Despite a myriad of advances in the understanding and development of vaccine formulations, safe and effective vaccines have yet to be discovered for many pathogens. An excellent example of such is the malarial parasite Plasmodium vivax. Not only does this parasite transition between both extracellular and intracellular states during infection, but it can remain dormant in the liver and have greater transmission potential with lower titers than its more notorious counterpart, Plasmodium falciparum (1). As a consequence, it is important for a candidate vaccine to elicit both cellular (Th1) and humoral (Th2) immune responses that are potent and long-lived. Although vaccine antigens have been identified for the malarial sporozoites (2), the resulting immune responses elicited are short-lived and limited in scope, which is not uncommon for subunit vaccines that do not contain all of the components of a live-attenuated vaccine (3). For this reason, the malarial subunit vaccine is a hallmark example of a formulation that will require an appropriate adjuvant capable of boosting the most relevant immune responses to be effective. In PNAS, Moon et al. (4) describe a unique, lipid-based nanoparticle adjuvant (called an interbilayer-crosslinked multilamellar vesicle, or ICMV) that could not only be a promising candidate for prophylaxis against P. vivax but may even provide clues to how protective immunity to malaria is acquired.
Nanoparticle adjuvants seem to be well suited for making this particularly challenging vaccine formulation effective. Indeed, nano- and microparticles are particularly flexible adjuvants that can serve as a point source for antigen retention and release in a sustained or even triggered fashion (5–7). Furthermore, as shown in the study by Moon et al. (4), synthetic particles can also be engineered to exhibit repetitive orientation of antigen on the surface. The multivalency of this surface antigen presentation has the potential to generate B-cell receptor cross-linking and enhanced activation, a phenomenon that has likely been acquired through evolution to recognize the repetitive nature of surface antigen on live pathogens (8). The result of this synthetic, multivalent presentation of subunit antigen on a nanoparticle surface is almost a full order of magnitude increase in antibody titers compared with using ICMVs that only encapsulate and release antigen but do not orient the antigen on the surface (4). Multivalent display also seems to lead to a more balanced Th1/Th2 response and may also play some role in the expansion of antigen-specific, follicular helper T cells, which are important in developing B-cell memory. Furthermore, achieving comparable antibody responses (with any of the conventional adjuvants used in this study) required 10 times the amount of unoriented, soluble antigen compared with particle-based orientation of that same antigen. Even then, responses were short-lived compared with those elicited by multivalent display of the antigen on a particle surface. It even seems that the majority of germinal centers (where B-cell responses are initiated) nucleate directly adjacent (within 100 μm) to the particle deposition centers in the draining lymph nodes. All of these observations seem consistent with the mechanisms of a particle-based adjuvant that would orient pathogen-based immunological cues around a point source and maintain persistent presentation, effectively mimicking how a “particle” or piece of sporozoite would be recognized (albeit in a circumscribed capacity).
Another fascinating observation is that oriented presentation of antigen on the particle surface seems to confer some diversity in terms of the specificity of antibodies produced (i.e., what regions of the sporozoite should be bound by antibodies). In effect, the nanoparticle adjuvant leads to the production not only of antibodies that bind to epitopes previously identified to be required for malarial protection, but also antibodies for the region I domain that effectively could deter sporozoite internalization by hepatocytes. It is unclear exactly how the nanoparticle adjuvant produces this effect, but it is speculated that lower-avidity B cells are provided with an opportunity to compete for activation when antigen is displayed multivalently on a particle surface (4). The ability of various adjuvants to determine the diversity of epitope recognition has also been seen in the past with CD4+ T-cell responses, with depot/particle-based presentation being suggested as a necessary condition (9). Regardless, the result of vaccination with the ICMVs with surface-bound antigen is a multimodal immune response that would block multiple stages of a parasite's attempt to infect its host.
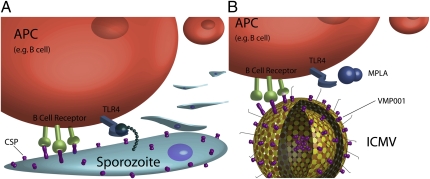
The choice of “conventional” adjuvant (monophophoryl lipid A, or MPLA) used in this study is also quite interesting. This particular pathogen-associated molecular pattern is a chemically modified derivative of bacterial lipopolysaccharide (10) that binds to the innate immune activating receptor TLR4. These types of “danger signals” can provide an additional piece of information to effectively inform the immune system “how” it should mount a defense. Unlike other conventional adjuvants, such as Montanide (incomplete Freund's adjuvant) and alum (aluminum hydroxide), which have both been traditionally suggested to aid in antigen depot and release (11), the MPLA danger signal may be more appropriate in the context of a malaria vaccine because TLR4 is likely activated by malarial surface moieties during sporozoite infection (12) (Fig. 1A). As further evidence, a prior study suggests that TLR4 activation is a critical event in the development of protection from malarial infection, with polymorphisms in TLR4 being responsible for significantly increased risk of severe malaria in children (13).
Fig. 1.
Multivalent display of antigen and TLR4 agonist to an antigen presenting cell (APC) such as a B cell by a malarial sporozoite and a nanoparticle/adjuvant formulation. (A) P. vivax displays circumsporozoite proteins (CSP) prominently on its surface, as well as structures that likely serve as TLR4 agonists (12, 13). Clustering of B-cell receptors is achieved as a result of the natural, repetitive display of the CSP. This combination of persistent, multivalent antigen presentation in context with particular, parasite-associated “danger signals” would be recognized by the immune system in a way that would produce immune responses that are well-suited to combat the parasite. (B) Synthetic ICMVs [as described in PNAS (4)] can be designed to display a subunit CSP antigen (VMP001) through both sustained release and multivalent presentation on their surface. When administered along with the TLR4 agonist MPLA, these nanoparticles produce an immune response that is better suited to combat malaria than when antigen and conventional adjuvant are delivered alone.
Taken collectively, these observations may have greater implications than simply suggesting a viable vaccine formulation for P. vivax. Specifically, the particle-based formulation put forth by Moon et al. in PNAS (4) represents an interesting form of adjuvancy whereby the individual components are rationally “reorganized” to mimic the natural presentation of the individual components of a target pathogen,
Synthetic particles are particularly attractive candidates for the next generation of rationally designed adjuvants.
resulting in more appropriate immune responses. In other words, synthetic particles can serve as a template on which a pathogen-derived “packet of information” can be “encoded” through some combination of sustained presence at a point source, oriented presentation on the surface, and/or addition of relevant danger signals (Fig. 1B). Once encoded into the particle, this “packet of information” would be “decoded” by the immune system through recognition mechanisms that have been naturally selected to provide life-saving protection against natural pathogen particles. Beyond presentation of various chemical stimuli, even the geometry and aspect ratio of the particle itself may be recognized as important information by the immune system (14). Overall, synthetic particles are particularly attractive candidates for the next generation of rationally designed adjuvants, given their flexibility in terms of what can be encoded by the engineer.
Presently, combinations of synthetic particles and well-characterized conventional adjuvants (such as the combination put forth by Moon et al.) may be a superb way to engineer subunit vaccine formulations to tackle a wide variety of other pathogens for which safe and effective vaccines are currently unavailable. Most certainly, our ever-increasing understanding of how our own bodies recognize pathogens will be the key to unlock even more advanced adjuvant formulations that can be increasingly more specific for producing a response to a given challenge. However, until synthetic adjuvants can be engineered to fully encode the necessary information to produce robust and customized immune responses, these synthetic, particle-based formulations with intentionally selected conventional adjuvants seem to be one step closer to tricking the body into believing that it has been exposed to the real thing.
Acknowledgments
I thank Mintai Peter Hwang for help with graphic design of the figure. Work on biomimetic particles and drug delivery in my laboratory is supported by National Institutes of Health Grants 1R01DE021058-01 A1 and 5KL2 RR024154 02, National Science Foundation Grant 0941260, the Arnold and Mabel Beckman Foundation, and the Wallace H. Coulter Foundation.
Footnotes
The author declares no conflict of interest.
See companion article on page 1080.
References
- 1.Price RN, Douglas NM, Anstey NM. New developments in Plasmodium vivax malaria: Severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis. 2009;22:430–435. doi: 10.1097/QCO.0b013e32832f14c1. [DOI] [PubMed] [Google Scholar]
- 2.Bell BA, et al. Process development for the production of an E. coli produced clinical grade recombinant malaria vaccine for Plasmodium vivax. Vaccine. 2009;27:1448–1453. doi: 10.1016/j.vaccine.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: Putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moon JJ, et al. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci USA. 2012;109:1080–1085. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirosue S, Kourtis IC, van der Vlies AJ, Hubbell JA, Swartz MA. Antigen delivery to dendritic cells by poly(propylene sulfide) nanoparticles with disulfide conjugated peptides: Cross-presentation and T cell activation. Vaccine. 2010;28:7897–7906. doi: 10.1016/j.vaccine.2010.09.077. [DOI] [PubMed] [Google Scholar]
- 6.Little SR, et al. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc Natl Acad Sci USA. 2004;101:9534–9539. doi: 10.1073/pnas.0403549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon JJ, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Chen Y-H. High epitope density in a single protein molecule significantly enhances antigenicity as well as immunogenicity: A novel strategy for modern vaccine development and a preliminary investigation about B cell discrimination of monomeric proteins. Eur J Immunol. 2005;35:505–514. doi: 10.1002/eji.200425749. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner CK, Malherbe LP. Regulation of CD4 T-cell receptor diversity by vaccine adjuvants. Immunology. 2010;130:16–22. doi: 10.1111/j.1365-2567.2010.03265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans JT, et al. Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi.529. Expert Rev Vaccines. 2003;2:219–229. doi: 10.1586/14760584.2.2.219. [DOI] [PubMed] [Google Scholar]
- 11.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gowda DC. TLR-mediated cell signaling by malaria GPIs. Trends Parasitol. 2007;23:596–604. doi: 10.1016/j.pt.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Mockenhaupt FP, et al. Toll-like receptor (TLR) polymorphisms in African children: Common TLR-4 variants predispose to severe malaria. Proc Natl Acad Sci USA. 2006;103:177–182. doi: 10.1073/pnas.0506803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]