Background: Centrosomal microtubule nucleation is important in the formation of the microtubule cytoskeleton.
Results: An integrin mutant that suppresses MEK/ERK signaling substantially reduces microtubule nucleation. Expression of activated RAF-1 restores nucleation inhibited by the mutant integrin.
Conclusion: Integrins promote microtubule nucleation by regulating MEK/ERK signaling.
Significance: Environmental cues provided by cell-matrix adhesion contribute to the regulation of the microtubule nucleating activity of the centrosome.
Keywords: Centrosome, ERK, Extracellular Matrix, Integrin, Microtubules, Src
Abstract
Microtubule nucleation is an essential step in the formation of the microtubule cytoskeleton. We recently showed that androgen and Src promote microtubule nucleation and γ-tubulin accumulation at the centrosome. Here, we explore the mechanisms by which androgen and Src regulate these processes and ask whether integrins play a role. We perturb integrin function by a tyrosine-to-alanine substitution in membrane-proximal NPIY motif in the integrin β1 tail and show that this mutant substantially decreases microtubule nucleation and γ-tubulin accumulation at the centrosome. Because androgen stimulation promotes the interaction of the androgen receptor with Src, resulting in PI3K/AKT and MEK/ERK signaling, we asked whether these pathways are inhibited by the mutant integrin and whether they regulate microtubule nucleation. Our results indicate that the formation of the androgen receptor-Src complex and the activation of downstream pathways are significantly suppressed when cells are adhered by the mutant integrin. Inhibitor studies indicate that microtubule nucleation requires MEK/ERK but not PI3K/AKT signaling. Importantly, the expression of activated RAF-1 is sufficient to rescue microtubule nucleation inhibited by the mutant integrin by promoting the centrosomal accumulation of γ-tubulin. Our data define a novel paradigm of integrin signaling, where integrins regulate microtubule nucleation by promoting the formation of androgen receptor-Src signaling complexes to activate the MEK/ERK signaling pathway.
Introduction
During interphase, the microtubule cytoskeleton determines the subcellular localization of organelles, promotes vesicular transport, and directs cell migration. The centrosome is a major site for the nucleation and organization of microtubules (1–3). It includes two centrioles embedded in pericentriolar material. The assembly of the microtubule cytoskeleton is a highly regulated process initiated by nucleation. Newly nucleated microtubules become anchored at the centrosome and exhibit growth, shrinkage, and stabilization in response to extracellular signals.
γ-Tubulin is essential for microtubule nucleation. It associates with other γ complex proteins in the cytoplasm to form γ-tubulin ring complexes (γ-TuRCs),2 which then accumulate at the centrosome to serve as templates for microtubule nucleation (3). NEDD1, also known as GCP-WD, is the last protein to associate with the complex and is required for its centrosomal localization (4, 5). A similar role has recently been proposed for GCP8, a newly identified component of γ-TuRC (6).
Accumulation of γ-tubulin at the centrosome increases dramatically at the onset of mitosis, and this correlates with increases in nucleation rates (7, 8). Current evidence indicates that Plk1 and Cdk1 promote enhanced centrosomal localization of γ-tubulin at mitosis and mitotic spindle formation in part by phosphorylating NEDD1 (9, 10). We recently described a role for the androgen receptor and Src signaling in promoting the centrosomal accumulation of γ-tubulin and microtubule nucleation during interphase (11). Thus, these processes are dynamically regulated throughout the cell cycle.
Numerous reports have identified steroid hormone receptors as initiators of cytoplasmic signaling responses, in addition to their classical roles as transcription factors. Initial studies identified a signaling complex containing the estrogen receptor and Src that leads to the activation of the MEK/ERK pathway (12). These observations were confirmed in a variety of cell systems and have been extended to include the progesterone and androgen receptors (13–15). Later, it was demonstrated that these receptor-Src complexes also lead to activation of the PI3K/AKT pathway (16–19). We recently showed that androgen stimulation promotes microtubule nucleation through a pathway requiring the androgen receptor and Src (11).
Cell-matrix adhesion provides critical positional information important in cell fate decisions. Integrins are α/β heterodimeric adhesion receptors that regulate cellular processes by directly activating signaling pathways or by affecting the signaling activity of other cell surface receptors (20–22). Cross-talk between integrin and tyrosine kinase and cytokine receptors contributes to many normal and pathological processes, including cell migration, proliferation, and survival, in addition to tissue differentiation and tumor progression (22, 23). However, it is not known whether integrins affect signaling pathways initiated by steroid hormone receptors such as the androgen receptor.
Here, we ask whether integrins regulate microtubule nucleation and, if so, whether they do so by affecting the ability of androgen to activate cytoplasmic signaling pathways. Integrin β subunit cytoplasmic domains (β tails) are critical to most aspects of integrin function (24–28). They act as scaffolds for a number of cytoskeletal and signaling proteins connecting integrins to the actin filament cytoskeleton and to a variety of signaling pathways (29). Studies from several laboratories, including our own, have demonstrated that a tyrosine-to-alanine mutation in the membrane-proximal NPIY motif of the β1 tail (Y783A) inhibits integrin activity and downstream signaling (25, 30–32). We recently showed that this mutation inhibits the formation of the microtubule cytoskeleton focused at the centrosome during interphase, the assembly of a bipolar spindle at mitosis, and cytokinesis (33, 34). In this study, we use the Y783A mutant to demonstrate that intact integrin function is required to promote microtubule nucleation and γ-tubulin accumulation at the centrosome by regulating the formation of the androgen receptor-Src complex to activate the MEK/ERK pathway.
EXPERIMENTAL PROCEDURES
Cell Culture
Chinese hamster ovary (CHO-K1) cell lines expressing the αIIb-5β3–1WT (WT cells) or αIIb-5β3–1Y783A (YA cells) chimeric integrins (32, 33) were cultured in Ham's F-12 supplemented with 10% FBS, 1% l-glutamine, and 1% penicillin/streptomycin (complete Ham's F-12). Human foreskin fibroblasts (HFFs; Vec Technologies) were cultured in DMEM supplemented with 10% FBS, 1% l-glutamine, and 1% penicillin/streptomycin (complete DMEM). Experiments were performed in CCM1 (Hyclone), serum-free Ham's F-12, or serum-free DMEM as indicated.
Generation of GFP-γ-Tubulin and RFP-EB3-expressing Cells
Stable cell lines were generated using the Lentiviral Gateway expression system (Invitrogen). GFP-γ-tubulin was amplified from the pEGFP-N1/γ-tubulin plasmid (35) by PCR with the forward primer 5′ CACCATGCCGAGGGAAATCATCAC-CCTA 3′ and reverse primer 5′ TTACTTGTACAGCTCGTCCATGCC 3′, and RFP-EB3 was amplified by PCR using the forward 5′ CACCATGGCCGTCAATGTGTACTCC 3′ and reverse 5′ TTAGGCGCCGGTGGAGTGGCGGCC 3′ primers from the pEGFP-N1/EB3 plasmid in which GFP was swapped with RFP (36). PCR products were inserted into pENTR/D-TOPO (Invitrogen) and then transferred to pLenti6/V5-DEST (Invitrogen) by site-specific recombination as described by the supplier. Successful generation of pLenti6/V5-DEST/GFP-γ-tubulin and pLenti6/V5-DEST/RFP-EB3 was confirmed by DNA sequence analysis. A lentiviral stock was then produced by co-transfection of the 293FT cell line with the expression construct and the ViraPower packaging mix (Invitrogen) and used to transduce WT and YA CHO cells. Cells with appropriate levels of RFP-EB3, with or without GFP-γ-tubulin, were isolated by fluorescence-activated cell sorting using the BD-FACS Aria high speed cell sorter.
Microtubule Regrowth
Similar passages of WT and YA CHO cells were grown to ∼80% confluence in complete Ham's F-12. To assay cells in G1 of the cell cycle, mitotic cells were isolated and replated onto fibrinogen or fibronectin in either CCM1 or serum-free Ham's F-12 for 3 h at 37 °C. HFFs were serum starved for 18 h and then replated onto fibronectin in either CCM1, complete DMEM, or serum-free DMEM for 3 h at 37 °C. Cells were then treated at 4 °C with 10 μg/ml nocodazole (Calbiochem) for 4 h to depolymerize microtubules. Cells were washed with cold PBS to remove the drug, and microtubule regrowth was allowed for 5 min in CCM1, complete DMEM (HFF), serum-free DMEM (HFF), or serum-free Ham's F-12 (CHO). In some experiments androgen, R1881 (PerkinElmer Life Sciences), was added at 10 nm to serum-free medium during the regrowth period. Where indicated, 10 μm SU6656 (Calbiochem) was added during the last half-hour of nocodazole treatment and to all solutions thereafter. Additional experiments included treatment with 1, 5, or 10 μm U0126 (Cell Signaling) or 25, 50, or 100 μm LY294002 (Invitrogen) as indicated. Only cells containing one centrosome were included in our analyses. This was determined by γ-tubulin or GFP-centrin as indicated in the figure legends. Similar results were obtained using either parameter.
Immunofluorescence Microscopy
To visualize microtubules, cells were permeabilized for 30 s in 80 mm PIPES, pH 6.8, 5 mm EGTA, pH 8.0, 1 mm MgCl2, and 0.5% Triton X-100, fixed for 10 min in the same buffer containing 5% glutaraldehyde, and then incubated for 7 min in 1% sodium borohydrate in PBS. Cells were then stained with antibodies to α-tubulin (DM1α, Sigma) and γ-tubulin (AK-15, Sigma) as described previously (33). Alexa-Fluor 488- and 594-conjugated secondary antibodies were from Invitrogen. Samples were analyzed using an inverted Nikon TE2000-E microscope equipped with phase contrast and epifluorescence, a digital camera (CoolSNAP HQ; Roper Scientific), a Ludl rotary encoded stage, and a 37 °C incubator. NIS Elements (Nikon) and AutoQuant deconvolution software (AutoQuant Imaging, Inc.) packages were used. Images were taken on the best centrosomal plane. Exposure time was set and kept constant throughout each independent experiment so that the fluorescent signal from the brightest microtubule array was not saturated. The α-tubulin and γ-tubulin signals were measured in each cell using a region of interest with a radius of 1 μm using NIS Elements software (Nikon). Background fluorescence using a circle of corresponding size was subtracted from each measurement.
Transient Transfection
WT and YA CHO cell lines were plated to ∼60% confluence for 18 h in complete Ham's F-12. The cells were then transfected using TransIT CHO transfection kit (Mirus) with the following expression vectors: FLAG-androgen receptor (18); WT-Src (37); ca-Src (37); dn-CSK (38), or ca-RAF (39). GFP-centrin (40) was used to identify transfected cells where indicated. After 18 h, mitotic cells were knocked off and replated for analysis.
Immunoprecipitation and Western Blotting
To assay the association of the androgen receptor and Src, transfected cells were serum starved for 18 h and then replated onto fibrinogen-coated plates for 3 h. Cells were then lysed with modified RIPA buffer, and the FLAG-androgen receptor of human origin was immunoprecipitated with a FLAG-M2 antibody conjugated to mouse agarose beads (Sigma). As a control, mouse IgG (Santa Cruz Biotechnology) and mouse agarose beads (Sigma) were used. All samples were then analyzed by Western blotting with antibodies to pSrc-Y416 (Cell Signaling) or total Src (Cell Signaling). Blots were stripped and reprobed with antibodies to the human androgen receptor (Santa Cruz Biotechnology) as a control.
To determine activation and expression levels of signaling and other recombinant proteins, a subset of isolated mitotic cells was replated onto fibrinogen-coated plates for 3 h, lysed with modified RIPA buffer, and assayed by Western blotting. Antibodies to pERK1/2 (E10), pAKT-S473 (D9E), and total AKT were purchased from Cell Signaling. Antibodies to total ERK (C-14), total FAK (C-20), EB3 (KT53), and CSK (C-20) were from Santa Cruz Biotechnology, and an antibody to total Src (GD11) was from Upstate Biotechnology Inc.
Microtubule Nucleation Visualized by Live Cell Imaging
Similar passages of WT and YA CHO cells stably expressing RFP-EB3 with or without GFP-γ-tubulin were grown to ∼80% confluence in complete Ham's F-12. Mitotic cells were isolated and replated for 3 h onto fibrinogen-coated glass bottom tissue culture plates (MatTek Corp.) in CCM1 supplemented with 10 mm HEPES, pH 7.4, at 37 °C. Plates were then transferred to a microscope equipped with a heated (37 °C) chamber, and images of RFP (EB3) and GFP-γ-tubulin or GFP-centrin (centrosome) were recorded every 3 s for a total of 30 s. Images were compiled, deconvolved, and then analyzed using ImageJ software. Where indicated, cells were transiently transfected with GFP-centrin with or without ca-Src, dn-CSK, or ca-RAF expression vectors. In some experiments, cells were incubated with 5 μm U0126 for 5 min prior to being transferred to the microscope for filming resulting in a maximal exposure to 5 μm U0126 for 15 min. Again, only cells containing one centrosome were included in or analyses. This was determined by γ-tubulin or GFP-centrin as indicated in the figure legends. Similar results were obtained using either parameter.
Statistical Analysis
To control for independent variation between experiments, all quantitative analysis represents normalized mean values ± S.D. All p values ≤0.05 were deemed as being statistically significant and denoted with an asterisk or hash mark. All analysis was performed using MiniTab 15 Statistical Software. In microtubule nucleation experiments, the mean value for each condition was normalized to the mean value for WT cells replated onto fibrinogen in CCM1. Exceptions are noted in the figure legends (see Figs. 3, 6, and 7). A test of equal variance, followed by either a one- or two-way analysis of variance, was performed and comparisons were made using Fisher's LSD test. To determine the fold changes in Src activation, the mean values were normalized to WT cells replated in CCM1. The fold changes in androgen-triggered Src, AKT, and ERK phosphorylation were calculated by normalizing the mean values to serum starved cells. Differences in the normalized mean values were deemed statistically significant using a test for normal distribution and a single sample t test.
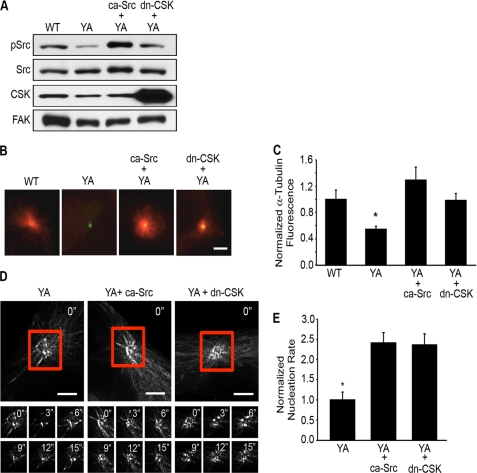
FIGURE 3.
Activation of Src signaling restores microtubule nucleation. A–E, WT or YA cells co-transfected with GFP-centrin with or without ca-Src or dn-CSK were replated onto fibrinogen in CCM1 and then assayed for Src activation and microtubule nucleation. A, Src activation was assayed by Western blotting using a phospho-specific antibody and reprobed for total Src as a loading control. C-terminal Src kinase (CSK) and focal adhesion kinase (FAK) levels were also assayed. B, representative images of microtubule regrowth stained for α-tubulin (red). C, quantification of the microtubule density at the centrosome during a regrowth assay. Values are the normalized fluorescence intensities ± S.D. calculated from 150 cells per condition (n = 3). D, time-lapse imaging of RFP-EB3 was used to track newly nucleated microtubules. Shown are results from representative cells where images were taken every 3 s. E, normalized nucleation rate ± S.D. calculated from 10 cells per condition (n = 3). For this experiment, values were normalized to control YA cells. *, p < 0.05 for control YA cells compared with all other conditions. Scale bar, 3 μm.
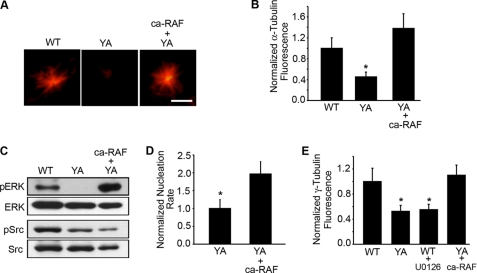
FIGURE 6.
Restoring MEK signaling in cells adhered by the YA mutant integrin promotes microtubule nucleation. A–E, WT and YA cells co-transfected with GFP-centrin with or without ca-RAF were replated onto fibrinogen in CCM1. A, shown are representative images of rapid microtubule regrowth with α-tubulin staining (red). B, quantification of the microtubule density at the centrosome where the values are the normalized fluorescence intensities of the α-tubulin signal ± S.D. calculated from 150 cells per condition (n = 3). *, p < 0.05. C, representative Western blot of ERK and Src phosphorylation using antibodies specific for activated ERK and Src, respectively. Blots were reprobed for total protein levels. D, normalized nucleation rate ± S.D. calculated from 10 cells per condition (n = 3). Values were normalized to control YA cells. *, p < 0.05. E, MEK/ERK signaling promotes γ-tubulin accumulation at the centrosome. Quantification of the normalized fluorescence intensities of the γ-tubulin signal at the centrosome from microtubule regrowth assays. Values are the normalized fluorescence intensities ± S.D. calculated from 150 cells per condition (n = 3). *, p < 0.05. Scale bar, 3 μm.
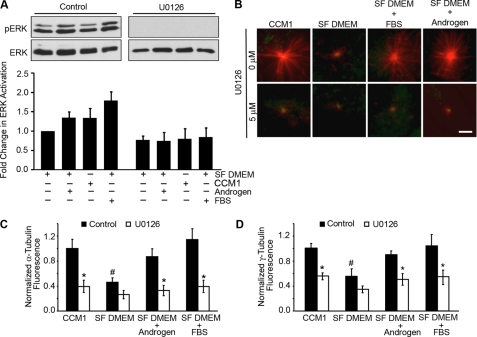
FIGURE 7.
Regulation of microtubule nucleation and the centrosomal accumulation of γ-tubulin is not cell type-specific. A–D, HFFs were replated onto fibronectin in CCM1, DMEM plus FBS, serum-free DMEM, or serum-free DMEM plus androgen, with androgen added for only 5 min. Treatment with the MEK inhibitor, U0126, for 5 min suppressed ERK activation. A, representative Western blot of ERK phosphorylation using antibodies specific for activated ERK and reprobed for total ERK. B, shown are representative images of rapid microtubule regrowth stained for α-tubulin (red) and γ-tubulin (green). C, quantification of the microtubule density at the centrosome. Values are the normalized fluorescence intensities of the α-tubulin signal ± S.D. calculated from 150 cells per condition (n = 3). D, quantification of the normalized fluorescence intensities of the γ-tubulin signal at the centrosome ± S.D. calculated from 150 cells per condition (n = 3). *, p < 0.05 for cells treated with U0126 compared with control. #, p < 0.05 for cells adhered in serum-free (SF) DMEM compared with the CCM1 control, serum-free DMEM plus androgen, and serum-free DMEM plus FBS. Scale bar, 3 μm.
RESULTS
YA Mutation Substantially Reduces Microtubule Nucleation from the Centrosome
To determine whether integrins regulate microtubule nucleation from the centrosome, we inhibited integrin function using a previously characterized Y783A mutation in the integrin β1 tail (25, 30–33). We used CHO cell lines stably expressing chimeric integrins containing either the wild-type β1 tail (WT) or the Y783A mutant β1 tail (YA) in the context of the αIIb-5β3-1 heterodimeric chimeric integrins (32, 33). These chimeras contain the extracellular and transmembrane domains of the αIIbβ3 fibrinogen-binding receptor connected to the cytoplasmic tails of the α5β1 fibronectin receptor (Fig. 1A). To isolate the function of the recombinant integrins (WT or YA), we adhered these cells to fibrinogen in CCM1, a serum-free growth-promoting medium.
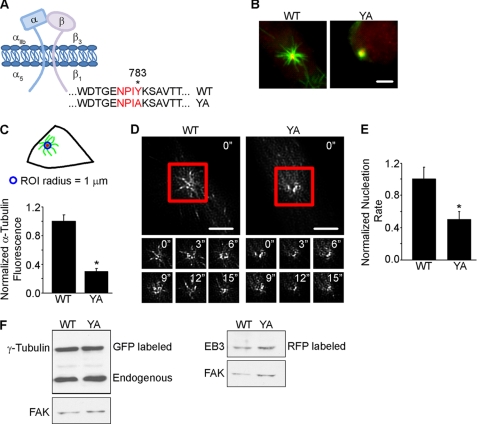
FIGURE 1.
YA mutation substantially decreases microtubule nucleation from the centrosome. A, schematic of the recombinant integrins. B–F, WT and YA cells were replated onto fibrinogen in CCM1. B–C, microtubule nucleation was assayed at 5 min of regrowth. B, micrographs of representative cells stained for α-tubulin (green) and γ-tubulin (red). C, quantification of the microtubule density at the centrosome using a region of interest (ROI) with a radius of 1 μm. Values are the normalized fluorescence intensities of the α-tubulin signal ± S.D. calculated from 150 cells per condition (n = 3). Top, schematic of experimental design. D and E, time-lapse imaging was used to track newly nucleated microtubules in cells stably co-expressing RFP-EB3 and GFP-γ-tubulin. D, shown are images taken every 3 s of EB3 comets emanating from the centrosome in a representative cell. See supplemental Fig. S1 for an example of how the appearance of newly nucleated microtubules was quantified by the appearance of new EB3 comets. E, normalized nucleation rate ± S.D. calculated from 10 cells per condition (n = 3). F, Western blot analysis of γ-tubulin and EB3 protein levels. *, p < 0.05; FAK, focal adhesion kinase. Scale bar, 3 μm.
The ability of cells to promote microtubule nucleation was assayed using two independent approaches. First, we assayed the density of microtubules at the centrosome during a microtubule regrowth assay, as a burst of nucleation occurs at the centrosome following nocodazole washout. We determined the density of newly nucleated microtubules by measuring the fluorescence intensity of the α-tubulin signal using a region of interest with a radius of 1 μm centered at the centrosome. The results indicate that the fluorescence intensity of the α-tubulin signal at the centrosome is significantly decreased when cells are adhered to fibrinogen by the YA mutant integrin compared with the WT integrin (Fig. 1, B and C).
As a complementary approach, we used live cell imaging to determine the rate at which newly nucleated microtubules emanate from the centrosome (8, 11, 41). For these studies, we generated cells stably expressing low levels of RFP-microtubule end-binding protein 3 (RFP-EB3) to label the plus end of growing microtubules and GFP-γ-tubulin to ensure centrosome-orientated microtubule nucleation. The results indicate that cells adhered by the WT integrin have an average nucleation rate of 19.6 ± 4.2 comets/min, whereas cells adhered by the YA mutant integrin have an average nucleation rate of only 10.1 ± 3.0 comets/min (Fig. 1, D and E and supplemental Fig. 1). Importantly, both WT and YA cells expressed similar levels of RFP-EB3 and GFP-γ-tubulin (Fig. 1F). Thus, cells adhered by the YA mutant integrin exhibit substantially reduced microtubule nucleation from the centrosome.
YA Mutation Inhibits the Association of Androgen Receptor with Src
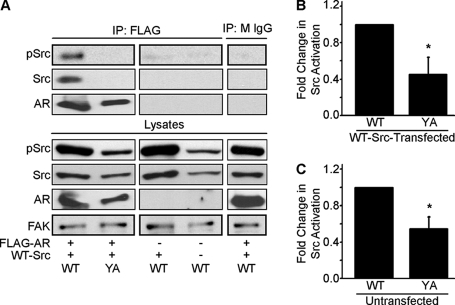
We previously showed that androgen is the main component in CCM1 medium that promotes microtubule nucleation and that it does so in an androgen receptor-dependent manner (11). It is known that the androgen receptor cytoplasmic signaling cascade is initiated by its interaction with Src (17, 18). To begin to dissect the mechanism by which integrins regulate microtubule nucleation, we compared the ability of the androgen receptor-Src signaling complex to form in cells adhered by either the WT or mutant integrin. To facilitate the detection of the complex by co-immunoprecipitation, we transiently co-expressed wild-type Src (WT-Src) and FLAG-tagged androgen receptor (FLAG-AR). Using the FLAG epitope as bait, we were able to detect an interaction between the androgen receptor and Src in cells adhered by the WT integrin; however, it was not observed when cells were adhered by the mutant integrin (Fig. 2A and supplemental Fig. 2). Importantly, WT-Src transfected cells adhered by the mutant integrin showed decreased levels of Src activation compared with cells adhered by the WT integrin (Fig. 2B). This was not specific to transfected cells; similar results were obtained when we assayed endogenous Src using untransfected cells (Fig. 2C). To our knowledge, this is the first report that integrins regulate androgen receptor signaling.
FIGURE 2.
YA mutation inhibits the association of the androgen receptor with Src. A, WT and YA cells were either co-transfected with FLAG-tagged androgen receptor (FLAG-AR) and wild-type Src (WT-Src), with WT-Src alone, or left untransfected as indicated. Transfected cells were serum starved and then replated onto fibrinogen in CCM1 to assay the association of the AR and Src. Antibodies directed against the FLAG tag (left panels) or a mouse antibody control (right panel) were used for immunoprecipitation (IP). Immunoprecipitates were analyzed by Western blotting with antibodies specific for activated Src. Blots were stripped and reprobed for total Src and the AR. Lysates were also analyzed for each condition by Western blotting for the AR, phosphorylated Src, and total Src levels and reprobed for total focal adhesion kinase (FAK) as a loading control (bottom panels). Shown is a representative experiment (n = 3). B and C, cells adhered by the YA mutant integrin in CCM1 are inhibited in Src activation. Quantification of the fold change in Src activity ± S.D. (n = 3) from WT Src-transfected cells (B) or untransfected cells (C). *, p < 0.05.
Activating Src Signaling Rescues Microtubule Nucleation
We previously showed that Src signaling is required for androgen to promote microtubule nucleation at the centrosome in human foreskin fibroblasts (11). Thus, as expected, the addition of androgen to serum-free medium promoted microtubule nucleation in cells adhered by the WT but not YA mutant integrins (supplemental Fig. 3, A and B), and adhering YA cells to fibronectin by their endogenous α5β1 integrins rescued androgen-triggered microtubule nucleation (supplemental Fig. 4). In addition, androgen stimulation promoted Src activation in WT cells, although the Src kinase inhibitor SU6656 inhibited androgen-triggered microtubule nucleation (supplemental Fig. 3, A–D).
To determine whether activating Src signaling is sufficient to promote nucleation in cells adhered by the mutant integrin, we experimentally activated Src by expressing either a constitutively active Src mutant (ca-Src), containing a tyrosine-to-phenylalanine substitution (Y527F) at the regulatory tyrosine residue (42), or a dominant negative (kinase-dead, K222R) CSK mutant (dn-CSK) to increase endogenous Src activation (38). The results indicate that expression of either ca-Src or dn-CSK in YA cells activates Src signaling and promotes microtubule nucleation (Fig. 3, A–E). A similar rescue in microtubule nucleation was observed when we assayed the density of microtubules at the centrosome and when we measured the rate at which microtubules emanated from the centrosome. Together, these data demonstrate the importance of a functional integrin to promote Src signaling and microtubule nucleation. Thus, perturbing integrin function by the YA mutation suppresses microtubule nucleation due to decreased Src signaling.
PI3K/AKT Signaling Does Not Regulate Microtubule Nucleation
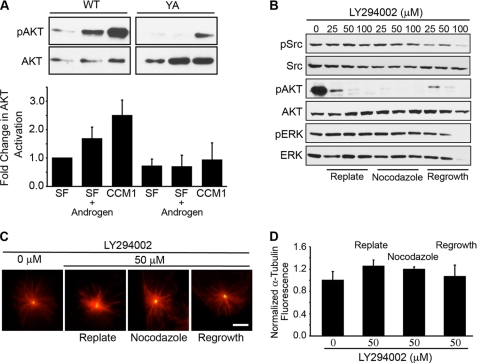
Because we determined that cells adhered by the YA mutant are inhibited in the formation of an androgen receptor-Src complex, we next asked whether signals downstream of Src are required for microtubule nucleation. We first examined the PI3K/AKT axis and, as expected, found that androgen stimulation promoted AKT phosphorylation in cells adhered by the WT but not the mutant integrin (Fig. 4A). To determine whether PI3K/AKT signaling regulates nucleation, we used the PI3K inhibitor, LY294002, in cells adhered in CCM1 by the WT integrin. The results indicate that treatment with LY294002 effectively inhibits AKT phosphorylation but does not suppress microtubule nucleation (Fig. 4, B–D). Thus, PI3K/AKT activity is not required to promote microtubule nucleation, at least in this context.
FIGURE 4.
PI3K/AKT signaling does not regulate microtubule nucleation. A, cells adhered by the YA mutant integrin are inhibited in androgen-triggered AKT activation. AKT activation was analyzed by Western blotting using a phospho-specific antibody and then reprobed for total AKT. Quantification of the fold change in AKT activity ± S.D. (n = 3). B–D, PI3K inhibitor, LY294002, specifically inhibits AKT phosphorylation but does not inhibit microtubule nucleation. WT cells were replated onto fibrinogen in CCM1 and treated with varying concentrations of LY294002 (0, 25, 50, or 100 μm) either as cells were replated with the addition of nocodazole or only during the regrowth period. B, Src, AKT, and ERK were analyzed using phospho-specific antibodies and reprobed for the total protein levels. C, representative images of microtubule regrowth in cells adhered by WT integrins and treated with 50 μm LY294002 are stained for α-tubulin (red) and γ-tubulin (green). D, quantification of the microtubule density at the centrosome. Values are the normalized fluorescence intensities of the α-tubulin signal ± S.D. from 150 cells per condition (n = 3). Scale bar, 3 μm.
MEK/ERK Signaling Promotes Microtubule Nucleation and Centrosomal Accumulation of γ-Tubulin
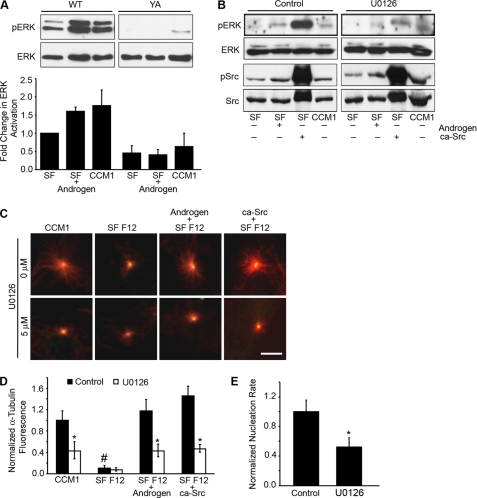
Cells adhered by the YA mutant integrin are also inhibited in androgen-triggered ERK activation as expected (Fig. 5A). To examine the role of the RAF/MEK/ERK pathway, we treated cells adhered by WT integrins with the MEK inhibitor, U0126. The results indicate that the inhibition of ERK activation significantly suppressed microtubule nucleation as indicated by the decrease in the α-tubulin signal at the centrosome (Fig. 5, B–D). Microtubule nucleation was also assayed by calculating the nucleation rate using live cell imaging. WT cells treated with U0126 had an average nucleation rate of 11.7 ± 2.8 comets/min, whereas the average nucleation rate of untreated cells was 22.3 ± 3.5 comets/min (Fig. 5E and supplemental Fig. 5A). Thus, MEK signaling is required to promote microtubule nucleation from the centrosome. Furthermore, ca-Src did not promote nucleation when MEK was inhibited, which is consistent with the role of Src upstream of MEK (Fig. 5, B–D).
FIGURE 5.
MEK/ERK signaling promotes microtubule nucleation. A, cells adhered by the YA mutant integrin are inhibited in androgen-triggered ERK activation. ERK phosphorylation was analyzed by Western blotting using an antibody specific to activated ERK and reprobed for total ERK. Quantification of the fold change in ERK activity ± S.D. (n = 3). B–E, MEK inhibitor, U0126, specifically inhibits ERK phosphorylation and microtubule nucleation. B–D, WT cells co-transfected with GFP-centrin with or without ca-Src were replated onto fibrinogen in CCM1, serum-free (SF) F-12, or serum-free F-12 plus androgen, with androgen added only for 5 min. ERK activity was suppressed by treating cells with 5 μm U0126 for only 5 min. B, representative Western blot of ERK and Src phosphorylation using antibodies specific for activated ERK and Src, respectively. Blots were reprobed for total protein levels. C, shown are representative images of cells during rapid microtubule regrowth with α-tubulin staining (red). D, quantification of the microtubule density at the centrosome where the values represent the normalized fluorescence intensities of the α-tubulin signal ± S.D. calculated from 150 cells per condition (n = 3). *, p < 0.0 5 for cells treated with U0126 compare with control. #, p < 0.01 for WT cells adhered in SF-12 compared with the CCM1 control, serum-free F-12 plus androgen and serum-free F-12 plus ca-Src. E, normalized nucleation rate ± S.D. calculated from 10 cells per condition (n = 3). *, p < 0.05. Scale bar, 3 μm.
To determine whether activation of MEK/ERK signaling is sufficient to promote microtubule nucleation in cells adhered by the mutant integrin, we expressed a constitutively active RAF-1 mutant (ca-RAF) containing a deletion of the amino-terminal regulatory domain (43). Microtubule nucleation was assayed by measuring the fluorescence intensity of the α-tubulin signal at the centrosome and by counting the number of EB3 comets emanating from the centrosome per unit of time. Expression of ca-RAF activated MEK/ERK signaling and restored the level of α-tubulin signal at the centrosome to that of cells adhered by WT integrins (Fig. 6, A–C), and it increased the nucleation rate to 22.3 ± 3.9 comets/min compared with 11.3 ± 2.8 comets/min observed in untransfected cells adhered by the YA mutant integrin (Fig. 6D and supplemental Fig. 5B). These data reveal a role for MEK/ERK signaling in microtubule nucleation and indicate that activating this pathway is sufficient to restore microtubule nucleation in cells adhered by the mutant integrin. Importantly, activating MEK/ERK signaling restored microtubule nucleation without affecting cell morphology (supplemental Fig. 5C). Furthermore, inhibition of MEK in the presence of either androgen or the constitutive activation of Src suppressed microtubule nucleation (Fig. 5, B–D), and inhibition of Src abrogated ERK activation (supplemental Fig. 6). Taken together our results define a novel mechanism of integrin signaling with integrins as regulators of microtubule nucleation acting through an androgen-Src-MEK-ERK pathway.
Increased levels of γ-tubulin at the centrosome are indicative of increased nucleation rates (7, 8, 11). To determine whether the decreased nucleation rates observed when cells were adhered by the mutant integrin are due to altered γ-tubulin levels at the centrosome, we measured the fluorescence intensity of the γ-tubulin signal at the centrosome. The results indicate that cells adhered by the YA mutant have lower levels of centrosomal γ-tubulin compared with WT cells (Fig. 6E). In addition, U0126 inhibited the accumulation of γ-tubulin at the centrosome when cells were adhered by WT integrins. Conversely, activating MEK signaling with the ca-RAF mutant restored levels of centrosomal γ-tubulin when cells were adhered by the mutant integrin. Thus, MEK/ERK signaling may promote microtubule nucleation during interphase by regulating the centrosomal localization of γ-tubulin.
Regulation of Microtubule Nucleation by MEK/ERK Signaling Is Not Cell Type-specific
We next asked whether the requirement for MEK/ERK signaling is specific to CHO cells. Previous data from our laboratory demonstrated that androgen and Src signaling regulates microtubule nucleation in HFFs (11). Therefore, we examined the effects of the U0126 MEK inhibitor when HFFs were adhered to fibronectin in CCM1, in serum-free DMEM with or without androgen, or in serum-containing DMEM. The data indicate that inhibition of the MEK/ERK pathway results in decreased microtubule nucleation (Fig. 7, A–C) and decreased accumulation of γ-tubulin at the centrosome (Fig. 7D). Together, these data demonstrate that MEK/ERK signaling regulates microtubule nucleation and γ-tubulin accumulation at the centrosome by a mechanism that is independent of cell type and culture conditions.
DISCUSSION
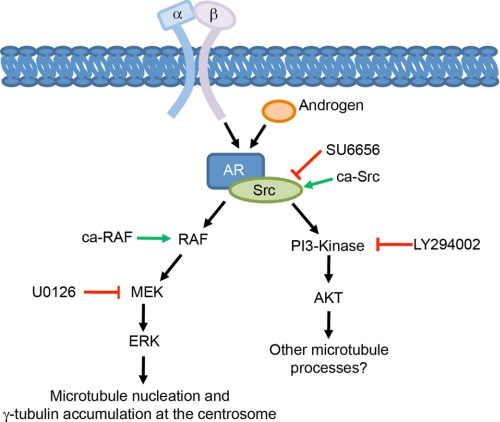
Microtubule nucleation at the centrosome is a fundamental cellular process, and thus, understanding the mechanisms involved is biologically important. In this study, we identified integrins and the MEK/ERK signaling pathway as major regulators of this process and propose that their downstream target is the centrosomal accumulation of γ-tubulin. We demonstrate that normal integrin function is required for the formation of a complex containing the androgen receptor and Src that is required for androgen-triggered ERK activation. Thus, we have defined a novel pathway whereby integrins promote microtubule nucleation through an androgen receptor-Src-MEK-ERK pathway (Fig. 8).
FIGURE 8.
Model. Our data are consistent with published reports that the androgen receptor and Src form a signaling module that activates MEK/ERK and PI3K/AKT signaling in response to stimulation with androgen. We show that integrins regulate the formation of the androgen receptor-Src complex and the ability of androgen to trigger the activation of AKT and ERK. Additionally, our results demonstrate that MEK/ERK signaling promotes the centrosomal accumulation of γ-tubulin and microtubule nucleation independent of cell type and culture conditions. Inhibiting MEK/ERK signaling with U0126 inhibits microtubule nucleation when cells are adhered by wild-type integrins, and expression of ca-RAF to activate MEK/ERK signaling is sufficient to rescue microtubule nucleation when integrin function is inhibited. Although inhibiting PI3K/AKT signaling does not effect microtubule nucleation, this pathway may regulate other processes required for the assembly of the microtubule cytoskeleton.
In principle, microtubule nucleation could be regulated at multiple steps, the assembly of γ-TuRCs, their centrosomal localization, or their nucleating activity once at the centrosome. Current evidence indicates the centrosomal accumulation of γ-TuRCs is a major point of control. Most studies have focused on the regulation of this process at the onset of mitosis because there is a dramatic increase in the centrosomal accumulation of γ-tubulin and microtubule nucleation in response to centrosome maturation (7, 8, 44). Several reports highlight the importance of Plk1 and its centrosomal localization in this process (9, 10, 45).
Our studies have identified integrins and MEK/ERK signaling as regulators of the centrosomal localization of γ-tubulin and microtubule nucleation during interphase. We previously hypothesized that Src might phosphorylate a member of the γ-TuRC to promote assembly of the complex and/or its localization to the centrosome (11). Here, we used a MEK inhibitor to demonstrate a role for the MEK/ERK pathway, and we further showed that expression of a constitutively active RAF-1 mutant is sufficient to promote microtubule nucleation during interphase. Our data also indicate that Src activity is required for androgen-triggered ERK activation. Therefore, we propose that Src activity promotes centrosomal accumulation of γ-tubulin and microtubule nucleation by activating the MEK/ERK pathway. At present, ERK is the only known substrate of MEK (46); thus, identifying the relevant ERK substrates will likely provide critical mechanistic insight.
It is likely that MEK/ERK signaling downstream of a number of different cell surface receptors, including tyrosine kinase and G-protein-coupled receptors, will also promote microtubule nucleation. In this regard, we showed that serum stimulation also promoted microtubule nucleation by a MEK/ERK-dependent mechanism. Furthermore, the activation of MEK/ERK signaling is common among steroid hormone receptors, as both progesterone and estrogen receptors can trigger this pathway (12, 13). In addition, estrogen and androgen are known to activate signaling in cells outside the reproductive organs (47). Thus, activation of MEK/ERK signaling downstream of androgen, progesterone, estrogen, or a variety of cell surface receptors is likely to be involved in the regulation of microtubule nucleation in many biological contexts.
Interestingly, MEK/ERK signaling can suppress microtubule stability (48, 49), in addition to promoting microtubule nucleation. Whether these two processes are interdependent regulatory events is subject to further experimentation. It is possible that a decrease in microtubule stability could result in increased levels of unpolymerized tubulin, which might enhance the rate of microtubule nucleation. We have not yet compared microtubule stability in cells adhered by WT and the YA mutant integrin. This is an important future direction.
Integrins regulate a number of other signaling proteins that target microtubule dynamics, such as the Rho family of GTPases, which influence microtubule growth and stability (50–56). Although the activity of these GTPases has not yet been assayed in our system, we know that the mutant integrin inhibits cell spreading, which is a Rac1-dependent process (26, 32), and Rac1 has been shown to promote microtubule growth (54, 55). Another potential integrin target is GSK-3β, which inhibits the activity of multiple microtubule-binding proteins that promote microtubule anchorage and growth (54, 57–60). Because AKT phosphorylates GSK-β to suppress its activity, the inhibition of AKT by the mutant integrin may also lead to defects in microtubule anchorage and growth. Thus, the restoration of microtubule nucleation may not be sufficient to maintain a microtubule cytoskeleton focused at the centrosome when cells are adhered by the mutant integrin. If this is the case, it will be important to identify additional signaling pathways needed.
Other laboratories have analyzed the mechanisms that regulate the formation of the androgen receptor-Src complex. These studies have demonstrated that androgen stimulation promotes the association of Src with the androgen receptor through the binding of Src homology 3 domain to a proline-rich domain in the androgen receptor (61); this mechanism also mediates the association of Src with the progesterone receptor (14). These interactions are presumably allowed by the ligand-induced conformational change in the androgen receptor and lead to Src activation by suppressing the autoinhibitory conformation of Src (16). Consistent with this model, we observe a stimulation of Src activity with the addition of androgen to cells adhered by WT integrins. Our results taken together strongly suggest that androgen-triggered Src activation triggers MEK/ERK signaling and microtubule nucleation in our system and that cells adhered by the mutant integrin are inhibited in these processes. Importantly, integrins are also known to regulate MEK/ERK signaling downstream of tyrosine kinase receptors, albeit by a different mechanism (22). Thus, integrin-regulated MEK/ERK signaling is likely to regulate microtubule nucleation in multiple biological contexts.
Subcellular localization is an important factor in the formation of signaling complexes. Integrins are known to nucleate the formation of multimolecular complexes containing Src and other signaling proteins. Consequently, much of integrin signaling can be localized to adhesion sites (21). Interestingly, the YA mutation inhibits the formation of focal adhesions (32) as well as the formation of the androgen receptor-Src complex, suggesting these processes may be linked. Our preliminary observations suggest that the androgen receptor does not localize at focal adhesion sites. However, the ability of integrins to regulate the subcellular localization of the androgen receptor needs further analysis.
Integrins have also been shown to regulate the surface expression of lipid rafts by a mechanism that depends both on the microtubule and actin cytoskeletons (62), which are defective in cells adhered by the mutant integrin (32, 33). Furthermore, the ability of integrins to regulate lipid rafts has important consequences for integrin signaling events (63). Because the androgen receptor and Src are known to associate with lipid rafts at the plasma membrane (64–66), it is possible that the mutant integrin inhibits the formation of the androgen receptor-/Src complex and downstream signaling by preventing the surface expression of lipid rafts.
The importance of the cytoplasmic signaling activities of the androgen receptor in normal and pathophysiological processes is just beginning to be fully appreciated. Our results demonstrating that integrins are co-regulators of these activities indicate that spatial cues provided by cell-matrix adhesion may help limit the activation of these pathways to the appropriate time and place. It will be important to determine how cancer cells bypass this mechanism of control.
Supplementary Material
Acknowledgments
We thank Dr. J. Brugge for the Src retroviral vectors; Dr. S. Nada for the dn-CSK expression vector; Dr. J. Salisbury for the GFP-centrin expression vector; Dr. J. Q. Cheng for the FLAG-androgen receptor expression vector; Dr. A. Khodjakov for helpful discussions, and Dr. Peter Vincent for assistance with statistical analysis. We also thank Dr. S. Mathew for critically reading this manuscript and Dr. C. Lim at the flow cytometry core, Ordway Institute.
This work was supported, in whole or in part, by National Institutes of Health Grant GM51540 (to S. E. L.).

This article contains supplemental Figs. S1–S6 and an additional reference.
- γ-TuRC
- γ-tubulin ring complex
- RFP
- red fluorescent protein
- HFF
- human foreskin fibroblast
- AR
- androgen receptor
- ca
- constitutively active
- dn
- dominant negative.
REFERENCES
- 1. Bornens M. (2002) Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14, 25–34 [DOI] [PubMed] [Google Scholar]
- 2. Doxsey S., McCollum D., Theurkauf W. (2005) Centrosomes in cellular regulation. Annu. Rev. Cell Dev. Biol. 21, 411–434 [DOI] [PubMed] [Google Scholar]
- 3. Lüders J., Stearns T. (2007) Microtubule-organizing centers: a re-evaluation. Nat. Rev. Mol. Cell Biol. 8, 161–167 [DOI] [PubMed] [Google Scholar]
- 4. Haren L., Remy M. H., Bazin I., Callebaut I., Wright M., Merdes A. (2006) NEDD1-dependent recruitment of the γ-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 172, 505–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lüders J., Patel U. K., Stearns T. (2006) GCP-WD is a γ-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8, 137–147 [DOI] [PubMed] [Google Scholar]
- 6. Teixidó-Travesa N., Villén J., Lacasa C., Bertran M. T., Archinti M., Gygi S. P., Caelles C., Roig J., Lüders J. (2010) The γ-TuRC revisited. A comparative analysis of interphase and mitotic human γ-TuRC redefines the set of core components and identifies the novel subunit GCP8. Mol. Biol. Cell 21, 3963–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khodjakov A., Rieder C. L. (1999) The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle do not require microtubules. J. Cell Biol. 146, 585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piehl M., Tulu U. S., Wadsworth P., Cassimeris L. (2004) Centrosome maturation. Measurement of microtubule nucleation throughout the cell cycle by using GFP-tagged EB1. Proc. Natl. Acad. Sci. U.S.A. 101, 1584–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haren L., Stearns T., Lüders J. (2009) Plk1-dependent recruitment of γ-tubulin complexes to mitotic centrosomes involves multiple PCM components. PLoS One 4, e5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang X., Chen Q., Feng J., Hou J., Yang F., Liu J., Jiang Q., Zhang C. (2009) Sequential phosphorylation of Nedd1 by Cdk1 and Plk1 is required for targeting of the γ-TuRC to the centrosome. J. Cell Sci. 122, 2240–2251 [DOI] [PubMed] [Google Scholar]
- 11. Colello D., Reverte C. G., Ward R., Jones C. W., Magidson V., Khodjakov A., LaFlamme S. E. (2010) Androgen and Src signaling regulate centrosome activity. J. Cell Sci. 123, 2094–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Domenico M., Castoria G., Bilancio A., Migliaccio A., Auricchio F. (1996) Estradiol activation of human colon carcinoma-derived Caco-2 cell growth. Cancer Res. 56, 4516–4521 [PubMed] [Google Scholar]
- 13. Migliaccio A., Piccolo D., Castoria G., Di Domenico M., Bilancio A., Lombardi M., Gong W., Beato M., Auricchio F. (1998) Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 17, 2008–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boonyaratanakornkit V., Scott M. P., Ribon V., Sherman L., Anderson S. M., Maller J. L., Miller W. T., Edwards D. P. (2001) Progesterone receptor contains a proline-rich motif that directly interacts with Src homology 3 domains and activates c-Src family tyrosine kinases. Mol. Cell 8, 269–280 [DOI] [PubMed] [Google Scholar]
- 15. Peterziel H., Mink S., Schonert A., Becker M., Klocker H., Cato A. C. (1999) Rapid signaling by androgen receptor in prostate cancer cells. Oncogene 18, 6322–6329 [DOI] [PubMed] [Google Scholar]
- 16. Migliaccio A., Castoria G., Di Domenico M., de Falco A., Bilancio A., Lombardi M., Barone M. V., Ametrano D., Zannini M. S., Abbondanza C., Auricchio F. (2000) Steroid-induced androgen receptor-oestradiol receptor β-Src complex triggers prostate cancer cell proliferation. EMBO J. 19, 5406–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Migliaccio A., Castoria G., Di Domenico M., De Falco A., Bilancio A., Auricchio F. (2002) Src is an initial target of sex steroid hormone action. Ann. N.Y. Acad. Sci. 963, 185–190 [DOI] [PubMed] [Google Scholar]
- 18. Sun M., Yang L., Feldman R. I., Sun X. M., Bhalla K. N., Jove R., Nicosia S. V., Cheng J. Q. (2003) Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85α, androgen receptor, and Src. J. Biol. Chem. 278, 42992–43000 [DOI] [PubMed] [Google Scholar]
- 19. Baron S., Manin M., Beaudoin C., Leotoing L., Communal Y., Veyssiere G., Morel L. (2004) Androgen receptor mediates nongenomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J. Biol. Chem. 279, 14579–14586 [DOI] [PubMed] [Google Scholar]
- 20. Hynes R. O. (2002) Integrins. Bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 21. Berrier A. L., Yamada K. M. (2007) Cell-matrix adhesion. J. Cell Physiol. 213, 565–573 [DOI] [PubMed] [Google Scholar]
- 22. Streuli C. H., Akhtar N. (2009) Signal cooperation between integrins and other receptor systems. Biochem. J. 418, 491–506 [DOI] [PubMed] [Google Scholar]
- 23. Schwartz M. A., Ginsberg M. H. (2002) Networks and cross-talk. Integrin signaling spreads. Nat. Cell Biol. 4, E65–E68 [DOI] [PubMed] [Google Scholar]
- 24. Ylänne J., Chen Y., O'Toole T. E., Loftus J. C., Takada Y., Ginsberg M. H. (1993) Distinct functions of integrin α and β subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J. Cell Biol. 122, 223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bodeau A. L., Berrier A. L., Mastrangelo A. M., Martinez R., LaFlamme S. E. (2001) A functional comparison of mutations in integrin β cytoplasmic domains. Effects on the regulation of tyrosine phosphorylation, cell spreading, cell attachment, and β1 integrin conformation. J. Cell Sci. 114, 2795–2807 [DOI] [PubMed] [Google Scholar]
- 26. Berrier A. L., Martinez R., Bokoch G. M., LaFlamme S. E. (2002) The integrin β tail is required and sufficient to regulate adhesion signaling to Rac1. J. Cell Sci. 115, 4285–4291 [DOI] [PubMed] [Google Scholar]
- 27. Hirsch E., Barberis L., Brancaccio M., Azzolino O., Xu D., Kyriakis J. M., Silengo L., Giancotti F. G., Tarone G., Fässler R., Altruda F. (2002) Defective Rac-mediated proliferation and survival after targeted mutation of the β1 integrin cytodomain. J. Cell Biol. 157, 481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tadokoro S., Shattil S. J., Eto K., Tai V., Liddington R. C., de Pereda J. M., Ginsberg M. H., Calderwood D. A. (2003) Talin binding to integrin β tails. A final common step in integrin activation. Science 302, 103–106 [DOI] [PubMed] [Google Scholar]
- 29. Legate K. R., Fässler R. (2009) Mechanisms that regulate adaptor binding to β-integrin cytoplasmic tails. J. Cell Sci. 122, 187–198 [DOI] [PubMed] [Google Scholar]
- 30. O'Toole T. E., Ylanne J., Culley B. M. (1995) Regulation of integrin affinity states through an NPXY motif in the β subunit cytoplasmic domain. J. Biol. Chem. 270, 8553–8558 [DOI] [PubMed] [Google Scholar]
- 31. Kääpä A., Peter K., Ylänne J. (1999) Effects of mutations in the cytoplasmic domain of integrin β(1) to talin binding and cell spreading. Exp. Cell Res. 250, 524–534 [DOI] [PubMed] [Google Scholar]
- 32. Nieves B., Jones C. W., Ward R., Ohta Y., Reverte C. G., LaFlamme S. E. (2010) The NPIY motif in the integrin β1 tail dictates the requirement for talin-1 in outside-in signaling. J. Cell Sci. 123, 1216–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reverte C. G., Benware A., Jones C. W., LaFlamme S. E. (2006) Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis. J. Cell Biol. 174, 491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. LaFlamme S. E., Nieves B., Colello D., Reverte C. G. (2008) Integrins as regulators of the mitotic machinery. Curr. Opin. Cell Biol. 20, 576–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khodjakov A., Rieder C. L., Sluder G., Cassels G., Sibon O., Wang C. L. (2002) De novo formation of centrosomes in vertebrate cells arrested during S phase. J. Cell Biol. 158, 1171–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Efimov A., Kharitonov A., Efimova N., Loncarek J., Miller P. M., Andreyeva N., Gleeson P., Galjart N., Maia A. R., McLeod I. X., Yates J. R., 3rd, Maiato H., Khodjakov A., Akhmanova A., Kaverina I. (2007) Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell 12, 917–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmidt J. W., Brugge J. S., Nelson W. J. (1992) pp60src tyrosine kinase modulates P19 embryonal carcinoma cell fate by inhibiting neuronal but not epithelial differentiation. J. Cell Biol. 116, 1019–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takayama Y., Nada S., Nagai K., Okada M. (1997) Role of Csk in neural differentiation of the embryonic carcinoma cell line P19. FEBS Lett. 406, 11–16 [DOI] [PubMed] [Google Scholar]
- 39. Bajaj A., Zheng Q., Adam A., Vincent P., Pumiglia K. (2010) Activation of endothelial ras signaling bypasses senescence and causes abnormal vascular morphogenesis. Cancer Res. 70, 3803–3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. White R. A., Pan Z., Salisbury J. L. (2000) GFP-centrin as a marker for centriole dynamics in living cells. Microsc. Res. Tech. 49, 451–457 [DOI] [PubMed] [Google Scholar]
- 41. Salaycik K. J., Fagerstrom C. J., Murthy K., Tulu U. S., Wadsworth P. (2005) Quantification of microtubule nucleation, growth, and dynamics in wound-edge cells. J. Cell Sci. 118, 4113–4122 [DOI] [PubMed] [Google Scholar]
- 42. Hirai H., Varmus H. E. (1990) Site-directed mutagenesis of the SH2- and SH3-coding domains of c-src produces varied phenotypes, including oncogenic activation of p60c-src. Mol. Cell Biol. 10, 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beeram M., Patnaik A., Rowinsky E. K. (2005) Raf. A strategic target for therapeutic development against cancer. J. Clin. Oncol. 23, 6771–6790 [DOI] [PubMed] [Google Scholar]
- 44. Meraldi P., Nigg E. A. (2002) The centrosome cycle. FEBS Lett. 521, 9–13 [DOI] [PubMed] [Google Scholar]
- 45. Casenghi M., Meraldi P., Weinhart U., Duncan P. I., Körner R., Nigg E. A. (2003) Polo-like kinase 1 regulates Nlp, a centrosome protein involved in microtubule nucleation. Dev. Cell 5, 113–125 [DOI] [PubMed] [Google Scholar]
- 46. Roberts P. J., Der C. J. (2007) Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26, 3291–3310 [DOI] [PubMed] [Google Scholar]
- 47. Kousteni S., Bellido T., Plotkin L. I., O'Brien C. A., Bodenner D. L., Han L., Han K., DiGregorio G. B., Katzenellenbogen J. A., Katzenellenbogen B. S., Roberson P. K., Weinstein R. S., Jilka R. L., Manolagas S. C. (2001) Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors. Dissociation from transcriptional activity. Cell 104, 719–730 [PubMed] [Google Scholar]
- 48. Harrison R. E., Turley E. A. (2001) Active erk regulates microtubule stability in H-ras-transformed cells. Neoplasia 3, 385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tolg C., Hamilton S. R., Morningstar L., Zhang J., Zhang S., Esguerra K. V., Telmer P. G., Luyt L. G., Harrison R., McCarthy J. B., Turley E. A. (2010) RHAMM promotes interphase microtubule instability and mitotic spindle integrity through MEK1/ERK1/2 activity. J. Biol. Chem. 285, 26461–26474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Daub H., Gevaert K., Vandekerckhove J., Sobel A., Hall A. (2001) Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J. Biol. Chem. 276, 1677–1680 [DOI] [PubMed] [Google Scholar]
- 51. Etienne-Manneville S., Hall A. (2003) Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature 421, 753–756 [DOI] [PubMed] [Google Scholar]
- 52. Palazzo A. F., Eng C. H., Schlaepfer D. D., Marcantonio E. E., Gundersen G. G. (2004) Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 303, 836–839 [DOI] [PubMed] [Google Scholar]
- 53. Wen Y., Eng C. H., Schmoranzer J., Cabrera-Poch N., Morris E. J., Chen M., Wallar B. J., Alberts A. S., Gundersen G. G. (2004) EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat. Cell Biol. 6, 820–830 [DOI] [PubMed] [Google Scholar]
- 54. Wittmann T., Waterman-Storer C. M. (2005) Spatial regulation of CLASP affinity for microtubules by Rac1 and GSK3β in migrating epithelial cells. J. Cell Biol. 169, 929–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grigoriev I., Borisy G., Vorobjev I. (2006) Regulation of microtubule dynamics in 3T3 fibroblasts by Rho family GTPases. Cell Motil. Cytoskeleton 63, 29–40 [DOI] [PubMed] [Google Scholar]
- 56. Wickström S. A., Lange A., Hess M. W., Polleux J., Spatz J. P., Krüger M., Pfaller K., Lambacher A., Bloch W., Mann M., Huber L. A., Fässler R. (2010) Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev. Cell 19, 574–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zumbrunn J., Kinoshita K., Hyman A. A., Näthke I. S. (2001) Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 β phosphorylation. Curr. Biol. 11, 44–49 [DOI] [PubMed] [Google Scholar]
- 58. Akhmanova A., Hoogenraad C. C., Drabek K., Stepanova T., Dortland B., Verkerk T., Vermeulen W., Burgering B. M., De Zeeuw C. I., Grosveld F., Galjart N. (2001) Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 104, 923–935 [DOI] [PubMed] [Google Scholar]
- 59. Watanabe T., Noritake J., Kakeno M., Matsui T., Harada T., Wang S., Itoh N., Sato K., Matsuzawa K., Iwamatsu A., Galjart N., Kaibuchi K. (2009) Phosphorylation of CLASP2 by GSK-3β regulates its interaction with IQGAP1, EB1, and microtubules. J. Cell Sci. 122, 2969–2979 [DOI] [PubMed] [Google Scholar]
- 60. Fumoto K., Hoogenraad C. C., Kikuchi A. (2006) GSK-3β-regulated interaction of BICD with dynein is involved in microtubule anchorage at centrosome. EMBO J. 25, 5670–5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Migliaccio A., Varricchio L., De Falco A., Castoria G., Arra C., Yamaguchi H., Ciociola A., Lombardi M., Di Stasio R., Barbieri A., Baldi A., Barone M. V., Appella E., Auricchio F. (2007) Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene 26, 6619–6629 [DOI] [PubMed] [Google Scholar]
- 62. Balasubramanian N., Scott D. W., Castle J. D., Casanova J. E., Schwartz M. A. (2007) Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat. Cell Biol. 9, 1381–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Echarri A., Del Pozo M. A. (2006) Caveolae internalization regulates integrin-dependent signaling pathways. Cell Cycle 5, 2179–2182 [DOI] [PubMed] [Google Scholar]
- 64. Freeman M. R., Cinar B., Lu M. L. (2005) Membrane rafts as potential sites of nongenomic hormonal signaling in prostate cancer. Trends Endocrinol. Metab. 16, 273–279 [DOI] [PubMed] [Google Scholar]
- 65. Cinar B., Mukhopadhyay N. K., Meng G., Freeman M. R. (2007) Phosphoinositide 3-kinase-independent nongenomic signals transit from the androgen receptor to Akt1 in membrane raft microdomains. J. Biol. Chem. 282, 29584–29593 [DOI] [PubMed] [Google Scholar]
- 66. Sandilands E., Frame M. C. (2008) Endosomal trafficking of Src tyrosine kinase. Trends Cell Biol. 18, 322–329 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.