Abstract
The mammalian gut harbors a dense microbial community interacting in multiple ways, including horizontal gene transfer (HGT). Pangenome analyses established particularly high levels of genetic flux between Gram-negative Enterobacteriaceae. However, the mechanisms fostering intraenterobacterial HGT are incompletely understood. Using a mouse colitis model, we found that Salmonella-inflicted enteropathy elicits parallel blooms of the pathogen and of resident commensal Escherichia coli. These blooms boosted conjugative HGT of the colicin-plasmid p2 from Salmonella enterica serovar Typhimurium to E. coli. Transconjugation efficiencies of ∼100% in vivo were attributable to high intrinsic p2-transfer rates. Plasmid-encoded fitness benefits contributed little. Under normal conditions, HGT was blocked by the commensal microbiota inhibiting contact-dependent conjugation between Enterobacteriaceae. Our data show that pathogen-driven inflammatory responses in the gut can generate transient enterobacterial blooms in which conjugative transfer occurs at unprecedented rates. These blooms may favor reassortment of plasmid-encoded genes between pathogens and commensals fostering the spread of fitness-, virulence-, and antibiotic-resistance determinants.
Keywords: bacterial evolution, hospital-acquired infection, mucosal immune response, plasmid spread
The mammalian gut harbors a very dense microbial community, the “microbiota” (>1012 bacteria/g), which has profound effects on the host's nutrition, physiology, and immune system (1). In humans, the microbiota is generally composed of several hundred different bacterial phylotypes. Its composition differs between individuals forming a “collective microbiome” of more than 100,000 genes (2, 3). Much of the microbiota's genome plasticity is thought to be attributable to horizontal gene transfer (HGT), the most effective mechanism of which is conjugation, the exchange of plasmids (4–6). Conjugational plasmid transfer is consistently fueling the emergence of hypervirulent or antibiotic-resistant pathogens (7, 8), as illustrated by Escherichia coli O104:H4, which has recently caused an outbreak in Germany (9). However, key questions about the maintenance and the transfer of conjugative plasmids in natural bacterial populations have remained unanswered. Here, we describe the discovery of a mechanism driving efficient conjugation between Enterobacteriaceae in the host's intestine. Enterobacteriaceae include many pathogenic as well as commensal species (i.e., Salmonella enterica and E. coli). Plasmid profiling, enterobacteriaceal genome sequencing, and microbiome analyses identified a large and highly diverse plasmid-encoded accessory gene pool indicative of efficient conjugative HGT (10). However, in the normal gut, the Enterobacteriaceae are generally present in very low densities (<<108 cfu/g). These densities are way too low for efficient conjugative plasmid transfer, as this process hinges on direct physical contact between the donor and the acceptor bacterium (11).
In the mammalian intestine, the vast majority of intestinal bacteria consist of obligate anaerobic members of the Firmicutes and Bacteroidetes phyla. It is thought that these anaerobes keep the overall density of facultative anaerobic bacteria (i.e., Enterobacteriaceae) rather low (<<108 cfu/g), a condition termed colonization resistance (CR) (12–14). Thus, in complex bacterial ecosystems, low densities of donor and recipient bacteria may lower the frequency of direct bacterial encounters and thus decrease the chance of conjugation-mediated HGT. Several studies reported inefficient enterobacterial HGT in the normal mammalian gut (15–17), whereas others identified higher rates of HGT (18, 19). This suggested that particular conditions exist that might favor plasmid exchange between Enterobacteriaceae. The factors influencing the efficiency of conjugative HGT in this bacterial family are incompletely understood to date.
Inflammatory host responses triggered by the gut immune system (in inflammatory bowel disease patients) or by pathogens such as Salmonella spp. or pathogenic E. coli strains can suppress the anaerobic microbiota and boost enterobacterial colonization densities (20–24). Here, we show that disease-triggered enterobacterial blooms can fuel HGT between two prominent members of this family, Salmonella enterica serovar Typhimurium (S. Tm) and E. coli.
Results and Discussion
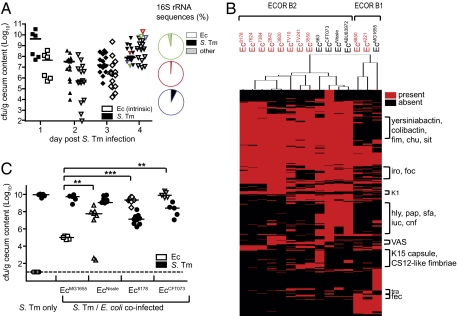
We have frequently observed parallel blooms (≥108 cfu/g each) of S. Tm strain SL1344 (S. Tmwt) and commensal E. coli in our experiments using the streptomycin mouse model for Salmonella diarrhea. Fig. 1 shows the presence of commensal E. coli recorded during routine screening of S. Tmwt infected mice by plating on selective agar (Fig. 1A) (21, 25, 26). In some cases, E. coli accounted for >80% of the total intestinal bacteria as determined by 16S rRNA sequencing (Fig. 1A; red, green, and blue symbols at day 4 p.i.). Based on this result, we hypothesized that these high enterobacterial densities might favor HGT. Our aim was thus to investigate whether HGT had taken place between Salmonella and E. coli during these parallel blooms. We began our study by randomly isolating 10 “co-blooming” E. coli strains from S. Tm infected mice during the last 8 y in our laboratory (Table S1). Comparative genome analysis using an E. coli phylogenic microarray (27) and multilocus sequence typing (MLST; Fig. 1B and Fig. S1A) established that the E. coli isolates were genetically distinct and most isolates belonged to the phylogenetic group ECOR B2 (Table S1). We decided to focus more closely on one of the isolates and randomly choose the ECOR B2 strain Ec8178 for further analysis. Ec8178 was isolated from the animal depicted in green in Fig. 1A. S. Tm coinfection experiments in mice lacking intrinsic E. coli showed that Ec8178 and two other ECOR B2 strains (EcNissle and EcCFT073), but not EcMG1655, were as well adapted to growth in the inflamed gut (Fig. 1C and Fig. S1B). Interestingly, Ec8178 also bloomed in mice suffering from T cell-induced gut inflammation, which had been demonstrated previously for S. Tm (Fig. S2; ref. 21). Identifying the genetic traits underlying adaptation to growth in the inflamed gut will be an interesting topic for future research.
Fig. 1.
Blooms of commensal E. coli in S. Tm infected mice. (A) Colonization levels of commensal E. coli in cecal contents of S. Tm infected mice. E. coli CFU were detected by routine screening on MacConkey without antibiotics by colony morphology and lactose-positive phenotype. Pie plots on the right show microbiota composition in three mice (marked in green, red, and blue). Here, total DNA was extracted, and bacterial 16S rRNA genes were PCR-amplified using universal bacterial primers, cloned, and sequenced (∼100 sequences per animal). (B) DNA microarray comparing the virulence gene content of 10 E. coli isolates collected from S. Tm infected mice from 2002 to 2007 (indicated in red) and different pathogenic and nonpathogenic E. coli reference strains. Genes were grouped with the CLUSTER software based on the presence (red) or absence (black) of genes. Groups of genes belonging to distinct islands or phages are indicated. (C) Coinfection experiments of S. Tm and different E. coli strains. Streptomycin-treated, E. coli-free mice were coinfected with 1:1 mixtures of wild type S. Tm and E. coli strains EcMG1655, or the ECOR B2 strains EcNissle, Ec8178 and EcCFT073 (total of 5 × 107; 1:1; intragastrically). E. coli (white) and S. Tm (black) colonization density in the cecum at day 4 p.i. (Log10 cfu/g). Bars show the median.
To uncover possible events of HGT, we generated a shotgun genome sequence of Ec8178. Analysis of the contigs showed that Ec8178 harbored about 1 Mbp extra DNA compared with EcMG1655 and that the closest sequenced relative was the uropathogenic strain EcCFT073 (28). However, the strain Ec8178 was negative for all tested typical virulence factors of pathogenic E. coli (Table S2). Curiously, Ec8178 harbored an 86-kb plasmid >99% identical to plasmid 2 (termed p2) from S. Tm strain SL1344 (http://www.sanger.ac.uk/resources/downloads/bacteria/salmonella.html), which is routinely used for infection studies by us and by others (Fig. 2A). P2 shows homology to R-64 and ColIb-P9-type conjugative plasmids of the IncI1 incompatibility group, a family of plasmids also encoding multiple antibiotic resistances (29–31) (Fig. 2B). However, p2 from S. Tmwt was by far the closest relative of the plasmid found in Ec8178. The only difference between the two plasmids was an inversion in the shufflon region encoding the variable pilus tip antigen (30) (Fig. 2A and Fig. S3). Shufflon rearrangements are frequently observed and are thought to adapt sex pilus binding specificity to the new recipient strain (32). We were concerned that modification of the shufflon region in Ec8178 p2 may have disrupted the pilus tip antigen essential for conjugative HGT. However, control experiments verified that the plasmid had retained its ability to conjugate into various E. coli and S. enterica strains, albeit at various efficiencies (Table S3).
Fig. 2.
Highly similar conjugative plasmid is present in S. Tm and commensal E. coli. (A) S. Tm and Ec8178 share a nearly identical conjugative plasmid. Sequence comparison of p2 S. Tm (outer circle) and p2 Ec8178 (inner circle); gene functions are color coded. (B) Phylogram showing relation of p2 to other R-64 and ColIb-P9-type conjugative plasmids (E. coli PEK104, S. Tm R64-1, S. Tm R64-2, S. Heidelberg pSL476, Shigella sonnei pColb-P9, S. Tm pNF1358, S. Kentucky CVM29188, E. coli SE11-1). The phylogram was generated using PHYLIB and the Clustal W multiple sequence alignment algorithm; branch length represents the relative nucleotide differences of each plasmid compared with the reference sequence p2. (C) p2 is detected by gel electrophoresis in 4 of 10 E. coli isolates collected from S. Tm-infected mice (from Fig. 1B).
These observations suggested that p2 was acquired by conjugative HGT during the infection experiment. In line with this hypothesis, S. Tmwt p2 carries a complete set of genes required for conjugative transfer. Furthermore, plasmids of similar size and structure, as revealed by gel electrophoresis, PCR and Southern blot, were detected in 4 of the 10 E. coli isolates from E. coli-blooms in S. Tmwt-infected mice (Fig. 2C and Fig. S4).
It should be mentioned that the C57BL/6 mouse colony used for Salmonella infection experiments is maintained under strict barrier conditions, monitored regularly for the absence of pathogens, and kept separate from the facility where the infection experiments are performed, making previous contact of Ec8178 to pathogens as S. Tmwt highly unlikely. This strongly suggested that p2 was indeed only acquired during the course of our infection experiments, i.e., by HGT from S. Tm. The fact that 4 of 10 independent E. coli isolates harbored this plasmid (Fig. 2C) provided a first hint that conjugative HGT might occur at extremely high rates if donor (S. Tm) and recipient (commensal E. coli) bloom in parallel.
The emergence of transconjugants might be affected by two different phenomena: plasmid-encoded fitness-factors favoring transconjugant growth and by the efficiency of the conjugation process itself. We have engineered appropriate plasmid mutants and bacterial strains to investigate the importance of either mechanism.
First, we analyzed the contribution of plasmid-encoded fitness factors. P2 contains a locus for colicin Ib production (cib) and immunity (imm) (Fig. 2A). Colicins are toxic proteins produced by and toxic for bacteria of the Enterobacteriaceae family (33, 34). Colicin-producing bacteria protect themselves from colicin-dependent killing by the expression of a cognate immunity protein. In the case of colicin Ib (Cib), the cognate immunity protein inserts into the inner bacterial membrane thus blocking Cib-mediated pore formation and killing (33). Thus, p2 might enhance bacterial fitness by killing of competing Enterobacteriaceae lacking the plasmid (e.g., EcNissle). Indeed, we found evidence for this hypothesis in vitro and in vivo. S. Tmwt produced colicin Ib in vitro. Colicin Ib of S. Tmwt killed susceptible E. coli strains such as EcMG1655 and EcNissle (Table S4). Killing was ablated if S. Tm harbored a cib imm deletion on p2 (S. Tm p2Δcib).
Similarly, we analyzed colicin Ib and Imm function in vivo. To this end, we chose EcNissle because it is sensitive to colicin Ib and naturally resistant to HGT (35). Mouse coinfection experiments comparing EcNissle vs. S. Tm growth in S. Tmwt or S. Tmwt p2Δcib infected mice demonstrated that colicin Ib production conferred a small, but significant competitive advantage in the gut (P < 0.05; Fig. 3A). In conclusion, colicin Ib production (cib) represents a fitness factor that may enhance the apparent transconjugant frequencies observed in the Salmonella-infected mouse gut.
Fig. 3.
Conjugative Salmonella plasmid can confer a fitness benefit and is efficiently transferred to commensal E. coli in vivo. (A) Colicin Ib production leads to superior growth of S. Tm in competition against EcNissle in the gut. Streptomycin-treated mice were coinfected with 1:1 mixtures (total of 5 × 107; 1:1; intragastrically) of EcNissle and S. Tmwt or S. Tmwt p2Δcib, respectively. Transfer of p2 into EcNissle could not be detected, which is in line with the established resistance against HGT of this strain. B., C. P2 is transferred to Ec8178_cured by conjugation with high efficiency. Streptomycin-treated mice were coinfected with 1:1 mixtures (total of 5 × 107; 1:1; intragastrically) of Ec8178_cured and S. Tmwt carrying p2kan or p2ΔoriTnikA, respectively. Densities of the S. Tm donor (black) and E. coli recipient strains (white) were determined in the feces at day 1 p.i. (B) and in the cecal contents at day 4 p.i. (C), and frequency of kanamycin-resistant E. coli transconjugants in cecal contents was determined by selective plating at day 4 p.i. (D). E. coli (white) and Salmonella (black) colonization densities (Log10 cfu/gram) were determined in the feces at day 1 p.i. (B) or cecum content at day 4 p.i. (A and C). Bars show the median.
Next, we analyzed the in vivo conjugation efficiency of the S. Tm p2. To measure the conjugation efficiency in the absence of cib-mediated fitness effects, we generated a p2-cured variant of Ec8178 as a recipient (Ec8178_cured; Fig. S4 and Table S2). Interestingly, this strain is insensitive to colicin Ib (Table S4). We speculate that this is due to spontaneous second site mutations in the chromosome, i.e., in the genes for colicin Ia binding and internalization: cirA, tonB, and exbCD (33). However, the precise location of this putative mutation could not be identified by comparison of the respective genes from resistant and sensitive strains so far. Anyhow, Ec8178_cured is a highly efficient recipient for conjugative transfer of a derivative of p2 (p2kan) genetically tagged with an antibiotic resistance marker (Table S3). The marker affected neither Cib production nor immunity of the donor strain.
To measure conjugative HGT in vivo, we coinfected streptomycin-treated mice with Ec8178_cured (recipient) and S. Tm p2kan (donor). Both strains bloomed to high density in the infected murine intestine (Fig. 3 B and C). Strikingly, the transconjugant frequency was extremely high, yielding almost 100% of Ec8178_cured harboring p2kan by day 4 p.i. (Fig. 3D). No transconjugants were detected in control infections using a S. Tmwt donor strain carrying an oriT-deficient, nonmobilizable plasmid variant (p2ΔoriTnikA; Fig. 3 B and C). In conclusion, the intrinsic rate of conjugative HGT of p2 is extremely high in vivo, even if a colicin-resistant E. coli strain is used as recipient.
We also wanted to determine whether other p2 genes besides cib would confer any detectable fitness benefit for the plasmid-bearing strain (i.e., S. Tm) in competition against plasmid-free Ec8178 in vivo. To test this, we had to use the conjugation-defective plasmid variant p2ΔoriTnikAΔcib to exclude conjugative transfer to Ec8178 during the competition experiment. We coinfected streptomycin-treated mice with S. Tm p2ΔoriTnikAΔcib and either Ec8178 or Ec8178-cured. The competitive index of S. Tm and E. coli did not differ significantly between both experiments. This verified that p2 does not confer any other detectable benefit to the recipient than those attributable to the cib/imm cassette (Fig. S5). Thus, in conclusion, extremely high transconjugant frequencies are observed in this system even in the absence of plasmid-conferred fitness benefits.
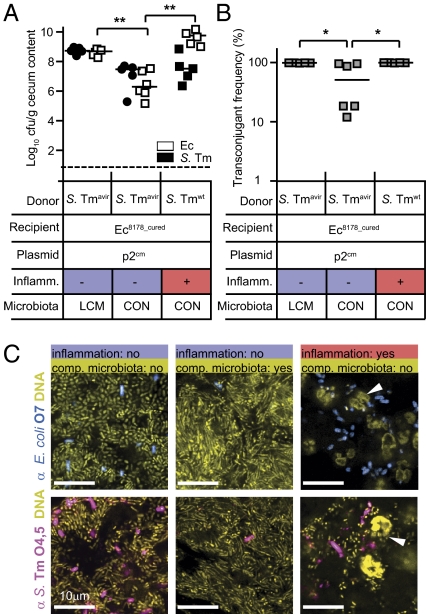
Our data suggested that conjugative HGT rates can be extremely high in pathogen-triggered enterobacterial blooms. However, it remained unclear whether high donor and recipient densities per se were sufficient. Alternatively, the inflammatory milieu in the gut might enhance HGT rates by some unknown mechanism. To address this, we analyzed conjugative transfer of p2 from S. Tm strains into Ec8178_cured under three different conditions:
i) In the first group, we coinfected animals with Ec8178_cured and an avirulent S. Tm SL1344 derivative (S. Tmavir p2cm), which does not trigger gut inflammation (Table S2 and refs. 21 and 36). For this group, we used low-complexity microbiota (LCM) mice, a colony harboring the commensals of the Altered Schaedler flora (37) in the gut. This gut flora allowed efficient Salmonella and E. coli gut colonization even in the absence of enteropathy or antibiotic treatment (26) (Fig. 4 A and C).
ii) In the second group, we infected streptomycin treated mice (normal gut microbiota) with the same strains as described above (i). In line with earlier work (21), the mice did not develop enteropathy, and regrowth of the normal gut microbiota of these mice reduced enterobacterial colonization levels by day 4 p.i. (Fig. 4 A and C).
iii) In the third group, we coinfected streptomycin treated mice (normal gut microbiota) with Ec8178_cured and S. Tmwt (p2cm). This elicited gut inflammation and triggered parallel blooms of the donor and the recipient (Fig. 4 A and C). The slight differences in total population sizes of donor and recipients might be attributable to minor differences in the blooming kinetics of both strains.
Fig. 4.
Inflammation boosts conjugative p2 transfer by increasing density of donors and recipients. (A) Streptomycin-treated low complexity microbiota (LCM; Left) or conventional (CON; Center and Right) mice were coinfected with 1:1 mixtures (total of 5 × 107; 1:1; intragastrically) of Ec8178_cured and S. Tmwt or S. Tmavir strains carrying p2cm, respectively. Densities of the S. Tm donor and E. coli recipient strains were determined in the cecal contents at day 4 p.i. (B) The frequency of chloramphenicol-resistant E. coli transconjugants in cecal contents was determined by selective plating at day 4 p.i. Bars show the median. (C) Competing gut microbiota hinders bacterial contact as shown by immunofluorescent staining of Ec8178 and S. Tm in the cecal lumen of a representative mouse of the experiment shown in a. Serial sections of PFA-fixed cecum tissue were stained with α-E. coli O7 (recolored blue) or α-S. Tm O4.5 antiserum (recolored magenta). Microbiota and host nuclear DNA was stained with Sytox green (recolored yellow). Arrows point at granulocytes in the cecal lumen of mice with gut inflammation. (Scale bar: 10 μm.)
In the first and the third group, conjugation efficiencies reached close to 100% and were significantly higher than in the second group (P < 0.05; Fig. 4B). Thus, a high rate of conjugative HGT required high donor- and acceptor densities. Of note, 100% transconjugation was achieved in LCM mice coinfected with the avirulent S. Tm strain. Therefore, HGT efficiency is not directly attributable to gut inflammation but rather to the high titers of donor and recipient bacteria.
To further substantiate this correlation, we manipulated the density of the S. Tm donor strain in the inflamed gut and analyzed the effects on the conjugation efficiency. To alter the density of the colicin-deficient S. Tm donor strain (S. Tmtet p2Δcib), we used a conjugation-inert but otherwise genetically identical S. Tm strain as “competitor” (S. Tm p2ΔoriTnikAΔcib) at various different concentrations. In addition, all S. Tm strains involved were deficient in colicin production. We coinfected groups of mice with Ec8178_cured (recipient) and the indicated ratio of S. Tmtet p2Δcib (donor) and S. Tm p2ΔoriTnikAΔcib (competitor; 10-, 100-, 1,000-, 10,000-, and 100,000-fold excess over the donor strain). These experiments revealed a positive correlation between the donor density and the yield of transconjugants at day 1 and 4 p.i. (Fig. 5 A and B; P < 0.05; Fig. S6 A and B). Similar results were observed in corresponding in vitro experiments (Fig. S6C). However, only 1% transconjugants could be maximally observed. We speculate that the extremely high transconjugant frequencies seen in vivo could be due to successive rounds of HGT during the 4-d-long infection period or to very high local E. coli/S. Tm densities at some site of the hosts intestine.
Fig. 5.
Intestinal density of S. Tm plasmid donors correlates with E. coli transconjugant frequency. Streptomycin-treated conventional mice (n = 20) were coinfected with mixtures of Ec8178_cured amp (recipient; ampR), S. Tmtet p2Δcib (donor; tetR; plasmid p2: kanR) and increasing amounts of S. Tmwt p2ΔoriTnikAΔcib (competitor; cmR) at no (n = 4), 10-fold (n = 3), 100-fold (n = 3), 1,000-fold (n = 3), 10,000-fold (n = 3), and 100,000-fold (n = 4) excess over the donor strain (total of 5 × 107; intragastrically). The density of the S. Tm donor (x axis) was plotted against E. coli transconjugant frequencies (fraction of ampR kanR E. coli of total ampR E. coli; y axis) as determined in the feces at day 1 p.i. (A) and cecal contents at day 4 p.i. (B). A linear relationship between cfu and transconjugant frequency could be predicted by linear regression analysis (P < 0.05).
In conclusion, we report the highly efficient HGT of a natural S. Tm plasmid to resident commensal E. coli in vivo. The extraordinary rate of transconjugant formation is partly explained by the cib-mediated fitness conferred by the plasmid but mostly by the high intrinsic efficiency of p2 transfer and by the capacity of the pathogen to trigger enteric inflammation. Inflammation elicits concomitant Salmonella and E. coli blooms, which can raise donor and acceptor densities to values >100-fold above those typically encountered in the normal mammalian intestine (14, 38). This fuels the reassortment of genetic material between different Enterobacteriaceae and suggests that infected patients might enhance the spread of plasmid-encoded fitness-, virulence- and antibiotic resistance-determinants (e.g., extended-spectrum β-lactamases; ref. 39). Our findings shift the current paradigm of the “separate” evolution of pathogens (i.e., Salmonella) and commensals (i.e., E. coli): Both are interconnected: Episodes of pathogen-inflicted disease boost not only the evolution of pathogens, but also that of the commensals. Our findings might be of central relevance for explaining the notorious emergence of new pathogenic and commensal strains with altered phenotypes.
Finally, our data may add to the question of how plasmids are maintained in bacterial populations over evolutionary time (“plasmid persistence puzzle”; see SI Text for detailed discussion) (40). Based on theoretical considerations, carriage of beneficial genes is insufficient to account for plasmid maintenance. Due to fitness costs, plasmids should be lost and beneficial genes should move to the chromosome. To explain the paradox of plasmid existence, several mechanisms had been proposed: high-rate conjugative HGT (“infectious transmission”), plasmid shuttling between bacterial strains, ecotypes or species (40) and the existence of “amplifyer strains,” exhibiting extraordinarily high transconjugation efficiencies (41). However, so far, the evidence supporting these hypotheses was mostly limited to theoretical models and in vitro data. Our analysis of p2 transfer in the infected gut provides evidence that “infectious transmission” as well as plasmid shuttling between bacterial strains contribute to plasmid maintenance in nature. This must occur at very high frequencies, as 4 of 10 independent strains analyzed in our initial experiments had acquired p2 (Fig. S4). Thus, plasmid transfer in enterobacterial blooms comprising different bacterial species is most likely a frequent event. Pathogenesis-inflicted blooms may be an important feature of enterobacterial plasmid biology.
Materials and Methods
Bacteria and Plasmids.
Bacterial strains and plasmids used are listed in Table S2. For infections, E. coli and S. Tm strains were grown as described (42). Generation of bacterial mutants and plasmids, E. coli phylogenic microarray, genome sequencing, sequence comparisons and alignments, broad-range bacterial 16S rRNA gene sequence analysis and plasmid isolation procedures are described in SI Materials and Methods.
Isolation and Characterization of E. coli Strains From Frozen Tissue.
Sections of cecum tissues embedded in O.C.T. (Sakura) were prepared on a previously sterilized cryotome. Sections were suspended in PBS and live bacteria recovered by plating on MacConkey agar plates. Lactose-positive colonies were further characterized using the Enterotube system (Becton Dickinson). ECOR typing was performed using a well established triplex-PCR (43). MLST analysis was done as described by sequencing of the adk, fumC, gyrB, icd, mdh, purA, rEcA, and ST gene fragments and corresponding allocation to sequence types (STs).
Animal Experiments.
All mice used in the study were on C57BL/6 background and bred at the Rodent Center at the ETH Zürich under SPF conditions in individually ventilated cages. LCM mice colonized with the Altered Schaedler flora were described (26). Conventional, E. coli-free mice (CON) were initially obtained from Janvier, bred at the RCHCI and routinely tested for the absence of E. coli in the feces. For S. Tm infections, mice were treated with streptomycin (20 mg per animal 24 h before Salmonella infection) and infected by gavage with 5 × 107 cfu S. Tm or mixtures of S. Tm and E. coli as described. Live bacterial loads in the cecal content were determined by plating on MacConkey-agar (Oxoid) with respective antibiotics (streptomycin 100 μg/mL; kanamycin 30 μg/mL and chloramphenicol 30 μg/mL; ampicillin 100 μg/mL; tetracycline 12 μg/mL). Histology was done at necropsy. Briefly, tissues were embedded in O.C.T. (Sakura) and flash frozen. H&E-stained cecum cryosections were scored as described: Evaluating submucosal edema, PMN infiltration, goblet cells, and epithelial damage yielded a total score of 0–13 points.
For induction of acute colitis, CD8+ T cells from CL4-TCR transgenic mice that express an α/βT cell receptor recognizing an epitope of the HA protein presented by MHC class I (the H-2Kd:HA512-520 complex) were adoptively transferred into VILLIN-HA mice that express the A/PR8/34 HA epitope from influenza virus A under control of the enterocyte-specific villin promoter (44). Single-cell suspensions were prepared from the spleen of CL4-TCR transgenic mice. Cell suspensions were depleted of CD4+, CD11b+, CD45R+, DX5+, and Ter-119+ cells by using the MACS CD8 T cell isolation kit (Miltenyi Biotec). CL4-TCR T cells were purified by negative selection according to the manufacturer's instructions. Isolated CD8+ T cells were washed once in PBS and resuspended (4 × 107 cells per mL of PBS). Then, 4 × 106 purified CL4-TCR transgenic T cells were injected i.v. into VILLIN-HA transgenic mice. Disease symptoms (weight loss and diarrhea) were observed 4–5 d after adoptive transfer. Animal experiments were approved (licenses 201/2004 and 201/2007 Kantonales Veterinäramt Zürich) and performed according to local guidelines (TschV, Zurich) and the Swiss animal protection law (TschG).
Immunofluorescence.
For detecting S. Tm and Ec8178 in the gut lumen in situ, cecal tissues were recovered and treated as described (45). Briefly, the tissue was fixed in paraformaldehyde (4% in PBS, pH 7.4 overnight, 4 °C), washed with PBS, equilibrated in PBS (20% sucrose, 0.1% NaN3 overnight, 4 °C), embedded in O.C.T. (Sakura), snap-frozen in liquid nitrogen and stored at −80 °C. Cryosections (7 μm) were air-dried for 2 h at room temperature, fixed in 4% paraformaldehyde (5 min), washed, and blocked in 10% (wt/vol) normal goat serum in PBS for 1 h. S. Tm and Ec8178 were detected by staining for 1 h with a polyclonal rabbit α-Salmonella-O-antigen group B serum (factors 4, 5; Difco; 1:300) or with a polyclonal rabbit α-E. coli-O7 serum (Statens Serum Institut; 1:100) and DyLight-549-conjugated secondary goat-α-rabbit antibody (Jackson). DNA was stained with Sytox-green (1:10,000; Invitrogen). Sections were mounted with Vectashield hard set (Vector Laboratories) and analyzed using a Leica SP5 confocal laser scanning microscope (Leica Microsystems).
Statistical Analysis.
Statistical analysis was performed using the exact Mann-Whitney U test (Graphpad Prism Version 5.01). P values less than 0.05 (two-tailed) were considered statistically significant.
Colicin Assay.
For measuring production of colicin and colicin sensitivity, the colicin-producing strain was grown overnight as small spot (ø5 mm) on LB agar containing 2 μg/mL Mitomycin C (Sigma). The plate was overlaid with the tester strain in Top-agar (0.75% agarose). Growth of the tester strain was analyzed after 24 h. Halo formation around the producer indicated sensitivity of the tester strain.
Mating Experiments.
An overnight culture of donor and recipient strains was grown in LB media with appropriate antibiotics (37 °C, 180 rpm). The recipient strain was marked with antibiotic resistance by either carrying pWKS30 (46) (Amp) or pACYC184 (New England Biolabs; Cm). A total of 1 mL of each overnight culture was washed three times in PBS and concentrated (300 μL PBS final). Donor and recipient strains were mixed at a ratio of 1:1. Eighty microliters of the mixture was spread on LB Agar plate (surface mating), and matings were incubated for 4 h at 37 °C. Bacteria were recovered and plated onto respective selective agar to quantify transconjugant frequencies (cfu of plasmid-positive/total recipient cfu).
Supplementary Material
Acknowledgments
We are grateful to Yvonne Lötscher (Zürich), Manuel Diehl, Lubov Nedialkova (Münich), and Barbara Plaschke (Würzburg) for experimental support. We thank members of the W.-D.H. laboratory, Sam Brown, and Mathias Heikenwälder for helpful comments and discussion. Work was supported by UBS AG (Zürich) on behalf of a customer (W.-D.H.), Wellcome Trust Grant 076964 (to N.R.T., D.J.P., M.J.S., and A.W.W.), and Deutsche Forschungsgemeinschaft DFG SFB 479 TP A1 and DO 789/4-1 (to U.D.) and DFG STE 1971/2-1 (to B.S.).
Footnotes
The authors declare no conflict of interest.
Database deposition: The 16S rRNA gene sequences reported in this paper have been deposited in the GenBank [accession nos. HE582402–HE582620 (ENA)].
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113246109/-/DCSupplemental.
References
- 1.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin J, et al. MetaHIT Consortium A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arumugam M, et al. Enterotypes of the human gut microbiome. Nature. 2011;470:510–512. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treangen TJ, Rocha EP. Horizontal transfer, not duplication, drives the expansion of protein families in prokaryotes. PLoS Genet. 2011;7:e1001284. doi: 10.1371/journal.pgen.1001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hehemann JH, et al. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. (Translated from eng) Nature. 2009;464:908–912. doi: 10.1038/nature08937. [DOI] [PubMed] [Google Scholar]
- 6.Nelson KE, et al. Human Microbiome Jumpstart Reference Strains Consortium A catalog of reference genomes from the human microbiome. Science. 2010;328:994–999. doi: 10.1126/science.1183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulsen IT, et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science. 2003;299:2071–2074. doi: 10.1126/science.1080613. [DOI] [PubMed] [Google Scholar]
- 8.Johnson TJ, et al. Horizontal gene transfer of a ColV plasmid has resulted in a dominant avian clonal type of Salmonella enterica serovar Kentucky. PLoS ONE. 2010;5:e15524. doi: 10.1371/journal.pone.0015524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielaszewska M, et al. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infect Dis. 2011;11:671–676. doi: 10.1016/S1473-3099(11)70165-7. [DOI] [PubMed] [Google Scholar]
- 10.Tenaillon O, Skurnik D, Picard B, Denamur E. The population genetics of commensal Escherichia coli. Nat Rev Microbiol. 2010;8:207–217. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- 11.Scott KP. The role of conjugative transposons in spreading antibiotic resistance between bacteria that inhabit the gastrointestinal tract. Cell Mol Life Sci. 2002;59:2071–2082. doi: 10.1007/s000180200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohnhoff M, Drake BL, Miller CP. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc Soc Exp Biol NY. 1954;86:132–137. doi: 10.3181/00379727-86-21030. [DOI] [PubMed] [Google Scholar]
- 13.van der Waaij D, Berghuis-de Vries JM, Lekkerkerk Lekkerkerk-v Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 1971;69:405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 15.Licht TR, Christensen BB, Krogfelt KA, Molin S. Plasmid transfer in the animal intestine and other dynamic bacterial populations: the role of community structure and environment. Microbiology. 1999;145:2615–2622. doi: 10.1099/00221287-145-9-2615. [DOI] [PubMed] [Google Scholar]
- 16.Daniels JB, Call DR, Besser TE. Molecular epidemiology of blaCMY-2 plasmids carried by Salmonella enterica and Escherichia coli isolates from cattle in the Pacific Northwest. Appl Environ Microbiol. 2007;73:8005–8011. doi: 10.1128/AEM.01325-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smet A, et al. In situ ESBL conjugation from avian to human Escherichia coli during cefotaxime administration. J Appl Microbiol. 2011;110:541–549. doi: 10.1111/j.1365-2672.2010.04907.x. [DOI] [PubMed] [Google Scholar]
- 18.Moubareck C, Bourgeois N, Courvalin P, Doucet-Populaire F. Multiple antibiotic resistance gene transfer from animal to human enterococci in the digestive tract of gnotobiotic mice. Antimicrob Agents Chemother. 2003;47:2993–2996. doi: 10.1128/AAC.47.9.2993-2996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faure S, Perrin-Guyomard A, Delmas JM, Chatre P, Laurentie M. Transfer of plasmid-mediated CTX-M-9 from Salmonella enterica serotype Virchow to Enterobacteriaceae in human flora-associated rats treated with cefixime. Antimicrob Agents Chemother. 2010;54:164–169. doi: 10.1128/AAC.00310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lupp C, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Stecher B, et al. Salmonella enterica serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. (Translated from Eng) PLoS Biol. 2007;5:e244. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pédron T, Sansonetti P. Commensals, bacterial pathogens and intestinal inflammation: an intriguing ménage à trois. Cell Host Microbe. 2008;3:344–347. doi: 10.1016/j.chom.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Winter SE, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darfeuille-Michaud A, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 25.Barthel M, et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stecher B, et al. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog. 2010;6:e1000711. doi: 10.1371/journal.ppat.1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brzuszkiewicz E, et al. How to become a uropathogen: comparative genomic analysis of extraintestinal pathogenic Escherichia coli strains. Proc Natl Acad Sci USA. 2006;103:12879–12884. doi: 10.1073/pnas.0603038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welch RA, et al. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc Natl Acad Sci USA. 2002;99:17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SR, Komano T. The plasmid R64 thin pilus identified as a type IV pilus. J Bacteriol. 1997;179:3594–3603. doi: 10.1128/jb.179.11.3594-3603.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komano T, Yoshida T, Narahara K, Furuya N. The transfer region of IncI1 plasmid R64: Similarities between R64 tra and legionella icm/dot genes. Mol Microbiol. 2000;35:1348–1359. doi: 10.1046/j.1365-2958.2000.01769.x. [DOI] [PubMed] [Google Scholar]
- 31.Sampei G, et al. Complete genome sequence of the incompatibility group I1 plasmid R64. Plasmid. 2010;64:92–103. doi: 10.1016/j.plasmid.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Komano T, Kubo A, Nisioka T. Shufflon: Multi-inversion of four contiguous DNA segments of plasmid R64 creates seven different open reading frames. Nucleic Acids Res. 1987;15:1165–1172. doi: 10.1093/nar/15.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cascales E, et al. Colicin biology. Microbiol Mol Biol Rev. 2007;71:158–229. doi: 10.1128/MMBR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerr B, Riley MA, Feldman MW, Bohannan BJ. Local dispersal promotes biodiversity in a real-life game of rock-paper-scissors. Nature. 2002;418:171–174. doi: 10.1038/nature00823. [DOI] [PubMed] [Google Scholar]
- 35.Sonnenborn U, Schulze J. The non-pathogenic Escherichia coli strain Nissle 1917 - features of a versatile probiotic. Microb Ecol Health Dis. 2009;21:122–158. [Google Scholar]
- 36.Hapfelmeier S, et al. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J Immunol. 2005;174:1675–1685. doi: 10.4049/jimmunol.174.3.1675. [DOI] [PubMed] [Google Scholar]
- 37.Dewhirst FE, et al. Phylogeny of the defined murine microbiota: Altered Schaedler flora. Appl Environ Microbiol. 1999;65:3287–3292. doi: 10.1128/aem.65.8.3287-3292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stecher B, Hardt WD. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol. 2010;14:82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Pfeifer Y, Cullik A, Witte W. Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. Int J Med Microbiol. 2010;300:371–379. doi: 10.1016/j.ijmm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Bergstrom CT, Lipsitch M, Levin BR. Natural selection, infectious transfer and the existence conditions for bacterial plasmids. Genetics. 2000;155:1505–1519. doi: 10.1093/genetics/155.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dionisio F, Matic I, Radman M, Rodrigues OR, Taddei F. Plasmids spread very fast in heterogeneous bacterial communities. Genetics. 2002;162:1525–1532. doi: 10.1093/genetics/162.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hapfelmeier S, et al. Role of the Salmonella pathogenicity island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect Immun. 2004;72:795–809. doi: 10.1128/IAI.72.2.795-809.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;66:4555–4558. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westendorf AM, et al. Autoimmune-mediated intestinal inflammation-impact and regulation of antigen-specific CD8+ T cells. Gastroenterology. 2006;131:510–524. doi: 10.1053/j.gastro.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Stecher B, et al. Motility allows S. Typhimurium to benefit from the mucosal defence. Cell Microbiol. 2008;10:1166–1180. doi: 10.1111/j.1462-5822.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang RF, Kushner SR. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.