Abstract
Infections with HIV, hepatitis B virus, and hepatitis C virus can turn into chronic infections, which currently affect more than 500 million patients worldwide. It is generally thought that virus-mediated T-cell exhaustion limits T-cell function, thus promoting chronic disease. Here we demonstrate that natural killer (NK) cells have a negative impact on the development of T-cell immunity by using the murine lymphocytic choriomeningitis virus. NK cell-deficient (Nfil3−/−, E4BP4−/−) mice exhibited a higher virus-specific T-cell response. In addition, NK cell depletion caused enhanced T-cell immunity in WT mice, which led to rapid virus control and prevented chronic infection in lymphocytic choriomeningitis virus clone 13- and reduced viral load in DOCILE-infected animals. Further experiments showed that NKG2D triggered regulatory NK cell functions, which were mediated by perforin, and limited T-cell responses. Therefore, we identified an important role of regulatory NK cells in limiting T-cell immunity during virus infection.
Keywords: virus persistence, effector T cells, virus elimination, NK cell activation, regulatory innate immunity
Natural Killer (NK) cells are a subset of lymphocytes that provide innate effector mechanisms against viruses and tumor cells through direct cytotoxic effects on target cells and cytokine production (1). NK cells detect microbial insults by Toll-like receptor stimulation or in response to proinflammatory cytokines predominantly produced by dendritic cells (2, 3). Notably, type I interferons (IFN-I) are potent cytokines that trigger NK cell activation during a viral infection (4). NK cells can also sense microbial and nonmicrobial signals from target cells through a variety of activating and inhibitory receptors. These receptors influence the cytotoxicity toward virus-infected or cancerous cells and may be involved in disease progression (5). Activating receptors, such as NKG2D or Ly49H, are associated with adaptor molecules that promote NK cell activation. Conversely, NK cell tolerance to self-tissue is maintained by inhibitory receptors, such as Ly49a, which interact with self-MHC class I (MHCI) molecules and prevent autoimmunity. However, MHCI-independent inhibitory and stimulatory signals also contribute to NK cell recognition, namely through NK cell receptor P1b (NKR-P1b) and 2B4 (CD244), respectively (6–8).
NK cells play a vital role in limiting viral replication. Studies have shown that NK cells are able to produce perforin, which is responsible for NK cell-mediated cytotoxic effects (9). In addition, NK cells can limit viral replication by binding to and sequestering specific virus-encoded proteins. For example, activating receptor Ly49H is able to engage the m157 viral glycoprotein encoded by mouse CMV such that a deficiency in Ly49H leads to enhanced mouse CMV replication (10). Moreover, the importance of NK cells for viral clearance is underscored by the observation that NK cell deficiencies in humans correlate with recurrent infections with the varicella zoster virus and severe infections with herpes simplex virus (11–13).
An immunoregulatory role for NK cells during virus infection is also beginning to be elucidated. For example, antigen presentation is enhanced after NK cell depletion and infection with lymphocytic choriomeningitis virus (LCMV) (14), supporting an inhibitory role of NK cells on adaptive immunity. Furthermore, NK cell depletion has also been reported to improve memory T-cell formation (15). NK cells are able to produce interleukin 10 (IL-10) after activation, which was demonstrated to contribute to virus-mediated T-cell exhaustion (16, 17). Finally, a recent report showed that the absence of the inhibitory receptor 2B4 on NK cells resulted in a reduced virus-specific CD8+ T-cell response that led to longer virus persistence (18).
The present study has identified a negative regulatory role for NK cells during both an acute and a chronic virus infection. Our data demonstrate that regulatory NK cells (NKreg) limit virus-specific CD8+ T-cell immunity and promote chronic virus infection or immune pathology. These studies establish a critical negative role for NK cells in virus-induced immunity in vivo.
Results
NK Cells Show Enhanced Cytotoxicity During LCMV Infection.
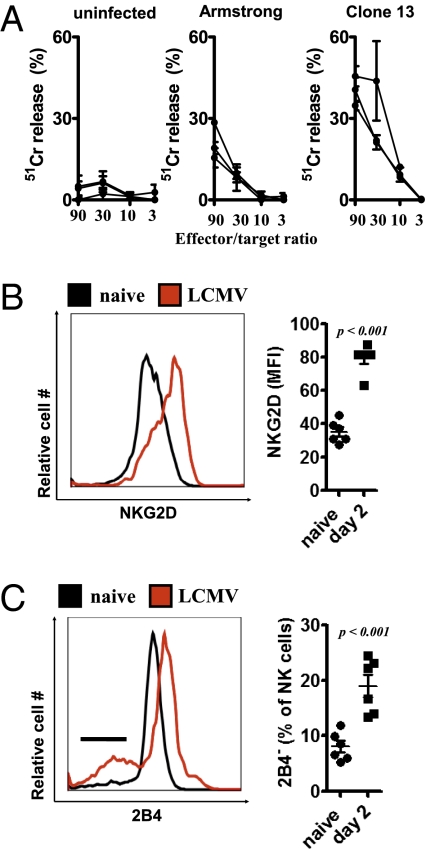
To establish whether NK cell activity was induced after infection with LCMV, we evaluated cytotoxic activity with YAC-1 target cells, which are susceptible to NK cell-mediated lysis (19). After coincubation with splenocytes from mice infected with LCMV clone 13 or LCMV-Armstrong, NK cell cytotoxicity was observed; these data are consistent with previous reports (4, 14). Acute LCMV infection with the Armstrong strain exhibited lower cytotoxicity than that of clone 13, but both clearly induced cytotoxicity compared with naïve controls (Fig. 1A). This cyto-toxicity is mediated by perforin (9). Next, the surface expression of activating and inhibitory receptors on NK cells was examined after LCMV infection. Expression of the activating receptor NKG2D was up-regulated on NK cells from WT mice after infection with 2 × 106 pfu of LCMV strain WE (LCMV WE) (Fig. 1B). In contrast, expression of the inhibitory receptor 2B4 was down-regulated in a proportion of NK cells after infection (Fig. 1C). Altogether, these data show that NK cells are strongly activated after LCMV infection.
Fig. 1.
NK cells are activated after LCMV infection. (A) Splenocytes from naïve (Left), LCMV-Armstrong–infected (Center), and LCMV clone 13-infected (Right) mice were incubated with YAC-1 cells at the indicated effector/target ratios. 51Cr release at different effector/target ratios is shown (n = 3, mean ± SEM of duplicates). (B and C) NK1.1+CD3e− splenocytes were analyzed by flow cytometry for surface expression of NKG2D (B) and 2B4 (C) at 2 d after infection with 2 × 106 pfu of LCMV WE (n = 6; Student's t test). NKG2D expression (mean fluorescence intensity) (B) and 2B4 negative (%) (C) NK1.1+CD3− cells are shown (n = 6; Student's t test).
NK Cell Depletion Leads to an Enhanced CD8+ T-Cell Immune Response.
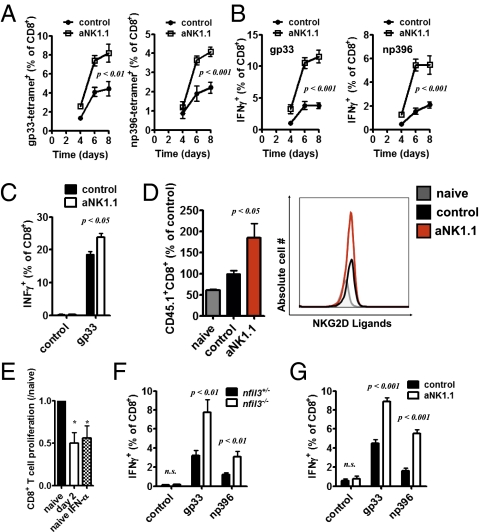
Given that 2B4 is an inhibitory receptor on NK cells (18), we wanted to determine whether NK cells influenced the CD8+ T-cell response during an infection with a high dose of virus. To examine T-cell immunity in the absence of NK cells, we used the NK1.1-depleting antibody (Fig. S1A) (20). Interestingly, an increase in gp33-tetramer+ CD8+ T cells as well as np396-tetramer+ CD8+ T cells was observed in NK cell-depleted mice compared with LCMV-infected, NK cell-competent, mice (Fig. 2A). Consistently, IFN-γ production from LCMV-specific CD8+ T cells was significantly increased in the absence of NK cells throughout the infection with LCMV (Fig. 2B and Fig. S1B). Similar results were also seen when NK cell-depleted mice were infected with 200 pfu of LCMV WE (Fig. 2C). Next, we adoptively transferred LCMV-specific transgenic T cells (P14) (21) into control and NK cell-depleted mice at 1 d before infection with 2 × 106 pfu of LCMV. After 3 d, we measured 30.3% (±9.2%, P < 0.01, n = 7) more LCMV-specific CD8+ T cells in NK cell-depleted mice compared with NK cell-competent mice. Consistently, when we transferred negatively sorted T cells from a P14+ animal (21) into control and NK1.1-treated animals infected with 200 pfu of LCMV WE, we observed a larger P14 population in NK cell-depleted animals (Fig. 2D). Interestingly, LCMV-specific T cells expressed more NKG2D ligands in infected mice than in uninfected animals (Fig. 2D), suggesting that LCMV-induced T-cell activation induces expression of NKG2D ligands on T cells. In NK cell-depleted mice, NKG2D ligand expression did not differ from control mice (Fig. 2D).
Fig. 2.
NK cells limit CD8+ T-cell response in vivo and in vitro. WT and NK cell-depleted mice were infected with 2 × 106 pfu of LCMV WE. (A) gp33-specific (Left) and np396-specific (Right) tetramer response in the spleen (% of CD8+ T cells) at the indicated time (days) after infection are shown (P < 0.01 for day 6 and P < 0.001 for day 8 gp33 tetramers; P < 0.001 for day 6 and 8 for np396 tetramers; two-way ANOVA, n = 6 of two independent experiments). (B) IFN-γ+CD8+ of all CD8+ splenocytes are shown after restimulation with gp33 (Left) or np396 (Right) peptide (P < 0.001 for days 6 and 8 postinfection, two-way ANOVA, n = 6). (C) Control or NK cell-depleted mice were infected with 200 pfu of LCMV WE. At 8 d postinfection, splenocytes were restimulated with the LCMV-specific epitope gp33, and IFN-γ production was determined (n = 7–8; two-way ANOVA). (D) A total of 2 × 106 negatively sorted, CD45.1+ P14 T cells were transferred at 2 d postinfection with 200 pfu of LCMV WE into control and NK cell-depleted mice. CD45.1+CD8+ population was analyzed (Left; n = 6; P < 0.05 for aNK1.1 from naïve and control group using one-way ANOVA), and NKG2D–hIgG expression was measured (one representative of n = 3–6 is shown). (E) Negatively sorted T cells were activated with anti-CD3/CD28 antibodies and coincubated with purified NK cells from naïve or LCMV-infected mice (day 2, 2 × 106 pfu) or naïve NK cells stimulated with IFN-α4. CD8+ T-cell number was compared after 72 h (n = 3–4; *P < 0.05, one-way ANOVA). (F) Nfil3−/− and nfil3+/− mice were infected with 2 × 105 pfu of LCMV WE. At 6 d after infection, splenocytes were restimulated in vitro with the virus-specific peptides gp33 and np396, and IFN-γ production was determined by intracellular cytokine staining and FCM analysis (n = 5–6 of two independent experiments; P < 0.01 for gp33 and np396, two-way ANOVA). (G) WT or NK cell-depleted mice were infected with 2 × 106 pfu of LCMV clone 13. Splenocytes from control or NK cell-depleted mice were restimulated ex vivo with the virus-derived peptides gp33 and np396 at 10 d after infection. Intracellular production of IFN-γ by CD8+ T cells was measured by FCM analysis (n = 6 of two independent experiments; P < 0.001 for gp33 and np396, two-way ANOVA).
To evaluate whether NK cells could modulate effector T-cell function, we coincubated purified NK cells from naïve and LCMV-infected mice with in vitro-activated T cells. Indeed, NK cells from infected animals could limit T-cell expansion, as evidenced by the reduction in the number of T cells when LCMV-activated NK cells were present (Fig. 2E). Because NK cells can be activated by IFN-I after viral infection (4), we wanted to determine whether the presence of IFN-α4 could trigger NK cell-mediated inhibition of T-cell expansion. To this end, we cocultured naïve NK cells with IFN-α4 and observed a similar reduction in the T-cell population (Fig. 2E). Nfil3−/− (E4bp4−/−) mice, which have a severe paucity of NK cells but normal proportion of NKT cells (22, 23), were also examined to further evaluate the impact of NK cells in LCMV-induced T-cell immunity. Consistent with our findings, Nfil3−/− mice displayed significantly enhanced IFN-γ production after LCMV infection (Fig. 2F). Altogether, these data suggest that the presence of NK cells limits the expansion of T cells in vitro and in vivo.
Because the presence of NK cells has a negative impact on CD8+ T-cell function, we examined whether NK cells play a role in establishing chronic viral infections. NK cell-depleted and NK cell-competent mice were infected with LCMV clone 13, and the function of virus-specific T cells was examined at 10 d after infection. Strikingly, virus-induced cytokine production was not exhausted in NK cell-depleted animals (Fig. 2G). Therefore, NK cells were able to limit the expansion of virus-specific T cells during both acute and chronic infection.
NKG2D Triggers Regulatory Functions of NK Cells.
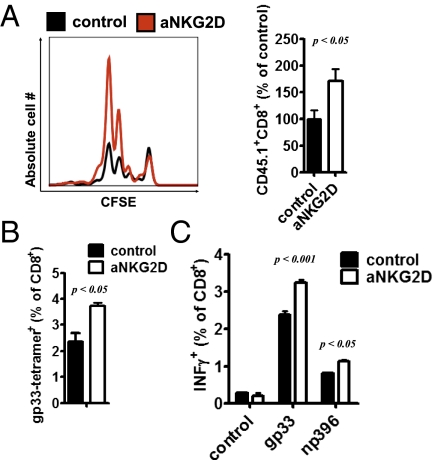
Because NK cells exhibited higher NKG2D expression and virus-specific T cells consistently showed enhanced expression of NKG2D ligands after infection, we next addressed whether NKG2D triggers regulatory functions of NK cells. First, we treated one group of mice with a NKG2D-blocking antibody (clone CX5) (24, 25) and a second group of control animals with an isotype control. When negatively sorted, 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled P14+ T cells were transferred into both groups, we observed an increased protortion of P14 cells in anti–NKG2D-treated animals compared with control mice (Fig. 3A). Next we wanted to determine whether NKG2D blockade could improve endogenous T-cell immunity during infection with 2 × 106 pfu of LCMV WE. Consistently, more gp33-tetramer+CD8+ T cells were detected in anti–NKG2D-treated animals at 8 d after infection (Fig. 3B). In addition, a larger population of CD8+ T cells from anti–NKG2D-injected mice was able to produce IFN-γ after restimulation with the LCMV-specific peptides gp33 and np396 compared with control animals (Fig. 3C). Altogether, these data support a critical role of NKG2D in limiting T-cell immunity.
Fig. 3.
NKG2D triggers NKreg functions. (A) Mice were treated with NKG2D-blocking antibody or isotype control (day −1, day 2 postinfection) and infected with 200 pfu of LCMV WE (day 0). On day 2, 2 × 106 negatively sorted, CFSE-labeled, and congenically marked P14 T cells were transferred into animals of both groups. On day 4 after infection, CFSE expression and expansion of CD45.1+CD8+ cells were determined (n = 3–4; Student's t test). (B and C) Mice were treated with NKG2D-blocking antibody or an isotype control (day −1, 12 h postinfection) and infected with 2 × 106 pfu of LCMV WE. gp33-tetramer+ was measured at 8 d after infection (n = 3; Student's t test) (B), and IFN-γ production was determined after restimulation with the virus-specific epitopes gp33 and np396 (C) (n = 3; two-way ANOVA).
NK Cells Limit T-Cell Immunity by Perforin Production.
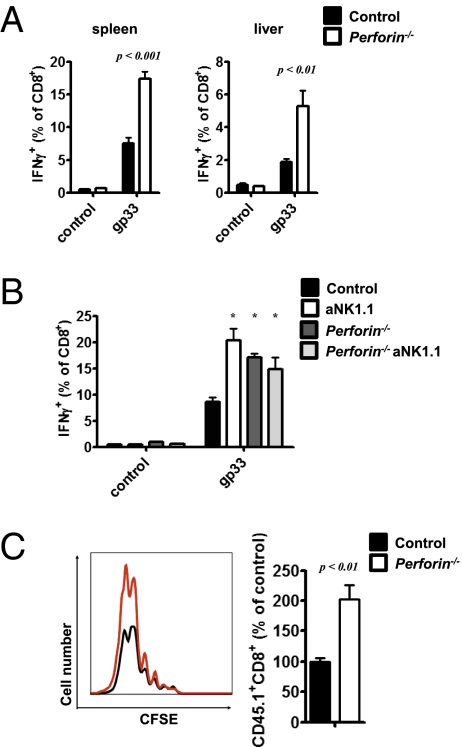
Previous studies have shown that cytotoxic function of NK cells can be mediated via NKG2D (26–29) and perforin (9). Experiments were done to determine whether NK cells from virus-infected mice limited CD8+ T cell function via a perforin-dependent mechanism. Because perforin is a major effector molecule of CD8+ T cells, we provided functional T cells to WT and perforin−/− mice by transferring 5 × 104 splenocytes from congenically marked (CD45.1+) P14 transgenic mice (21). Interestingly, virus-mediated IFN-γ production of CD8+ T cells was enhanced in single-cell suspensions from perforin−/− spleen and liver tissue after restimulation with the LCMV-specific epitope gp33 (Fig. 4A). Accordingly, after NK cell depletion, perforin−/− mice exhibited similar virus-induced IFN-γ production (Fig. 4B). To determine whether NK cell-mediated perforin production affected T-cell proliferation, we transferred 2 × 106 negatively sorted, CFSE-labeled P14 CD8+ T cells into perforin−/− and WT mice. Consistent with the data on NK cell-depleted and anti–NKG2D-treated mice, an increased number of P14 cells was observed in perforin−/− animals (Fig. 4C). Altogether, these data suggest that perforin-mediated NK cell cytotoxicity affects virus-specific CD8+ T-cell immunity.
Fig. 4.
NK cell-mediated perforin production limits T-cell immunity. (A and B) Splenocytes (50,000) from P14+CD45.1+ mice were transferred into perforin−/− and WT mice and infected with 2 × 106 pfu of LCMV WE. (A) At 8 d postinfection, IFN-γ production of CD8+ T cells after restimulation with gp33 was determined in single-cell suspensions of spleen and liver tissue (n = 6; P < 0.01, Student's t test). (B) One group was treated with an NK cell-depleting antibody, and IFN-γ production was monitored (n = 5–6; *P < 0.05 for aNK1.1, perforin−/−, and aNK1.1 perforin−/− vs. control, Newman–Keuls one-way ANOVA). (C) A total of 2 × 106 negatively sorted, CFSE-labeled T cells from P14+CD45.1+ mice were transferred into WT and perforin−/− mice on day 2 postinfection with 200 pfu of LCMV WE. (Left) After 48 h, CFSE expression of the CD45.1+CD8+ population was analyzed. (Right) Relative amount (%) of CD45.1+CD8+ cells in perforin−/− mice compared with WT mice (n = 4; P < 0.01, Student's t test).
NK Cells Influence Chronic Viral Infection and Virus-Induced Immuno-pathology.
To investigate the impact of NK cells on LCMV infection, we first analyzed whether NK cells affected virus replication. At 4 d after infection, NK cell-depleted mice showed similar virus titers and virus distribution in liver and spleen tissue (Fig. S2 A and B) and had comparable IFN-I levels in the serum to those in control animals (Fig. S2C). IFN-I–regulated genes were also efficiently up-regulated in control and NK cell-depleted animals (Fig. S2D), suggesting that NK cells did not directly act on virus replication.
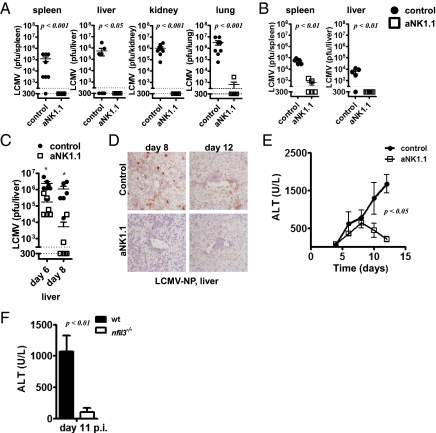
Because CD8+ T-cell immunity is crucial for LCMV clearance (30, 31), we hypothesized that NK cells might promote chronic infection by limiting CD8+ T-cell function. LCMV clone 13 and LCMV DOCILE establish chronic virus infections resulting in T-cell exhaustion followed by virus persistence (32, 33). Because we observed enhanced T-cell immunity in the absence of NK cells, we wanted to investigate whether NK cell depletion could prevent viral persistence. When we measured virus titers at 10 d after infection with LCMV clone 13, animals depleted of NK cells exhibited diminished viral titers compared with control mice in all compartments analyzed (Fig. 5A). Infections with a high dose of LCMV DOCILE showed that NK cell-depleted mice were also able to eliminate the virus from spleen and liver tissue (Fig. 5B), and they exhibited reduced titers in kidney and lung tissue (Fig. S3A). Importantly, these data demonstrate that the presence of NK cells has the ability to promote chronic viral infections.
Fig. 5.
NK cells promote development of chronic infection and immunopathology. (A) WT or NK cell-depleted mice were infected with 2 × 106 pfu of LCMV clone 13. Virus titers were determined from spleen, liver, kidney, and lung tissue at 10 d after infection [n = 8 of two independent experiments; P < 0.001 except liver (P < 0.05) of 10log(Titer), Student's t test]. (B) Control and NK cell-depleted mice were infected with 105 pfu of LCMV DOCILE. Virus titers were measured after 33 d (n = 5, Student's t test). (C–E) WT or NK cell-depleted mice were infected with 2 × 106 pfu of LCMV WE. (C) At 6 and 8 d postinfection, virus titers were analyzed in spleen tissue. (n = 6). *P < 0.05, significant difference for 10log(Titer), Student's t test. (D) Liver from control and NK cell-depleted mice were analyzed for LCMV nucleoprotein expression by immunohistology. One representative picture is shown (n = 5). (E) WT mice or NK cell-depleted mice were infected with 2 × 106 pfu of LCMV. Hepatitis induction was assessed by measuring ALT activity in the sera at different time points (n = 2–11; P < 0.05 for day 10 and P < 0.01 for day 12 postinfection, two-way ANOVA). (F) Nfil3−/− and nfil3+/− mice were infected with 2 × 105 pfu of LCMV WE. ALT activity in the serum of nfil3−/− and nfil3+/− mice was determined at 11 d postinfection (n = 7–8; P < 0.01 using Student's t test).
Next, we wanted to determine whether NK cells could affect virus-induced hepatitis, which was previously reported in mice infected with 2 × 106 pfu of LCMV WE (30, 31). Indeed, virus titers in the liver were reduced in mice depleted of NK cells (Fig. 5C). Consistently, LCMV WE was eliminated rapidly in the absence of NK cells in the spleen, lung, and kidney (Fig. S3 B and C). Accordingly, immunohistology revealed that the number of infected cells was highly reduced in liver (Fig. 5D) and spleen (Fig. S3D) tissue of NK cell-depleted mice, whereas LCMV was still readily observed in control mice (Fig. 5D and Fig. S3D). Importantly, NK cell-depleted mice showed reduced virus-dependent liver cell damage as measured by alanine aminotransferase (ALT) activity in the sera (Fig. 5E). In addition, Nfil3−/− mice exhibited reduced ALT activity in the serum (Fig. 5F). In conclusion, these data demonstrate that the presence of NK cells promotes immunopathology, including viral persistence and virus-induced hepatitis.
Discussion
Our study examined the regulatory function of NK cells in antiviral response to a noncytopathic murine virus, LCMV. NK cell depletion promoted virus-induced CD8+ T-cell immunity, which led to rapid viral clearance. We demonstrated that infection with LCMV clone 13 or LCMV DOCILE, which normally establishes a chronic infection, was cleared within 10 d in the absence of NK cells. Consistent with this finding, virus-induced immunopathologies were also abolished in NK cell-deficient mice because the improved CD8+ T-cell response eliminated LCMV in the liver. Mechanistically, NKreg functions were activated by NKG2D, which may have triggered perforin-mediated killing of T cells.
Although it is well established that NK cells produce IL-10 (16), the involvement of NKG2D-mediated perforin production remains unclear. Our data show that NKG2D expression by NK cells increased during infection with LCMV and that NKG2D blockade enhanced T-cell immunity. It is likely that NKreg functions are mediated by perforin because perforin−/− mice exhibited an enhanced antiviral T-cell response. Previous studies have shown that perforin−/− mice succumb to infection of even low-dose infection with LCMV because of increased IFN-γ production (9, 34). By limiting T-cell immunity, NK cell activation may provide an evolutionary advantage by preventing excessive immunopathology. In our setting, however, NK cell depletion led to highly increased T-cell immunity followed by virus elimination and antigen control. Thus, perforin-mediated regulatory functions might be critical to preventing lethal immunopathology or autoimmunity under some circumstances.
Our data add to the existing evidence that NK cells provide immunoregulatory functions during chronic virus infections. Infections with hepatitis B virus, hepatitis C virus (HCV), and HIV often lead to chronic infections. The involvement of NK cells during these virus infections has been discussed but poorly understood (35–37). Data generated from patient studies support a regulatory role of NK cells during chronic infections. These reports have shown that NK cells are activated in patients suffering from chronic infection with HCV (38, 39). However, HCV does not interfere with NK cell activity in vitro (38). In humans, the killer cell Ig-like receptors (KIRs) represent a diverse family of activating and inhibitory receptors that bind to the MHCI ligands. The KIR haplotypes vary in type and number of genes. Interestingly, the homozygous expression of the inhibitory NK cell receptor gene KIR2DL3 and its ligands, the HLA-C group 1 alleles, correlate with clearance of HCV (40). In turn, the activating receptor gene KIR2DS3 is associated with elevated transaminases and seropositive HCV infection (41). Both studies suggest that reduced NK cell activity might have a beneficial impact on HCV clearance. Recent evidence also suggest that NK cell activity induced during chronic HCV infection contributes to liver injury through cytotoxicity, although changes in IFN-γ production were not detected (39). In light of our findings, NK cells could limit virus-specific T-cell immunity in chronically infected patients and thus trigger higher viral load and enhanced immunopathology.
Our conclusion, that NK cells may enhance the immunopathology associated with chronic virus infection, brings an interesting aspect to hepatitis virus therapy. A recent report demonstrated that the amount of degranulated NK cells correlates with ALT elevation in HCV-infected patients (39). Moreover, a previous report showed that IFN-αs can not only inhibit virus replication but also activate NK cells (4). Our in vitro data showed that IFN-α was able to activate NKreg functions, which resulted in limited T-cell expansion. Thus, IFN-α therapy might suppress the virus-specific CD8+ T-cell response as long as NK cells are present. However, a therapeutic regimen that combines IFN-α and NK cell depletion or NKG2D blockage might be much more powerful in eliminating hepatitis viruses.
Materials and Methods
Mice, Viruses, and Virus Titration.
LCMV WE was originally obtained from F. Lehmann-Grube (Heinrich Pette Institute, Hamburg, Germany) and was propagated in L929 cells as described (42). LCMV clone 13 (a generous gift from Sam Basta, Queens University, Kingston, ON, Canada) and LCMV-Armstrong (a generous gift from Rolf Zinkernagel (University of Zurich, Zurich, Switzerland) was grown in L929 cells (32, 43). Virus titers were measured with a plaque-forming assay as described (44). Mice were infected i.v. with 2 × 106 pfu of LCMV WE or LCMV clone 13 or 105 pfu of LCMV DOCILE. Acute infections were established by infecting mice with 200 pfu of LCMV WE. All mice used in this study were maintained on the C57BL/6 genetic background. All experiments were performed in single ventilated cages. Animal experiments were carried out in accordance with the guidelines of the Ontario Cancer Institute and the German law for animal protection. Nfil3−/− mice and P14 T-cell receptor transgenic mice have been previously described (21, 23). NK cells were depleted with i.v. injection of anti-NK1.1 [clone PK136, kindly provided by Yonghong Wan (McMaster University, Hamilton, ON, Canada) and purchased with corresponding isotype control at BD Pharmingen] antibody at 3 d and 1 d before infection (0.2 mg per mouse) (Fig. S1A) (20). Anti-NKG2D antibody (clone CX5) and corresponding isotype control (eBioscience) was given on day −1 and 2 d after infection with 200 pfu and day −1 and 12 h after infection with 2 × 106 pfu of LCMV WE.
NK Cell Cytotoxicity Assay.
Details of the NK cell cytotoxicity assay are described in SI Materials and Methods.
IFN Production.
IFN-α ELISA was performed according to the manufacturer's instructions (PBL).
Purification of T Cells and NK Cells.
For T-cell and NK cell purification, single-cell suspended splenocytes were enriched following the manufacturer's instructions (pan T-cell MACS kit, NK cell MACS kit; Miltenyi).
T-Cell Proliferation.
T-cell proliferation is described in SI Materials and Methods.
ALT serum levels were measured with a serum multiple biochemical analyzer (Ektachem DTSCII; Johnson & Johnson Inc.).
Quantitative RT-PCR Measurement.
RT-PCR analysis was performed as previously described (45). Detailed procedures are described in SI Materials and Methods.
Histology.
Histological analysis was performed on snap-frozen tissue as described (46). Antibodies against mouse CD8 (clone YTS169.4) and LCMV NP (clone VL4) were used. A color reaction was developed with an alkaline phosphatase system.
Flow Cytometry (FCM) Analysis.
Tetramer was provided by the National Institutes of Health (NIH) Tetramer Core Facility at the Emory University. Tetramer, surface, and intracellular FCM staining were performed as described previously (45). NKG2D–human IgG (hIgG) was used to indicate NKG2D ligand expression (47). Detailed procedures are described in SI Materials and Methods.
Gradient Centrifugation.
Detailed gradient centrifugation procedures (48) are described in SI Materials and Methods.
Statistical Analysis.
Data are expressed as mean ± SEM. Statistically significant differences between two different groups were analyzed with Student's t test. Statistical difference between several groups was tested with one-way ANOVA with additional Bonferroni, Newman–Keuls, or Dunnett's test. Statistically significant differences between groups in experiments involving more than one analysis time point were calculated with two-way ANOVA (repeated measurements). P < 0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgments
We thank Olivia Chan for critical reading of the manuscript. This study was supported by Canadian Institute for Health Research Grant CIHR-MOP-106529 (to P.S.O.). This study was supported by Alexander von Humboldt Foundation Grants SKA2008 (to K.S.L.) and SKA2010 (to P.A.L.); Deutsche Forschungsgemeinschaft Grant LA1419/3-1; and the Strategic Research Fund of the Heinrich Heine University. Tetramer was provided by the NIH Tetramer Core Facility at Emory University. Further support came from the Collaborative Research Center (SFB575) “Experimental Hepatology” (coordinator D.H.). M.R. was supported by Grant PASMP3-127678/1 from the Swiss National Science Foundation/Swiss Foundation for Medical-Biological Scholarships. P.S.O. holds a Canada Research Chair in Autoimmunity and Tumor Immunity. This research was funded in part by the Ontario Ministry of Health and Long-Term Care.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118834109/-/DCSupplemental.
References
- 1.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 2.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “L'union fait la force”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 3.Sivori S, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: Induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biron CA, Nguyen KB, Pien GC. Innate immune responses to LCMV infections: Natural killer cells and cytokines. Curr Top Microbiol Immunol. 2002;263:7–27. doi: 10.1007/978-3-642-56055-2_2. [DOI] [PubMed] [Google Scholar]
- 5.Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 6.Lanier LL. Up on the tightrope: Natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlyle JR, et al. Missing self-recognition of Ocil/Clr-b by inhibitory NKR-P1 natural killer cell receptors. Proc Natl Acad Sci USA. 2004;101:3527–3532. doi: 10.1073/pnas.0308304101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KM, et al. 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J Exp Med. 2004;199:1245–1254. doi: 10.1084/jem.20031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kägi D, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 10.Fodil-Cornu N, et al. Ly49h-deficient C57BL/6 mice: A new mouse cytomegalovirus-susceptible model remains resistant to unrelated pathogens controlled by the NK gene complex. J Immunol. 2008;181:6394–6405. doi: 10.4049/jimmunol.181.9.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orange JS. Human natural killer cell deficiencies. Curr Opin Allergy Clin Immunol. 2006;6:399–409. doi: 10.1097/ACI.0b013e3280106b65. [DOI] [PubMed] [Google Scholar]
- 12.Vossen MT, et al. Absence of circulating natural killer and primed CD8+ cells in life-threatening varicella. J Infect Dis. 2005;191(2):198–206. doi: 10.1086/426866. [DOI] [PubMed] [Google Scholar]
- 13.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 14.Su HC, et al. NK cell functions restrain T cell responses during viral infections. Eur J Immunol. 2001;31:3048–3055. doi: 10.1002/1521-4141(2001010)31:10<3048::aid-immu3048>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Soderquest K, et al. Cutting edge: CD8+ T cell priming in the absence of NK cells leads to enhanced memory responses. J Immunol. 2011;186:3304–3308. doi: 10.4049/jimmunol.1004122. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Kim KS, Fodil-Cornu N, Vidal SM, Biron CA. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med. 2009;206:2235–2251. doi: 10.1084/jem.20082387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks DG, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waggoner SN, Taniguchi RT, Mathew PA, Kumar V, Welsh RM. Absence of mouse 2B4 promotes NK cell-mediated killing of activated CD8+ T cells, leading to prolonged viral persistence and altered pathogenesis. J Clin Invest. 2010;120:1925–1938. doi: 10.1172/JCI41264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan GS, Mittrücker HW, Kägi D, Matsuyama T, Mak TW. The transcription factor interferon regulatory factor-1 is essential for natural killer cell function in vivo. J Exp Med. 1996;184:2043–2048. doi: 10.1084/jem.184.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo GC, Peppard JR. Establishment of monoclonal anti-Nk-1.1 antibody. Hybridoma. 1984;3:301–303. doi: 10.1089/hyb.1984.3.301. [DOI] [PubMed] [Google Scholar]
- 21.Pircher H, Bürki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 22.Gascoyne DM, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 23.Kamizono S, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J Exp Med. 2009;206:2977–2986. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lodoen M, et al. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J Exp Med. 2003;197:1245–1253. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogasawara K, et al. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18(1):41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 26.Hayakawa Y, et al. Cutting edge: Tumor rejection mediated by NKG2D receptor-ligand interaction is dependent upon perforin. J Immunol. 2002;169:5377–5381. doi: 10.4049/jimmunol.169.10.5377. [DOI] [PubMed] [Google Scholar]
- 27.Smyth MJ, et al. NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J Exp Med. 2004;200:1325–1335. doi: 10.1084/jem.20041522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabinovich BA, et al. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol. 2003;170:3572–3576. doi: 10.4049/jimmunol.170.7.3572. [DOI] [PubMed] [Google Scholar]
- 29.Diefenbach A, et al. Ligands for the murine NKG2D receptor: Expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1(2):119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 30.Zinkernagel RM, et al. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J Exp Med. 1986;164:1075–1092. doi: 10.1084/jem.164.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang PA, et al. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med. 2008;14:756–761. doi: 10.1038/nm1780. [DOI] [PubMed] [Google Scholar]
- 32.Salvato M, Borrow P, Shimomaye E, Oldstone MB. Molecular basis of viral persistence: A single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J Virol. 1991;65:1863–1869. doi: 10.1128/jvi.65.4.1863-1869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 34.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104:735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, et al. Activation and function of hepatic NK cells in hepatitis B infection: An underinvestigated innate immune response. J Viral Hepat. 2005;12(1):38–45. doi: 10.1111/j.1365-2893.2005.00543.x. [DOI] [PubMed] [Google Scholar]
- 36.Cheent K, Khakoo SI. Natural killer cells and hepatitis C: Action and reaction. Gut. 2011;60(2):268–278. doi: 10.1136/gut.2010.212555. [DOI] [PubMed] [Google Scholar]
- 37.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: Paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 38.Yoon JC, Shiina M, Ahlenstiel G, Rehermann B. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009;49(1):12–21. doi: 10.1002/hep.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahlenstiel G, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325–335.e2. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khakoo SI, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 41.Paladino N, et al. Increased frequencies of activating natural killer receptors are associated with liver injury in individuals who do not eliminate hepatitis C virus. Tissue Antigens. 2007;69(Suppl 1):109–111. doi: 10.1111/j.1399-0039.2006.762_7.x. [DOI] [PubMed] [Google Scholar]
- 42.Welsh RM, Seedhom MO. Current Protocols in Microbiology. New York: Wiley; 2008. Lymphocytic choriomeningitis virus (LCMV): Propagation, quantitation, and storage. Chap 15, Unit 15A.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Battegay M, et al. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J Virol Methods. 1991;33(1–2):191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 45.Lang PA, et al. Hematopoietic cell-derived interferon controls viral replication and virus-induced disease. Blood. 2009;113:1045–1052. doi: 10.1182/blood-2007-10-117861. [DOI] [PubMed] [Google Scholar]
- 46.Recher M, et al. Extralymphatic virus sanctuaries as a consequence of potent T-cell activation. Nat Med. 2007;13:1316–1323. doi: 10.1038/nm1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cerwenka A, et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 48.Lang PA, et al. Tissue macrophages suppress viral replication and prevent severe immunopathology in an interferon-I-dependent manner in mice. Hepatology. 2010;52(1):25–32. doi: 10.1002/hep.23640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.