Background: The gap junctional protein connexin 43 (Cx43) has a 1–3 h half-life, and both the proteasome and lysosome have been implicated in Cx43 turnover.
Results: Use of lysine to arginine mutants and kinase inhibitors showed that Akt activation stabilizes gap junctions.
Conclusion: Akt phosphorylation of Cx43 stabilizes gap junctions.
Significance: Linkage of Akt to gap junction stability may mechanistically explain their cell survival/growth roles.
Keywords: Akt PKB, Connexin, Gap Junctions, Intracellular Trafficking, Ubiquitin
Abstract
The pore-forming gap junctional protein connexin 43 (Cx43) has a short (1–3 h) half-life in cells in tissue culture and in whole tissues. Although critical for cellular function in all tissues, the process of gap junction turnover is not well understood because treatment of cells with a proteasomal inhibitor results in larger gap junctions but little change in total Cx43 protein whereas lysosomal inhibitors increase total, mostly nonjunctional Cx43. To better understand turnover and identify potential sites of Cx43 ubiquitination, we prepared constructs of Cx43 with different lysines converted to arginines. However, when transfected into cells, a mutant version of Cx43 with all lysines converted to arginines behaved similarly to wild type in the presence of proteasomal and lysosomal inhibitors, indicating that ubiquitination of Cx43 did not appear to be playing a role in gap junction stability. Through the use of inhibitors and dominant negative constructs, we found that Akt (protein kinase B) activity controlled gap junction stability and was necessary to form larger stable gap junctions. Akt activation was increased upon proteasomal inhibition and resulted in phosphorylation of Cx43 at Akt phosphorylation consensus sites. Thus, we conclude that Cx43 ubiquitination is not necessary for the regulation of Cx43 turnover; rather, Akt activity, probably through direct phosphorylation of Cx43, controls gap junction stability. This linkage of a kinase involved in controlling cell survival and growth to gap junction stability may mechanistically explain how gap junctions and Akt play similar regulatory roles.
Introduction
The passage of ions and small metabolites between adjacent cells occurs via gap junction-mediated intercellular communication (1–3). Vertebrate gap junctions, composed of integral membrane proteins from the connexin gene family, are critically important in regulating development, coordinated contraction of excitable cells, tissue homeostasis, and controlled cell growth and differentiation (2, 3). Connexin mutations have been linked to several diseases (4–6) including oculodentodigital dysplasia, a disease caused by connexin 43 (Cx43)2 mutations that can lead to craniofacial abnormalities, atrioseptal defects, and arrhythmias (7). Cx43, the most ubiquitous connexin, oligomerizes into a hexamer or “connexon” in the trans-Golgi network (8) and, after transport to the plasma membrane and association with other connexons in neighboring cell membranes, can form a gap junction.
Cx43 is highly phosphorylated, and many of the modifications appear to increase gap junction formation whereas others inhibit formation or decrease gap junction channel open time (9–11). Given the unusually short half-life (1–3 h) of Cx43 in cell culture and tissues, Cx43 turnover also appears to be highly regulated (12–16). Cx43 degradation, mostly based on the use of inhibitors, has been reported to involve both the proteasome and lysosome (17–21). Several reports have shown that a ubiquitin ladder can be observed upon immunoprecipitation of Cx43 (17, 22–25). Furthermore, interaction of Cx43 with the ubiquitin ligase Nedd4 and the endocytic adapter protein Eps15 also indicates that Cx43 might be ubiquitinated and degraded via the proteasomal or endocytic pathway (22, 26). Reports indicate that endoplasmic reticulum-associated degradation is involved in the turnover of misfolded Cx43 and is sensitive to heat shock and cytosolic stress (27). However, a recent report using a mutant version of Cx43 with all lysines converted to arginines indicated that this process does not involve ubiquitination of Cx43 (28). Several other observations are not consistent with polyubiquitination of Cx43 leading to proteasomal degradation. Treatment with proteasomal inhibitors often leads to increased levels of Cx43 within gap junctions (i.e. they become larger) with increased phosphorylation, but little change in total Cx43, whereas treatment with lysosomal inhibitors leads to increased levels of the protein (>95% of cell surface Cx43 was retained for up to 6 h after lysosomal inhibition) (29). A clear polyubiquitin ladder co-labeled with Cx43 and ubiquitin antibodies has not been shown nor has a specific lysine acceptor for ubiquitin been identified in Cx43 that when mutated to arginine prevents ubiquitination and subsequent internalization and degradation.
Our laboratories have spent significant time looking for Cx43 ubiquitination and the possible lysine targets for this process. Among several other mutations, we created a construct representing full-length Cx43 with all of the lysines converted to arginines that maintains the same net charge but that could not be ubiquitinated at lysine residues. When expressed in cells that did not express wild-type Cx43, this mutant version trafficked to the plasma membrane, formed gap junctions, and responded to proteasomal inhibitors in a manner similar to wild-type Cx43, i.e. junctions became larger in immunofluorescence studies, and slower migrating Cx43 was observed in immunoblots, essentially demonstrating that direct Cx43 ubiquitination was not necessary to observe the effects of proteasomal inhibition on gap junction size. We then turned our search to other proteins that might be regulated by ubiquitination that could in turn regulate Cx43 localization within the plasma membrane. We found that Akt (protein kinase B) is the most likely candidate for the following reasons: Akt becomes ubiquitinated and phosphorylated (activated) to translocate to the plasma membrane and phosphorylate membrane proteins (30). Proteasomal inhibition led to increased phosphorylation of Akt substrates including Cx43. Inhibition of Akt with specific Akt inhibitors or with a dominant negative version of Akt (either of which dramatically reduce Akt activity) resulted in loss of the proteasomal inhibitor effect, i.e. junctions remained smaller, and less phosphorylated Cx43 was observed. Our data support a model where ubiquitination of Akt leads to increased Akt activity and direct phosphorylation of Cx43, resulting in increased junctional size.
EXPERIMENTAL PROCEDURES
Antibodies and Other Reagents
All general chemicals, unless otherwise noted, were purchased from Fisher Scientific. 12-O-tetradecanoylphorbol-13-acetate (TPA, 75 nm final concentration) was purchased from Sigma Co. Akt VIII and MG132 were purchased from EMD Biosciences (Gibbstown, NJ) and both used at 10 μm. Mouse anti-Cx43 antibodies, Cx43CT1 and Cx43IF, prepared against amino acids 360–382 of Cx43 and antibody Cx43NT1 against amino acids 1–20 (Cx43NT1) of Cx43 (described in Refs. 31–33) were made at the Fred Hutchinson Cancer Research Center Hybridoma Development Facility (Seattle, WA). Anti-hemagglutinin (HA)-tagged monoclonal antibody was from Covance (Princeton, NJ). A rabbit anti-Cx43 (PNRF) was obtained from A. Boynton (34). Akt, phospho-Akt (Ser-473), and phospho-(Ser/Thr) Akt substrate antibodies were purchased from Cell Signaling (Danvers, MA) or Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Cell Line Maintenance, Treatment, cDNA Transfection, and Microinjection
Madin-Darby canine kidney (MDCK) cells that do not express Cx43 (35) and normal rat kidney (NRK)-52E cells (ATTC) were cultured in DMEM (Mediatech, Pittsburgh PA) supplemented with 10% FCS and antibiotics in a humidified 5% CO2 environment. Transfections with wild-type and K/R mutant Cx43 (resulting in all lysines in the sequence being converted to arginines; prepared as described by Su et al.) (28), HA-tagged ubiquitin (a gift from James Roberts, Fred Hutchinson Cancer Research Center), or dominant negative Akt (Akt-K197A) (36), a gift from Michael Quon, University of Maryland School of Medicine, constructs into the MDCK or NRK cell lines were performed by electroporation with a Nucleofector apparatus (Amaxa Biosystems, Gaithersburg, MD), and cells were analyzed 24–48 h later. MDCK cells stably expressing the lysine to arginine mutations (MDCK43K/R) and wild-type Cx43 (MDCK43) were isolated using cloning rings. MG132, Akt VIII, and/or NH4Cl were added to cells for 3 h in OptiMEM 1 (Invitrogen) except where indicated. Where MG132 treatment was followed by TPA incubation, cells were pretreated with MG132 for 30 min followed by TPA for 1 h. For experiments where cells were treated with Akt VIII followed by MG132, cells were pretreated with 10 μm Akt VIII inhibitor for 30 min followed by 10 μm MG132 for 3 h. Cells were microinjected with the gap junction-permeable dye Alexa Fluor 488 (Invitrogen) as described previously (37) and are reported as the number of recipient cells receiving dye in 3 min.
Immunoblotting
Cells were lysed in sample buffer containing 50 mm NaF, 500 μm Na3VO4, 2 mm PMSF, and 1× Complete Protease inhibitor (Roche Applied Science), and cellular proteins were separated by SDS-PAGE (10% polyacrylamide gel). After electrophoresis, protein was transferred to nitrocellulose, the membrane was blocked, and antibodies were incubated as indicated previously (32). IRDye800 donkey anti-mouse (Rockland Immunochemicals, Gilbertsville, PA) and Alexa Fluor 680 goat anti-rabbit (Invitrogen) secondary antibodies were used to visualize rabbit and mouse primary antibodies, respectively, simultaneously and were quantified directly using the Li-Cor Biosciences Odyssey infrared imaging system and associated software (Lincoln, NE). Statistical differences were calculated via a paired two-tailed t test.
Immunofluorescence
Cells were washed twice in PBS, and fixed in cold methanol/acetone (50:50) for 1 min followed by a 1-h block in 1% BSA in PBS. Cells were incubated with a mouse anti-Cx43 antibody (Cx43IF1) or rabbit anti-Cx43 in blocking solution for 1 h. Following several PBS washes, the cultures were incubated with Alexa Fluor 546-conjugated goat anti-rabbit antibody and/or Alexa Fluor 488-conjugated goat anti-mouse antibody for 30–60 min and counterstained with DAPI (Molecular Probes), followed by several washes in PBS. The coverslips were mounted onto slides with DABCO anti-fade medium (25 mg/ml of 1,4-diazobicyclo-(2,2,2)octane (Sigma) diluted in 90% glycerol and 10% PBS, pH 8.6) and viewed with a Zeiss LSM 510 laser scanning fluorescence microscope.
Immunoprecipitation
Anti-HA-tagged antibody inked to agarose (Syd Laboratories, Malden, MA) or the PNRF anti-Cx43 antibody was used in immunoprecipitation reactions as described previously (38). Briefly, cells were lysed in radioimmuneprecipitation assay buffer (0.5% deoxycholate, 0.5% Triton X-100, 100 mm NaCl, 10 mm EDTA, 50 mm NaF, 500 μm Na3VO4, 2 mm PMSF, and 1× Complete Protease inhibitor, 25 mm Tris-HCl, pH 7.2), precleared, incubated with the HA-antibody-coated agarose or PNRF and protein A beads, washed three times with cold radioimmuneprecipitation assay buffer, and eluted with sample buffer prior to SDS-PAGE and immunoblotting.
RESULTS
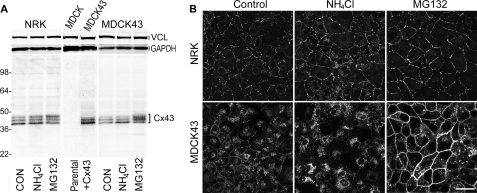
Several studies have shown that treatment of Cx43-expressing cells with proteasomal inhibitors increases the level of Cx43 present in gap junctions often without increasing the total level of Cx43 in the cells. On the other hand, treatment with lysosomal inhibitors leads to an increase in the P0 or fastest migrating form of Cx43. Results consistent with these observations are illustrated in Fig. 1 for NRK and MDCK cells expressing Cx43. NRK cells endogenously express Cx43 and have been a common cell line to examine Cx43 trafficking and gap junction assembly (e.g. 39). In these cells, treatment with the lysosomal degradation inhibitor ammonium chloride (NH4Cl) led to a 33% statistically significant (p < 0.001, n = 9) increase in the fastest migrating Cx43 species compared with control in SDS-PAGE whereas the proteasomal inhibitor MG132 led to a 53% statistically significant increase (p < 0.001, n = 9) in the slower migrating isoforms at the expense of the faster migrating bands (Fig. 1A). The MDCK cells used in this study do not express endogenous Cx43 (35) (Fig. 1A, middle panel) and hence were transfected with a construct containing wild-type Cx43 (MDCK43). These cells showed similar responses to these drugs as the NRK cells with increases of 36 and 45% in the fastest migrating and slower migrating Cx43 species in response to NH4Cl and MG132 treatment, respectively, compared with control (both p < 0.001, n = 9).
FIGURE 1.
Lysosomal and proteasomal inhibitors affect Cx43 protein levels and distribution. A, representative Western immunoblot of Cx43 in NRK and MDCK43 cells treated with ammonium chloride (NH4Cl) or MG132 for inhibition of lysosomal and proteasomal function, respectively. Similar banding patterns were observed in nine independent experiments. In the middle panel, the Cx43 contents of MDCK parental and Cx43 transfected cells are shown. The position of prestained standards of the designated molecular mass in kDa is indicated on the left. Vinculin (VCL) and GAPDH controls were included on the top of each panel as loading controls. B, immunofluorescence microscopy detection of Cx43 in NRK (upper panels) and MDCK43 cells (lower panels) either untreated or treated with NH4Cl or MG132. Scale bar, 25 μm.
Immunofluorescence studies to localize Cx43 in control, NH4Cl-, and MG132-treated NRK (Fig. 1B, top) and MDCK43 (Fig. 1B, bottom) cells showed little change in junctional Cx43 upon NH4Cl treatment and a readily apparent increase in punctate and continuous junctional staining in the presence of MG132.
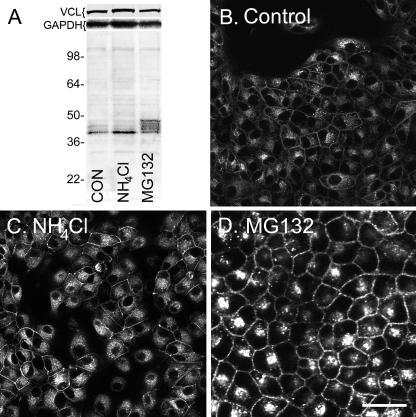
We were interested in determining whether and at which lysine residue Cx43 is ubiquitinated and created several lysine to arginine mutants including a construct of Cx43 where all of the 27 lysine residues in Cx43 were converted to arginines. We stably expressed this K/R mutant version of Cx43 in MDCK cells (termed MDCK43K/R) and examined the protein localization and ability to dye transfer. When MDCK43K/R cells were examined in immunoblots after treatment with proteasomal and lysosomal inhibitors (Fig. 2A), we were surprised to find that the mutant Cx43 reacted very similarly to wild-type Cx43 (MDCK43 cells). Significant increases of 23 and 68% in the amount of the fastest migrating and slower migrating Cx43 species compared with control were observed in SDS-PAGE in response to NH4Cl and MG132 treatment, respectively (both p < 0.001, n = 9). Similarly, Cx43 immunolocalization in MDCK43K/R cells appeared generally similar. Fig. 2B shows the expression of the K/R mutant version of Cx43 at apparent ER/Golgi and gap junctional membranes (CON) via immunofluorescence. NH4Cl led to few overt changes whereas MG132 caused an obvious increase in the amount of junctional Cx43 (Fig. 2, B–D). To examine whether gap junctions formed by the K/R mutant were functional, Alexa Fluor 488 dye transfer was measured 3 min after microinjection. MDCK43K/R cells transferred dye to 3.3 ± 1.7 (mean ± S.D.) cells in contrast to 6.0 ± 3.6 in MDCK cells expressing wild-type Cx43. Because there could be differences in Cx43 expression between the clones, we cannot calculate reliable differences in efficiency due to the mutations but can conclude that the mutated version is capable of forming functional channels.
FIGURE 2.
Lysosomal and proteasomal inhibitors similarly affect Cx43 protein levels and distribution in MDCK43K/R cells. A, representative immunoblot of Cx43 in MDCK43K/R cells untreated or treated with ammonium chloride (NH4Cl) or MG132. Although varying in the magnitude of the shift, similar banding patterns were observed in nine independent experiments. Positions of prestained standards of the designated molecular mass in kDa are indicated on the left. Vinculin (VCL) and GAPDH controls were included on the top of the panel as loading controls. B–D, immunofluorescence microscopy for Cx43 in MDCK43K/R cells either untreated (B) or treated with NH4Cl (C) or MG132 (D). Scale bar, 25 μm.
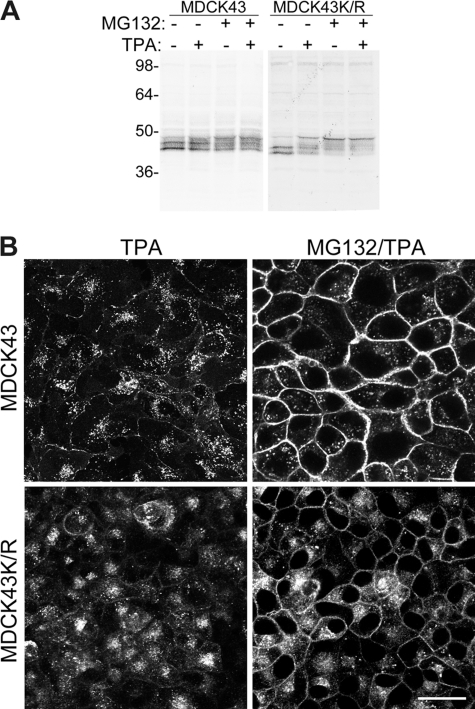
Reagents that activate MAPK and PKC such as EGF and TPA have been reported to lead to increased turnover of gap junctions and increased Cx43 ubiquitination (24) and could possibly represent an alternative pathway for Cx43 ubiquitination and turnover. Therefore, we decided to see whether TPA treatment alone or in combination with proteasomal inhibition affected MDCK43 and MDCK43K/R cells differently. As expected, either TPA or MG132 treatment decreased the mobility of Cx43 in SDS-PAGE (Fig. 3A), although each reagent probably resulted in phosphorylation at different serines in Cx43 (24, 40). The mobility of Cx43 from TPA/MG132-treated cells did not appear to vary from MG132 treatment alone for either MDCK43 or MDCK43K/R cells. This “elimination” of the effect of TPA was more apparent in immunofluorescence studies. TPA treatment led to a near complete loss of gap junctional structures whereas a combined treatment of TPA and MG132 not only retained gap junctions but did not appear to vary from MG132 treatment alone (compare Fig. 3B, right column, with Figs. 1B and 2B) for either MDCK43 or MDCK43K/R cells. Because Cx43 in the MDCK43K/R cells could not be ubiquitinated, these results led us to question the relevance of Cx43 ubiquitination for gap junctional stability and whether proteasomal inhibition was actually affecting the activity of a different protein that regulated gap junctional localization of Cx43.
FIGURE 3.
Cx43 in MDCK43 and MDCK43K/R cells treated with TPA and MG132. A, representative immunoblot for Cx43 in cells treated with TPA (1 h) or MG132 (30 min) followed by TPA addition (1 h). Similar banding patterns were observed in four independent experiments. Positions of prestained standards of the designated molecular mass in kDa are indicated on the left. B, immunofluorescence microscopy for Cx43 in cells treated with TPA or MG132 followed by TPA. Scale bar, 25 μm.
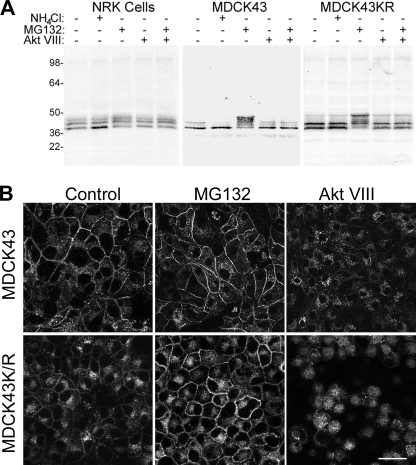
Given that Cx43 localization to junctions is regulated via phosphorylation, we tested the ability of a variety of inhibitors of kinases known to phosphorylate Cx43 to see whether they affected its localization during proteasomal inhibition. Akt has been previously shown to phosphorylate Cx43 (41), and an inhibitor of Akt named Akt VIII (42) completely eliminated the MG132-dependent shift in Cx43 migration found in SDS-PAGE of NRK, MDCK43, and MDCK43K/R cells (Fig. 4A). Furthermore, 3 h of Akt VIII treatment caused Cx43 to localize to cytoplasmic membranes and almost completely eliminated Cx43 in junctional structures (Fig. 4B).
FIGURE 4.
Cx43 in NRK, MDCK43, and MDCK43K/R cells with or without lysosomal, proteasomal, and Akt activity inhibitors. A, representative immunoblot for Cx43 in cells treated with NH4Cl, MG132, or Akt VIII inhibitor. Similar banding patterns were observed in nine independent experiments. Positions of prestained standards of the designated molecular mass in kDa are indicated on the left. B, immunofluorescence microscopy for Cx43 in cells treated with NH4Cl, MG132, or Akt VIII inhibitor. Scale bar, 25 μm.
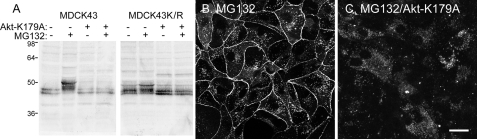
Although Akt VIII is reported to be highly selective (42), we wanted to decrease Akt activity by an alternate method and chose to use a dominant negative Akt-K197A construct (36). When Akt-K197A was expressed in either NRK or MDCK43 cells, the cells no longer responded to MG132 with a shift to the slower migrating Cx43 species in SDS-PAGE compared with controls (Fig. 5A). Similar to Akt VIII treatment, Cx43 immunofluorescence in Akt-K197A-transfected MDCK43 cells treated with MG132 showed mostly cytoplasmic localization (Fig. 5C).
FIGURE 5.
Cx43 in MDCK43 cells overexpressing a dominant negative version of Akt (Akt-K179A). A, immunoblot of MDCK43 and MDCK43K/R cells expressing or not expressing Akt-K179A with or without MG132 treatment. Similar banding patterns were observed in four independent experiments. Positions of prestained standards of the designated molecular mass in kDa are indicated on the left. B and C, immunofluorescence microscopy of MG132-treated cells expressing Akt-K179A (C) or control (B). Scale bar, 25 μm.
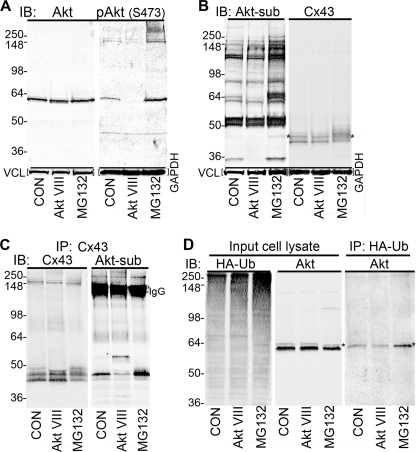
Ubiquitination of Akt has been reported to cause translocation of Akt to the plasma membrane and increase its activity via phosphorylation at Ser-473 (43, 44). We wanted to determine whether MG132 treatment led to increased Akt activity, phosphorylation/activation at Ser-473, increased Akt substrate phosphorylation in general, and increased Cx43 phosphorylation in our cellular systems, thereby making the mechanistic connection between drug treatment and effect. When MDCK43 cells were treated with Akt VIII or MG132, Akt phosphorylation at Ser-473 was dramatically decreased or increased, respectively, as detected with the phospho-Akt (Ser-473) phosphospecific antibody (Fig. 6A; Akt is ∼60 kDa).
FIGURE 6.
Detection of Akt, phospho-Akt (Ser-473) and ubiquitin levels in MDCK43 cells treated with the Akt VIII inhibitor or MG132. A, Akt and phospho-Akt (Ser-473) levels in control (CON) cells or cells treated with Akt VIII or MG132. B, Akt activity and Cx43 in MDCK cells with and without Akt VIII or MG132 treatment as measured by substrate phosphorylation using an antibody that reacts with proteins phosphorylated at Akt consensus phosphorylation sequences (left panel) or an antibody to Cx43 (right panel) in separate optical channels. Asterisks indicate a band that overlays both the phospho-(Ser/Thr) Akt substrate (left panel) and Cx43 (right panel) antibodies. Vinculin (VCL) and GAPDH loading controls are shown below the blot (note that both left and right panels in A and B are from the same blot scanned at different wavelengths). C, immunoprecipitation of Cx43 followed by immunoblotting (IB) with the phospho-(Ser/Thr) Akt substrate antibody (right panel) and Cx43 (left panel; same blot scanned at different wavelengths). D, levels of HA-tagged ubiquitin (HA-Ub; left panel) and Akt in lysates (middle panel) and Akt isolated with HA-antibody-coated agarose (right panel). Asterisks indicate putative ubiquitinated Akt. Positions of prestained standards of the designated molecular mass in kDa are indicated on the left in each panel.
Akt functional activity (i.e. the ability to phosphorylate substrates at Akt consensus sequences) can be assayed via an antibody (45) that recognizes the Akt substrate consensus sequence K/RXK/RXXS/T where S/T is phosphorylated (named phospho-(Ser/Thr) Akt substrate antibody). Akt activity was 52% higher (p = 0.004, n = 6) upon MG132 treatment when densitometry is performed on all bands in an immunoblot of cell lysates probed with the phospho-(Ser/Thr) Akt substrate antibody (i.e. compared with control) (Fig. 6B). Akt VIII treatment showed a nonsignificant 10% decrease in Akt activity as defined by the phospho-(Ser/Thr) Akt substrate antibody compared with control and a statistically significant (p = 0.009) 62% decrease versus MG132 treatment (Fig. 6B, left panel). However, some bands (three in particular at the position of the 64- and the 36-kDa standards and the band indicated by an asterisk) appeared to be more responsive to the 3-h Akt VIII treatment, which most likely represented species that turn over more rapidly or are subject to more acute phosphatase activity. When the same immunoblot was probed with an antibody to Cx43 (Fig. 6B, right panel), a band corresponding to a phosphorylated Cx43 species that overlays with the phospho-(Ser/Thr) Akt substrate antibody (indicated by the asterisk) was detected. When the level of this Akt-phosphorylated species of Cx43 was compared, MG132 treatment led to a 3.3× and 10.0× increase over control and Akt VIII-treated samples (p < 0.001 and p = 0.014, n = 6), respectively. To ensure that the overlaying band was indeed a Cx43 isoform, we immunoprecipitated Cx43 from control, Akt VIII-, or MG132-treated cells and performed immunoblotting with the phospho-(Ser/Thr) Akt substrate antibody or anti-Cx43. As before, MG132 enriched the amount of the slower migrating Cx43 isoforms, most of which reacted with the phospho-(Ser/Thr) Akt substrate antibody, indicating that Akt-dependent phosphorylation of Cx43 was increased in response to MG132.
To show that Akt could be ubiquitinated in our cellular system, as has been previously observed in others (46), we transfected cells with HA-tagged ubiquitin to determine whether Akt could be isolated with HA-antibody-coated beads. In Fig. 6D, we show that MG132 treatment resulted in increased total HA-ubiquitin signal (left panel) and the appearance of a band that overlays exactly with a faint band observed when probed with an anti-Akt antibody in the input cell lysate (marked with an asterisk, this is the same lane blotted with the two different antibodies and detected in different optical channels). When the HA-bead immunoprecipitate was probed with the Akt antibody, MG132 increased the quantity of Akt immunoprecipitated whereas Akt VIII treatment decreased the amount. These results and the molecular size of the apparent ubiquitinated Akt band are essentially the same as reported by Adachi et al. (46).
DISCUSSION
We sought to determine how Cx43 phosphorylation and ubiquitination were linked. However, after trying and failing to discover either sites of Cx43 ubiquitination or a functional need for ubiquitination in the cell types we analyzed, we hypothesized that another ubiquitinated protein that was degraded by the proteasome might in fact regulate gap junctional Cx43 retention and/or removal. We now believe that Akt is the protein that regulates Cx43 gap junctional retention for the following reasons: Akt membrane localization, activation, and phosphorylation of membrane protein substrates are regulated by Akt ubiquitination (30). Ubiquitinated Akt is degraded via the proteasome to limit signaling, a process that is inhibited by blocking proteasomal degradation (46). Our Cx43 K/R mutant protein that contains no lysine acceptors for ubiquitination behaved very similarly to wild-type Cx43 in terms of the effects of TPA treatment and proteasomal inhibition, indicating that ubiquitination is not necessary for the enhanced degradation or stabilization of gap junctional Cx43 caused by these reagents, respectively. Moreover, Akt VIII treatment or transfection with a dominant negative Akt that dramatically reduced Akt ubiquitination/activity abrogated the effect of the proteasomal inhibitor on Cx43 in gap junctions and Cx43 phosphorylation and dramatically reduced the amount of larger stable junctions. Cx43 phosphorylation at Akt sites was increased by proteasomal inhibition and decreased by Akt VIII treatment. Akt has been shown previously to phosphorylate and potentially interact with Cx43 particularly at the edges of gap junction plaques (41). We hypothesize that Akt-dependent Cx43 phosphorylation could result in gap junction stabilization, and we are currently testing this linkage.
Cx43 has a very short half-life (1–3 h) compared with most integral membrane proteins. Many factors apparently influence gap junction assembly and degradation, including cell-cell adhesion and protein kinase activation. For example, protein kinase A (47, 48) and casein kinase 1 (31) activation have been shown to increase the amount of Cx43 within gap junctions whereas MAPK (49) activity decreases junctional Cx43. Treatment of cells with proteasomal inhibitors increases the level of phosphorylated Cx43 and the amount of Cx43 within gap junctions (50, 51). Early results with inhibitors led to speculation that the proteasome might actively degrade ubiquitinated Cx43 that had been in the membrane (17). Because subsequent results more clearly implicated the lysosome in Cx43 degradation (18, 51, 52), Cx43 monoubiquitination has been proposed based on ubiquitin antibody specificity (24). Thus, a current model suggests that Cx43 ubiquitination is involved in regulation of early endosome/lysosome sorting in the endocytic pathway (20, 22, 23).
In the cell types we studied, proteasomal inhibition had a dramatic effect on gap junction plaques, but this effect was not apparently dependent on Cx43 ubiquitination. We do not claim that Cx43 ubiquitination cannot occur, particularly in cells that overexpress components of the ubiquitin conjugation pathway, rather that it is not necessary for the regulation of Cx43 gap junctional stability that is affected negatively and positively by TPA or proteasomal inhibitor treatment, respectively. In addition, we do not discount the role that ubiquitin modification may have in the interactions of Nedd4, Hrs, and Tsg101 with Cx43 (20, 22, 26) because these proteins have also been reported to affect Akt regulation (53–55). Importantly, in our cellular systems, we could not find Cx43 ubiquitination despite significant efforts. On the other hand, Akt ubiquitination and activation, as had been shown previously in other cellular systems (46), was readily apparent and changed in the manner expected for gap junctional stabilization. We did observe an ∼50-kDa band resulting from Cx43 phosphorylation that could explain a similar band observed in other studies in the range for ubiquitinated Cx43. We found that Akt and Cx43 could co-immunoprecipitate (data not shown), also possibly explaining ubiquitin-positive bands.
We have shown that Akt activity is necessary for MG132-dependent stabilization of gap junctions using two molecularly distinct methods for reducing Akt activity. Thus, although Cx43 ubiquitination is not necessary, Akt activity is required for gap junction stabilization induced by proteasomal inhibition. We conclude that inhibition of proteasomal degradation leads to ubiquitination and phosphorylation/activation of Akt, direct Akt phosphorylation of Cx43 and other proteins at Akt substrate sites, and the stabilization of gap junction structures in the plasma membrane. We hypothesize that some of the proposed roles for gap junctional communication may be partially explained by the PI3K/Akt1/mTOR signaling pathways because gap junctional communication and Akt both play roles in the control of apoptosis suppression, inhibition of stress-regulated kinase cascades, and cell cycle regulation (56). This interplay between a proto-oncogene and a tumor suppressor protein has some interesting biological implications and could help explain why gap junctions appear to act as tumor suppressors and yet can promote growth under other conditions (57).
This work was supported, in whole or in part, by National Institutes of Health Grants GM55632-16 (to P. D. L.) and CA052098-18 (to A. F. L.). This work was also supported by American Heart Association Grant 11POST5460028 (to V. S.).
- Cx43
- connexin 43
- MDCK
- Madin-Darby canine kidney
- NRK
- normal rat kidney
- TPA
- 12-O-tetradecanoylphorbol-13-acetate.
REFERENCES
- 1. Laird D. W. (2010) The gap junction proteome and its relationship to disease. Trends Cell Biol. 20, 92–101 [DOI] [PubMed] [Google Scholar]
- 2. Söhl G., Willecke K. (2004) Gap junctions and the connexin protein family. Cardiovasc. Res. 62, 228–232 [DOI] [PubMed] [Google Scholar]
- 3. Scemes E., Suadicani S. O., Dahl G., Spray D. C. (2007) Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 3, 199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kelsell D. P., Dunlop J., Stevens H. P., Lench N. J., Liang J. N., Parry G., Mueller R. F., Leigh I. M. (1997) Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387, 80–83 [DOI] [PubMed] [Google Scholar]
- 5. Bergoffen J., Scherer S. S., Wang S., Scott M. O., Bone L. J., Paul D. L., Chen K., Lensch M. W., Chance P. F., Fischbeck K. H. (1993) Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 262, 2039–2042 [DOI] [PubMed] [Google Scholar]
- 6. Laird D. W. (2006) Life cycle of connexins in health and disease. Biochem. J. 394, 527–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paznekas W. A., Boyadjiev S. A., Shapiro R. E., Daniels O., Wollnik B., Keegan C. E., Innis J. W., Dinulos M. B., Christian C., Hannibal M. C., Jabs E. W. (2003) Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet. 72, 408–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Musil L. S., Goodenough D. A. (1993) Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin 43, occurs after exit from the ER. Cell 74, 1065–1077 [DOI] [PubMed] [Google Scholar]
- 9. Solan J. L., Lampe P. D. (2009) Connexin 43 phosphorylation: structural changes and biological effects. Biochem. J. 419, 261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Solan J. L., Lampe P. D. (2007) Key connexin 43 phosphorylation events regulate the gap junction life cycle. J. Membr. Biol. 217, 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moreno A. P. (2005) Connexin phosphorylation as a regulatory event linked to channel gating. Biochim. Biophys. Acta 1711, 164–171 [DOI] [PubMed] [Google Scholar]
- 12. Lampe P. D. (1994) Analyzing phorbol ester effects on gap junctional communication: a dramatic inhibition of assembly. J. Cell Biol. 127, 1895–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laird D. W., Puranam K. L., Revel J. P. (1991) Turnover and phosphorylation dynamics of connexin 43 gap junction protein in cultured cardiac myocytes. Biochem. J. 273, 67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crow D. S., Beyer E. C., Paul D. L., Kobe S. S., Lau A. F. (1990) Phosphorylation of connexin 43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol. Cell. Biol. 10, 1754–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beardslee M. A., Laing J. G., Beyer E. C., Saffitz J. E. (1998) Rapid turnover of connexin 43 in the adult rat heart. Circ. Res. 83, 629–635 [DOI] [PubMed] [Google Scholar]
- 16. Musil L. S., Beyer E. C., Goodenough D. A. (1990) Expression of the gap junction protein connexin 43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J. Membr. Biol. 116, 163–175 [DOI] [PubMed] [Google Scholar]
- 17. Laing J. G., Beyer E. C. (1995) The gap junction protein connexin 43 is degraded via the ubiquitin proteasome pathway. J. Biol. Chem. 270, 26399–26403 [DOI] [PubMed] [Google Scholar]
- 18. Laing J. G., Tadros P. N., Westphale E. M., Beyer E. C. (1997) Degradation of connexin 43 gap junctions involves both the proteasome and the lysosome. Exp. Cell Res. 236, 482–492 [DOI] [PubMed] [Google Scholar]
- 19. Leithe E., Brech A., Rivedal E. (2006) Endocytic processing of connexin43 gap junctions: a morphological study. Biochem. J. 393, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leithe E., Kjenseth A., Sirnes S., Stenmark H., Brech A., Rivedal E. (2009) Ubiquitylation of the gap junction protein connexin 43 signals its trafficking from early endosomes to lysosomes in a process mediated by Hrs and Tsg101. J. Cell Sci. 122, 3883–3893 [DOI] [PubMed] [Google Scholar]
- 21. Kjenseth A., Fykerud T., Rivedal E., Leithe E. (2010) Regulation of gap junction intercellular communication by the ubiquitin system. Cell. Signal. 22, 1267–1273 [DOI] [PubMed] [Google Scholar]
- 22. Girão H., Catarino S., Pereira P. (2009) Eps15 interacts with ubiquitinated Cx43 and mediates its internalization. Exp. Cell Res. 315, 3587–3597 [DOI] [PubMed] [Google Scholar]
- 23. Leithe E., Rivedal E. (2004) Epidermal growth factor regulates ubiquitination, internalization and proteasome-dependent degradation of connexin 43. J. Cell Sci. 117, 1211–1220 [DOI] [PubMed] [Google Scholar]
- 24. Leithe E., Rivedal E. (2004) Ubiquitination and down-regulation of gap junction protein connexin 43 in response to 12-O-tetradecanoylphorbol-13-acetate treatment. J. Biol. Chem. 279, 50089–50096 [DOI] [PubMed] [Google Scholar]
- 25. Huang T., Zhu Y., Fang X., Chi Y., Kitamura M., Yao J. (2010) Gap junctions sensitize cancer cells to proteasome inhibitor MG132-induced apoptosis. Cancer Sci. 101, 713–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leykauf K., Salek M., Bomke J., Frech M., Lehmann W. D., Dürst M., Alonso A. (2006) Ubiquitin protein ligase Nedd4 binds to connexin 43 by a phosphorylation-modulated process. J. Cell Sci. 119, 3634–3642 [DOI] [PubMed] [Google Scholar]
- 27. Musil L. S. (2009) Biogenesis and degradation of gap junctions, in Connexins: A Guide (Harris A. L., Locke D., eds) pp. 225–240, Humana Press, New York [Google Scholar]
- 28. Su V., Nakagawa R., Koval M., Lau A. F. (2010) Ubiquitin-independent proteasomal degradation of endoplasmic reticulum-localized connexin 43 mediated by CIP75. J. Biol. Chem. 285, 40979–40990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. VanSlyke J. K., Musil L. S. (2005) Cytosolic stress reduces degradation of connexin 43 internalized from the cell surface and enhances gap junction formation and function. Mol. Biol. Cell 16, 5247–5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang W. L., Wang J., Chan C. H., Lee S. W., Campos A. D., Lamothe B., Hur L., Grabiner B. C., Lin X., Darnay B. G., Lin H. K. (2009) The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science 325, 1134–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cooper C. D., Lampe P. D. (2002) Casein kinase 1 regulates connexin 43 gap junction assembly. J. Biol. Chem. 277, 44962–44968 [DOI] [PubMed] [Google Scholar]
- 32. Lampe P. D., Cooper C. D., King T. J., Burt J. M. (2006) Analysis of Connexin 43 phosphorylated at Ser-325, Ser-328, and Ser-330 in normoxic and ischemic heart. J. Cell Sci. 119, 3435–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sosinsky G. E., Solan J. L., Gaietta G. M., Ngan L., Lee G. J., Mackey M. R., Lampe P. D. (2007) The C-terminus of connexin 43 adopts different conformations in the Golgi and gap junction as detected with structure-specific antibodies. Biochem. J. 408, 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hossain M. Z., Ao P., Boynton A. L. (1998) Rapid disruption of gap junctional communication and phosphorylation of connexin 43 by platelet-derived growth factor in T51B rat liver epithelial cells expressing platelet-derived growth factor receptor. J. Cell. Physiol. 174, 66–77 [DOI] [PubMed] [Google Scholar]
- 35. Jordan K., Solan J. L., Dominguez M., Sia M., Hand A., Lampe P. D., Laird D. W. (1999) Trafficking, assembly, and function of a connexin 43-green fluorescent protein chimera in live mammalian cells. Mol. Biol. Cell 10, 2033–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cong L. N., Chen H., Li Y., Zhou L., McGibbon M. A., Taylor S. I., Quon M. J. (1997) Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol. Endocrinol. 11, 1881–1890 [DOI] [PubMed] [Google Scholar]
- 37. Richards T. S., Dunn C. A., Carter W. G., Usui M. L., Olerud J. E., Lampe P. D. (2004) Protein kinase C spatially and temporally regulates gap junctional communication during human wound repair via phosphorylation of connexin 43 on serine 368. J. Cell Biol. 167, 555–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Solan J. L., Marquez-Rosado L., Sorgen P. L., Thornton P. J., Gafken P. R., Lampe P. D. (2007) Phosphorylation at Ser-365 is a gatekeeper event that changes the structure of connexin 43 and prevents down-regulation by PKC. J. Cell Biol. 179, 1301–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Musil L. S., Goodenough D. A. (1991) Biochemical analysis of connexin 43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J. Cell Biol. 115, 1357–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Solan J. L., Fry M. D., TenBroek E. M., Lampe P. D. (2003) Connexin 43 phosphorylation at Ser-368 is acute during S and G2/M and in response to protein kinase C activation. J. Cell Sci. 116, 2203–2211 [DOI] [PubMed] [Google Scholar]
- 41. Park D. J., Wallick C. J., Martyn K. D., Lau A. F., Jin C., Warn-Cramer B. J. (2007) Akt phosphorylates connexin 43 on Ser-373, a “mode-1” binding site for 14-3-3. Cell Commun. Adhes. 14, 211–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lindsley C. W., Zhao Z., Leister W. H., Robinson R. G., Barnett S. F., Defeo-Jones D., Jones R. E., Hartman G. D., Huff J. R., Huber H. E., Duggan M. E. (2005) Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg. Med. Chem. Lett. 15, 761–764 [DOI] [PubMed] [Google Scholar]
- 43. Feng J., Park J., Cron P., Hess D., Hemmings B. A. (2004) Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 279, 41189–41196 [DOI] [PubMed] [Google Scholar]
- 44. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 45. Alessi D. R., Caudwell F. B., Andjelkovic M., Hemmings B. A., Cohen P. (1996) Molecular basis for the substrate specificity of protein kinase B: comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 399, 333–338 [DOI] [PubMed] [Google Scholar]
- 46. Adachi M., Katsumura K. R., Fujii K., Kobayashi S., Aoki H., Matsuzaki M. (2003) Proteasome-dependent decrease in Akt by growth factors in vascular smooth muscle cells. FEBS Lett. 554, 77–80 [DOI] [PubMed] [Google Scholar]
- 47. Atkinson M. M., Lampe P. D., Lin H. H., Kollander R., Li X. R., Kiang D. T. (1995) Cyclic AMP modifies the cellular distribution of connexin 43 and induces a persistent increase in the junctional permeability of mouse mammary tumor cells. J. Cell Sci. 108, 3079–3090 [DOI] [PubMed] [Google Scholar]
- 48. Burghardt R. C., Barhoumi R., Sewall T. C., Bowen J. A. (1995) Cyclic AMP induces rapid increases in gap junction permeability and changes in the cellular distribution of connexin 43. J. Membr. Biol. 148, 243–253 [DOI] [PubMed] [Google Scholar]
- 49. Ruch R. J., Trosko J. E., Madhukar B. V. (2001) Inhibition of connexin 43 gap junctional intercellular communication by TPA requires ERK activation. J. Cell. Biochem. 83, 163–169 [DOI] [PubMed] [Google Scholar]
- 50. Girão H., Pereira P. (2003) Phosphorylation of connexin 43 acts as a stimulus for proteasome-dependent degradation of the protein in lens epithelial cells. Mol. Vis. 9, 24–30 [PubMed] [Google Scholar]
- 51. Musil L. S., Le A. C., VanSlyke J. K., Roberts L. M. (2000) Regulation of connexin degradation as a mechanism to increase gap junction assembly and function. J. Biol. Chem. 275, 25207–25215 [DOI] [PubMed] [Google Scholar]
- 52. Rivedal E., Leithe E. (2005) Connexin 43 synthesis, phosphorylation, and degradation in regulation of transient inhibition of gap junction intercellular communication by the phorbol ester TPA in rat liver epithelial cells. Exp. Cell Res. 302, 143–152 [DOI] [PubMed] [Google Scholar]
- 53. Dai B., Pieper R. O., Li D., Wei P., Liu M., Woo S. Y., Aldape K. D., Sawaya R., Xie K., Huang S. (2010) FoxM1B regulates NEDD4–1 expression, leading to cellular transformation and full malignant phenotype in immortalized human astrocytes. Cancer Res. 70, 2951–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fallon L., Bélanger C. M., Corera A. T., Kontogiannea M., Regan-Klapisz E., Moreau F., Voortman J., Haber M., Rouleau G., Thorarinsdottir T., Brice A., van Bergen En Henegouwen P. M., Fon E. A. (2006) A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI3K-Akt signaling. Nat. Cell Biol. 8, 834–842 [DOI] [PubMed] [Google Scholar]
- 55. Kwak Y. D., Wang B., Pan W., Xu H., Jiang X., Liao F. F. (2010) Functional interaction of phosphatase and tensin homologue (PTEN) with the E3 ligase NEDD4–1 during neuronal response to zinc. J. Biol. Chem. 285, 9847–9857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Franke T. F. (2008) PI3K/Akt: getting it right matters. Oncogene 27, 6473–6488 [DOI] [PubMed] [Google Scholar]
- 57. Naus C. C., Laird D. W. (2010) Implications and challenges of connexin connections to cancer. Nat. Rev. Cancer 10, 435–441 [DOI] [PubMed] [Google Scholar]