Background: Heme attachment to cytochrome c is a catalyzed post-translational modification.
Results: We identify a ternary complex of the cytochrome c biogenesis protein CcmE, heme, and a cytochrome, and demonstrate its functional significance.
Conclusion: The complex is a trapped catalytic intermediate at the point of heme transfer from the cytochrome biogenesis apparatus to the cytochrome.
Significance: An insight into biosynthesis of heme proteins.
Keywords: Cytochrome c, Cytochromes, Energy Metabolism, Enzymes, Heme, CcmE, Cytochrome c Biogenesis, Post-translational Modification
Abstract
c-Type cytochromes are widespread proteins, fundamental for respiration or photosynthesis in most cells. They contain heme covalently bound to protein in a highly conserved, highly stereospecific post-translational modification. In many bacteria, mitochondria, and archaea this heme attachment is catalyzed by the cytochrome c maturation (Ccm) proteins. Here we identify and characterize a covalent, ternary complex between the heme chaperone CcmE, heme, and cytochrome c. Formation of the complex from holo-CcmE occurs in vivo and in vitro and involves the specific heme-binding residues of both CcmE and apocytochrome c. The enhancement and attenuation of the amounts of this complex correlates completely with known consequences of mutations in genes for other Ccm proteins. We propose the complex is a trapped catalytic intermediate in the cytochrome c biogenesis process, at the point of heme transfer from CcmE to the cytochrome, the key step in the maturation pathway.
Introduction
c-Type cytochromes are widespread proteins, essential for respiration and/or photosynthesis in mitochondria, chloroplasts, most bacteria, and some archaea. They contain heme, covalently bound to the polypeptide chain. With very few exceptions, this attachment is between the vinyl groups of the heme and the cysteine thiols of a Cys-Xxx-Xxx-Cys-His heme-binding motif (Fig. 1); the histidine becomes a ligand to the heme iron atom. This heme attachment is a post-translational modification with strictly conserved stereospecificity (1). However, it is catalyzed by multiple, completely distinct, biogenesis proteins in different organisms and organelles (reviewed by Refs. 2–8). The cytochrome c maturation (Ccm)6 system is the biogenesis apparatus of many Gram-negative bacteria, archaea, and the mitochondria of land plants, red algae, and some protozoa. The paradigm Ccm system is from Escherichia coli, where it consists of eight essential core proteins, all membrane bound, and several accessory factors (9) (Fig. 1). The system functions in the periplasm where all bacterial c-type cytochromes are located.
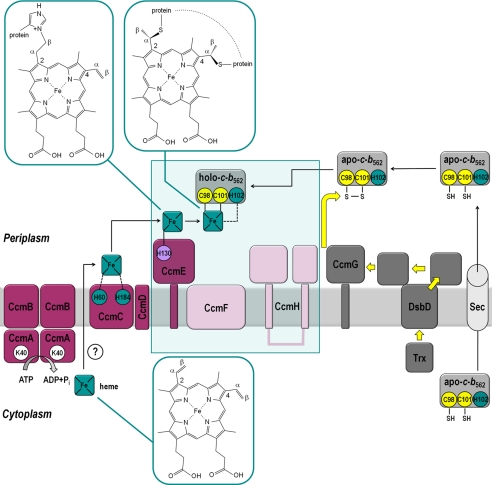
FIGURE 1.
Scheme illustrating the Ccm system in E. coli and the structures of heme attachment to c-type cytochromes and CcmE. All Ccm system components are membrane anchored or integral membrane proteins. Apocytochrome c (apo-c-b562) is synthesized in the cytoplasm and transported to the periplasm (where all bacterial c-type cytochromes are matured and located) by the Sec apparatus. The heme-binding cysteines (highlighted in yellow) are oxidized in the periplasm by the nonspecific oxidant DsbA to form an intramolecular disulfide bond. Heme is also synthesized in the cytoplasm and transported to the periplasm by an unknown route (indicated by a question mark). Heme becomes associated with CcmC (15, 16) and is then passed to CcmE, where it binds covalently to a histidine residue (H130, lilac) (10). The disulfide bond in the apocytochrome is reduced by electron transfer from CcmG (and/or CcmH). Electrons are first passed (yellow arrows) from the cytoplasmic thioredoxin (Trx) through the transmembrane protein DsbD to CcmG (proteins in this part of the pathway colored dark gray). Release of heme-bound CcmE from a tight complex with CcmC requires the ATPase activity of CcmAB (13, 14). CcmD is implicated in formation/stabilization of the CcmCE complex. Proteins involved in heme delivery and binding to holo-CcmE are colored dark pink. Subsequent holocytochrome (holo-c-b562) formation (boxed) involves heme transfer from CcmE to the reduced apocytochrome; this requires the hemoprotein CcmF and CcmH (light pink) (10, 17, 18), but the mechanism is not known. E. coli CcmH consists of two functionally distinct domains that are found as separate proteins (CcmH and CcmI) in most Ccm-containing bacteria (38). Key amino acids for experiments in this work are highlighted. His-130 of CcmE (lilac), and Cys-98 and Cys-101 of cytochrome c-b562 (yellow), covalently bind to heme. His-102 of cytochrome c-b562 is a ligand to the heme iron atom in the holocytochrome (iron ligation indicated by a dashed line and residues in green). His-60 and His-184 of CcmC have been implicated in heme provision to CcmE (15, 16). Lys-40 of CcmA (in white) is part of the Walker A motif of the protein and therefore crucial for the ATPase activity of CcmAB (13). Structures of free heme and heme as attached to proteins are shown enlarged. Free heme (Fe-protoporphyrin IX) is at the bottom of the figure. The vinyl substituents are in the 2- and 4-positions and the α- and β-carbon atoms of the vinyl groups are indicated. Heme as covalently attached to CcmE (11) is shown in the top left. It was not possible to determine whether the 2- or 4-vinyl group of heme formed the covalent bond to the Nδ1 of the histidine (His-130 in E. coli) (11); here, only attachment to the 2-vinyl group is shown. Heme as attached to the cysteine thiols of the CXXCH heme-binding motif of a typical c-type cytochrome is shown in the upper middle of the figure. The stereospecificity of this attachment is universally conserved. The histidine of the CXXCH heme-binding motif becomes a ligand to the iron atom.
CcmE is the pivotal protein in the Ccm system. It binds heme covalently, through a histidine residue, as an intermediate in the cytochrome c biogenesis pathway (Fig. 1) (10). This heme attachment is to the β-carbon of the vinyl group of heme, whereas the cysteine thiol of the c-type cytochrome attaches to the α-carbon (Fig. 1) (11). CcmA, -B, -C, and -D are all involved in forming holo (i.e. heme-bound) CcmE in a state that can transfer the heme to apocytochrome c (12–16); CcmF and -H are involved in heme transfer from CcmE to form the holocytochrome (Fig. 1) (10, 17, 18). However, the mechanisms of most of these steps remain unresolved. Interactions have been identified between apocytochrome c and each of CcmF, CcmG, and CcmH by yeast two-hybrid analysis or in vitro (17, 19, 20). However, no interaction has been observed between CcmE and the apocytochrome, even though current models of the Ccm pathway predict that such an interaction is crucial.
Cytochrome b562 is a soluble, ∼12-kDa periplasmic E. coli protein. A variant of this cytochrome containing a CXXCH c-type cytochrome heme-binding motif is matured efficiently and correctly by the Ccm apparatus (21, 22). Variants containing a single cysteine residue also covalently bind heme (22), but single cysteine c-type cytochromes are very poor substrates for the Ccm system (23, 24). Exceptionally, apocytochrome b562 is also stable (3, 22); normally apocytochromes c are subject to rapid degradation in vivo.
In this work, we describe experiments to identify novel interactions between components of the Ccm system and (apo)cytochrome c. We have used c-type cytochrome b562 variants to maximize the chances of isolating such interacting partners. We report a covalent, ternary complex between CcmE, heme, and cytochrome, and propose this represents a critical intermediate in the Ccm pathway, at the point of heme transfer from the biogenesis system to the product cytochrome.
EXPERIMENTAL PROCEDURES
Construction of Plasmids
Plasmids used in this study are listed in supplemental Table S3. Plasmid pb562R98CStrep was produced by site-directed mutagenesis (ExSite, Stratagene) using plasmid pb562R98C (22) as the template. The same method was used to insert a thrombin cleavage site, with additional Gly residues (GLVPRGSG) into pE221 (25). The plasmid produced, pE225, expresses the periplasmic region of CcmE (from Ser-32) with an N-terminal cleavable pelB signal sequence. Plasmids pb562R98CH102R and pFR015 were produced by site-directed mutagenesis (QuikChange, Stratagene) using plasmids pb562R98C (22) and pEC86 (9) as templates, respectively. DNA manipulations were conducted using standard methods. KOD Hot Start DNA polymerase (Novagen) was used for all PCRs and all constructs were sequenced before use.
Cell Growth and Fractionation
Bacterial strains used in this study are listed in supplemental Table S4. Routine cell growth was conducted in 100 ml of 2× TY medium (16 g liter−1 of peptone, 10 g liter−1 of yeast extract, 5 g liter−1 of NaCl) in 2.5-liter flasks. Cultures were inoculated from single colonies and incubated at 37 °C for 15–18 h with shaking at 200 rpm. Fully aerobic growth conditions prevented expression of the endogenous E. coli Ccm system. 1 mm Isopropyl 1-thio-β-d-galactopyranoside was added to the cultures from inoculation. 100 μg ml−1 of ampicillin and 34 μg ml−1 of chloramphenicol were used.
For the isolation of the crude membrane fraction a French press was used. Disruption of the cells was performed at 16,000 p.s.i. followed by centrifugation at 257,000 × g for 1 h at 4 °C. The membrane fraction was resuspended in ∼25 ml of 50 mm Tris-HCl, 150 mm NaCl (pH 7.5) and was re-centrifuged as above. The washed crude membrane fraction was resuspended in 1–2 ml of 50 mm Tris-HCl, 150 mm NaCl (pH 7.5).
SDS-PAGE Analysis
SDS-PAGE analysis was carried out on 10% BisTris NuPAGE gels (Invitrogen) with prestained molecular weight markers (SeeBlue Plus 2, Invitrogen, or ColorPlus Prestained Protein Marker, New England Biolabs). Samples containing membrane fractions were denatured by incubation at 42 °C for 5 min. All other samples were denatured at 100 °C for 2 min. 2-Mercaptoethanol and urea were added at final concentrations of 5% (v/v) and 8 m, respectively, where appropriate. Gel loadings were normalized according to total protein content, and determined using the Pierce BCA Reducing Agent Compatible Protein Assay Kit (ThermoScientific); 5–20 μg of protein were loaded per lane. Proteins with covalently bound heme were detected on gels using the method of Goodhew et al. (26) and quantification of heme-bound species was performed by densitometry using GeneSnap (SYNGENE).
Western blotting was carried out following SDS-PAGE by transferring onto nitrocellulose (Hybond C-Extra, Amersham Biosciences). Blocking was with 5% (w/v) skimmed milk powder in Tris-buffered saline (50 mm Tris-HCl, 120 mm NaCl, 0.1% (v/v) Tween 20 (pH 7.5)). The primary antibodies used (Covalab) were rabbit antiserum raised against E. coli cytochrome b562 (dilution 1:1000) and rabbit antiserum raised against E. coli CcmE (dilution 1:1000). In the in vitro studies antibody raised against a CcmE peptide (10) was used. Goat anti-rabbit alkaline phosphatase-conjugated antibody (Sigma) was used as secondary antibody (dilution 1:30000). Development was carried out using a SigmaFast BCIP/NBT tablet.
Sample Preparation for Proteomics Analysis
Proteomics analysis of the CcmE-heme-cytochrome c-b562 R98C complex was carried out on two different samples.
Co-immunoprecipitation
Crude membrane fractions from E. coli JCB387 cells transformed with plasmids pEC86 and pb562R98C were solubilized in 1% (w/v) n-dodecyl β-d-maltoside (DDM) (Melford) under gentle agitation at 4 °C for 1 h. Rabbit antiserum raised against E. coli cytochrome b562 was bound to beads from the Dynabeads-Protein G Immunoprecipitation Kit (Invitrogen). The pulled-down product was analyzed by SDS-PAGE. The gel was silver-stained using the SilverQuest Silver Staining Kit (Invitrogen) and the band corresponding to the CcmE-heme-cytochrome c-b562 R98C complex was excised and destained.
Purification of CcmE-Heme-Cytochrome c-b562 R98C Complex Using a Streptavidin II Tag
Crude membrane fraction from E. coli JCB387 cells, transformed with plasmids pEC86 and pb562R98CStrep, was solubilized in 20 mm Tris-HCl, 300 mm NaCl, 20% (v/v) glycerol, 1% (w/v) DDM (pH 7.5) under gentle agitation at 4 °C for 1 h at a protein concentration of 5 mg ml−1. Unsolubilized material was removed by centrifugation at 257,000 × g for 30 min at 4 °C. The supernatant was diluted 10-fold with 20 mm Tris-HCl, 150 mm NaCl (pH 7.5) and applied to 7 ml of Strep-Tactin Sepharose (IBA GmbH) pre-equilibrated with 20 mm Tris-HCl, 150 mm NaCl, 0.1% (w/v) DDM (pH 7.5). The column was washed with 20 mm Tris-HCl, 1 m NaCl, 0.1% (w/v) DDM (pH 7.5) and protein was eluted using 20 mm Tris-HCl, 150 mm NaCl, 0.1% (w/v) DDM, 2.5 mm desthiobiotin (IBA) (pH 7.5). The eluent was exchanged into 20 mm Tris-HCl, 50 mm NaCl, 0.03% (w/v) DDM (pH 7.5) and concentrated. The protein solution was analyzed by SDS-PAGE in the presence of dithiothreitol (DTT). The gel was stained using SimplyBlue Safestain (Invitrogen) and the band corresponding to the CcmE-heme-cytochrome c-b562 R98C complex was excised.
Proteomics Analysis
Identification of the CcmE-heme-cytochrome c-b562 R98C complex components was achieved by proteomics analysis. Blue-stained gel bands were excised, destained in 25 mm ammonium bicarbonate in 50% acetonitrile, and then reduced with DTT and alkylated with iodoacetamide before being digested with trypsin overnight. Tryptic peptides were extracted and then desalted on an in-house manufactured C18 tip. Samples were analyzed on a Thermo LTQ XL Orbitrap coupled to a Dionex U3000 nano-LC system run in direct injection configuration. Peptides were resolved on an in-house manufactured reverse-phase column made by packing a Picotip (New Objective) with C18 resin (Reprosil-Pur, C18-Aq, 3-μm beads). Samples were typically resolved on 40- or 120-min LC-MS/MS gradients depending on sample complexity. The Orbitrap was operated in a “Top 5” method in which 1+ ions were not selected for fragmentation and dynamic exclusion was applied. Precursor mass accuracy tolerance was set at ±20 ppm and the MS/MS fragment ion tolerance at ±0.5 Da. Data were searched using Mascot against data base NCBI nr/nr version 2010.03.21, specifying E. coli as the taxonomy. The fixed modification was defined as carbamidomethyl cysteine and variable modifications were oxidized Met, N-terminal acetylation, and deamidation on Asn and Glu.
For peptide mapping, stained gel bands containing CcmE-heme-cytochrome c-b562 R98C complex were subjected to in-gel digestion with trypsin as described elsewhere (27) or chymotrypsin (Roche Diagnostics) used under identical conditions. Digested samples were subjected to analysis by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) using an Ultraflex mass spectrometer (Bruker Daltonics) as described (28). The MS data (peptide mass fingerprinting) was analyzed using FlexAnalysis software version 2.4 (Bruker Daltonics) and searched against the SwissProt data base combined with the E. coli CcmE and cytochrome c-b562 R98C sequences using an in-house Mascot server (Matrixscience). In addition, the same sample was also analyzed by LC-MS/MS using a U3000 nano-LC (Dionex) coupled to a high capacity trap tandem mass spectrometer (Bruker Daltonics) as described previously (27). MS/MS data were also searched using Mascot as described above, allowing for carbamidomethylation on Cys, oxidation on Met, deamidation on Glu and Asn, and heme addition on Cys and His, as modifications.
In Vitro Formation and Characterization of CcmE-Heme-Cytochrome c Complex
Soluble holo-CcmE was produced by coexpressing plasmids pE225 and pEC86 in E. coli JM109(DE3) cells. Cultures were incubated at 37 °C with shaking at 200 rpm in LB medium to mid-exponential phase and induced with 1 mm isopropyl 1-thio-β-d-galactopyranoside overnight at 30 °C. Cells were harvested and resuspended in 50 mm Tris-HCl, 300 mm NaCl (pH 7.5). The periplasmic fraction containing holo-CcmE was isolated and the protein was purified as described previously (25). Thrombin cleavage of the polyhistidine tag was performed using the Sigma CleanCleave Kit. Hydrogenobacter thermophilus apocytochrome c552 and variants thereof were produced as described previously (29, 30). N-terminal sequencing was carried out from protein samples in SDS-PAGE gels that were electrophoretically transferred to a PVDF membrane and stained with Coomassie Brilliant Blue. The protein bands were excised and subjected to automated Edman sequencing using an Applied Biosystems 494A Procise protein sequencer. A PerkinElmer Lambda 2 spectrophotometer was used to collect absorption spectra. Pyridine hemochrome spectra were obtained according to the method of Bartsch (31) using 5 μm protein in 19% (v/v) pyridine and 0.15 m NaOH.
H. thermophilus apocytochrome c552, C11A, C14A, and C11A/C14A apocytochrome variants (15–25 μm) were incubated with E. coli holo-CcmE* (5–10 μm) in 50 mm potassium phosphate buffer (pH 7.0) at 25 °C for up to 20 h. Samples were reduced by the addition of disodium dithionite. Solutions contained 10 mm Tris(2-carboxyethyl)phosphine and were thoroughly sparged with humidified argon. Reactions were carried out in the dark.
Inhibition of the Ccm System
E. coli JCB387 cells were transformed with the following combinations of plasmids (supplemental Table S3): pEG278 + pb562EV; pEG278 + pb562R98C; pRZ001 + pb562EV; and pRZ001 + pb562R98C. Cell growth, fractionation, and analysis of the periplasmic fractions was performed as described in Ref. 32. Autoinduction was used for expression of cytochrome c-b562 R98C and cytochrome cd1. The production of both the endogenous cytochrome NrfA and the exogenous cytochrome cd1 was quantified (33).
RESULTS AND DISCUSSION
A Complex between CcmE, Heme, and Cytochrome
The Ccm system must interact with apocytochrome c to catalyze heme attachment and hence holocytochrome formation; we set out to identify such interactions using variants of cytochrome b562 as the test cytochromes. E. coli strain JCB387 cells were co-transformed with a plasmid from which the Ccm proteins are constitutively expressed (pEC86), and plasmids encoding variants of cytochrome b562 with CXXCH (R98C/Y101C), CXXXH (R98C), and XXXCH (Y101C) heme-binding motifs. Similar results to those in this article were also obtained using E. coli strains MC1000 and MC1061 (supplemental Table S4). In this work, we refer to the c-type cytochrome variants of cytochrome b562 as cytochrome c-b562. Cells were fractionated into soluble and membrane extracts. These fractions were analyzed by Western blotting with a cytochrome b562 antibody. Of particular note was a band in the blot of the membrane fraction, observed at ∼32 kDa (Fig. 2A, lanes 3–5). This band was not observed in the soluble fraction (not shown). The cytochrome c-b562 variants themselves ran at ∼12 kDa and a dimer was also observed at ∼30 kDa (Fig. 2A); these bands arise from cytochrome b562 that remains bound to the membranes even after extensive washing.
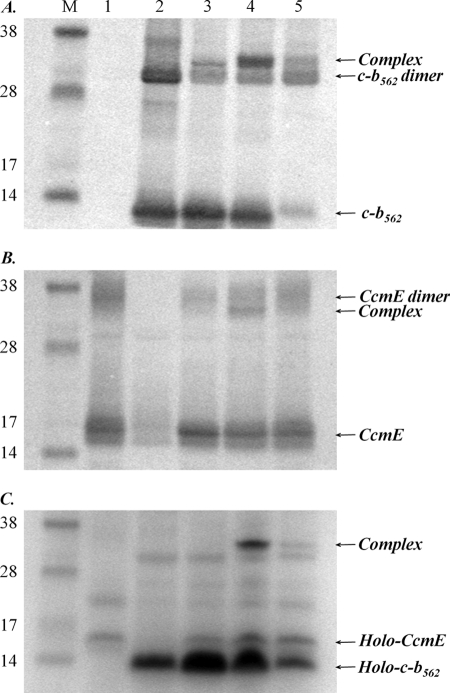
FIGURE 2.
Detection of a complex between CcmE, heme, and cytochrome c-b562. A, Western blot of cell membranes using a cytochrome b562 antibody. B, Western blot of cell membranes using a CcmE antibody. C, SDS-PAGE of cell membranes stained for proteins containing covalently bound heme. For each panel, the lane order is: M, molecular mass markers (as indicated, in kDa); lanes 1, cells expressing the Ccm system from pEC86; 2, cells expressing cytochrome c-b562 R98C/Y101C; 3, cells expressing the Ccm system from pEC86 and cytochrome c-b562 R98C/Y101C; 4, cells expressing the Ccm system from pEC86 and cytochrome c-b562 R98C; 5, cells expressing the Ccm system from pEC86 and cytochrome c-b562 Y101C. The position of the CcmE-heme-cytochrome c-b562 complex, which runs just above a cytochrome c-b562 dimer and just below the CcmE dimer, is indicated. An equal amount of total protein was loaded in each lane.
From the molecular masses of the Ccm proteins, we reasoned that the 32-kDa band might be a complex between (apo)cytochrome c-b562 (molecular mass = 12 kDa) and CcmE (18 kDa), which is a membrane-anchored protein. The band was only observed when both the Ccm system and a cytochrome c-b562 variant were co-expressed (Fig. 2, compare lanes 3–5 with lanes 1 and 2). We therefore probed the E. coli membrane fraction using a CcmE antibody. The 32-kDa band was again observed (Fig. 2B, lanes 3–5), running just below a band corresponding to a CcmE dimer. The CcmE monomer was observed at ∼16 kDa. To establish whether heme was involved in the complex with CcmE and cytochrome c-b562, we ran the cell membrane fractions on denaturing SDS-PAGE, which was stained for proteins containing covalently bound heme (Fig. 2C). Once again, the 32-kDa band was apparent. Densitometry measurements of the heme-stained gel (Fig. 2C), which was normalized for total protein loading, indicate the largest amount of the complex formed with the R98C (CXXXH) variant cytochrome (lane 4). The relative yield was ∼70% lower with the Y101C (XXXCH) protein (lane 5). With the CXXCH variant, no band was visible on the heme-stained gel (Fig. 2C, lane 3) (sensitivity ≥ 1 pmol of holocytochrome (33)); it was barely detectable in the Western blots (Fig. 2, A and B, lanes 3). Membranes from cells expressing the R98C variant cytochrome c-b562 were therefore selected for further analysis.
The 32-kDa band remained even after treatment of the solubilized membrane proteins in harshly denaturing conditions such as boiling (supplemental Fig. S1, lanes 2 and 4) or 8 m urea (data not shown), indicating that the CcmE-heme-cytochrome c-b562 R98C complex is very stable and that the components are covalently linked. The complex was insensitive to treatment with 2-mercaptoethanol, which would reduce any disulfide bonds present (supplemental Fig. S1, lanes 3 and 4). Following treatment with 2-mercaptoethanol, the intensity of the heme-staining bands arising from each of the complex, holo-CcmE and holocytochrome c-b562 R98C, decreased (supplemental Fig. S1C, compare lane 1 with 3). Such reducing agents cause a portion of the iron to dissociate from heme, which reduces the intrinsic peroxidase activity of the heme that gives rise to the heme stain. However, it is clear from the Western blots using both cytochrome b562 and CcmE antibodies (supplemental Fig. S1, A and B, compare lanes 3 and 4 with lane 1) that treatment with 2-mercaptoethanol did not decrease the total amount of complex.
To isolate the complex, solubilized membranes were immunoprecipitated using the cytochrome b562 antibody. The 32-kDa species was visible on a silver-stained SDS-PAGE of the immunoprecipitate. The relevant band was excised from the gel, trypsin digested, and assessed by high-resolution mass spectrometry (LC-MS/MS). Peptides from both cytochrome c-b562 and CcmE were detected in this analysis, including two from cytochrome c-b562 and one from CcmE, each of ≥12 amino acids, and that could be assigned with high confidence (supplemental Table S1A). To verify this analysis, we co-expressed a C-terminal streptavidin II-tagged form of cytochrome c-b562 R98C with the Ccm proteins. The tag facilitated small scale purification of the putative complex, which could be seen on protein-stained SDS-PAGE. Again, bands were excised, trypsin digested and analyzed by LC-MS/MS. Multiple peptides were identified from both cytochrome c-b562 R98C and CcmE, each with >95% confidence (but often much higher); sequence coverage in identified peptides (supplemental Table S1A) was 64% for cytochrome b562 and 44% for CcmE.
We conclude that we have resolved a stable complex containing cytochrome c-b562, CcmE, and heme, all linked covalently. This is the first complex to be identified between CcmE and a cytochrome. Such a complex is a critical intermediate in current models of the Ccm system-mediated pathway of cytochrome c biogenesis (10). The small amount of complex formed from CXXCH containing cytochrome c (Fig. 2, A and B, lane 3) presumably represents the steady-state level of the complex as a reaction intermediate in the normally functioning Ccm pathway. In contrast, with single cysteine (XXXCH or CXXXH) cytochromes, the complex accumulated as a trapped intermediate.
Organization of the Complex
What is the structural arrangement of this ternary complex between cytochrome c-b562, CcmE, and heme? We investigated its formation using site-directed variants of CcmE and cytochrome c-b562 R98C. No complex was observed when wild-type cytochrome b562 (which contains no cysteine residues) was co-expressed with the Ccm system (Fig. 3, A and B, compare lane 3 with lane 4). Similarly, no complex was observed when His-102, the proximal heme iron ligand in cytochrome c-b562 R98C (i.e. the histidine residue of the heme-binding motif, which binds noncovalently and axially to the heme iron atom), was mutagenized to produce cytochrome c-b562 R98C/H102R (Fig. 3C, lane 2). The histidine of the CXXCH motif has previously been shown to be essential for c-type cytochrome maturation by the Ccm system (34, 35). E. coli CcmE binds heme covalently through residue His-130 as an intermediate in Ccm system-dependent cytochrome c biogenesis (Fig. 1) (10). No complex between CcmE, heme, and cytochrome c-b562 R98C was observed for the H130A variant of CcmE (Fig. 3C, lane 3). As anticipated, this mutation also prevented accumulation of holo-CcmE. Apo-CcmE was clearly abundant on Western blots (not shown).
FIGURE 3.
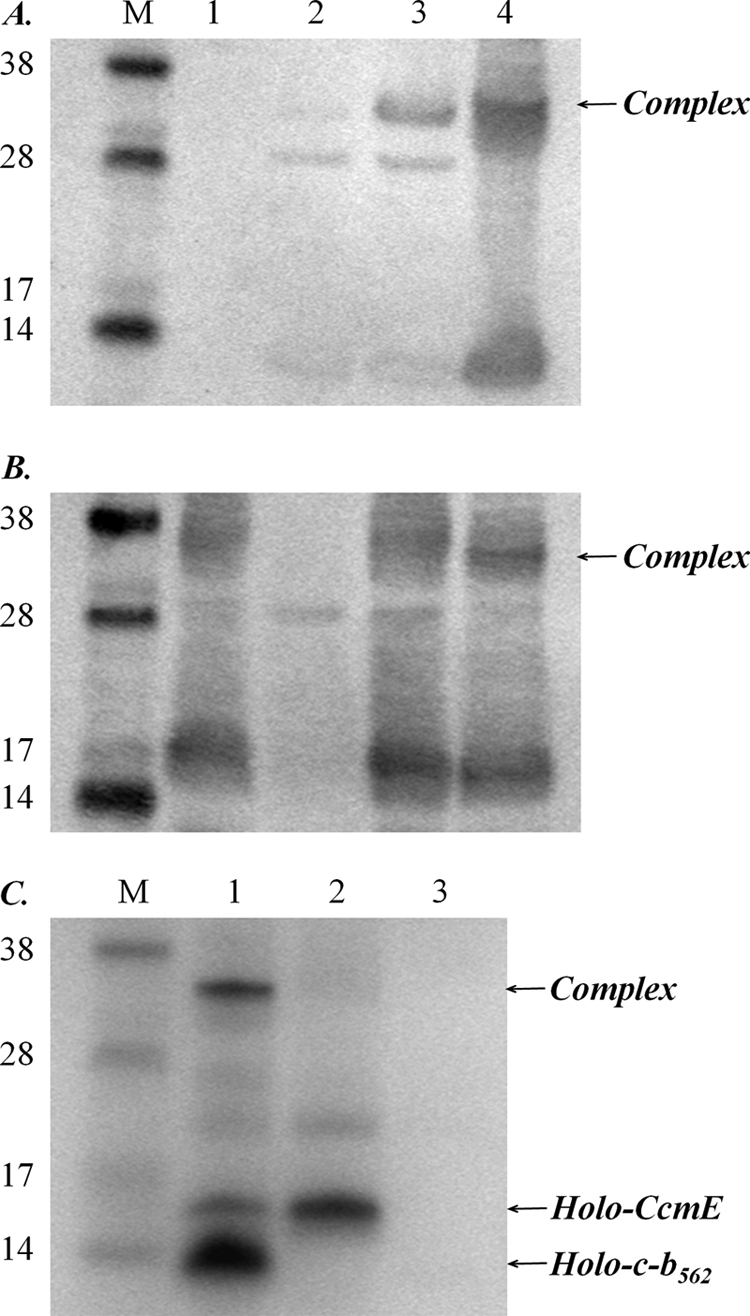
Formation of the complex between CcmE, heme, and cytochrome c-b562 requires the heme-binding histidine residue of CcmE, a heme-binding cysteine residue from cytochrome c-b562, and the heme-iron-ligating histidine of cytochrome c-b562. A, Western blot of cell membranes using a cytochrome b562 antibody. B, Western blot of cell membranes using a CcmE antibody. For panels A and B the lane order is: M, molecular mass markers (as indicated, in kDa); lanes 1, cells expressing the Ccm system from pEC86; 2, cells expressing wild-type cytochrome b562; 3, cells expressing the Ccm system from pEC86 and wild-type cytochrome b562; 4, cells expressing the Ccm system from pEC86 and cytochrome c-b562 R98C. The position of the CcmE-heme-cytochrome c-b562 complex, which runs just above a cytochrome b562 dimer (visible in lanes 2 and 3 of panel A) and just below the CcmE dimer (visible in lane 3 of panel B), is indicated by an arrow; it is clearest when panels A and B are viewed together. An equal amount of total protein was loaded in each lane. C, SDS-PAGE of cell membranes stained for proteins containing covalently bound heme. For panel C the lane order is: M, molecular mass markers; lanes 1, cells expressing the wild-type Ccm system and cytochrome c-b562 R98C; 2, cells expressing the wild-type Ccm system and cytochrome c-b562 R98C/H102R; 3, cells expressing the Ccm system with H130A variant CcmE and cytochrome c-b562 R98C. An equal amount of total protein was loaded in each lane.
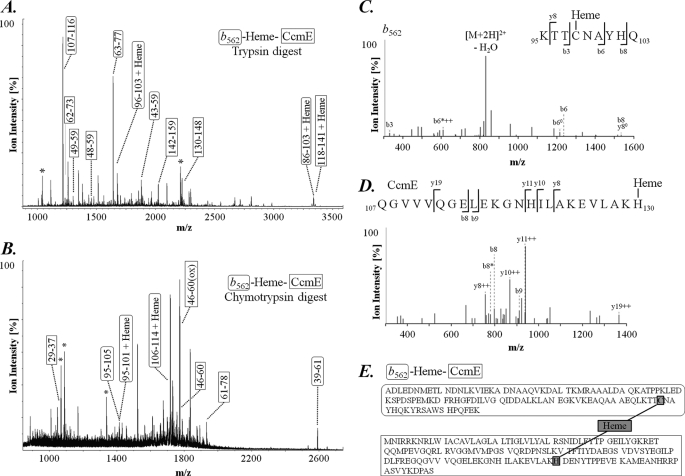
To investigate the heme-binding sites in greater detail, we used a peptide mapping approach. CcmE-heme-cytochrome c-b562 R98C complex was purified using the streptavidin tag, separated by SDS-PAGE, and excised gel slices were digested using trypsin or chymotrypsin. Peptide mass fingerprinting by mass spectrometry identified a heme-binding peptide fragment of CcmE (residues 114–141), which included His-130 (supplemental Table S1B and Fig. 4A). In addition, we detected four heme-binding peptide fragments from cytochrome c-b562, comprising residues 96–103, 86–103, 95–101, and 106–114, three of which included Cys-98 (supplemental Table S1B and Fig. 4, A and B). Heme binding to the latter peptide (cytochrome c-b562 R98C residues 106–114) is very likely to be an artifact because residues 107–114 are part of the streptavidin II tag we added to the C terminus of cytochrome c-b562 R98C to facilitate purification of the complex. We have only used streptavidin-tagged protein to isolate CcmE-heme-cytochrome c-b562 for mass spectrometry analysis; all other experiments in this article used untagged protein. Thus formation of the CcmE-heme-cytochrome c-b562 complex is independent of the streptavidin tag (e.g. Figs. 2, 3, and 5). The trypsin and chymotrypsin-based peptide mapping did not definitively determine which residues in the various fragments had heme bound. Therefore, we further analyzed the peptides obtained from the digests by LC-MS/MS. Despite the presence of the heme group reducing ionization and fragmentation efficiency, MS/MS analysis showed a peptide fragment consisting of residues 95–103 from cytochrome c-b562 R98C had heme bound to Cys-98, and a fragment consisting of residues 107–130 from CcmE had heme attached to His-130 (Fig. 4, C and D). We were unable to identify a single cross-linked fragment containing heme and a peptide from both CcmE and cytochrome c-b562, possibly because the ion intensity of such a species would be impaired by the presence of the heme group as well as its expected size (>4000 Da), where the sensitivity of mass spectrometry-based detection is suboptimal.
FIGURE 4.
Mapping the heme binding sites in the CcmE-heme-cytochrome c-b562 R98C complex. CcmE-heme-cytochrome c-b562 complex was separated by SDS-PAGE, excised, subjected to digestion with trypsin or chymotrypsin, and analyzed by mass spectrometry. A and B, MALDI-TOF spectra of the CcmE-heme-cytochrome c-b562 R98C complex digested with trypsin (A) or chymotrypsin (B). CcmE-derived peptides are indicated in square boxes, cytochrome c-b562 R98C-derived peptides in rounded boxes. Peptides modified with heme are indicated and trypsin/chymotrypsin-derived peptides are marked (*). The peptide sequences are listed in supplemental Table S1B. C, MS/MS spectrum of the cytochrome c-b562 R98C-derived peptide 95–103 KTTC(heme)NAYHQ with a precursor ion mass of m/z 840.9 [M + 2H]2+ and a calculated molecular mass of 1680.6 Da (δ mass = 0.8 Da). The identified b and y fragment ions are indicated, where o represents a loss of H2O, ++ indicates a doubly charged ion and * represents loss of NH2. D, MS/MS spectrum of the CcmE-derived peptide 107–130, QGVVVQGELEKGNHILAKEVLAKH(heme), with a precursor ion mass of m/z 1071.3 [M + 3H]3+ and a calculated of molecular mass 3211.6 Da (δ mass = 0.7 Da). The identified b and y fragment ions are indicated, where ++ indicates a doubly charged ion and * represents loss of NH2. E, the complete amino sequences of cytochrome c-b562 R98C (including the C-terminal streptavidin tag) and CcmE, with the heme-binding sites highlighted.
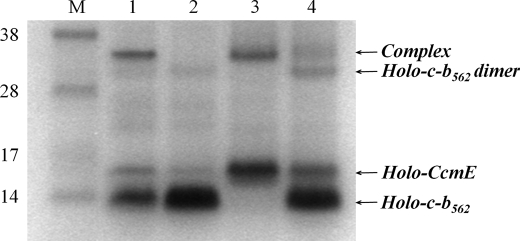
FIGURE 5.
More CcmE-heme-cytochrome c-b562 complex accumulates in cells where ccmFGH are not expressed. SDS-PAGE of cell membranes stained for proteins containing covalently bound heme. The lane order is: M, molecular mass markers (as indicated, in kDa); lanes 1, cells expressing the whole Ccm system (from pEC86) and cytochrome c-b562 R98C; 2, cells expressing the whole Ccm system and cytochrome c-b562 R98C/Y101C; 3, cells expressing ccmABCDE and cytochrome c-b562 R98C; 4, cells expressing ccmABCDE and cytochrome c-b562 R98C/Y101C. An equal amount of total protein was loaded in each lane.
The simplest interpretation of our mutagenesis and mass spectrometry data is that the CcmE-heme-cytochrome c-b562 R98C complex consists of two proteins cross-linked by heme covalently bound to both His-130 of CcmE and Cys-98 of cytochrome c-b562 (Fig. 4E). It has recently been suggested (6) that heme might be released from holo-CcmE by a reverse Michael reaction before becoming attached to apocytochrome c. This would restore free heme, with the vinyl group formerly bound to CcmE once again unsaturated. Our data, showing a heme-transfer intermediate with heme bound simultaneously to both CcmE and the cytochrome casts doubt on this proposal, although a reverse Michael reaction could release the heme vinyl group from CcmE once the other vinyl group has reacted with the apocytochrome.
Accumulation of the CcmE-Heme-Cytochrome c-b562 Complex Reflects Phenotypes of Mutants of the Ccm System
If the complex described above is an intermediate in the Ccm pathway, one would expect its abundance to change predictably in characterized variants of the Ccm system. CcmF and -H function late in the pathway after heme becomes attached covalently to CcmE and are involved in transferring heme from holo-CcmE to apocytochrome c to form the holocytochrome (Fig. 1) (10, 17, 18). When cytochrome c-b562 R98C was co-expressed in E. coli with a plasmid encoding a partial Ccm system with ccmFGH deleted (i.e. expressing ccmABCDE), more CcmE-heme-cytochrome c-b562 R98C complex was observed than when CcmFGH were present (Fig. 5, compare lane 1 with lane 3). When cytochrome c-b562 R98C/Y101C (i.e. with the CXXCH heme-binding motif) was co-expressed with the Ccm system lacking ccmFGH, the complex also accumulated and became visible on a heme-stained gel (Fig. 5, lane 4). In contrast, it was undetectable by heme stain when cytochrome c-b562 R98C/Y101C was co-expressed with the entire Ccm system (Figs. 2C, lane 3, and 5, lane 2), although low levels were observed on Western blots (Fig. 2, A and B, lane 3). Western blots of membranes from cells expressing the ΔccmFGH Ccm system (not shown) confirmed the results from the heme-stained gel. Note that, as expected (10), holo-CcmE accumulates in the ΔccmFGH cells (Fig. 5, compare lanes 3 and 4 with lanes 1 and 2). Our results indicate that (at least some of) CcmFGH resolve (i.e. breakdown) the ternary CcmE-heme-cytochrome c-b562 complex, consistent with the role of CcmF and -H in heme transfer from CcmE to apocytochrome c (which would therefore occur after formation of the ternary intermediate between them). Moreover, our data show that the CcmE-heme-cytochrome adduct forms in the absence of CcmF, -G, and -H.
Heme attachment to CcmE requires heme transfer from CcmC. Histidine residues of CcmC (His-60, His-184) have been implicated in heme provision to CcmE (15, 16) (Fig. 1). When membranes from cells expressing the Ccm system with a CcmC H60A/H184A variant were analyzed, very little holo-CcmE was seen (Fig. 6A, compare lane 1 with lane 2), although apo-CcmE was observed to be abundant on Western blots (Fig. 6B, lane 2). When cytochrome c-b562 R98C was co-expressed with the CcmC H60A/H184A variant Ccm system, no CcmE-heme-cytochrome c-b562 complex was observed (Fig. 6, A and B, lane 4). This result is, again, entirely consistent with what is known about the Ccm system: the two CcmC histidines are required for heme provision to CcmE (15); in their absence, CcmE does not become significantly charged with heme. In the absence of holo-CcmE, the complex between CcmE, heme, and cytochrome c-b562 does not form. Importantly, this result indicates that the CcmE-heme-cytochrome c-b562 R98C complex forms after, and from, holo-CcmE, rather than by reaction between holocytochrome c-b562 and apo-CcmE.
FIGURE 6.
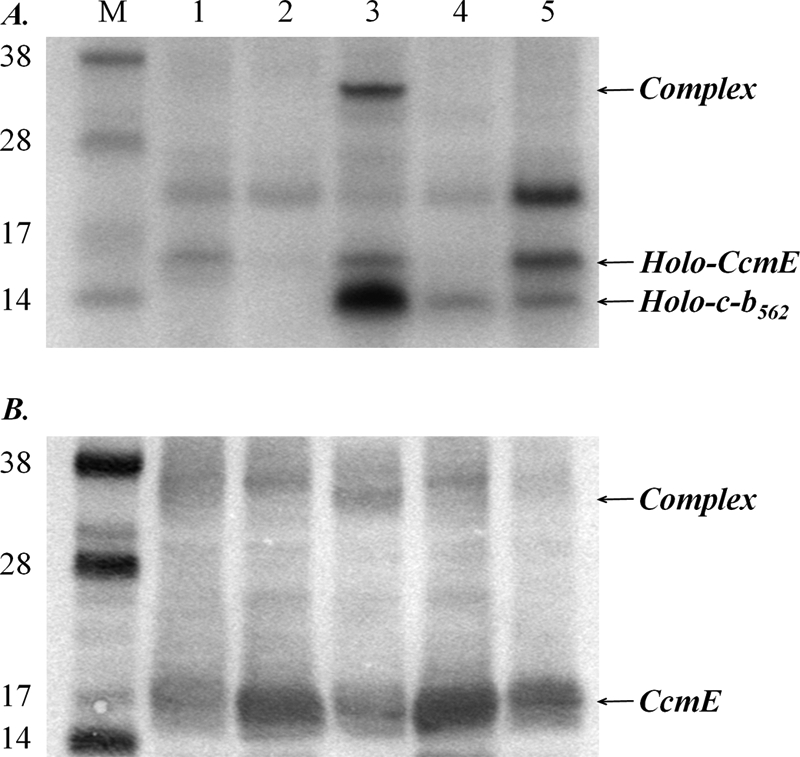
The CcmE-heme-cytochrome c-b562 complex does not form in cells carrying mutations in CcmA and CcmC that disrupt formation of holo-CcmE. A, SDS-PAGE of cell membranes stained for proteins containing covalently bound heme. B, Western blot of cell membranes using a CcmE antibody. The lane order is: M, molecular mass markers (as indicated, in kDa); lanes 1, cells expressing the wild-type Ccm system from pEC86; 2, cells expressing the Ccm system with H60A/H184A variant CcmC; 3, cells expressing the wild-type Ccm system and cytochrome c-b562 R98C; 4, cells expressing the Ccm system with H60A/H184A variant CcmC and cytochrome c-b562 R98C; 5, cells expressing the Ccm system with K40D variant CcmA and cytochrome c-b562 R98C. An equal amount of total protein was loaded in each lane.
CcmA is a component of an ATPase (13); a K40D mutation in the Walker A motif of E. coli CcmA causes loss of ATPase activity and loss of cytochrome c biogenesis activity by the Ccm system (13, 14) (Fig. 1). In this variant of the system, heme becomes covalently attached to CcmE but is not transferred to apocytochrome c. Feissner et al. (14) proposed that holo-CcmE remained tightly bound to CcmC unless released by the ATPase activity of CcmA. We have investigated formation of the CcmE-heme-cytochrome c-b562 complex when the cytochrome was coexpressed with the Ccm system including CcmA K40D. In these conditions, no detectable complex formation was observed (Fig. 6, lane 5). This is consistent with the ATPase activity of CcmA being involved in releasing holo-CcmE from CcmC; presumably this step must occur before the ternary complex can form, and again suggests that the complex forms from, and after, “free” holo-CcmE. In combination, our experiments with various ccm mutants demonstrate that the chronology of formation of the complex is correct for an on-pathway intermediate; it forms from (i.e. after) holo-CcmE and is broken down by CcmFGH.
A further prediction is that if the CcmE-heme-cytochrome c-b562 complex is an intermediate in cytochrome c maturation by the Ccm system, the system overall will be inhibited if the complex accumulates (because at least some CcmE will be blocked and unavailable for cytochrome c maturation). To test this, we co-expressed cytochrome c-b562 R98C (to accumulate the complex) and another c-type cytochrome, Paracoccus pantotrophus cytochrome cd1. Control experiments were also performed using appropriate combinations of plasmids, including otherwise identical expression vectors lacking the cytochrome genes. The cells were grown anaerobically on a nitrate-rich medium to induce expression of the endogenous E. coli ccm genes (pEC86 was not used in this experiment). The endogenous E. coli c-type cytochrome NrfA (a nitrite reductase) is also induced in these conditions. We assessed the yields of cytochrome cd1 and NrfA for each combination of plasmids using densitometry of heme-stained SDS-PAGE (e.g. supplemental Fig. S2). The relative holocytochrome yields are given in Table 1. The yields of both cytochrome cd1 and NrfA were significantly reduced when co-expressed with cytochrome c-b562 R98C (supplemental Fig. S2), but not when the equivalent expression vector lacking the cytochrome c-b562 gene was used instead. Thus, consistent with the prediction, accumulation of the CcmE-heme-cytochrome c-b562 complex does inhibit overall flux through the Ccm system.
TABLE 1.
Inhibition of the Ccm system by expression of cytochrome c-b562 R98C, which forms a cytochrome c-b562-heme-CcmE complex
Various combinations of cytochromes were expressed as shown. E. coli NrfA is endogenous and was expressed in all cases. Where a cytochrome was not expressed, an equivalent expression vector lacking the cytochrome gene was expressed instead, so all cells were grown in identical conditions under the same antibiotic selection. Relative holocytochrome yields were assessed by densitometry of heme-stained SDS-PAGE (e.g. supplemental Fig. S2). Values are the mean of at least 6 biological replicates. Yields of cytochrome cd1 and NrfA are given relative to that particular cytochrome and cannot be compared with each other.
| Combination of cytochromes expressed | Relative yield of NrfA | Relative yield of cytochrome cd1 |
|---|---|---|
| NrfA only (dummy cytochrome c-b562 and cytochrome cd1 expression vectors) | 100% | NAa |
| NrfA and cytochrome cd1 (dummy cytochrome c-b562 expression vector) | 88% | 100% |
| NrfA and cytochrome c-b562 R98C (dummy cytochrome cd1 expression vector) | 56% | NA |
| NrfA, cytochrome cd1 and cytochrome c-b562 R98C | 40% | 18% |
a NA = not applicable.
In Vitro Formation of a CcmE-Heme-Cytochrome c Complex
Previous work has investigated cytochrome c biogenesis by exploring the reactions of apocytochromes c, CcmE, and heme in vitro, using purified proteins and in the absence of other Ccm system components (25, 29, 30, 36, 37). In the light of the results above, we have investigated in vitro formation of a complex involving apocytochrome c, CcmE, and heme. We were not able to form a complex in vitro using cytochrome c-b562 or variants. We believe this is because the apocytochrome is fully folded in vitro (as judged by NMR).7 We were, however, able to investigate in vitro formation of the complex using H. thermophilus apocytochrome c552, which forms a molten globule.8 This apocytochrome has been used extensively in previous in vitro studies of cytochrome c biogenesis (30, 36, 37).
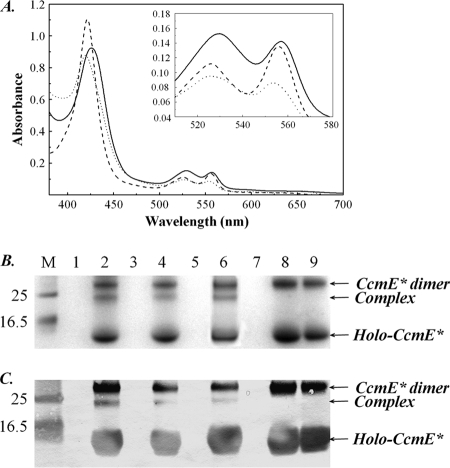
Holo-CcmE lacking its membrane anchor and with its C-terminal polyhistidine purification tag cleaved (denoted CcmE* in this work) was mixed with H. thermophilus apocytochrome c552, which has a typical CXXCH heme-binding motif, in reducing conditions. Over several hours, a change in the visible absorption spectrum of the mixture was observed, indicating that a reaction was taking place (Fig. 7A and supplemental Table S2). The pyridine hemochrome and absorption maxima blue-shifted (supplemental Table S2), as would be expected for saturation of the free vinyl group of the heme bound to CcmE (Fig. 1) if it were forming an adduct with cytochrome c552.
FIGURE 7.
The in vitro reaction between E. coli holo-CcmE* and H. thermophilus apocytochrome c552. A, visible absorption spectra of reduced holo-CcmE* with and without addition of apocytochrome c552. Dashed line, reduced holo-CcmE*. Solid line, the spectrum of the holo-CcmE*-cytochrome c mixture obtained after a 10-min incubation in reducing conditions. Dotted line, the reaction product from holo-CcmE* and apocytochrome after incubation for 10 h. All samples were in 50 mm potassium phosphate buffer (pH 7.0) containing 10 mm Tris(2-carboxyethyl)phosphine and disodium dithionite. B, SDS-PAGE stained for proteins containing covalently bound heme, and C, Western blot using a CcmE antibody showing various forms of H. thermophilus cytochrome c with and without incubation with E. coli holo-CcmE*. The lane order for both panels is: M, molecular mass markers (as indicated, in kDa); lanes 1, wild-type H. thermophilus apocytochrome c552; 2, the reaction mixture of wild-type apocytochrome c552 with holo-CcmE*; 3, C11A variant apocytochrome c552; 4, the reaction mixture of C11A variant apocytochrome c552 with holo-CcmE*; 5, C14A variant apocytochrome c552; 6, the reaction mixture of C14A variant apocytochrome c552 with holo-CcmE*; 7, C11A/C14A variant apocytochrome c552; 8, the reaction mixture of C11A/C14A variant apocytochrome c552 with holo-CcmE*; 9, holo-CcmE* incubated by itself under otherwise identical reaction conditions. All reactions were carried out in 50 mm potassium phosphate buffer (pH 7.0) containing 10 mm Tris(2-carboxyethyl)phosphine and disodium dithionite. The incubation time was 20 h.
Supporting this interpretation, when the mixture was analyzed by denaturing SDS-PAGE stained for proteins with covalently bound heme, an additional band appeared compared with that from holo-CcmE* alone, its mass (∼26 kDa) corresponding to that of holo-CcmE* (16 kDa) plus apocytochrome c (8 kDa) (Fig. 7B, lane 2). The additional band (from an equivalent protein-stained gel) was subjected to N-terminal sequencing; the band contained the sequences of both H. thermophilus cytochrome and E. coli CcmE* in a 1:1 ratio (Table 2). The presence of CcmE* in this complex was further confirmed by Western blotting (Fig. 7C).
TABLE 2.
Comparison of the N-terminal amino acid sequences of E. coli CcmE and H. thermophilus cytochrome c552 with the sequencing data (in bold) from the protein complex obtained after incubation of Holo-CcmE* with H. thermophilus apocytochrome c552in vitro for 20 h
Yields are given in picomoles.
| Protein | Amino acid sequence at the N terminus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CcmE* | M | D | S | N | I | D | L | F | Y | T | |
| Cytochrome c552 | M | N | E | Q | L | A | K | Q | K | G | |
| Protein complex | M | D | S | N | I | D | L | F | Y | T | |
| Yield | 3.2a | 1.8 | 1.7 | 1.8 | 1.7 | 1.5 | |||||
| M | N | E | Q | L | A | K | Q | K | G | ||
| Yield | 1.8 | 1.8 | 2.0 | 1.6 | 1.5 | ||||||
a Indicates the first cycle of sequencing and represents the combined yield of both proteins since Met is the first residue in each.
The results from the optical spectroscopy, SDS-PAGE, Western blotting, and Edman sequencing therefore all indicate formation in vitro of a covalent, ternary complex between CcmE, heme, and cytochrome. Surprisingly, in these experiments little heme transfer to the apocytochrome c (to produce holocytochrome) was observed. This is in contrast to the observation that a His-tagged version of holo-CcmE used in previous studies was capable of transferring most of its heme to yield holocytochrome c (25). Isolation of this stable CcmE-heme-cytochrome c552 complex in vitro is also consistent with the role of CcmF, -G and/or -H in breaking down the complex, which was apparent in our experiments in vivo (Fig. 5).
No complex formed when a C11A/C14A variant of H. thermophilus apocytochrome c552, which lacks the heme-binding cysteines, was used in experiments similar to those above (Fig. 7, B and C, lane 8). However, a complex did form when either the C11A (XXXCH heme-binding motif) or C14A (CXXXH) variants were used (Fig. 7, B and C, lanes 4 and 6), indicating that both cysteines of the apocytochrome are capable of binding covalently to CcmE* in vitro. In structurally characterized c-type cytochromes, the stereochemistry of heme attachment is invariant; the N-terminal cysteine of the CXXCH motif is bound covalently to the 2-vinyl group of the heme, and the C-terminal cysteine to the 4-vinyl group (1) (Fig. 1). The structure of heme attachment to His-130 of CcmE has been determined from a heme-containing fragment of the protein (Fig. 1), but the heme vinyl group forming the covalent bond was not identified (11). It is often assumed that heme binding to CcmE is stereo- and regiospecific, leaving one heme vinyl group free and oriented for stereospecific attachment to cytochrome c. However, the CcmE-heme-cytochrome complex reported here formed from apocytochromes with both XXXCH and CXXXH heme-binding motifs, both in vivo and in vitro (Figs. 2 and 7). It may be that stereochemical control is favored in vivo for a cytochrome with a CXXCH heme-binding motif, i.e. for the genuine substrate of the Ccm system, but not for the single cysteine cytochromes, which are poor substrates. We have been unable to produce enough CcmE-heme-cytochrome c-b562 R98C/Y101C complex to investigate this point (Fig. 2, lane 3). It may also be that, contrary to expectations, heme binding to CcmE is not the site of stereochemical control by the Ccm system.
Concluding Remarks
Here we describe detection and analysis of the first identified complex between CcmE and cytochrome c. This membrane-bound complex includes the two proteins both covalently linked to heme, via the heme-binding histidine of CcmE and a heme-binding cysteine of cytochrome c. Multiple lines of evidence suggest this CcmE-heme-cytochrome complex is an intermediate in the catalytic pathway of the Ccm system. Identification of this complex provides a new focus for experiments on the Ccm apparatus. Analysis of formation and breakdown of the complex is also a means by which the phenotypes of new Ccm system mutants can be assessed.
Supplementary Material
Acknowledgments
We thank Feng Rao for the use of the plasmid pFR015. We are grateful to Nicola Ternette (Nuffield Dept. of Clinical Medicine, Oxford), Benjamin Thomas (Dunn School of Pathology, Oxford) and Antony C. Willis (MRC Immunochemistry Unit, Oxford) for assistance with proteomics analyses. We acknowledge the Computational Biology Research Group, Medical Sciences Division, Oxford for use of their services in this project.
This work was supported in part by Biotechnology and Biological Sciences Research Council Grants BB/D019753/1 and BB/H017887/1, Wellcome Trust Grant 092532, and a Value-in-People Award.

This article contains supplemental Figs. S1 and S2 and Tables S1–S4.
D. A. I. Mavridou, S. J. Ferguson, and C. Redfield, unpublished data.
S. J. Ferguson and C. Redfield, unpublished data.
- Ccm
- cytochrome c maturation
- cytochrome c-b562
- c-type variant of cytochrome b562
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- DDM
- n-dodecyl β-d-maltoside.
REFERENCES
- 1. Barker P. D., Ferguson S. J. (1999) Still a puzzle. Why is heme covalently attached in c-type cytochromes? Structure 7, R281–290 [DOI] [PubMed] [Google Scholar]
- 2. Allen J. W. (2011) Cytochrome c biogenesis in mitochondria, systems III and V. FEBS J. 278, 4198–4216 [DOI] [PubMed] [Google Scholar]
- 3. Allen J. W., Daltrop O., Stevens J. M., Ferguson S. J. (2003) c-Type cytochromes, diverse structures and biogenesis systems pose evolutionary problems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358, 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Vitry C. (2011) Cytochrome c maturation system on the negative side of bioenergetic membranes, CCB or system IV. FEBS J. 278, 4189–4197 [DOI] [PubMed] [Google Scholar]
- 5. Hamel P., Corvest V., Giegé P., Bonnard G. (2009) Biochemical requirements for the maturation of mitochondrial c-type cytochromes. Biochim. Biophys. Acta 1793, 125–138 [DOI] [PubMed] [Google Scholar]
- 6. Kranz R. G., Richard-Fogal C., Taylor J. S., Frawley E. R. (2009) Cytochrome c biogenesis, mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol. Mol. Biol. Rev. 73, 510–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simon J., Hederstedt L. (2011) Composition and function of cytochrome c biogenesis system II. FEBS J. 278, 4179–4188 [DOI] [PubMed] [Google Scholar]
- 8. Stevens J. M., Mavridou D. A., Hamer R., Kritsiligkou P., Goddard A. D., Ferguson S. J. (2011) Cytochrome c biogenesis system I. FEBS J. 278, 4170–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arslan E., Schulz H., Zufferey R., Künzler P., Thöny-Meyer L. (1998) Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem. Biophys. Res. Commun. 251, 744–747 [DOI] [PubMed] [Google Scholar]
- 10. Schulz H., Hennecke H., Thöny-Meyer L. (1998) Prototype of a heme chaperone essential for cytochrome c maturation. Science 281, 1197–1200 [DOI] [PubMed] [Google Scholar]
- 11. Lee D., Pervushin K., Bischof D., Braun M., Thöny-Meyer L. (2005) Unusual heme-histidine bond in the active site of a chaperone. J. Am. Chem. Soc. 127, 3716–3717 [DOI] [PubMed] [Google Scholar]
- 12. Ahuja U., Thöny-Meyer L. (2005) CcmD is involved in complex formation between CcmC and the heme chaperone CcmE during cytochrome c maturation. J. Biol. Chem. 280, 236–243 [DOI] [PubMed] [Google Scholar]
- 13. Christensen O., Harvat E. M., Thöny-Meyer L., Ferguson S. J., Stevens J. M. (2007) Loss of ATP hydrolysis activity by CcmAB results in loss of c-type cytochrome synthesis and incomplete processing of CcmE. FEBS J. 274, 2322–2332 [DOI] [PubMed] [Google Scholar]
- 14. Feissner R. E., Richard-Fogal C. L., Frawley E. R., Kranz R. G. (2006) ABC transporter-mediated release of a heme chaperone allows cytochrome c biogenesis. Mol. Microbiol. 61, 219–231 [DOI] [PubMed] [Google Scholar]
- 15. Richard-Fogal C., Kranz R. G. (2010) The CcmC-heme-CcmE complex in heme trafficking and cytochrome c biosynthesis. J. Mol. Biol. 401, 350–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schulz H., Fabianek R. A., Pellicioli E. C., Hennecke H., Thöny-Meyer L. (1999) Heme transfer to the heme chaperone CcmE during cytochrome c maturation requires the CcmC protein, which may function independently of the ABC-transporter CcmAB. Proc. Natl. Acad. Sci. U.S.A. 96, 6462–6467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rayapuram N., Hagenmuller J., Grienenberger J. M., Bonnard G., Giegé P. (2008) The three mitochondrial encoded CcmF proteins form a complex that interacts with CCMH and c-type apocytochromes in Arabidopsis. J. Biol. Chem. 283, 25200–25208 [DOI] [PubMed] [Google Scholar]
- 18. Reid E., Eaves D. J., Cole J. A. (1998) The CcmE protein from Escherichia coli is a heme-binding protein. FEMS Microbiol. Lett. 166, 369–375 [DOI] [PubMed] [Google Scholar]
- 19. Meyer E. H., Giegé P., Gelhaye E., Rayapuram N., Ahuja U., Thöny-Meyer L., Grienenberger J. M., Bonnard G. (2005) AtCCMH, an essential component of the c-type cytochrome maturation pathway in Arabidopsis mitochondria, interacts with apocytochrome c. Proc. Natl. Acad. Sci. U.S.A. 102, 16113–16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turkarslan S., Sanders C., Ekici S., Daldal F. (2008) Compensatory thio-redox interactions between DsbA, CcdA, and CcmG unveil the apocytochrome c holdase role of CcmG during cytochrome c maturation. Mol. Microbiol. 70, 652–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allen J. W., Barker P. D., Ferguson S. J. (2003) A cytochrome b562 variant with a c-type cytochrome CXXCH heme-binding motif as a probe of the Escherichia coli cytochrome c maturation system. J. Biol. Chem. 278, 52075–52083 [DOI] [PubMed] [Google Scholar]
- 22. Barker P. D., Nerou E. P., Freund S. M., Fearnley I. M. (1995) Conversion of cytochrome b562 to c-type cytochromes. Biochemistry 34, 15191–15203 [DOI] [PubMed] [Google Scholar]
- 23. Allen J. W., Tomlinson E. J., Hong L., Ferguson S. J. (2002) The Escherichia coli cytochrome c maturation (Ccm) system does not detectably attach heme to single cysteine variants of an apocytochrome c. J. Biol. Chem. 277, 33559–33563 [DOI] [PubMed] [Google Scholar]
- 24. Goddard A. D., Stevens J. M., Rondelet A., Nomerotskaia E., Allen J. W., Ferguson S. J. (2010) Comparing the substrate specificities of cytochrome c biogenesis systems I and II. FEBS J. 277, 726–737 [DOI] [PubMed] [Google Scholar]
- 25. Daltrop O., Stevens J. M., Higham C. W., Ferguson S. J. (2002) The CcmE protein of the c-type cytochrome biogenesis system. Unusual in vitro heme incorporation into apo-CcmE and transfer from holo-CcmE to apocytochrome. Proc. Natl. Acad. Sci. U.S.A. 99, 9703–9708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goodhew C. F., Brown K. R., Pettigrew G. W. (1986) Haem staining in gels, a useful tool in the study of bacterial c-type cytochromes. Biochim. Biophys. Acta 852, 288–294 [Google Scholar]
- 27. Batycka M., Inglis N. F., Cook K., Adam A., Fraser-Pitt D., Smith D. G., Main L., Lubben A., Kessler B. M. (2006) Ultra-fast tandem mass spectrometry scanning combined with monolithic column liquid chromatography increases throughput in proteomic analysis. Rapid Commun. Mass Spectrom. 20, 2074–2080 [DOI] [PubMed] [Google Scholar]
- 28. Kramer H. B., Lavender K. J., Qin L., Stacey A. R., Liu M. K., di Gleria K., Simmons A., Gasper-Smith N., Haynes B. F., McMichael A. J., Borrow P., Kessler B. M. (2010) Elevation of intact and proteolytic fragments of acute phase proteins constitutes the earliest systemic antiviral response in HIV-1 infection. PLoS Pathog. 6, e1000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daltrop O., Ferguson S. J. (2003) Cytochrome c maturation. The in vitro reactions of horse heart apocytochrome c and Paracoccus dentrificans apocytochrome c550 with heme. J. Biol. Chem. 278, 4404–4409 [DOI] [PubMed] [Google Scholar]
- 30. Daltrop O., Smith K. M., Ferguson S. J. (2003) Stereoselective in vitro formation of c-type cytochrome variants from Hydrogenobacter thermophilus containing only a single thioether bond. J. Biol. Chem. 278, 24308–24313 [DOI] [PubMed] [Google Scholar]
- 31. Bartsch R. G. (1971) Cytochromes: Bacterial. Methods Enzymol. 23, 344–363 [Google Scholar]
- 32. Mavridou D. A., Saridakis E., Kritsiligkou P., Goddard A. D., Stevens J. M., Ferguson S. J., Redfield C. (2011) Oxidation state-dependent protein-protein interactions in disulfide cascades. J. Biol. Chem. 286, 24943–24956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goddard A. D., Stevens J. M., Rao F., Mavridou D. A., Chan W., Richardson D. J., Allen J. W., Ferguson S. J. (2010) c-Type cytochrome biogenesis can occur via a natural Ccm system lacking CcmH, CcmG, and the heme-binding histidine of CcmE. J. Biol. Chem. 285, 22882–22889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allen J. W., Ferguson S. J. (2003) Variation of the axial heme ligands and heme-binding motif as a probe of the Escherichia coli c-type cytochrome maturation (Ccm) system. Biochem. J. 375, 721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Allen J. W., Leach N., Ferguson S. J. (2005) The histidine of the c-type cytochrome CXXCH heme-binding motif is essential for heme attachment by the Escherichia coli cytochrome c maturation (Ccm) apparatus. Biochem. J. 389, 587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Daltrop O., Allen J. W., Willis A. C., Ferguson S. J. (2002) In vitro formation of a c-type cytochrome. Proc. Natl. Acad. Sci. U.S.A. 99, 7872–7876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Daltrop O., Ferguson S. J. (2004) In vitro studies on thioether bond formation between Hydrogenobacter thermophilus apocytochrome c(552) with metalloprotoporphyrin derivatives. J. Biol. Chem. 279, 45347–45353 [DOI] [PubMed] [Google Scholar]
- 38. Robertson I. B., Stevens J. M., Ferguson S. J. (2008) Dispensable residues in the active site of the cytochrome c biogenesis protein CcmH. FEBS Lett. 582, 3067–3072 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.