A 51-year-old woman presented to her local emergency room with a 6-month history of intermittent palpitations, which had worsened that day. She described her pulse as fast (it was approximately 140 beats per minute) but regular. She also reported recent worsening fatigue, heat intolerance, and an 18-kg (40-lb) weight loss despite a good appetite. She reported no dyspnea, orthopnea, syncopal episodes, headaches, tremors, or profuse sweating. She did not have a history of cardiovascular disease.
The patient’s pulse of 140 beats per minute is consistent with sinus tachycardia. The occurrence of palpitations in a relatively young woman without structural heart disease or atherosclerotic risk factors suggests noncardiac causes. Noncardiac causes of palpitations include metabolic derangements, use of medications or substances that may increase the heart rate (e.g., cocaine, adrenergic drugs, caffeine, or alcohol), and psychiatric disorders. Associated weight loss without anorexia suggests a hypermetabolic state (thyrotoxicosis, uncontrolled diabetes mellitus, or pheochromocytoma), rather than cancer or a chronic infection.
The patient had had a spinal cord injury at T12 from a car accident at the age of 21 years. The injury resulted in paraplegia with associated fecal and urinary incontinence, for which she underwent a colostomy and a urinary diversion with a Koch pouch (a continent ileal reservoir) that required self-catheterization several times daily. She had a history of kidney stones, which developed less frequently after urinary diversion, but she still had urinary tract infections intermittently, necessitating antibiotic therapy. The patient also had osteoporosis, for which she was being treated with recombinant human parathyroid hormone, calcium, and vitamin D. She reported no personal or family history of thyroid disease. She was independent in a wheelchair and was able to drive and maintain a full-time job. She reported no neck pain or swelling, choking sensations, or difficulty swallowing.
On physical examination, her pulse was 99 beats per minute, and her blood pressure was normal. The patient appeared well nourished and comfortable. There was no tremor. Her thyroid gland was slightly enlarged, nontender, and firm on palpation, with a pebbly surface and no discrete palpable nodules. There was no exophthalmos, extraocular muscle weakness, or lid lag. The skin examination was normal. Cardiovascular examination revealed a regular tachycardia with normal heart sounds and no murmurs. The abdomen was soft and nontender. The sites of her colostomy and ileal conduit appeared normal. The rest of the examination was unremarkable.
Despite a lack of physical findings that are strongly suggestive of thyrotoxicosis, this diagnosis remains a good possibility in view of the chronic weight loss, fatigue, and tachycardia. A thyrotropin measurement should be obtained; if the level is abnormal, peripheral-blood thyroid hormone levels should also be measured. To confirm the patient’s cardiac rhythm, an electrocardiogram should be obtained. Urinalysis is also indicated to rule out a urinary tract infection, given the patient’s long-term use of urinary catheterization; such an infection could explain her fatigue and tachycardia. However, an acute infection would not account for the longstanding nature of her presenting symptoms.
An electrocardiogram revealed sinus tachycardia without other abnormalities. A complete blood count, urinalysis, and a metabolic screen were normal. The serum thyrotropin level was undetectable. The serum thyroxine (T4 ) level was 14.2 μg per deciliter (183 nmol per liter) (reference range, 4.5 to 10.9 μg per deciliter [58 to 140 nmol per liter]), the triiodothyronine (T3) level 197 ng per deciliter (3.0 nmol per liter) (reference range, 60 to 181 ng per deciliter [0.9 to 2.8 nmol per liter]), the T3 resin uptake 37% (reference range, 22.5 to 37.0), and the free T4 index 4.9 (reference range, 1.0 to 4.0).
The laboratory-test results are consistent with thyrotoxicosis. The most common cause of this condition in a woman of this age is Graves’ disease. Although Graves’ disease commonly presents with an increased ratio of T3 to T4 and in some cases is associated with ophthalmopathy and, less frequently, dermopathy, the absence of these findings does not rule out the diagnosis. Measurement of anti–thyroid peroxidase antibodies may be helpful, because they are usually present in Graves’ disease; they are also common in silent lymphocytic thyroiditis, but the 6-month duration of symptoms is longer than is consistent with this diagnosis. Toxic multinodular goiter and a toxic adenoma remain part of the differential diagnosis. An evaluation of radioactive iodine (iodine-123) uptake and scan of the thyroid would help to distinguish Graves’ disease or other diagnoses associated with increased uptake (i.e., toxic multinodular goiter and toxic adenoma) from conditions in which the uptake is low or zero.
Tests of thyroid peroxidase antibodies, thyroglobulin antibodies, and thyroid-stimulating immunoglobulin were negative. Ultrasonography of the thyroid gland showed that it was slightly enlarged but diffusely hypoechoic, findings suggestive of autoimmune thyroid disease. There was no evidence of a marked increase in intraglandular vascular flow, which is characteristic of Graves’ disease. A 0.7-cm hypoechoic nodule without increased intranodular vascularity was seen in the left thyroid lobe. The iodine-123 uptake at 24 hours was 1.8% (normal range, 15 to 30); the images were of poor quality because of the low uptake.
This additional information narrows the differential diagnosis to causes of thyrotoxicosis associated with a low uptake of radioactive iodine. The patient was not taking any medications associated with low-uptake thyrotoxicosis, such as amiodarone, interferon alfa, or lithium. Although ultrasonography of the thyroid provided some additional information, this test is generally not necessary, in the absence of a palpable nodule, for the purpose of distinguishing among causes of thyrotoxicosis. The negative tests for thyroid peroxidase and thyroglobulin antibodies provide further evidence against silent lymphocytic thyroiditis, and the negative thyroid-stimulating immunoglobulin titer makes Graves’ disease less likely.
The patient should be carefully questioned to rule out exposures to excess iodine and exogenous thyroid hormone, both of which result in a low iodine-123 uptake. Serum thyroglobulin could be measured, because a low concentration in a patient with thyrotoxicosis is virtually diagnostic of thyrotoxicosis factitia in the absence of thyroglobulin antibodies.
The patient reported no ingestion of exogenous thyroid hormone, thyroid extract, or other dietary supplements that may have contained iodine. She had not undergone any recent radiographic studies that required the use of iodine-containing contrast agents. However, the diagnosis of iodine-induced hyperthyroidism was still considered highly likely. On detailed questioning regarding possible sources of excess iodine, the patient stated that she had been using povidone–iodine swabs for many years immediately before urinary self-catheterization four to five times daily. The serum thyroglobulin level was 69.9 ng per milliliter (reference range, 1 to 55).
The slightly elevated thyroglobulin level in the absence of thyroglobulin antibodies essentially rules out thyrotoxicosis factitia, and the patient’s long-standing use of povidone–iodine swabs was suspected as the source of excess iodine. Urinary iodine values would not be useful in this patient, because the specimen would be contaminated by the locally applied povidone–iodine swabs. However, serum iodine values would be extremely helpful for confirming the presence of excess iodine absorption.
Shortly after the patient used a povidone–iodine swab and performed self-catheterization, the serum inorganic iodide concentration, calculated as the difference between total and protein-bound (primarily T4) iodine concentrations, was measured in our laboratory. The value was markedly elevated, at 57 μg per liter (normal range, <2).
This test result confirms excess iodine exposure as the apparent cause of the patient’s thyrotoxicosis.
A beta-adrenergic blocker was prescribed to manage the patient’s tachycardia. She was asked to stop using povidone–iodine swabs for urinary self-catheterization. After she substituted chlorhexidine swabs, the serum inorganic iodide concentration reverted to the normal range, and a random urinary iodine concentration was not elevated. Eight weeks after discontinuation of the povidone–iodine swabs, the thyroid hormone level had normalized and the thyrotropin level became detectable (0.11 IU per milliliter; reference range, 0.35 to 5.50). Twenty-four-hour thyroid uptake of iodine-123 was evaluated again approximately 12 weeks after iodine exposure had been discontinued, and the result was normal, at 21.1%. On repeat testing, the thyrotropin concentration had returned to the normal range, and the serum T4 concentration, free T4 index, and total T3 concentration remained normal. All symptoms of thyrotoxicosis had abated.
COMMENTARY
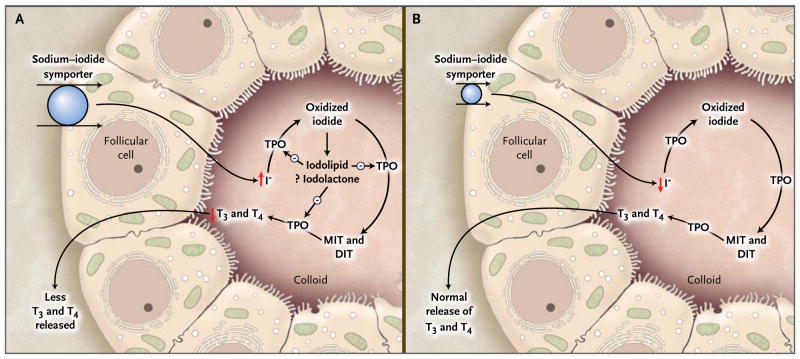
The thyroid gland has an intrinsic regulatory mechanism to maintain normal thyroid function in the presence of excess iodine, as described by Wolff and Chaikoff 1 (Fig. 1). Thyroid hormone synthesis decreases transiently over a period of 24 to 48 hours as a result of an increased concentration of intrathyroidal iodine, termed the acute Wolff–Chaikoff effect. Escape from the acute Wolff–Chaikoff effect occurs because of a decrease in the expression of the sodium–iodide symporter,2 leading to a decrease in the transport of iodide into the thyroid and resumption of normal thyroid function. Excess iodine results in hypothyroidism if the acute Wolff–Chaikoff effect persists (as it may in patients with Hashimoto’s thyroiditis or other thyroid disorders associated with subtle defects in hormone synthesis). Hyperthyroidism may develop in persons whose autoregulatory mechanism fails to prevent an iodine-induced increase in thyroid hormone synthesis (most often, euthyroid patients with a nodular goiter or an autonomous thyroid nodule). Studies suggest that excess iodine exposure in normal, euthyroid persons may also reduce thyroid hormone release, leading to small reductions in serum T4 and T3 levels and a compensatory rise in the thyrotropin level, but with all values still within reference ranges.3
Figure 1. The Wolff–Chaikoff Effect.
Panel A shows a proposed mechanism of the acute Wolff–Chaikoff effect. During the first day of iodine exposure, the sodium–iodide symporter transports the excess iodine into the thyroid, resulting in transient inhibition of thyroid peroxidase (TPO) and a decrease in thyroid hormone synthesis. Panel B shows the mechanism that turns off the acute Wolff–Chaikoff effect: a dramatic decrease in sodium–iodide symporter expression results in decreased iodine transport and the subsequent resumption of thyroid hormone synthesis. DIT denotes diiodotyrosine, I− iodide, MIT monoiodotyrosine, T3 triiodothyronine, and T4 thyroxine.
Findings typical of the various causes of thyrotoxicosis are presented in Table 1. The thyroid iodine-123 uptake is most useful in the differential diagnosis of thyrotoxicosis but should not be carried out during pregnancy. Iodine-induced hyperthyroidism, also known as the Jod–Basedow phenomenon, is much more common in areas where dietary iodine is insufficient than in areas where the diet contains sufficient iodine. The classic description of the disorder involved persons who resided in areas where dietary iodine was insufficient, including the midwestern United States in the 1920s, and who were receiving iodine supplementation for the treatment of goiter.4 However, cases of iodine-induced thyrotoxicosis have also been reported in areas where dietary iodine has been sufficient, as it has been in the United States over the past several decades.5 Whereas this condition is more common in patients with underlying thyroid disease (those with nontoxic nodular goiter, an autonomous nodule, or euthyroid Graves’ disease, especially after treatment with antithyroid drugs), it also occurs in patients with no recognized underlying thyroid disease, such as the patient described here.
Table 1.
Differential Diagnosis of Thyrotoxicosis and Tests That May Help Distinguish among the Various Causes.
| Cause | Thyroid Iodine-123 Uptake | Thyroid Peroxidase Antibodies | Thyroid-Stimulating Immunoglobulin | Thyroglobulin |
|---|---|---|---|---|
| Graves’ disease | Elevated | Usually positive | Usually positive | Increased |
| Toxic nodular goiter | Elevated | Negative | Negative | Increased |
| Thyrotropin-secreting pituitary adenoma | Elevated | Negative | Negative | Increased |
| Trophoblastic tumor | Elevated | Negative | Negative | Increased |
| Hyperemesis gravidarum | Elevated | Negative | Negative | Increased |
| Painful thyroiditis | Low | Negative | Negative | Increased |
| Silent lymphocytic thyroiditis | Low | Usually positive | Negative | Increased |
| Drugs (amiodarone, interferon alfa, and lithium) | Low | Usually negative | Negative | Increased |
| Iodine-induced hyperthyroidism | Low | Usually negative | Negative | Increased |
| Struma ovarii | Low | Negative | Negative | Increased |
| Thyrotoxicosis factitia | Low | Negative | Negative | Decreased |
Common sources of excess iodine include iodinated radiographic contrast agents and iodine-containing medications (e.g., amiodarone), topical antiseptics, and dietary supplements (e.g., kelp). Iodine-induced hyperthyroidism has developed in travelers and military personnel who drank water enriched with iodine for purification purposes.6 Hyperthyroidism can result from the extremely large iodine load in many of these sources, as compared with the recommended iodine intake for healthy, nonpregnant adults (150 μg per day). The estimated tolerable upper level of iodine intake in most persons is 1100 μg per day.7 A 200-mg tablet of amiodarone contains approximately 75 mg of iodine, and 1 ml of most intravenous radiocontrast mediums contains 320 to 370 mg of iodine. Because of its long half-life and high lipid solubility, amiodarone may cause iodine-induced thyrotoxicosis long after its discontinuation.8
Povidone–iodine in the form of a 10% topical solution (containing 10 mg of iodine per milliliter) is one of the most widely used antiseptics. Multiple studies have shown systemic absorption of iodine from povidone–iodine, both through mucosal surfaces, such as during vaginal douching9 or mouth rinsing,10 and at areas of skin breakage, such as surgical wounds11 or pressure ulcers.12 Iodine excess in our patient probably occurred as a result of systemic iodine absorption at the mucosal opening of her ileal pouch during urinary self-catheterization. In most patients, the diagnosis of iodine-induced thyrotoxicosis can be confirmed by an elevated iodine concentration in a random or 24-hour urine specimen. For this patient, however, urinary iodine measurement would not have been reliable because of iodine contamination from the povidone–iodine swabs used for urinary self-catheterization. To establish the diagnosis of iodine-induced thyrotoxicosis, we relied on the measurement of serum inorganic iodide13; however, this test is not readily available in clinical laboratories.
Therapy for iodine-induced hyperthyroidism includes discontinuation of the source of excess iodine and initiation of beta-adrenergic blockers to provide symptomatic relief. High doses of antithyroid drugs (preferably methimazole rather than propylthiouracil) may be required in patients with severe, persistent thyrotoxicosis. If treatment fails, oral sodium or potassium perchlorate, which inhibits the thyroid sodium–iodide symporter and thereby prevents the entry of iodide into the thyroid, may be used at doses of approximately 200 mg every 8 hours. Although perchlorate capsules are no longer manufactured in the United States, they can be produced by pharmacies with compounding machines. Iodine-induced thyrotoxicosis is self-limited and resolves spontaneously over a variable period, once the source of excess iodine is withdrawn, as in our case.
Susceptible patients, especially elderly patients with nodular goiters, should be counseled regarding the risk, albeit low (3%), of iodine-related thyroid dysfunction after radiologic studies using iodine-containing contrast agents.14 Prophylactic treatment with methimazole or perchlorate before exposure to contrast agents in patients with thyroid autonomy is possible15 but not routinely recommended because of the potential side effects of these drugs and the low risk of hyperthyroidism.
This case emphasizes the importance of carefully assessing the cause of thyrotoxicosis when the diagnosis is not evident. The low thyroid uptake of iodine-123 was an extremely useful finding, because there are only a few causes of low uptake. The negative test for thyroid antibodies and the absence of both a painful thyroid and a history of excess use of thyroid hormone or other relevant medications made autoimmune or drug-induced thyrotoxicosis unlikely and pointed toward a diagnosis of iodine-induced hyperthyroidism. In the absence of an obvious source of excess iodine, a careful history taking led to a “hidden solution” that explained this diagnostic dilemma and, ultimately, the patient’s thyrotoxicosis.
Acknowledgments
We are indebted to Dr. Susan Bergman, who referred this patient to us.
Footnotes
Dr. Malabanan reports receiving speaker’s bureau fees from Novartis and Procter & Gamble/Sanofi-Aventis. Dr. Pearce reports receiving compensation from DuPont for testimony for the defense regarding the development of hypothyroidism after exposure to radioactive iodine released by the Hanford Nuclear Reservation. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
In this Journal feature, information about a real patient is presented in stages (boldface type) to an expert clinician, who responds to the information, sharing his or her reasoning with the reader (regular type). The authors’ commentary follows.
References
- 1.Wolff J, Chaikoff IL. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem. 1948;174:555–64. [PubMed] [Google Scholar]
- 2.Eng PH, Cardona GR, Fang SL, et al. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology. 1999;140:3404–10. doi: 10.1210/endo.140.8.6893. [DOI] [PubMed] [Google Scholar]
- 3.Paul T, Meyers B, Witorsch RJ, et al. The effect of small increases in dietary iodine on thyroid function in euthyroid subjects. Metabolism. 1988;37:121–4. doi: 10.1016/s0026-0495(98)90004-x. [DOI] [PubMed] [Google Scholar]
- 4.Stanbury JB, Ermans AE, Bourdoux P, et al. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid. 1998;8:83–100. doi: 10.1089/thy.1998.8.83. [DOI] [PubMed] [Google Scholar]
- 5.Vagenakis AG, Wang CA, Burger A, Maloof F, Braverman LE, Ingbar SH. Iodide-induced thyrotoxicosis in Boston. N Engl J Med. 1972;287:523–7. doi: 10.1056/NEJM197209142871101. [DOI] [PubMed] [Google Scholar]
- 6.Liel Y, Alkan M. ‘Travelers’ thyrotoxicosis’: transitory thyrotoxicosis induced by iodinated preparations for water purification. Arch Intern Med. 1996;156:807–10. doi: 10.1001/archinte.156.7.807. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Dietary reference intakes. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 8.Cohen-Lehman J, Dahl P, Danzi S, Klein I. Effects of amiodarone therapy on thyroid function. Nat Rev Endocrinol. 2010;6:34–41. doi: 10.1038/nrendo.2009.225. [DOI] [PubMed] [Google Scholar]
- 9.Safran M, Braverman LE. Effect of chronic douching with polyvinylpyrrolidone-iodine on iodine absorption and thyroid function. Obstet Gynecol. 1982;60:35–40. [PubMed] [Google Scholar]
- 10.Ader AW, Paul TL, Reinhardt W, et al. Effect of mouth rinsing with two polyvinylpyrrolidone-iodine mixtures on iodine absorption and thyroid function. J Clin Endocrinol Metab. 1988;66:632–5. doi: 10.1210/jcem-66-3-632. [DOI] [PubMed] [Google Scholar]
- 11.Rajatanavin R, Safran M, Stoller WA, Mordes JP, Braverman LE. Five patients with iodine-induced hyperthyroidism. Am J Med. 1984;77:378–84. doi: 10.1016/0002-9343(84)90726-5. [DOI] [PubMed] [Google Scholar]
- 12.Shetty KR, Duthie EH., Jr Thyrotoxicosis induced by topical iodine application. Arch Intern Med. 1990;150:2400–1. [PubMed] [Google Scholar]
- 13.Benotti J, Benotti N, Pino S, Gardyna H. Determination of total iodine in urine, stool, diets, and tissue. Clin Chem. 1965;11:932–6. [PubMed] [Google Scholar]
- 14.Martin FI, Tress BW, Colman PG, Deam DR. Iodine-induced hyperthyroidism due to nonionic contrast radiography in the elderly. Am J Med. 1993;95:78–82. doi: 10.1016/0002-9343(93)90235-h. [DOI] [PubMed] [Google Scholar]
- 15.Nolte W, Müller R, Siggelkow H, Emrich D, Hüfner M. Prophylactic application of thyrostatic drugs during excessive iodine exposure in euthyroid patients with thyroid autonomy: a randomized study. Eur J Endocrinol. 1996;134:337–41. doi: 10.1530/eje.0.1340337. [DOI] [PubMed] [Google Scholar]