Background: MKK4 and MKK7 have important development functions that are not fully understood.
Results: In vitro ESC differentiation studies show that MKK4 is required for p38 activation and cardiomyocyte differentiation, while MKK4 and MKK7 both regulate Jun N-terminal kinase (JNK) and differentiating cell survival.
Conclusion: MKK4 and MKK7 have complementary and distinct roles in ESC differentiation.
Significance: Understanding signaling mechanisms underlying early developmental programs.
Keywords: Cell Signaling, Differentiation, Embryonic Stem Cell, Jun N-terminal Kinase (JNK), MAP Kinases (MAPKs), p38, MKK4, MKK7
Abstract
Signal transduction pathways are integral components of the developmental regulatory network that guides progressive cell fate determination. MKK4 and MKK7 are upstream kinases of the mitogen-activated protein kinases (MAPKs), responsible for channeling physiological and environmental signals to their cellular responses. Both kinases are essential for survival of mouse embryos, but because of embryonic lethality, their precise developmental roles remain largely unknown. Using gene knock-out mouse ESCs, we studied the roles of MKK4 and MKK7 in differentiation in vitro. While MKK4 and MKK7 were dispensable for ESC self-renewal and pluripotency maintenance, they exhibited unique signaling and functional properties in differentiation. MKK4 and MKK7 complemented each other in activation of the JNK-c-Jun cascades and loss of both led to senescence upon cell differentiation. On the other hand, MKK4 and MKK7 had opposite effects on activation of the p38 cascades during differentiation. Specifically, MKK7 reduced p38 activation, while Mkk7(−/−) ESCs had elevated phosphorylation of MKK4, p38, and ATF2, and increased MEF2C expression. Consequently, Mkk7(−/−) ESCs had higher expression of MHC and MLC and enhanced formation of contractile cardiomyocytes. In contrast, MKK4 was required for p38 activation and Mkk4(−/−) ESCs exhibited diminished p-ATF2 and MEF2C expression, resulting in impaired MHC induction and defective cardiomyocyte differentiation. Exogenous MKK4 expression partially restored the ability of Mkk4(−/−) ESCs to differentiate into cardiomyocytes. Our results uncover complementary and interdependent roles of MKK4 and MKK7 in development, and identify the essential requirement for MKK4 in p38 activation and cardiomyocyte differentiation.
Introduction
The MAP kinases (MAPKs)4 are a family of cytosolic kinases responsible for relaying extracellular signals to intracellular responses. In mammalian systems, there are three major MAPK subgroups: ERK (ERK1/2), JNK (1/2/3), and p38 (α/β/γ/δ), all activated through a conserved three-component MAP3K-MAP2K-MAPK signaling cascade (1). Within this cascade, the MAP3Ks activate the MAP2Ks, which in turn catalyze MAPK phosphorylation and thereby their activation. There are six major MAPKKs with distinct specificity for MAPK phosphorylation (2). In principle, MEK1 and MEK2 are responsible for phosphorylation of ERK; MKK4 and MKK7 phosphorylate preferentially JNK, whereas, MKK3 and MKK6 phosphorylate p38.
Gene knock-out studies in mice reveal that several of the MAPK signaling events are essential for embryonic development, while analyses of knock-out ESCs provide additional information on how each MAPK contributes to the developmental programs. Collectively, these studies show that the MAPKs are dispensable for ESC self-renewal and pluripotency, but have developmental stage-specific roles in lineage commitment. ERK2 signaling is essential for embryonic survival by regulating trophectoderm and mesoderm differentiation (3–7). p38α regulates erythrogenesis and myotube formation in vivo, but in vitro it promotes cardiomyogenesis and prevents early stage neurogenesis, while inhibiting adipogenesis at a late stage of differentiation (8, 9). JNKs, on the other hand, are required for neural tube closure in vivo and early mesoderm differentiation in vitro (10–12). Additionally, the JNK1 isoform is shown to repress Wnt4, Wnt6, and BMP4 expression, thereby potentiating neuronal differentiation while blocking epithelial differentiation (13).
MKK4 and MKK7 are upstream kinases responsible for phosphorylation of JNK at the Thr and Tyr residues located in the activation loop. Specifically, MKK4 was shown to phosphorylate JNK on Tyr, while MKK7 phosphorylates Thr, and MKK4 and MKK7 together cause dual phosphorylation of JNK thus, optimal activation (14, 15). Mice lacking either MKK4 or MKK7 display an analogous embryonic lethal phenotype that may be attributed, at least in part, to insufficient JNK activation (16–19). On the other hand, MKK4 and MKK7 have distinct expression pattern and their knock-out embryos exhibit certain unique phenotypic features, suggesting that these MAPKKs have distinct downstream targets in addition to their common effects on JNK. For example, MKK4, but not MKK7, has been shown to phosphorylate p38; however, MKK4 in this case is redundant to MKK3 and MKK6, and the MKK4-p38 cascade is therefore considered non-essential for any biological processes (2, 20). While the gene knock-out studies have certainly establish that MKK4 and MKK7 are essential for embryogenesis, the knock-out fetuses die at an early stage of development, precluding a clear understanding of how these kinases are involved in developmental processes.
Embryonic development starts with fertilization of the ovum, followed by rapid mitotic division and differentiation of ESCs. The ESCs first commit to ectoderm, mesoderm and endoderm lineages, followed by more restricted differentiation toward specific fates (21). These processes ultimately lead to the generation of over 220 different mammalian cell types that are organized into tissues to provide all the functions required for viability and reproduction. The ESCs captured from the pre-implantation embryo can be expanded in vitro for extended periods of time (22). These cells are able to either self-renew while they maintain pluripotency or differentiate to give arise to a broad spectrum of lineages (23, 24). Because many of the regulatory machineries effective for embryonic development in vivo also control ESC differentiation in vitro, the in vitro system has emerged as a valuable tool to investigate basic mechanisms in development. The in vitro system is particularly useful to analyze specific gene function in conjunction with knock-out cell lines, especially in the cases when the gene product is required for survival of the embryos in vivo.
In the present study, we combined the in vitro ESC differentiation and gene knock-out systems and investigated the roles of MKK4 and MKK7 in mesoderm lineage differentiation. Our results uncovered complementary and unique signaling and functional properties of MKK4 and MKK7 in lineage commitment and differentiation of cardiomyocytes. Results from this work provided new insights into the roles the stress-activated MKK4 and MKK7 play in directing differentiation and govern tissue development.
EXPERIMENTAL PROCEDURES
Reagents, Antibodies, and Plasmids
Cell culture reagents were purchased from Mediatech; ESGRO (LIF) was from Millipore; fetal bovine serum (FBS) and FBS knock-out serum replacement were from Invitrogen. Chemical inhibitors for JNK (SP600125), p38 (SB202190), and ERK (PD98059), and hygromycin were from Calbiochem; mitomycin C, G418, and puromycin were from Sigma. Antibodies for phospho- and/or total-p38, ERK, c-Jun, ATF2, MKK4, and MKK7 were from Cell Signaling Technology; antibodies for phospho-JNK were from Promega; antibodies for total-JNK, MEF2C and c-Fos were from Santa Cruz Biotechnology; antibody for β-actin was from Pharmingen and anti-MHC was from Neumarkers. The bacterially expressed GST-fusion proteins, including MKK4, MKK7, p38, and c-Jun, and MKK4 mammalian expression vector, were as described (25, 26).
The pNkx2.5PuroIRES2eGFP plasmid was constructed by amplifying the 500 bp of the mouse Nkx2.5 basal promoter (from −500 to +1) and 2 kb of the enhancer region (from −9507 to −7419) from mouse genomic DNA. The PCR products were sequentially inserted upstream of the puromycin-resistance gene in the pPuroIRES2eGFP vector (27), a kind gift from Drs. Agapios Sachinidis and Michael Jesudoss, Institute of Neurophysiology, University of Cologne, Germany.
Cell Culture and Embryoid Body (EB) Formation
The wild type, Mkk4(−/−), Mkk7(−/−), and Mkk4(−/−)/Mkk7(−/−) mouse ESCs were provided by Dr. Nishina and were described elsewhere (28–30). The mESCs were maintained on feeder cell line STO pretreated with mitomycin C (10 μg/ml) in ES medium with 103 units/ml LIF. For in vitro differentiation, the mESCs were cultured in the absence of feeder cells and followed the EB protocol as described (31). Briefly, the ES cells were trypsinized and resuspended in differentiation medium at 3.75 × 104 cells/ml. The cells (20 μl) were applied to the lid of bacterial plates and cultured as “hanging drops” in a CO2 incubator for 3 days to form EBs. The EBs were transferred to a 10 cm2 bacterial plate at ∼100 EBs per plate for 2 days, after which (on day 5), the EBs were transferred to either 10 cm2 or 24-well plates at 1 EB/well. The EBs were observed under microscope and the contractile phenotype was quantified, and RNA and proteins extracted at different days of culture were examined.
Transient Transfection and Luciferase Assay
ESCs at various differentiation days were transfected with 0.3 μg of 2xMef2c-TATA-firefly luc/pGL2 and 0.1 μg β-actin-Renilla luciferase reporter plasmids. The cell lysates were examined at 24 h after transfection for firefly and Renilla luciferase activities, according to the manufacture's manual (Invitrogen). The relative luciferase activity was calculated based on the ratio of firefly to Renilla activities, with the values in undifferentiated ESCs set as 1.
Establishing Stable Cell Lines
To generate Nkx2.5-ES cell line, pNkx2.5PuroIRES2eGFP was transfected into wild type ES cells with Lipofectamine (Invitrogen). Transfected cells were selected with 600 μg/ml G418 for 2 weeks. To reconstitute MKK4 expression, the Mkk4(−/−) ESCs were co-transfected with MKK4/SRα and hygromycine/pUC plasmids. Transfected cells were selected with 200 μg/ml hygromycine for 3 days, and then in 150 μg/ml for 2 weeks.
After selection, single clones were isolated and maintained in 300 μg/ml G418 or 150 μg/ml hygromycine, respectively. Integration of the plasmid DNA was confirmed by PCR genotyping of the genomic DNA isolated from individual stable clones (data not shown).
GST Pull-down, Western Blot Analyses, and in Vitro Kinase Assays
Experiments were done as described previously (25). Briefly, the GST-fusion proteins were incubated with 500 μg of cell lysates at 4 °C for 2–4 h, and in some experiments, 100 μg of cell lysates were used for an in vitro kinase assay using GST-MKK4 as a substrate. The fusion proteins were purified with GST Sepharose beads, and were either directly subjected to Western blotting analyses, or used to phosphorylate GST-p38 in an in vitro kinase assay, followed by a Western blotting analysis.
RNA Isolation, Reverse Transcription, Chromatin Immunoprecipitation (ChIP), and Real-time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from undifferentiated ESCs and EBs at different days of differentiation. The RNAs were used for reverse transcription to generate cDNA, while cDNA was used for real-time PCR as described before (32). Relative cycle differences in qRT-PCR were determined using ΔCt. The ΔCt value for each sample was determined using the cycle threshold (CT) value of the specific gene normalized to the CT of Gapdh. The fold change was calculated based on the expression in differentiated versus un-differentiated (control) samples, designated as 1.
ChIP was performed following procedures described (33) with minor modifications. The precipitated DNA was analyzed by real-time PCR, using 6 sets of primers that cover the region between −2.0 kbp and +1 bp of the mouse Mhca promoters. The ΔCt value for each sample was determined using the cycle threshold (CT) value of ChIP DNA normalized to the CT of input DNA. The ΔΔCT was calculated by subtracting control ΔCT values from the corresponding experimental ΔCT. The qRT-PCR results are shown as fold change in specific antibody immunoprecipitation over IgG nonspecific immunoprecipitation controls.
Immunofluorescent Staining
The ES cells and EBs were grown on gelatin-coated glass coverslip for different days and fixed with 4% paraformaldehyde for 20 min at 4 °C. The cells were then permeabilized with 0.1% Triton X-100 at 4 °C for 20 min. After blocking with 5% bovine serum albumin (BSA) for 1 h at room temperature, the cells were incubated with primary antibodies at 4 °C overnight, followed by incubation with rhodamine isothiocyanate- or FITC-conjugated secondary antibodies (Molecule Probes) and Hoechst for nuclei staining at room temperature for 1 h. The cells were examined and pictures were captured using a Zeiss Axio microscope. The fluorescence intensity was quantified, while the relative intensity of rhodamine and FITC was calculated by comparison to that of Hoechst.
Statistical Analyses
Data represent the average ± S.D. from at least three independent experiments. Statistical comparisons were performed using analysis of ANOVA or Student's two-tailed paired t test. *, p value <0.05; **, p < 0.01; and ***, p < 0.001 are considered significant.
RESULTS
Redundant Roles of MKK4 and MKK7 in Survival of Differentiated Cells
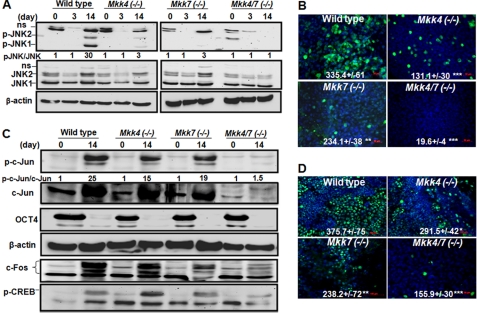
To evaluate the roles of MKK4 and MKK7 in development, we examined the growth and differentiation of wild type and knock-out ESCs lacking MKK4, MKK7 or both. The knock-out ESCs were similar to the wild type in growth rate and expression of pluripotent markers OCT4, SOX2, and NANOG under stem cell growth conditions (supplemental Fig. S1, A and B). Expression of OCT4 was diminished in ESCs of all the genotypes under differentiation conditions, while expression of MKK4 and MKK7 was consistent with the genotype of the cells and was unaffected by differentiation. Additionally, all the ESCs displayed about 70% efficiency of forming EB regardless their genotype (supplemental Fig. S1C). While the wild type, Mkk4(−/−) and Mkk7(−/−) EBs were morphologically indistinguishable, forming spread and multi-layered colonies, the Mkk4(−/−)/Mkk7(−/−) EBs were clearly different. The compound mutant EBs began to detach on day 6 and displayed significantly reduced density and thinner cell layers on day 13 (Fig. 1A).
FIGURE 1.
MKK4 and MKK7 have complementary roles in survival of differentiated cells. The wild type, Mkk4(−/−), Mkk7(−/−), and Mkk4/7(−/−) ESCs were either undifferentiated or subjected to EB differentiation. A, EBs at 13 days of differentiation were stained by Hoechst and observed under fluorescence microscopy. The multiple cell layers were observed and quantified after reconstruction of the three-dimensional pictures. B, expression of senescence markers were determined by real-time RT-PCR. The relative expression was calculated based on the levels of Gapdh in each sample and fold induction was determined by comparison to the levels in un-differentiated cells, set as 1. Results are shown as mean ± S.D. from triplicates of at least three experiments. Statistical analyses were done by comparing to the level in wild type cells, *, p < 0.05; **, p < 0.01; and ***, p < 0.001 were considered significant.
To determine why the Mkk4(−/−)/Mkk7(−/−) EBs had reduced density, we examined cell proliferation and apoptosis. The EB colonies of different genotypes displayed the same extent of apoptosis; however, the Mkk4(−/−)Mkk7(−/−) EBs had reduced cell number while a marked increase of senescence associated gene expression (Fig. 1B and supplemental Fig. S1D). Specifically, Mkk4(−/−)Mkk7(−/−) EBs had significantly higher expression of Transgelin (Sm22), cyclin-dependent kinase inhibitor 1A (p21) and prosaposin, than wild type, Mkk4(−/−), and Mkk7(−/−) EBs. As these genes are all markers of senescence (34, 35), our results suggest that loss of MKK4 and MKK7, while having no effects on stem cell survival, potentiates senescence upon differentiation.
MKK4 and MKK7 Are Implicated in Activation of the JNK-c-Jun Cascades during Differentiation
The morphological features of the Mkk4(−/−)Mkk7(−/−) EBs are remarkably similar to those described for the Jnk1(−/−)Jnk2(−/−), which are fully competent for self-renewal, but are unable to proliferate and become senescent upon differentiation (12). Such similarity raises the possibility that loss of MKK4 and MKK7 impairs activation of the JNK cascades. To test this possibility, we examined phosphorylation of JNK in wild type and knock-out ESCs at various days of in vitro differentiation. By Western blotting analyses, we found that phosphorylation of JNK in wild type was undetectable in undifferentiated and early stage differentiated ESCs (day 3), whereas it became readily detectable at later stage (day 9–14) differentiation (Fig. 2A). Ablation of MKK4 or MKK7 markedly reduced p-JNK, while ablation of both completely abolished it. Consistent with the Western blotting data, immunostaining detected p-JNK positive cells being most abundant in wild type, less so in Mkk4(−/−) and Mkk7(−/−), and almost completely absent in Mkk4(−/−)Mkk7(−/−) EBs at 13 days of differentiation (Fig. 2B). One consequence of JNK activation is the phosphorylation of c-Jun at Ser-63 and 73 (36), and accordingly, p-c-Jun was absent in undifferentiated ESCs while it was present in late stage differentiated wild type ESCs (Fig. 2C). Compared with its level in wild type ESCs, p-c-Jun was slightly reduced in cells lacking either MKK4 or MKK7, but markedly decreased in cells lacking both (Fig. 2, C and D). The JNK-c-Jun cascades therefore are quiescent in undifferentiated ESCs, but become activated upon differentiation in a MKK4/MKK7-dependent manner.
FIGURE 2.
MKK4 and MKK7 contribute to activation of the JNK-c-Jun pathways during in vitro differentiation. Wild type, Mkk4(−/−), Mkk7(−/−), and Mkk4/7(−/−) ESCs were either maintained under stem cell condition or subjected to EB protocol for differentiation. The cell lysates were collected at various days of differentiation as indicated and subjected to Western blotting for (A) p-JNK, total JNK and β-actin, and (C) p-c-Jun, total-c-Jun, p-CREB, c-Fos, Oct4, and β-actin. The immunobloting was captured with UVP bioimaging system and the relative intensity was calculated based on that in un-differentiated cells of each genotype, set as 1. The EBs at 13 days of differentiation were subjected to immunostaining using (B) anti-p-JNK and (D) anti-p-c-Jun, followed by FITC-conjugated secondary antibodies (green), while the nuclei were marked by Hoechst staining (blue). Images were captured under fluorescence microscopy and the relative fluorescence density was calculated by comparing the values of FITC to those of Hoechst. The scale bars represent 50 μm. Results represent the mean ± S.D. from at least three slides of biological samples. Statistical analyses were done by compared with the levels in wild type cells, with *, p < 0.05; **, p < 0.01; and ***, p < 0.001 considered significant.
In addition to activation of the JNK-c-Jun cascade, phosphorylation of the cAMP response element-binding protein (CREB), implicated in neurogenesis and cardiogenesis (37–39) was increased upon EB differentiation, but much less so in the double knock-out cells (Fig. 2C). Furthermore, expression of c-Jun and c-Fos, known as differentiation factors induced by the AP-1 transcriptional complex (40), was increased upon differentiation. The basal and induced levels of c-Jun and c-Fos were similar in wild type, Mkk4(−/−) and Mkk7(−/−) cells, but were undetectable in Mkk4(−/−)Mkk7(−/−) cells. On the other hand, expression of JNK and β-actin remained constant regardless of differentiation or gene knock-out status, suggesting that lack of c-Jun and c-Fos in the double knock-out cells was not due to global reduction of cellular proteins. Collectively, these results describe complementary roles of MKK4 and MKK7 in the regulation of several differentiation-associated events, including c-Jun and c-Fos expression and CREB phosphorylation, in addition to activation of the JNK-c-Jun cascades.
MKK4 is required for cardiomyocyte differentiation, while MKK7 represses it. In vitro differentiation through formation of EBs gives rise to derivatives of all three-embryonic germ layers, but the majority of the cells tend to differentiate along the mesodermal lineages, which further undergo myogenesis and cardiogenesis (41, 42) (supplemental Fig. S2A). Cardiogenesis can be traced by microscopic examination the EB colonies forming a contractile phenotype, known as “beating cardiomyocytes.” In wild type cells, the onset of the contractile phenotype was evident at day 10 and reached a plateau at day 14 of differentiation (Fig. 3A). Strikingly, the Mkk4(−/−) and Mkk4(−/−)Mkk7(−/−) cells completely failed to form beating cardiomyocytes, while exogenous MKK4 expression in the Mkk4(−/−) cells restored the beating phenotype (Fig. 3B). In contrast, the Mkk7(−/−) cells formed more abundant beating foci than the wild type cells.
FIGURE 3.
Opposite effects of MKK4 and MKK7 in MHC expression and cardiomyocyte differentiation. The ESCs were subjected to EB differentiation for various days as indicated. (A) The number of EBs in wild type, Mkk4(−/−), Mkk7(−/−), and Mkk4/7(−/−) that developed rhythmic beating was counted under light microscopy, and (B) EBs that developed rhythmic beating in wild type, Mkk4(−/−), and Mkk4(−/−) reconstituted with MKK4 expression was quantified at day 13 of differentiation The percentage of beating versus total number of EBs was calculated. Results are the average of at least four experiments. The RNAs isolated from the EBs were subjected to reverse transcription and real-time PCR to determine the expression of (C) Mlc, Mhca, and Mhcb, as indicated. The relative values of expression were normalized to the levels of Gapdh in each sample and compared with the levels in un-differentiated cells, set as 1. Results are shown as mean ± S.D. from triplicates of at least three experiments. D, EBs at 13 days of differentiation were subjected to immunostaining using anti-MHC (green) and nuclei were labeled by Hoechst staining (blue). The staining was observed under fluorescence microscopy and photographs were taken. The relative fluorescence density was quantified by comparing the values of FITC to those of Hoechst, and results are means ± S.D. of at least three slides. The scale bars represent 100 μm. Statistical analyses in all experiments were done by comparing to the values in wild type cells at a given differentiate stage, with *, p < 0.05; **, p < 0.01; and ***, p < 0.001 considered significant.
To understand at which differentiation stage MKK4 and MKK7 affected cardiogenesis, we performed real-time RT-PCR to trace the expression of mesodermal cell type-specific genes. Induction of primitive mesodermal markers, such as FGF5, NODAL, and T (Brachyury), was detected at earlier time points of differentiation, while loss of MKK4 or MKK7 had no significant effect on their expression (supplemental Fig. S2). Expression of NKX-2.5, a cardiac homeobox transcription factor and marker of mesoendoderm, was also unaffected in the knock-out cells.
The cardiac stem cells further differentiate, giving arise to three main cardiac lineages: cardiac myocytes, smooth muscle cells and endothelial cells (43). Expression of endothelial cell marker (Vimentin) increased only slightly upon differentiation, suggesting that the in vitro system did not favor endothelial lineage differentiation and produced only a few, if any, endothelial cells (supplemental Fig. S2B). On the other hand, expression of α-smooth muscle actin and cardiac muscle specific genes, including myosin light chain (Mlc) and myosin heavy chain α (Mhca) and β (Mhcb), was slight induced at day 5, but markedly increased at day 9 and reached the highest levels at day 14 in wild type ESCs (Figs. 3C and supplemental Fig. S2B). While loss of MKK4 or MKK7 did not affect the induction of α-smooth muscle actin (supplemental Fig. S2B), it differentially affected cardiac muscle gene expression. Specifically, loss of MKK4 significantly blocked the induction of Mhca and Mhcb, but had no effect on expression of Mlc. In contrast, loss of MKK7 significantly elevated the expression of Mlc, Mhca, and Mhcb (Fig. 3C).
To confirm this observation, we examined MHC expression by immunostaining. Compared with wild type cells, Mkk7(−/−) had enhanced, whereas Mkk4(−/−) and double knock-out cells had significantly reduced level of MHC (Fig. 3D). These results together suggest that while MKK4 and MKK7 are dispensable for ESC commitment to mesodermal lineages and early myogenesis, they have opposite effects on MHC expression.
Differential Regulation of p38 by MKK4 and MKK7
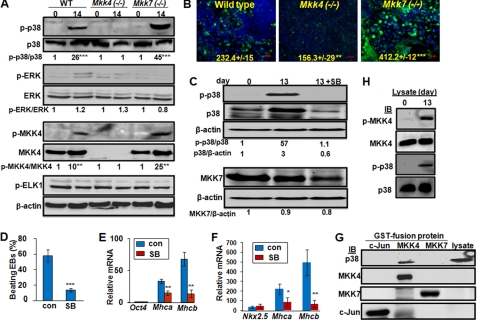
We have shown before that MKK4 and MKK7 are complementary in activation of JNK, but this mechanism cannot explain their opposite effects on cardiomyocyte differentiation. We suggest that MKK4 and MKK7 may have distinct effects on other MAPKs. To test this possibility, we examined phosphorylation of p38 and ERK in differentiating EBs. Phosphorylation of ERK and its downstream target ELK1 was similar in undifferentiated and differentiated ESCs of all the genotypes examined (Fig. 4A). In contrast, phosphorylation of p38 was minimal in undifferentiated ESCs, but was significantly increased in differentiating wild type cells (Fig. 4, A and B). Interestingly, compared with that in wild type, induction of p-p38 was significantly higher in Mkk7(−/−), but almost completely abolished in Mkk4(−/−). Correspondingly, the p-MKK4 was more abundant in Mkk7(−/−), while absent in Mkk4(−/−). Hence, MKK4 is essential for p38 activation, whereas, MKK7 may negatively regulate the MKK4-p38 axis during differentiation.
FIGURE 4.
MKK4 is required for p38 activation, while MKK7 attenuates it. Wild type, Mkk4(−/−) and Mkk7(−/−) ESCs were subjected to EB protocol, and (A) cell lysates were obtained at days 0 and 14 of differentiation, and were subjected to Western blotting using antibodies as indicated. The relative levels of phospho- versus total-proteins were calculated based on the immunoblotting intensity of four experiments, and were compared with the level in undifferentiated wild type ESCs, set as 1. Statistical analyses were done by comparing the values in differentiated to that in un-differentiated cells of each genotype. B, EBs at 14 days of differentiation were subjected to immunostaining for p-p38 while nuclei were stained by Hoechst (blue). Pictures were taken under fluorescence microscopy. The relative fluorescence density was quantified by comparing intensity of FITC to that of Hoechst, and results are means ± S.D. of at least three slides. Statistic analyses were done by comparing to the relative fluorescence in wild type cells. Scale bars represent 50 μm. The wild type EBs were either un-treated or treated with the p38 inhibitor, SB202190 (5 μm), at days 6–13 of differentiation, and (C) cell lysates were analyzed by Western blotting using antibodies as indicated, and the relative band intensity was labeled based on calculations using that in undifferentiated wild type ESCs set as 1, (D) number of EBs developed rhythmic beating was counted under light microscopy and the percentage of beating versus total EBs was calculated, and (E) expression of Oct4, Mhca and Mhcb was determined by real-time RT-PCR. F, pNkx2.5PuroIRES2eGFP ESCs were either un-treated or treated with the p38 inhibitor at days 6–13 of differentiation, and expression of Nkx2.5, Mhca, and Mhcb was determined by real-time RT-PCR at 13 day of differentiation. The relative expression values were normalized to Gapdh and results shown are mean ± S.D. from at least three experiments. Statistic analyses were done by comparing to that in cells without p38 inhibitor treatment. All results are means ± S.D. of at least three experiments. Statistical analyzes were done by comparing the values in SB treated samples to the control. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 were considered significant. G, lysates (500 μg) from Mkk4(−/−)Mkk7(−/−) ESCs were mixed with bacterially expressed and purified GST-c-Jun, GST-MKK4, and GST-MKK7, as labeled, followed by the GST-beads pull-down assays. The pull-down proteins were examined by Western blotting using antibodies as indicated and cell lysates (50 μg) were loaded directly to the gel as a positive control. H, lysates (100 μg) of either undifferentiated or differentiated wild type ESCs were mixed with GST-MKK4 for an in vitro kinase assay. The GST-MKK4 was then purified by GST-beads and was either directly examined by Western blotting using anti-p-MKK4 and anti-MKK4 (top 2 panels), or applied to an in vitro kinase assay using GST-P38 as a substrate. The kinase reaction was loaded onto SDS-PAGE and examined using anti-p-p38 and total p38, as indicated (bottom 2 panels).
The differential effects of MKK4 and MKK7 on p38 were similar to those on cardiogenesis, raising the possibility that p38 was involved in cardiogenesis. To evaluate this, we treated wild type EBs with a chemical inhibitor of p38, SB202190. Although the inhibitor had a minimal effect on p38 and MKK7 expression, it totally blocked p38 but not JNK activation (Figs. 4C and supplemental Fig. S3A). Importantly, SB202190 treatment significantly reduced beating cardiomyocyte formation and Mhca and Mhcb expression (Fig. 4, D and E). By contrast, neither the JNK inhibitor SP600125 nor the ERK inhibitor PD98059 had much, if any, effect on cardiomyocyte differentiation (supplemental Fig. S3, B and C).
Despite that the differentiating EBs consist multiple cell types of mixed lineages, the gene expression patterns strongly suggest that p38 is required for differentiation of myogenic precursors (Nkx2.5-positive) to cardiomyocytes (Mhc-positive). To confirm this observation, we established a stable ESC line, in which expression of puromycin and GFP was controlled by the Nkx-2.5 promoter, activated specifically in myogenic precursor cells. Puromycin treatment enriched the GFP-positive myogenic precursor cells in the differentiating EBs (supplemental Fig. S4). Within this population, inhibition of p38 did not affect the number of puromycin-resistant GFP cells, but significantly inhibited MHC expression (Fig. 4F and supplemental Fig. S2B).
To understand how MKK4 was specifically connected to p38, we characterized the ability of MKK4 to interact and activate p38 (44, 45). We first showed that GST-MKK4 could interact and bring down p38, whereas neither GST-MKK7 nor GST-c-Jun was able to pull-down p38 from the ESC lysates (Fig. 4G). We further showed that lysates of differentiated EBs were able to phosphorylate GST-MKK4 in an in vitro kinase assay, while the GST-MKK4 isolated from the above reaction could in turn phosphorylate p38 in vitro (Fig. 4H). By contrast, lysates of undifferentiated ESCs were unable to activate this cascade of phosphorylation reactions. Taken together, our results suggest that MKK4 and MKK7 differentially regulate p38, whereas, the MKK4-p38 axis is an intrinsic signaling mechanism required by the myogenic precursor cells to differentiate into cardiomyocyte.
Opposite Roles of MKK4 and MKK7 in ATF2 and MEF2C Activity during Cardiogenesis
One downstream target of p38 is ATF2, which was phosphorylated in wild type ESCs, more so in Mkk7(−/−), but less so in Mkk4(−/−) cells (Fig. 5A). Another well-known downstream target of p38 is MEF2C, a crucial transcription factor responsible for activation of cardiac-specific embryonic genes in early development (46). While expression of MEF2C was gradually increased upon differentiation of wild type ESCs, it was significantly potentiated in Mkk7(−/−), but decreased in Mkk4(−/−) cells (Fig. 5B). Correspondingly, the transcriptional activity of MEF2C was elevated upon differentiation of wild type ESCs, and was higher in Mkk7(−/−), but remained at basal level in Mkk4(−/−) cells (Fig. 5C).
FIGURE 5.
Differential effects of MKK4 and MKK7 on MEF2C and ATF2. Wild type and Mkk4(−/−), Mkk7(−/−), and Mkk4/7(−/−) ESCs were either maintained under stem cell condition or subjected to EB protocol for 14 days. A, EBs were subjected to immunostaining for p-ATF2 (green) and Hoechst (blue) staining for nuclei. Pictures were taken under fluorescence microscopy. The relative fluorescence density was quantified by comparing the values of FITC to those of Hoechst, and results are means ± S.D. of at least three slides. Statistic analyses were done by comparing to the relative fluorescence in wild type cells. The scale bars represent 50 μm. Wild type, Mkk4(−/−), Mkk7(−/−), and Mkk4/7(−/−) ESCs either undifferentiated or differentiated for various days were used for (B) isolation of total RNA and the expression of Mef2c was determined by real-time RT-PCR. The relative Mef2c expression was normalized to Gapdh and results are mean ± S.D. from at least three experiments. Statistical analyses were done by comparing to values in wild type cells, and (C) transient transfection with 6x-Mef2-firefly Luc and β-actin-Renilla luc plasmids. The relative luciferase activity was calculated based on firefly/Renilla units and the values in undifferentiated cells of each genotype were set as 1. Results represent the mean values ± S.E. from at least three independent experiments. Statistical analyses were done by comparing to the mean values in wild type cells.
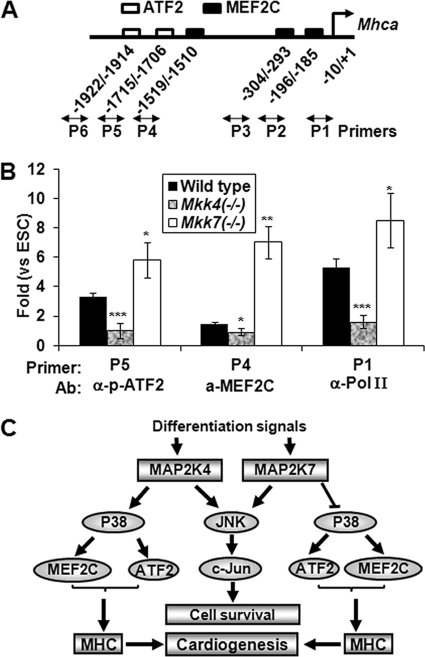
Differential regulation of Mhca expression by MKK4 and MKK7 is therefore likely mediated through ATF2 and MEF2C, because within the 2 kb region upstream of the transcription initiation sites of Mhca, there were two putative ATF2 binding sites and three MEF2C binding sites (Fig. 6A). To test this possibility, we examined undifferentiated ESC and EBs at day 13 of differentiation by chromatin immunoprecipitation (ChIP) assays. Compared with the undifferentiated cells, the wild type EBs had a significant increase of p-ATF2 binding to the P5 site while MEF2C binding to the P4 site, paralleled by RNA polymerase II recruitment (P1) to the Mhca promoter (Fig. 6B). Interestingly, the Mkk4(−/−) EBs had a marked decrease of p-ATF2 binding, while conversely, the Mkk7(−/−) EBs displayed a significantly increased binding of both p-ATF2 and MEF2C, consistent with RNA pol II occupancy at the Mhca promoter. These results suggest that ATF2 and MEF2C may be the molecular links connecting MKK4 and MKK7 to MHC expression during cardiomyocyte differentiation.
FIGURE 6.
MKK4 and MKK7 differentially regulate the Mhca promoter through MEF2C and ATF2. A, sequence analyses the −2000 to +1 bp 5′ to the transcriptional initiation sites of the Mhca promoter reveal putative binding sites for MEF2C and ATF2, and the primers correspond to the promoter sequences are as indicated. B, wild type, Mkk4(−/−), and Mkk7(−/−) ESCs were differentiated for 13 days and subjected to ChIP analyses using antibodies as indicated. IgG was used as a negative control. The precipitated DNA was examined by real-time PCR, using primers encompassing the Mhca promoter regions and the relative protein recruitment to DNA (fold induction) was calculated in comparison to the levels in undifferentiated cell and to the IgG control. Statistical analyses were done by comparing the mean values of at least 4 samples to those in wild type EBs. C, schematic diagram indicates that MKK4 and MKK7 complement each other in activation of the JNK-c-Jun cascades and loss of both lead to senescence of the differentiated cells. While MKK4 is essential for activation of the p38-ATF2/MEF2C cascades, MKK7 attenuates this pathway. Consequently, the Mkk4(−/−) ESCs are defective in MHC induction and cardiomyocyte differentiation, but Mkk7(−/−) ESCs have enhanced MHC expression and cardiogenesis.
DISCUSSION
Combining gene knock-out and in vitro embryonic stem cell differentiation, we show that while both MKK4 and MKK7 are dispensable for ESC self-renewal and survival, they have important signaling and functional properties for differentiation (Fig. 6C). MKK4 and MKK7 both contribute to activation of the JNK-c-Jun cascades. Consequently, loss of both enzymes together abolishes JNK activation and leads to senescence of differentiated cells, whereas loss of either alone does not have this effect (Fig. 6C). Given that the senescence feature of the Mkk4(−/−)Mkk7(−/−) cells resembles that of the JNK-null cells (12), we suggest that this phenotype is attributed, at least in part, to impairment of JNK signaling. MKK4 and MKK7 also exhibit redundant roles in several molecular events associated with differentiation, such as phosphorylation of CREB, and induction of c-Jun and c-Fos expression, but whether these molecular changes depend on JNK has remained to be determined. Nevertheless, in vivo studies have shown that Mkk4(−/−)Mkk7(−/−) mutant fetuses die at E9.5, much earlier than the Jnk1(−/−)Jnk2(−/−) fetuses, which die at E11, raising the possibility that MKK4 and MKK7 have other molecular targets in addition to JNK (10, 11, 47). Conversely, some early differentiation defects, such as impaired expression of Brachyury, observed in the Jnk1(−/−)Jnk2(−/−) are not found in the Mkk4(−/−)Mkk7(−/−) cells, suggesting that JNK also have developmental functions independent of MKK4/MKK7.
A unique MKK4 target identified by our work is p38. Previous in vitro and in vivo studies show that MKK4 contributes to p38 activation in response to selective stimuli, but MKK4 acts in a manner redundant to MKK3 and MKK6 (2, 20, 26). The MKK4-p38 axis is therefore considered dispensable for any biological functions. This however is not the case in ESC differentiation. During differentiation, MKK4 is essentially required for p38 activation and that the MKK4-p38 axis is implicated in MHC induction and cardiomyocyte differentiation (Fig. 6C). Most importantly, this novel signaling and functional property of MKK4 is unique and cannot be compensated by other MAP2Ks. In contrast to Mkk4(−/−), Mkk7(−/−) ESCs show elevated expression of MLC and MHC, and enhanced cardiogenesis upon in vitro differentiation. Corresponding to the higher MEF2C expression and activity and increased recruitment of MEF2C and p-ATF2 to the Mhca promoters in the Mkk7(−/−) EB, there was increased phosphorylation of MKK4 and p38. It is thus possible that MKK7 may negatively regulate the MKK4-p38 axis to modulate cardiogenesis.
The involvement of p38 in cardiogenesis is further supported by the findings that the chemical p38 inhibitor significantly reduces cardiogenesis in vitro, an observation that agrees with work by others (44, 48). Blocking cardiogenesis by the inhibitor, however, has never been as effective as inhibition resulting from Mkk4 gene ablation. One possible explanation is that the chemical inhibitors have limited stability and therefore are not effective for the entire process of in vitro differentiation. Alternatively, MKK4 may regulate cardiogenesis via multiple pathways, while p38 is only one of the downstream events. On the other hand, in contrast to the Mkk4(−/−) ESCs defective specifically in cardiogenesis, the p38α-null ESCs are impaired in a broad spectrum of mesodermal differentiation, incapable of commitment into cardiac, endothelial, smooth muscle and skeletal muscular lineages, and lacking expression of the early differentiation marker Brachyury (49). MKK4 is therefore likely a cell type- and developmental stage-specific upstream regulator for p38, while additional MAP2Ks are responsible for p38 activities associated with other developmental processes.
Activation of the MKK4-p38 axis leads to ATF2 phosphorylation and MEF2C activation. Interestingly, the Mkk4(−/−) cells display a remarkable decrease of p-ATF2 recruitment to the 2kb Mhca promoter, paralleled with significantly less RNA pol II binding, while binding of MEF2C is not much affected. These observations, together with the findings that expression of Mlc, a well-known MEF2C target gene, is not affected by MKK4 ablation, emphasize the importance of the p38-ATF2 cascades downstream of MKK4 in cardiomytocyte differentiation. Consistent with this idea, ATF-2 was shown to regulate MHC expression under pathological conditions that lead to cardiac hypertrophy, characterized by the cardiomyocytes reentering a growth program very similar to the one observed during embryogenesis (50–52). By contrast however the ATF-2 knock-out mice do not have apparent cardiac developmental problems, suggesting that the roles of ATF2 in cardiogenesis can be compensated by other factors in vivo (53). On the other hand, p38 is responsible for MEF2C phosphorylation, which in turn induces cardiac-specific embryonic genes (45, 46, 54–56), and accordingly, the Mef2c knock-out mice are embryonic lethal due to profound cardiovascular defects (56, 57). It is hence possible that while MKK4 ablation does not affect MEF2C binding to the Mhca promoter, it decreases MEF2C phosphorylation thereby its ability to recruit RNA pol II to the promoter and transcriptional gene activation. The MKK4-p38 axis therefore may act through both MEF2C and ATF2 in the regulation of cardiogenesis.
Under conditions of in vitro differentiation that lead to predominant production of mesoderm lineages, we show that differentiation does not alter the expression of MKK4, MKK7, and MAPKs, but induces activation of the MKK4/7-JNK and MKK4-p38 signaling pathways. While the former signaling pathway prevents senescence of differentiated cells, the later is required for cardiomyocyte differentiation. Our studies further identify a window of development, in which MKK4 is particularly crucial for activation of p38 and differentiation of myogenic precursors to cardiomyocytes. This important developmental role of MKK4, however, has not been discovered by using in vivo gene knock-out mice. In vivo deletion of MKK4 in cardiac myocytes does not lead to overt abnormality, suggesting that MKK4 is dispensable for cardiac muscle cell survival once cardiogenesis is accomplished (58). On the other hand, deletion of MKK4 globally leads to early embryonic lethality, prior to the beginning of heart development, suggesting that MKK4 has other developmental roles. In this context, the in vitro system described here has its unique advantage than the in vivo system. The in vitro system allows detailed characterization of differentiation and developmental functions at multiple endpoints without the requirement of tissue viability. In addition, it affords more controlled methods to present morphogenetic and environmental cues in the microenvironment and allows direct assessment of the differentiated cell phenotypes. Furthermore, the in vitro system can be easily manipulated so that molecular biological analyses can be carried out in relatively pure cell types and with easily readout markers. Since MKK4 and MKK7 are evolutionarily conserved protein kinases exhibiting diverse developmental functions, such as dorsal closure, dorsoventral patterning and somitogenesis in multiple species (59–61), the in vitro ESC systems may be adaptable to investigate other differentiation processes in which MKK4 and MKK7 may play a critical role.
Supplementary Material
Acknowledgments
We thank Drs. Nishina (Tokyo Women's Medical University, Japan), Wylie, Gu, and Molkentin (Cincinnati Children's Hospital), and Sachinidis and Jesudoss (Institute of Neurophysiology, University of Cologne, Germany) for cell lines and plasmids.
This work was supported, in whole or in part, by funding from the National Institutes of Health, Grant EY15227 (to J. W., L. C., and Y. X.), NIEHS ES006273 (to C. K., and A. P.), and NIEHS P30 ES006096 (to J. W., L. C., C. K., A. P., and Y. X.), and Ryan Foundation (to L. C.).

This article contains supplemental Figs. S1–S4.
- MAPK
- MAP kinase
- ESC
- embryonic stem cell
- EB
- embryoid body
- FITC
- fluorescein isothiocyanate
- MKK
- mitogen-activated protein kinase kinase.
REFERENCES
- 1. Davis R. J. (1993) The mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem. 268, 14553–14556 [PubMed] [Google Scholar]
- 2. Dérijard B., Raingeaud J., Barrett T., Wu I. H., Han J., Ulevitch R. J., Davis R. J. (1995) Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267, 682–685 [DOI] [PubMed] [Google Scholar]
- 3. Bélanger L. F., Roy S., Tremblay M., Brott B., Steff A. M., Mourad W., Hugo P., Erikson R., Charron J. (2003) Mek2 is dispensable for mouse growth and development. Mol. Cell Biol. 23, 4778–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bissonauth V., Roy S., Gravel M., Guillemette S., Charron J. (2006) Requirement for Map2k1 (Mek1) in extra-embryonic ectoderm during placentogenesis. Development 133, 3429–3440 [DOI] [PubMed] [Google Scholar]
- 5. Giroux S., Tremblay M., Bernard D., Cardin-Girard J. F., Aubry S., Larouche L., Rousseau S., Huot J., Landry J., Jeannotte L., Charron J. (1999) Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 9, 369–372 [DOI] [PubMed] [Google Scholar]
- 6. Saba-El-Leil M. K., Vella F. D., Vernay B., Voisin L., Chen L., Labrecque N., Ang S. L., Meloche S. (2003) An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 4, 964–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yao Y., Li W., Wu J., Germann U. A., Su M. S., Kuida K., Boucher D. M. (2003) Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc. Natl. Acad. Sci. U.S.A. 100, 12759–12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tamura K., Sudo T., Senftleben U., Dadak A. M., Johnson R., Karin M. (2000) Requirement for p38α in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell 102, 221–231 [DOI] [PubMed] [Google Scholar]
- 9. Aouadi M., Bost F., Caron L., Laurent K., Le Marchand, Brustel Y., Binétruy B. (2006) p38 mitogen-activated protein kinase activity commits embryonic stem cells to either neurogenesis or cardiomyogenesis. Stem Cells 24, 1399–1406 [DOI] [PubMed] [Google Scholar]
- 10. Kuan C. Y., Yang D. D., Samanta Roy D. R., Davis R. J., Rakic P., Flavell R. A. (1999) The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22, 667–676 [DOI] [PubMed] [Google Scholar]
- 11. Sabapathy K., Hu Y., Kallunki T., Schreiber M., David J. P., Jochum W., Wagner E. F., Karin M. (1999) JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr. Biol. 9, 116–125 [DOI] [PubMed] [Google Scholar]
- 12. Xu P., Davis R. J. (2010) c-Jun NH2-terminal kinase is required for lineage-specific differentiation but not stem cell self-renewal. Mol. Cell Biol. 30, 1329–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amura C. R., Marek L., Winn R. A., Heasley L. E. (2005) Inhibited neurogenesis in JNK1-deficient embryonic stem cells. Mol. Cell Biol. 25, 10791–10802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lawler S., Fleming Y., Goedert M., Cohen P. (1998) Synergistic activation of SAPK1/JNK1 by two MAP kinase kinases in vitro. Curr. Biol. 8, 1387–1390 [DOI] [PubMed] [Google Scholar]
- 15. Wada T., Nakagawa K., Watanabe T., Nishitai G., Seo J., Kishimoto H., Kitagawa D., Sasaki T., Penninger J. M., Nishina H., Katada T. (2001) Impaired synergistic activation of stress-activated protein kinase SAPK/JNK in mouse embryonic stem cells lacking SEK1/MKK4: different contribution of SEK2/MKK7 isoforms to the synergistic activation. J. Biol. Chem. 276, 30892–30897 [DOI] [PubMed] [Google Scholar]
- 16. Ganiatsas S., Kwee L., Fujiwara Y., Perkins A., Ikeda T., Labow M. A., Zon L. I. (1998) SEK1 deficiency reveals mitogen-activated protein kinase cascade crossregulation and leads to abnormal hepatogenesis. Proc. Natl. Acad. Sci. U.S.A. 95, 6881–6886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishina H., Vaz C., Billia P., Nghiem M., Sasaki T., De la Pompa J. L., Furlonger K., Paige C., Hui C., Fischer K. D., Kishimoto H., Iwatsubo T., Katada T., Woodgett J. R., Penninger J. M. (1999) Defective liver formation and liver cell apoptosis in mice lacking the stress signaling kinase SEK1/MKK4. Development 126, 505–516 [DOI] [PubMed] [Google Scholar]
- 18. Wada T., Penninger J. M. (2004) Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23, 2838–2849 [DOI] [PubMed] [Google Scholar]
- 19. Yang D., Tournier C., Wysk M., Lu H. T., Xu J., Davis R. J., Flavell R. A. (1997) Targeted disruption of the MKK4 gene causes embryonic death, inhibition of c-Jun NH2-terminal kinase activation, and defects in AP-1 transcriptional activity. Proc. Natl. Acad. Sci. U.S.A. 94, 3004–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brancho D., Tanaka N., Jaeschke A., Ventura J. J., Kelkar N., Tanaka Y., Kyuuma M., Takeshita T., Flavell R. A., Davis R. J. (2003) Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 17, 1969–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Downs K. M., Davies T. (1993) Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development 118, 1255–1266 [DOI] [PubMed] [Google Scholar]
- 22. Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., Jones J. M. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 [DOI] [PubMed] [Google Scholar]
- 23. Smith A. G. (2001) Embryo-derived stem cells: of mice and men. Annu. Rev. Cell Dev. Biol. 17, 435–462 [DOI] [PubMed] [Google Scholar]
- 24. Keller G. (2005) Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 19, 1129–1155 [DOI] [PubMed] [Google Scholar]
- 25. Xia Y., Wu Z., Su B., Murray B., Karin M. (1998) JNKK1 organizes a MAP kinase module through specific and sequential interactions with upstream and downstream components mediated by its amino-terminal extension. Genes Dev. 12, 3369–3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin A., Minden A., Martinetto H., Claret F. X., Lange-Carter C., Mercurio F., Johnson G. L., Karin M. (1995) Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science 268, 286–290 [DOI] [PubMed] [Google Scholar]
- 27. Doss M. X., Winkler J., Chen S., Hippler-Altenburg R., Sotiriadou I., Halbach M., Pfannkuche K., Liang H., Schulz H., Hummel O., Hübner N., Rottscheidt R., Hescheler J., Sachinidis A. (2007) Global transcriptome analysis of murine embryonic stem cell-derived cardiomyocytes. Genome Biol. 8, R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nishina H., Fischer K. D., Radvanyi L., Shahinian A., Hakem R., Rubie E. A., Bernstein A., Mak T. W., Woodgett J. R., Penninger J. M. (1997) Stress-signaling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature 385, 350–353 [DOI] [PubMed] [Google Scholar]
- 29. Nishitai G., Shimizu N., Negishi T., Kishimoto H., Nakagawa K., Kitagawa D., Watanabe T., Momose H., Ohata S., Tanemura S., Asaka S., Kubota J., Saito R., Yoshida H., Mak T. W., Wada T., Penninger J. M., Azuma N., Nishina H., Katada T. (2004) Stress induces mitochondria-mediated apoptosis independent of SAPK/JNK activation in embryonic stem cells. J. Biol. Chem. 279, 1621–1626 [DOI] [PubMed] [Google Scholar]
- 30. Sasaki T., Wada T., Kishimoto H., Irie-Sasaki J., Matsumoto G., Goto T., Yao Z., Wakeham A., Mak T. W., Suzuki A., Cho S. K., Zuniga-Pflucker J. C., Oliveira-dos-Santos A. J., Katada T., Nishina H., Penninger J. M. (2001) The stress kinase mitogen-activated protein kinase kinase (MKK)7 is a negative regulator of antigen receptor and growth factor receptor-induced proliferation in hematopoietic cells. J. Exp. Med. 194, 757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gribaldo L., Alison M., Andrews P. W., Bremer S., Donovan P. J., Knaän-Shanzer S., Mertelsmann R., Spielmann H., Testa N. G., Triffitt J. T., Zipori D., de Wynter E. (2002) Meeting summary: European Workshop on Stem Cells, European Centre for the Validation of Biomedical Testing Methods, Institute for Health and Consumer Protection, Joint Research Centre, Ispra, Italy, November 21–23, 2001. Exp. Hematol. 30, 628–633 [DOI] [PubMed] [Google Scholar]
- 32. Peng Z., Peng L., Fan Y., Zandi E., Shertzer H. G., Xia Y. (2007) A critical role for IkappaB kinase beta in metallothionein-1 expression and protection against arsenic toxicity. J. Biol. Chem. 282, 21487–21496 [DOI] [PubMed] [Google Scholar]
- 33. Schnekenburger M., Peng L., Puga A. (2007) HDAC1 bound to the Cyp1a1 promoter blocks histone acetylation associated with Ah receptor-mediated trans-activation. Biochim. Biophys. Acta 1769, 569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu A. L., Birke K., Moriniere J., Welge-Lüssen U. (2010) TGF-{β}2 induces senescence-associated changes in human trabecular meshwork cells. Invest Ophthalmol. Vis. Sci. 51, 5718–5723 [DOI] [PubMed] [Google Scholar]
- 35. Kim N. Y., Woo A. M., Kim J. R., Lee C. (2009) Exploration of senescence-associated genes by differential display reverse transcription polymerase chain reaction: prosaposin as a novel senescence-associated gene. Arch. Pharm. Res. 32, 737–745 [DOI] [PubMed] [Google Scholar]
- 36. Hibi M., Lin A., Smeal T., Minden A., Karin M. (1993) Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 7, 2135–2148 [DOI] [PubMed] [Google Scholar]
- 37. Wang J., Ma H., Tong C., Zhang H., Lawlis G. B., Li Y., Zang M., Ren J., Nijland M. J., Ford S. P., Nathanielsz P. W., Li J. (2010) Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. FASEB J. 24, 2066–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dworkin S., Malaterre J., Hollande F., Darcy P. K., Ramsay R. G., Mantamadiotis T. (2009) cAMP response element binding protein is required for mouse neural progenitor cell survival and expansion. Stem Cells 27, 1347–1357 [DOI] [PubMed] [Google Scholar]
- 39. Chen A. E., Ginty D. D., Fan C. M. (2005) Protein kinase A signaling via CREB controls myogenesis induced by Wnt proteins. Nature 433, 317–322 [DOI] [PubMed] [Google Scholar]
- 40. Grigoriadis A. E., Schellander K., Wang Z. Q., Wagner E. F. (1993) Osteoblasts are target cells for transformation in c-fos transgenic mice. J. Cell Biol. 122, 685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Höpfl G., Gassmann M., Desbaillets I. (2004) Differentiating embryonic stem cells into embryoid bodies. Methods Mol. Biol. 254, 79–98 [DOI] [PubMed] [Google Scholar]
- 42. Itskovitz-Eldor J., Schuldiner M., Karsenti D., Eden A., Yanuka O., Amit M., Soreq H., Benvenisty N. (2000) Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 6, 88–95 [PMC free article] [PubMed] [Google Scholar]
- 43. Di F., V, De L. A., Colorito M. L., Montalbano A., Ardizzone N. M., Macaluso F., Gammazza A. M., Cappello F., Zummo G. (2009) Cardiac stem cell research: an elephant in the room? Anat. Rec. 292, 449–454 [DOI] [PubMed] [Google Scholar]
- 44. Davidson S. M., Morange M. (2000) Hsp25 and the p38 MAPK pathway are involved in differentiation of cardiomyocytes. Dev. Biol. 218, 146–160 [DOI] [PubMed] [Google Scholar]
- 45. Eriksson M., Leppä S. (2002) Mitogen-activated protein kinases and activator protein 1 are required for proliferation and cardiomyocyte differentiation of P19 embryonal carcinoma cells. J. Biol. Chem. 277, 15992–16001 [DOI] [PubMed] [Google Scholar]
- 46. Harvey R. P. (1999) Seeking a regulatory roadmap for heart morphogenesis. Semin. Cell Dev. Biol. 10, 99–107 [DOI] [PubMed] [Google Scholar]
- 47. Asaoka Y., Nishina H. (2010) Diverse physiological functions of MKK4 and MKK7 during early embryogenesis. J. Biochem. 148, 393–401 [DOI] [PubMed] [Google Scholar]
- 48. Li Z., Ma J. Y., Kerr I., Chakravarty S., Dugar S., Schreiner G., Protter A. A. (2006) Selective inhibition of p38alpha MAPK improves cardiac function and reduces myocardial apoptosis in rat model of myocardial injury. Am. J. Physiol. Heart Circ. Physiol 291, H1972-H1977 [DOI] [PubMed] [Google Scholar]
- 49. Barruet E., Hadadeh O., Peiretti F., Renault V., Hadjal Y., Bernot D., Tournaire R., Negre D., Juhan-Vague I., Alessi M. C., Binetruy B. (2010) Stem Cells Dev. 20, 1233–1246 [DOI] [PubMed] [Google Scholar]
- 50. Lim J. Y., Park S. J., Hwang H. Y., Park E. J., Nam J. H., Kim J., Park S. I. (2005) TGF-β1 induces cardiac hypertrophic responses via PKC-dependent ATF-2 activation. J. Mol. Cell Cardiol. 39, 627–636 [DOI] [PubMed] [Google Scholar]
- 51. Sugden P. H., Clerk A. (1998) Cellular mechanisms of cardiac hypertrophy. J. Mol. Med. 76, 725–746 [DOI] [PubMed] [Google Scholar]
- 52. Zechner D., Thuerauf D. J., Hanford D. S., McDonough P. M., Glembotski C. C. (1997) A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization, and cardiac-specific gene expression. J. Cell Biol. 139, 115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maekawa T., Bernier F., Sato M., Nomura S., Singh M., Inoue Y., Tokunaga T., Imai H., Yokoyama M., Reimold A., Glimcher L. H., Ishii S. (1999) Mouse ATF-2 null mutants display features of a severe type of meconium aspiration syndrome. J. Biol. Chem. 274, 17813–17819 [DOI] [PubMed] [Google Scholar]
- 54. Han J., Jiang Y., Li Z., Kravchenko V. V., Ulevitch R. J. (1997) Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature 386, 296–299 [DOI] [PubMed] [Google Scholar]
- 55. Aikawa R., Nagai T., Kudoh S., Zou Y., Tanaka M., Tamura M., Akazawa H., Takano H., Nagai R., Komuro I. (2002) Integrins play a critical role in mechanical stress-induced p38 MAPK activation. Hypertension 39, 233–238 [DOI] [PubMed] [Google Scholar]
- 56. Lin Q., Schwarz J., Bucana C., Olson E. N. (1997) Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276, 1404–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bi W., Drake C. J., Schwarz J. J. (1999) The transcription factor MEF2C-null mouse exhibits complex vascular malformations and reduced cardiac expression of angiopoietin 1 and VEGF. Dev. Biol. 211, 255–267 [DOI] [PubMed] [Google Scholar]
- 58. Liu W., Zi M., Jin J., Prehar S., Oceandy D., Kimura T. E., Lei M., Neyses L., Weston A. H., Cartwright E. J., Wang X. (2009) Cardiac-specific deletion of mkk4 reveals its role in pathological hypertrophic remodeling but not in physiological cardiac growth. Circ. Res. 104, 905–914 [DOI] [PubMed] [Google Scholar]
- 59. Seo J., Asaoka Y., Nagai Y., Hirayama J., Yamasaki T., Namae M., Ohata S., Shimizu N., Negishi T., Kitagawa D., Kondoh H., Furutani-Seiki M., Penninger J. M., Katada T., Nishina H. (2010) Negative regulation of wnt11 expression by Jnk signaling during zebrafish gastrulation. J. Cell Biochem. 110, 1022–1037 [DOI] [PubMed] [Google Scholar]
- 60. Glise B., Noselli S. (1997) Coupling of Jun amino-terminal kinase and Decapentaplegic signaling pathways in Drosophila morphogenesis. Genes Dev. 11, 1738–1747 [DOI] [PubMed] [Google Scholar]
- 61. Rui Y., Xu Z., Xiong B., Cao Y., Lin S., Zhang M., Chan S. C., Luo W., Han Y., Lu Z., Ye Z., Zhou H. M., Han J., Meng A., Lin S. C. (2007) A β-catenin-independent dorsalization pathway activated by Axin/JNK signaling and antagonized by aida. Dev. Cell 13, 268–282 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.