Abstract
Previous studies in our laboratory have shown that mixed lineage kinase 3 (MLK3) can be activated following global ischemia. In addition, other laboratories have reported that the activation of MLK3 may be linked to the accumulation of free radicals. However, the mechanism of MLK3 activation remains incompletely understood. We report here that MLK3, overexpressed in HEK293 cells, is S-nitrosylated (forming SNO-MLK3) via a reaction with S-nitrosoglutathione, an exogenous nitric oxide (NO) donor, at one critical cysteine residue (Cys-688). We further show that the S-nitrosylation of MLK3 contributes to its dimerization and activation. We also investigated whether the activation of MLK3 is associated with S-nitrosylation following rat brain ischemia/reperfusion. Our results show that the administration of 7-nitroindazole, an inhibitor of neuronal NO synthase (nNOS), or nNOS antisense oligodeoxynucleotides diminished the S-nitrosylation of MLK3 and inhibited its activation induced by cerebral ischemia/reperfusion. In contrast, 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine (an inhibitor of inducible NO synthase) or nNOS missense oligodeoxynucleotides did not affect the S-nitrosylation of MLK3. In addition, treatment with sodium nitroprusside (an exogenous NO donor) and S-nitrosoglutathione or MK801, an antagonist of the N-methyl-d-aspartate receptor, also diminished the S-nitrosylation and activation of MLK3 induced by cerebral ischemia/reperfusion. The activation of MLK3 facilitated its downstream protein kinase kinase 4/7 (MKK4/7)-JNK signaling module and both nuclear and non-nuclear apoptosis pathways. These data suggest that the activation of MLK3 during the early stages of ischemia/reperfusion is modulated by S-nitrosylation and provides a potential new approach for stroke therapy whereby the post-translational modification machinery is targeted.
Keywords: Apoptosis, Calcium Calmodulin-dependent Protein Kinase (CaMK), Jun N-terminal kinase (JNK), MAPKs, Neurotransmitter Receptors, JNK3, MLK3, S-Nitrosylation, Cerebral Ischemia, Neuroprotection
Introduction
As a free radical, NO is an endogenous cell signaling molecule involved in the regulation of many physiological and pathophysiological processes (1). NO and NO-related compounds exert both protective and cytotoxic effects, depending on the cellular context and the nature of the NO group. The multifaceted actions of the NO group can be classified into two categories, cGMP-dependent and cGMP-independent. The cGMP-dependent actions play critical roles in a variety of physiological processes, including NO-mediated vasodilation. In contrast, cGMP-independent, nitrosative protein modifications are postulated to be involved in both physiological and pathological responses (2).
Endogenous NO is synthesized from l-arginine by NO synthase (NOS) and is associated with S-nitrosylation. S-Nitrosylation, the modification of the covalent attachment to the side chain of cysteine by an NO group, is considered as an important post-translational modification that has profound effects on protein function (3). Three subtypes of NOS have been identified, endothelial NOS, iNOS,3 and nNOS. Glutamate-induced excitotoxicity, which is mainly associated with an excessive release of glutamate and subsequent influx of Ca2+ via the N-methyl-d-aspartate (NMDA) subtype of glutamate receptor, has been implicated in various kinds of neuronal degenerative diseases, including seizure, Alzheimer disease, and stroke (4, 5). Ca2+ targets include protein phosphatases, protein kinases, and NOS (6–8). nNOS and iNOS are present in the brain, and the difference between them is that nNOS mediates early neuronal injury, and iNOS contributes to late neuronal injury (9). Moreover, nNOS is expressed mainly in neurons, whereas iNOS is primarily induced under pathological conditions in macrophages, microglia, neurons, and astrocytes. In addition, nNOS is constitutively expressed and responds to calcium-calmodulin signaling. In contrast, iNOS is induced by inflammatory mediators and is coupled to an activated calmodulin that does not require calcium for activation (9, 10). Other evidence has also shown that NO is biosynthesized by nNOS in brain tissue, which following the increase of the binding of calcium and calmodulin is activated by calcium influx through NMDAR (11).
The mitogen-activated protein kinase (MAPK) signaling pathway is considered to be a critical mediator of neuronal survival and apoptosis (12, 13). This pathway consists of MAPK kinase kinases (MAPKKK), MAPK kinases (MAPKK), and MAPKs (14). MLK3, a member of the serine/threonine MAPKKKs, is composed of an N-terminal Src homology 3 domain, a middle kinase domain, and a C-terminal proline-rich region. MLK3 phosphorylates and activates MAPKKs, including MKK4 and MKK7, which in turn activates JNKs (members of the MAPK family), at both Thr-183 and Tyr-185 residues (15). Many studies have documented that MLK3 activation facilitates the assembly of the glutamate receptor 6 (GluR6)·postsynaptic density protein 95 (PSD-95)·MLK3 signaling module in brain ischemia and reperfusion (16). In addition, it has been reported that homodimerization and the autophosphorylation of Thr-277 and Ser-281 is the principal mechanism that regulates the activity of MLK3 (17) and that MLK3 can be regulated by the small GTP-binding proteins, Cdc42 and Rac1 (17, 18). In addition, our early reports that the antioxidant N-acetylcysteine significantly blocks the activation peaks of MLK3 in the early stages of ischemia/reperfusion (19), have indicated that activated MLK3 also associates with free radicals in the signaling events that follow brain ischemia. However, the mechanism underlying MLK3 activation remains incompletely understood. Previous studies have shown that JNK3 can be activated by the upstream protein MLK3 during ischemia-reperfusion in the rat hippocampus (16, 17, 20). Consequently, activated JNK may translocate into the nucleus, leading to cell apoptosis via the phosphorylation of c-Jun and enhancement of the expression of Fas ligand (FasL). At the same time, activated JNK can cause mitochondrion-mediated apoptosis by regulating the activity of Bcl-2 and inducing the release of cytochrome c (16, 20). Consequently, FasL and cytochrome c can activate caspase-3 and ultimately induce apoptosis.
Based upon the evidence mentioned above, we hypothesize that following brain ischemia/reperfusion, Ca2+ influx via NMDAR leads to the formation of calcium-calmodulin signaling compounds, which activate nNOS and increase the release of endogenous NO. This augmentation of endogenous NO may S-nitrosylate MLK3 by adding NO to the thiol moiety of reactive cysteine residues and activating this protein MLK3, which in turn facilitates the activation of the JNK signaling pathway and finally exacerbates ischemic neuron death. In this study, we initially examined whether MLK3, which is overexpressed in HEK293 cells, is S-nitrosylated by reaction with GSNO at Cys-688 and whether this contributes to its dimerization and activation. Second, we show that cerebral ischemia induces the S-nitrosylation of MLK3 at different time points of reperfusion. When treated with 7-NI and exogenous NO donors (SNP and GSNO), the activation of nNOS was inhibited. This leads to a decrease in the levels of S-nitrosylated MLK3 during the early stages of reperfusion, further down-regulates the downstream signaling pathway of JNK, and finally induces neuroprotection in the hippocampal CA1 region.
MATERIALS AND METHODS
Antibody and Reagents
The following primary antibodies were used: anti-p-JNKs (sc-6254), anti-p-c-Jun (sc-16312), anti-MKK4 (sc-837), anti-FasL (sc-6237), anti-Fas (sc-716), anti-actin (sc-10731), and anti-Bcl-2 (sc-492). All were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-nNOS (4234) (used for Western blotting), anti-p-MLK3 (2811), anti-MLK3 (2817), anti-p-MKK4 (9156), anti-p-MKK7 (4171), anti-MKK7 (4172), anti-c-Jun (9162), anti-cytochrome c (4272), anti-COX IV (4844), and anti-caspase-3 (9662) were acquired from Cell Signaling Biotechnology (Boston). The secondary anti-mouse IgG (A1682) or anti-rabbit IgG (T6778) used in our experiments were purchased from Sigma. Nitrocellulose filters were acquired from Amersham Biosciences. 5-Bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium were obtained from Promega (Madison, WI). CHAPS, SNP, GSNO, MK801, 7-NI, AMT, N-ethylmaleimide (NEM), dl-dithiothreitol (DTT), methyl methanethiosulfonate, streptavidin-agarose, and other chemicals were all from Sigma. The RT-PCR primers used to amplify MLK3, mutant MLK3 plasmids, nNOS AS-ODN, and MS-ODN were synthesized by the Shanghai Sangon Biological Engineering Technology & Services Co., Ltd.
Plasmids
A full-length rat pME18s-nNOS expression plasmid was obtained from Professor Yasuo Watanabe (Department of Pharmacology and Ophthalmology, Nagoya University School of Medicine, Showa-ku, Nagoya, Japan). The pcDNA3.1-MLK3 plasmid was constructed as follows. Total RNA was isolated from fresh rat hippocampi. The MLK3 insert was amplified by RT-PCR using the following primers: primer 1 (sense primer, with an NheI site), 5′-TAGCTAGCATGGAGCCCTTGAAGAACC-3′, and primer 2 (antisense primer with EcoR I site), 5′-CGAATTCGGGCCCTGCTTCTGGTGC-3′. The PCR product and the pCDNA3.1 vector were then digested with NheI and EcoRI and ligated to generate pCDNA3.1-MLK3. This construct was used to transfect HEK293 cells.
Site-directed Mutagenesis of MLK3
Mutant MLK3 plasmids were constructed using the pCDNA3.1-MLK3 plasmid as a template. Each cysteine residue of MLK3 was mutated to glycine. The following sets of forward and reverse primers were used to introduce mutations at Cys-110, -184, -191, -232, -328, -348, -359, -564, -688, and -831: C110G, GTGGAGGCCCACCCCCCGGTGAGGTGGCCAGCTTC (forward) and GAAGCTGGCCACCTCACCGGGGGGTGGGCCTCCAC (reverse); C184G, CCTCAAAGCTGTGGGCCTGGAGGAGCC (forward) and GGCTCCTCCAGGCCCACAGCTTTGAGG (reverse); C191G, CCTCAAAGCTGTGGGCCTGGAGGAGCC (forward) and GGCTCCTCCAGGCCCACAGCTTTGAGG (reverse); C232G, GGGATGCACTACCTGCACGGTGAGGCTCTAGTGCCTG (forward) and CAGGCACTAGAGCCTCACCGTGCAGGTAGTGCATCCC (reverse); C328G, CCCTACCGTGGTATCGACGGTCTTGCGGTAGCCTATG (forward) and CATAGGCTACCGCAAGACCGTCGATACCACGGTAGGG (reverse); C348G, CCCATCCCATCCACTGGCCCTGAGCCCTTCG (forward) and CGAAGGGCTCAGGGCCAGTGGATGGGATGGG (reverse); C359G, CACAACTCATGGCTGACGGCTGGGCACAGGACCCC (forward) and GGGGTCCTGTGCCCAGCCGTCAGCCATGAGTTGTG (reverse); C564G, GGAGAGAGGCGTGCTGGCTGGGCCTGGGGTC (forward) and GACCCCAGGCCCAGCCAGCACGCCTCTCTCC (reverse); C688G, CACAGATGGCTTCACCCGGTCCACCTGACCTGCCC (forward) and GGGCAGGTCAGGTGGACCGGGTGAAGCCATCTGTG (reverse); and C831G, CGGGGAGGCTCCCAGGACGGCAGGACGCAGACCAAAG (forward) and CTTTGGTCTGCGTCCTGCCGTCCTGGGAGCCTCCCCG (reverse). All mutant plasmids were constructed using the QuikChange II kit (Stratagene, La Jolla, CA). Mutagenesis was confirmed by automated nucleotide sequencing.
Cell Culture and Plasmid Transfection Analysis
HEK293 cells were grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal calf serum (37 °C, 5% CO2). Wild-type (WT) or mutant pCDNA3.1-MLK3 plasmids were transfected into HEK293 cells using Lipofectamine 2000 and Opti-MEM I reagents (Invitrogen) in accordance with the manufacturer's instructions (achieving an ∼60% transfection efficiency as determined by GFP labeling). After transfection, cells were cultured at 37 °C with 5% CO2 for 48 h before treatment with either 500 μm GSNO or 250 μm NEM and then treatment with GSNO 30 min later. The cells were then collected and homogenized in ice-cold HEN buffer containing 250 mm Hepes-NaOH, pH 7.7, 1 mm EDTA, and 0.1 mm neocuproine. The homogenates were centrifuged at 800 × g for 10 min at 4 °C. Supernatants were collected, and protein concentrations were determined by the method of Lowry et al. (21). Samples were stored at −80 °C until use. To further test the possibility that MLK3 is S-nitrosylated by endogenous NO, HEK293 cells were transiently transfected with pCDNA3.1-MLK3 and pME18s-nNOS plasmids in a 1:1 ratio. After 48 h, the cells were exposed to either 10 μm A23187 or 7-NI (10 μm) and then A23187 alone (10 μm) for 1 h and were then collected. GSNO, NEM, A23187, and 7-NI were prepared as 20 mm stock solutions in DMEM.
Induction of Ischemia
Adult male Sprague-Dawley rats weighing 200–250 g were used (Shanghai Experimental Animal Center, Chinese Academy of Science, Shanghai, China). Transient cerebral ischemia was induced using the four-vessel occluded method as described previously (22). Briefly, rats were anesthetized with chloral hydrate (300 mg/kg, intraperitoneally) before both vertebral arteries were occluded permanently by electrocautery and both carotid arteries were then isolated. Then rats were allowed to recover for 24 h, and both carotid arteries were occluded with aneurysm clips to induce cerebral ischemia without the administration of chloral hydrate. After occlusion for 15 min, the aneurysm clips were removed for reperfusion. Rats that lost their righting reflex within 30 s and those whose pupils were dilated and unresponsive to light were selected for the experiments. The rectal temperature was maintained at 36.5–37.5 °C during ischemia (15 min) and a 2-h reperfusion. Sham control rats were treated using the same surgical procedures except that the carotid arteries were not occluded.
Administration of Drugs
The rats were injected intraperitoneally three times with SNP dissolved in 0.9% NaCl at a dose of 5 mg/kg and with an interval of 1.5 h. The first injection was given 30 min prior to the induction of ischemia. MK801 (3 mg/kg) or AMT (0.65 mg/kg) dissolved in 0.9% NaCl were given intraperitoneally 20 min before ischemia. 7-NI (25 mg/kg) dissolved in 1% dimethyl sulfoxide (DMSO) was intraperitoneally injected 20 min before ischemia. DTT, dissolved in 0.9% NaCl at a dose of 10 mm, and GSNO (100 μg/μg), dissolved in 0.9% NaCl, were administered intracerebroventricularly (10 μl; bregma, 1.5 mm lateral, 0.8 mm posterior, 3.8 mm deep). 10 nmol of nNOS antisense oligodeoxynucleotides and missense oligodeoxynucleotides in 10 μl of TE buffer (10 mm Tris-HCl, pH 8.0, 1 mm EDTA) were given to the rats every 24 h for 3 days. Control rats were intraperitoneally or intracerebroventricularly given the corresponding solvent, i.e. 0.9% NaCl, 1% DMSO, or TE buffer. The sequences for nNOS AS-ODN and MS-ODN were 5′-ACGTGTTCTCTTCCAT-3′ and 5′-AAAGGGAGAACACGT-3′, respectively.
Sample Preparation
Rats were decapitated immediately after reperfusion with different methods, and the hippocampal CA1 was isolated and flash-frozen in liquid nitrogen. These issues were then homogenized in an ice-cold buffer containing 50 mm MOPS, pH 7.4, 100 mm KCl, 320 mm sucrose, 50 mm NaF, 0.5 mm MgCl2, 0.2 mm DTT (free when S-nitrosylation was tested), 1 mm EDTA, 1 mm EGTA, 1 mm Na3VO4, 20 mm sodium pyrophosphate, 20 mm phosphoglycerol, 1 mm p-nitrophenyl phosphate, 1 mm benzamidine, 1 mm phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, 5 μg/ml aprotinin, and 5 μg/ml pepstatin A. The homogenates were then centrifuged at 800 × g for 10 min at 4 °C. Supernatants were collected, and the protein concentration was determined by the method of Lowry. Samples were stored at −80 °C until single use.
When necessary, the hippocampal CA1 regions were immediately isolated to prepare mitochondrial fractions. All procedures were conducted in a cold room. Generally, unfrozen brain tissue was used to prepare mitochondrial fractions as freezing causes the release of cytochrome c from the mitochondria. The hippocampal CA1 samples were homogenized in a 1:10 (w/v) ice-cold buffer. The homogenates were then centrifuged at 800 × g for 10 min at 4 °C. The pellets were discarded, and supernatants were centrifuged at 17,000 × g for 20 min at 4 °C to obtain the cytosolic fraction in the supernatants and a crude mitochondrial fraction in the pellets. The protein concentrations were determined by the method of Lowry.
Nuclear Extraction
The homogenates were centrifuged at 800 × g for 10 min at 4 °C. Supernatants (cytosolic fraction) were collected, and protein concentrations were determined. The nuclear pellets were extracted for 30 min at 4 °C with 20 mm Hepes, pH 7.9, 20% glycerol, 420 mm NaCl, 0.5 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 1 mm dithiothreitol, and enzyme inhibitors with constant agitation. After centrifugation at 12,000 × g for 15 min at 4 °C, supernatants (nuclear fraction) were collected, and protein concentrations were determined. Samples were stored at −80 °C until single use.
Immunoprecipitation and Immunoblotting
Tissue homogenates (400 μg of protein) were diluted 4-fold with immunoprecipitation buffer (50 mm Hepes buffer, pH 7.4, containing 10% glycerol, 150 mm NaCl, 1% Triton X-100, 0.5% Nonidet P-40, 1 mm EDTA, 1 mm EGTA, 1 mm phenylmethylsulfonyl fluoride, and 1 mm Na3VO4). Samples were preincubated for 1 h with 20 μl of protein A-Sepharose CL-4B (Amersham Biosciences) at 4 °C and centrifuged to remove proteins that had adhered nonspecifically to protein A. The supernatants were then incubated with 1–2 μg of primary antibodies for 4 h or overnight at 4 °C. Protein A was added to the tube for a further 2 h of incubation. Samples were then centrifuged at 10,000 × g for 2 min at 4 °C, and the pellets were washed three times with immunoprecipitation buffer. Bound proteins were eluted by boiling at 100 °C for 5 min in SDS-PAGE loading buffer and then isolated by centrifugation. The supernatants were separated on polyacrylamide gels and then electrotransferred to a nitrocellulose membrane (Amersham Biosciences). After blocking for 3 h in Tris-buffered saline with 0.1% Tween 20 (TBST) and 3% bovine serum albumin, membranes were incubated overnight at 4 °C with primary antibodies in TBST containing 1% bovine serum albumin. The filters were then washed and incubated with alkaline phosphatase-conjugated secondary antibodies in TBST for 2 h and developed using nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate color substrate (Promega, Madison, WI). The density of the bands on the membrane was scanned and analyzed with LabWorks image analysis software (UVP, Inc.).
When necessary, to examine monomer and dimeric forms of MLK3, tissues were homogenized in ice-cold homogenization buffer and protein 2× SDS sample buffer (100 mm Tris-HCl, pH 6.8, 4% SDS, 0.2% bromphenol blue, and 20% glycerol) with or without both the reducing agent DTT (final concentration, 200 mm) and β-mercaptoethanol (β-ME, final concentration, 0.715 m). After boiling for 3 min, the samples were separated by SDS-PAGE and transferred to nitrocellulose membranes. The remaining samples were treated using the blotting process described above.
Determination of Protein S-Nitrosylation
Measurement of S-nitrosylated MLK3 was performed using the biotin switch assay as described previously by Jaffrey et al. (23), under light-free conditions and using opaque tubes. Briefly, the cells or hippocampi were homogenized in HEN buffer. Free thiols were blocked by methylation with methyl methanethiosulfonate. Unreacted methyl methanethiosulfonate was removed by protein precipitation in 10 volumes of acetone (−20 °C). Cysteine residues that had been S-nitrosylated by NO were converted to free thiols with sodium ascorbate (1 mm final concentration), which does not alter the methylated thiols. The free thiols were then biotinylated with biotin-hexyl pyridyldithiopropionamide at 25 °C for more than 1 h. Thus, the S-nitrosylated cysteines were switched for biotin. Proteins were precipitated by chilled acetone, and the pellet was resuspended in HENS buffer (250 mm Hepes, pH 7.7, 1 mm EDTA, 0.1 mm neocuproine, 1% SDS). Biotinylated proteins were precipitated with streptavidin-agarose and eluted from the beads with a solution containing 20 mm Hepes-NaOH, pH 7.7, 100 mm NaCl, 1 mm EDTA and 100 mm β-ME.
Histological Analysis and Immunohistochemistry
Rats were perfusion-fixed with 4% paraformaldehyde in 0.1 m sodium phosphate buffer, pH 7.4, under anesthesia. Brains were removed quickly and further fixed with the same fixation solution overnight at 4 °C. Post-fixed brains were embedded in paraffin, followed by preparation of coronal sections (5 μm thick) using a microtome. The paraffin-embedded brain sections were deparaffinized with xylene and rehydrated with ethanol at graded concentrations of 100 to 70% (v/v), followed by washing with water. The sections were stained with 0.1% (w/v) cresyl violet and examined by light microscopy. The number of surviving hippocampal CA1 pyramidal cells (1 mm length) was counted as the neuronal density.
Immunoreactivity was determined using the avidin/biotin/peroxidase method. Briefly, sections were deparaffinized with xylene and rehydrated with ethanol at graded concentrations in distilled water. High temperature antigen retrieval was performed in 1 mm citrate buffer. To block endogenous peroxidase activity, sections were incubated for 30 min in 1% H2O2. After treating with rabbit polyclonal anti-phospho-c-Jun or anti-FasL antibody (1:50 dilution) at 4 °C for 2 days, these sections were then incubated overnight with biotinylated goat anti-rabbit secondary antibody and subsequently with avidin-conjugated horseradish peroxidase for 1 h at 37 °C. Finally, sections were incubated with the peroxidase substrate diaminobenzidine until the desired stain intensity developed.
Measurement of NO Production
Total NO production in the cell lysates was determined by measuring the concentration of nitrate and nitrite, a stable metabolite of NO, by the modified Griess reaction method. The procedure involved use of the total nitric oxide assay kit (Beyotime Institute of Biotechnology).
Data Analysis and Statistics
Values are expressed as the means ± S.D. and were obtained from at least six independent rats. Statistical analysis of the results was carried out by one-way analysis of variance, followed by the Duncan's new multiple range method or the Newman-Keuls test. p values of < 0.05 were considered significant.
RESULTS
S-Nitrosylation of MLK3 by GSNO or nNOS-derived NO in Vitro
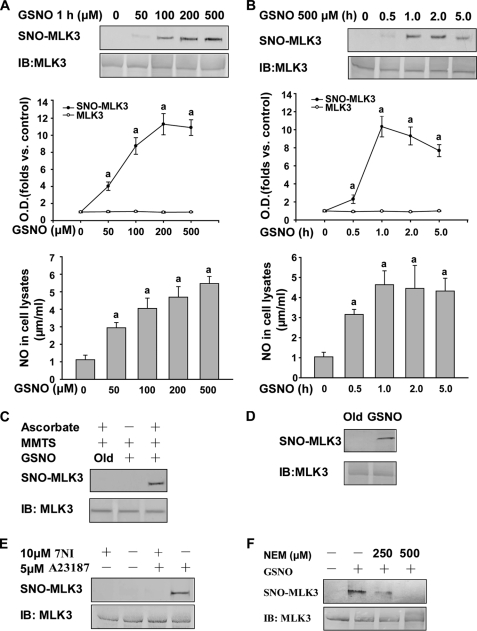
We obtained initial evidence that MLK3, expressed in HEK293 cells, is S-nitrosylated by GSNO using the biotin-switch assay. In this assay, an SNO-protein is identified on Western blots after replacing the SNO group with a more stable biotin group by chemical reduction with ascorbate. GSNO markedly enhanced the levels of S-nitrosylated MLK3 (SNO-MLK3) in cell lysates (Fig. 1D). In intact cells, GSNO-treated MLK3 resulted in significant SNO-protein formation in a concentration- and time-dependent manner. The contents of nitrate/nitrite in the cell lysates have similar pattern. (Fig. 1, A and B). In contrast, negative controls under the same conditions, using decayed (old) GSNO or without ascorbate for the detection of nitrosylation in vitro, did not enhance the level of SNO-MLK3 (Fig. 1C).
FIGURE 1.
MLK3 can be S-nitrosylated by exogenous and endogenous NO in vitro. Upper gels show SNO-MLK3, and lower gels show total MLK3 in cells analyzed by Western blotting. Variable levels of NO were produced after treating with GSNO, and the contents of nitrate/nitrite in the cell lysates were determined by Griess assay. A, HEK293 cells expressing MLK3 were incubated with various concentrations of GSNO at 37 °C and assayed for SNO-MLK3 and the contents of nitrate/nitrite. a, p < 0.05 versus control. B, time course of S-nitrosylated MLK3 production and the contents of nitrate/nitrite in the presence of the NO donor GSNO. a, p < 0.05 versus control. C, HEK293 cells expressing MLK3 were exposed to 500 μm GSNO followed by an assay for S-nitrosylation. Control samples were subjected to decayed (old) GSNO or GSNO with no ascorbate. MMTS, methyl methanethiosulfonate. D, cell lysates from HEK293 cells expressing MLK3 were incubated with GSNO or degraded GSNO at room temperature, followed by an assay for SNO-MLK3. E, HEK293 cells expressing nNOS and MLK3 were assayed for endogenous SNO-MLK3. nNOS was activated by the addition of 5 μm of the Ca2+ ionophore A23187 in the presence or absence of 7-NI. Activation of nNOS increased the levels of endogenous SNO-MLK3, and 7-NI prevented this increase. F, NEM can block the SNO-MLK3 increase in the presence of GSNO. IB, immunoblot.
To next test the possibility that the S-nitrosylation of MLK3 occurs via NO, we evaluated the effects of NEM, which is known to covalently modify protein sulfhydryl groups making them incapable of nitrosylation. The addition of NEM to intact HEK293 cells expressing MLK3 completely eliminated the direct S-nitrosylation of MLK3 by GSNO (Fig. 1F).
Additionally, to further determine whether endogenous NO could S-nitrosylate MLK3, we coexpressed nNOS and MLK3 in HEK293 cells. MLK3 was nitrosylated by NO produced by endogenous nNOS activity when A23187 was present, and this reaction was inhibited by 7-NI (Fig. 1E).
S-Nitrosylation of Cys-688 Is Critical for the Activation of MLK3 by GSNO in Vitro
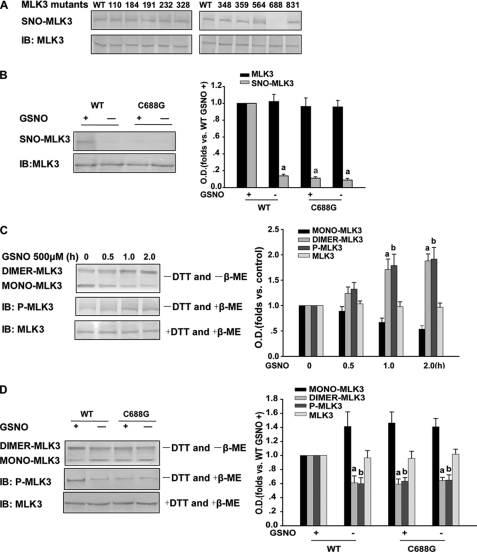
Mammalian MLK3 contains 10 cysteine residues in total. To determine the target site(s) of S-nitrosylation on MLK3, we performed the biotin-switch assay using HEK293 cells transfected with wild-type or mutant MLK3 species (harboring cysteine-to-glycine mutations). The density of the bands on the membrane for the C688G mutant was decreased by about 85% compared with the wild type, indicating that cysteine 688 is a target of S-nitrosylation (Fig. 2, A and B).
FIGURE 2.
MLK3 S-nitrosylation contributes to its dimerization and phosphorylation. A and B, HEK293 cells overexpressing WT or mutant MLK3 products were exposed to 500 μm GSNO for 1 h (upper gel), and SNO-MLK3 was detected using the biotin-switch assay with an anti-MLK3 antibody. Lower gel, Western blotting using an anti-MLK3 antibody. Mutation of a critical cysteine thiol on MLK3 (C688A) prevents S-nitrosylation by GSNO. a, p < 0.05 versus WT GSNO+. C, upper gel, in the absence of DTT in ice-cold homogenization buffer and absence of β-ME in 2× SDS sample buffer, HEK293 cells expressing MLK3 pretreated with GSNO show MLK3 dimerization. Middle gel, under the same conditions, in the absence of DTT in ice-cold homogenization buffer but in 2× SDS sample buffer with β-ME, GSNO enhances the phosphorylation of MLK3. However, in the presence of DTT and β-ME, the dimerization of MLK3 could not be detected (lower gel). a, p < 0.05 versus control; b, p < 0.05 versus control. D, wild-type MLK3 and its C688G mutant were expressed in HEK293 cells and treated with GSNO for 1 h. Cells were collected, and MLK3 dimerization and phosphorylation was examined by Western blotting as above. a and b, p < 0.05 versus WT and GSNO+, respectively. IB, immunoblot.
A previous study has confirmed that dimerization is a prerequisite for MLK3 autophosphorylation (17). To determine whether S-nitrosylation affects MLK3 function, we monitored the dimerization and phosphorylation of MLK3 expressed in HEK293 cells treated with GSNO. Dimerization and phosphorylation were assessed using a standard assay as described previously (25). The data shown in Fig. 2C suggest that S-nitrosylation contributes to the functional activities of wild-type MLK3. Next, we evaluated both the dimerization and phosphorylation levels of the MLK3 C688G mutant after treatment with GSNO. Wild-type MLK3 and the C688G mutant were expressed in HEK293 cells which were then treated with GSNO for 1 h. As shown in Fig. 2D, the dimerization and phosphorylation of the C688G mutant were decreased compared with wild-type MLK3.
MLK3 S-Nitrosylation Is Mediated by Endogenous NO during Cerebral Ischemia-Reperfusion
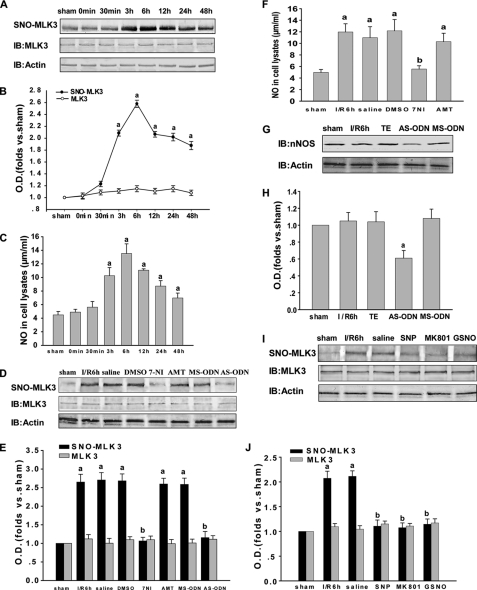
We have reported previously that MLK3 is activated during the assembly of the GluR6·PSD-95·MLK3 signaling module following brain ischemia and reperfusion and that its activation may also associate with free radicals in the signaling events that take place following brain ischemia. To investigate whether MLK3 is S-nitrosylated and to further characterize the alteration of S-nitrosylated MLK3 at different time points after reperfusion, we examined the time course of MLK3 S-nitrosylation. As shown in Fig. 3, A and B, MLK3 is S-nitrosylated at significantly elevated levels after 3, 6, and 12 h and 1 day of reperfusion and is recovered at 2 days. The content of nitrate/nitrite in the cell lysates is similar to it (Fig. 3C).
FIGURE 3.
Effects of NOS inhibitors and an NMDAR antagonist on S-nitrosylated MLK3. A–C, time course of S-nitrosylated MLK3 and the contents of nitrate/nitrite in the rat hippocampal CA1 derived from sham-treated animals or rats subjected to 15 min of ischemia at various time points after reperfusion. (a) p < 0.05 versus sham. D and I, effects on the S-nitrosylation of MLK3 of treatment with 7-NI, AMT, MS-ODN, AS-ODN, SNP, MK801, or GSNO. F, contents of nitrate/nitrite in the cell lysates of treatment with 7-NI, AMT. G, effects on total nNOS of treatment with MS-ODN or AS-ODN. B, E, H, and J, bands were scanned, and the intensities are represented as the fold changes versus sham treatment. Data are the means ± S.D. from four independent experiments. E and J, (a) p < 0.05 versus sham; (b) p < 0.05 versus saline groups. H, (a) p < 0.05 versus TE. IB, immunoblot.
It is known that S-nitrosylation requires NO. To elucidate whether the S-nitrosylation of MLK3 is induced by endogenous NO, we utilized inhibitors of NOS. Because nNOS and iNOS are the main NO synthases present in the brain and nNOS-derived endogenous NO is found during cerebral ischemia-reperfusion, 7-NI, AMT, nNOS AS-ODN, and nNOS MS-ODN were used in the experimental treatments. As shown in Fig. 3, D and E, the administration of 7-NI and nNOS AS-ODN markedly suppressed the S-nitrosylation of MLK3, whereas the treatments with AMT and nNOS MS-ODN were ineffective. 7-NI also decreased the NO production (Fig. 3F). The data shown in Fig. 3, G and H, further indicate that AS-ODN and MS-ODN may influence the S-nitrosylation of MLK3 by affecting the protein expression of nNOS, a factor that is critical in regulating the S-nitrosylation of proteins. Besides the effects of nNOS-derived endogenous NO, we investigated whether exogenous NO also influences the S-nitrosylation of MLK3. Hence, exogenous NO donors (SNP and GSNO) were administered to observe their effects on S-nitrosylation of MLK3 after 6 h of reperfusion. Subsequent Western blotting (Fig. 3, I and J) revealed that treatment with SNP and GSNO reduces the S-nitrosylation of MLK3.
Much evidence to date shows that brain ischemia-reperfusion promotes the assembly of NMDAR and nNOS with PSD-95. Thus, we administered an antagonist of NMDAR (MK801) to observe whether S-nitrosylation of MLK3 is also mediated by NMDAR. Our results clearly showed that the S-nitrosylation of MLK3 was suppressed by the treatment with MK801 (Fig. 3, I and J). These results suggest that the NMDAR/nNOS signaling module is likely to be involved in MLK3 S-nitrosylation, which is mediated mainly by nNOS but not iNOS.
S-Nitrosylation of MLK3 Promotes Its Activation and That of Its Downstream Signaling Kinases during Cerebral Ischemia-Reperfusion
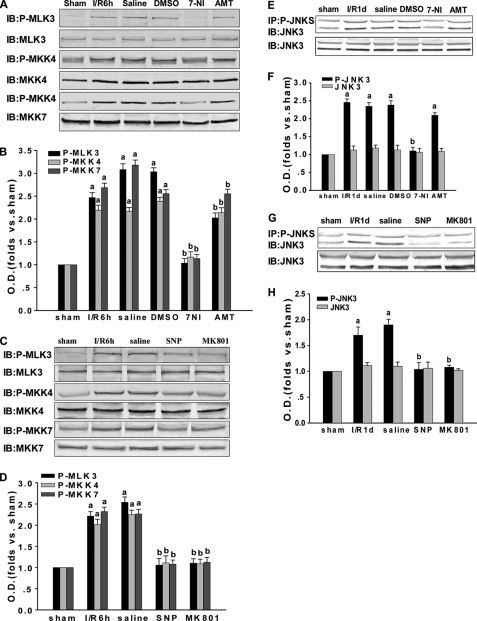
To investigate whether MLK3 downstream proteins were also affected by the S-nitrosylation of MLK3, we injected 7-NI, AMT, SNP, and MK801 into the animals and analyzed the phosphorylated downstream proteins in this pathway. Previous studies have indicated that the activation of JNK3 peaks initially at 30 min and again at 3 days during brain ischemia-reperfusion, and the latter peak is considered to account for apoptosis. Thus, the 1 day of reperfusion time point was chosen to investigate the activation of JNK3, on the basis that after 2–3 days of reperfusion cell death occurs for the hippocampal CA1 pyramidal neurons. As indicated in Fig. 4, A–H, 7-NI, SNP, and MK801 inhibit p-MLK3, p-MKK4, p-MKK7, and p-JNK3, whereas AMT showed no effect. The protein expression levels of MLK3, MKK4, MKK7, and JNK3 were unchanged by these treatments. These results suggest that the S-nitrosylation of MLK3 promotes its activation and affects the phosphorylation, and consequently the activation, of its downstream proteins.
FIGURE 4.
Effect of NOS inhibitors and exogenous NO and NMDAR antagonists on the MLK3-mediated downstream signaling pathway. Rats were treated with 7-NI, AMT, SNP, or MK801 to observe the effects of these molecules on the assembly of the MLK3·MKK4/7·JNK signaling module induced by transient brain ischemia followed by 6 h or 1 day of reperfusion in the rat hippocampal CA1. A and C, homogenized samples of the rat hippocampal CA1 region were examined by immunoblotting (IB) separately with anti-p-MLK3, anti-p-MKK4, anti-p-MKK7, anti-MLK3, anti-MKK4, and anti-MKK7 antibodies. E and G, sample proteins were examined by immunoprecipitation (IP) with anti-p-JNK antibodies followed by immunoblotting with an anti-JNK3 antibody or directly immunoblotted with the anti-JNK3 antibody. B, D, F, and H, bands were scanned, and the intensities represent the fold changes versus the sham control. Data are the means ± S.D. from four independent experiments. a, p < 0.05 versus sham treatment; b, p < 0.05 versus saline groups.
S-Nitrosylated MLK3 Influences Its Downstream JNK-mediated Nucleic Signaling Pathway during Cerebral Ischemia-Reperfusion
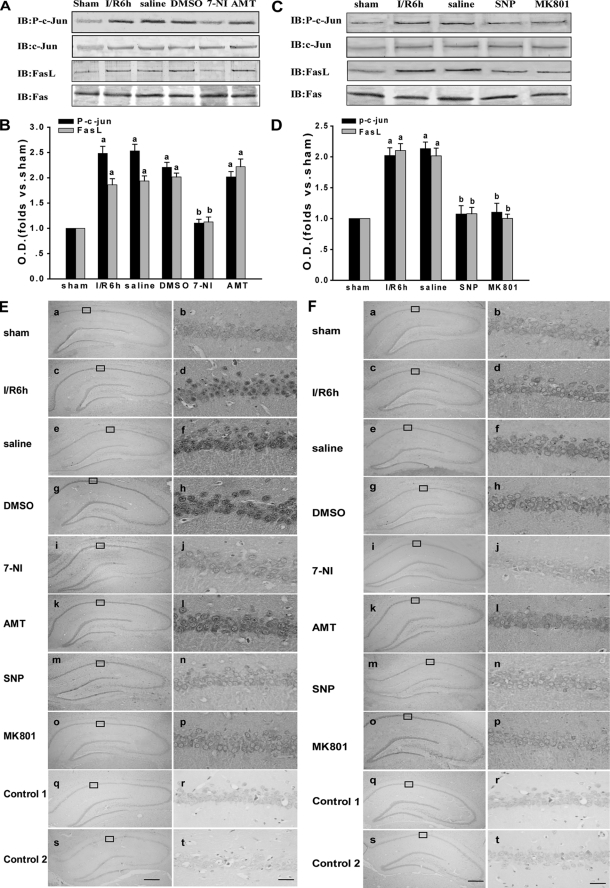
To further investigate whether JNK downstream proteins are also influenced by the S-nitrosylation of MLK3, we administered various targeting drugs. Because the activation of JNK can activate c-Jun and increase FasL expression, we tested the effects of 7-NI, AMT, SNP, and MK801 on these molecules. As shown in Fig. 5, A–D, p-c-Jun and FasL are suppressed by treatment with 7-NI, SNP, and MK801 but not by AMT treatment. Similar results were observed by immunohistochemistry (Fig. 5, E and F). The results demonstrate that the administration of 7-NI, SNP, and MK801 significantly decreases the immunoactivity of p-c-Jun and FasL in the nuclei of hippocampal CA1 pyramidal layer cells. This was in contrast to the 6-h reperfusion, saline, and DMSO treatment groups, where treatment with AMT did not show an obvious change in immunoactivity. Our results thus indicate that S-nitrosylated MLK3 regulates its downstream JNK-mediated nucleic signaling pathway.
FIGURE 5.
Effects of NOS inhibitors and exogenous NO and NMDAR antagonists on downstream JNK nuclear signaling pathway. A and C, rats were treated with 7-NI, AMT, SNP, or MK801 to observe the effects of these inhibitors on the JNK nuclear signaling pathway induced by transient brain ischemia followed by 6 h of reperfusion in the rat hippocampal CA1. Protein samples were separately examined by immunoblotting (IB) with anti-p-c-Jun, anti-c-Jun, anti-FasL, and anti-Fas. B and D, corresponding Western bands were scanned, and the intensities are represented as the fold changes versus sham treatment. Data are the means ± S.D. from four independent experiments. a, p < 0.05 versus the sham group; b, p < 0.05 versus saline groups. E and F, immunohistochemical analysis of the expression and subcellular localization of p-c-Jun and FasL after different treatments. Representative immunohistochemically stained sections are shown of hippocampi from sham-operated rats (panels a and b), rats subjected to 15 min of ischemia followed by 6 h of reperfusion (panels c and d), and rats subjected to 15 min of ischemia followed by 6 h of reperfusion and treated with saline (panels e and f), DMSO (panels g and h), 7-NI (panels i and j), AMT (panels k and l), SNP (panels m and n), or MK801 (panels o and p). Control only used primary antibody alone (panels q and r) or secondary antibody alone (panels s and t) in immunohistochemical analysis. Boxed areas in the left column are shown at a higher magnification than in the right column. The original magnification is ×40 in panels a, c, e, g, i, k, m, o, q, and s; scale bar, 300 μm; the original magnification is ×400 in panels b, d, f, h, j, l, n, p, r, and t; scale bar, 30 μm.
S-Nitrosylated MLK3 Influences Its Downstream JNK-mediated Mitochondrial signaling Pathway during Cerebral Ischemia-Reperfusion
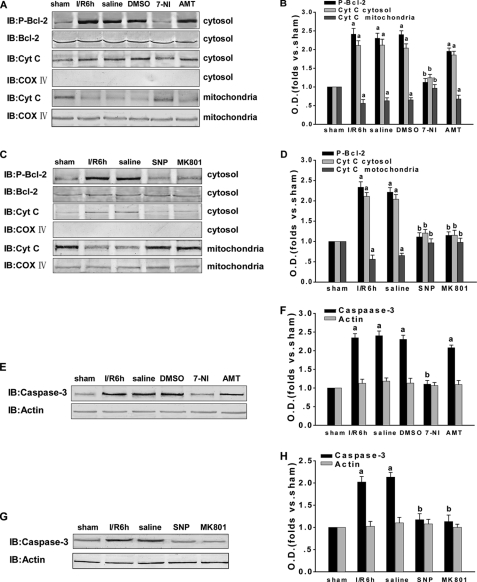
To determine whether JNK downstream proteins are also influenced by the S-nitrosylation of MLK3, we once again examined those proteins following the administration of various drugs. It is well known that, after translocation to the mitochondria, p-Bcl-2 increases the release of cytochrome c from the mitochondria to the cytosol. Hence, cytochrome c and this JNK non-nuclear substrate (Bcl-2) were examined to further confirm our earlier results. The cytochrome c oxidase subunit IV (COX IV) was detected to preclude the participation of other mitochondrial proteins. As shown in Fig. 6, A–D, the administration of 7-NI, SNP, and MK801 suppressed the phosphorylation of Bcl-2, whereas the treatment with AMT had little effect. Moreover, 7-NI, SNP, and MK801 each inhibited the release of cytochrome c to cytosol at 6 h after reperfusion. This contrasts with the marked increase in the levels of cytochrome c in the cytosol under the same conditions following AMT treatment. However, COX IV was found to exist only in the mitochondrial fraction but not in the cytosol. These results indicate that 7-NI, SNP, and MK801 all down-regulate the JNK pathway downstream of MLK3, which is probably caused by the reduced S-nitrosylation of MLK3.
FIGURE 6.
Effect of NOS inhibitors and exogenous NO and NMDAR antagonists on the JNK downstream mitochondrial signaling pathway. A, C, E, and G, rats were treated with 7-NI, AMT, SNP, or MK801 to observe their effects on the JNK downstream mitochondrial signaling pathway proteins and on the apoptotic response induced by transient brain ischemia followed by 6 h of reperfusion in the hippocampal CA1. Protein samples were separately examined by immunoblotting (IB) with anti-p-Bcl-2, anti-Bcl-2, anti-Cyt c, anti-COX IV, anti-caspase-3, and anti-actin antibodies. B, D, F, and H, Western bands were scanned, and the intensities are represented as the fold changes versus the sham treatment. Data are the means ± S.D. from four independent experiments. a, p < 0.05 versus the sham group; b, p < 0.05 versus the saline group.
Many reports have now demonstrated that the cell death pathway can be facilitated by either caspase-independent necrosis or caspase-dependent apoptosis, depending on the intensity of the excitotoxic injury. In our current experiments, we assayed cleaved caspase-3. As shown in Fig. 6, E–H, treatment with 7-NI, SNP, and MK801 attenuates the ischemia-induced increase in caspase-3 at 6 h of reperfusion and then exerts a neuroprotective effect against cerebral ischemia-reperfusion injury. These results suggest that S-nitrosylated MLK3 regulates the JNK-mediated mitochondrial signaling pathway that operates downstream.
Neuroprotective Effects of Drugs That Attenuate the S-Nitrosylation of MLK3 Caused by Ischemic Brain Injury in Vivo
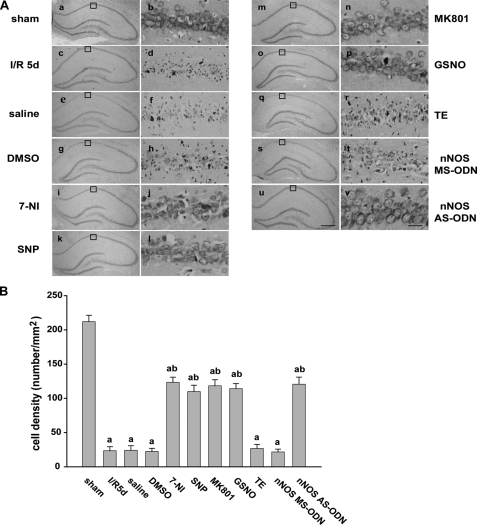
Our initial results showed that treatment with 7-NI, SNP, and MK801 attenuates the ischemia-induced increase of caspase-3 at 6 h of reperfusion. Cresyl violet staining was used to test the neuroprotective effects of the drugs that attenuate the S-nitrosylation of MLK3 caused by ischemic brain injury in vivo through the detection of survival of hippocampal CA1 pyramidal cells at 5 days after the induction of ischemia. Normal cells show rounded and weakly stained nuclei (Fig. 7A, panels a and b), whereas shrunken cells with pyknotic nuclei (Fig. 7A, panels c and d) were regarded as dead cells. As shown in Fig. 7, the sham group (Fig. 7A, panels a and b) showed normal nuclei, whereas dead cells in the ischemia-reperfusion and vehicle treatment group showed pyknotic nuclei (Fig. 7A, panels c and h). The administration of 7-NI (Fig. 7A, panels i and j), SNP (Fig. 7A, panels k and l), MK801 (Fig. 7A, panels m and n), or GSNO (Fig. 7A, panels o, and p) dramatically decreased neuronal degeneration. The group (Fig. 7A, panels u and v) treated with nNOS AS-ODN showed evidence of functional rescue from cell death, whereas the groups of animals treatment with nNOS MS-ODN (Fig. 7A, panels s and t) and TE buffer (Fig. 7A, panels q and r) showed no such rescue. The numbers of surviving pyramidal cells counted within a 1-mm length of the CA1 region were 212.1 ± 9.2, 23.4 ± 5.9, 24.1 ± 6.8, 22.3 ± 4.6, 123.4 ± 7.5, 109.8 ± 9.4, 118.3 ± 8.9, 114.2 ± 7.4, 26.7 ± 6.1, 21.6 ± 4.2, and 120.6 ± 10.4, respectively (Fig. 7B).
FIGURE 7.
Effect of NOS inhibitors and exogenous NO and NMDAR antagonists on the survival of CA1 pyramidal neurons. A, cresyl violet staining was performed on sections from the hippocampi of sham-operated rats (panels a and b) and of rats subjected to 5 days of reperfusion after global ischemia (panels c and d), and the administration of saline (panels e and f), DMSO (panels g and h), 7-NI (panels i and j), SNP (panels k and l), MK801 (panels m and n), GSNO (panels o and p), TE (panels q and r), MS-nNOS (panels s and t), and AS-nNOS (panels u and v) before or after ischemia. Cresyl violet staining data were obtained from six independent animals, and a typical experiment is presented. B, cell density was expressed as the number of cells per 1-mm length of the CA1 pyramidal cells counted under a light microscope. Data are the mean ± S.D. (n = 6). Scale bars, 300 μm (panels a, c, e, g, i, k, m, o, q, s, and u); 30 μm (panels b, d, f, h, j, l, n, p, r, t, and v). a, p < 0.05 versus the sham groups; b, p < 0.05 versus the saline groups.
DISCUSSION
We here report for the first time that MLK3, expressed in HEK293 cells, is S-nitrosylated (forming SNO-MLK3) by reaction with GSNO, an exogenous NO donor, at cysteine 688. This facilitates its dimerization and activation. Moreover, we further report herein that MLK3 can be S-nitrosylated during the early stages of reperfusion. This event likely enhances the activation of MLK3, which in turn activates the JNK signaling pathway. In an in vivo animal model of global ischemic/reperfusion, treatment with an nNOS inhibitor (7-NI) and exogenous NO donors (SNP and GSNO) can attenuate the S-nitrosylation of MLK3 induced by global ischemia/reperfusion. This subsequently inhibits the activation of the MLK3·MKK4/7·JNK3·c-Jun pathway, Bcl-2 phosphorylation, the expression of Fas-L, the release of cytochrome c from mitochondria, and the activation of caspase-3 during the early stages of reperfusion after global ischemia. Meanwhile, the administration of an inhibitor of iNOS (AMT) did not produce the above results, which suggests that the S-nitrosylation of MLK3 is associated with nNOS but not iNOS.
MLK3, a member of the MAPKKK family, phosphorylates mitogen-activated protein kinase kinase 4/7, which in turn phosphorylates JNK at its dual specific residues and activates this protein (15). Meanwhile, ASK1, as a similar kinase to MAPKKK, has been reported to undergo S-nitrosylation (26). At the same time, GluR6 (an upstream protein in the MLK3 pathway) and JNK3 (a downstream protein of MLK3) can also be S-nitrosylated during brain ischemia/reperfusion (27, 28). Based on these findings, we speculated that MLK3 might be S-nitrosylated under the pathophysiological conditions induced by ischemia-reperfusion. As shown as Figs. 1 and 3, we confirmed that MLK3, expressed in HEK293 cells or in vivo in rat, can be S-nitrosylated. The levels of S-nitrosylation were significantly elevated at 3, 6, and 12 h and at 1 day post-reperfusion and recovered at 2 days. These results suggest that the S-nitrosylation of MLK3 can be reversibly modified via the addition or removal of NO at the thiol moiety of reactive cysteine residues present in the MLK3 protein.
In our previous studies, MLK3 was found to be activated during the assembly of the GluR6·PSD-95·MLK3 signaling module under conditions of brain ischemia and reperfusion (16). The activation of MLK3, followed by homodimerization and the autophosphorylation of Thr-277 and Ser-281, was also found to associate with free radicals in the signaling events that were induced following brain ischemia (17–19). In this study, we report that MLK3 is S-nitrosylated (forming SNO-MLK3) via a reaction with GSNO or exogenous NO donors and that this contributes to its dimerization and activation. We next demonstrated that MLK3 can be S-nitrosylated during the early stages of reperfusion and that may enhance the activation of MLK3, which in turn activates the JNK signaling pathway.
MLK3 and other MLK kinases are characterized by the presence of multiple protein-protein interaction domains, including a tandem leucine/isoleucine zipper motif. Leucine zippers are known to mediate protein dimerization and function as the dimerization motifs for MLK3 (29). Furthermore, MLK3 dimerization via its leucine/isoleucine zipper motif is a prerequisite for its autophosphorylation, which is followed by activation of the JNK signaling pathway (9, 25, 29). In this study, we examined whether MLK3 S-nitrosylation affects the dimerization and phosphorylation of this protein. Indeed, we found that the time course of MLK3 dimerization and phosphorylation in HEK293 cells correlates with its S-nitrosylation at the Cys-688 residue. Our studies in rats further showed that after treatment with the inhibitor of nNOS (7-NI) and exogenous NO donors, a reduced S-nitrosylation corresponded to the phosphorylation of MLK3. This indicates that the S-nitrosylation of MLK3 likely provides a critical checkpoint for its activation. Our previous study has shown that JIP1 can maintain MLK3 in an inactive and unphosphorylated state. However, following the induction of excitotoxicity by ischemic reperfusion, a calcium influx through glutamate receptors occurs, especially through the NMDA receptor. This subsequently triggers an intracellular signaling cascade and induces JNK recruitment to JIP1, which induces MLK3 dissociation from JIP1 and accumulation at PSD-95, allowing MLK3 to dimerize and autophosphorylate (25). Here, our results present a possible mechanism in which MLK3 S-nitrosylation affects its dimerization and phosphorylation. However, whether the activation of nitrosylated MLK3 facilitates the dimerization of MLK3 via its leucine/isoleucine zipper motif and the transformation of its association with JIP1 requires further study.
It is known that both nNOS and iNOS are present in the brain. The most significant difference between them is that nNOS contributes to early neuronal injury, whereas iNOS is expressed mainly under pathological conditions, mediating late neuronal injury (31). The induction of iNOS in vitro results in delayed neuronal cell death and can also exacerbate glutamate excitotoxicity (32). As described previously, to examine nNOS during ischemic brain injury without interference from iNOS, the 6-h time point was adopted in our current experiments. We hypothesized that the inhibitor of iNOS (AMT) might not be involved in the regulation of protein S-nitrosylation during the early stages of transient ischemia-reperfusion in the rat brain but may play a critical role in the rescue of cell death after iNOS is induced by inflammatory mediators during the late stages of transient ischemia-reperfusion. As shown by all of the findings presented herein, AMT does not decrease the levels of S-nitrosylation and phosphorylation of MLK3 at the 6-h time point. However, the results of cresyl violet staining for AMT, which was used to verify the survival of hippocampal CA1 pyramidal cells (data not shown), indicate that AMT can inhibit cell death more efficiently than 7-NI, which is in agreement with previous results from in our laboratory (33). This result strongly suggests also that iNOS has no effect on the production of endogenous NO or on the activation of MLK3 induced by ischemia-reperfusion during the early stages of transient ischemia-reperfusion in the brain, but it has significant effects on the rescue from cell death in hippocampal CA1 cells after 5 days of reperfusion. This conclusion is based on the fact that iNOS is only detected under pathological inflammatory conditions. Taken together, we conclude from our current data and previous findings that the S-nitrosylation of MLK3 is mediated by nNOS through its production of endogenous NO.
In summary, the activation of MLK3 and its downstream proteins involved in apoptotic pathways is connected to the S-nitrosylation of MLK3 following ischemia-reperfusion in vivo. Similar studies have reported the activation of caspase-3 (34), Bcl-2 (35), and JNK (36, 37) in this context. Meanwhile, more studies (24, 30, 38) support the notion that S-nitrosylation, which is the modification of a cysteine thiol by a nitric oxide group, is an important post-translational modification for the function of various signaling proteins. As shown in this study, administration of inhibitors of nNOS and exogenous NO donors (SNP and GSNO) suppresses neuronal apoptosis by inhibiting the S-nitrosylation of MLK3 and subsequently decreasing the phosphorylation of MLK3, JNK, c-Jun, and Bcl-2. The results of this study thus provide new insights into the activation of MAPK and JNK signaling pathways and suggest a potential new therapeutic approach to the future treatment of transient cerebral ischemia injury.
This work was supported by Project of National Natural Science Foundation of China Grants 30870543 and 90608015, Natural Science Foundation of Jiangsu Grant BK2010171, Jiangsu Educational Science Foundation Grant 10KJA310053, and Xuzhou Medical College Science Foundation Grant 2010KJZ12.
- iNOS
- inducible NO synthase
- GSNO
- S-nitrosoglutathione
- 7-NI
- 7-nitroindazole
- nNOS
- neuronal NO synthase
- SNP
- sodium nitroprusside
- AS-ODN
- antisense oligodeoxynucleotide
- MS-ODN
- missense oligodeoxynucleotide
- AMT
- 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine
- NMDAR
- NMDA receptor
- MAPKK
- MAPK kinase
- MAPKKK
- MAPK kinase kinase
- NEM
- N-ethylmaleimide
- β-ME
- β-mercaptoethanol.
REFERENCES
- 1. Rahman M. A., Senga T., Ito S., Hyodo T., Hasegawa H., Hamaguchi M. (2010) S-Nitrosylation at cysteine 498 of c-Src tyrosine kinase regulates nitric oxide-mediated cell invasion. J. Biol. Chem. 285, 3806–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yasukawa T., Tokunaga E., Ota H., Sugita H., Martyn J. A., Kaneki M. (2005) s-Nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J. Biol. Chem. 280, 7511–7518 [DOI] [PubMed] [Google Scholar]
- 3. Derakhshan B., Hao G., Gross S. S. (2007) Balancing reactivity against selectivity. The evolution of protein S-nitrosylation as an effector of cell signaling by nitric oxide. Cardiovasc. Res. 75, 210–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lipton S. A., Rosenberg P. A. (1994) Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 330, 613–622 [DOI] [PubMed] [Google Scholar]
- 5. Zorumski C. F., Olney J. W. (1993) Excitotoxic neuronal damage and neuropsychiatric disorders. Pharmacol. Ther. 59, 145–162 [DOI] [PubMed] [Google Scholar]
- 6. Almeida A., Bolaños J. P., Medina J. M. (1999) Nitric oxide mediates glutamate-induced mitochondrial depolarization in rat cortical neurons. Brain Res. 816, 580–586 [DOI] [PubMed] [Google Scholar]
- 7. Choi D. W. (1995) Calcium. Still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 18, 58–60 [PubMed] [Google Scholar]
- 8. Lipton S. A., Nicotera P. (1998) Calcium, free radicals, and excitotoxins in neuronal apoptosis. Cell Calcium 23, 165–171 [DOI] [PubMed] [Google Scholar]
- 9. Samdani A. F., Dawson T. M., Dawson V. L. (1997) Nitric-oxide synthase in models of focal ischemia. Stroke 28, 1283–1288 [DOI] [PubMed] [Google Scholar]
- 10. Parratt J. R. (1998) Nitric oxide in sepsis and endotoxaemia. J. Antimicrob. Chemother. 41, 31–39 [DOI] [PubMed] [Google Scholar]
- 11. Christopherson K. S., Hillier B. J., Lim W. A., Bredt D. S. (1999) PSD-95 assembles a ternary complex with the N-methyl-d-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J. Biol. Chem. 274, 27467–27473 [DOI] [PubMed] [Google Scholar]
- 12. Yuan Z. Q., Feldman R. I., Sussman G. E., Coppola D., Nicosia S. V., Cheng J. Q. (2003) AKT2 inhibition of cisplatin-induced JNK/p38 and Bax activation by phosphorylation of ASK1. Implication of AKT2 in chemoresistance. J. Biol. Chem. 278, 23432–23440 [DOI] [PubMed] [Google Scholar]
- 13. Simpson L., Parsons R. (2001) PTEN. Life as a tumor suppressor. Exp. Cell Res. 264, 29–41 [DOI] [PubMed] [Google Scholar]
- 14. Vazquez F., Sellers W. R. (2000) The PTEN tumor suppressor protein. An antagonist of phosphoinositide 3-kinase signaling. Biochim. Biophys. Acta 1470, M21–M35 [DOI] [PubMed] [Google Scholar]
- 15. Gallo K. A., Mark M. R., Scadden D. T., Wang Z., Gu Q., Godowski P. J. (1994) Identification and characterization of SPRK, a novel Src-homology 3 domain-containing proline-rich kinase with serine/threonine kinase activity. J. Biol. Chem. 269, 15092–15100 [PubMed] [Google Scholar]
- 16. Pei D. S., Sun Y. F., Guan Q. H., Hao Z. B., Xu T. L., Zhang G. Y. (2004) Postsynaptic density protein 95 antisense oligodeoxynucleotides inhibits the activation of MLK3 and JNK3 via the GluR6.PSD-95.MLK3 signaling module after transient cerebral ischemia in rat hippocampus. Neurosci. Lett. 367, 71–75 [DOI] [PubMed] [Google Scholar]
- 17. Gallo K. A., Johnson G. L. (2002) Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell Biol. 3, 663–672 [DOI] [PubMed] [Google Scholar]
- 18. Teramoto H., Coso O. A., Miyata H., Igishi T., Miki T., Gutkind J. S. (1996) Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J. Biol. Chem. 271, 27225–27228 [DOI] [PubMed] [Google Scholar]
- 19. Tian H., Zhang Q., Li H., Zhang G. (2003) Antioxidant N-acetylcysteine and AMPA/KA receptor antagonist DNQX inhibited mixed lineage kinase-3 activation following cerebral ischemia in rat hippocampus. Neurosci. Res. 47, 47–53 [DOI] [PubMed] [Google Scholar]
- 20. Gu Z., Jiang Q., Zhang G. (2001) c-Jun N-terminal kinase activation in hippocampal CA1 region was involved in ischemic injury. Neuroreport 12, 897–900 [DOI] [PubMed] [Google Scholar]
- 21. Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 22. Pulsinelli W. A., Brierley J. B. (1979) A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke 10, 267–272 [DOI] [PubMed] [Google Scholar]
- 23. Jaffrey S. R., Erdjument-Bromage H., Ferris C. D., Tempst P., Snyder S. H. (2001) Protein S-nitrosylation. A physiological signal for neuronal nitric oxide. Nat. Cell Biol. 3, 193–197 [DOI] [PubMed] [Google Scholar]
- 24. Pei D. S., Sun Y. F., Song Y. J. (2009) S-Nitrosylation of PTEN Involved in ischemic brain injury in rat hippocampal CA1 region. Neurochem. Res. 34, 1507–1512 [DOI] [PubMed] [Google Scholar]
- 25. Zhang Q. X., Pei D. S., Guan Q. H., Sun Y. F., Liu X. M., Zhang G. Y. (2007) Cross-talk between PSD-95 and JIP1-mediated signaling modules. The mechanism of MLK3 activation in cerebral ischemia. Biochemistry 46, 4006–4016 [DOI] [PubMed] [Google Scholar]
- 26. Park H. S., Yu J. W., Cho J. H., Kim M. S., Huh S. H., Ryoo K., Choi E. J. (2004) Inhibition of apoptosis signal-regulating kinase 1 by nitric oxide through a thiol redox mechanism. J. Biol. Chem. 279, 7584–7590 [DOI] [PubMed] [Google Scholar]
- 27. Yu H. M., Xu J., Li C., Zhou C., Zhang F., Han D., Zhang G. Y. (2008) Coupling between neuronal nitric-oxide synthase and glutamate receptor 6-mediated c-Jun N-terminal kinase signaling pathway via S-nitrosylation contributes to ischemia neuronal death. Neuroscience 155, 1120–1132 [DOI] [PubMed] [Google Scholar]
- 28. Stamler J. S., Lamas S., Fang F. C. (2001) Nitrosylation. The prototypic redox-based signaling mechanism. Cell 106, 675–683 [DOI] [PubMed] [Google Scholar]
- 29. Leung I. W., Lassam N. (1998) Dimerization via tandem leucine zippers is essential for the activation of the mitogen-activated protein kinase kinase kinase, MLK-3. J. Biol. Chem. 273, 32408–32415 [DOI] [PubMed] [Google Scholar]
- 30. Pi X., Wu Y., Ferguson J. E., 3rd, Portbury A. L., Patterson C. (2009) SDF-1α stimulates JNK3 activity via eNOS-dependent nitrosylation of MKP7 to enhance endothelial migration. Proc. Natl. Acad. Sci. U.S.A. 106, 5675–5680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boje K. M., Arora P. K. (1992) Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 587, 250–256 [DOI] [PubMed] [Google Scholar]
- 32. Hewett S. J., Csernansky C. A., Choi D. W. (1994) Selective potentiation of NMDA-induced neuronal injury following induction of astrocytic iNOS. Neuron 13, 487–494 [DOI] [PubMed] [Google Scholar]
- 33. Song Y. J., Zhang G. Y. (2005) 7-NI and AMT diminish ischaemic brain injury of rat. Acta Acad. Med. Xuzhou 25, 385–386 [Google Scholar]
- 34. Tenneti L., D'Emilia D. M., Lipton S. A. (1997) Suppression of neuronal apoptosis by S-nitrosylation of caspases. Neurosci. Lett. 236, 139–142 [DOI] [PubMed] [Google Scholar]
- 35. Azad N., Vallyathan V., Wang L., Tantishaiyakul V., Stehlik C., Leonard S. S., Rojanasakul Y. (2006) S-Nitrosylation of Bcl-2 inhibits its ubiquitin-proteasomal degradation. A novel antiapoptotic mechanism that suppresses apoptosis. J. Biol. Chem. 281, 34124–34134 [DOI] [PubMed] [Google Scholar]
- 36. Pei D. S., Song Y. J., Yu H. M., Hu W. W., Du Y., Zhang G. Y. (2008) Exogenous nitric oxide negatively regulates c-Jun N-terminal kinase activation via inhibiting endogenous NO-induced S-nitrosylation during cerebral ischemia and reperfusion in rat hippocampus. J. Neurochem. 106, 1952–1963 [DOI] [PubMed] [Google Scholar]
- 37. Park H. S., Huh S. H., Kim M. S., Lee S. H., Choi E. J. (2000) Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proc. Natl. Acad. Sci. U.S.A. 97, 14382–14387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsang A. H., Lee Y. I., Ko H. S., Savitt J. M., Pletnikova O., Troncoso J. C., Dawson V. L., Dawson T. M., Chung K. K. (2009) S-Nitrosylation of XIAP compromises neuronal survival in Parkinson's disease. Proc. Natl. Acad. Sci. U.S.A. 106, 4900–4905 [DOI] [PMC free article] [PubMed] [Google Scholar]