Background: The intracellular accumulation of compatible osmolytes in hypertonic conditions reduces macromolecular crowding and ionic strength.
Results: Compatible osmolytes disassemble mRNA stress granules. Hypertonic-preconditioning and gap-junction communication favor cell survival via compatible osmolyte accumulation.
Conclusion: Macromolecular crowding regulates stress granule assembly and, thus, the cell fate after osmotic stress.
Significance: Compatible osmolytes can promote cell survival through their action on stress granules.
Keywords: Biophysics, Cell Biology, Kidney, Renal Physiology, RNA Processing, Betaine, Compatible Osmolytes, Hypertonicity, Macromolecular Crowding, Stress Granules
Abstract
The massive uptake of compatible osmolytes such as betaine, taurine, and myo-inositol is a protective response shared by all eukaryotes exposed to hypertonic stress. Their accumulation results mostly from the expression of specific transporters triggered by the transcriptional factor NFAT5/TonEBP. This allows the recovery of the cell volume without increasing intracellular ionic strength. In this study we consider the assembly and dissociation of mRNA stress granules (SGs) in hypertonic-stressed cells and the role of compatible osmolytes. In agreement with in vitro results obtained on isolated mRNAs, both macromolecular crowding and a high ionic strength favor the assembly of SGs in normal rat kidney epithelial cells. However, after hours of constant hypertonicity, the slow accumulation in the cytoplasm of compatible osmolytes via specific transporters both reduces macromolecular crowding and ionic strength, thus leading to the progressive dissociation of SGs. In line with this, when cells are exposed to hypertonicity to accumulate a large amount of compatible osmolytes, the formation of SGs is severely impaired, and cells increase their chances of survival to another hypertonic episode. Altogether, these results indicate that the impact of compatible osmolytes on the mRNA-associated machineries and especially that associated with SGs may play an important role in cell resistance and adaption to hyperosmolarity in many tissues like kidney and liver.
Introduction
After hypertonic exposure, the immediate response of mammalian cells relies on changes in membrane ion channel and transporter activity (1), water efflux, and remodeling of the cell cytoskeleton. Such a cascade of events leads to a decrease of cell volume and increase in intracellular ionic strength (2–5). The accumulation of ions then produces an inverse osmosis-driven water movement that allows a partial cell volume recovery. This situation is, however, temporary as cells cannot sustain a high intracellular ionic strength for long as it affects most enzymatic reactions. In mammalian cells long term protection and adaptation to hypertonicity is triggered by translocation of NFAT5 (or TonEBP) (6, 7), a transcription factor that activates genes leading to the synthesis of neutral compatible osmolytes (sorbitol (8), glycerophosphorylcholine (9)) or their active uptake from the extracellular medium (betaine (10), taurine (11), and myo-inositol (12)). As a high intracellular ionic strength enhances the NFAT5 activity (13), the consecutive accumulation of these neutral organic compounds in place of the inorganic ions allows the reduction of the intracellular ionic strength while equilibrating the extracellular/intracellular osmotic balance. Nevertheless, the time required to complete such a massive accumulation is long, about 12–24 h (14–16) whatever the nature of the mammalian cell involved. Meantime, the intracellular environment is not optimal to keep the cellular machineries going (3, 17). For DNA-associated processes, the appearance of DNA breakages after hypertonic shock has been particularly under focus as well as the impairment of the DNA repair machineries. Surprisingly, when kidney epithelial cells become adapted to long term exposures to high salinity, DNA breakages persist even though compatible osmolytes have been accumulated (18).
Regarding mRNA-associated processes that are investigated in this study, the presence of compatible osmolytes in the cytoplasm may have a profound impact, as observed in cell-free protein synthesis systems (19). In cells, an acute hypertonic shock rapidly leads to a translational arrest (20–22) and thus to the formation of stalled preinitiation complexes composed of mRNAs and multiple protein partners like initiation factors. The subsequent assembly of these complexes finally leads to the formation of stress granules (SGs)2 in the cell cytoplasm (22–24). This assembly is probably promoted by the shuttling from the nucleus to the cytoplasm of some mRNA-binding proteins like TIA-1 (25), as observed for stresses mediated by arsenite, hypoxia, and hyperthermia (26). However, there are no data regarding the role of compatible osmolyte accumulation on SG assembly/disassembly, which might be critical for the recovery and the protection of the mRNA-associated machineries under chronic hypertonic exposure.
In this study we first analyzed by atomic force microscopy (AFM) the importance of macromolecular crowding (27–29) and ionic strength. Both are theoretically expected to promote self-attraction between liked-charged surfaces (30–33) and, thus, the association of anionic mRNAs into SGs or, as the control, anionic microtubules (MTs) into bundles (34), a prediction confirmed here by the results. Indeed, using MT bundling as an indicator of exacerbated excluded volume interactions (30) in the cell cytoplasm, the results show that macromolecular crowding and ionic strength are critical for the formation of mRNA granules in hypertonic-stressed normal rat kidney cells (NRK). We then analyzed whether the uptake of compatible osmolytes like betaine, taurine, and myo-inositol, which reduces excluded volume interactions, could lead to SG disassembly. In addition, in cells preconditioned to hypertonicity, we investigated the putative impact of compatible osmolyte accumulation on SG assembly and, as a consequence, their resistance to osmotic challenge. In light of the results, we propose that the maintenance of cell homeostasis, thanks to compatible osmolytes, may be of particular importance in preventing the formation of SGs and maintaining the mRNA-associated machineries under chronic hypertonicity, with possible applications in many tissues. The kidney and especially the inner medulla are known to be exposed to varying hyperosmolarity (35), but among other examples, the liver, which responds to hydration changes by altering cell volume (36), and the cornea of dry eye (37) are also potentially exposed to varying osmolarity.
MATERIALS AND METHODS
Cell Culture
NRK (proximal tubule)-52E cells (ATCC, Manassas, VA) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% (v/v) fetal bovine serum (FBS), 2 mm l-glutamine, and 1% antibiotics (penicillin and streptomycin) in a humidified 5% CO2 atmosphere at 37 °C. The osmolality of the DMEM with 5% (v/v) FBS is considered to be 320 mosmol/kg (referred here as isotonic). The hypertonicity of the medium was adjusted by adding NaCl to the osmolalities as indicated on the figures. Cells were counted using a Malassez hemocytometer. The number of living cells was estimated by trypan blue exclusion.
Atomic Force Microscopy
mRNAs and MTs were imaged by AFM under macromolecular crowding conditions as previously described (32). Briefly, 5 μl of solutions containing mRNA molecules (2 nm) or taxol-stabilized MTs (20 μm tubulin, 5 μm taxol) in 20 mm Tris-HCl, pH 7.5, and different polyethylene glycol (PEG) and KCl concentrations were deposited onto NiCl2 pretreated mica for 1 min. Samples were then thoroughly rinsed with 0.02% uranyl acetate. Finally, the mica was dried with a filter paper. AFM imaging was carried out using a Nanoscope IIIa AFM (Veeco Instrument) in tapping mode with silicon cantilevers AC160TS (Olympus). The scan frequency was typically 1 Hz per line, and the modulation amplitude was of about a few nanometers.
Tubulin Preparation
Tubulin was purified from sheep brain crude extracts. Before use, a cycle of tubulin polymerization was performed in 50 mm MES-KOH, pH 6.8, 0.5 mm dithiothreitol, 0.5 mm EGTA, 0.5 mm EDTA, 6 mm MgCl2, 0.6 mm GTP, 30% glycerol. MTs were sedimented by centrifugation (52,000 × g, 30 min at 37 °C), at the end of which the MT pellet was resuspended in 25 mm MES-KOH, pH 6.8, 0.5 mm EGTA, 1 mm DTT, and disassembled at 4 °C for 20 min. Tubulin aggregates were finally eliminated by a further centrifugation at 4 °C (52,000 × g, 20 min). Tubulin concentration was determined by spectrophotometry using an extinction coefficient of 1.2 mg−1 × cm2 at 278 nm.
Synthesis of mRNA
Plasmid pSP72–2Luc was used as a template for synthesis by T7 polymerase of 2Luc mRNAs (3000 nucleotides). After transcription, unincorporated NTPs were removed by gel filtration through a NAP-5 column (GE Healthcare), and mRNAs were further isolated with RNAble (Eurobio) following the manufacturer's recommendation.
Quantification of Betaine by Solution NMR Spectroscopy
NRK cells were grown to confluency and exposed to hypertonicity (uptake) or to isotonicity (efflux) under conditions specified in the figure captions. Cells were rapidly washed with adjusted PBS to maintain a constant osmolarity and avoid the release of organic osmolytes. Cells were then scraped off in 200 μl of PBS and analyzed by one-dimensional 1H NMR spectroscopy without further treatment. A 10-μl aliquot was used to quantify by hemacytometry the number of cells analyzed by NMR. NMR spectra were acquired at 20 °C on a Bruker Avance 600 spectrometer. All experiments were performed on 60-μl samples with a MATCH system (Cortecnet, Paris, France). The water signal was suppressed using the Watergate method. Each spectrum was obtained after 64 scans. Spectra were processed with Topspin 2.0 (Bruker). To have statistically relevant results, three different samples were used for each condition. 2,2-Dimethyl-2-silapentane-5-sulfonic acid was added as an external reference in pure D20. The area of betaine at 3.25 ppm peak was calculated relative to this normalized area and then divided by the number of cells (arbitrary units (a.u.); 1 a.u. corresponds to 72 fmol of betaine per NRK cell for all figures).
Immunofluorescence
NRK cells grown on plastic dishes were fixed with 4% paraformaldehyde in PBS, 200 mm sucrose for 20 min at 37 °C. After fixation, cells were then washed and incubated for 1 h with mouse monoclonal anti-tubulin antibody E7 (1:2000 dilution), a mouse anti-HuR antibody (Molecular Probes, 10 μg/ml), and a rabbit anti-YB-1 (produced as described in Davydova et al. (38)). Cells were washed extensively in PBS and incubated for 1 h with fluorochrome (Alexa Fluor®488 and -555)-coupled secondary antibodies (Invitrogen) in blocking solution. For statistics, the mean granule area was measured using ImageJ over at least 500 granules coming from at least 10 different representative cells.
Immunoblotting
Cells were lysed in 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.1% Triton X-100, 1 mm EDTA, and protease inhibitor mixture (Roche Applied Science). Lysates were centrifuged at 14,000 × g for 15 min at 4 °C, and supernatants were collected. Proteins were separated on 12% SDS-PAGE gels and transferred onto a PVDF membrane (Invitrogen). The membranes were blocked in 5% (w/v) nonfat dried milk, PBS for 30 min at room temperature (20 °C) and incubated for 1 h at room temperature with primary antibodies (anti-phospho-eIF2α antibodies (Cell Signaling, 1:2000 dilution) and anti-GAPDH (Abcam, 1:5000). Bound antibodies were detected and quantified using anti-rabbit-IRDye 800 or anti-mouse-IRDye 680 secondary antibodies (Odyssey, 1:5000 dilution) with an Odyssey imaging system (LI-COR Biosciences).
Plasmid Construction and Transfection
The cDNA encoding the full-length YB-1 was amplified by PCR and cloned into the XhoI and BamH1 sites of the pEGFP-C3 vector (Clontech). PCR-amplified products were then sequenced. NRK cells were transfected with plasmid DNA by using the NucleofectorTM technology.
RESULTS
Excluded Volume Interactions Mediated by Macromolecular Crowding and High Ionic Strength Trigger mRNA and MT Self-association
Theoretical Prerequisites
All macromolecular complexes in the cell cytoplasm are a priori sensitive to exacerbated excluded volume interactions (39), which is an important aspect of macromolecular crowding. However, in the case of hypertonicity, charged macromolecules are likely to be more sensitive due to the uptake of inorganic ions (K+, Na+, Cl−), which neutralize charged surfaces and screen electrostatic repulsion. Consequently, in contrast with oppositely charged biomacromolecules, excluded volume interactions between two like-charged biomacromolecules are promoted by high ionic strength (31). Among these, mRNA and MTs may be particularly sensitive because of their highly negatively charged surface. The expected results of a self-attraction mediated by excluded volume interactions are the formation of mRNA granules and MT bundles, respectively.
High Ionic Strength and Macromolecular Crowding Promote mRNA Granule Formation and MT Bundling in Vitro
To illustrate these theoretical predictions, we analyzed by AFM in vitro formation of MT bundles and mRNA granules under macromolecular crowding condition using PEG, a neutral polymer, to mimic macromolecular crowding (Fig. 1A). We found that high molecular weight PEG Mr 35,000 (PEG 35K) induced mRNA aggregation and MT bundling, whereas PEG Mr 1000, which is not large enough to trigger macromolecular crowding-induced attraction, was inefficient. Macromolecular crowding can then induce MT bundling and mRNA granule formation via excluded volume interactions. In addition, as stated above, ionic strength is also an important parameter that promotes self-attraction of like-charged macromolecules. Accordingly, we observed that at PEG 35K concentrations below the threshold values for mRNA granule assembly and MT bundling, respectively, increasing KCl concentration leads to the appearance of mRNA granules and MT bundles (Fig. 1B). In contrast, the addition of betaine to obtain a similar osmolality without increasing the ionic strength fails to induce mRNA aggregation and MT bundling.
FIGURE 1.
High resolution AFM imaging reveals mRNA assembly into granules and MT bundling under macromolecular crowding environment. A, at moderate ionic strength (20 mm Tris-HCl, pH 7.4, 50 mm KCl), 1% PEG 35K (w/v), a crowding agent, triggers the assembly of MTs into thick bundles, and 20% PEG 35K triggers that of 2Luc mRNA into granules. In contrast, PEG 1K was unable to induce mRNA self-assembly, as expected for excluded volume interactions because of its small size. Incubation time, 30 min. Scale bars, 2.5 μm (AFM images of MTs) and 1 μm (AFM images of mRNAs). B, with 0.5 and 15% PEG 35K at moderate ionic strength, no MT bundles or mRNA aggregates were detected, respectively. However, increasing KCl concentration led to the progressive appearance of MT bundles or mRNA granules. When betaine, a neutral osmolyte, was used instead of KCl to increase the osmolality without increasing the ionic strength, no mRNA granules or MT bundles were observed. Incubation time, 30 min. Scale bars, 2.5 μm (AFM images of MTs) and 1 μm (AFM image of mRNAs).
In addition to these results, we can remark that MT bundling occurs at very low PEG 35K concentrations (∼1% w/v) compared with that required for mRNA granule formation (∼20%). This could be due to the large interacting surface between MTs in bundles (30) compared with that of mRNAs and the heterogeneous charge distribution of MTs with some positive charge on the MT body (40) and a highly negatively charged C-terminal tail that facilitates short-ranged electrostatic attraction.
We may then wonder why MTs do not naturally form bundles in the cell cytoplasm, which is naturally crowded with macromolecules (27–29). In fact, MT diffusion in the cell cytoplasm is hindered by obstacles like actin filaments (41), which can slow down the bundling rate (42), and MTs are highly dynamical polymers, which reduces their chance to form bundles because of their limited lifetime. In line with this, taxol, an agent that stabilizes MTs, promotes the formation of MT bundles in cells (43). Interestingly, hypertonicity also favors the formation of MT bundles in taxol-treated cells, whereas hypotonicity inhibits their formation (supplemental Fig. S1).
Regarding the high PEG 35K concentration required to form mRNA granules in vitro, we should note that multiple mRNA protein partners are associated with mRNAs in SGs, like the prion-like protein TIA-1, which may act in combination with macromolecular crowding to favor the formation of SGs in cells. For example, when YB-1, a major mRNA-binding protein (44), is mixed with mRNA to form ribonucleoprotein particles, the PEG concentration required to trigger in vitro the compaction is lower than that needed for naked mRNAs (supplemental Fig. S2).
Hypertonicity Promotes SG Assembly and MT Bundling in NRK Cells
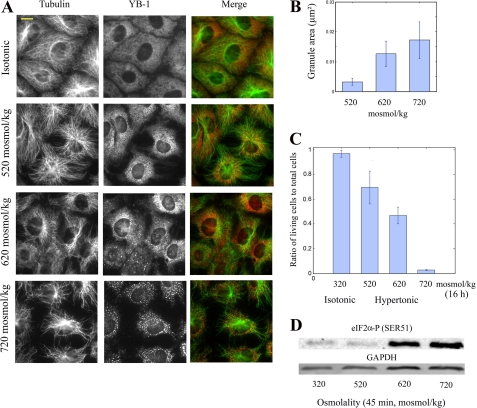
Given the AFM results, we explored how hypertonicity leads to the formation of SGs in NRK cells and whether MT bundling occurs simultaneously. To detect the formation of SGs, we used YB-1, which is an interesting marker to track the formation of SGs because of its cytoplasmic location and its binding to mRNA before and after stress (45). In control experiments (supplemental Fig. S3) we confirmed that YB1 is indeed present in SGs and co-localizes with other SGs markers like HuR (46). SGs were detected for NaCl- and sucrose-mediated hypertonic shocks but not with molecules that cross the cell membrane like urea (Fig. 2), thus indicating that these granules were truly of osmotic origin. The formation of YB-1-containing SGs was observed above 520 mosmol/kg (Fig. 3A). These granules shared the typical properties of other SGs related to oxidative stress or hyperthermia (47). Cycloheximide, a polysome stabilizer, prevents their formation (supplemental Fig. S4), whereas puromycin, which induces premature chain termination during translation, has no effect on the assembly mechanism (data not shown). The extracellular osmolality modulates the size of SGs, which significantly increases from 520 to 720 mosmol/kg (Fig. 3B). A similar pattern was obtained for MT bundling, which starts to be significant above 620 mosmol/kg. This suggests that a similar mechanism may be involved in these two processes, i.e. excluded volume interactions. To investigate whether the appearance of SGs could provide clues to the cell fate, we measured the rate of cell survival after 6 h of hypertonicity and found a sharp decrease of cell viability at osmolalities higher than 520 mosmol/kg (Fig. 3C). In addition, we noticed that a significant phosphorylation of eIF2α, which was reported to promote apoptosis upon hypertonic stress (22), also occurs at a critical osmolality of 620 mosmol/kg (Fig. 3D).
FIGURE 2.
NaCl and sucrose induce SG assembly and MT bundling in NRK cells in contrast with membrane-permeable molecules like urea. NRK cells labeled with anti-YB-1 were exposed to a hypertonic environment (720 mosmol/kg), in which osmolality has been adjusted with urea, NaCl, or sucrose. Scale bar, 15 μm.
FIGURE 3.
Hypertonicity induces SG assembly and MT bundling in cells. A, NRK cells were incubated at the indicated osmolalities for 45 min and then fixed for immunostaining. Anti-YB-1 labeling of NRK cells reveals at 620 mosmol/kg the formation of SGs, which appear even larger at 720 mosmol/kg. Similarly, anti-tubulin labeling indicates a significant tendency for MTs to assemble into bundles, which is especially pronounced at 720 mosmol/kg. Scale bar, 10 μm. B, mean granule area was obtained from A. The results indicate that SG assembly is promoted by increasing the extracellular osmolality above 520 mosmol/kg. Results are the mean ± S.D. (see “Materials and Methods”). C, the rate of cell survival after 16 h of hypertonicity was measured using a hemacytometer with viability determined by trypan blue exclusion. Above 620 mosmol/kg, cells poorly survive to hypertonicity. Results are the mean ± S.D. D, Western blotting of NRK cell extracts show the phosphorylation of the initiation factor eIF2α, triggered after hypertonic treatment for 45 min.
Extracellular Osmolality Modulates Assembly of SGs Triggered by Oxidative Stress
Albeit there is a concomitant appearance of MT bundles and SGs, this does not signify that macromolecular crowding and high ionic strength drive the forces behind SG assembly. To further address this issue, NRK cells were incubated in media of osmolality ranging from pronounced hypotonicity (170 mosmol/kg) to high hypertonicity (620 mosmol/kg) for 45 min. The cells were then exposed to varying concentrations of arsenite for 45 min to trigger the formation of SGs via oxidative stress (25). Arsenite, which is probably taken up in NRK cells via aquaglyceroporins (48), induces the rapid formation of SGs under isotonic conditions (45) at lethal doses (LD50 about 100–300 μm for 1–2 h exposition in hepatoma cells and mouse fibroblasts (49, 50)). As shown in Fig. 4A, increasing the extracellular osmolality promotes the formation of arsenite-mediated SGs. On the other hand, hypotonicity inhibits their assembly. We may, however, wonder if hypotonic conditions impair the formation of SGs through other effects than decreasing macromolecular crowding and lowering ionic strength, for instance, by preventing the shuttling of critical proteins from cell nucleus to cytoplasm. To explore this idea, we first treated cells with arsenite to trigger the shuttling of the critical proteins and the formation of SGs and then exposed cells to varying osmolalities for 45 min before observation. Here again hypotonicity promotes SG dissociation, whereas hypertonicity preserves SG assembly (Fig. 4B). In addition, for cells first exposed to arsenite under hypotonic conditions, which does not lead to SG assembly, increasing the extracellular osmolality leads to their formation even in the absence of arsenite. Then the extracellular osmolality modulates the ability of oxidative stress agents to form SGs, most probably via its influence on both macromolecular crowding and the intracellular ionic strength. To finish, SGs and MT bundles formed under acute hypertonicity disappeared within minutes upon return to isotonicity (Fig. 4C), thus indicating that excluded volume interactions are mandatory to preserve SG assembly.
FIGURE 4.
The extracellular osmolality regulates the assembly of SGs in arsenite-stressed cells. A, NRK cells were pretreated at the indicated osmolalities for 45 min before the addition of arsenite for another 45 min. SGs were detected via anti-YB1 labeling. We observed that hypertonicity favors SG assembly in contrast to hypotonic conditions. Scale bar, 15 μm. Statistical analysis clearly indicates a positive correlation between SG formation and extracellular osmolality. B, NRK cells were first pretreated with arsenite for 45 min in iso- or hypotonic conditions and then exposed to various osmolalities in the absence of arsenite. Hypotonic exposure dissociates SGs, in contrast with hypertonicity, which preserves preformed SGs or can make them appear (pretreatment under hypotonic environment). Insets, NRK cells fixed at the end of the 45 min arsenite pretreatment. We observed the presence of SGs in iso- but not hypotonic environment. SGs were detected via anti YB1-labeling. Scale bar, 15 μm. C, NRK cells displayed a homogenous distribution of MTs and YB1 protein under isotonic conditions. SGs and MT bundles appeared after 45 min of hypertonic treatment, and interestingly, upon return to isotonicity for 5 min, both SGs and MT bundles were dissociated. SGs and MTs were observed via anti-YB1 and anti-tubulin staining, respectively. Scale bar, 15 μm.
The Accumulation of Compatible Osmolytes Is Required for Dissociation of SGs
When cells grown in isotonic conditions are exposed to hypertonicity, the first SGs appear after about 15 min of hypertonicity (supplemental Fig. S5). Such a time lapse is required for critical proteins like TIA-1 to shuttle from the cell nucleus to the cytoplasm and promote SG assembly (25). In addition, the aggregation of mRNA-containing particles into large SGs occurs at a slow rate due to hindered diffusion in the cytoplasm (45). For hypertonic stress, the SGs are rather homogeneously distributed in the cytoplasm, and in contrast with arsenite-mediated SGs (45), their size and localization weakly depended on the presence of MTs (supplemental Fig. S6), probably a consequence of MT bundling.
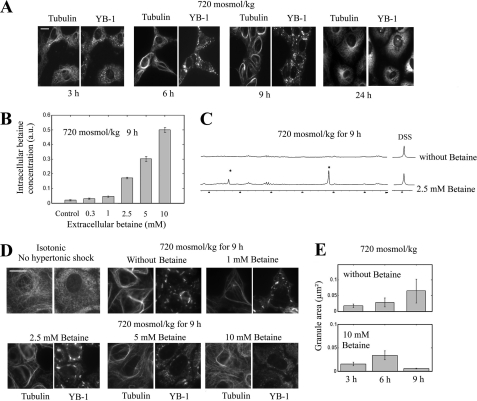
In contrast with SG assembly, SG dissociation under constant hypertonicity is long and occurs between 9 and 24 h in the absence of extracellular osmolytes (Fig. 5A). Such a long time may be linked to the slow accumulation of synthesized compatible osmolytes, sorbitol and GPC, which requires between 12 to 24 h to complete (8, 51). To test whether the accumulation of compatible osmolytes is rate-limiting in the dissociation process, we examined the time-course of SG dissociation in the presence of extracellular compatible osmolytes (here betaine). In parallel, we measured the intracellular content of betaine to estimate its accumulation in NRK cells. We found that the betaine uptake may be accelerated in the presence of a high extracellular concentration of betaine, as detected by NMR after 9 h (Fig. 5, B and C). Indeed there are both high affinity (Km = 0.12 mm) and low affinity (Km = 5.6 mm) betaine transport sites (52); the latter may then explain the accelerated accumulation. In this case the disassembly of SGs indeed occurs significantly earlier (Fig. 5, D and E). Here again, MT bundling follows the same trend, as thick MT bundles were observed in the absence of betaine after 9 h of hypertonicity, whereas these bundles were already dissociated with 10 mm betaine. Altogether, these results indicate that compatible osmolytes play a key role in the disassembly of SGs and MT bundles. Interestingly, a high extracellular betaine concentration (in the millimolar range) was already shown to have a protective effect to hypertonicity (53, 54).
FIGURE 5.
The accumulation of compatible osmolytes after hypertonic exposure promotes SG disassembly. A, shown is time-lapse immunolabeling of NRK cells labeled with anti-YB1 and anti-tubulin after hypertonic shock. Up to 9 h of hypertonic exposure, SG size grew with time, and MT bundles were thicker. Between 9 and 24 h, SGs and MT bundles dissociated in the few cells that survived. Scale bar, 15 μm. B, we analyzed by NMR the intracellular betaine accumulation in NRK cells after a hypertonic shock with various extracellular concentrations of betaine for 9 h. The results show that betaine transport in NRK cells under hypertonicity is accelerated in the presence of extracellular betaine. Results are the means ± S.D., and 1 a.u. corresponds to 72 fmol of betaine per NRK cell (see “Materials and Methods”). C, two typical NMR spectra were obtained as described in B. In the presence of increasing betaine concentration, the area of cellular betaine peaks (see the asterisks) significantly increases. D, NRK cells were exposed for 9 h to constant hypertonicity in the presence of varying concentrations of betaine. Betaine, above 2.5 mm, promotes the disassemblies of SGs and MT bundles. SGs and MTs were detected with anti-YB1 and anti-tubulin immunostaining, respectively. Scale bar, 15 μm. E, shown are statistical measurements of the mean SG area obtained from the analysis of NRK immunostained with anti-YB1 after the indicated treatment. Although SG size increased between 6 and 9 h for hypertonic-stressed cells in the absence of betaine, a net decrease in SG size was detected in the presence of 10 mm betaine. Results are the means ± S.D.
Cell Preconditioning to Hypertonicity and Inhibition of SG Assembly
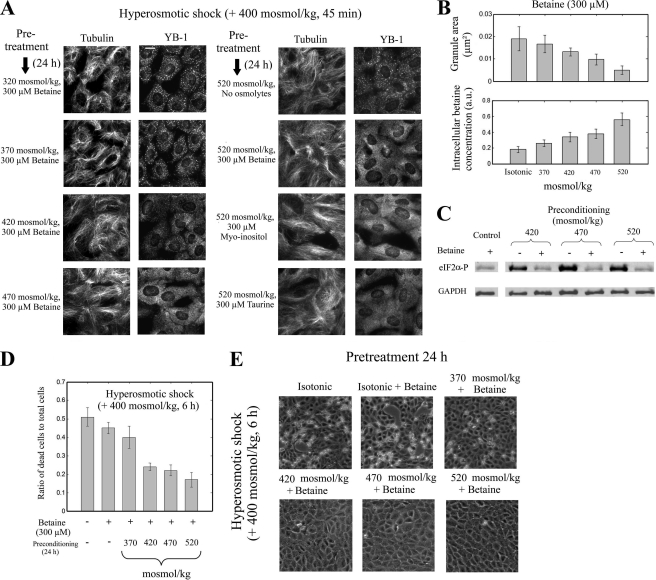
As compatible osmolyte accumulation leads to the dissociation of SGs, the idea is to accumulate compatible osmolytes in cells and to analyze whether this accumulation inhibits SG assembly under such condition. To that end, NRK cells were first preconditioned under moderate hypertonic conditions for 24 h to accumulate compatible osmolytes in the presence of betaine and then exposed to an additional hypertonic shock of large amplitude, +400 mosmol/kg, whatever the osmolality of the pretreatment. The results clearly show that cells pre-exposed to hypertonicities higher than 370 mosmol/kg displayed significantly smaller granules than those grown under isotonic condition (Fig. 6A). In fact, the mean granule size decreases with the intracellular accumulation of betaine, which is monitored by the extracellular osmolality of the pretreatment medium (Fig. 6B). The impaired SG assembly in cells preconditioned to hypertonicity is not specific to betaine but is shared by the other compatible osmolytes like taurine and myo-inositol (Fig. 6A). We also noticed that cell preconditioning inhibits the formation of MT bundles, which as usual indicates that MT bundling and SG assembly follow the same pattern of association and dissociation.
FIGURE 6.
Hypertonic preconditioning inhibits SG assembly and promotes cell survival. A, NRK cells were pretreated for 24 h as indicated. Then, NaCl osmotic shock was applied for 45 min by increasing the osmolality by 400 mosmol/kg with respect to the osmolality of the pretreatment medium. In the first column, we observed that increasing the osmolality of the pretreatment in the presence of betaine leads to the progressive impairment of SG assembly. In the second column, we observed that not only betaine but also myo-inositol and taurine can also impair SG assembly. These same remarks are also valid for the impairment of MT bundling. SGs and MTs were detected with anti-YB1 and anti-tubulin immunostaining. Scale bar, 10 μm. B, statistical analysis of the SG areas obtained from A and NMR measurement of the betaine accumulation in cells after 24 h of hypertonicity is shown. As expected, the magnitude of the intracellular betaine accumulation is dictated by the extracellular osmolality, and there is a negative correlation between the SG size and the amount of intracellular betaine. Results are the mean ± S.D. (1 a.u. corresponds to 72 fmol of betaine per NRK cell). C, Western blotting of cells extracts from preconditioned NRK cells exposed to an increase of osmolality (+400 mosmol/kg) for 45 min is shown. The phosphorylation of the initiation factor eIF2α is significantly reduced for cells preconditioned in the presence of betaine (10 mm). Control, NRK cells under isotonic condition with 10 mm betaine without hypertonic shock. GAPDH was used as a loading control. D, shown is the rate of cell death after hypertonic shock (+400 mosmol/kg for 6 h) applied to preconditioned NRK cells as determined via trypan blue exclusion. Cell preconditioning under hypertonic environment in the presence of betaine (300 μm) promotes cell resistance to hypertonic aggression. E, shown is phase contrast optical microscopy of NRK cells preconditioned as indicated and exposed to a hypertonic shock (+400 mosmol/kg for 6 h). We observed that without hypertonic preconditioning the integrity of the epithelia was affected, and many cells were detached, in contrast to preconditioned epithelia, which were protected from these damages. Together, these results confirm that hypertonic preconditioning in the presence of betaine promotes cell resistance to hyperosmolarity. See also supplemental Fig. S7 for the effects of other extracellular compatible osmolytes (taurine and myo-inositol).
The presence of SGs may have a negative impact on cell survival due to their reported proapoptotic influence (22) and the necessity to recover mRNA translation for cell adaptation to chronic hypertonicity. In this context the disassembly of SGs mediated by compatible osmolytes may have a positive impact on cell survival. In agreement with this, cell preconditioning in the presence of betaine before the hypertonic shock also prevents the phosphorylation of eIF2α (Fig. 6C) and increases the survival rate (Fig. 6, D and E). Similar protective effects were also observed for taurine and myo-inositol (supplemental Fig. S7).
Osmolyte Efflux Induces Formation of SGs in Preconditioned Cells
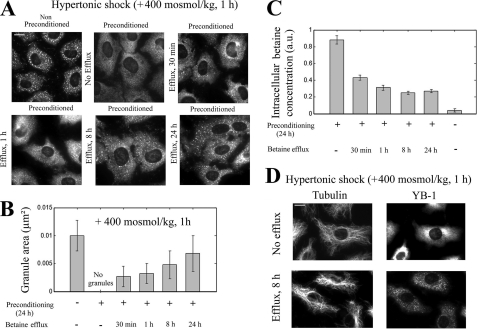
To further explore the role of compatible osmolytes in impaired SG assembly after hypertonic preconditioning, we analyzed SG formation during the return to isotonic conditions and its relationship with compatible osmolyte efflux that occurs under such conditions (55). To this end, after cell preconditioning for 24 h in hypertonic medium in the presence of betaine, cells were returned to isotonicity to allow the efflux of accumulated osmolytes for varying times and were again exposed to hypertonicity (+400 mosmol/kg). The results clearly indicate that the osmolyte efflux promotes the formation of SGs (Fig. 7A), and the size of the granules increases with the magnitude of the efflux (Fig. 7, B and C). We note, however, that even after 24 h of efflux, SGs were smaller than for non-preconditioned cells, which may be due to the partial osmolyte removal that persists for days (55). We also note that the osmolyte efflux promotes MT bundling, thus indicating exacerbated excluded volume interactions in the cell cytoplasm (Fig. 7D).
FIGURE 7.
Betaine efflux in isotonic condition allows the recovery of SGs in preconditioned cells. A, NRK cells were preconditioned for 24 h in the presence of betaine (300 μm) with an extracellular osmolarity of 520 mosmol/kg for 24 h. Cells were then returned to isotonic condition s(320 mosmol/kg) in the absence of betaine for varying times as indicated before being exposed to hypertonic shock (+400 mosmol/kg) for 45 min. In contrast with preconditioned cells, cells returned to isotonic conditions displayed SGs. and their size increased with the time spent in isotonic conditions. Scale bar, 10 μm. B, statistical analysis of the SG areas obtained from A is shown. Results are the means ± S.D. Note that even after long efflux (24 h), SGs remains smaller than those of non preconditioned cells. C, shown is NMR quantification of the intracellular betaine content in preconditioned NRK cells after their return to isotonicity for indicated time. The results show that the rate of betaine efflux is rather rapid during the first hour but then considerably slows down with time. The betaine content is still significant after 24 h. Results are the means ± S.D. (1 a.u. corresponds to 72 fmol of betaine per NRK cell). D, betaine efflux also promotes MT bundling after hypertonic shock in preconditioned NRK cells. SGs and MTs were observed with anti-YB1 and anti-tubulin immunostaining. Scale bar, 10 μm.
Cell-Cell Interactions Allow Rapid Preconditioning of Epithelial Cells
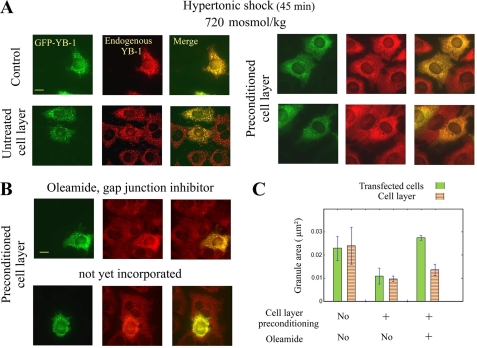
Compatible osmolytes are small neutral molecules that can be easily exchanged between cells communicating via gap junctions in NRK cells (56). We then explored the idea that cell preconditioning, if monitored by compatible osmolytes, can be transmitted from cell to cell. For this purpose, non-preconditioned NRK cells were transfected with GFP-YB1 and then deposited on a layer of NRK cells that had been preconditioned to hypertonic stress or not. After 4 h, to allow the incorporation of transfected cells in the cell monolayer and the formation of gap junctions (57), samples were exposed to acute hypertonic shock to eventually form SGs. The cell transfection and the expression of GFP-YB1 by themselves did not inhibit the formation of SGs, and tagged YB-1 also co-localized with SGs (Fig. 8A). As a control, the incorporation of transfected cells into the non-preconditioned monolayer of NRK cells did not change the SG appearance, with both transfected and non-transfected cells displaying large granules. On the other hand, their incorporation into a monolayer of preconditioned cells led to a significant inhibition of SG assembly. In addition, when transfected cells were not yet fully incorporated in the cell monolayer or in the presence of oleamide, an inhibitor of gap junction communications (58, 59), larger SGs were observed (Fig. 8, B and C). Altogether, these results indicate that cell-cell interactions, at least partly via gap junction communications, allow preconditioned cells to share with non-preconditioned cells their aptitude to limit the formation of SGs, presumably through the transit of compatible osmolytes.
FIGURE 8.
Intercellular interactions between preconditioned and non-preconditioned NRK cells prevent SG formation after hypertonic shock in non-preconditioned cells. A, GFP-YB1-transfected NRK cells were loaded onto layers of control or preconditioned NRK cells. Preconditioned cells were returned to isotonic conditions just before the addition of transfected cells. After 4 h, to let the transfected cells incorporate the cell layers, cells were exposed to hypertonic shock (720 mosmol/kg for 45 min) and fixed. GFP fluorescence was used to distinguish transfected cells and anti-YB-1 immunostaining for the detection of SGs in all cells. Isolated transfected cells (control) or transfected cells that had incorporated a non-preconditioned monolayer of cells displayed typical SGs. On the other hand, for transfected cells incorporated into a preconditioned layer, cell-cell interactions led to the inhibition of SG assembly. Scale bar, 10 μm. B, conditions were the same as A with a preconditioned cell layer. Transfected cells not yet fully incorporated in the monolayer of cells or when treated with oleamide, a gap junction inhibitor (50 μm for the 4 h required for transfected cells to incorporate the cell monolayer), displayed large SGs, whereas the assembly of SGs was inhibited in the preconditioned cell monolayer. Scale bar, 10 μm. C, statistical analysis of the SG areas after the treatments indicated in A and B. Results are the means ±S.D.
DISCUSSION
Biophysics of SG Assembly after Hypertonic Shock
We show in this study that SG assembly is triggered by hypertonic shock provided that the variation of osmolality is larger than 200 mosmol/kg (Fig. 3). For such acute hypertonic shocks, cell volume can shrink within minutes to ∼30–40% that of its initial value or even more depending of the amplitude of the hypertonic shock (60), which increases the intracellular macromolecule concentration (61), noted below as c. The ensuing increase of macromolecular crowding may then promote the attraction between macroscopic bodies like mRNA-containing particles or MTs (30, 62). We can estimate the impact of cell shrinkage on excluded-volume attractions by considering the benefit of the free energy due to the association between two mRNA-containing particles, F, which scales like δV × c9/4 (63), where δV is considered constant and represents the excluded volume overlap between the two particles due to their association. Consequently, F increases between 2.2 and 3.1 times due to cell shrinkage (30–40%) and may thus initiate the formation of SGs via excluded volume interactions. In agreement with this, we observed that SGs appear after about 10–15 min of hypertonicity (supplemental Fig. S5). However, with the entry of inorganic ions like Na+, K+, and Cl−, cell volume mostly recovers its initial value by about 1 h (60). Another mechanism should then come into play to keep SGs for hours under constant hypertonicity, as observed for NRK cells. Besides cell shrinkage, a high intracellular ionic strength also promotes excluded volume interactions by reducing the electrostatic repulsion between like-charged macromolecules (63) or surfaces (32) like that of mRNA and MTs. As the recovery of a normal ionic strength requires the uptake of compatible osmolytes and thus is very long (between 12 and 24 h), this explains why the dissociation of SGs takes several hours under constant hypertonicity (Fig. 5). In line with this, when cells were returned to isotonic conditions, both the cell volume and the ionic strength returned to normal values, and the SGs dissociated within minutes (Fig. 4D). We can then conclude, as observed in vitro by AFM (Fig. 1), that both macromolecular crowding and high ionic strength promote the assembly of SGs in hypertonic-stressed cells and may favor the assembly of SGs triggered by other stresses (Fig. 4).
Compatible Osmolytes Promote SG Dissociation and Cell Survival
The accumulation of compatible osmolytes in the cell cytoplasm after cell preconditioning for 24 h in hypertonic medium impairs the assembly of SGs (Fig. 6). We also show that the phosphorylation level of eIF2α is significantly reduced (Fig. 6C). eIF2α phosphorylation is considered as an important step regarding the formation of stalled preinitiation complexes and their subsequent assembly into SGs in various stressful conditions (64, 65) even if SG assembly can also occur via other pathways (66). In the case of hypertonicity, impaired eIF2α phosphorylation cannot explain inhibition of SG assembly, as mutants deficient in eIF2α phosphorylation are resistant to hypertonicity but are still able to form SGs (22). The results presented here, however, indicate that SG disassembly is most probably due to weaker excluded volume interactions in the cell cytoplasm of preconditioned cells. For instance, the efflux of compatible osmolytes before osmotic shock leads to the reappearance of small SGs (Fig. 7).
Another important point of this study is that preconditioned cells acquire a resistance to hyperosmotic challenge (Fig. 6, D and E). SGs are not inert aggregates of mRNA molecules and associated proteins. They fulfill many functions like mRNA storing, sorting, and degradation (22–24) and may then influence the positive or negative outcome after stress. In the case of hypertonicity, the phosphorylation of eIF2α allows the cytoplasmic accumulation in SGs of heterogeneous nuclear ribonucleoprotein A1, which may induce cell apoptosis (22). The inhibition of eIF2α phosphorylation can thus explain the acquired resistance to hypertonic shock. However, besides specific molecular mechanisms related to SGs, the long term presence of SGs generally indicates that the translational machineries are mostly arrested, a situation that cells cannot sustain for long under chronic hyperosmolarity. Compatible osmolytes can also promote cell survival by SG-independent mechanisms. The recovery of normal cell cytoskeleton after osmotic shock (56, 67), such as the MT bundle disassembly observed after betaine accumulation, may play a role in hypertonic adaption. In addition, compatible osmolytes counteract the negative effect of ionic strength regarding protein aggregation (68, 69) and have a positive impact on many enzymatic reactions (17) and mRNA translation (19) without considering the DNA- or membrane-related processes.
Is Cell Preconditioning by Compatible Osmolytes Relevant in Vivo?
The kidney and especially the renal medulla are permanently exposed to varying hypertonicities, for example, during diuresis and antidiuresis (70). In line with this, NFAT5-null mice display a profound atrophy of the kidney medulla, thus indicating that the accumulation of compatible osmolytes triggered by the NFAT5 transcriptional response is most probably mandatory for normal renal functions (35). However, many other tissues can experience hypertonicity in pathological conditions like the brain (71) and the liver (72), for which, respectively, taurine and betaine contents are osmo-regulated (73, 74). In addition, inflammatory disorders also induce local hypertonicity like in the intestine and cornea (75).
Besides the information about the fundamental processes associated to SG assembly and disassembly in hypertonic environment, here are three examples of possible insights into in vivo osmo-protection suggested by the following results. (i) Upon decrease in environmental osmolality, compatible osmolyte concentrations in kidney cells decrease sharply due a transient and rapid efflux occurring during the first 3 h and then significantly more slowly for days due to the slow decrease of active influx (76). A partial removal of compatible osmolytes can provide a “memory,” i.e. a long term protection in case of future rise in extracellular osmolality. (ii) Interestingly, the lymphoid environments of the liver but also the spleen and thymus are moderately hypertonic (∼+40 mosmol/kg) (77), which could stimulate NFAT5-related osmolyte uptake. As indicated in this study, even a moderate hypertonic pretreatment may significantly increase the cell resistance to hypertonicity, which in this case may apply to lymphocytes or other cells exposed to this environment. (iii) We also show that the beneficial effect of cell preconditioning can be transferred from cell to cell provided that they are interacting via gap junctions (Fig. 8). Indeed cells exposed to constant hypertonicity can accumulate compatible osmolytes and then share these precious osmolytes with adjacent communicating cells even if these cells do not express compatible osmolyte transporters. This way, epithelia can sense a potential osmotic aggression coming. Such a strategy, if any, may be useful in many tissues and especially in the kidney, which abundantly expresses connexins (78).
Supplementary Material
Acknowledgment
We gratefully acknowledge the Genopole EVRY for constant support of the laboratory.
This work was supported by funds from the INSERM.

This article contains supplemental Figs. S1–S7.
- SG
- stress granule
- AFM
- atomic force microscopy
- MT
- microtubule
- NRK
- rat kidney cells
- a.u.
- arbitrary unit
- PEG 35K
- PEG Mr 35,000.
REFERENCES
- 1. Strange K. (2004) Cellular volume homeostasis. Adv. Physiol. Educ 28, 155–159 [DOI] [PubMed] [Google Scholar]
- 2. Wehner F., Olsen H., Tinel H., Kinne-Saffran E., Kinne R. K. (2003) Cell volume regulation. Osmolytes, osmolyte transport, and signal transduction. Rev. Physiol. Biochem. Pharmacol 148, 1–80 [DOI] [PubMed] [Google Scholar]
- 3. Burg M. B., Ferraris J. D. (2008) Intracellular organic osmolytes. Function and regulation. J. Biol. Chem. 283, 7309–7313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grinstein S., Woodside M., Sardet C., Pouyssegur J., Rotin D. (1992) Activation of the Na+/H+ antiporter during cell volume regulation. Evidence for a phosphorylation-independent mechanism. J. Biol. Chem. 267, 23823–23828 [PubMed] [Google Scholar]
- 5. Strange K., Emma F., Jackson P. S. (1996) Cellular and molecular physiology of volume-sensitive anion channels. Am. J. Physiol. 270, C711–C730 [DOI] [PubMed] [Google Scholar]
- 6. Ferraris J. D., Williams C. K., Ohtaka A., García-Pérez A. (1999) Functional consensus for mammalian osmotic response elements. Am. J. Physiol. 276, C667–C673 [DOI] [PubMed] [Google Scholar]
- 7. Burg M. B., Kwon E. D., Kültz D. (1997) Regulation of gene expression by hypertonicity. Annu. Rev. Physiol. 59, 437–455 [DOI] [PubMed] [Google Scholar]
- 8. Moriyama T., Garcia-Perez A., Burg M. B. (1989) Osmotic regulation of aldose reductase protein synthesis in renal medullary cells. J. Biol. Chem. 264, 16810–16814 [PubMed] [Google Scholar]
- 9. Kwon E. D., Jung K. Y., Edsall L. C., Kim H. Y., García-Pérez A., Burg M. B. (1995) Osmotic regulation of synthesis of glycerophosphocholine from phosphatidylcholine in MDCK cells. Am. J. Physiol. 268, C402–C412 [DOI] [PubMed] [Google Scholar]
- 10. Yamauchi A., Uchida S., Kwon H. M., Preston A. S., Robey R. B., Garcia-Perez A., Burg M. B., Handler J. S. (1992) Cloning of a Na+- and Cl−-dependent betaine transporter that is regulated by hypertonicity. J. Biol. Chem. 267, 649–652 [PubMed] [Google Scholar]
- 11. Ito T., Fujio Y., Hirata M., Takatani T., Matsuda T., Muraoka S., Takahashi K., Azuma J. (2004) Expression of taurine transporter is regulated through the TonE (tonicity-responsive element)/TonEBP (TonE-binding protein) pathway and contributes to cytoprotection in HepG2 cells. Biochem. J. 382, 177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamauchi A., Uchida S., Preston A. S., Kwon H. M., Handler J. S. (1993) Hypertonicity stimulates transcription of gene for Na+-myo-inositol cotransporter in MDCK cells. Am. J. Physiol. 264, F20–F23 [DOI] [PubMed] [Google Scholar]
- 13. Rødgaard T., Schou K., Friis M. B., Hoffmann E. K. (2008) Does the intracellular ionic concentration or the cell water content (cell volume) determine the activity of TonEBP in NIH3T3 cells? Am. J. Physiol. Cell Physiol 295, C1528–C1534 [DOI] [PubMed] [Google Scholar]
- 14. Strange K., Morrison R., Heilig C. W., DiPietro S., Gullans S. R. (1991) Up-regulation of inositol transport mediates inositol accumulation in hyperosmolar brain cells. Am. J. Physiol. 260, C784–790 [DOI] [PubMed] [Google Scholar]
- 15. Navarro P., Chiong M., Volkwein K., Moraga F., Ocaranza M. P., Jalil J. E., Lim S. W., Kim J. A., Kwon H. M., Lavandero S. (2008) Osmotically-induced genes are controlled by the transcription factor TonEBP in cultured cardiomyocytes. Biochem. Biophys. Res. Commun. 372, 326–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miyakawa H., Woo S. K., Dahl S. C., Handler J. S., Kwon H. M. (1999) Tonicity-responsive enhancer-binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl. Acad. Sci. U.S.A. 96, 2538–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. (1982) Living with water stress. Evolution of osmolyte systems. Science 217, 1214–1222 [DOI] [PubMed] [Google Scholar]
- 18. Dmitrieva N. I., Cai Q., Burg M. B. (2004) Cells adapted to high NaCl have many DNA breaks and impaired DNA repair both in cell culture and in vivo. Proc. Natl. Acad. Sci. U.S.A. 101, 2317–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brigotti M., Petronini P. G., Carnicelli D., Alfieri R. R., Bonelli M. A., Borghetti A. F., Wheeler K. P. (2003) Effects of osmolarity, ions, and compatible osmolytes on cell-free protein synthesis. Biochem. J. 369, 369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel J., McLeod L. E., Vries R. G., Flynn A., Wang X., Proud C. G. (2002) Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur. J. Biochem. 269, 3076–3085 [DOI] [PubMed] [Google Scholar]
- 21. Morley S. J., Naegele S. (2002) Phosphorylation of eukaryotic initiation factor (eIF) 4E is not required for de novo protein synthesis after recovery from hypertonic stress in human kidney cells. J. Biol. Chem. 277, 32855–32859 [DOI] [PubMed] [Google Scholar]
- 22. Bevilacqua E., Wang X., Majumder M., Gaccioli F., Yuan C. L., Wang C., Zhu X., Jordan L. E., Scheuner D., Kaufman R. J., Koromilas A. E., Snider M. D., Holcik M., Hatzoglou M. (2010) eIF2α phosphorylation tips the balance to apoptosis during osmotic stress. J. Biol. Chem. 285, 17098–17111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guil S., Long J. C., Cáceres J. F. (2006) hnRNP A1 relocalization to the stress granules reflects a role in the stress response. Mol. Cell. Biol. 26, 5744–5758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dewey C. M., Cenik B., Sephton C. F., Dries D. R., Mayer P., 3rd, Good S. K., Johnson B. A., Herz J., Yu G. (2011) TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor. Mol. Cell. Biol. 31, 1098–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gilks N., Kedersha N., Ayodele M., Shen L., Stoecklin G., Dember L. M., Anderson P. (2004) Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell 15, 5383–5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson P., Kedersha N. (2008) Stress granules. The Tao of RNA triage. Trends Biochem. Sci. 33, 141–150 [DOI] [PubMed] [Google Scholar]
- 27. Ellis R. J. (2001) Macromolecular crowding. Obvious but underappreciated. Trends Biochem. Sci. 26, 597–604 [DOI] [PubMed] [Google Scholar]
- 28. Zimmerman S. B., Minton A. P. (1993) Macromolecular crowding. Biochemical, biophysical, and physiological consequences. Annu. Rev. Biophys. Biomol. Struct. 22, 27–65 [DOI] [PubMed] [Google Scholar]
- 29. Minton A. P. (2000) Implications of macromolecular crowding for protein assembly. Curr. Opin Struct. Biol. 10, 34–39 [DOI] [PubMed] [Google Scholar]
- 30. Marenduzzo D., Finan K., Cook P. R. (2006) The depletion attraction. An underappreciated force driving cellular organization. J. Cell Biol. 175, 681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang J. X., Ito T., Tao T., Traub P., Janmey P. A. (1997) Opposite effects of electrostatics and steric exclusion on bundle formation by F-actin and other filamentous polyelectrolytes. Biochemistry 36, 12600–12607 [DOI] [PubMed] [Google Scholar]
- 32. Pastré D., Hamon L., Mechulam A., Sorel I., Baconnais S., Curmi P. A., Le Cam E., Piétrement O. (2007) Atomic force microscopy imaging of DNA under macromolecular crowding conditions. Biomacromolecules 8, 3712–3717 [DOI] [PubMed] [Google Scholar]
- 33. Munishkina L. A., Cooper E. M., Uversky V. N., Fink A. L. (2004) The effect of macromolecular crowding on protein aggregation and amyloid fibril formation. J. Mol. Recognit. 17, 456–464 [DOI] [PubMed] [Google Scholar]
- 34. Liu Y., Guo Y., Valles J. M., Jr., Tang J. X. (2006) Microtubule bundling and nested buckling drive stripe formation in polymerizing tubulin solutions. Proc. Natl. Acad. Sci. U.S.A. 103, 10654–10659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. López-Rodríguez C., Antos C. L., Shelton J. M., Richardson J. A., Lin F., Novobrantseva T. I., Bronson R. T., Igarashi P., Rao A., Olson E. N. (2004) Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc. Natl. Acad. Sci. U.S.A. 101, 2392–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Häussinger D. (1996) The role of cellular hydration in the regulation of cell function. Biochem. J. 313, 697–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Foulks G. N. (2007) The correlation between the tear film lipid layer and dry eye disease. Surv. Ophthalmol. 52, 369–374 [DOI] [PubMed] [Google Scholar]
- 38. Davydova E. K., Evdokimova V. M., Ovchinnikov L. P., Hershey J. W. (1997) Overexpression in COS cells of p50, the major core protein associated with mRNA, results in translation inhibition. Nucleic Acids Res. 25, 2911–2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Minton A. P. (1997) Influence of excluded volume upon macromolecular structure and associations in “crowded” media. Curr. Opin Biotechnol. 8, 65–69 [DOI] [PubMed] [Google Scholar]
- 40. Baker N. A., Sept D., Joseph S., Holst M. J., McCammon J. A. (2001) Electrostatics of nanosystems. Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U.S.A. 98, 10037–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luby-Phelps K. (2000) Cytoarchitecture and physical properties of cytoplasm. Volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 192, 189–221 [DOI] [PubMed] [Google Scholar]
- 42. Hamon L., Savarin P., Curmi P. A., Pastré D. (2011) Rapid assembly and collective behavior of microtubule bundles in the presence of polyamines. Biophys. J. 101, 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Brabander M., Geuens G., Nuydens R., Willebrords R., De Mey J. (1981) Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc. Natl. Acad. Sci. U.S.A. 78, 5608–5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Evdokimova V., Ruzanov P., Imataka H., Raught B., Svitkin Y., Ovchinnikov L. P., Sonenberg N. (2001) The major mRNA-associated protein YB-1 is a potent 5′ cap-dependent mRNA stabilizer. EMBO J. 20, 5491–5502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chernov K. G., Barbet A., Hamon L., Ovchinnikov L. P., Curmi P. A., Pastré D. (2009) Role of microtubules in stress granule assembly. Microtubule dynamical instability favors the formation of micrometric stress granules in cells. J. Biol. Chem. 284, 36569–36580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tourrière H., Chebli K., Zekri L., Courselaud B., Blanchard J. M., Bertrand E., Tazi J. (2003) The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 160, 823–831 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47. Kedersha N., Cho M. R., Li W., Yacono P. W., Chen S., Gilks N., Golan D. E., Anderson P. (2000) Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 151, 1257–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu Z., Shen J., Carbrey J. M., Mukhopadhyay R., Agre P., Rosen B. P. (2002) Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc. Natl. Acad. Sci. U.S.A. 99, 6053–6058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McEwen E., Kedersha N., Song B., Scheuner D., Gilks N., Han A., Chen J. J., Anderson P., Kaufman R. J. (2005) Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J. Biol. Chem. 280, 16925–16933 [DOI] [PubMed] [Google Scholar]
- 50. Wiegant F. A., Souren J. E., van Rijn H., van Wijk R. (1993) Arsenite induced sensitization and self-tolerance of Reuber H35 hepatoma cells. Cell Biol. Toxicol 9, 49–59 [DOI] [PubMed] [Google Scholar]
- 51. Gallazzini M., Ferraris J. D., Kunin M., Morris R. G., Burg M. B. (2006) Neuropathy target esterase catalyzes osmoprotective renal synthesis of glycerophosphocholine in response to high NaCl. Proc. Natl. Acad. Sci. U.S.A. 103, 15260–15265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nakanishi T., Turner R. J., Burg M. B. (1990) Osmoregulation of betaine transport in mammalian renal medullary cells. Am. J. Physiol. 258, F1061–F1067 [DOI] [PubMed] [Google Scholar]
- 53. Petronini P. G., De Angelis E. M., Borghetti P., Borghetti A. F., Wheeler K. P. (1992) Modulation by betaine of cellular responses to osmotic stress. Biochem. J. 282, 69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Horio M., Ito A., Matsuoka Y., Moriyama T., Orita Y., Takenaka M., Imai E. (2001) Apoptosis induced by hypertonicity in Madin Darley canine kidney cells. Protective effect of betaine. Nephrol Dial. Transplant. 16, 483–490 [DOI] [PubMed] [Google Scholar]
- 55. Nakanishi T., Burg M. B. (1989) Osmoregulatory fluxes of myo-inositol and betaine in renal cells. Am. J. Physiol. 257, C964–C970 [DOI] [PubMed] [Google Scholar]
- 56. Desforges B., Savarin P., Bounedjah O., Delga S., Hamon L., Curmi P. A., Pastre D. (2011) Gap junctions favor normal rat kidney epithelial cell adaptation to chronic hypertonicity. Am. J. Physiol. Cell Physiol. 301, C705—C716 [DOI] [PubMed] [Google Scholar]
- 57. Ko K., Arora P., Lee W., McCulloch C. (2000) Biochemical and functional characterization of intercellular adhesion and gap junctions in fibroblasts. Am. J. Physiol. Cell Physiol 279, C147–157 [DOI] [PubMed] [Google Scholar]
- 58. Boger D. L., Patterson J. E., Guan X., Cravatt B. F., Lerner R. A., Gilula N. B. (1998) Chemical requirements for inhibition of gap junction communication by the biologically active lipid oleamide. Proc. Natl. Acad. Sci. U.S.A. 95, 4810–4815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guan X., Cravatt B. F., Ehring G. R., Hall J. E., Boger D. L., Lerner R. A., Gilula N. B. (1997) The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J. Cell Biol. 139, 1785–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roger F., Martin P. Y., Rousselot M., Favre H., Féraille E. (1999) Cell shrinkage triggers the activation of mitogen-activated protein kinases by hypertonicity in the rat kidney medullary thick ascending limb of the Henle's loop. Requirement of p38 kinase for the regulatory volume increase response. J. Biol. Chem. 274, 34103–34110 [DOI] [PubMed] [Google Scholar]
- 61. Lang F., Busch G. L., Ritter M., Völkl H., Waldegger S., Gulbins E., Häussinger D. (1998) Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 78, 247–306 [DOI] [PubMed] [Google Scholar]
- 62. Minton A. P. (2001) The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 276, 10577–10580 [DOI] [PubMed] [Google Scholar]
- 63. de Vries R. (2001) Flexible polymer-induced condensation and bundle formation of DNA and F-actin filaments. Biophys. J. 80, 1186–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kedersha N. L., Gupta M., Li W., Miller I., Anderson P. (1999) RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2α to the assembly of mammalian stress granules. J. Cell Biol. 147, 1431–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kimball S. R., Horetsky R. L., Ron D., Jefferson L. S., Harding H. P. (2003) Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am. J. Physiol. Cell Physiol. 284, C273–C284 [DOI] [PubMed] [Google Scholar]
- 66. Mazroui R., Sukarieh R., Bordeleau M. E., Kaufman R. J., Northcote P., Tanaka J., Gallouzi I., Pelletier J. (2006) Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2α phosphorylation. Mol. Biol. Cell 17, 4212–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Di Ciano C., Nie Z., Szászi K., Lewis A., Uruno T., Zhan X., Rotstein O. D., Mak A., Kapus A. (2002) Osmotic stress-induced remodeling of the cortical cytoskeleton. Am. J. Physiol. Cell Physiol. 283, C850–C865 [DOI] [PubMed] [Google Scholar]
- 68. Ignatova Z., Gierasch L. M. (2006) Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant. Proc. Natl. Acad. Sci. U.S.A. 103, 13357–13361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Natalello A., Liu J., Ami D., Doglia S. M., de Marco A. (2009) The osmolyte betaine promotes protein misfolding and disruption of protein aggregates. Proteins 75, 509–517 [DOI] [PubMed] [Google Scholar]
- 70. Saikia T. C. (1965) Composition of the renal cortex and medulla of rats during water diuresis and antidiuresis. Q. J. Exp. Physiol. Cogn Med. Sci. 50, 146–157 [DOI] [PubMed] [Google Scholar]
- 71. Loyher M. L., Mutin M., Woo S. K., Kwon H. M., Tappaz M. L. (2004) Transcription factor tonicity-responsive enhancer-binding protein (TonEBP) which transactivates osmoprotective genes, is expressed and up-regulated after acute systemic hypertonicity in neurons in brain. Neuroscience 124, 89–104 [DOI] [PubMed] [Google Scholar]
- 72. Schäfer C., Hoffmann L., Heldt K., Lornejad-Schäfer M. R., Brauers G., Gehrmann T., Garrow T. A., Häussinger D., Mayatepek E., Schwahn B. C., Schliess F. (2007) Osmotic regulation of betaine homocysteine-S-methyltransferase expression in H4IIE rat hepatoma cells. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1089–G1098 [DOI] [PubMed] [Google Scholar]
- 73. Wade J. V., Olson J. P., Samson F. E., Nelson S. R., Pazdernik T. L. (1988) A possible role for taurine in osmoregulation within the brain. J. Neurochem. 51, 740–745 [DOI] [PubMed] [Google Scholar]
- 74. Slow S., Lever M., Chambers S. T., George P. M. (2009) Plasma-dependent and -independent accumulation of betaine in male and female rat tissues. Physiol. Res. 58, 403–410 [DOI] [PubMed] [Google Scholar]
- 75. Neuhofer W. (2010) Role of NFAT5 in inflammatory disorders associated with osmotic stress. Curr. Genomics 11, 584–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nakanishi T., Turner R. J., Burg M. B. (1989) Osmoregulatory changes in myo-inositol transport by renal cells. Proc. Natl. Acad. Sci. U.S.A. 86, 6002–6006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Go W. Y., Liu X., Roti M. A., Liu F., Ho S. N. (2004) NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc. Natl. Acad. Sci. U.S.A. 101, 10673–10678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Barajas L., Liu L., Tucker M. (1994) Localization of connexin43 in rat kidney. Kidney Int. 46, 621–626 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.