Background: Formation of a functional precursor B cell receptor (pre-BCR) is dependent on N-glycosylation of μ-heavy chains (μHC).
Results: Lack of core fucosylation of μHC attenuated the interaction between μHC and λ5.
Conclusion: Core fucosylation of μHC mediates the assembly of pre-BCR.
Significance: Learning how Fut8 regulates the assembly of pre-BCR is crucial for understanding pre-B cell development.
Keywords: Cell Biology, Glycobiology, Glycoprotein, Immunology, Lymphocyte
Abstract
α1,6-Fucosyltransferase (Fut8) knock-out (Fut8−/−) mice showed an abnormality in pre-B cell generation. Membrane assembly of pre-BCR is a crucial checkpoint for pre-B cell differentiation and proliferation in both humans and mice. The assembly of pre-BCR on the cell surface was substantially blocked in the Fut8-knockdown pre-B cell line, 70Z/3-KD cells, and then completely restored by re-introduction of the Fut8 gene to 70Z/3-KD (70Z/3-KD-re) cells. Moreover, loss of α1,6-fucosylation (also called core fucosylation) of μHC was associated with the suppression of the interaction between μHC and λ5. In contrast to Fut8+/+ CD19+CD43− cells, the subpopulation expressing the μHC·λ5 complex in the Fut8−/− CD19+CD43− cell fraction was decreased. The pre-BCR-mediated tyrosine phosphorylation of CD79a and activation of Btk were attenuated in Fut8-KD cells, and restored in 70Z/3-KD-re cells. The frequency of CD19lowCD43− cells (pre-B cell enriched fraction) was also reduced in Fut8−/− bone marrow cells, and then the levels of IgM, IgG, and IgA of 12-week-old Fut8−/− mice sera were significantly lower than those of Fut8+/+ mice. Our results suggest that the core fucosylation of μHC mediates the assembly of pre-BCR to regulate pre-BCR intracellular signaling and pre-B cell proliferation.
Introduction
Early B lymphocytes in the bone marrow (BM)2 can be divided into stepwise subsets from hematopoietic stem cells to mature B cells, based on the rearrangement of immunoglobulin genes and the expression of B cell receptor (BCR) and particular cell surface markers. The intermediate steps consist of pro-B cells (CD19+CD43+ cells), in which the V(D)J rearrangement of the μ-heavy chain (μHC) gene is in process, and pre-B cells (CD19+CD43− cells), in which the gene rearrangement of light chains takes place. The first, critical checkpoint in early B lymphocyte development is transition from pro-B to pre-B cells. Only when the gene rearrangement of μHC is productive are pre-B cell antigen receptors (pre-BCR) expressed on their surface. A deficiency in pre-BCR formation results in severe impairment of B cell differentiation in both humans and mice (1). Thus, the early B-lymphocyte development relies on the assembly and expression of pre-BCR.
In a functional pre-BCR, immunoglobulin (Ig) μHC assembles with the surrogate light chain (SLC) and the signal-transducing heterodimer Igα/Igβ (CD79a and CD79b) (2, 3). The μHC (GI:90956) consists of the variable region of Ig HC (VH) and the constant portion of the Ig HC (CH). The SLC consists of 2 invariant polypeptides: λ5 (GI:54887631) and Vpre-B. Vpre-B and λ5 proteins are noncovalently associated and together form a SLC on the surface of B cell precursors. However, λ5 is covalently coupled to the CH1 domain of μHC via a carboxyl-terminal cysteine (4).
GDP-l-Fuc:N-acetyl-β-d-glucosaminide α1,6-fucosyltransferase (Fut8) catalyzes the transfer of a fucose residue from GDP-fucose to the innermost N-acetylglucosamine (GlcNAc) residue of hybrid and complex N-glycans via an α1,6-linkage (core fucosylation) in the Golgi apparatus in mammals (5) as shown in supplemental Fig. S1. The presence of core fucose in the N-linked glycoprotein has been shown to be important in glycoprotein processing and recognition. A lack of core fucosylation of the transforming growth factor-β1 (TGF-β1) receptor and/or epidermal growth factor (EGF) receptor consequently results in the marked dysregulation of their activation, due to a decreased ligand affinity for the receptor (5–7). Deletion of the core fucose from the Cγ2 of IgG1 enhanced antibody-dependent cell-mediated cytotoxity up to 50–100-fold (8). Recently, Pinho et al. (9) reported that the modification of Fut8 on E-cadherin affected the adhesive function of this adhesion molecule. More recently, it has been reported that Fut8−/− mice exhibited multiple behavioral abnormalities associated with a schizophrenia-like phenotype (10). Taken together, these results suggest that the core fucose plays a key role in regulating important physiological functions via the modification of functional proteins.
The μHC is a glycoprotein, containing 5 potential N-glycosylation sites: N46 (CH1), N211 (CH2), N243 (CH3), N258 (CH3), and N281 (CH3) in mice (11). It has been reported that N-glycosylation of IgM was related to serum half-life, complement activation, and IgM oligomerization (12, 13). Haimovich et al. (14) demonstrated that N-glycosylation in μHC plays a role in its activation. Recently, Ubelhart et al. (11) also reported that the formation of a functional pre-BCR was strictly dependent on a specific N-glycosylation site in the CH1 domain of μHC. The core fucose is present in several classes of N-linked glycans and could affect the conformation and flexibility of the antenna of N-linked biantennary oligosaccharides (15). However, the role of core fucosylation of μHC in pre-BCR assembly has not yet been addressed.
In our previous study, the loss of core fucosylation of very late antigen 4 and vascular cell adhesion molecule 1 led to the low interaction between pre-B cells and stromal cells, which accounts for an abnormality in the development of B cell progenitors (16). In the present study, we further explored a new mechanism of B lymphopoietic failure at the pre-B cell stage in Fut8−/− mice, and found that core fucosylation of μHC was required for the assembly of pre-BCR and intracellular signaling via pre-BCR.
EXPERIMENTAL PROCEDURES
Mice
Fut8−/− mice were generated as previously described (6) and were backcrossed eight times to the BALB/cA background. Homozygous wild (Fut8+/+) and knock-out (Fut8−/−) mice were obtained by crossing heterozygous Fut8+/− mice. All animal procedures complied with the institutional animal protocol guidelines. Peripheral blood was collected by cardiac puncture. The component of peripheral blood was analyzed by the Research Foundation for Microbial Diseases of Osaka University.
Antibodies
FITC-labeled anti-IgM (II/41), anti-erythroid (TER-119), anti-CD79b (HM79-16), PE-labeled anti-CD43 (S7), anti-IgD(11–26), PE-Cy5-labeled anti-CD19 (MB19-1), anti-Gr-1 (RB6–8C5), and APC-labeled anti-CD11b (Mac-1, M1/70) and anti-CD45R (RA3–6B2) monoclonal antibody (mAb) were obtained from e-Bioscience. Biotin-conjugated anti-mouse pre-BCR mAb (SL156), streptavidin-PE Cy5, and λ5 (LM34) were purchased from BD Bioscience. Anti-phosphotyrosine antibody (Ab) (PY20) was from BD Transduction Laboratories. Abs specific to Vpre-B (M-17; sc-25014), rat/mouse β-actin (sc-8432), was purchased from Santa Cruz; anti-mouse CD79a mAb were obtained from Beckman-Coulter-Immunotech. A mouse anti-Fut8 mAb (15C6) was obtained from Fujirebio Inc. (Japan); a rabbit anti-mouse IgG HRP-conjugate was from ICN Pharmaceuticals, Inc. (Aurora, OH). The anti-μHC mAb was from Southern Biotech.
Cells and Culture Conditions
The 70Z/3 cells, a pre-B lymphoma line, were purchased from ATCC. 70Z/3 derivative cell lines, stably transfected with the pSINsi-mU6 plasmid expressing siRNA that targeted Fut8 are referred to hereafter as “70Z/3-KD.” Fut8 restored 70Z/3-KD cells, 70Z/3-KD-re cells were established as previously described (16). The cells were grown in RPMI 1640 supplemented with 2 mm glutamine, 50 μm 2-mercaptoethanol (Fluka, Buchs, Switzerland), 5% FCS, 100 units/ml of penicillin, 100 μg/ml of streptomycin.
Flow Cytometry and Cell Sorting
BM cells and 70Z/3 cells in subconfluent conditions were harvested using phosphate-buffered saline (PBS) containing 0.2% EDTA and centrifuged at 1,000 × g for 5 min. The cell pellets were suspended in PBS(−) (5 × 106 cells) and incubated with an anti-CD16/CD32 (2.4G2) mAb to block Fc receptors and then stained on ice for 15 min with several combinations of mAbs, as indicated in the figure legends. Flow cytometry was performed on a FACS-Calibur (BD Biosciences), and the data were analyzed with CellQuest (BD Biosciences).
For cell sorting, BM cells were obtained by crushing two femurs and two tibia of 1-week-old mice. The crude mixture was filtered through nylon mesh, and resuspended at 1 × 107 cells/ml. BM cells were stained with PE-labeled anti-CD43 Ab and PE-Cy5-labeled anti-CD19 Ab and subpopulations were sorted with a FACStar Plus (BD Biosciences) instrument.
Fut8 Enzyme Activity Assay
The enzyme activity of Fut8 was determined using a synthetic substrate, 4-(2-pyridylamino)butylamine-labeled oligosaccharide as a substrate. Cells grown to subconfluence were washed with PBS(−) once, and the cell pellet was suspended in 200 μl of lysis buffer containing 10 mm Tris-HCl (pH 7.4), 150 mm NaCl, and 1% Triton X-100. The cell lysate was then assayed for Fut8 activity by high-performance liquid chromatography (HPLC) as described previously (17).
Western Blot and Lectin Blot Analysis
Cells were solubilized in 1% Triton X-100 lysis buffer (20 mm Tris-HCl (pH 7.4), 10 mm EGTA, 10 mm MgCl2, 1 mm benzamidine, 60 mm β-glycerophosphate, 1 mm Na3VO4, 20 mm NaF, 2 μg/ml of aprotinin, 5 μg/ml of leupeptin, 0.1 mm phenylmethylsulfonyl fluoride) and then centrifuged at 15,000 × g for 15 min. The supernatants were collected, and protein concentrations were determined using a protein assay BCA kit (Pierce). Equal amounts of protein were run on 10% SDS-PAGE under reducing conditions and then transferred to PVDF membranes (Millipore Corp.). Blots were blocked for 2 h with 5% skim milk in TBS-T (TBS-T; 10 mm Tris-HCl (pH 7.5), 150 mm NaCl, and 0.1% Tween 20) for immunoblot or with 3% BSA in TBS-T for lectin blot. Following incubation with the appropriate primary antibodies or 0.5 μg/ml of biotin-conjugated Aspergillus oryzae lectin (AOL) (18), which preferentially recognizes core fucosylation on N-glycans overnight, and then the membranes were washed. After washing, the blots were incubated with the corresponding secondary antibodies conjugated with horseradish peroxidase or ABC reagent (Vector Laboratories) for the AOL blot. Finally, specific proteins were visualized using an ECL system (Amersham Biosciences). These membranes were stripped and reprobed with an antibody against the corresponding total proteins to confirm equal loading.
Cell Surface Biotinylation and Immunoprecipitation
Cells were surface labeled by a sulfosuccinimido-biotin (sulfo-NHS-biotin) (Pierce) procedure (19). Briefly, after three washes the cells were suspended in PBS with 0.2 mg/ml of sulfo-NHS-biotin. After a 1-h incubation at 4 °C with occasional shaking, the cells were washed three times with chilled PBS and solubilized in lysis buffer.
Cell lysates (about 500 μg of protein) were incubated with the indicated antibodies overnight at 4 °C with gentle rocking and then added to 20 μl of protein G-Sepharose (50% slurry) (Amersham Biosciences) for another incubation of 2 h at 4 °C. The immunoprecipitate was washed three times with lysis buffer. The biotinylated proteins were visualized using the Vectastain ABC and ECL kits.
Assays for Frequencies of Pre-B Cells
The frequencies of pre-B cells growing dependent on SL156 plus interleukin 7 (IL-7) (clonable pre-B cells) were evaluated by means of a clonable pre-B cell assay with some modifications (16). The enriched CD19lowCD43− cells were seeded into methylcellulose media containing 10 ng/ml of recombinant human IL-7 (Stemcell Technologies Inc.) and 10 ng/ml of SL156. Colonies were counted after 7 days of culture. Aggregates consisting of >40 cells were differentially scored as colonies. All assays were performed in triplicate.
Assay for Molecular Interaction
Real-time molecular analysis was performed with an optical biosensor (Affinity Sensors, Cambridge, UK). Biotinylated λ5 peptide (NH2-wyvfgggtqltilgqpksdplvtlflpslknlqptrphvvclvsefypgtlvvdwkvdgvpvtqgvettqpskqtnnkymvssyltlisdqwmphsryscrvthegntveksvspaecs-COOH) was obtained by solid synthesis (Takara Bio). The biotinylated λ5 was immobilized on a biotinyl cuvette via streptavidin. Then, various concentrations of μHC purified from the lysates of 70Z/3, 70Z/3-KD, and 70Z/3-KD-re cells were placed in the cuvette.
Real-time PCR
Real-time PCR analyses were performed using a Smart Cycler II System (Cephied, Sunnyvale, CA) as described (6, 18).
Enzyme-linked Immunosorbent Assay (ELISA) for Immunoglobulin Typing
ELISA was carried out using mouse monoclonal antibody isotyping reagents (Sigma). The sera from 2- and 12-week-old mice (three per group) were used in this experiment.
Statistical Analysis
The results are expressed as mean ± S.D. Statistical analyses were carried out using Student's t test. A p value of less than 0.05 was considered statistically significant.
RESULTS
Impaired Pre-B Cell Population in Fut8−/− BM Cells
To determine the effects of targeting Fut8 on the hematolymphopoietic system, we analyzed peripheral blood cells of Fut8−/− mice. In the peripheral blood test, the numbers of white blood cells (WBC) in Fut8−/− mice were 2.7 ± 0.7 × 106/ml, whereas those in Fut8+/+ mice were 3.8 ± 1.2 × 106/ml (Table 1). Because the Fut8 product, a core-fucosylated N-glycan, is ubiquitously expressed in the BM microenvironment of Fut8+/+ BM, as confirmed by fucose lectin, AOL (16), a decrease of peripheral white blood cells in Fut8−/− mice suggest that Fut8 plays some positive roles in the regulation of hematopoiesis in the BM. Flow cytometry analysis revealed that the population of CD45+CD19+ cells was markedly reduced in Fut8−/− BM (Fig. 1 and Table 1). Also, the CD19+CD43− (pre-B enriched) and CD19+IgM+ (immature B enriched) populations were significantly decreased in Fut8−/− BM, whereas the CD19+CD43+ (pro-B enriched) population was sustained (Fig. 1 and Table 1). The populations of CD11b+ myeloid cells and TER119+ erythroid cells were relatively increased in Fut8−/− BM (Fig. 1 and Table 1), probably reflecting the reduction of B-lineage fractions. Development of B-1 cells, natural killer cells, and natural killer T cells were relatively normal in Fut8−/− BM (Table 1). Our results showed that disruption of Fut8 led to an abnormality in the development of the pre-B cell stage.
TABLE 1.
Comparison of BM cell compositions between Fut8+/+ and Fut8−/− mice
Data are representative of the mean ± S.D. of four mice per genotype.
| Genotype | Fut8+/+ mice | Fut8−/− mice | p values |
|---|---|---|---|
| WBC (106/ml) | 3.8 ± 1.2 | 2.7 ± 0.7 | 0.01 < p < 0.05 |
| CD19+CD45R+ (%) | 36.5 ± 2.6 | 16.2 ± 2.6 | p < 0.01** |
| CD19+CD43+ (%) | 5.9 ± 1.6 | 5.2 ± 2.1 | p > 0.05 |
| CD19+CD43− (%) | 30.2 ± 4.6 | 10.8 ± 5.5 | p < 0.01** |
| CD19+IgM+ (%) | 6.8 ± 1.7 | 3.7 ± 1.1 | 0.01 < p < 0.05 |
| CD11b+Gr-1− (%) | 4.9 ± 3.4 | 7.9 ± 1.5 | 0.01 < p < 0.05 |
| TER119+ (%) | 30.5 ± 3.5 | 47.7 ± 2.1 | p < 0.01** |
| DX5+CD3− (%) | 1.1 ± 0.3 | 1.2 ± 0.5 | p > 0.05 |
| DX5+CD3+ (%) | 0.8 ± 0.5 | 0.9 ± 0.2 | p > 0.05 |
| IgM+CD5+ (%) | 1.8 ± 1.0 | 1.7 ± 1.2 | p > 0.05 |
FIGURE 1.
FACS analysis of the proportion of CD45R+CD19+, CD19+CD43−, CD19+CD43+, CD19+IgM−, CD19+IgM+, CD11b+, CD11c+, Gr-1+, and TER119+ cells in Fut8−/− BM. BM cells were obtained by crushing 2 femurs and 2 tibia of 1-week-old mice. The crude mixture was filtered through nylon mesh, and resuspended at 1 × 107 cells/ml. Upper panels indicate Fut8+/+ BM cell subsets and the lower panels indicate Fut8−/− BM cell subsets. Numbers indicate the percentage of the total BM cells within this quadrant, and 10,000 events were acquired for each analysis. The results of 1 of 4 representative experiments are shown.
Membrane Assembly of Pre-BCR Requires μHC Core Fucosylation
In our previous study, we established Fut8 knockdown 70Z/3 cells, namely 70Z/3-KD cells, and Fut8 restored 70Z/3-KD cells (70Z/3-KD-re cells) (16). As shown in Fig. 2A, the expression of Fut8 mRNA was significantly reduced in 70Z/3-KD cells, and then re-introduction of the Fut8 gene into 70Z/3-KD cells resulted in recovery of Fut8 expression. Again, Fut8 enzyme activity analysis reflected the results of Fut8 gene expression. Fut8 activities were barely detectable in 70Z/3-KD cells, and were restored in 70Z/3-KD-re cells (Fig. 2B). Again, an AOL blot analysis reflected the results of the mRNA expressions and enzyme activities (supplemental Fig. S2), suggesting that the post-translational modification by core fucosylation on N-glycans is only catalyzed by the Fut8 gene. No apparent changes were found in the expressions of other glycosyltransferase genes, such as GnTIII and β4GalT-I (Fig. 2C).
FIGURE 2.
Characterization of 70Z/3-KD and 70Z/3-KD-re cells. A, gene-silencing effects of siRNA on the Fut8 mRNA expression were determined by real-time PCR, and normalized by the levels of GAPDH (n = 3). B, analyses of Fut8 activity. Fut8 activity was examined using a fluorescence-labeled sugar chain, GnGn-Asn-PABA (4-(2-pyridylamino)butylamine), as an acceptor substrate, as described under “Experimental Procedures.” The substrate (S) and Fut8 product (P) were eluted at 10 and 20 min, respectively, in mock and restored cells but not in 70Z/3-KD cells. ND, not detectable. Restored, re-introduction of Fut8 gene into 70Z/3-KD cells. C, gene expressions of Fut8, GnTIII, and β4GalT-I by real-time PCR analysis. RNAs were isolated from 70Z/3 and 70Z/3-KD cells. All values were normalized to that of the GAPDH gene. 70Z/3-KD cell, Fut8 knockdown 70Z/3 cell; 70Z/3-KD-re cell, Fut8 restored 70Z/3-KD cells. Data were representative of the mean ± S.D. of 3 per genotype (**, p < 0.01).
Membrane assembly of the pre-BCR is a crucial checkpoint for B cell differentiation and proliferation in both humans and mice (20–23). The μHC produced by pre-B cells performs a very important role to form the pre-BCR, which is composed of μHC and SLC (22). The CH1 of μHC reportedly contained the N-linked glycosylation site N46, and the conserved N46-glycosylation site is important for pre-BCR function (11). To elucidate the effects of core fucosylation in the assembly of pre-BCR, we examined the expression level of pre-BCR on the cell surface by biotin labeling. As shown in Fig. 3A, the expression of pre-BCR on the cell surface was down-regulated in 70Z/3-KD, and was restored in 70Z/3-KD-re cells. In the densitometric analysis, in contrast to 70Z/3 cells, pre-BCR expression on the cell surface was down-regulated by a factor of 5.2 in 70Z/3-KD cells. However, no significant differences in the expression levels of intracellular precursor μHC were found among the 3 cell types (Fig. 3B). The levels of core fucosylation in μHC were abolished in 70Z/3-KD cells, and they were rescued by reintroduction of Fut8 (Fig. 3B), suggesting that μHC is the target of Fut8. In the immunoprecipitation assay, we also found that loss of core fucosylation of μHC impaired the interaction between μHC and λ5, but not between μHC and Vpre-B (Fig. 3C). There were no differences in the expression levels of λ5 among the 3 cell types. Because λ5 is covalently coupled to the CH1 domain of μHC via a carboxyl-terminal cysteine (4), we synthesized a peptide, the COOH-terminal portion of λ5, and detected the binding affinity of λ5 to μHC. In the binding assay using an optical biosensor IAsys, the purified μHC from 70Z/3-KD cells showed impaired adhesion to the synthetic λ5 peptide (0.05, 0.1, and 0.2 μm), by comparison with mock cells. The reintroduction of Fut8 restored the binding affinity of μHC to λ5 (Fig. 3D). These results indicated that core fucosylation of μHC is required for the functional interaction between μHC and λ5 to complete the assembly of the pre-BCR.
FIGURE 3.
Membrane assembly of pre-BCR requires μHC core fucosylation. A, cell surface biotinylation and immunoprecipitation of pre-BCR from 70Z/3, 70Z/3-KD, and 70Z/3-KD-re cells. The surface cellular proteins of the indicated cells were biotinylated before cell lysis. Whole lysates were immunoprecipitated (IP) with anti-pre-BCR antibody. The samples were subjected to 10% SDS-PAGE. After Western blotting (WB), the membrane was incubated with streptavidin-HRP. Quantification of expression levels of pre-BCR was analyzed by NIH Image 1.63. B, core defucosylation of μHC in 70Z/3-KD cells. The cell lysates were immunoprecipitated by anti-μHC Ab, and the immunoprecipitates were resolved by SDS-PAGE on 7.5% gel, transferred to a PVDF membrane, and probed with the anti-μHC Ab (upper panel) and AOL (lower panel). Abrogation of the core fucose of μHC was detected by AOL staining. C, impaired interaction between μHC and λ5 in 70Z/3-KD cells. Cell lysates were immunoprecipitated with anti-μHC Ab and analyzed by Western blot with antibody to λ5 (upper panel) and cell lysates were immunoprecipitated with anti-λ5 Ab and analyzed by Western blot with antibody to λ5 (lower panel). D, molecular interactions between λ5 and μHC, detected by an optical biosensor (n = 3). The μHCs were extracted from the lysates of 70Z/3, 70Z/3-KD, and 70Z/3-KD-re cells. Monobiotinylated λ5 were coated. The data reflect the proportion of μHC associated with λ5.
Next, we examined the surface expressions of μHC, λ5, pre-BCR, and CD79b on CD19+CD43− cells (enriched pre-B) by flow cytometry. Pre-B cells express cell surface CD19 and cell surface μHCs associated with SLCs, whereas Pro-B cells are those B-lineage cells that express cell surface CD19 but do not express cytoplasmic or cell surface μHCs (28). In contrast with Fut8+/+ CD19+CD43− cells, the subpopulation expressing the μHC·λ5 complex in the Fut8−/− CD19+CD43− cell fraction was significantly decreased (Fig. 4). The percentage of Fut8−/− pre-BCR+CD79blow cells was 2.6 ± 0.5% of the pre-B cells (CD19+CD43− cells), whereas that of Fut8+/+ pre-BCR+CD79blow cells was 5.7 ± 1.2% (Fig. 4). No expression of either pre-BCR or CD79b was found in CD19+CD43+ cells (enriched pro-B). These results suggested that inefficient assembly of the pre-BCR due to core defucosylated μHC accounted for low levels of pre-BCR expression on the Fut8−/− and Fut8 knockdown cell surface.
FIGURE 4.
Impaired generation of μHC+λ5+ cells and pre-BCR+CD79blow in Fut8−/− CD19+CD43− pre-B cells. CD19+CD43− pre-B cells (R2) and CD19+CD43+ pro-B cells (R1) were gated. Upper panels indicate Fut8+/+ cell subsets and lower panels indicate Fut8−/− cell subsets. Numbers adjacent to the boxed areas indicate the frequency of μHC+λ5+ cells and pre-BCR+CD79blow cells in each as a percentage of the total cells. Data were representative of the mean ± S.D. of 3 per genotype (**, p < 0.01). Data were analyzed using the FLOWJO software program.
Loss of Fut8 Reduced Transduction of Pre-BCR Signaling
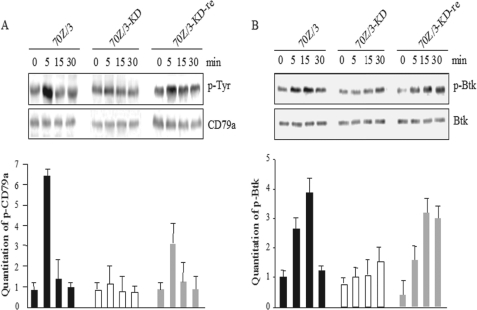
Signaling events initiated by the pre-BCR regulate several biological functions including cell proliferation and differentiation (4). The monoclonal antibody SL156 is specific for total pre-BCR and could cross-link the pre-BCR on the cellular surface to induce sustained intracellular signaling (37). Pre-BCR activation begins by the phosphorylation of the immunoreceptor tyrosine-based activatory motif present on the cytoplasmic tails of the CD79a and CD79b molecules and relies on the sequential activation of Src protein-tyrosine kinases Lyn, Syk, and the Bruton tyrosine kinase (Btk) (24). To address the effects of core fucosylation in pre-BCR mediated signaling, the cells were stimulated by SL156, and then the cell lysates were immunoprecipitated with anti-CD79a antibody. The kinetics of CD79a phosphorylation were quite similar in the 3 cell lines, and reached the maximal level at 5 min and returned to the basal level within 15 min after the stimulation of monoclonal antibody SL156. However, compared with 70Z/3 cells, the phosphorylation level of CD79a at 5 min was attenuated by a factor of 6.7 in 70Z/3-KD cells, whereas down-regulation of phosphorylation was partly restored in 70Z/3-KD-re cells (Fig. 5A). Moreover, the expression level of CD79a was reduced in 70Z/3-KD cells, compared with 70Z/3 and 70Z/3-KD-re cells (Fig. 5A). Also, the phosphorylation of Btk at 15 min was reduced by a factor of 3.8 in 70Z/3-KD cells, whereas those of Btk were rescued in the 70Z/3-KD-re cells (Fig. 5B). It is noteworthy that incomplete pre-BCR complex formation was seen in Fut8−/− mice, which were expected to have compromised signaling capacity.
FIGURE 5.
Down-regulation of phosphorylated CD79a and Btk in 70Z/3-KD cells. The serum-starved cells of 70Z/3, 70Z/3-KD, or 70Z/3-KD-re were treated with anti-pre-BCR antibody (SL156) at the indicted concentrations for 5 min and solubilized in lysis buffer as described under “Experimental Procedures.” Whole cell lysates were subjected to 10% SDS-PAGE. A, protein expression and phosphorylated forms of CD79a. The cell lysates were immunoprecipitated by anti-CD79a Ab, and the immunoprecipitates were resolved by SDS-PAGE on a 7.5% gel, transferred to a PVDF membrane, and probed with the PY20 (upper panel) and CD79a (lower panel). Increased phosphorylation of CD79a was maximal by 5 min. The quantitative data of phosphorylated CD79a were represented as the mean ± S.D. (n = 3). B, protein expression and phosphorylated forms of Btk are indicated in Western blot. The blots were probed with anti-p-Btk Ab (upper panel) or anti-Btk Ab (lower panel). The quantitative data of phosphorylated Btk are represented as mean ± S.D. (n = 3).
Loss of Fut8 Reduced Frequency of Pre-B Cells and Production of IgM, IgA, and IgG
To determine whether the core-fucosylated N-glycans functionally compromised pre-B cell colony formation, we cultivated 70Z/3, 70Z/3-KD, and 70Z/3-KD-re cells in Complete methylcellulose medium in the presence of SL156 plus IL-7. In the 70Z/3-KD cells the frequency of clonable pre-B cell progenitors was decreased, in comparison with mock cells and 70Z/3-KD-re cells (Fig. 6A). Moreover, colony formation of Fut8−/− CD19lowCD43− cells (pre-B cell enriched) were suppressed, compared with those of Fut8+/+ CD19lowCD43− cells (Fig. 6B). Our previous study showed that colony formation of pre-B cells in Complete methylcellulose medium in response to IL-7 alone (CFU-IL-7) was indistinguishable between Fut8+/+ pre-B cells and Fut8−/− pre-B cells (16), indicating that core fucosylation is critically important for pre-BCR-dependant cell proliferation.
FIGURE 6.
The pre-B colonies in the Complete methylcellulose medium in the presence of SL156 and IL-7. A, comparison of the frequencies of 70Z/3, 70Z/3-KD, and 70Z/3-KD-re cells in response to SL156 + IL-7. B, comparison of the frequencies of pre-B cells between Fut8+/+ CD19lowCD43− and Fut8−/− CD19lowCD43− cells in response to SL156 and IL-7. CD19lowCD43− pre-B cells were isolated using a FACStar Plus (BD Biosciences) instrument. Sorted cell populations were routinely re-analyzed and showed more than 92% purity. The pre-B colonies counted were cultured for 7 days in Complete methylcellulose medium in the presence of SL156 and IL-7. Data are presented as mean ± S.D. of pre-B colony-forming units.
We further examined the amount of each immunoglobulin isotype by ELISA. In 2-week-old Fut8−/− mice, the levels of IgM and IgG3 were significantly lower than those in Fut8+/+ mice, whereas the amounts of other immunoglobulins, IgG1, IgG2a, IgG2b, and IgA, were relatively normal. However, in 12-week-old Fut8−/− mice sera, the levels of IgG1, IgG2a, IgG2b, IgA, IgG3, and IgM were significantly reduced (Fig. 7). These data indicate that Fut8−/− mice exhibited defective humoral immune responses in addition to impaired early B cell development, in transition from pro-B to pre-B cells.
FIGURE 7.
Comparison of the levels of serum immunoglobulin isotypes in Fut8+/+ and Fut8−/− mice. The sera from 2- and 12-week-old mice (three per group) are used in this experiment. The concentrations of immunoglobulin isotypes are measured by ELISA kit (Sigma). ELISA was carried out using mouse monoclonal antibody isotyping reagents. **, p < 0.01 and *, p < 0.05 were considered statistically significant.
DISCUSSION
A selective and profound reduction in the pre-B cell populations and no concomitant change in the population containing pro-B cells were observed in Fut8−/− BM (Fig. 1 and Table 1). In agreement with this result, in 70Z/3-KD cells, the frequency of clonable pre-B cell progenitors was reduced, compared with mock cells and 70Z/3-KD-re cells. The present study is the first to clearly demonstrate that core fucosylation of μHC influences the pre-BCR assembly so as to regulate pre-BCR signaling and pre-B cell proliferation.
It is well known that glycoprotein expression can be regulated at post-translational levels. The mammalian glycans produced in the Golgi modulate the endocytosis of cell-surface glycoproteins, thereby controlling protein expression (25–27). The μHC have been described through the Golgi system, and are transported to the cell surface after modification of the carbohydrate moieties (28). The μHCs associated with the SLC were typically obligatory for pre-BCR transport to the surface (29–31). λ5 is covalently coupled to the CH1 domain of μHC via a carboxyl-terminal cysteine (4). λ5 has no potential N-glycosylation sites, whereas the CH1 domain of μHC has a single N-glycosylation site, N46. Ubelhart et al. (11) also found that a conserved N46-glycosylation site in the CH1 domain of μHC was the crucial element that regulates the interaction of μHC and λ5, followed by pre-BCR formation. Fut8 could modify multiple proteins and the core fucosylation of protein is an important post-translational process, which regulates protein folding, stability, and functional expression (5–10). To investigate an abnormality in pre-B cell development in Fut8−/− mice, here, we focused only on the role of core-fucosylated μHC during the assembly of pre-BCR. As anticipated, loss of core fucosylation of μHC impaired the interaction between μHC and λ5 (Fig. 3, C and D). The formation of pre-BCR on the cell surface was down-regulated in 70Z/3-KD, and was restored in 70Z/3-KD-re cells (Fig. 3A). This notion was also supported by evidence showing that in Fut8−/− BM the subpopulation of μHC+λ5+ cells was lower than Fut8+/+ BM. Indeed, not all rearranged μHCs can pair with the SLC to form a pre-BCR: only half of the in-frame rearranged μHCs pair correctly with SLCs (32). Only those B cells that express a μHC capable of pairing with an SLC undergo clonal expansion. Because the core fucosylation of μHC is required for the assembly of pre-BCR (11), and because the expression of the Fut8 gene was dramatically up-regulated at the developmental stage from pro-B to pre-B cells (16), it is conceivable that Fut8 regulates the level of core fucosylation of μHC, followed by assembly of pre-BCR and B cell clonal expansion. In addition to interaction between λ5 and the CH1 domain of μHC, in a pre-BCR complex, the interaction occurs between Vpre-B and the VH domain. Indeed, the Vpre-B is stabilized by a salt bridge between Vpre-B residue Glu106 and VH residue Arg59 (3). The VH domain, Vpre-B, and λ5 do not have any potential N-glycosylation sites, the interaction between the VH domain of μHC and Vpre-B belongs to the protein-protein interaction, whereas the interaction between the CH1 domain of μHC and λ5 is a protein-glycoprotein interaction. It is reasonable that the loss of core fucosylation of the CH1 domain of μHC could suppress the interaction between CH1 and λ5, but could not influence the formation of a salt bridge between the Vpre-B and the μHC.
The pro-B/pre-B I cells, freshly isolated from the BM, are able to differentiate and divide 2–5 times in the absence of stromal cells and IL-7, whereas the corresponding cells from λ5-deficient mice are unable to proliferate in such conditions, showing that surface expression of a complete pre-BCR is necessary and sufficient to provide constitutive cell signaling (33). Mutations in genes encoding subunits of the pre-BCR and molecules involved in pre-BCR signaling culminate in X-linked and non-X-linked agammaglobulinemia (34). For example, the μHC-affected patients were characterized by a normal level of pro-B cells but an absence of pre-B cells in the BM, and they presented severe hypogammaglobulinemia (35), λ5-, Vpre-B-, and CD79b-deficient mice exhibited a block in B cell development at the pre-B cell stage (20–22), then terminated pre-BCR expression and VJ(L) rearrangement by small pre-B cells destined to become B cells (2). The Btk-null mice also showed a developmental delay of B cells (36). In the present study, in contrast with 70Z/3 cells, the CD79a signaling intensity was attenuated by a factor of 6.7 in 70Z/3-KD cells. Also, in 70Z/3-KD cells, pre-BCR expression on the cell surface was down-regulated by a factor of 5.2 (Fig. 3A). It is conceivable that decreased signaling in 70Z/3-KD cells was proportional to the decreased expression of pre-BCR on the cell surface. It is also noteworthy that CD79a phosphorylation was preceded by the sequential activation of Btk at 15 min. The reintroduction of the Fut8 gene to 70Z/3-KD cells potently rescued pre-BCR-mediated signaling, which was impaired in 70Z/3-KD cells (Fig. 5). In turn, the attenuated signal transduction accounted for the low pre-B cell proliferation in Fut8−/− cells and in Fut8 knockdown cells (Fig. 6). In our previous study, the combined down-regulation of those genes: CD79a, CD79b, Ebf1, and Tcfe2a, which promote the activation of B cells, were down-regulated in Fut8−/− B progenitors (16). In 12-week-old Fut8−/− mice sera, the levels of IgG1, IgG2a, IgG2b, IgA, IgG3, and IgM were significantly reduced. However, in 2-week-old Fut8−/− mice sera, IgG1, IgG2a, and IgG2b levels were relatively normal, but levels of IgM and IgG3 were reduced by Fut8 deficiency, resembling the phenotype of Btk−/− mice (38). As to why the levels of IgGs were relatively normal in 2-week-old Fut8−/− mice, one possible explanation involves the transfer of IgGs from the pregnant Fut8+/− mice through the placenta to the offspring of Fut8−/− mice. In our previous study, the existence of core-fucosylated IgG in the serum of early Fut8−/− mice was confirmed by mass spectrographic analysis (data not shown).
In conclusion, the phenotype of Fut8−/− mice combined with our in vitro data presented an intriguing possibility that the core fucose is involved in the appropriate interactions of μHC and λ5, and the assembly of pre-BCR, which is required and sufficient for transduction of pre-BCR intracellular signaling and proliferation. Our results provide insight into the molecular mechanisms of Fut8-regulated pre-B cell differentiation and proliferation.
Supplementary Material
Acknowledgment
We gratefully acknowledge Katsuhiko Ishihara (Kawasaki Medical University, Japan) for critical reading of the manuscript.
This work was supported by National Nature Science Foundation of China Grant 30972675, the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (SRF for ROCS, SEM), and Science and Technology Planning Project of Dalian City, China, Grant 2010J21DW011.

This article contains supplemental Figs. S1 and S2.
- BM
- bone marrow
- Fut8
- core fucosyltransferase or α1,6-fucosyltransferase
- pre-BCR
- precursor B cell antigen receptor
- μHC
- μ heavy chain
- SLC
- surrogate light chain
- VLA-4
- very late antigen 4
- AOL
- A. oryzae lectin
- GnTIII
- N-acetylglucosaminyl transferase III
- Btk
- Bruton tyrosine kinase
- 70Z/3-KD cell
- Fut8 knockdown 70Z/3 cell
- 70Z/3-KD-re cell
- Fut8 restored 70Z/3-KD cells
- PE
- phycoerythrin.
REFERENCES
- 1. Conley M. E., Rohrer J., Rapalus L., Boylin E. C., Minegishi Y. (2000) Defects in early B-cell development: comparing the consequences of abnormalities in pre-BCR signaling in the human and the mouse. Immunol. Rev. 178, 75–90 [DOI] [PubMed] [Google Scholar]
- 2. Burrows P. D., Stephan R. P., Wang Y. H., Lassoued K., Zhang Z., Cooper M. D. (2002) The transient expression of pre-B cell receptors governs B cell development. Semin. Immunol. 14, 343–349 [DOI] [PubMed] [Google Scholar]
- 3. Bankovich A. J., Raunser S., Juo Z. S., Walz T., Davis M. M., Garcia K. C. (2007) Structural insight into pre-B cell receptor function. Science 316, 291–294 [DOI] [PubMed] [Google Scholar]
- 4. Espeli M., Rossi B., Mancini S. J., Roche P., Gauthier L., Schiff C. (2006) Initiation of pre-B cell receptor signaling: common and distinctive features in human and mouse. Semin. Immunol. 18, 56–66 [DOI] [PubMed] [Google Scholar]
- 5. Wang X., Inoue S., Gu J., Miyoshi E., Noda K., Li W., Mizuno-Horikawa Y., Nakano M., Asahi M., Takahashi M., Uozumi N., Ihara S., Lee S. H., Ikeda Y., Yamaguchi Y., Aze Y., Tomiyama Y., Fujii J., Suzuki K., Kondo A., Shapiro S. D., Lopez-Otin C., Kuwaki T., Okabe M., Honke K., Taniguchi N. (2005) Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 102, 15791–15796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li W., Nakagawa T., Koyama N., Wang X., Jin J., Mizuno-Horikawa Y., Gu J., Miyoshi E., Kato I., Honke K., Taniguchi N., Kondo A. (2006) Down-regulation of trypsinogen expression is associated with growth retardation in alpha1,6-fucosyltransferase-deficient mice: attenuation of proteinase-activated receptor 2 activity. Glycobiology 16, 1007–1019 [DOI] [PubMed] [Google Scholar]
- 7. Wang X., Gu J., Ihara H., Miyoshi E., Honke K., Taniguchi N. (2006) Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J. Biol. Chem. 281, 2572–2577 [DOI] [PubMed] [Google Scholar]
- 8. Shinkawa T., Nakamura K., Yamane N., Shoji-Hosaka E., Kanda Y., Sakurada M., Uchida K., Anazawa H., Satoh M., Yamasaki M., Hanai N., Shitara K. (2003) The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466–3473 [DOI] [PubMed] [Google Scholar]
- 9. Pinho S. S., Seruca R., Gärtner F., Yamaguchi Y., Gu J., Taniguchi N., Reis C. A. (2011) Modulation of E-cadherin function and dysfunction by N-glycosylation. Cell Mol. Life Sci. 68, 1011–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fukuda T., Hashimoto H., Okayasu N., Kameyama A., Onogi H., Nakagawasai O., Nakazawa T., Kurosawa T., Hao Y., Isaji T., Tadano T., Narimatsu H., Taniguchi N., Gu J. (2011) Alpha1,6-fucosyltransferase-deficient mice exhibit multiple behavioral abnormalities associated with a schizophrenia-like phenotype: importance of the balance between the dopamine and serotonin systems. J. Biol. Chem. 286, 18434–18443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ubelhart R., Bach M. P., Eschbach C., Wossning T., Reth M., Jumaa H. (2010) N-linked glycosylation selectively regulates autonomous precursor BCR function. Nat. Immunol. 11, 759–765 [DOI] [PubMed] [Google Scholar]
- 12. Wang F., Nakouzi A., Angeletti R. H., Casadevall A. (2003) Site-specific characterization of the N-linked oligosaccharides of a murine immunoglobulin M by high-performance liquid chromatography/electrospray mass spectrometry. Anal. Biochem. 314, 266–280 [DOI] [PubMed] [Google Scholar]
- 13. Wright J. F., Shulman M. J., Isenman D. E., Painter R. H. (1990) C1 binding by mouse IgM. The effect of abnormal glycosylation at position 402 resulting from a serine to asparagine exchange at residue 406 of the μ-chain. J. Biol. Chem. 265, 10506–10513 [PubMed] [Google Scholar]
- 14. Haimovich J., Ben Moshe N., Raviv Y., Hollander N. (2010) All oligosaccharide moieties of the μ chains in the pre-BCR are of the high-mannose type. Mol. Immunol. 48, 351–355 [DOI] [PubMed] [Google Scholar]
- 15. Stubbs H. J., Lih J. J., Gustafson T. L., Rice K. G. (1996) Influence of core fucosylation on the flexibility of a biantennary N-linked oligosaccharide. Biochemistry 35, 937–947 [DOI] [PubMed] [Google Scholar]
- 16. Li W., Ishihara K., Yokota T., Nakagawa T., Koyama N., Jin J., Mizuno-Horikawa Y., Wang X., Miyoshi E., Taniguchi N., Kondo A. (2008) Reduced alpha4beta1 integrin/vascular cell adhesion molecule-1 interactions lead to impaired pre-B cell repopulation in alpha1,6-fucosyltransferase deficient mice. Glycobiology 18, 114–124 [DOI] [PubMed] [Google Scholar]
- 17. Uozumi N., Teshima T., Yamamoto T., Nishikawa A., Gao Y. E., Miyoshi E., Gao C. X., Noda K., Islam K. N., Ihara Y., Fujii S., Shiba T., Taniguchi N. (1996) A fluorescent assay method for GDP-L-Fuc:N-acetyl-beta-D-glucosaminide alpha 1–6fucosyltransferase activity, involving high performance liquid chromatography. J. Biochem. 120, 385–392 [DOI] [PubMed] [Google Scholar]
- 18. Ishida H., Moritani T., Hata Y., Kawato A., Suginami K., Abe Y., Imayasu S. (2002) Molecular cloning and overexpression of fleA gene encoding a fucose-specific lectin of Aspergillus oryzae. Biosci. Biotechnol. Biochem. 66, 1002–1008 [DOI] [PubMed] [Google Scholar]
- 19. Miyake K., Weissman I. L., Greenberger J. S., Kincade P. W. (1991) Evidence for a role of the integrin VLA-4 in lymphohemopoiesis. J. Exp. Med. 173, 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hess J., Werner A., Wirth T., Melchers F., Jäck H. M., Winkler T. H. (2001) Induction of pre-B cell proliferation after de novo synthesis of the pre-B cell receptor. Proc. Natl. Acad. Sci. U.S.A. 98, 1745–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mårtensson I. L., Almqvist N., Grimsholm O., Bernardi A. I. (2010) The pre-B cell receptor checkpoint. FEBS Lett. 584, 2572–2579 [DOI] [PubMed] [Google Scholar]
- 22. Kawano Y., Yoshikawa S., Minegishi Y., Karasuyama H. (2006) Pre-B cell receptor assesses the quality of IgH chains and tunes the pre-B cell repertoire by delivering differential signals. J. Immunol. 177, 2242–2249 [DOI] [PubMed] [Google Scholar]
- 23. Wasserman R., Li Y. S., Shinton S. A., Carmack C. E., Manser T., Wiest D. L., Hayakawa K., Hardy R. R. (1998) A novel mechanism for B cell repertoire maturation based on response by B cell precursors to pre-B receptor assembly. J. Exp. Med. 187, 259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cambier J. C. (1995) Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM). J. Immunol. 155, 3281–3285 [PubMed] [Google Scholar]
- 25. Partridge E. A., Le Roy C., Di Guglielmo G. M., Pawling J., Cheung P., Granovsky M., Nabi I. R., Wrana J. L., Dennis J. W. (2004) Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306, 120–124 [DOI] [PubMed] [Google Scholar]
- 26. Wyss D. F., Choi J. S., Li J., Knoppers M. H., Willis K. J., Arulanandam A. R., Smolyar A., Reinherz E. L., Wagner G. (1995) Conformation and function of the N-linked glycan in the adhesion domain of human CD2. Science 269, 1273–1278 [DOI] [PubMed] [Google Scholar]
- 27. Dwek R. A. (1995) Glycobiology: more functions for oligosaccharides. Science 269, 1234–1235 [DOI] [PubMed] [Google Scholar]
- 28. Rabinovich E., Bar-Nun S., Amitay R., Shachar I., Gur B., Taya M., Haimovich J. (1993) Different assembly species of IgM are directed to distinct degradation sites along the secretory pathway. J. Biol. Chem. 268, 24145–24148 [PubMed] [Google Scholar]
- 29. Corcos D., Dunda O., Butor C., Cesbron J. Y., Lorès P., Bucchini D., Jami J. (1995) Pre-B-cell development in the absence of lambda 5 in transgenic mice expressing a heavy-chain disease protein. Curr. Biol. 5, 1140–1148 [DOI] [PubMed] [Google Scholar]
- 30. Shaffer A. L., Schlissel M. S. (1997) A truncated heavy chain protein relieves the requirement for surrogate light chains in early B cell development. J. Immunol. 159, 1265–1275 [PubMed] [Google Scholar]
- 31. Muljo S. A., Schlissel M. S. (2002) The variable, C(H)1, C(H)2 and C(H)3 domains of Ig heavy chain are dispensable for pre-BCR function in transgenic mice. Int. Immunol. 14, 577–584 [DOI] [PubMed] [Google Scholar]
- 32. ten Boekel E., Melchers F., Rolink A. G. (1997) Changes in the V(H) gene repertoire of developing precursor B lymphocytes in mouse bone marrow mediated by the pre-B cell receptor. Immunity 7, 357–368 [DOI] [PubMed] [Google Scholar]
- 33. Gauthier L., Rossi B., Roux F., Termine E., Schiff C. (2002) Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc. Natl. Acad. Sci. U.S.A. 99, 13014–13019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. LeBien T. W. (2000) Fates of human B-cell precursors. Blood 96, 9–23 [PubMed] [Google Scholar]
- 35. Lopez Granados E., Porpiglia A. S., Hogan M. B., Matamoros N., Krasovec S., Pignata C., Smith C. I., Hammarstrom L., Bjorkander J., Belohradsky B. H., Casariego G. F., Garcia Rodriguez M. C., Conley M. E. (2002) Clinical and molecular analysis of patients with defects in micro heavy chain gene. J. Clin. Invest. 110, 1029–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Middendorp S., Dingjan G. M., Hendriks R. W. (2002) Impaired precursor B cell differentiation in Bruton's tyrosine kinase-deficient mice. J. Immunol. 168, 2695–2703 [DOI] [PubMed] [Google Scholar]
- 37. Winkler T. H., Rolink A., Melchers F., Karasuyama H. (1995) Precursor B cells of mouse bone marrow express two different complexes with the surrogate light chain on the surface. Eur. J. Immunol. 25, 446–450 [DOI] [PubMed] [Google Scholar]
- 38. Khan W. N., Alt F. W., Gerstein R. M., Malynn B. A., Larsson I., Rathbun G., Davidson L., Müller S., Kantor A. B., Herzenberg L. A., et al. (1995) Defective B cell development and function in Btk-deficient mice. Immunity 3, 283–299 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.