Abstract
Somatic hypermutation (SHM) of Ig variable region (IgV) genes requires both IgV transcription and the enzyme activation-induced cytidine deaminase (AID). Identification of a cofactor responsible for the fact that IgV genes are much more sensitive to AID-induced mutagenesis than other genes is a key question in immunology. Here, we describe an essential role for a splice isoform of the prototypical serine/arginine-rich (SR) protein SRSF1, termed SRSF1-3, in AID-induced SHM in a DT40 chicken B-cell line. Unexpectedly, we found that SHM does not occur in a DT40 line lacking SRSF1-3 (DT40-ASF), although it is readily detectable in parental DT40 cells. Strikingly, overexpression of AID in DT40-ASF cells led to a large increase in nonspecific (off-target) mutations. In contrast, introduction of SRSF1-3, but not SRSF1, into these cells specifically restored SHM without increasing off-target mutations. Furthermore, we found that SRSF1-3 binds preferentially to the IgV gene and inhibits processing of the Ig transcript, providing a mechanism by which SRSF1-3 makes the IgV gene available for AID-dependent SHM. SRSF1 not only acts as an essential splicing factor but also regulates diverse aspects of mRNA metabolism and maintains genome stability. Our findings, thus, define an unexpected and important role for SRSF1, particularly for its splice variant, in enabling AID to function specifically on its natural substrate during SHM.
Keywords: gene conversion, genomic instability
Somatic hypermutation (SHM) and class switch recombination (CSR) of Ig genes are key processes needed to generate functional antibodies (Abs) with high affinity in humoral immune responses. Activation-induced cytidine deaminase (AID) is crucial for both SHM and CSR (1, 2), which are initiated by AID-catalyzed deamination of dC to dU in the variable (V) region and the switch (S) region DNA strands, respectively (3, 4). However, AID has been recently shown to bind numerous chromosomal locations genome-wide and to mutate non-Ig genes, although the mutation frequency of non-Ig genes is much lower than that of the Ig genes (5, 6). It has been proposed that specific cofactors interact with AID and regulate its activity in a target-specific manner (7). AID has been reported to associate with several proteins including RPA (8, 9), protein kinase A (10), RNA polymerase II (Pol II) (11), DNA-PKcs (12), MDM2 (13), Spt6 (14), 14–3-3 (15), Spt5 (16), CTNNBL1 (17), PTBP2 (18), RNA exosome subunits (19), KAP1 (20), and HP1 (20). However, these investigations were mostly focused on CSR, and, thus, the identified factors themselves are not sufficient to explain how AID activity is specifically targeted to the IgV genes during SHM.
SHM occurs in IgV region exons starting 100–200 bp downstream of the promoter and extending over 1–2 kb, preferentially targeting WRC/GYW hotspot motifs (3, 4). Importantly, the activity of the SHM machinery strictly depends on transcription of the IgV genes (21, 22). In vitro experiments have shown that replication protein A (RPA) binds to and stabilizes single-stranded (ss)DNA bubbles in transcribed artificial substrate DNAs harboring SHM hotspot motifs, thus enabling AID-mediated deamination (8, 23–25). In addition, it has recently been reported that depletion of the THO-TREX complex, which functions as the interface between transcription and mRNA export, enhances mutation in AID-expressing yeast cells (26). However, cofactors that are essential for generating and stabilizing ssDNA bubbles in the IgV genes in vivo are not well defined, although these recent reports suggest that transcription-coupled factors have a role in generating AID substrates.
Chicken DT40 B cells provide an excellent system to analyze AID cofactors responsible for SHM. These cells spontaneously undergo Ig hypermutation by AID-dependent gene conversion (GCV) and point mutation (27–30), the latter being equivalent to SHM observed in human and murine B cells (31). Interestingly, it has been shown that genetic depletion of a serine/arginine (SR)-rich protein splicing factor SRSF1 in a DT40 cell line (DT40-ASF) results in enhanced genomic instability because of generation of ssDNA in transcribed regions genome-wide (32). A prototypical SR protein, SRSF1 [formerly ASF/SF2 (33)], not only acts as an essential splicing factor but also exerts biological roles in diverse aspects of RNA metabolism, including mRNA nuclear export, mRNA stability, mRNA quality control, and regulation of translation (34, 35).
In this study, we used DT40 cell lines to analyze requirements for accurate and efficient AID-dependent SHM. Significantly, we found that SRSF1, and in particular a specific splice isoform, SRSF1-3, is necessary for the AID-dependent SHM machinery to target the IgV genes. Our results, thus, not only identify a cofactor necessary for ensuring accurate SHM but also expand the role of SR proteins to include a function in the immune response.
Results
Hypermutation Is Deficient in DT40-ASF Cells.
In DT40-ASF cells, the endogenous SRSF1 gene is homozygously disrupted, and a human SRSF1 cDNA under the control of a tetracycline (Tet)-repressible promoter is present as the only source of SRSF1 (36). When DT40-ASF cells are treated with Tet or its more active analog, doxycycline (Dox), most of cells die because of depletion of SRSF1, an essential factor in diverse aspects of RNA metabolism. However, a small fraction of cells proliferate as Tet (or Dox)-resistant colonies, in which SRSF1 expression escaped from the control of the Tet-Off promoter (32). This has been shown to reflect enhanced DNA rearrangement and translocation of the exogenous SRSF1 gene, resulting from the formation of cotranscriptional R loops under SRSF1-deficient conditions. To explore a role for SRSF1 in the formation of transcription bubbles during SHM, we first analyzed a hypermutation profile of the IgV gene in DT40-ASF cells that has been maintained in the absence of Tet and Dox. Unexpectedly, we found that the DT40-ASF cell population bore a virtually monoclonal IgV gene (Fig. S1A), indicating that DT40-ASF has a critical defect in GCV and SHM. To further confirm the defect of IgV diversification in DT40-ASF cells, hypermutation frequencies in the IgVL gene were compared between wild-type DT40 and DT40-ASF cells after culture for 30 d. Strikingly, DT40-ASF cells were unable to introduce mutations (Fig. 1A and Fig. S1B), despite comparable expression of AID and SRSF1, relative to the mutation-competent wild type (Fig. 1B).
Fig. 1.
DT40-ASF cells lack IgV hypermutation activity even in the presence of AID expression. (A) IgVL sequence analysis of wild-type DT40 and DT40-ASF cells after culture for 30 d. The numbers of mutated and nonmutated genes are indicated in each pie chart. Segment sizes in the satellite chart depict the proportion of sequences containing 1–5 mutations. (B) Quantitative RT-PCR (Left) and Western blot (Right) analyses of AID and SRSF1 expression. Primers that can bind to both chicken and human SRSF1 genes were used. Data are means of triplicates; error bar, SD. (C) The structure of the chicken SRSF1 locus (NC_006106.2) and its products. Splice variants are generated from the chicken SRSF1. RRM, RNA-recognition motif; RS, arginine/serine-rich region; S, start; T, termination. (D) RT-PCR of chicken SRSF1 and SRSF1-3 transcripts. Fourfold serially diluted cDNAs were used. Primers are shown as arrowheads in A. (E) Expression of chicken SRSF1 and SRSF1-3 transcripts in the bone marrow (BM), bursa of Fabricius (BF), spleen (SP), and thymus (TH) cells removed from a chicken and in the wild-type DT40 and DT40-ASF cells. Data were collected using quantitative RT-PCR and normalized to the levels of the β-actin and gapdh transcripts.
An SRSF1 Splice Isoform, SRSF1-3, but Not SRSF1, Is Required for Hypermutation in the IgV Genes.
We next sought to investigate the possible basis for the defect in SHM in DT40-ASF cells. One possibility may reflect the fact that these cells possess only human SRSF1 instead of the chicken ortholog (Fig. 1B) (36). However, hypermutation potency was not restored when the chicken SRSF1 was reintroduced in DT40-ASF at a comparable level to that observed in the wild type (Fig. 2 A and B and Table S1). The chicken SRSF1 locus, as shown in human and mouse cell lines, can generate not only full-length SRSF1 but also splice variants including SRSF1-2 and SRSF1-3 (formerly ASF-2 and ASF-3, respectively), the functions of which remain unknown (37, 38) (Fig. 1C). We confirmed that DT40-ASF cells did not express the chicken isoforms of these transcripts (Fig. 1D), whereas wild-type DT40 cells, as well as chicken and mouse lymphoid tissues, expressed SRSF1 and SRSF1-3 transcripts (Fig. 1 D and E and Fig. S2). Thus, we considered the possibility that a splice variant other than SRSF1 is necessary for hypermutation in DT40 cells.
Fig. 2.
The SRSF1 splice isoform SRSF1-3 restores hypermutation in DT40-ASF cells. (A) Levels of chicken SRSF1 and SRSF1-3 transcripts in DT40-ASF cells transfected with a mock vector or the chicken SRSF1 or SRSF1-3 cDNA (Vector, SRSF-1R, or SRSF1-3R, respectively). SRSF1 and SRSF1-3 transcript levels were estimated by quantitative PCR using primers specific for chicken SRSF1 and SRSF1-3. Data were normalized to levels of the β-actin transcript. Two independent clones were analyzed. (B) IgVL sequence analysis of transfected DT40-ASF cells. The mutation frequency is the mean ± SD (n = 2 or 3). Pie charts are displayed as in Fig. 1A. (C) Mutated sequences are illustrated as horizontal bars. Sticks and balls, point mutations; bold bars, GCV tracts. The number of independent mutants in total analyzed clones is shown below. (D) Levels of the AID transcript and protein were assessed with quantitative RT-PCR and Western blotting, respectively. β-actin was used as a control. Relative protein levels to Vector are indicated below.
To test the above idea, we first transfected DT40-ASF cells with a chicken splice variant cDNA, preSRSF1. The preSRSF1 cDNA can produce SRSF1 and SRSF1-2 after splicing and also directly generate SRSF1-3 without splicing (Fig. 1C). This led to a significant restoration of both GCV and SHM (Fig. S3). Importantly, when DT40-ASF cells were transfected with a CMV promoter-driven SRSF1-3 encoding cDNA, the frequency of both GCV and SHM in the IgVL gene was completely restored to the level observed in wild-type cells (Fig. 2 and Table S1). However, an SRSF1-3 cDNA mutant in which the ATG start codon was changed to CTG failed to repair the defect of IgV hypermutation in DT40-ASF. Overexpression of SRSF1-3 did not increase the expression level of AID (Fig. 2D), and restoration of hypermutation activity was also observed in the IgVH gene in the SRSF1-3-reconstituted DT40-ASF cells (Fig. S4).
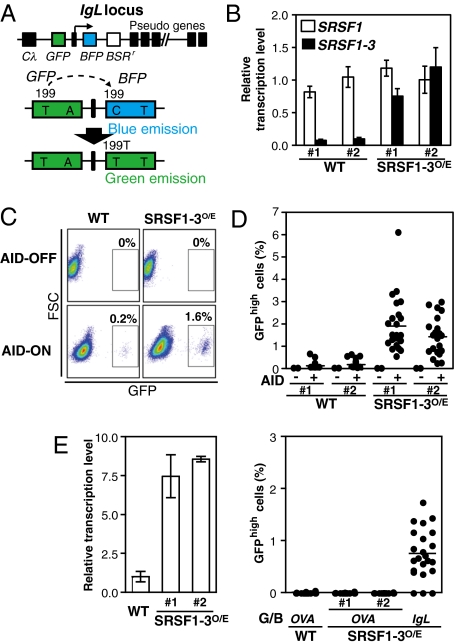
We next sought to determine whether increased expression of SRSF1-3 also enhances hypermutation in wild-type DT40 cells and whether the enhancement of hypermutation depends on AID. To this end, we examined the effect of overexpression of SRSF1-3 on GCV frequency in an engineered line, DT40-SW, which harbors an artificial GCV substrate (39) and the intact SRSF1 locus and in which AID expression can be switched on and off (40). In this cell line, a GCV event in each cell can be monitored as the change of fluorescence color from blue to green (Fig. 3A). When the GCV substrate, G/B construct, was placed in the IgVL locus in DT40-SW cells, overexpression of SRSF1-3 led to enhancement of GCV frequency in an AID-dependent fashion (Fig. 3 B–D). Transfection with the SRSF1-2 cDNA was, however, ineffective. Taken together, we conclude that the SRSF1-3 protein has a critical role in inducing AID-dependent hypermutation in the IgV genes.
Fig. 3.
SRSF1-3 enhances AID-dependent hypermutation on the IgV locus in DT40 cells bearing the intact SRSF1 locus. (A) An artificial GCV substrate, G/B construct. G/B construct was introduced in the IgL locus in DT40-SW cells. Conversion of 199C to T in the transcribed EBFP gene by GCV with the upstream untranscribed EGFP gene results in fluorescence shift from blue to green. Expression of the EBFP gene is driven by the CMV promoter and a synthetic intron. (B) The levels of chicken SRSF1 and SRSF1-3 transcripts in DT40-SW cells transfected with the SRSF1-3 cDNA were assessed by quantitative RT-PCR. Two independent transfectants (indicated as SRSF1-3O/E) were analyzed. Data were normalized to the level of the β-actin transcript. (C) Flow-cytometric analysis of GCV frequency. AID-expressing (AID-ON) cells were cultured for 30 d and analyzed for occurrence of GCV (GFPhigh cells). (D) SRSF1-3 overexpression enhanced mutation frequency in the IgVL locus. Single-cell-sorted subclones of DT40-SW cells bearing the G/B construct in the IgVL locus (AID-OFF cells, n = 2; AID-ON cells, n = 21 to ∼32) were analyzed. (E) SRSF1-3 overexpression did not induce hypermutation in a non-Ig locus. Wild-type DT40 cells in which the G/B construct was introduced in the ovalbumin locus were transfected with the SRSF1-3 cDNA and single cells (n = 24) were analyzed after culture for 3 wk (Right). The level of the SRSF1-3 transcript in transfectants was determined by quantitative RT-PCR (Left).
AID Functions in SRSF1 Depletion-Induced Genomic Instability.
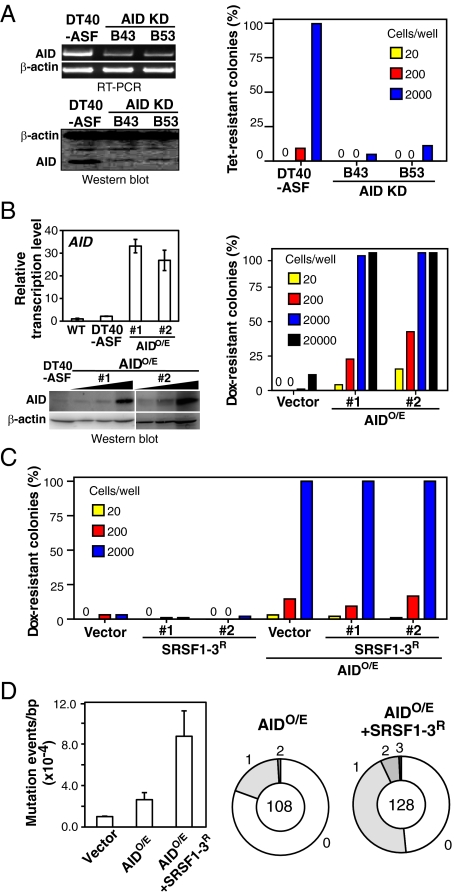
In addition to diversifying the Ig genes, AID can also target a variety of non-Ig genes (5), in some cases causing malignant transformation by initiating chromosome translocations (41). By treating DT40-ASF cells with Tet or Dox, DNA rearrangement and translocation of the exogenous SRSF1 gene can be induced under SRSF1-deficient conditions, resulting from the formation of cotranscriptional R loops (32). Although we have recently shown that this reflects, in part, defects in DNA replication (42), we next examined whether AID also contributes to SRSF1 depletion-induced genomic instability, which can be observed as occurrence of Tet- or Dox-resistant colonies. When AID levels were reduced by stable expression of AID-specific shRNAs, the appearance of Tet-resistant clones following SRSF1 depletion was suppressed (Fig. 4A). On the other hand, in DT40-ASF cells overexpressing AID, the frequency of Dox-resistant colony formation sharply increased (Fig. 4B), possibly because of an increased attack of AID to non-Ig targets (ssDNA regions or R-loops) generated under SRSF1-deficient conditions. The resultant colonies expressed SRSF1 even in the presence of Dox. The apparent lower frequency of colony formation in the presence of Dox (Fig. 4 B and C, Vector) may reflect the fact that Dox is ∼100-times more effective than Tet (43) and more readily causes cell death at the concentrations used. In any event, these results are consistent with previous findings that AID is involved in translocation of the c-myc gene to the IgH locus in B lymphomas (41) and that AID expression leads to induction of genome-wide mutations (5). Thus, our data suggest that SRSF1 naturally protects against the off-target action of AID, likely by preventing appearance of ssDNA as a result of R-loop formation.
Fig. 4.
SRSF1-3 does not contribute to an off-target activity of AID but induces hypermutation specifically in the IgV gene. (A) AID is important for SRSF1 depletion-induced genomic instability. Two AID knockdown derivatives of DT40-ASF, B43 and B53, were established by stable transfection of shRNA targeting the AID gene. Steady-state levels of AID mRNA and protein were determined by RT-PCR and Western blot (Left). SRSF1-depeletion assay was carried out using DT40-ASF and AID knock-down cells (Right). (B) Overexpression of AID enhances genomic instability. AID was overexpressed in DT40-ASF cells by stable transfection of AID cDNA. Steady-state levels of AID mRNA and protein were determined by quantitative RT-PCR and Western blot (Left) (5- and 10-fold diluted samples were also loaded). SRSF1-depeletion assay was carried out using AID-overexpressing DT40-ASF cells (AIDO/E) (Right). (C) Overexpression of SRSF1-3 does not affect AID-induced genomic instability. DT40-ASF cells overexpressing AID and/or SRSF1-3 (AIDO/E, SRSF1-3R, or AIDOE+SRSF1-3R, respectively) were used for SRSF1-depeletion assays. Two representative transfectants were assessed. DT40-ASF and AIDOE cells transfected with a mock vector (Vector and AIDOE+Vector, respectively) were used as controls. Cells were treated with 1 μg/mL Tet (A) or 50 ng/mL Dox (B and C). The number 0 in the charts indicates that no Tet or Dox-resistant colonies formed. (D) SRSF1-3 but not AID induces hypermutation efficiently in the IgV gene in DT40-ASF cells. Two or three independent clones of DT40-ASF cells overexpressing AID and/or SRSF1-3 (Vector, AIDOE, and AIDOE+SRSF1-3R, respectively) were cultured for 30 d and IgVL sequences were analyzed.
SRSF1-3 Has a Critical Role in Targeted Induction of Hypermutation in the IgV Genes.
We next investigated whether SRSF1-3 contributes to genetic alteration of non-Ig targets. We used an SRSF1-depletion assay in DT40-ASF cells overexpressing AID and/or SRSF1-3. In contrast to overexpression of AID, enhanced SRSF1-3 expression did not further increase the translocation frequency either in the original or AID-overexpressing cells under SRSF1-depleted conditions (Fig. 4C). If overexpression of SRSF1-3 causes genomic instability in the presence of SRSF1, drug-resistant cells should accumulate spontaneously during maintenance in culture without addition of Dox. However, this was not the case, as observed in SRSF1-3-reconstituted DT40-ASF cells in comparison with the vector control (Fig. 4C). In addition, reporter-based assays for GCV using the G/B construct showed that overexpression of SRSF1-3 in wild-type DT40 cells did not induce GCV in the reporter construct placed in the ovalbumin locus, in which the AID-dependent hypermutation machinery has been shown to be inactive (39) (Fig. 3E). These results indicate that SRSF1-3 did not contribute to off-target mutation induced by AID either in the presence or absence of SRSF1.
On the other hand, when DT40-ASF cells were treated with Dox for 48 h followed by culture in the absence of Dox for 1 mo, mutations were not found in the IgV genes of these cells, although the Dox treatment has been shown sufficient to cause genomic instability (32). In addition, despite the enormous increase of translocation, AID-overexpressing DT40-ASF cells showed only a marginal increase in IgVL mutations, most of which were limited to point mutations (Fig. 4D and Fig. S5). However, introduction of SRSF1-3 into these cells resulted in restoration of the hypermutation frequency to the level of the wild-type cells, in terms of both GCV and SHM (Fig. 4D and Fig. S5). Taken together, our data suggest that SRSF1-3 has a role in the specific induction of AID activity in the IgV genes.
SRSF1-3 Collaborates with AID to Inhibit Splicing of Ig Transcripts.
How does SRSF1-3 direct AID to act on the IgV gene? To address this issue, we examined whether AID and SRSF1-3 are recruited to the IgV gene. To discriminate between SRSF1 and SRSF1-3 in DT40 cells, we introduced Flag-tagged SRSF1-3 into DT40-ASF cells. Chromatin immunoprecipitation (ChIP) analysis using an anti-Flag mAb revealed that SRSF1-3 localized weakly but preferentially to the IgV gene (Fig. 5 A and C; the weak signal likely reflects a suppressed level of SRSF1-3 protein in the presence of SRSF1; Fig. S6). With respect to AID recruitment to the IgV gene, in vivo evidence has been limited except for experiments using Ramos human lymphoma cells (44). By careful optimization of ChIP conditions, a weak AID signal was detected on the IgV gene, and this was not further enhanced by SRSF1-3 expression (Fig. 5 B and C). These results suggest that SRSF1-3 does not participate in AID recruitment to the IgV genes.
Fig. 5.
SRSF1-3 is localized on the IgV genes but does not enhance recruitment of AID to the IgV gene. (A) Expression of Flag-tagged SRSF1-3 in DT40-ASF. Flag-SRSF1-3 was detected by Western blot using anti-Flag antibody. ChIP analysis for SRSF1-3. Crosslinked chromatin was extracted from DT40-ASF cells expressing Flag-SRSF1-3 (F-SRSF1-3R), precipitated using anti-Flag antibody (clone M2), and examined for the IgVH and control genes by PCR. Fourfold serially diluted ChIP DNAs were used for PCR. (B) ChIP analysis for AID. Chromatin obtained from SRSF1-3R cells using anti-AID or anti-acetylated H4 antibodies was analyzed for the IgVH and H2A genes. Data are representative of two or more experiments. (C) Quantitative PCR analysis of ChIP DNA for Flag-tagged SRSF1-3 and AID. Quantitative PCR was carried out for the IgVH and IgVL genes for chromatin precipitated using an anti-Flag antibody (clone 1E6) and for the IgVH gene for chromatin obtained using an anti-AID antibody. Data were normalized for input DNA. Means ± SD of triplicate assays are displayed. Data are representative of two experiments.
In vitro biochemical analyses have shown that SRSF1-3 not only lacks splicing activity but also inhibits the activity of SRSF1 (45). Thus, SRSF1-3 might function as a competitive inhibitor of SRSF1 in vivo. Indeed, introduction of SRSF1-3 resulted in an increased nuclear accumulation of unspliced nascent Ig transcripts in DT40-ASF cells (Fig. 6A). Interestingly, the unspliced RNA accumulation was largely dependent on AID expression (Fig. 6B), implying a close relationship between unspliced Ig RNA generation and the presence of AID. Below, we discuss how accumulation of unspliced nascent Ig RNA facilitates AID-dependent SHM.
Fig. 6.
SRSF1-3 inhibits splicing of the Ig transcripts in an AID-dependent manner. (A) SRSF1-3 expression increased unspliced IgH RNAs in the nucleus. Nuclear RNAs extracted from SRSF1-3R cells were used for analysis. IgH transcripts with unspliced L-V and J-C introns or only J-C intron (designated as L-V/J-C and J-C, respectively) were analyzed by RT-PCR (Left). Positions of a sense primer for L exon (indicated as a) and antisense primers for J-C intron (indicated as b) are shown as arrowheads. Fourfold serially diluted cDNAs were used for PCR. RNAs without reverse transcription were used as controls (RT-). The level of unspliced transcript was also quantified by quantitative PCR (Right). (B) The increase of unspliced Ig transcripts depends on AID. RT-PCR of unspliced Ig transcripts was carried out using SRSF1-3-overexpressing DT40-SW cells (SRSF1-3O/E) with or without AID expression (Left). The level of unspliced transcript was also quantified by quantitative PCR (Right). Data are representative of two or more experiments.
Discussion
AID-dependent SHM is largely limited to the IgV genes, although off-target mutations and translocations that depend on AID are also found at much lower frequency (5, 6). What directs the SHM machinery to its targets is an important issue for understanding not only SHM but also the mechanisms of AID-linked oncogenic genomic alterations. However, reliable culture conditions have not been established that enable naïve human or murine B cells to undergo SHM ex vivo. Thus, DT40 cells, which spontaneously undergo hypermutation, are useful for analyzing mechanisms of SHM, because the Ig diversification process in chicken and mammals is similar (31). The present results have established an essential role for a splicing isoform of the SR protein SRSF1, SRSF1-3, in AID-induced SHM in the IgV genes in DT40 cells. The specific action and localization of SRSF1-3 to the IgV genes leads us to the conclusion that SRSF1-3 plays a critical role in the targeted induction of AID activity specifically in the IgV genes, probably by providing AID with substrate ssDNA on the IgV genes by reducing Ig RNA splicing.
Several AID cofactors have been reported to be essential for AID-dependent SHM, GCV and CSR. However, there is little evidence for cofactors that restrict the activity of AID specifically to the Ig genes, although Liu and Schatz have proposed that non-Ig loci are protected from AID-induced SHM by high-fidelity DNA repair (46). The present study showed that constitutive expression of AID was not sufficient for SHM induction in DT40-ASF cells, although the amount of AID was enough to cause translocations when SRSF1 was depleted. Thus, in the absence of SRSF1-3, the IgV genes behave similarly to non-Ig loci and are not subject to SHM. Because SRSF1-3 appeared not to enhance recruitment of AID to the IgV genes, the SRSF1-3-induced activation of SHM specifically on the IgV genes must result from another activity of SRSF1-3, and we suggest that this reflects its inhibitory effect on SRSF1 (45).
We suggest that the enhanced accumulation of unspliced Ig transcripts by SRSF1-3 results from inhibition of SRSF1 activity by SRSF1-3. Depletion of SRSF1 results in the generation of cotranscriptional R loops, likely, at least in part, from an increase in unspliced nascent transcripts, suggesting that SRSF1 suppresses transcription-linked ssDNA formation to maintain genomic stability (32). Thus, an SRSF1-3-induced increase of unspliced transcripts likely also generates R loops, leading to the generation and/or stabilization of ssDNA substrates for AID through SRSF1-3-mediated inhibition of SRSF1 specifically on the IgV genes. The AID-dependent increase of unspliced RNA might result from stalling and/or dissociation of elongating transcription complexes by the action of AID that causes DNA lesions, as initially proposed by Peters and Storb (21). Similarly, disruption of cotranscriptional mRNA packaging by mutations in the THO/TREX complex has been shown to promote R-loop formation that can be accessible by AID (26). In addition, it has been recently reported that Spt5, a factor associated with stalled Pol II and ssDNA, is required for recruiting AID to target sites during CSR (16). During immune responses, centroblasts, a major B-cell population undergoing SHM in germinal centers, are surface Ig-dim or negative (47). This in vivo observation is in good agreement with recent reports and our findings in the context of regulation of transcription and posttranscriptional processes of the Ig genes.
Hypermutation in DT40 cells has been estimated to occur approximately every 40 cell cycles (27). That is probably attributable to a low level of SRSF1-3 protein. SRSF1 has been shown to autoregulate its own expression (48), and, thus, a relatively large amount of SRSF1 will suppress expression of SRSF1-3, consistent with our finding that the level of SRSF1-3 was drastically increased when SRSF1 was depleted. As a result, the level of unspliced Ig RNA in the nuclei of DT40 cells appeared to be very low. In good agreement with this, overexpression of SRSF1-3 in wild-type DT40 cells enhanced GCV frequency and generation of unspliced Ig RNA in an AID-dependent manner. Thus, regulation of SRSF1-3 expression to low levels might result in stochastic occurrence of hypermutation in DT40 cells.
Alternative splicing enables particular genes to produce two or more mRNAs, each of which can have different physiological functions. SRSF1 was originally described as an alternative splicing factor, but more recent studies have elucidated its roles in many aspects of RNA metabolism (34, 35). The present study not only reveals a function of SRSF1 in AID-dependent hypermutation, but also establishes a function for the splice isoform SRSF1-3, whose function has been hitherto unknown. Thus, our findings have provided insights into both the mechanisms of AID-dependent hypermutation and the extended functions of SR proteins in cellular metabolism.
Materials and Methods
Analysis of Hypermutation in the IgV Gene.
DT40 cells were propagated from single subclones for 1 mo. The IgVL and IgVH genes were amplified from genomic DNA by PCR and analyzed to find mutations. Mutations were categorized as GCV or point mutation by comparing mutated sequences with published V pseudogene sequences as reported previously (49). The frequency of mutation events was calculated by dividing all mutation events by the total analyzed nucleotide numbers. An artificial GCV substrate, G/B construct was also used to analyze hypermutation frequency in DT40-SW cells as described previously (39). AID expression in DT40-SW cells were switched on, and single cells expressing AID (AID-ON cells) were sorted as described previously (40). Colonies were transferred to 24-well plate and maintained for 1 mo between ∼1 × 105 and 1 × 106 cells. Frequency of cells with strong green fluorescence was analyzed using FACS Calibur (BD Biosciences).
SRSF1-Depletion Assay.
Genomic instability caused by depletion of SRSF1 in DT40-ASF cells was assessed using Tet or a Tet analog, Dox (Sigma-Aldrich) (32). After 1μg/mL Tet or 50 ng/mL Dox treatment for 2 d, DT40-ASF cells were seeded on 96-well plates at 2, 20, 200, 2,000, or 20,000 cells/well. Cells were maintained in the presence of 1μg/mL Tet or 50 ng/mL Dox, respectively, for 10 d, and the surviving clones were scored.
ChIP.
ChIP assays were carried out according to a recently reported method with modifications (6). After cells were fixed and lysed, nuclear fractions were sonicated and separated by immunoprecipitation using mouse anti-AID mAb (clone ZA001, Invitrogen), rabbit anti-acetylated histone H4 (Millipore), anti-Flag [clone M2 (Sigma-Aldrich) or clone 1E6 for quantitative PCR (Wako)], or isotype-matched control (mouse IgG1 clone G3A1, Cell Signaling Technology), and protein G-conjugated magnetic beads (Dynabeads Protein G; Invitrogen Dynal AS). DNAs extracted from precipitates were analyzed by PCR.
Analysis of Nuclear Unspliced RNA.
Total RNA was extracted from the nuclear fraction of DT40 cells using TRIzol Reagent (Invitrogen) and treated with DNase I (Takara Bio). cDNA was synthesized from total RNA using random hexamer primers and the SuperScript II Reverse Transcriptase (Invitrogen). Spliced and unspliced IgH transcripts were amplified using KOD FX DNA polymerase (Toyobo).
Primers used are listed in Table S2. Detailed experimental procedures are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank N. Matsumoto for technical assistance, Y. Kondo for chicken experiments, and J. M. Beurstedde and H. Arakawa for plox-bsr and pExpress vectors. This work was supported, in part, by grants from the Ministry of Education, Culture, Sports, Science and Technology and the New Energy and Industrial Development Technology Organization of Japan. J.L.M. acknowledges support from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120368109/-/DCSupplemental.
References
- 1.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 2.Revy P, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 3.Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 4.Peled JU, et al. The biochemistry of somatic hypermutation. Annu Rev Immunol. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- 5.Liu M, et al. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 2008;451:841–845. doi: 10.1038/nature06547. [DOI] [PubMed] [Google Scholar]
- 6.Yamane A, et al. Deep-sequencing identification of the genomic targets of the cytidine deaminase AID and its cofactor RPA in B lymphocytes. Nat Immunol. 2011;12:62–69. doi: 10.1038/ni.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shinkura R, et al. Separate domains of AID are required for somatic hypermutation and class-switch recombination. Nat Immunol. 2004;5:707–712. doi: 10.1038/ni1086. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhuri J, Khuong C, Alt FW. Replication protein A interacts with AID to promote deamination of somatic hypermutation targets. Nature. 2004;430:992–998. doi: 10.1038/nature02821. [DOI] [PubMed] [Google Scholar]
- 9.Vuong BQ, et al. Specific recruitment of protein kinase A to the immunoglobulin locus regulates class-switch recombination. Nat Immunol. 2009;10:420–426. doi: 10.1038/ni.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu U, et al. The AID antibody diversification enzyme is regulated by protein kinase A phosphorylation. Nature. 2005;438:508–511. doi: 10.1038/nature04255. [DOI] [PubMed] [Google Scholar]
- 11.Nambu Y, et al. Transcription-coupled events associating with immunoglobulin switch region chromatin. Science. 2003;302:2137–2140. doi: 10.1126/science.1092481. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Geraldes P, Platt JL, Cascalho M. The double-edged sword of activation-induced cytidine deaminase. J Immunol. 2005;174:934–941. doi: 10.4049/jimmunol.174.2.934. [DOI] [PubMed] [Google Scholar]
- 13.MacDuff DA, Neuberger MS, Harris RS. MDM2 can interact with the C-terminus of AID but it is inessential for antibody diversification in DT40 B cells. Mol Immunol. 2006;43:1099–1108. doi: 10.1016/j.molimm.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Okazaki I-M, et al. Histone chaperone Spt6 is required for class switch recombination but not somatic hypermutation. Proc Natl Acad Sci USA. 2011;108:7920–7925. doi: 10.1073/pnas.1104423108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z, et al. 14-3-3 adaptor proteins recruit AID to 5'-AGCT-3'-rich switch regions for class switch recombination. Nat Struct Mol Biol. 2010;17:1124–1135. doi: 10.1038/nsmb.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavri R, et al. Activation-induced cytidine deaminase targets DNA at sites of RNA polymerase II stalling by interaction with Spt5. Cell. 2010;143:122–133. doi: 10.1016/j.cell.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conticello SG, et al. Interaction between antibody-diversification enzyme AID and spliceosome-associated factor CTNNBL1. Mol Cell. 2008;31:474–484. doi: 10.1016/j.molcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Nowak U, Matthews AJ, Zheng S, Chaudhuri J. The splicing regulator PTBP2 interacts with the cytidine deaminase AID and promotes binding of AID to switch-region DNA. Nat Immunol. 2010;12:160–166. doi: 10.1038/ni.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu U, et al. The RNA exosome targets the AID cytidine deaminase to both strands of transcribed duplex DNA substrates. Cell. 2011;144:353–363. doi: 10.1016/j.cell.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeevan-Raj BP, et al. Epigenetic tethering of AID to the donor switch region during immunoglobulin class switch recombination. J Exp Med. 2011;208:1649–1660. doi: 10.1084/jem.20110118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters A, Storb U. Somatic hypermutation of immunoglobulin genes is linked to transcription initiation. Immunity. 1996;4:57–65. doi: 10.1016/s1074-7613(00)80298-8. [DOI] [PubMed] [Google Scholar]
- 22.Fukita Y, Jacobs H, Rajewsky K. Somatic hypermutation in the heavy chain locus correlates with transcription. Immunity. 1998;9:105–114. doi: 10.1016/s1074-7613(00)80592-0. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhuri J, et al. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- 24.Yu K, Chedin F, Hsieh C-L, Wilson TE, Lieber MR. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat Immunol. 2003;4:442–451. doi: 10.1038/ni919. [DOI] [PubMed] [Google Scholar]
- 25.Ramiro AR, Stavropoulos P, Jankovic M, Nussenzweig MC. Transcription enhances AID-mediated cytidine deamination by exposing single-stranded DNA on the nontemplate strand. Nat Immunol. 2003;4:452–456. doi: 10.1038/ni920. [DOI] [PubMed] [Google Scholar]
- 26.Gómez-González B, Aguilera A. Activation-induced cytidine deaminase action is strongly stimulated by mutations of the THO complex. Proc Natl Acad Sci USA. 2007;104:8409–8414. doi: 10.1073/pnas.0702836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buerstedde J-M, et al. Light chain gene conversion continues at high rate in an ALV-induced cell line. EMBO J. 1990;9:921–927. doi: 10.1002/j.1460-2075.1990.tb08190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S, Humphries EH, Tjoelker L, Carlson L, Thompson CB. Ongoing diversification of the rearranged immunoglobulin light-chain gene in a bursal lymphoma cell line. Mol Cell Biol. 1990;10:3224–3231. doi: 10.1128/mcb.10.6.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arakawa H, Hauschild J, Buerstedde J-M. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science. 2002;295:1301–1306. doi: 10.1126/science.1067308. [DOI] [PubMed] [Google Scholar]
- 30.Harris RS, Sale JE, Petersen-Mahrt SK, Neuberger MS. AID is essential for immunoglobulin V gene conversion in a cultured B cell line. Curr Biol. 2002;12:435–438. doi: 10.1016/s0960-9822(02)00717-0. [DOI] [PubMed] [Google Scholar]
- 31.Arakawa H, Buerstedde J-M. Activation-induced cytidine deaminase-mediated hypermutation in the DT40 cell line. Philos Trans R Soc Lond B Biol Sci. 2009;364:639–644. doi: 10.1098/rstb.2008.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Manley JL. Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell. 2005;122:365–378. doi: 10.1016/j.cell.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Manley JL, Krainer AR. A rational nomenclature for serine/arginine-rich protein splicing factors (SR proteins) Genes Dev. 2010;24:1073–1074. doi: 10.1101/gad.1934910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long JC, Caceres JF. The SR protein family of splicing factors: Master regulators of gene expression. Biochem J. 2009;417:15–27. doi: 10.1042/BJ20081501. [DOI] [PubMed] [Google Scholar]
- 35.Zhong X-Y, Wang P, Han J, Rosenfeld MG, Fu X-D. SR proteins in vertical integration of gene expression from transcription to RNA processing to translation. Mol Cell. 2009;35:1–10. doi: 10.1016/j.molcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Takagaki Y, Manley JL. Targeted disruption of an essential vertebrate gene: ASF/SF2 is required for cell viability. Genes Dev. 1996;10:2588–2599. doi: 10.1101/gad.10.20.2588. [DOI] [PubMed] [Google Scholar]
- 37.Ge H, Zuo P, Manley JL. Primary structure of the human splicing factor ASF reveals similarities with Drosophila regulators. Cell. 1991;66:373–382. doi: 10.1016/0092-8674(91)90626-a. [DOI] [PubMed] [Google Scholar]
- 38.Tacke R, Boned A, Goridis C. ASF alternative transcripts are highly conserved between mouse and man. Nucleic Acids Res. 1992;20:5482. doi: 10.1093/nar/20.20.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanayama N, Todo K, Takahashi S, Magari M, Ohmori H. Genetic manipulation of an exogenous non-immunoglobulin protein by gene conversion machinery in a chicken B cell line. Nucleic Acids Res. 2006;34:e10. doi: 10.1093/nar/gnj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanayama N, Todo K, Reth M, Ohmori H. Reversible switching of immunoglobulin hypermutation machinery in a chicken B cell line. Biochem Biophys Res Commun. 2005;327:70–75. doi: 10.1016/j.bbrc.2004.11.143. [DOI] [PubMed] [Google Scholar]
- 41.Ramiro AR, et al. AID is required for c-myc/IgH chromosome translocations in vivo. Cell. 2004;118:431–438. doi: 10.1016/j.cell.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Gan W, et al. R-loop-mediated genomic instability is caused by impairment of replication fork progression. Genes Dev. 2011;25:2041–2056. doi: 10.1101/gad.17010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gossen M, et al. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 44.Maeda K, et al. GANP-mediated recruitment of activation-induced cytidine deaminase to cell nuclei and to immunoglobulin variable region DNA. J Biol Chem. 2010;285:23945–23953. doi: 10.1074/jbc.M110.131441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuo P, Manley JL. Functional domains of the human splicing factor ASF/SF2. EMBO J. 1993;12:4727–4737. doi: 10.1002/j.1460-2075.1993.tb06161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M, Schatz DG. Balancing AID and DNA repair during somatic hypermutation. Trends Immunol. 2009;30:173–181. doi: 10.1016/j.it.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 47.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 48.Sun S, Zhang Z, Sinha R, Karni R, Krainer AR. SF2/ASF autoregulation involves multiple layers of post-transcriptional and translational control. Nat Struct Mol Biol. 2010;17:306–312. doi: 10.1038/nsmb.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magari M, et al. Enhancement of hypermutation frequency in the chicken B cell line DT40 for efficient diversification of the antibody repertoire. Biochem Biophys Res Commun. 2010;396:353–358. doi: 10.1016/j.bbrc.2010.04.096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.