Abstract
Ocular dominance (OD) plasticity in the visual cortex is a classic model system for understanding developmental plasticity, but the visual cortex also shows plasticity in adulthood. Whether the plasticity mechanisms are similar or different at the two ages is not clear. Several plasticity mechanisms operate during development, including homeostatic plasticity, which acts to maintain the total excitatory drive to a neuron. In agreement with this idea, we found that an often-studied substrain of C57BL/6 mice, C57BL/6JOlaHsd (6JOla), lacks both the homeostatic component of OD plasticity as assessed by intrinsic signal imaging and synaptic scaling of mEPSC amplitudes after a short period of dark exposure during the critical period, whereas another substrain, C57BL/6J (6J), exhibits both plasticity processes. However, in adult mice, OD plasticity was identical in the 6JOla and 6J substrains, suggesting that adult plasticity occurs by a different mechanism. Consistent with this interpretation, adult OD plasticity was normal in TNFα knockout mice, which are known to lack juvenile synaptic scaling and the homeostatic component of OD plasticity, but was absent in adult α-calcium/calmodulin-dependent protein kinase II;T286A (αCaMKIIT286A) mice, which have a point mutation that prevents autophosphorylation of αCaMKII. We conclude that increased responsiveness to open-eye stimulation after monocular deprivation during the critical period is a homeostatic process that depends mechanistically on synaptic scaling during the critical period, whereas in adult mice it is mediated by a different mechanism that requires αCaMKII autophosphorylation. Thus, our study reveals a transition between homeostatic and long-term potentiation–like plasticity mechanisms with increasing age.
The visual cortex demonstrates a clear critical period for ocular dominance (OD) plasticity (1, 2), which in the mouse spans approximately postnatal days (P) 19–32 (3). During the critical period, monocular deprivation (MD) causes neurons in the binocular zone of the primary visual cortex (V1) to undergo an initial long term depression (LTD)-like decrease in responsiveness to the closed eye (4), followed by a delayed increase in responsiveness to both the open and closed eye (5). Many previous studies have searched for factors that might limit the critical period to this early developmental time window (for reviews see refs. 6 and 7). This search is complicated by recent studies showing that in mice, OD plasticity also occurs in the adult visual cortex (8, 9) although it declines beyond 110 d of age (10). There are notable phenomenologic differences between plasticity in the critical period and adulthood; in adult mice, the response to MD is slower, is restricted to an increase in open-eye responsiveness, and is more pronounced in the hemisphere contralateral to the deprived eye (11). These findings raise the question of whether adult plasticity mechanisms are the same as or different than those in operation during the critical period. If the mechanisms are the same, then the critical period is simply a stage of development during which the gain or sensitivity of the plasticity mechanism is greatest; if different, then either more plasticity mechanisms are present during the critical period than in adulthood or new plasticity mechanisms replace critical period plasticity mechanisms in adulthood.
Homeostatic plasticity has been shown to play a role in synaptic development (12) and in OD plasticity during the critical period (13, 14). TNFα is an important factor for synaptic scaling (15) and is required for the increased open-eye responsiveness observed during juvenile OD plasticity (13). OD plasticity after MD in the adult visual cortex lacks the depression of the closed-eye responses observed during the critical period and is instead characterized by increased cortical responsiveness to the open eye (9). Whether this potentiation depends on the same homeostatic plasticity mechanism(s) that operate during the critical period is not known, however. One study has suggested that a form of synaptic scaling persists into adulthood in layer 2/3 (L2/3) of the visual cortex (16). On the other hand, studies in the hippocampus have shown that homeostatic plasticity, and synaptic scaling in particular, are down-regulated during development (17, 18).
We report a pronounced homeostatic plasticity deficit in the commonly used 6JOla substrain of C57BL/6 mice. During the critical period, these mice exhibit impairments in both the MD-induced increase in open-eye responsiveness and synaptic scaling induced by dark exposure (DE). In contrast in adult 6JOla animals, the MD-induced increase in open-eye responsiveness was normal, as was OD plasticity in adult TNFα KO (TNFα−/−) mice, which are also known to lack synaptic scaling and the juvenile homeostatic component of OD plasticity. Furthermore, adult OD plasticity was entirely absent in T286A mice. Taken together, our data suggest that with aging, visual cortex plasticity becomes increasingly dependent on a mechanism that requires α-calcium/calmodulin-dependent protein kinase II (αCaMKII) autophosphorylation and less dependent on the homeostatic mechanisms that are critical to juvenile plasticity.
Results
The Homeostatic Component of OD Plasticity Is Absent in 6JOla Mice.
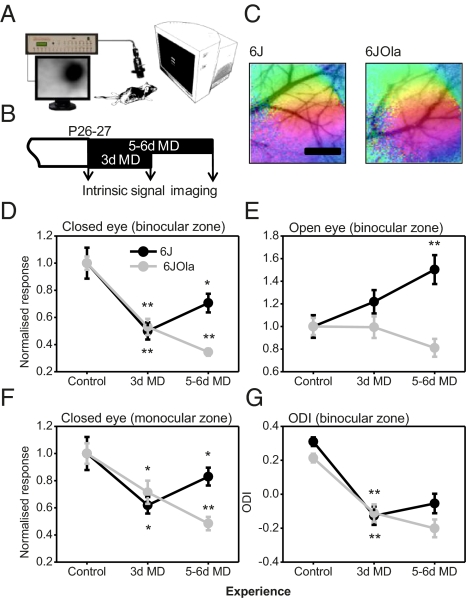
A 3-d period of MD is known to result in an LTD-like loss of cortical responsiveness to the closed eye with no change in open-eye responses (13). If MD continues for another 2 or 3 d, then the cortical response to the closed eye increases slightly from its low point but is still depressed overall, and the response to the open eye increases substantially (13). There is evidence indicating that both these increases depend on a homeostatic mechanism (13). We used intrinsic signal imaging (Fig. 1A) to compare OD plasticity in vivo in the primary visual cortex of 6J and 6JOla mice after 3 d or 5–6 d of MD (Fig. 1B) and analyzed the open-eye and closed-eye responses separately (3). A two-way ANOVA of closed- and open-eye response magnitudes found a significant interaction between substrain and deprivation, indicating that MD had different effects on the two substrains of C57BL/6 mice [closed eye: P < 0.01, F2,60 = 6.33 (Fig. 1D); open eye: P < 0.01, F2,60 = 5.04 (Fig. 1E)] prompting further investigation.
Fig. 1.
In vivo intrinsic signal imaging reveals the absence of the homeostatic component of OD plasticity during the critical period in the 6JOla substrain. (A) Schematic of the intrinsic signal imaging setup. (B) Deprivation and imaging timeline. (C) Representative retinotopic maps from the two C57BL/6 substrains investigated overlaid over the cortical vasculature. (Scale bar: 1 mm.) (D) Binocular zone responsiveness to closed-eye stimulation during MD. (E) Binocular zone responsiveness to open-eye stimulation during MD. (F) Monocular zone responsiveness to closed-eye stimulation during MD. (G) ODI in the binocular zone during MD. For 6J: control, n = 10; 3 d MD, n = 8; 5–6 d MD, n = 9. For 6JOla: control, n = 22; 3 d MD, n = 6; 5–6 d MD, n = 11. **P < 0.01; *P < 0.05 for comparisons between time points. In D–F, response values are mean ΔR/R normalized to control values ± SEM.
In both the 6J and 6JOla substrains, 3 d of MD resulted in a robust OD shift toward the open eye, mediated principally by a loss of responsiveness to the closed eye (6J: control, 1.16 ± 0.13; 3 d MD, 0.58 ± 0.08; P < 0.01; t = 3.55; 6JOla: control, 1.40 ± 0.07; 3 d MD, 0.75 ± 0.07; P < 0.01; t = 4.83) (Fig. 1 D and G). However, the cortical response to MD diverged between the two substrains after 5–6 d. In the 6J substrain, 5–6 d of MD resulted in a homeostatic increase in the cortical response to stimulation of the open eye, as has been reported previously (5, 13) (6J: control, 0.60 ± 0.06; 5–6 d MD, 0.90 ± 0.08; P < 0.05; t = 3.15) (Fig. 1E). In contrast, the homeostatic increase in open-eye responsiveness was absent in the 6JOla substrain after 5–6 d of MD (6JOla: control, 0.96 ± 0.07; 5–6 d MD, 0.77 ± 0.08; P = 0.09; t = 1.79) (Fig. 1E).
An indication that a homeostatic process is induced during OD plasticity is the otherwise paradoxical “rebound” in cortical responsiveness to the deprived eye input between 3 d and 5–6 d of MD (5, 13). This has been observed previously in mice as a slight increase in cortical responsiveness to the closed eye in both the binocular cortex and the monocular cortex, which receives input only from the deprived (contralateral) eye (13). In 6J mice, homeostatic recovery of deprived eye responses occurred in both the binocular cortex and the monocular cortex after 5–6 d of MD [binocular: 3 d MD, 0.58 ± 0.08; 5–6 d MD, 0.82 ± 0.08; P < 0.05; t = 2.16 (Fig. 1D); monocular: 3 d MD, 0.83 ± 0.08; 5–6 d MD, 1.12 ± 0.09; P < 0.05; t = 2.39 (Fig. 1F)]. Strikingly, the opposite trend was observed in 6JOla mice, whose deprived-eye responses continued to decrease significantly between 3 d and 5–6 d MD (binocular: 3 d MD, 0.75 ± 0.07; 5–6 d MD, 0.50 ± 0.07; P < 0.05; t = 3.30; monocular: 3 d MD, 1.07 ± 0.13; 5–6 d MD, 0.70 ± 0.07; P < 0.05; t = 2.65) (Fig. 1F). Despite the apparent absence of this homeostatic plasticity mechanism in 6JOla mice, intrinsic signal response magnitude was comparable in the two substrains (control 6J contralateral: 2.43 ± 0.45; 6JOla contralateral: 2.92 ± 0.27; P = 0.37; t = 0.94) (Fig. S1A), as was retinotopic map organization (6J scatter: 3.02 ± 0.43; 6JOla scatter: 2.90 ± 0.36; P = 0.84; t = 0.21) (Fig. 1C and Fig. S1B).
One known genetic difference between the two substrains is that the gene encoding α-synuclein, a neuronally expressed protein associated with neurodegeneration but of uncertain function, is absent in the 6JOla strain (19). Therefore, we assessed the homeostatic component of OD plasticity (i.e., the up-regulation of open-eye and deprived-eye responses between 3 d and 5–6 d of MD) in α-synuclein KO mice and compared them with WT littermates of the C57BL/6JCrl substrain (which exhibit juvenile OD plasticity identical to that of the 6J substrain, including the homeostatic component). We found that the homeostatic component of OD plasticity was normal in the α-synuclein KO mice (Fig. S2), indicating that some other genetic difference is likely responsible for the absence of homeostatic plasticity in the 6JOla substrain.
These results indicate that 6JOla mice have a robust OD plasticity deficit that is specific to the homeostatic component that manifests itself at the 5–6 d MD time point. Accordingly, the increase in cortical responsiveness to the nondeprived eye is completely absent in 6JOla mice during the critical period.
Homeostatic Synaptic Scaling Is Absent in 6JOla Mice.
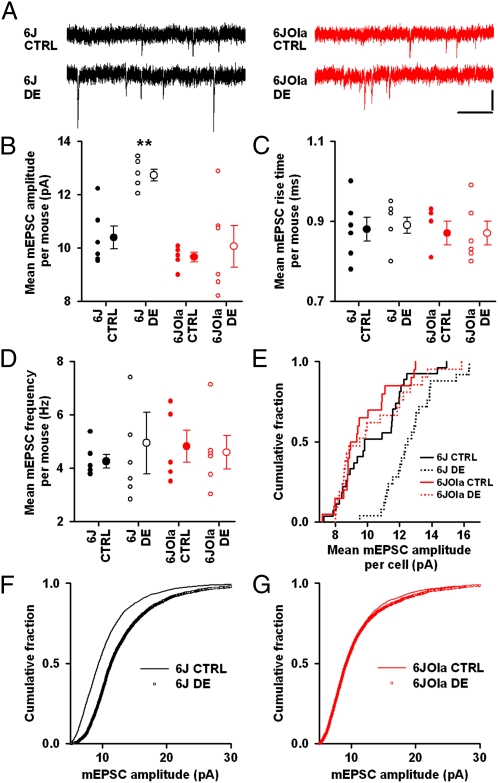
Synaptic scaling, a key mechanism underlying response homeostasis in the brain, is typically assayed by measuring the amplitude of miniature excitatory postsynaptic currents (mEPSCs), which result from the spontaneous release of single vesicles of a neurotransmitter. There is some evidence indicating that synaptic scaling might underlie the homeostatic component of OD plasticity that occurs between brief (3 d) and a longer period (5–6 d) of MD in the mouse visual cortex (13). However, this had not been previously tested directly by measuring the effect of visual deprivation on mEPSC amplitudes in the visual cortex of mice lacking homeostatic plasticity. Therefore, because the homeostatic component of OD plasticity was absent in 6JOla mice (Fig. 1), we tested whether homeostatic synaptic scaling of mEPSC amplitudes was present in the 6JOla substrain after visual deprivation during the critical period. A previous study showed that brief periods (1–3 d) of DE result in scaling-up of mEPSC amplitude in V1 of juvenile rats and 6J mice, which can be detected ex vivo in acute brain slices (16, 20). We chose DE over MD because the latter would be expected to have different effects on different synapses depending on whether they represent the open eye or the closed eye, whereas the former would be expected to affect the synapses of both eyes on a given neuron similarly. Therefore, we recorded mEPSCs from L2/3 pyramidal neurons in V1 of acute brain slices from 6J and 6JOla mice that had been either dark-exposed for 3 d (P25–P28) or reared on a normal 12-h light/12-h dark cycle (Fig. 2A).
Fig. 2.
Ex vivo homeostatic synaptic scaling is absent in 6JOla mice. (A) Example traces showing mEPSCs recorded in brain slices from 6J and 6JOla control (CTRL) and dark-exposed (DE) mice. (Scale bars: 10 pA; 200 ms.) (B) Effect of DE on mEPSC amplitude. Data are shown as mean for each mouse (small symbols) and grand mean ± SEM (large symbols); n = 6 mice/group. **P < 0.01 for 6J DE vs. all other groups. (C) Effect of DE on mEPSC rise time. Symbols are as in B. (D) Effect of DE on mEPSC frequency. Symbols are as in B. (E) Cumulative distribution plot of mean mEPSC amplitude for individual neurons in the four groups. 6J control, n = 27; 6J DE, n = 25; 6JOla control, n = 20; 6JOla DE, n = 21. (F) Cumulative distribution plot showing the effect of DE on raw mEPSC amplitude for 6J mice. n = 50 mEPSCs/neuron. mEPSCs >30 pA are not shown. (G) As in F for 6JOla mice.
Two-way ANOVA indicated significant effects of DE (P = 0.008; F1,20 = 8.91) and substrain (P = 0.002; F1,20 = 13.66), as well as an interaction between DE and substrain (P = 0.049; F1,20 = 4.39). Using post hoc pairwise comparisons, we found that, as expected, mEPSC amplitude was significantly larger in the dark-exposed 6J mice compared with age-matched 6J controls, indicating the presence of synaptic scaling (6J control: 10.4 ± 0.4 pA; 6J dark-exposed: 12.7 ± 0.2 pA; 22% increase; P = 0.010; t = 3.68) (Fig. 2 A and B). In contrast, there was no difference in mEPSC amplitude between dark-exposed and control 6JOla mice (6JOla control: 9.7 ± 0.2 pA; 6JOla dark-exposed: 10.1 ± 0.7 pA; 4% increase; P = 0.55, t = 0.62), indicating a lack of synaptic scaling in this substrain (Fig. 2 A and B).
The difference in DE-induced mEPSC scaling between the substrains could not be explained by differences in baseline mEPSC amplitudes, which were similar in the 6J and 6JOla mice (P = 0.30; t = 1.11) (Fig. 2B). Neither substrain nor DE had any effect on mEPSC rise time (effect of substrain: P = 0.87, F1,20 = 0.02; effect of DE: P = 0.79, F1,20 = 0.07; interaction: P = 0.46; F1,20 = 0.57; two-way ANOVA) (Fig. 2C) or mEPSC frequency (effect of substrain: P = 0.51 F1,20 = 0.44; effect of DE: P = 0.97, F1,20 = 0.00; interaction, P = 0.71, F1,20 = 0.14; two-way ANOVA) (Fig. 2D). Resting membrane potential and input resistance were also similar across all groups (Table S1).
The conclusions derived from the statistical comparisons between individual animals described above were corroborated by further analysis of the distributions of mEPSC amplitudes between the 6J and 6JOla substrains. The distribution of mEPSC amplitudes for individual neurons was shifted toward larger values after DE in 6J mice [D = 0.49; P = 0.002, Kolmogorov–Smirnov (KS) test] (Fig. 2E), but not in 6JOla mice (D = 0.23; P = 0.60, KS test). We also found that the distribution of raw mEPSC amplitudes was shifted toward larger values in dark-exposed 6J mice than in control 6J mice (D = 0.24; P < 0.001, KS test) (Fig. 2F). In contrast, the distribution of mEPSC amplitudes was similar in dark-exposed and control 6JOla mice (D = 0.03; P = 0.32, KS test) (Fig. 2G). In summary, DE resulted in robust homeostatic synaptic scaling in the 6J mice, but not in the 6JOla mice.
Homeostatic synaptic scaling is thought to involve a proportional change in the strength of all inputs to a neuron, referred to as multiplicative scaling (12). Multiplicative scaling has been reported in cultured neurons after pharmacologic activity blockade (12) and in L2/3 pyramidal neurons in the visual cortex after DE during the critical period, but not in adulthood (16). Therefore, we investigated whether the increase in mEPSC amplitude in dark-exposed 6J mice that we report here resulted from multiplicative scaling. Mean mEPSC amplitude was 22% larger in the 6J dark-exposed mice compared with the 6J control mice; thus, we multiplied 6J control mEPSC amplitudes by 1.22 to generate a scaled distribution (6J CTRLscaled), and compared these values with the amplitudes of mEPSCs recorded in brain slices from dark-exposed 6J mice (Fig. S3). There was no significant difference between the 6J dark-exposed and 6J CTRLscaled distributions (D = 0.03; P = 0.22, KS test) (Fig. S3), consistent with multiplicative scaling-up of inputs to L2/3 pyramidal neurons in the 6J mice after DE during the critical period.
Normal Adult OD Plasticity in 6JOla and TNFα−/− Mice.
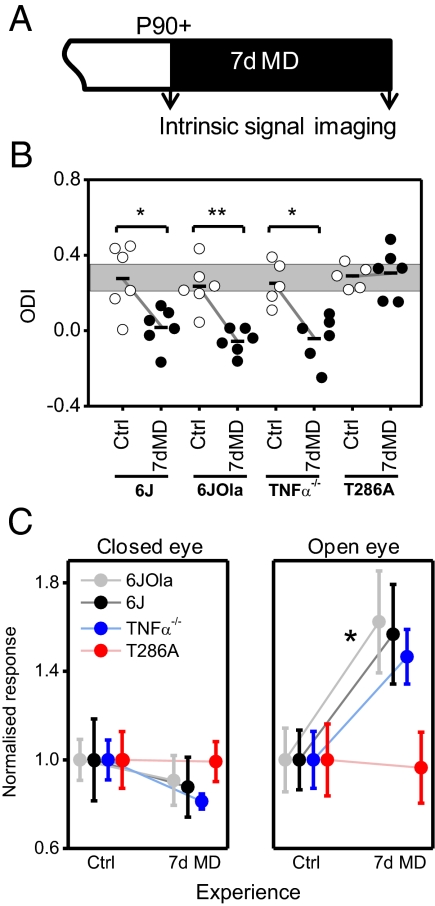
Induction of adult OD plasticity requires a longer period of altered visual experience and is mediated primarily by an increased cortical response to the open eye (9, 11, 21). Having found that 6JOla mice lack a (homeostatic) increase in open-eye responsiveness during the critical period, we tested whether the same deficit persisted into adulthood (Fig. 3A). Interestingly, the two substrains showed nearly identical plasticity profiles in adulthood after 7 d of MD (substrain-deprivation interaction: P = 0.69; F1,20 = 0.16) (Fig. 3B), with a clear shift in the OD index (ODI) in both substrains (deprivation effect: P < 0.001, F1,20 = 28.18; substrain effect: P = 0.24, F1,20 = 1.46; two-way ANOVA). The shift in ODI was mediated purely by an increase in the open-eye response (6J: control, 1.29 ± 0.17; 7 d MD, 2.05 ± 0.28; P < 0.05; t = 2.29; 6JOla: control, 1.86 ± 0.26; 7 d MD, 3.02 ± 0.43; P < 0.05; t = 2.31) (Fig. 3C), whereas the deprived eye responses were unchanged in both strains (6J: control, 2.43 ± 0.45; 7 d MD, 2.13 ± 0.33; P = 0.60; t = 0.54; 6JOla: control, 2.92 ± 0.66; 7 d MD, 2.65 ± 0.33; P = 0.54; t = 0.64). No change in deprived monocular area responses was observed in either the 6J mice (control, 2.41 ± 0.32; 7 d MD, 2.40 ± 0.17) or the 6JOla mice (control, 3.12 ± 0.24; 7 d MD, 2.81 ± 0.44).
Fig. 3.
Adult OD plasticity is normal in 6JOla and TNFα−/− mice but absent in T286A mice. (A) Adult deprivation and imaging timeline. (B) ODI shift after 7 d of MD. (C) Responsiveness to closed-eye (Left) and open-eye (Right) stimulation before and after 7 d of MD. For 6J and 6JOla control and 7 d MD, n = 6; T286A control, n = 5; 7 d MD, n = 6; TNFα−/− control, n = 5; 7 d control, n = 5; 7 d MD, n = 6. **P < 0.01; *P < 0.05. Response values are mean ΔR/R normalized to control values ± SEM. Gray box indicates control 6J mean ± SEM.
These findings suggest that the increase in the open-eye response in adults might operate by a mechanism distinct from the homeostatic process that appears to underlie increased open-eye responsiveness during the critical period. To further explore this possibility, we measured adult OD plasticity in mice lacking TNFα (bred on a 6J background), which has been shown to be required for the homeostatic component of OD plasticity in juvenile mice and homeostatic synaptic scaling in organotypic slice cultures (13, 15). Strikingly, OD plasticity appeared completely normal in adult TNFα−/− mice, further indicating that adult OD plasticity is not mediated by the same homeostatic mechanisms that operate during juvenile plasticity (ODI: control, 0.25 ± 0.05; 7 d MD, -0.04 ± 0.05; P < 0.01; t = 4.04; deprived eye: control, 3.61 ± 0.32; 7 d MD, 2.93 ± 0.12; P = 0.11; t = 1.95; open eye: control, 2.20 ± 0.28; 7 d MD, 3.23 ± 0.27; P < 0.05; t = 2.60) (Fig. 3 B and C).
Previous studies have shown that αCaMKII autophosphorylation is critical for experience-dependent potentiation in adult somatosensory cortex (22), and that the dependence of cortical long-term potentiation (LTP) on αCaMKII increases with age in the visual cortex (23). This raises the possibility that the experience-dependent cortical plasticity seen in adult visual cortex depends on αCaMKII. Thus, we examined adult plasticity in the visual cortex of αCaMKII mutants that lack the ability to autophosphorylate at T286. We found a complete absence of adult OD plasticity in T286A mice (ODI: control, 0.29 ± 0.03; 7 d MD, 0.31 ± 0.05; P = 0.76; t = 0.32; deprived eye: control, 2.34 ± 0.30; 7 d MD, 2.32 ± 0.21; P = 0.96; t = 0.05; open eye: control, 1.33 ± 0.22; 7 d MD, 1.28 ± 0.21; P = 0.88; t = 0.15) (Fig. 3 B and C), supporting the concept that αCaMKII autophosphorylation is vital for the increase in responsiveness to the open eye in monocularly deprived adults. The fact that our T286A mice had been bred on a mixed 6J and 6JOla background is unlikely of consequence here, given that the WT in both the 6J and 6JOla substrains exhibited normal adult OD plasticity (in contrast to the T286A mice).
Discussion
In this study, we have shown that a previously unknown homeostatic plasticity deficit exists in a commonly studied substrain of C57BL/6 mouse, the 6JOla substrain. The deficit in this substrain was observed both at the systems level using intrinsic signal imaging and at the cellular level using acute brain slices ex vivo, and could be revealed by either DE or monocular eyelid closure. Therefore this study demonstrates directly a mechanistic association between visual deprivation-induced homeostasis at the systems level and visual deprivation-induced synaptic scaling at the cellular level. We exploited this finding to ask whether adult visual cortex plasticity, although phenomenologically similar to juvenile plasticity, is mechanistically distinct. Although the increased cortical responsiveness to open-eye stimulation is entirely absent in juvenile 6JOla mice, it appears to be normal in adult 6JOla mice, indicating that the two processes occur by different mechanisms. Consistent with this interpretation, we found normal adult OD plasticity in TNFα−/− mice, which were previously shown to completely lack the increase in both open-eye and closed-eye responsiveness after 5–6 d of MD during the critical period, and also to lack synaptic scaling (13). Conversely, visual cortex plasticity as assessed by 4 d of MD during the critical period is markedly impaired but not abolished in T286A mice, which lack autophosphorylatable αCaMKII (24, 25), whereas we show here that OD plasticity in response to MD is entirely absent in adult T286A mice, indicating a difference in the degree of involvement of αCaMKII autophosphorylation in juvenile and adult plasticity. Thus, our data are consistent with a model in which both TNFα and αCaMKII autophosphorylation are involved in juvenile plasticity, but αCaMKII autophosphorylation is dominant in adult plasticity.
Strain Differences in Cortical Plasticity.
C57BL/6 substrains are often considered interchangeable, and a lack of reference to which specific substrain was used is common in experimental methodologies. However, there is growing awareness of the significant genetic and phenotypic differences between even closely related mouse strains and substrains (19, 26–28), and the present study demonstrates the complete absence in a C57BL/6 substrain of what is generally considered a ubiquitous plasticity mechanism in both mice and rats (14, 16, 20, 29). This finding underscores the importance of reporting mouse background substrains and of using appropriate littermate controls. This finding also prompts the question of the critical genetic difference in the 6JOla substrain. The most prominent genetic difference between the two substrains is the absence of α-synuclein in 6JOla mice; however, we have shown that this protein is not required for the homeostatic component of OD plasticity. The numerous other genetic differences between the two substrains include other absent or mutated genes and a multitude of copy number variations (26, 27, 30). The analysis of these differences may allow identification of a unique molecular mechanism of homeostatic plasticity as more is discovered about the signaling pathways involved.
Homeostasis in Critical Period Cortical Plasticity.
Several previous studies have provided evidence for the existence of homeostatic plasticity in response to reduced neuronal drive in a number of central brain regions, including the hippocampus and neocortex (12–14, 20, 31). Theoretically, a homeostatic response to visual deprivation could occur through a change in intrinsic membrane properties (32), a change in the excitatory/inhibitory balance at the circuit level (33), or scaling of the efficacy of individual synapses at presynaptic (34, 35) and/or postsynaptic loci (15, 36–41). Although there is evidence indicating that each of these mechanisms exists, our data (showing a change in mEPSC amplitude but not in frequency) imply that postsynaptic scaling at excitatory to excitatory cell synapses is an essential mechanism for homeostatic plasticity in the visual cortex. This conclusion is consistent with studies showing that TNFα−/− mice lack homeostatic plasticity in the visual cortex during the critical period, and that organotypic slice cultures derived from the same mutant lack synaptic scaling (13, 15). Similarly, all aspects of OD plasticity, including homeostatic plasticity, are absent in Arc KO mice during the critical period, and Arc also has been strongly implicated in synaptic scaling (42, 43).
The apparent normality of visual response magnitudes and visual cortex map formation, as well as baseline mEPSC amplitude, in the 6JOla mice is surprising given the apparent severity of the homeostatic plasticity deficit that we observed. Curiously, this is a recurrent theme in studies of homeostatic plasticity in vivo, because TNFα and Arc knockouts are also reported to develop normally (13, 42), which might reflect the fact that multiple homeostatic mechanisms operate in parallel (33). In this regard, it may be noteworthy that although we have found evidence indicating that the 6JOla mouse lacks a homeostatic mechanism that up-regulates synaptic gain, this mouse may have an intact mechanism for down-regulation (12, 44). Homeostatic down-regulation may mitigate the more significant risk of excitotoxicity during the intense synaptogenesis that occurs during early postnatal development.
Distinct Mechanisms of Adult Visual Cortical Plasticity.
The consensus opinion is that induction of adult mouse OD plasticity requires ∼7 d and is mediated by an increased cortical response to the open eye (9, 11). We have shown that 6JOla mice lack both the MD-induced increase in open eye responsiveness and synaptic scaling during the critical period for OD plasticity, but have normal open-eye potentiation in adults. We interpret these findings to indicate that juvenile OD plasticity operates through a different mechanism than adult OD plasticity. An alternative explanation could be that homeostatic plasticity matures later in 6JOla mice than in 6J mice, and that adult open-eye potentiation in both strains occurs via a homeostatic mechanism. However, we believe that this is unlikely for at least two reasons. First, we observed no homeostatic increase in the response of deprived adult monocular cortex in either substrain, which would be predicted if adult plasticity were due to homeostatic plasticity (13, 20). Second, delayed development seems unlikely, given that previous studies in the mouse visual cortex have found that synaptic scaling is typically present in mice at least 2 wk younger than those in the present study. Visual deprivation-induced synaptic scaling in the cortex has been observed as early as P14 in rats and P21 in mice, with earlier observations complicated by the fact that the eyes do not open until P14 (16, 20).
There is evidence suggesting that the capacity for synaptic scaling might be down-regulated during postnatal development. For example, in the rat hippocampus, a period of in vivo activity blockade by TTX application results in scaling-up of pyramidal neuron mEPSC amplitudes in juvenile animals, but has no effect on mEPSC amplitude in adults (18), whereas the susceptibility to TTX incubation-induced scaling-up of mEPSC amplitude declines significantly between P4 and P8 (17). Interestingly, a nonmultiplicative form of DE-induced synaptic plasticity, which comprises a nonuniform increase in synaptic weights, has been reported to persist into adulthood in L2/3 of the mouse visual cortex (16). Which subset of synapses are preferentially strengthened by this mechanism is unknown, although one possibility is that the subset is more active (in the case of DE, active because of spontaneous activity). In the case of adult MD, the more active inputs are those relaying input from the open eye, which would be scaled preferentially. This interpretation is reminiscent of the idea of “metaplasticity” and a homeostatically regulated threshold for synaptic modification (45), and is consistent with previous experimental findings of enhanced LTP and adult OD plasticity after dark rearing (46, 47). Regardless of the nonmultiplicative scaling mechanism that supports adult OD plasticity, our data demonstrate that multiplicative scaling dependent on TNFα is not required. A developmentally regulated transition between synaptic scaling during the critical period and homosynaptic LTP in adulthood is also consistent with the increased importance of αCaMKII activity in adult experience-dependent plasticity, given the known crucial role of αCaMKII in several forms of cortical LTP (48).
Materials and Methods
Subjects.
All animal procedures were performed in accordance with the U.K. Animals (Scientific Procedures) Act 1986. The C57BL/6J (Jackson) mice were supplied by Charles River Laboratories. The C57BL/6JOlaHsd (Harlan) mice were supplied by Harlan. The α-synuclein knockout mice were kindly supplied by Prof. Vladimir Buchman (Cardiff University, Cardiff, United Kingdom.) and were congenic C57BL/6JCrl (backcrossed for 12 generations). TNFα−/− mice were kindly supplied by Dr. Denise McDonald (Queens University, Belfast, Northern Ireland) and were inbred on a homozygous C57BL/6J strain originally sourced from Bantin & Kingman and generated by targeting C57BL/6 ES cells. T286A mice were obtained from Prof. Alcino Silva (University of California, Los Angeles, CA). These mice were originally congenic C57BL/6J (backcrossed for five generations) and were then inbred (cousin matings) over 14 y, during which time they were outbred with C57BL/6JOlaHsd mice on three separate occasions. For the ex vivo experiments, six mice per group were used (24 mice total). For in vivo experiments, a total of 92 juvenile mice and 43 adult mice were used.
Visual Deprivation.
Mice were normally reared on a 12-h light/12-h dark cycle. For in vivo experiments, mice were monocularly deprived by eyelid suture under isoflurane anesthesia (2% in O2, 0.6 L/min). For critical period studies, deprivation began at P26–P27 and lasted for 3 d or 5–6 d, whereas for adult studies, deprivations began at P90–P120 and lasted for 7 d. The integrity of the deprivation was checked daily and immediately before intrinsic signal imaging. The experiment was discontinued if the deprivation was impaired. For ex vivo experiments, mice were housed in a dark room for 3 d beginning at P25–P28. Night vision goggles were used for animal care. On the experimental day, mice were anesthetized with isoflurane in the dark before brain slice preparation.
Ex Vivo Electrophysiology.
Mice were anesthetized with isoflurane and decapitated. Brain slices were prepared and mEPSC recordings from L2/3 pyramidal neurons in V1 were made as described previously (20, 49). Then ≥100 mEPSCs per cell were detected and measured with the MiniAnalysis program (Synaptosoft). All experiments and analyses were performed blind to the sensory experience and background strain of the animal.
In Vivo Intrinsic Signal Imaging.
Acute intrinsic signal imaging was performed in the primary visual cortex contralateral to the deprived eye. The visual cortex was imaged transcranially using 0.8–1% isoflurane in O2 at 0.3 L/min, supplemented with 25 μg chlorprothixene as described previously to measure OD (13, 21) and retinotopic map organization (13, 21). For quantification of OD, visual responses were elicited using a 0.03-cycles/degree square-wave grating drifting at 2 cycles per second presented in the binocular or monocular visual field, with the stimulated eye determined by computer-controlled eye shutters. The ODI was calculated by the formula (C − I)/(C + I), where C and I are the contralateral and ipsilateral response magnitudes, respectively. Response magnitudes are presented as ΔR/R values, where R is light reflected. For measurement of retinotopic map organization, the stimulus was a white horizontal bar of height 1–2 degrees drifting at a rate of 0.125 Hz. Map scatter was calculated by comparing each pixel in the map with its immediate neighbors in a 5 × 5 pixel box as described previously (50, 51).
Statistics.
For ex vivo mEPSC data, mean values were calculated for each neuron, and grand mean ± SEM values were then calculated for each mouse. In vivo data are shown as mean ± SEM. Datasets were compared using two-way ANOVA, the t test, or the KS test. All tests were two-tailed, and α was set at 0.05.
Supplementary Material
Acknowledgments
We thank Thomas Mrsic-Flogel for critical comments on the manuscript. This work was supported by funding from European Community's Seventh Framework Programme FP2007-2013, under Grant Agreement 223326 (to F.S.), the Medical Research Council (to K.F.), and National Institute of Mental Health's Conte Center Grant P50-MH0779720 (to K.F.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112204109/-/DCSupplemental.
References
- 1.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olson CR, Freeman RD. Profile of the sensitive period for monocular deprivation in kittens. Exp Brain Res. 1980;39:17–21. doi: 10.1007/BF00237065. [DOI] [PubMed] [Google Scholar]
- 3.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heynen AJ, et al. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat Neurosci. 2003;6:854–862. doi: 10.1038/nn1100. [DOI] [PubMed] [Google Scholar]
- 5.Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Hensch TK. Critical period mechanisms in developing visual cortex. Curr Top Dev Biol. 2005;69:215–237. doi: 10.1016/S0070-2153(05)69008-4. [DOI] [PubMed] [Google Scholar]
- 7.Daw NW, Reid SN, Wang XF, Flavin HJ. Factors that are critical for plasticity in the visual cortex. Ciba Found Symp. 1995;193:258–276. doi: 10.1002/9780470514795.ch13. [DOI] [PubMed] [Google Scholar]
- 8.Tagawa Y, Kanold PO, Majdan M, Shatz CJ. Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nat Neurosci. 2005;8:380–388. doi: 10.1038/nn1410. [DOI] [PubMed] [Google Scholar]
- 9.Sawtell NB, et al. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann K, Löwel S. Age-dependent ocular dominance plasticity in adult mice. PLoS ONE. 2008;3:e3120. doi: 10.1371/journal.pone.0003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato M, Stryker MP. Distinctive features of adult ocular dominance plasticity. J Neurosci. 2008;28:10278–10286. doi: 10.1523/JNEUROSCI.2451-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-α mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mrsic-Flogel TD, et al. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 16.Goel A, Lee HK. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci. 2007;27:6692–6700. doi: 10.1523/JNEUROSCI.5038-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huupponen J, Molchanova SM, Taira T, Lauri SE. Susceptibility for homeostatic plasticity is down-regulated in parallel with maturation of the rat hippocampal synaptic circuitry. J Physiol. 2007;581:505–514. doi: 10.1113/jphysiol.2007.130062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Echegoyen J, Neu A, Graber KD, Soltesz I. Homeostatic plasticity studied using in vivo hippocampal activity-blockade: Synaptic scaling, intrinsic plasticity and age-dependence. PLoS ONE. 2007;2:e700. doi: 10.1371/journal.pone.0000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Specht CG, Schoepfer R. Deletion of the α-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci. 2001;2:11. doi: 10.1186/1471-2202-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- 21.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Prior experience enhances plasticity in adult visual cortex. Nat Neurosci. 2006;9:127–132. doi: 10.1038/nn1610. [DOI] [PubMed] [Google Scholar]
- 22.Glazewski S, Giese KP, Silva A, Fox K. The role of α-CaMKII autophosphorylation in neocortical experience-dependent plasticity. Nat Neurosci. 2000;3:911–918. doi: 10.1038/78820. [DOI] [PubMed] [Google Scholar]
- 23.Kirkwood A, Silva A, Bear MF. Age-dependent decrease of synaptic plasticity in the neocortex of α-CaMKII mutant mice. Proc Natl Acad Sci USA. 1997;94:3380–3383. doi: 10.1073/pnas.94.7.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taha SA, Stryker MP. Ocular dominance plasticity is stably maintained in the absence of α calcium calmodulin kinase II (αCaMKII) autophosphorylation. Proc Natl Acad Sci USA. 2005;102:16438–16442. doi: 10.1073/pnas.0508185102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taha S, Hanover JL, Silva AJ, Stryker MP. Autophosphorylation of αCaMKII is required for ocular dominance plasticity. Neuron. 2002;36:483–491. doi: 10.1016/s0896-6273(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 26.Zurita E, et al. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011;20:481–489. doi: 10.1007/s11248-010-9403-8. [DOI] [PubMed] [Google Scholar]
- 27.Egan CM, Sridhar S, Wigler M, Hall IM. Recurrent DNA copy number variation in the laboratory mouse. Nat Genet. 2007;39:1384–1389. doi: 10.1038/ng.2007.19. [DOI] [PubMed] [Google Scholar]
- 28.Heimel JA, Hermans JM, Sommeijer JP, Levelt CN. Neuro-Bsik Mouse Phenomics Consortium Genetic control of experience-dependent plasticity in the visual cortex. Genes Brain Behav. 2008;7:915–923. doi: 10.1111/j.1601-183X.2008.00431.x. [DOI] [PubMed] [Google Scholar]
- 29.Turrigiano GG. The self-tuning neuron: Synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Specht CG, Schoepfer R. Deletion of multimerin-1 in α-synuclein–deficient mice. Genomics. 2004;83:1176–1178. doi: 10.1016/j.ygeno.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Breton JD, Stuart GJ. Loss of sensory input increases the intrinsic excitability of layer 5 pyramidal neurons in rat barrel cortex. J Physiol. 2009;587:5107–5119. doi: 10.1113/jphysiol.2009.180943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han EB, Stevens CF. Development regulates a switch between post- and presynaptic strengthening in response to activity deprivation. Proc Natl Acad Sci USA. 2009;106:10817–10822. doi: 10.1073/pnas.0903603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao C, Dreosti E, Lagnado L. Homeostatic synaptic plasticity through changes in presynaptic calcium influx. J Neurosci. 2011;31:7492–7496. doi: 10.1523/JNEUROSCI.6636-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci. 2009;29:6479–6489. doi: 10.1523/JNEUROSCI.3753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goel A, et al. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat Neurosci. 2006;9:1001–1003. doi: 10.1038/nn1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goel A, et al. Phosphorylation of AMPA receptors is required for sensory deprivation-induced homeostatic synaptic plasticity. PLoS ONE. 2011;6:e18264. doi: 10.1371/journal.pone.0018264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Brien RJ, et al. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- 41.Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCurry CL, et al. Loss of Arc renders the visual cortex impervious to the effects of sensory experience or deprivation. Nat Neurosci. 2010;13:450–457. doi: 10.1038/nn.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shepherd JD, et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goold CP, Nicoll RA. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron. 2010;68:512–528. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bear MF. Bidirectional synaptic plasticity: From theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358:649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- 47.He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006;26:2951–2955. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 49.Kaneko M, et al. Constitutively active H-ras accelerates multiple forms of plasticity in developing visual cortex. Proc Natl Acad Sci USA. 2010;107:19026–19031. doi: 10.1073/pnas.1013866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith SL, Trachtenberg JT. The refinement of ipsilateral eye retinotopic maps is increased by removing the dominant contralateral eye in adult mice. PLoS ONE. 2010;5:e9925. doi: 10.1371/journal.pone.0009925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cang J, et al. Ephrin-As guide the formation of functional maps in the visual cortex. Neuron. 2005;48:577–589. doi: 10.1016/j.neuron.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.