Background: We use GbpC as a model protein of LRRK2, which is defective in Parkinson disease.
Results: GbpC, like LRRK2, translocates toward the membrane, which is needed for proper functional activity in vivo.
Conclusion: cAMP stimulation induces a cascade leading to activation and, independently, to GRAM-dependent translocation of GbpC toward the cell boundary.
Significance: The experiments provide new insights in the function of GbpC and LRRK2.
Keywords: Actin, Cyclic GMP (cGMP), Dictyostelium, LRRK2, Membrane
Abstract
GbpC is a multidomain Roco protein in Dictyostelium, involved in transduction of intracellular cGMP that is produced by chemotactic signals. We have shown previously that cGMP binding to GbpC induces an intramolecular signaling cascade by activating subsequently the GEF, Ras, and kinase domains. In this study, we report on the cellular localization of GbpC. In resting cells, the protein is present in the cytoplasm, but GbpC rapidly translocates to the cell boundary upon stimulation with the chemoattractant cAMP. Also, during the formation of cell-cell streams and osmotic shock, the protein localizes toward the plasma membrane and actin cytoskeleton. The translocation upon cAMP stimulation occurs downstream of heterotrimeric G proteins but is independent of guanylyl cyclases and the previously identified cGMP-induced intramolecular signaling cascade in GbpC. Mutations in the GRAM domain of GbpC lead to disturbed membrane association and inactivation of GbpC function during chemotaxis in vivo. Furthermore, we show that the GRAM domain itself associates with cellular membranes and binds various phospholipids in vitro. Together, the results show that GbpC receives multiple input signals that are both required for functional activity in vivo. cAMP-stimulation induces a cGMP-dependent signaling cascade, leading to activation of kinase activity, and, independently, cAMP induces a GRAM-dependent translocation of GbpC toward the plasma membrane and cell cortex, where it may locally phosphorylate effector proteins, which are needed for proper biological activity.
Introduction
Parkinson disease (PD)3 is a neurodegenerative disease that affects more than 5 million people worldwide and one in 100 people over the age of 60. PD is both a chronic and degenerative disorder that is characterized by loss of dopaminergic neurons in the substantia nigra, associated with the formation of fibrillar aggregates composed of α-synuclein and other proteins (1). Recently, missense mutations in LRRK2 have been linked to autosomal-dominant, late-onset PD (2, 3). LRRK2 is a member of the novel Roco family of complex Ras-like GTPases that have an unique domain architecture. Roco proteins are characterized by the presence of N-terminal leucine-rich repeats (LRR), a Ras-like G-domain called Roc (Ras of complex proteins), followed by a COR domain (C-terminal of Roc) and a kinase domain (4, 5). Next to this general domain composition of the Roco family, individual Roco proteins are found to be combined with a diversity of additional domains. Although the Roco proteins have been studied intensely because of the discovery of missense mutations in LRRK2 segregating with PD, the exact pathogenic role and molecular mechanism of how Roco proteins are activated and function are still not completely understood (6, 7). We have previously used Dictyostelium GbpC as model for the complex structure and regulatory mechanism of LRRK2. GbpC, also called Roco1, was originally identified in a bioinformatical screen for molecular targets of the second messenger cGMP and is the founding member of the Roco family of proteins (4, 8, 9). Besides the conserved Roco region, GbpC has an unique regulatory C-terminal region consisting of a Ras Exchange Motif (REM), DEP, CDC25, and two cyclic nucleotide binding domains with a GRAM domain inserted in between (10). Contrary to LRRK2, the cellular function of GbpC has been characterized in detail. GbpC is the only cGMP signal-transducing protein in Dictyostelium. cGMP binds with high affinity to its cyclic nucleotide binding domain, and cGMP-mediated GbpC activation is essential for the proper regulation of myosin II during chemotaxis, for cell-cell interactions during multicellular stream formation, and to resist osmotic stress (8, 10–13). Subsequent experiments showed that GbpC contains a complete intramolecular signal transduction pathway. cGMP binding to the cyclic nucleotide binding domain causes activation of the GEF domains, the subsequent GDP/GTP exchange of the Roc-COR domain leading to the activation of the mitogen-activated protein kinase kinase kinase domain, and phosphorylation of downstream targets (14). This intramolecular signaling cascade is essential for GbpC to mediate its signaling function in the cell. Analogous to the human Roco protein LRRK2, the LRRs of GbpC are vital for biological activity of GbpC but are not directly involved in the activation of the Roc and kinase domain (15).
Recent data suggest that the subcellular localization of LRRK2 is important for its activity and function. The protein is present both in the cytoplasm and at the membrane, and the membrane-associated LRRK2 dimer most likely represents the physiologically active form of the protein (16). The regulation of membrane association is not well understood but probably includes dimerization, posttranslational modification, and protein-protein interactions (16–18). Here we report that GbpC, like LRRK2, translocates toward the membrane. In resting cells, the protein is present uniformly in the cytoplasm, but during stream formation and under osmotic stress, the protein localizes toward the boundary of the cell. Furthermore, uniform stimulation with the chemoattractant cAMP induces a rapid translocation of the protein toward the cell boundary. To better understand the localization of Roco proteins in the cell, we studied the translocation of GbpC and its function for chemotaxis and cell streaming. Together, our results show that GbpC receives multiple input signals. cAMP stimulation induces a cGMP-dependent signaling cascade leading to kinase activity and, independently, GRAM-dependent translocation of GbpC toward the plasma membrane and cell cortex, which are both needed for proper functional activity in vivo.
EXPERIMENTAL PROCEDURES
Strains, Culture Conditions, and Western Blotting
All cells were cultured in HG5 medium (14.3 g/liter oxoid pepton, 7.15 g/liter bacto yeast extract, 1.36 g/liter Na2HPO4*12 H2O, 0.49 g/liter KH2PO4, 10 g/liter glucose), and 10 μg/ml G418 and hygromycin B were added to the medium for selection of cells with extrachromosomal plasmids. Western blotting was done as described previously (14).
Cloning of GbpC Mutants, DEP, and the GRAM Domain
The cloning process of GbpC mutants followed the same strategy as described before (14), with the following notes. Mutations in the GRAM domain of gbpC ORF were created using site-directed mutagenesis of the previously described GbpC part 6 and part 8. Novel amplified fragments of gbpC cDNA (carrying introduced mutations) were ligated into pBluescript first, and after sequencing, the inserts were subsequently exchanged with part 6 or part 8 of the previously described GbpC parts 6–8 in the pGemTeasy plasmid (Promega) using unique restriction sites. The last step of the cloning process (fusion of parts 6–8 with parts 1–5 in MB74-derived expression plasmids) was done as described previously (14). The primer pair used for expression of the GRAM domain (amino acids 2331–2470) was as follows: CGGATCCAAAAAAATGACGTCGACTTCACCATTG (the BamHI site is shown in boldface, followed by a Kozak sequence and an underlined start codon) and GGCGGCCGCTTAACTAGT AGCCAATTTATTTTTG (the SpeI site is shown in boldface). The PCR product was ligated in pBluescript, digested with BamHI/SpeI, and ligated in the BglII/SpeI digested Dictyostelium MB74GFP expression plasmid. The plasmids were coelectroporated with monomeric red fluorescent protein MARS (RFP) to gbpC-null cells (19).
Fluorescence Microscopy
All fluorescence experiments were carried out on a confocal laser scanning microscope (Zeiss ConfoCor 2-LSM 510 combination setup). For the cAMP-stimulations, vegetative cells were harvested, washed twice in 17 mm phosphate buffer (PB) (pH 6.5), and starved in 6-well plates (Nunc) on non-nutrient-agar (17 mm PB with 1.5% agar) overnight at 8 °C. The next day, cell aggregation was initiated by incubating the plates at room temperature. When cells formed streams, they were harvested and settled in a flow chamber. After 10 min, cells were stimulated with 1 μm cAMP, and fluorescence was recorded. To analyze GbpC translocation during osmotic stress, cells were resuspended in PB and incubated with 200 mm sorbitol. The line scans and image analyzes were done with ImageJ using a macro that measures the total fluorescence intensity of the cytoplasm in every frame (20). For each strain, 5–20 cells from at least two independent experiments were analyzed.
Phospholipid Filter Binding Assay
Lysates were made by collecting, washing, and resuspending 1.5 × 107 Dictyostelium cells in 1 ml of lysis buffer (20 mm HEPES (pH 7.0), 1% Triton, 100 mm KCl, 1 μg/ml crushed EDTA-free protease inhibitor tablets (Roche)). Samples were left on ice for 60 min, centrifuged (10 min at 4 °C, 14,000 × g), and the supernatants were collected. PIP strip membranes (Echelon) were blocked in blocking solution (3% BSA in TBST (25 mm Tris-HCl, 137 mm NaCl, 5 mm KCl, 0.05% Tween (pH 8.0)) for 1 h at room temperature. Next, the PIP strips were incubated for 1 h with 10 ml of TBST containing 50 μl of a lysate. After washing three times for 10 min with TBST, anti-GFP antibody (Santa Cruz Biotechnology, Inc., dilution 1:500) in blocking solution was added to the membranes for incubation overnight on a rocking platform at 4 °C. The next day, strips were first washed three times with TBST, and then secondary antibody in blocking solution was added (anti-mouse IgG, Santa Cruz Biotechnology, Inc., dilution 1:2000). Strips were incubated for 1 h at room temperature and subsequently washed three times with TBST. Bound protein was detected using Lumi-Light Plus Western blotting substrate (Roche) according to the supplier's information.
Protein input was visualized with Western blotting using 10 μl of lysate from cells expressing GRAM-GFP and GRAMG2378A-GFP. For direct comparison, both proteins were assayed on PIP strips in parallel and exposed on film together.
Chemotaxis, cGMP-binding Assay, and GTP-agarose Pull-down Assays
Chemotaxis was measured using a small population assay as described before (21). cGMP-binding was measured using a radioisotope binding assay, and GTP-binding was assayed using GTP-coupled agarose beads, both as described before (14).
RESULTS
GbpC Localizes to the Cell Boundary upon cAMP Stimulation and during Streaming
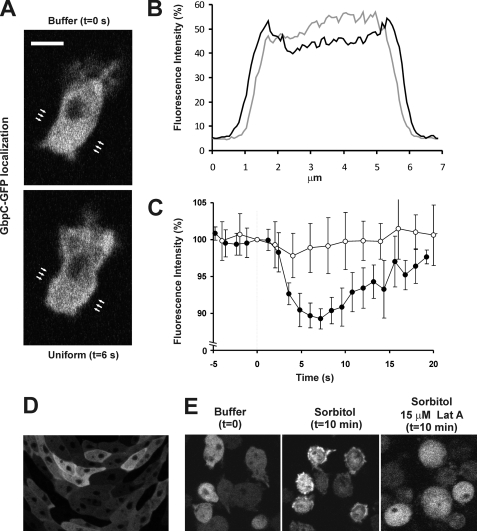
To study the localization of GbpC, we coexpressed GbpC fused at its C-terminal to GFP and monomeric red fluorescent protein MARS (RFP) in gbpC-null cells and simultaneously obtained confocal fluorescent images of both markers. The GbpC fusion protein binds cGMP and GTP in vitro and restores the gbpC-null phenotype (14), suggesting that the GFP tag does not affect correct folding and function of GbpC in vivo. In resting cells, GbpC-GFP has a uniform distribution in the cytoplasm. The protein is not enriched at the cell boundary, and it is absent from the nucleus (Fig. 1A). No difference in localization was observed between starved and unstarved cells. Global stimulation of starved cells with 10−6 m cAMP resulted in a translocation of GbpC-GFP to the cell boundary (Fig. 1A). Line scans through the cell reveal a decrease of fluorescence in the cytoplasm with a concomitant increase at the cell boundary (Fig. 1B). The kinetics of the cAMP-induced translocation of GbpC-GFP was more accurately monitored by computational analysis of fluorescence intensity depletion in the cytoplasm (Fig. 1C). The translocation starts very rapidly after cAMP-stimulation (about 2 s) and is maximal between 4 and 8 s, upon which GbpC gradually returns to the cytoplasm and reaches basal levels after ∼30 s (Fig. 1C). Stimulation with cAMP leads to an average depletion of cytoplasmatic GbpC of 11.4 ± 4.4% (n = 19), whereas the fluorescence intensity of the free RFP marker remains constant, indicating that the observed GbpC translocation is not due to a general change in cell shape or volume.
FIGURE 1.
GbpC translocates to the cell boundary and cell cortex upon cAMP-stimulation and osmotic stress and during cell streaming. Starved gbpC-null cells expressing GbpC-GFP were stimulated with 10−6 m cAMP. A, confocal images at the indicated times before and after cAMP-stimulation. B, line-scan images derived of the full image in A. Positions of scans are indicated by arrows in gray cells before and in black cells 3–6 s after stimulation. C, the decrease of the fluorescence intensity of GbpC-GFP (●) and cytosolic RFP (○) in the cytoplasm, which was analyzed using ImageJ. Data are mean ± S.D. (n = 6 cells). D, gbpC-null cells expressing GbpC-GFP were placed on non-nutrient agar plates and allowed to starve. Confocal images were taken after 6 h of aggregation. E, cells expressing GbpC-GFP were exposed to 200 mm sorbitol, and confocal pictures were taken before and after osmotic stress in the presence and absence of actin polymerization inhibitor latrunculin A.
The biological role of GbpC becomes more important in longer-starved cells and during cell streaming (22, 23). Consistently, whereas GbpC-GFP is cytosolic in starved single cells, GbpC becomes uniformly distributed at the cell boundary when cells make stable head-to-tail cell-cell contacts and are part of streams (Fig. 1D).
The Role of the F-actin Cytoskeleton for the Translocation of GbpC to the Cell Cortex
The production of cGMP and activation of GbpC is not only induced by cAMP but also by osmotic stress (11, 24). cGMP production occurs in the order of minutes after osmotic stress compared with a few seconds after cAMP stimulation (12). Therefore, we studied the localization of GbpC-GFP during osmoshock. After incubation with sorbitol, GbpC becomes enriched at the cell boundary and cortex (Fig. 1E). Consistent with the slow kinetics of the osmotic response, translocation of GbpC-GFP is maximal about 10 min after addition of sorbitol (data not shown). Because GbpC localization seem to be more fuzzy than only the defined plasma membrane (Fig. 1, A and E) and because GbpC is an important regulator of myosin II and thus the cytoskeleton (8, 10–13), we investigated a possible role of the cell cortex in regulating GbpC localization. Therefore, cells were incubated with 15 μm actin-polymerization inhibitor, latrunculin A. Under these conditions, GbpC does not translocate toward the cell boundary upon uniform cAMP stimulation or osmotic stress (Figs. 1E and 2A), indicating that a functional cytoskeleton is essential for GbpC translocation.
FIGURE 2.
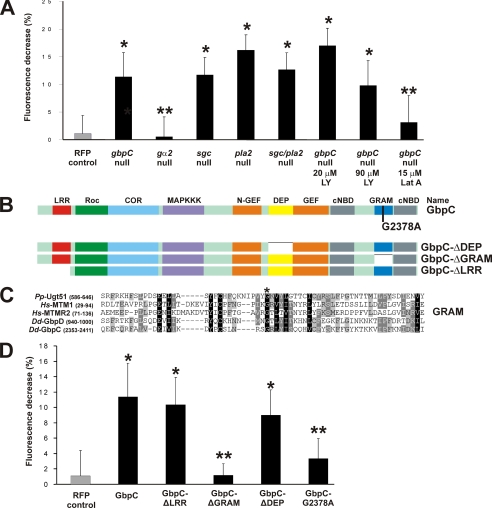
Expression of mutated GbpC. A, quantification of the maximal decrease of fluorescence intensity in the cytoplasm for GbpC-GFP in the indicated cell strains at 4–8 s after stimulation. Data are mean ± S.D. B, schematic view of the domain architecture of wild-type GbpC and several mutants used in this study. GbpC-G2378A is a point mutant in the GRAM domain, whereas GbpC-ΔGRAM lacks the GRAM domain. The GbpC-ΔLRR mutant misses amino acids 2–311. C, alignment of part of the GRAM domain of GbpC with a selection of other GRAM domains. Numbers refer to amino acids. The asterisk shows a conserved glycine that was mutated to an alanine to yield the GbpC-G2378A mutant. Dd, Dictyostelium discoideum; Hs, Homo sapiens; Pp, Pichia pastoris. D, quantification of the maximal cytoplasmatic fluorescence decrease for mutants in the LRR, GRAM, and DEP domain of GbpC expressed in gbpC-null cells. Cells were treated similarly as described in the legend to Fig. 1. *, p < 0.001 versus RFP control and not significantly different from GbpC-GFP; **, p < 0.001 versus GbpC-GFP and not significantly different from RFP control (A and D).
GbpC Translocation Is Regulated by G-protein Signaling but Does Not Depend on Previously Identified Chemotaxis Pathways
In Dictyostelium, cAMP binds to the cAMP receptor (cAR1), which results in activation of the heterotrimeric G-protein Gα2βγ (25). The activated G-protein subunits transduce the signal to the interior of the cell where they activate a complex network of signaling molecules. To assess if GbpC translocation is regulated by this pathway, GbpC-GFP was expressed in cells lacking gα2. The data show that the translocation of GbpC is completely abrogated (Fig. 2), indicating that localization is regulated by the heterotrimeric G-protein signaling cascade. So far, four signaling pathways have been implicated in chemotaxis: PI3K, TorC2, PLA2, and sGC. Because GbpC binds cGMP with high affinity (Kd ∼4 nm) (8) and because cGMP is produced rapidly after cAMP stimulation (26), it could well be that cGMP binding to GbpC regulates the localization of GbpC. To assess this hypothesis, GbpC-GFP was expressed in gc-null cells, which lack both guanylyl cyclases and thus cannot produce any cGMP (22). The kinetics and the maximal fluorescence decrease in the cytoplasm were not noticeably disturbed in these cells (11.7% ± 3.2%, n = 7), suggesting that GbpC translocates independently of guanylyl cyclases and their product cGMP (Fig. 2A). This was confirmed by the observation that addition of the membrane-permeable cGMP analog 8-Br-cGMP to cells did not change the distribution of GbpC (data not shown). Next, we tested the involvement of the other signaling pathways by analyzing GbpC translocation in disruption mutants and/or the presence of 20 μm LY294002 to inhibit PI3K and 90 μm LY294002 to inhibit PI3K and TORC2 (27) The fluorescence decrease in all mutants is similar to that of wild-type cells (Fig. 2A), indicating that none of the signaling enzymes (sGC, PLA2, PI3K, and TorC2) is essential for GbpC translocation.
The GRAM Domain Is Required for GbpC Translocation
In various other proteins, the LRR, DEP, and GRAM domains have been implicated in membrane association (28–31). Therefore, we expressed truncated proteins in which one of these three domains were deleted (Fig. 2B). Deletion of the LRR or DEP domain had no effect on the translocation of GbpC, whereas deletion of the GRAM domain abrogated translocation to the cell boundary (Fig. 2D).
To further investigate the role of the GRAM domain, we investigated point mutants that could potentially inactivate this domain (Fig. 2C). A characteristic glycine is conserved in all GRAM domains. Moreover, this residue was found mutated in myotubularin-related protein 2 (MTMR2), resulting in Charcot-Marie-Tooth disease (28, 29). This G103E mutation causes loss of membrane localization of MTMR2, and a crystal structure of MTMR2 reveals that this residue is positioned in the middle of an important β-sheet (28, 29). We mutated the corresponding glycine to an alanine, resulting in the GRAM mutant GbpC-G2378A (Fig. 2, B and C). Expression and correct folding of GbpC-G2378A is indicated by the high cGMP-binding activity (data not shown). Upon global cAMP-stimulation with 10−6 m cAMP, the GbpC-G2378A mutant showed a strongly disturbed protein translocation to the cell boundary, providing further support that the GRAM domain of GbpC is involved in translocation (Fig. 2D).
The GRAM Domain Binds Directly to Cellular Membranes
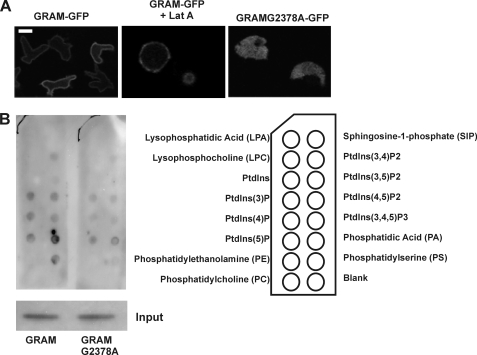
To further investigate the role of the GRAM domain in membrane targeting of GbpC, we expressed a GFP-tagged GRAM domain in gbpC-null cells and determined its localization. The GRAM domain (comprising amino acids 2331–2470) is localized nearly exclusively at the plasma membrane (Fig. 3A). This membrane association does not need a functional cytoskeleton but was disrupted when the G2378A mutation was introduced in this protein (Fig. 3A). Furthermore, a truncated GRAM domain (missing amino acids 2350–2437) was also cytoplasmatic (data not shown). Together, these results show that the G2378A mutation abolishes the membrane binding capacity of the GRAM domain.
FIGURE 3.
The GRAM domain of GbpC binds to the plasma membrane. A, confocal fluorescent images were taken from gbpC-null cells expressing GRAM-GFP in the absence and presence of latrunculin A or GRAMG2378A-GFP. The results show that GRAM-GFP localizes independently of F-actin at the plasma membrane, whereas the mutant GRAMG2378A-GFP shows a cytoplasmatic distribution. B, PIP strips containing spots with various phospholipids were incubated with lysates from cells expressing GRAM-GFP or GRAMG2378A-GFP. Binding of these proteins to phospholipids was visualized using an anti-GFP antibody. For direct comparison, both proteins were assayed on PIP strips in parallel and exposed on film together, and input protein was similar for both proteins.
GRAM domains were previously shown to bind phospholipids (31). To determine phospholipid binding potential for the GRAM domain of GbpC, we performed PIP-Strip assays, using lysates from cells that express GRAM-GFP (Fig. 3B). In this assay, membrane strips containing spots with various phospholipids are incubated with proteins (Fig. 3B). Protein binding to these phospholipids can be detected with antibodies, in this case against GFP to detect GRAM-GFP. We found that GRAM-GFP binds to several phospholipids: mono-PIPs, as well as PI(3,4)P, PI(4,5)P and PI(3,4,5)P are tolerated, but the strongest binding was found for phosphatidic acid and phosphatidylserine, which are the prevailing lipids in Dictyostelium (32). In a parallel assay in which the G2378A mutation was introduced, binding to phosphatidic acid and phosphatidylserine was severely reduced, whereas binding to other phospholipids was less disturbed (Fig. 3B).
Localization of GbpC Is Critical for Activity in Vivo
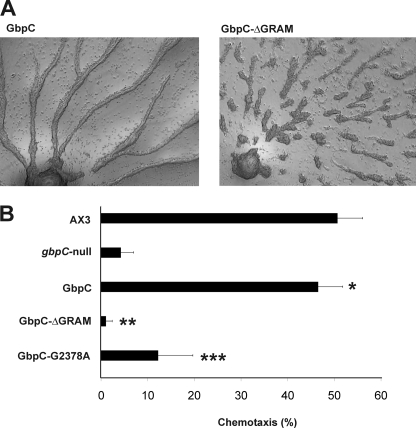
Upon starvation, Dictyostelium cells enter a developmental program. Cells begin to secrete cAMP and neighboring cells move toward the source of cAMP and relay the signal. Because of the resulting wave of cAMP that travels through the population, cells become polarized, connect to each other in a head-to-tail fashion, and form streams of cells. Cells lacking cGMP or GbpC have a severe streaming defect. These cells show extensive breaks of streams because of reduced cell elongation and the inability to maintain stable head-to-tail cell contacts (13). Whereas re-expression of GbpC in gbpC-null cells completely rescues this phenotype (Fig. 4A, left panel), cells expressing GbpC mutants that lack the GRAM domain or that contains the G2378A mutation still have the gbpC-null phenotype (Fig. 4A, right panel), indicating that the GRAM domain is essential in vivo.
FIGURE 4.
Development and chemotaxis of GbpC mutants. A, GbpC-GFP and GbpCΔGRAM-GFP were expressed in gbpC-null cells and starved on non-nutrient agar plates. Pictures of streaming cells were taken after 6 h of starvation. B, 7-h starved cells were monitored in a small population assay and scored for their ability to chemotax toward drops with 10−6 m cAMP. Experiments were performed in the presence of 50 μm LY (PI3K inhibitor) and p-bromophenylacyl bromide (PLA2 inhibitor), which causes chemotaxis to be critically dependent on the cGMP-pathway. The GbpC mutants were expressed in gbpC-null cells and compared with the chemotaxis data of AX3 and gbpC-null cells. The data presented are mean ± S.E. of at least three independent measurements on different days. *, p < 0.001 versus gbpC-null and not significantly different from AX3 at p > 0.05; **, p < 0.001 versus AX3 and not significantly different from gbpC-null; ***, significantly less than AX3 at p < 0.01 and significantly above gbpC-null at p < 0.01.
Chemotaxis in Dictyostelium cells can be monitored by a small-population/drop assay. Cells are placed on nutrient-free agar plates in small drops. Small drops of 10−6 m cAMP are placed close to these cells, and chemotactic activity toward cAMP is observed and scored. GbpC plays an important role in chemotaxis together with PI3K, TorC2, and PLA2 (23, 33, 34). The recognition that these parallel pathways mediate the transduction of chemotactic cAMP signals allowed us to develop an assay to specifically analyze the activity of GbpC in vivo. In this assay, chemotaxis is measured in the presence of LY294002 (an inhibitor of PI3K) and p-bromophenylacyl bromide (an inhibitor of PLA2). Under these circumstances, chemotaxis of 7-h starved cells is critically dependent on guanylyl cyclases and GbpC. In this assay, AX3 wild-type cells show around 50% chemotaxis, but cells lacking either guanylyl cyclases or GbpC have lost this remaining chemotactic activity. By reintroducing GbpC, chemotaxis is restored to wild-type levels (Fig. 4B), which indirectly allows screening for biological activity of GbpC mutants (23). The GbpC-ΔGRAM and GbpC-G2378A mutants were unable to chemotax in this assay, thus highlighting an essential role for the GRAM domain in biological activity of GbpC activity in vivo.
We investigated the localization of GbpC during chemotaxis. For careful quantification we coexpressed GbpC-GFP and cytosolic-RFP and obtained images of cells moving toward a pipette releasing cAMP. These images suggest a slight enrichment of GbpC-GFP in the front area of the cell (supplemental Fig. S1). We determined the average fluorescent intensity of GbpC-GFP in the cell cortex relative to the intensity in the cytoplasm using cytosolic-RFP as an internal control. The results show that in the cortex at the front of the cell GbpC-GFP is 8.3 ± 1.4% higher that the levels in the cytoplasm (mean ± S.E., n = 59, p < 0.005). The level of GbpC-GFP in the cortex at the side and the rear of the cell is not significantly increased relative to the cytoplasm (supplemental Fig. S1).
GbpC Translocation Is Uncoupled from the Intramolecular Signaling Cascade
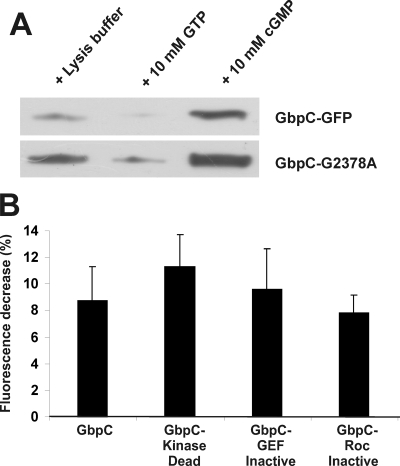
Correct signaling through the RasGEF, Roc, and mitogen-activated protein kinase kinase kinase domains of GbpC is essential for biological activity of GbpC (14). Because our results suggest that the GRAM is also critical for biological activity of GbpC, we hypothesized that the inactivated GRAM domain could potentially interfere with the intramolecular signaling cascade in GbpC, thereby inhibiting GbpC activity. One aspect of the intramolecular signaling cascade in GbpC involves cGMP-stimulated GTP binding to (and thus activation of) the Roc domain. This is visualized by pulling down GbpC-GFP with GTP-coupled agarose beads and subsequent Western blotting with a GFP-antibody. Using this assay, we found that the GRAM mutant GbpC-G2378A shows strong cGMP-stimulated GTP-binding activity, implying that a disturbed GRAM domain does not affect (part of) the intramolecular signaling cascade (Fig. 5A). We also tested cAMP-stimulated translocation in previously described mutants that are defective in this intramolecular signaling cascade (14). The results (Fig. 5B) show that mutants with disturbed RasGEF, Roc, or kinase domains translocate toward the cell boundary as the wild-type protein, indicating that translocation is independent of this signaling cascade. These results demonstrate that protein localization and cGMP-stimulated kinase activity of GbpC are mutually uncoupled processes.
FIGURE 5.
Protein localization and intramolecular signaling of GbpC are independent processes. A, GbpC-GFP and GbpC-G2378A-GFP were expressed in gbpC-null cells. Lysates were prepared, and proteins were pulled down with GTP-agarose beads in the absence or presence of cGMP or GTP, respectively. Bound proteins were visualized on a Western blot analysis using anti-GFP antibodies. cGMP-stimulated GTP binding was still present in the G2378A mutant at comparable levels with the wild-type protein. Representative data for at least three independent experiments on different days are presented. B, quantification of the maximal cytoplasmatic fluorescence decrease after cAMP stimulation for GbpC and the previously described GbpC-KinaseDead, GbpC-GefInactive, and GbpC-RocInactive mutants expressed in gbpC-null cells. Cells were treated as described in Fig. 1.
DISCUSSION
The Roco protein LRRK2 has been found to be thus far the most frequent cause of late-onset and idiopathic PD (2, 3). Detailed information about the activation mechanism of LRRK2 is missing, but all known pathogenic mutations in LRRK2 result in decreased GTPase activity and enhanced kinase activity, suggesting a possible PD-related gain of abnormal/toxic function (35–42). Recently it was shown that Roco proteins belong to the GAD class of molecular switches (G-proteins activated by nucleotide-dependent dimerization) (43, 44). It has been proposed that the juxtaposition of the G domains of two monomers in the complex across the GTP-binding sites activates the GTPase reaction and thereby regulate the biological function of these proteins. On the basis of the observed effects of PD mutations in LRRK2, it is thought that interaction with other proteins modify the dimer interactions, resulting in decreased GTPases and enhanced kinase activity (43, 44). Understanding how Roco proteins are activated will provide fundamental insights in this important class of proteins, which is also relevant for PD.
The strong and diverse phenotypes of the 11 Dictyostelium Roco disruption mutants provides a powerful tool to investigate the activation mechanisms of Roco proteins (9, 14). This resulted in the identification of an intramolecular signaling cascade in GbpC involving cGMP-stimulated RasGEF activity, subsequent Roc activation, and output kinase activity (14). This intramolecular cGMP signaling cascade appears to be essential for cGMP/GbpC-mediated activity during chemotaxis and cell stream formation in vivo.
Here, we extended the study by examining the localization of GbpC. We found that localization of GbpC to the cell boundary appears to be a second critical process for activity of GbpC in vivo. All mutants that have a disturbed protein translocation upon cAMP stimulation are also strongly disturbed in chemotaxis and cell stream formation. Translocation of GbpC to the cell boundary requires the GRAM domain of GbpC and a functional cytoskeleton. Translocation to the cell boundary is inhibited by the F-actin inhibitor latrunculin A. Furthermore, the localization of GbpC after cAMP stimulation, and especially after osmotic shock, strongly resembles the localization of F-actin, suggesting that GbpC may translocate to membrane and the F-actin rich cell cortex. In cells with a deleted or mutated GRAM domain, GbpC no longer translocates to the membrane or cell cortex after stimulation with cAMP or osmotic stress. The N-terminal part (LRR-Roc-COR-kinase) is localized in the cytoplasm, whereas the C-terminal part (GEF-GRAM-cNB) behaves as the full-length protein4. The GRAM domain itself associates with the plasma membrane and binds various phospholipids in vitro. These experiments suggest that stimulation with cAMP or osmotic stress induces a GRAM-dependent translocation to the plasma membrane and subsequent transient enrichment in the F-actin cytoskeleton that requires the C-terminal part of the protein.
Translocation of GbpC toward the cell boundary occurs independently of cGMP and the intramolecular signaling cascade in GbpC (14). GbpC still translocates toward the cell boundary in mutants that lack either cGMP production or a functional GEF, Roc, or kinase domain, whereas cGMP-stimulated Roc activity in the localization-defective mutants is not recognizably different from the activation of the wild-type protein.
Experiments on the role of the LRR domain of Roco proteins suggest that this domain is probably a third critical element for biological activity in vivo. Mutants of GbpC that lack this domain exhibit normal cAMP-stimulated translocation toward the membrane (Fig. 2D) and have normal cGMP-stimulated activation of the Roc domain (14) but nevertheless cannot support biological activity in vivo (14). Similarly in LRRK2, the LRR are essential in vivo but not for Roc-activated kinase activity in vitro (15).
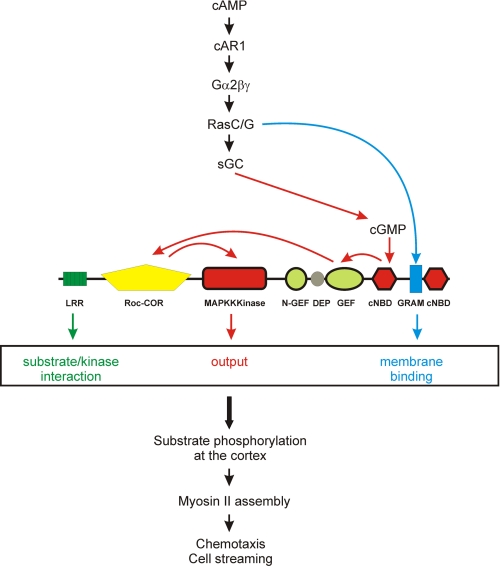
We propose that the activation mechanism of Roco proteins involves three independent processes (Fig. 6). 1) Regulation of its subcellular localization. Recent studies showed that LRRK2 is present both in the cytoplasm and at the cell boundary, that the fraction of LRRK2 dimer is enriched at the membrane, and that the membrane bound fraction binds GTP more efficiently and has a higher kinase activity than LRRK2 in the cytoplasm (16, 18). Together, these results suggest that the membrane-associated dimer most likely presents the physiologically active form of the protein and that, therefore, regulation of LRRK2 localization is important for its biological function. The exact mechanism and domain of LRRK2 that is required for membrane binding has not been identified. In Dictyostelium, the translocation of GbpC toward the cell boundary is critical for proper chemotaxis and cell streaming. The GRAM domain appears to be essential for membrane binding. Translocation of GbpC toward the cell boundary is induced by the chemoattractant cAMP through a signaling cascade that contains the cAMP-receptor cAR1 and heterotrimeric G-proteins but does not depend on one of the previously identified chemotaxis pathways (sGC, PLA2, PI3K, and TorC2). Although the membrane localization of the GRAM domain alone is independent of stimulation or a functional cytoskeleton, GbpC is localized in the cytoplasm before stimulation and translocation is disrupted in the presence of the actin-polymerization inhibitor latrunculin A. This suggests that translocation of GbpC is caused by a cAMP-mediated conformational change in GbpC, thereby resulting in the exposure of the GRAM domain, translocation to the membrane, and, subsequently, binding and phosphorylation of substrates at the cell cortex. Further studies are necessary to identify the upstream regulators and molecular mechanism of this conformational change, but they may well include dimerization and direct protein-protein interactions at the cell cortex. 2) Regulation of kinase activity. Roco proteins belong to the GAD class of molecular switches in which the Roc GTPase is regulated by homodimerization. Roco proteins have a low nucleotide affinity (in the μm range) and a relative high intrinsic nucleotide exchange rate (43, 44). Therefore, in contrast to Ras GTPases, regulation by GEFs and GAPs is not absolutely necessary for Roco proteins (43, 44). However, to be able to react rapidly on transient responses, additional stimulation by a GEF protein may be required. GbpC differs from the other Roco family members, in the sense that it already contains its own GEF. The cAMP-induced second messenger cGMP binds to GbpC, leading to stimulation of the RasGEF domain, GDP/GTP exchange at the Roc domain and activation of the output kinase domain. The net outcome of cGMP-binding to GbpC is phosphorylation molecular targets. iii) Substrate specificity. The LRR of LRRK2 and Dictyostelium GbpC are absolutely essential for activity of the protein in vivo (9, 14, 15), but they are not required for cGMP-stimulated Roc activity, nor for protein translocation. The Dictyostelium Roco proteins Roco3 (QkgA) and Roco4 are the product of a relatively recent gene duplication in Dictyostelium. Nevertheless, gene inactivations result in very different phenotypes (9). By expressing chimera proteins consisting of different combinations of domains from Roco3 and Roco4, it was found that specificity was attributed to the LRR and not to the Roc or kinase domains. Furthermore, On the basis of pull-down assays, the LRR interacts with the kinase domain5. These data suggest that LRR mediate input/output specificity, possibly by binding upstream proteins that activate the Roco protein and/or by selectively binding of the substrate, thereby bringing it in close proximity to the kinase.
FIGURE 6.
Model for the activation of GbpC. Extracellular cAMP binds to a G-protein-coupled receptor, cAR1, that stimulates a specific G-protein and Ras. A pathway downstream of Ras activation induces transient translocation of GbpC toward the membrane, which may be caused by either activation of the GRAM domain or formation of specific lipids in the membrane (blue). The produced cGMP induces an intramolecular signaling cascade involving GEF, Roc-COR, and activation of the kinase domain (red). The third activation signal comes from LRR, which are regulating substrate specificity (green). GbpC is only active when all three independent processes occur. Active GbpC phosphorylates so far unknown substrates at the cell cortex, which is important for myosin assembly into filaments and, subsequently, chemotaxis and cell streaming.
Together, our results give new insights in the complex activation mechanism of Roco proteins. Nevertheless, many important questions remain open, for example what are the upstream activators of Roco proteins, what are the output substrates of activated kinase, how does the Roc domain regulates the kinase activity, which role does COR plays in this process, and importantly how do the Parkinson disease-linked mutations alter the interactions between the different domains? We anticipate that addressing these questions in Dictyostelium might be instrumental and can thereby contribute to the understanding of the molecular mechanism of LRRK2 activation and how mutations of LRRK2 result in neuronal toxicity.
Supplementary Material

This article contains supplemental Fig. S1.
A. Kortholt, W. N. van Egmond, K. Plak, L. Bosgraaf, I. Keizer-Gunnink, and P. J. M. van Haastert, unpublished data.
- PD
- Parkinson disease
- LRR
- leucine-rich repeat(s)
- Roc
- Ras of complex proteins
- COR
- C-terminal of Roc
- GEF
- guanine nucleotide exchange factor
- GRAM
- glucosyltransferases, Rab-like GTPase activators, and myotubularins
- DEP
- Dishevelled, EgI-10, and pleckstrin
- RFP
- red fluorescent protein
- PB
- phosphate buffer.
REFERENCES
- 1. Lees A. J., Hardy J., Revesz T. (2009) Parkinson's disease. Lancet 373, 2055–2066 [DOI] [PubMed] [Google Scholar]
- 2. Paisán-Ruíz C., Jain S., Evans E. W., Gilks W. P., Simón J., van der Brug M., López, de Munain A., Aparicio S., Gil A. M., Khan N., Johnson J., Martinez J. R., Nicholl D., Carrera I. M., Pena A. S., de Silva R., Lees A., Martí-Massó J. F., Pérez-Tur J., Wood N. W., Singleton A. B. (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 44, 595–600 [DOI] [PubMed] [Google Scholar]
- 3. Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R. J., Calne D. B., Stoessl A. J., Pfeiffer R. F., Patenge N., Carbajal I. C., Vieregge P., Asmus F., Müller-Myhsok B., Dickson D. W., Meitinger T., Strom T. M., Wszolek Z. K., Gasser T. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607 [DOI] [PubMed] [Google Scholar]
- 4. Bosgraaf L., Van Haastert P. J. (2003) Roc, a Ras/GTPase domain in complex proteins. Biochim. Biophys. Acta 1643, 5–10 [DOI] [PubMed] [Google Scholar]
- 5. Marín I., van Egmond W. N., van Haastert P. J. (2008) The Roco protein family. A functional perspective. FASEB J. 22, 3103–3110 [DOI] [PubMed] [Google Scholar]
- 6. Cookson M. R., Bandmann O. (2010) Parkinson's disease. Insights from pathways. Hum. Mol. Genet. 19, R21-R27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cookson M. R. (2010) The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson's disease. Nat. Rev. Neurosci. 11, 791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bosgraaf L., Russcher H., Smith J. L., Wessels D., Soll D. R., Van Haastert P. J. (2002) A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. EMBO J. 21, 4560–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Egmond W. N., van Haastert P. J. (2010) Characterization of the Roco protein family in Dictyostelium discoideum. Eukaryot. Cell 9, 751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldberg J. M., Bosgraaf L., Van Haastert P. J., Smith J. L. (2002) Identification of four candidate cGMP targets in Dictyostelium. Proc. Natl. Acad. Sci. U.S.A. 99, 6749–6754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Araki T., van Egmond W. N., van Haastert P. J., Williams J. G. (2010) Dual regulation of a Dictyostelium STAT by cGMP and Ca2+ signalling. J. Cell Sci. 123, 837–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuwayama H., Ecke M., Gerisch G., Van Haastert P. J. (1996) Protection against osmotic stress by cGMP-mediated myosin phosphorylation. Science 271, 207–209 [DOI] [PubMed] [Google Scholar]
- 13. Veltman D. M., van Haastert P. J. (2008) The role of cGMP and the rear of the cell in Dictyostelium chemotaxis and cell streaming. J. Cell Sci. 121, 120–127 [DOI] [PubMed] [Google Scholar]
- 14. van Egmond W. N., Kortholt A., Plak K., Bosgraaf L., Bosgraaf S., Keizer-Gunnink I., van Haastert P. J. (2008) Intramolecular activation mechanism of the Dictyostelium LRRK2 homolog Roco protein GbpC. J. Biol. Chem. 283, 30412–30420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iaccarino C., Crosio C., Vitale C., Sanna G., Carrì M. T., Barone P. (2007) Apoptotic mechanisms in mutant LRRK2-mediated cell death. Hum. Mol. Genet. 16, 1319–1326 [DOI] [PubMed] [Google Scholar]
- 16. Berger Z., Smith K. A., Lavoie M. J. (2010) Membrane localization of LRRK2 is associated with increased formation of the highly active LRRK2 dimer and changes in its phosphorylation. Biochemistry 49, 5511–5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nichols R. J., Dzamko N., Morrice N. A., Campbell D. G., Deak M., Ordureau A., Macartney T., Tong Y., Shen J., Prescott A. R., Alessi D. R. (2010) 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson's disease-associated mutations and regulates cytoplasmic localization. Biochem. J. 430, 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sen S., Webber P. J., West A. B. (2009) Dependence of leucine-rich repeat kinase 2 (LRRK2) kinase activity on dimerization. J. Biol. Chem. 284, 36346–36356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bosgraaf L., Keizer-Gunnink I., Van Haastert P. J. (2008) PI3-kinase signaling contributes to orientation in shallow gradients and enhances speed in steep chemoattractant gradients. J. Cell Sci. 121, 3589–3597 [DOI] [PubMed] [Google Scholar]
- 20. Bosgraaf L., van Haastert P. J., Bretschneider T. (2009) Analysis of cell movement by simultaneous quantification of local membrane displacement and fluorescent intensities using Quimp2. Cell Motil. Cytoskeleton 66, 156–165 [DOI] [PubMed] [Google Scholar]
- 21. Konijn T. M. (1970) Microbiological assay of cyclic 3′,5′-AMP. Experientia 26, 367–369 [DOI] [PubMed] [Google Scholar]
- 22. Veltman D. M., Van Haastert P. J. (2006) Guanylyl cyclase protein and cGMP product independently control front and back of chemotaxing Dictyostelium cells. Mol. Biol. Cell 17, 3921–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Veltman D. M., Keizer-Gunnik I., Van Haastert P. J. (2008) Four key signaling pathways mediating chemotaxis in Dictyostelium discoideum. J. Cell Biol. 180, 747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Araki T., Tsujioka M., Abe T., Fukuzawa M., Meima M., Schaap P., Morio T., Urushihara H., Katoh M., Maeda M., Tanaka Y., Takeuchi I., Williams J. G. (2003) A STAT-regulated, stress-induced signalling pathway in Dictyostelium. J. Cell Sci. 116, 2907–2915 [DOI] [PubMed] [Google Scholar]
- 25. Van Haastert P. J., Devreotes P. N. (2004) Chemotaxis. Signalling the way forward. Nat. Rev. Mol. Cell Biol. 5, 626–634 [DOI] [PubMed] [Google Scholar]
- 26. Roelofs J., Van Haastert P. J. (2002) Characterization of two unusual guanylyl cyclases from Dictyostelium. J. Biol. Chem. 277, 9167–9174 [DOI] [PubMed] [Google Scholar]
- 27. Liao X. H., Majithia A., Huang X., Kimmel A. R. (2008) Growth control via TOR kinase signaling, an intracellular sensor of amino acid and energy availability, with cross-talk potential to proline metabolism. Amino Acids 35, 761–770 [DOI] [PubMed] [Google Scholar]
- 28. Begley M. J., Taylor G. S., Kim S. A., Veine D. M., Dixon J. E., Stuckey J. A. (2003) Crystal structure of a phosphoinositide phosphatase, MTMR2. Insights into myotubular myopathy and Charcot-Marie-Tooth syndrome. Mol. Cell 12, 1391–1402 [DOI] [PubMed] [Google Scholar]
- 29. Berger P., Schaffitzel C., Berger I., Ban N., Suter U. (2003) Membrane association of myotubularin-related protein 2 is mediated by a pleckstrin homology-GRAM domain and a coiled-coil dimerization module. Proc. Natl. Acad. Sci. U.S.A. 100, 12177–12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Rooij J., Rehmann H., van Triest M., Cool R. H., Wittinghofer A., Bos J. L. (2000) Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J. Biol. Chem. 275, 20829–20836 [DOI] [PubMed] [Google Scholar]
- 31. Tsujita K., Itoh T., Ijuin T., Yamamoto A., Shisheva A., Laporte J., Takenawa T. (2004) Myotubularin regulates the function of the late endosome through the gram domain-phosphatidylinositol 3,5-bisphosphate interaction. J. Biol. Chem. 279, 13817–13824 [DOI] [PubMed] [Google Scholar]
- 32. Weeks G., Herring F. G. (1980) The lipid composition and membrane fluidity of Dictyostelium discoideum plasma membranes at various stages during differentiation. J. Lipid Res. 21, 681–686 [PubMed] [Google Scholar]
- 33. Kamimura Y., Xiong Y., Iglesias P. A., Hoeller O., Bolourani P., Devreotes P. N. (2008) PIP3-independent activation of TorC2 and PKB at the cell's leading edge mediates chemotaxis. Curr. Biol. 18, 1034–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen L., Iijima M., Tang M., Landree M. A., Huang Y. E., Xiong Y., Iglesias P. A., Devreotes P. N. (2007) PLA2 and PI3K/PTEN pathways act in parallel to mediate chemotaxis. Dev. Cell 12, 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Greggio E., Jain S., Kingsbury A., Bandopadhyay R., Lewis P., Kaganovich A., van der Brug M. P., Beilina A., Blackinton J., Thomas K. J., Ahmad R., Miller D. W., Kesavapany S., Singleton A., Lees A., Harvey R. J., Harvey K., Cookson M. R. (2006) Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol. Dis. 23, 329–341 [DOI] [PubMed] [Google Scholar]
- 36. Guo L., Gandhi P. N., Wang W., Petersen R. B., Wilson-Delfosse A. L., Chen S. G. (2007) The Parkinson's disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity. Exp. Cell Res. 313, 3658–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ito G., Okai T., Fujino G., Takeda K., Ichijo H., Katada T., Iwatsubo T. (2007) GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson's disease. Biochemistry 46, 1380–1388 [DOI] [PubMed] [Google Scholar]
- 38. Lewis P. A., Greggio E., Beilina A., Jain S., Baker A., Cookson M. R. (2007) The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem. Biophys. Res. Commun. 357, 668–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li X., Tan Y. C., Poulose S., Olanow C. W., Huang X. Y., Yue Z. (2007) Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson's disease R1441C/G mutants. J. Neurochem. 103, 238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luzón-Toro B., Rubio de la Torre E., Delgado A., Pérez-Tur J., Hilfiker S. (2007) Mechanistic insight into the dominant mode of the Parkinson's disease-associated G2019S LRRK2 mutation. Hum. Mol. Genet. 16, 2031–2039 [DOI] [PubMed] [Google Scholar]
- 41. West A. B., Moore D. J., Biskup S., Bugayenko A., Smith W. W., Ross C. A., Dawson V. L., Dawson T. M. (2005) Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl. Acad. Sci. U.S.A. 102, 16842–16847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. West A. B., Moore D. J., Choi C., Andrabi S. A., Li X., Dikeman D., Biskup S., Zhang Z., Lim K. L., Dawson V. L., Dawson T. M. (2007) Parkinson's disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum. Mol. Genet. 16, 223–232 [DOI] [PubMed] [Google Scholar]
- 43. Gasper R., Meyer S., Gotthardt K., Sirajuddin M., Wittinghofer A. (2009) It takes two to tango. Regulation of G proteins by dimerization. Nat. Rev. Mol. Cell Biol. 10, 423–429 [DOI] [PubMed] [Google Scholar]
- 44. Gotthardt K., Weyand M., Kortholt A., Van Haastert P. J., Wittinghofer A. (2008) Structure of the Roc-COR domain tandem of C. tepidum, a prokaryotic homologue of the human LRRK2 Parkinson kinase. EMBO J. 27, 2239–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.