Abstract
Phagocytosis of Borrelia burgdorferi, the causative agent of Lyme disease, is a poorly understood process, despite its importance during the host immune response to infection. B. burgdorferi has been shown to bind to different receptors on the surface of phagocytic cells, including the β2 integrin, complement receptor 3 (CR3). However, whether these receptors mediate the phagocytosis of the spirochete remains unknown. We now demonstrate that CR3 mediates the phagocytosis of the spirochete by murine macrophages and human monocytes. Interaction of B. burgdorferi with the integrin is not sufficient, however, to internalize the spirochete; phagocytosis requires the interaction of CR3 with the GPI-anchored protein, CD14, independently of TLR/MyD88-induced or inside-out signals. Interestingly, the absence of CR3 leads to marked increases in the production of TNF in vitro and in vivo, despite reduced spirochetal uptake. Furthermore, the absence of CR3 during infection with B. burgdorferi results in the inefficient control of bacterial burdens in the heart and increased Lyme carditis. Overall, our data identify CR3 as a MyD88-independent phagocytic receptor for B. burgdorferi that also participates in the modulation of the proinflammatory output of macrophages. These data also establish a unique mechanism of CR3-mediated phagocytosis that requires the direct cooperation of GPI-anchored proteins.
Lyme disease, caused by Borrelia burgdorferi is the most prevalent arthropod-borne infection in the United States (1). Lyme disease typically begins with an expanding annular skin rash known as erythema migrans. If not eradicated with appropriate antimicrobial therapy, the spirochete can disseminate hematogenously and invade target organs, giving rise to clinical manifestations such as carditis, meningitis, and acute arthritis (1). It is generally believed that the pathology associated with infection results from the activation of locally recruited immune cells and the ensuing inflammatory response (2). In particular, macrophages are critical components of the response to B. burgdorferi, particularly during skin and cardiac infection (3). Macrophages phagocytose spirochetes, contributing to their elimination from infected tissues (2, 3). Phagocytosis, in turn, leads to the production of proinflammatory factors that contribute to tissue damage (2).
The identification of borrelial lipoproteins as pathogen-associated molecular patterns (PAMPs) with potent stimulatory capacity through their interaction with TLR1/2-CD14 complexes was a milestone in our understanding of macrophage responses to B. burgdorferi (4–8). These studies led to the notion that the activation of phagocytic cells occurs principally via surface TLR signaling and that the responses to isolated lipoproteins mimic those elicited by whole organisms. Recent reports, however, have demonstrated that the induction of a complete proinflammatory response to B. burgdorferi, including the induction of type I IFNs, requires internalization of the spirochete (9–12). Surprisingly, even though the signaling receptors, TLR2 and CD14, are not involved in the internalization of the spirochete (13, 14), MyD88-mediated signals are partly required for phagocytosis of B. burgdorferi (14, 15), although phagocytosis still occurs in their absence (13, 14). MyD88, therefore, serves a dual role during the response of macrophages to B. burgdorferi, affecting both their proinflammatory and phagocytic functions.
Integrins are heterodimers composed of α- and β-subunits that mediate multiple essential cellular processes, including phagocytosis of microorganisms (16). The β2-integrin complement receptor (CR) 3 (αMβ2, CD11b/CD18) mediates internalization of bacteria in the presence (17) or absence (17, 18) of complement-derived opsonins, such as iC3b. CR3 alone is sufficient to mediate the internalization of microorganisms (19), but the participation of coreceptors, often the GPI-anchored protein CD14, may enhance phagocytosis mediated by the integrin. CD14 in combination with TLR2 induces the generation of MyD88-dependent inside-out signals that result in the activation of CR3 and its conversion to a high-affinity conformation (20–22). CR3 has been reported to mediate the nonopsonic binding of B. burgdorferi to human neutrophils and CHO cells expressing the integrin (23, 24), but its contribution to the internalization of the spirochete by phagocytic cells and the mechanisms involved have not been addressed.
In this report, we demonstrate that CR3 is a phagocytic receptor for B. burgdorferi. Importantly, our results indicate that CR3-mediated internalization of B. burgdorferi is independent of inside-out signals but requires the participation of CD14 as an accessory receptor. Our data also show that CR3/CD14-mediated phagocytosis tempers the proinflammatory output of macrophages in response to the spirochete. These data demonstrate a unique mechanism of CR3-mediated phagocytosis that involves GPI-anchored proteins in the absence of TLR-mediated signals, acting as a critical regulator of the response of macrophages to B. burgdorferi and the development of inflammation.
Results and Discussion
CR3 Is a Phagocytic Receptor for B. burgdorferi.
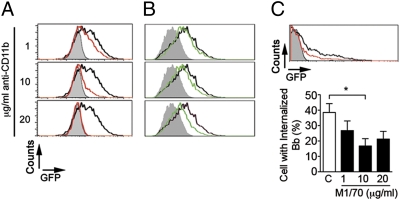
The β2 integrin complement receptor 3 has been shown to bind B. burgdorferi in human neutrophils and CHO cells (23, 24). Whether CR3 is associated with phagocytosis of the spirochete remains, however, unknown. To examine the role of CR3 in the phagocytosis of the spirochete, we first treated RAW264.7 cells with a blocking mAb against CD11b (clone M1/70) or CD11c (clone HL3, as a control) and tested their phagocytic activity in the absence of serum. The anti-CD11b mAb inhibited the phagocytosis of GFP-expressing B. burgdorferi (Bb914, strain 297 (25), as assessed by flow cytometry (Fig. S1A) and fluorescence microscopy (Fig. S1B). The effect of the anti-CD11b mAb was dose dependent, resulting in an almost complete abrogation of phagocytosis at a concentration of 20 μg/mL (Fig. 1A). The presence of the anti-CR3 mAb also resulted in a dose-dependent reduction of B. burgdorferi phagocytosis by bone marrow macrophages (BMMs) (Fig. 1B). However, even at high doses (20 μg/mL), the blocking effect of the mAb was incomplete (Fig. 1B). Similarly, the presence of M1/70 resulted in a partial reduction of phagocytosis by highly purified human monocytes (Fig. 1C).
Fig. 1.
CR3 mediates phagocytosis of B. burgdorferi. RAW264.7 cells (A) and BMMs (B) were incubated with Bb914 at an m.o.i. of 50 and 10, respectively, for 4 h in the absence (black histograms) or presence (green histograms) of increasing concentrations of anti-CD11b antibody; the gray histograms indicate a 4 °C control to determine background binding levels. The experiments shown are representative of at least three independent experiments. (C) Monocytes isolated from human peripheral blood were incubated with Bb914 (m.o.i. = 10) for 4 h in the presence of increasing concentrations of M1/70. (Upper) Representative flow cytometry histograms in the presence (red histogram) of 10 μg/mL of M1/70 or an isotype control (black histogram); the gray histogram indicates the 4 °C control. (Lower) The number of Bb914-containing monocytes assessed by epifluorescence microscopy. *Student's t test, P < 0.05.
CR3 Deficiency Results in Diminished Phagocytosis of B. burgdorferi.
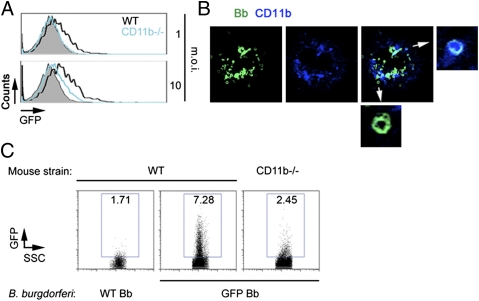
We next examined the capacity of CD11b-deficient BMMs to phagocytose B. burgdorferi. Compared with wild-type control (B6) macrophages, CD11b-deficient BMMs showed reduced phagocytic activity for B. burgdorferi, although internalization of spirochetes still occurred (Fig. 2A). The analysis of BMMs by confocal microscopy confirmed the colocalization of CD11b and GFP in some phagosomes, whereas others did not colocalize with the integrin (Fig. 2B), suggesting that internalization of the spirochete involves CR3 and other receptors. Although several surface proteins of the spirochete interact with integrins (26, 27), including OspA and OspB to CR3 (28), OspA/B-deficient spirochetes were phagocytosed by BMMs as efficiently as wild-type 297 (Fig. S2), indicating that other surface ligands mediate the interaction between CR3 and B. burgdorferi.
Fig. 2.
CR3 mediates phagocytosis of B. burgdorferi by BMMs. (A) Phagocytosis of Bb914 by BMMs generated from CD11b-deficient mice (blue histogram) or B6 controls (black histogram). The 4 °C binding control is represented by the gray histograms. The cells were tested at an m.o.i. of 1 (Upper) and 10 (Lower). (B) Confocal micrograph of a BMM showing colocalization of GFP (B. burgdorferi Bb914, green) and CD11b (blue). The small micrographs represent internalized B. burgdorferi (arrows) that displayed presence or absence of colocalization with CD11b. (C) In vivo phagocytosis of B. burgdorferi by CD11b-deficient and wild-type B6 mice injected intraperitoneally with 1 × 107 spirochetes. A B6 mouse was also injected with non-GFP (WT) B. burgdorferi to control for migration of inflammatory cells into the peritoneum. The peritoneal lavages were analyzed after 6 h by flow cytometry. Shown are representative results for a total of three CD11b-deficient and three control B6 mice.
We next performed in vivo phagocytosis assays (29). We injected 1 × 107 Bb914 intraperitoneally into CD11b-deficient and wild-type mice. After 6 h, the peritoneal lavage was analyzed by flow cytometry. CD11b-deficient macrophages contained lower levels of GFP-expressing B. burgdorferi than wild-type controls (Fig. 2C). The percentage of F4/80+ cells did not vary between wild-type and CD11b-deficient animals (WT, 21.2 ± 1%; CD11b−/−, 20.2 ± 3.1%), indicating that the difference was not due to a diminished capacity of CD11b-negative macrophages to migrate to the site of injection. Overall, these data demonstrate that CR3 is a phagocytic receptor for B. burgdorferi.
CR3 Mediates Binding but Not Internalization of B. burgdorferi.
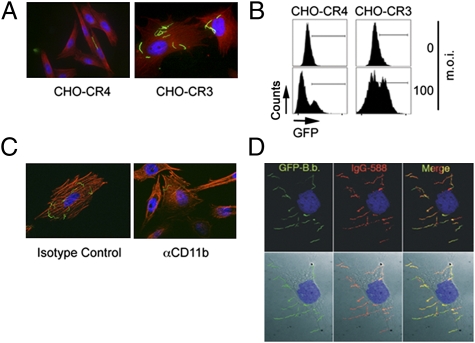
To confirm the interaction between B. burgdorferi and CR3, we used CHO cells transfected with human CR3 or CR4 (CD11c/CD18, control) (30). Binding of B. burgdorferi to CHO-CR3 cells was significantly higher than to CHO cells expressing CR4 (Fig. 3 A and B and Fig. S3A). The use of the anti-CD11b mAb M1/70 resulted in reduced levels of B. burgdorferi bound to CHO-CR3 cells (Fig. 3C), demonstrating that the interaction is CR3 specific. Compared with RAW264.7 cells and BMMs (Figs. 1B and 2B), organisms attached to CHO-CR3 cells retained their characteristic morphology (Fig. 3A), suggesting that they were not internalized. Indeed, spirochetes bound to unfixed, intact CHO-CR3 cells were accessible to specific antibodies (Fig. 3D). Furthermore, vigorous washing completely disrupted the association between spirochetes and cells (Fig. S3B). Thus, CR3 is not sufficient for phagocytosis of the spirochete.
Fig. 3.
CR3 alone can mediate binding but not internalization of B. burgdorferi by CHO cells. (A) Microscopic analysis of binding of B. burgdorferi by CHO-CR4 and CHO-CR3 cells. A total of 5 × 104 CHO-CR4 and CHO-CR3 cells were grown for 16 h in eight-well chamber slides and incubated with Bb914 for 6 h at an m.o.i. of 100. The cells were washed, fixed, and stained with rhodamine phalloidin (red) and DAPI (blue). (B) Flow cytometry of Bb914 interaction with CR4- and CR3-expressing CHO cells after 6 h at an m.o.i. of 100. The graph is representative of three independent experiments. (C) CHO-CR3 cells grown in eight-well chamber slides were incubated with Bb914 at an m.o.i. of 100 in the presence of 10 μg/ml of anti-CD11b blocking antibody or an antibody control. The cells were fixed and stained with phalloidin (red) and DAPI (blue) and analyzed by microscopy. (D) CHO-CR3 cells were grown in eight-well chamber slides and incubated with Bb914 as before at an m.o.i. of 100 for 6 h. The unfixed cells were incubated with murine-infected serum (1/400) followed by anti-mouse IgG Alexa Fluor 568. (Lower) DIC images.
CD14 Cooperates with CR3 to Promote Phagocytosis of B. burgdorferi Independently of TLR2 and MyD88-Induced Signals.
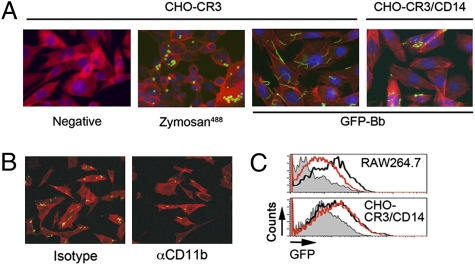
The previous results could be due to an intrinsic defect of the cells to internalize CR3-dependent targets or, alternatively, because CR3-dependent phagocytosis of spirochetes requires a coreceptor. CHO-CR3 cells were able to phagocytose zymosan particles (Fig. 4A), confirming that CR3 ectopically expressed in CHO cells is functional. To determine whether phagocytosis of B. burgdorferi mediated by CR3 requires the presence of a coreceptor, we used CHO-CR3 cells stably transfected with the GPI-anchored protein, CHO-CR3/CD14 (31). CHO-CR3/CD14 cells were readily able to phagocytose B. burgdorferi (Fig. 4A), which was dependent on the interaction with CR3, given that mAb M1/70 diminished the uptake of the spirochete (Fig. 4B). To assess whether CD14 cooperates with CR3 by participating in the binding of the spirochete, we compared CHO-CR3 and CHO-CR3/CD14 cells. Both cells bound spirochetes equivalently at increasing multiplicity of infection (m.o.i.) (Fig. S4), showing that CD14 does not contribute to the binding of B. burgdorferi.
Fig. 4.
CD14 cooperates with CR3 to promote phagocytosis of B. burgdorferi. (A) CHO-CR3 and CHO-CR3-CD14 cells were grown in eight-well chamber slides and incubated with Zymosan labeled with Alexa Fluor 488 (m.o.i. = 5) or Bb914 (m.o.i. = 25) for 4 h. The cells were washed, fixed, permeabilized, and stained with phalloidin Alexa Fluor 594, followed by analysis by Apotome fluorescence microscopy. (B) CHO-CR3/CD14 cells grown in chamber slides were incubated with Bb914 (m.o.i. = 25) for 4 h in the presence of M1/70 mAb or an isotype control. The cells were fixed, stained with phalloidin Alexa Fluor 594, and analyzed by confocal microscopy. (C) Flow cytometry of phagocytosis by CHO-CR3/CD14 (Lower) and RAW264.7 cells (Upper) in the presence of MyD88-BP (red histograms) or the control peptide (black histograms). The cells were preincubated with the peptides at 50 μM for 1 h, before adding Bb914 (m.o.i. = 25).
CD14 enhances CR3-mediated phagocytosis through its association with TLR2 (20–22) and involves PI3K activity (21). However, CHO cells lack a functional TLR2 (32), implying that the uptake of the spirochete by CHO-CR3/CD14 cells occurs without MyD88-mediated signaling. We analyzed phagocytosis of Bb914 by CHO-CR3/CD14 cells in the presence of a membrane-permeable MyD88-blocking peptide (33). The blocking peptide did not affect the uptake of B. burgdorferi by CHO-CR3/CD14 cells (Fig. 4C and Fig. S5A), in contrast to the uptake by RAW264.7 cells (14) (Fig. 4C). We also analyzed the uptake of Bb914 by CHO-CR3/CD14 cells in the presence of the PI3K inhibitors, LY294002 and wortmannin. In contrast to that by RAW264.7 cells (Fig. S5B), internalization of Bb914 by CHO-CR3/CD14 cells was unaffected by either inhibitor (Fig. S5B). Overall, these data show that in the CHO cell system, CD14 cooperates with CR3 for the phagocytosis of B. burgdorferi but does so independently of TLR2 and MyD88-derived signals, including PI3K activity. Our data show that MyD88-independent phagocytosis involves, at least partly, the integrin CR3 and imply the presence of other receptor(s) in macrophages that contribute to the internalization of the spirochete by macrophages, including those that are MyD88 dependent. Whether CD14 is unique among GPI-anchored proteins in its ability to promote CR3-dependent phagocytosis of spirochetes remains to be addressed. The lack of a noticeable effect in the phagocytic capacity of macrophages that lack CD14 against B. burgdorferi (13) suggests the redundant function of other GPI-anchored proteins in CR3-mediated phagocytosis of B. burgdorferi.
Phagocytosis of B. burgdorferi Is Enhanced by, but Can Occur Independently of, CR3 Activation.
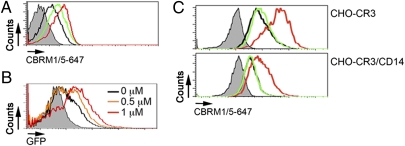
Integrins, including CR3, are normally exposed on the cell surface in a low-affinity conformation but undergo conformational changes that lead to increased affinity for their specific ligands (34). These inside-out signals can emanate from TLR2/CD14 engagement (20, 22) and other receptors, such as CD44 (35). We used the mAb clone CBRM1/5 as a marker of the conformational change by the integrin to a high-affinity state (21, 36). The incubation of RAW264.7 cells with 1 μM Mn2+ induced the expected increased binding of CBRM1/5 (36) (Fig. 5A), as did the stimulation with live B. burgdorferi (Fig. 5A). We next determined whether the activation of CR3 would increase the ability of RAW264.7 cells to phagocytose B. burgdorferi. The activation of CR3 by Mn+2 resulted in the increased internalization of the spirochete in a dose-dependent manner (Fig. 5B). We then assessed the effect of spirochete stimulation on CR3 affinity in both CHO-CR3 and CHO-CR3/CD14 cells. In both cases the stimulation with B. burgdorferi failed to induce the increased binding of CBRM1/5 (Fig. 5C), indicating that the activation of CR3 enhances, but is not required for, the internalization of B. burgdorferi.
Fig. 5.
Inside-out signals are not required for the phagocytosis of B. burgdorferi. (A) RAW264.7 cells were incubated with Bb914 (m.o.i. = 25, green histogram) or MnCl2 (1 μM, red histogram), washed, and stained with the mAb CBRM1/5. The cells were fixed and analyzed by flow cytometry. (B) RAW264.7 cells were incubated with increasing concentrations of MnCl2 (0.5 μM, orange histogram; 1 μM, red histogram) or left unstimulated (black histogram), washed, and assessed for their phagocytic activity using Bb914 (m.o.i. = 25). (C) CHO-CR3 (Upper) and CHO-CR3/CD14 cells (Lower) were incubated with Bb914 (green histograms) or MnCl2 (red histograms), as before. The cells were stained with the mAb CBRM1/5, fixed, and analyzed by flow cytometry.
Infection of CD11-Deficient Mice with B. burgdorferi Results in the Deficient Control of the Spirochete, Enhanced tnf Expression, and Increased Lyme Carditis.
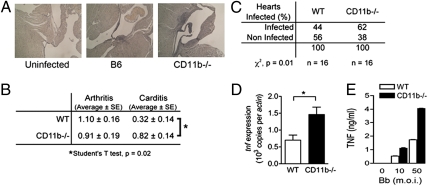
To determine the contribution of CR3 to spirochete control and proinflammatory cytokine induction, we infected CD11b-deficient mice with B. burgdorferi. The mice were analyzed at the peak of infection. CD11b-deficient mice developed similar levels of arthritis compared with wild-type controls (Fig. 6B). However, carditis was significantly enhanced in the absence of the integrin (Fig. 6 A and B). The absence of CD11b resulted in a significant increase in the percentage of infected hearts (Fig. 6C) as well as in the geometric mean of the number of bacteria per infected heart (WT, 24; CD11b deficient, 174 copies of spirochetal recA per copy of murine actin). Furthermore, the analysis of tnf gene expression levels in the infected heart by qRT-PCR revealed significantly increased levels of the cytokine transcripts in the absence of the integrin (Fig. 6D). In vitro, CD11b-deficient BMMs produced highly increased levels of TNF in response to the spirochete compared with wild-type controls, despite reduced phagocytosis (Fig. 6E). The observed phenotype in the absence of CR3 mimics that reported in CD14-deficient mice (37) and strongly supports a model in which the GPI-anchored protein cooperates with CR3 in the internalization of B. burgdorferi and the modulation of cytokine expression in response to the spirochete. Importantly, the IFN-γ–induced increase of phagocytosis of the spirochete (2) correlated with the induction of CR3 expression (Fig. S6 A and B) and could be blocked by M1/70 (Fig. S6 C and D), associating the protective capacity of IFN-γ (2, 38) with the control of the number of spirochetes during infection.
Fig. 6.
Lyme carditis in CD11b-deficient mice involves increased inflammation and deficient control of B. burgdorferi. CD11b-deficient and wild-type controls were infected with 1 × 105 spirochetes by s.c. injection in the midline of the back. (A and B) After being euthanized, the mice were analyzed histologically for signs of arthritis (B) and carditis (A and B). *Student's t test, P < 0.05. n = 11 in each group. (C) Percentages of hearts positive for B. burgdorferi recA targets by qPCR. *χ2, P < 0.05. n = 16 in each group. (D) The levels of tnf gene expression in the hearts of the infected mice were analyzed by qRT-PCR relative to actin levels. *Student's t test, P < 0.05. n = 11 in each group. The results represent two to three independent experiments with 5–6 mice per group. (E) TNF production by B6 and CD11b-deficient BMMs in response to B. burgdorferi stimulation. BMMs were stimulated with live spirochetes at increasing m.o.i. for 16 h, followed by the analysis of the stimulation supernatants for TNF by ELISA.
Overall, our results demonstrate a unique mechanism of CR3-mediated phagocytosis that requires the cooperation of the GPI-anchored protein, CD14, in the absence of MyD88-mediated signals. These data also reveal the modulatory capacity of CR3/CD14 in the proinflammatory cytokine output of macrophages and the development of cardiac inflammation during infection with the spirochete, situating CR3 as a central pathogen recognition receptor for the bacterium.
Materials and Methods
Mice.
C57BL/6 (B6) and CD11b-deficient mice were purchased from Jackson Laboratories. The Institutional Animal Care and Use Committee at University of Massachusetts, Amherst, approved all procedures involving animals.
Cells.
Bone marrow-derived macrophages were generated as described in ref. 2 and SI Materials and Methods. CHO cells transfected with human CR3, CD14, or CR4 were maintained in Ham's F-12 medium (Sigma Chemical). The macrophage-like RAW264.7 cell line was maintained in RPMI 1640. Peripheral blood human monocytes were purified as previously described from Lyme seronegative healthy volunteers (11). Experiments involving human monocytes were approved by the Institutional Review Board at the University of Connecticut Health Center.
Bacteria.
Bb914, a virulent strain 297 B. burgdorferi clone that contains a constitutively expressed gfp reporter stably inserted into cp26 (25), and wild-type B. burgdorferi 297 were used throughout.
CR3 Receptor Blocking.
Before coculture of B. burgdorferi with CHO cells, purified human monocytes, or murine macrophages, cells were preincubated for 15 min with 1, 10, or 20 μg/mL of rat anti-mouse CD11b clone M1/70 mAb (BD Biosciences), which reacts with both murine and human CD11b (39). As controls, cells were preincubated with the same concentration of Armenian Hamster anti-mouse CD11c clone HL3 or a rat IgG isotype control (BD Biosciences).
Phagocytosis Assay.
Phagocytosis of B. burgdorferi by CHO cells and monocytes/macrophages was analyzed as described in ref. 4 and SI Materials and Methods. To confirm phagocytosis, cells were examined by epifluorescence (ApoTome) and confocal microscopy (SI Materials and Methods).
In Vivo Phagocytosis.
Mice were injected intraperitoneally with 107 B. burgdorferi. The macrophages were harvested after 6 h by peritoneal lavage with 10 mL of ice-cold PBS, stained with mAb F4/80 conjugated with allophycocyanin (APC) (eBioscience) in PBS, 1% FCS for 30 min at 4 °C, and analyzed by flow cytometry.
TNF ELISA.
The levels of TNF produced by B. burgdorferi stimulation of B6 and CD11b-deficient BMMs were determined by capture ELISA according to manufacturer's recommendations, using the BD optEIA TNF set II kit (BD Bioscience).
Analysis of CR3 Affinity Conformation.
The mAb CBRM1/5 was used to establish the activation status of CR3, as described in SI Materials and Methods. To address the effect of CR3 activation on B. burgdorferi phagocytosis, 106 cells were incubated for 15 min at 37 °C with 0.5 or 1 μM MnCl2 and washed. The cells were then assessed for their phagocytic activity.
Infection with B. burgdorferi.
CD11b-deficient mice and wild-type littermates were infected with B. burgdorferi as described in ref. 2. The mice were killed at the peak of infection and analyzed for inflammation, bacterial burdens, and tnf gene expression levels in the heart (SI Materials and Methods).
Statistical Analysis.
The results are presented as means ± SE. Significant differences between means were calculated with Student's t test. The percentages of infected murine hearts as determined by qPCR were compared by χ2-analysis. P values ≤0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Star Dunham-Elms for helpful discussions. We also thank Frank Yang and Michael V. Norgard for reagents. This work was supported by National Institutes of Health Grants AI-29735 (to J.D.R. and M.J.C.), AI-090166 (to J.C.S.), AI-080615 (to U.P.), and M01RR006192 (to the University of Connecticut Health Center); the National Research Fund for Tick-Borne Diseases (M.J.C.); the Connecticut Children's Medical Center's Arrison and Burr Curtis Research Funds (to J.C.S.), and by the American Heart Association (C.M.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112078109/-/DCSupplemental.
References
- 1.Shapiro ED. Lyme disease. Adv Exp Med Biol. 2008;609:185–195. doi: 10.1007/978-0-387-73960-1_14. [DOI] [PubMed] [Google Scholar]
- 2.Olson CM, Jr, et al. Local production of IFN-γ by invariant NKT cells modulates acute Lyme carditis. J Immunol. 2009;182:3728–3734. doi: 10.4049/jimmunol.0804111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery RR, et al. Recruitment of macrophages and polymorphonuclear leukocytes in Lyme carditis. Infect Immun. 2007;75:613–620. doi: 10.1128/IAI.00685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aliprantis AO, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 5.Hirschfeld M, et al. Cutting edge: Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- 6.Alexopoulou L, et al. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002;8:878–884. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- 7.Sellati TJ, et al. Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J Immunol. 1999;163:2049–2056. [PubMed] [Google Scholar]
- 8.Wooten RM, et al. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J Immunol. 1998;160:5485–5492. [PubMed] [Google Scholar]
- 9.Moore MW, et al. Phagocytosis of Borrelia burgdorferi and Treponema pallidum potentiates innate immune activation and induces γ interferon production. Infect Immun. 2007;75:2046–2062. doi: 10.1128/IAI.01666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cervantes JL, et al. Phagosomal signaling by Borrelia burgdorferi in human monocytes involves Toll-like receptor (TLR) 2 and TLR8 cooperativity and TLR8-mediated induction of IFN-β. Proc Natl Acad Sci USA. 2011;108:3683–3688. doi: 10.1073/pnas.1013776108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salazar JC, et al. Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-β. PLoS Pathog. 2009;5:e1000444. doi: 10.1371/journal.ppat.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petzke MM, Brooks A, Krupna MA, Mordue D, Schwartz I. Recognition of Borrelia burgdorferi, the Lyme disease spirochete, by TLR7 and TLR9 induces a type I IFN response by human immune cells. J Immunol. 2009;183:5279–5292. doi: 10.4049/jimmunol.0901390. [DOI] [PubMed] [Google Scholar]
- 13.Sahay B, et al. CD14 signaling restrains chronic inflammation through induction of p38-MAPK/SOCS-dependent tolerance. PLoS Pathog. 2009;5:e1000687. doi: 10.1371/journal.ppat.1000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin OS, et al. Distinct roles for MyD88 and Toll-like receptors 2, 5, and 9 in phagocytosis of Borrelia burgdorferi and cytokine induction. Infect Immun. 2008;76:2341–2351. doi: 10.1128/IAI.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin OS, et al. Downstream signals for MyD88-mediated phagocytosis of Borrelia burgdorferi can be initiated by TRIF and are dependent on PI3K. J Immunol. 2009;183:491–498. doi: 10.4049/jimmunol.0900724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben Nasr A, et al. Critical role for serum opsonins and complement receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in phagocytosis of Francisella tularensis by human dendritic cells (DC): Uptake of Francisella leads to activation of immature DC and intracellular survival of the bacteria. J Leukoc Biol. 2006;80:774–786. doi: 10.1189/jlb.1205755. [DOI] [PubMed] [Google Scholar]
- 18.Mobberley-Schuman PS, Weiss AA. Influence of CR3 (CD11b/CD18) expression on phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun. 2005;73:7317–7323. doi: 10.1128/IAI.73.11.7317-7323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Cabec V, Cols C, Maridonneau-Parini I. Nonopsonic phagocytosis of zymosan and Mycobacterium kansasii by CR3 (CD11b/CD18) involves distinct molecular determinants and is or is not coupled with NADPH oxidase activation. Infect Immun. 2000;68:4736–4745. doi: 10.1128/iai.68.8.4736-4745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sendide K, et al. Cross-talk between CD14 and complement receptor 3 promotes phagocytosis of mycobacteria: Regulation by phosphatidylinositol 3-kinase and cytohesin-1. J Immunol. 2005;174:4210–4219. doi: 10.4049/jimmunol.174.7.4210. [DOI] [PubMed] [Google Scholar]
- 21.Oliva C, Turnbough CL, Jr, Kearney JF. CD14-Mac-1 interactions in Bacillus anthracis spore internalization by macrophages. Proc Natl Acad Sci USA. 2009;106:13957–13962. doi: 10.1073/pnas.0902392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harokopakis E, Hajishengallis G. Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur J Immunol. 2005;35:1201–1210. doi: 10.1002/eji.200425883. [DOI] [PubMed] [Google Scholar]
- 23.Cinco M, Murgia R, Perticarari S, Presani G. Surface receptors of neutrophils towards B. burgdorferi. Wien Klin Wochenschr. 1998;110:866–869. [PubMed] [Google Scholar]
- 24.Cinco M, Murgia R, Presani G, Perticarari S. Integrin CR3 mediates the binding of nonspecifically opsonized Borrelia burgdorferi to human phagocytes and mammalian cells. Infect Immun. 1997;65:4784–4789. doi: 10.1128/iai.65.11.4784-4789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunham-Ems SM, et al. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J Clin Invest. 2009;119:3652–3665. doi: 10.1172/JCI39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behera AK, et al. Borrelia burgdorferi BBB07 interaction with integrin α3β1 stimulates production of pro-inflammatory mediators in primary human chondrocytes. Cell Microbiol. 2008;10:320–331. doi: 10.1111/j.1462-5822.2007.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Defoe G, Coburn J. Delineation of Borrelia burgdorferi p66 sequences required for integrin α(IIb)β(3) recognition. Infect Immun. 2001;69:3455–3459. doi: 10.1128/IAI.69.5.3455-3459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia RC, Murgia R, Cinco M. Complement receptor 3 binds the Borrelia burgdorferi outer surface proteins OspA and OspB in an iC3b-independent manner. Infect Immun. 2005;73:6138–6142. doi: 10.1128/IAI.73.9.6138-6142.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amiel E, Acker JL, Collins RM, Berwin B. Uncoupling scavenger receptor A-mediated phagocytosis of bacteria from endotoxic shock resistance. Infect Immun. 2009;77:4567–4573. doi: 10.1128/IAI.00727-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medvedev AE, et al. Involvement of CD14 and complement receptors CR3 and CR4 in nuclear factor-kappaB activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J Immunol. 1998;160:4535–4542. [PubMed] [Google Scholar]
- 31.Golenbock DT, Liu Y, Millham FH, Freeman MW, Zoeller RA. Surface expression of human CD14 in Chinese hamster ovary fibroblasts imparts macrophage-like responsiveness to bacterial endotoxin. J Biol Chem. 1993;268:22055–22059. [PubMed] [Google Scholar]
- 32.Heine H, et al. Cutting edge: Cells that carry A null allele for toll-like receptor 2 are capable of responding to endotoxin. J Immunol. 1999;162:6971–6975. [PubMed] [Google Scholar]
- 33.Loiarro M, et al. Peptide-mediated interference of TIR domain dimerization in MyD88 inhibits interleukin-1-dependent activation of NF-κB. J Biol Chem. 2005;280:15809–15814. doi: 10.1074/jbc.C400613200. [DOI] [PubMed] [Google Scholar]
- 34.Liddington RC, Ginsberg MH. Integrin activation takes shape. J Cell Biol. 2002;158:833–839. doi: 10.1083/jcb.200206011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vachon E, et al. CD44-mediated phagocytosis induces inside-out activation of complement receptor-3 in murine macrophages. Blood. 2007;110:4492–4502. doi: 10.1182/blood-2007-02-076539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hristov M, Gümbel D, Lutgens E, Zernecke A, Weber C. Soluble CD40 ligand impairs the function of peripheral blood angiogenic outgrowth cells and increases neointimal formation after arterial injury. Circulation. 2010;121:315–324. doi: 10.1161/CIRCULATIONAHA.109.862771. [DOI] [PubMed] [Google Scholar]
- 37.Benhnia MR, et al. Signaling through CD14 attenuates the inflammatory response to Borrelia burgdorferi, the agent of Lyme disease. J Immunol. 2005;174:1539–1548. doi: 10.4049/jimmunol.174.3.1539. [DOI] [PubMed] [Google Scholar]
- 38.Bockenstedt LK, et al. CD4+ T helper 1 cells facilitate regression of murine Lyme carditis. Infect Immun. 2001;69:5264–5269. doi: 10.1128/IAI.69.9.5264-5269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan ST, Edgington TS. Integrin regulation of leukocyte inflammatory functions. CD11b/CD18 enhancement of the tumor necrosis factor-alpha responses of monocytes. J Immunol. 1993;150:2972–2980. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.