Abstract
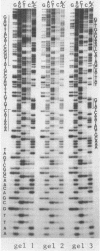
DNA sequence analysis was undertaken to investigate the structural basis of mutations showing different integration efficiencies in Streptococcus pneumoniae. Wild type, mutant and revertant sequences at two sites in the amiA locus were determined. It appears that markers which transform efficiently or inefficiently can result from single base pair changes. A low efficiency (LE) marker corresponds to a C:G to T:A change and a high efficiency (HE) marker to a G:C to T:A change. In the latter case, two mismatches, G/A and T/C, can exist at the heteroduplex stage in transformation; only T/C appears to be recognized by the hex system which controls transforming efficiencies in pneumococcus. Each of the recognized mismatches, T/G and C/A, which result from transitional change, and T/C appears to involve at least one pyrimidine. It is proposed that the mismatch repair system of S. pneumoniae is directed against mismatched pyrimidines. DNA sequence analysis also reveals that short deletions (33 or 34 bases long) behave as very high efficiency markers, confirming that deletions are not recognized by the hex system.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Claverys J. P., Louarn J. M., Sicard A. M. Cloning of Streptococcus pneumoniae DNA: its use in pneumococcal transformation and in studies of mismatch repair. Gene. 1981 Jan-Feb;13(1):65–73. doi: 10.1016/0378-1119(81)90044-5. [DOI] [PubMed] [Google Scholar]
- Claverys J. P., Roger M., Sicard A. M. Excision and repair of mismatched base pairs in transformation of Streptococcus pneumoniae. Mol Gen Genet. 1980 Apr;178(1):191–201. doi: 10.1007/BF00267229. [DOI] [PubMed] [Google Scholar]
- Ephrussi-Taylor H., Gray T. C. Genetic studies of recombining DNA in pneumococcal transformation. J Gen Physiol. 1966 Jul;49(6):211–231. doi: 10.1085/jgp.49.6.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi-Taylor H., Sicard A. M., Kamen R. Genetic Recombination in DNA-Induced Transformation of Pneumococcus. I. the Problem of Relative Efficiency of Transforming Factors. Genetics. 1965 Mar;51(3):455–475. doi: 10.1093/genetics/51.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh P. J., Schmeissner U., Hofer M., Miller J. H. Genetic studies of the lac repressor. VII. On the molecular nature of spontaneous hotspots in the lacI gene of Escherichia coli. J Mol Biol. 1978 Dec 25;126(4):847–857. doi: 10.1016/0022-2836(78)90023-2. [DOI] [PubMed] [Google Scholar]
- Galibert F., Hérissé J., Courtois G. Nucleotide sequence of the EcoRI-F fragment of adenovirus 2 genome. Gene. 1979 May;6(1):1–22. doi: 10.1016/0378-1119(79)90081-7. [DOI] [PubMed] [Google Scholar]
- Gasc A. M., Sicard A. M. Genetic studies of acridine-induced mutants in Streptococcus pneumoniae. Genetics. 1978 Sep;90(1):1–18. doi: 10.1093/genetics/90.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasc A. M., Sicard A. M. Mutagenèse induite par l'hydroxylamine sur l'ADN du pneumocoque. C R Acad Sci Hebd Seances Acad Sci D. 1972 Jul 10;275(2):285–287. [PubMed] [Google Scholar]
- Gasc A. M., Vacher J., Buckingham R., Sicard A. M. Characterization of an amber suppressor in Pneumococcus. Mol Gen Genet. 1979;172(3):295–301. doi: 10.1007/BF00271729. [DOI] [PubMed] [Google Scholar]
- Guild W. R., Shoemaker N. B. Mismatch correction in pneumococcal transformation: donor length and hex-dependent marker efficiency. J Bacteriol. 1976 Jan;125(1):125–135. doi: 10.1128/jb.125.1.125-135.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker W. D., Laskowski M., Sr Polynucleotide kinase: functional purification and use in the direct kinetic measurement of single- and double- strand cleavages of DNA by restriction and other endonucleases of limited action. Anal Biochem. 1977 May 1;79(1-2):63–72. doi: 10.1016/0003-2697(77)90379-7. [DOI] [PubMed] [Google Scholar]
- Lacks S. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics. 1966 Jan;53(1):207–235. doi: 10.1093/genetics/53.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lataste H., Claverys J. P., Sicard A. M. Physical and genetic characterization of deletions in Streptococcus pneumoniae. J Bacteriol. 1980 Oct;144(1):422–424. doi: 10.1128/jb.144.1.422-424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Lipsich L., Wigler M. Isolation of the chicken thymidine kinase gene by plasmid rescue. Nature. 1980 May 22;285(5762):207–210. doi: 10.1038/285207a0. [DOI] [PubMed] [Google Scholar]
- Roger M. Mismatch excision and possible polarity effects result in preferred deoxyribonucleic acid strand of integration in pneumococcal transformation. J Bacteriol. 1977 Jan;129(1):298–304. doi: 10.1128/jb.129.1.298-304.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Guild W. R. Destruction of low efficiency markers is a slow process occurring at a heteroduplex stage of transformation. Mol Gen Genet. 1974;128(4):283–290. doi: 10.1007/BF00268516. [DOI] [PubMed] [Google Scholar]
- Sicard A. M., Ephrussi-Taylor H. Genetic recombination in DNA-induced transformation of Pneumococcus. II. Mapping the amiA region. Genetics. 1965 Dec;52(6):1207–1227. doi: 10.1093/genetics/52.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard A. M., Ephrussi-Taylor H. Recombinaison génétique dans la transformation chez le pneumocoque. Etude des réversions au locus amiA. C R Acad Sci Hebd Seances Acad Sci D. 1966 May 23;262(21):2305–2308. [PubMed] [Google Scholar]
- Tiraby G., Sicard M. A. Integration efficiencies of spontaneous mutant alleles of amiA locus in pneumococcal transformation. J Bacteriol. 1973 Dec;116(3):1130–1135. doi: 10.1128/jb.116.3.1130-1135.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraby J. G., Fox M. S. Marker discrimination in transformation and mutation of pneumococcus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3541–3545. doi: 10.1073/pnas.70.12.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]