise specified, all other reagents were obtained from Sigma Aldrich. All data were obtained by investigators blinded to treatment status.
RV Pressure.
Closed-chest RV pressure was measured in anesthetized mice through an abdominal incision, as previously described (9). Mice were anesthetized with sodium pentobarbital (60 mg/kg), the diaphragm visualized through the abdomen, and RV pressure measured via a 23-gauge needle filled with heparinized saline and connected to a pressure transducer (model P10EZ; Spectramed). Correct localization of the puncture was verified by postmortem inspection. Pressure was recorded using Power Lab Software (ADI Instruments). Only mice in which stable tracings were obtained and RV puncture was verified were included in the analysis.
Hct and Digoxin Measurements.
Blood was collected from the LV and placed in EGTA-treated tubes. The blood was mixed and a small amount drawn into a capillary tube, the plasma separated via low-speed centrifugation, and Hct measured using a microhematocrit capillary tube reader chart. The remaining blood was centrifuged and the plasma collected and frozen at −80 °C until use. Plasma digoxin levels were determined using a commercially available kit (Monobind) according to manufacturer's instructions.
RVH Determination.
Under a dissecting microscope, the atria and extraneous vascular material were removed from the heart. The RV wall was carefully separated from the LV+S, and both portions were quickly blotted dry and weighed.
Isolation of PASMCs.
Intralobar pulmonary arteries (100–400 μm outer diameter) were dissected and cleaned of connective tissue in ice-cold Hepes-buffered saline solution (HBSS) containing (in mM): 130 NaCl, 5 KCl, 1.2 MgCl2, 10 Hepes, and 10 glucose with pH adjusted to 7.2 with 5 M NaOH, as previously described (2). The arteries were opened and the lumen gently rubbed to remove the endothelium. Cleaned arteries were allowed to recover for 30 min in cold (4 °C) HBSS followed by 20 min in reduced-Ca2+ HBSS (20 μM CaCl2) at room temperature. After recovery, the tissue was incubated for 10 min (mice) or 20 min (rats) at 37 °C in reduced-Ca2+ HBSS containing collagenase (type I; 1,750 U/mL), papain (9.5 U/mL), BSA (2 mg/mL), and DTT (1 mM). Single SMCs were dispersed by gentle trituration of the tissue in Ca2+-free HBSS. Cells were cultured in SMGM complete media (Lonza) supplemented with 10% FCS (HyClone) and 1% penicillin/streptomycin for 24–48 h and placed in basal media (SMBM plus 0.3% FCS and 1% penicillin/streptomycin) 24 h before experiments.
Measurement of [Ca2+]i and pHi.
PASMCs were incubated with 5 μM Fura-2 AM (Molecular Probes) for measurement of [Ca2+]i, or 5 μM BCECF-AM (Molecular Probes) for pH measurements, at 37 °C for 60 min. After incubation, PASMCs were placed in a heated cell chamber (Warner Instruments) and perfused with modified Kreb's solution containing (in mM) 118.3 NaCl, 4.7 KCl, 1.2 MgSO4, 25 NaHCO3, 11 glucose, and 1.2 KH2PO4, and gassed with 16% O2-5% CO2 for 15 min at 37 °C to remove extracellular dye. Fluorescence was measured using a workstation based on an inverted microscope. The collimated light beam from a xenon arc lamp was filtered at the appropriate wavelengths (340 and 380 nm for Fura-2 or 440 and 490 nm for BCECF), passed through a 20× fluorescence objective (Super Fluor 20; Nikon), and focused onto the field of PASMCs. Emitted fluorescence was returned through the objective and a bandpass filter and detected by an imaging camera at a rate of five images per minute. Protocols were executed and data collected online with InCyte software (Intracellular Imaging). Fura-2 fluorescence ratios (F340/F380) were used to estimate [Ca2+]i using a calibration curve created with solutions containing [Ca2+] ranging between 0 and 610 nM (Molecular Probes). [H+] was estimated from in situ calibration after each experiment. Cells were perfused with a solution containing (in mM) 105 KCl, 1 MgCl2, 1.5 CaCl2, 10 glucose, 20 Hepes·Tris, and 0.01 nigericin to allow [H+]i to equilibrate to external [H+] (4). A two-point calibration was created from fluorescence measured as pHi was adjusted with KOH from 6.5 to 7.5. pHi was determined from [H+]i using the following formula: pHi = −log ([H+]i).
Lung Histology.
A suture was used to occlude the right lung, which was removed for isolation of PASMCs. A cannula was inserted into the trachea, and the left lung was inflated with 0.5 mL of 10% formalin, transferred to 70% ethanol, embedded in paraffin, and sectioned into 5-μm slices. Sections were subjected to antigen retrieval, blocked, stained with SMA antibody (Sigma Aldrich) overnight at 4 °C, and incubated with CY3-conjugated secondary antibody (Molecular Probes) for 1 h at room temperature. Each lung section was evaluated by a blinded investigator for the presence of SMA-positive small-diameter vessels (outer diameter ≤100 μm) using confocal microscopy (LSM-510; Zeiss). Vessels were randomly identified via light microscopy and then scanned at 560 nm to evaluate the presence of SMA. For each lung, 20 randomly selected vessels were scored, and the percentage of SMA-positive vessels was calculated.
Real-Time RT-PCR.
The methods for isolation of total RNA and real-time RT-PCR have been previously described (41). Primer sequences specific for mouse TRPC1, NHE1, GLUT1 and cyclophilin mRNA were designed using Primer3 software (http://frodo.wi.mit.edu/primer3/) and are listed in Table S3. Specificity of quantitative PCR products was confirmed by the observation of a single peak in the melting curve performed after cDNA amplification and a single band of expected size on an agarose gel, which was then excised and sequenced at The Johns Hopkins Sequencing facility. The relative expression of each mRNA was determined using the Pfaffl method (42), whereby PCR detection threshold cycle (CT) values were calculated using iCycler (Bio-Rad) software and the efficiency of each primer pair determined from a five-point standard curve for each mRNA of interest. Data are expressed as the ratio of the mRNA of interest to a reference RNA within a sample.
Data Analysis.
All values are expressed as mean ± SEM. For physiological measurements, “n” refers to the number of animals. For experiments in which [Ca2+]i or pHi was measured, data were collected from 10 to 25 cells per animal, and the total number of cells and animals per experiment are indicated in the figure legends. Change in [Ca2+]i or pHi was computed by subtracting the average basal value, determined from 1 min of data collected immediately before beginning challenge, from the average of 1 min of data at the end of the challenge. pH values were converted to [H+], and fold change values were subjected to arctangent conversion, before statistical analysis. Unless otherwise noted, data were compared group-wise as a single analysis using a two-way ANOVA with a Holms-Sidak post hoc test to determine differences between groups. A P value <0.05 was accepted as statistically significant.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health Grants HL67191, HL75389, HL84762, and GM08074. G.L.S. is the C. Michael Armstrong Professor at The Johns Hopkins University School of Medicine.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120385109/-/DCSupplemental.
References
- 1.Tuder RM, Yun JH, Bhunia A, Fijalkowska I. Hypoxia and chronic lung disease. J Mol Med (Berl) 2007;85:1317–1324. doi: 10.1007/s00109-007-0280-4. [DOI] [PubMed] [Google Scholar]
- 2.Morrell NW, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1, Suppl):S20–S31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humbert M, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12, Suppl S):13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Rios EJ, Fallon M, Wang J, Shimoda LA. Chronic hypoxia elevates intracellular pH and activates Na+/H+ exchange in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L867–L874. doi: 10.1152/ajplung.00455.2004. [DOI] [PubMed] [Google Scholar]
- 5.Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L941–L949. doi: 10.1152/ajplung.00528.2005. [DOI] [PubMed] [Google Scholar]
- 6.Shimoda LA, Sham JS, Shimoda TH, Sylvester JT. L-type Ca(2+) channels, resting [Ca(2+)](i), and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol. 2000;279:L884–L894. doi: 10.1152/ajplung.2000.279.5.L884. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, et al. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006;98:1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 8.Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1α deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2001;281:L202–L208. doi: 10.1152/ajplung.2001.281.1.L202. [DOI] [PubMed] [Google Scholar]
- 9.Yu AY, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimoda LA, Semenza GL. HIF and the lung: Role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011;183:152–156. doi: 10.1164/rccm.201009-1393PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 13.Jaakkola P, et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 14.Ivan M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, et al. Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida T, et al. Digoxin inhibits retinal ischemia-induced HIF-1α expression and ocular neovascularization. FASEB J. 2010;24:1759–1767. doi: 10.1096/fj.09-145664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee K, et al. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci USA. 2009;106:17910–17915. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Chodera A, Feller K. Some aspects of pharmacokinetic and biotransformation differences in humans and mammal animals. Int J Clin Pharmacol Biopharm. 1978;16:357–360. [PubMed] [Google Scholar]
- 19.Greiner B, et al. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest. 1999;104:147–153. doi: 10.1172/JCI6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gan XT, et al. Sodium-hydrogen exchange inhibition attenuates glycoside-induced hypertrophy in rat ventricular myocytes. Cardiovasc Res. 2010;85:79–89. doi: 10.1093/cvr/cvp283. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol. 2007;293:C1489–C1497. doi: 10.1152/ajpcell.00158.2007. [DOI] [PubMed] [Google Scholar]
- 22.Huang L, Kometiani P, Xie Z. Differential regulation of Na/K-ATPase alpha-subunit isoform gene expressions in cardiac myocytes by ouabain and other hypertrophic stimuli. J Mol Cell Cardiol. 1997;29:3157–3167. doi: 10.1006/jmcc.1997.0546. [DOI] [PubMed] [Google Scholar]
- 23.Vitali SH, et al. Divergent cardiopulmonary actions of heme oxygenase enzymatic products in chronic hypoxia. PLoS ONE. 2009;4:e5978. doi: 10.1371/journal.pone.0005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weidemann A, et al. The glial cell response is an essential component of hypoxia-induced erythropoiesis in mice. J Clin Invest. 2009;119:3373–3383. doi: 10.1172/JCI39378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291:F271–F281. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng M, Huang L, Xie Z, Huang WH, Askari A. Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expressions of early-response genes in cardiac myocytes. J Biol Chem. 1996;271:10372–10378. doi: 10.1074/jbc.271.17.10372. [DOI] [PubMed] [Google Scholar]
- 27.Limas CJ, Cohn JN. Stimulation of vascular smooth muscle sodium, potassium—adenosinetriphosphatase by vasodilators. Circ Res. 1974;35:601–607. doi: 10.1161/01.res.35.4.601. [DOI] [PubMed] [Google Scholar]
- 28.Tagaya E, Tamaoki J, Kawatani K, Nagai A. Role of Na(+)-K(+)-ATPase in sodium nitroprusside-induced relaxation of pulmonary artery under hypoxia. Respiration. 2001;68:186–191. doi: 10.1159/000050490. [DOI] [PubMed] [Google Scholar]
- 29.Tamaoki J, Tagaya E, Yamawaki I, Konno K. Hypoxia impairs nitrovasodilator-induced pulmonary vasodilation: role of Na-K-ATPase activity. Am J Physiol. 1996;271:L172–L177. doi: 10.1152/ajplung.1996.271.1.L172. [DOI] [PubMed] [Google Scholar]
- 30.Farrukh IS, Michael JR. Cellular mechanisms that control pulmonary vascular tone during hypoxia and normoxia. Possible role of Ca2+ATPases. Am Rev Respir Dis. 1992;145:1389–1397. doi: 10.1164/ajrccm/145.6.1389. [DOI] [PubMed] [Google Scholar]
- 31.Voelkel NF. Calcium-induced pulmonary vasodilation: modification by meclofenamate and ouabain. Prostaglandins Leukot Med. 1984;15:359–373. doi: 10.1016/0262-1746(84)90135-5. [DOI] [PubMed] [Google Scholar]
- 32.Saito K, Kashima T, Kiyonaga K, Tanaka H. The effect of digoxin on cardiovascular responses to hypoxia and reoxygenation in anesthetized dogs. Eur J Pharmacol. 1982;80:237–242. doi: 10.1016/0014-2999(82)90060-7. [DOI] [PubMed] [Google Scholar]
- 33.Becker S, Moir LM, Snetkov VA, Aaronson PI. Hypoxic pulmonary vasoconstriction in intact rat intrapulmonary arteries is not initiated by inhibition of Na+-Ca2+ exchange. Am J Physiol Lung Cell Mol Physiol. 2007;293:L982–L990. doi: 10.1152/ajplung.00361.2006. [DOI] [PubMed] [Google Scholar]
- 34.Hoshino Y, Morrison KJ, Vanhoutte PM. Mechanisms of hypoxic vasoconstriction in the canine isolated pulmonary artery: role of endothelium and sodium pump. Am J Physiol. 1994;267:L120–L127. doi: 10.1152/ajplung.1994.267.2.L120. [DOI] [PubMed] [Google Scholar]
- 35.Tuder RM, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: Evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 36.Bonnet S, et al. An abnormal mitochondrial-hypoxia inducible factor-1α-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: Similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 37.Rich S, et al. The short-term effects of digoxin in patients with right ventricular dysfunction from pulmonary hypertension. Chest. 1998;114:787–792. doi: 10.1378/chest.114.3.787. [DOI] [PubMed] [Google Scholar]
- 38.McLaughlin VV, Rich S. Pulmonary hypertension. Curr Probl Cardiol. 2004;29:575–634. doi: 10.1016/j.cpcardiol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Green LH, Smith TW. The use of digitalis in patients with pulmonary disease. Ann Intern Med. 1977;87:459–465. doi: 10.7326/0003-4819-87-4-459. [DOI] [PubMed] [Google Scholar]
- 40.Beller GA, Smith TW, Abelmann WH, Haber E, Hood WB., Jr Digitalis intoxication. A prospective clinical study with serum level correlations. N Engl J Med. 1971;284:989–997. doi: 10.1056/NEJM197105062841801. [DOI] [PubMed] [Google Scholar]
- 41.Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L104–L113. doi: 10.1152/ajplung.00058.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.