Background: Plants possess conserved TOR PK but appear to display rapamycin resistance.
Results: Rapamycin effectively inactivates Arabidopsis TOR PK and retards glucose-mediated growth.
Conclusion: Integrative analyses with TOR-S6K phosphorylation, rapamycin, and estradiol-inducible tor and fkp mutants unravel the central roles of glucose-TOR signaling in diverse plant cells and organs.
Significance: Rapamycin and estradiol-inducible tor mutants facilitate chemical genetic dissection of plant TOR signaling networks.
Keywords: Allosteric Regulation, Antibiotic Resistance, Arabidopsis, Enzyme Inhibitors, Protein-Protein Interactions, S6 Kinase, TOR, TOR Complex (TORC), FK506-binding Protein 12, Rapamycin
Abstract
Target of rapamycin (TOR) kinase is an evolutionarily conserved master regulator that integrates energy, nutrients, growth factors, and stress signals to promote survival and growth in all eukaryotes. The reported land plant resistance to rapamycin and the embryo lethality of the Arabidopsis tor mutants have hindered functional dissection of TOR signaling in plants. We developed sensitive cellular and seedling assays to monitor endogenous Arabidopsis TOR activity based on its conserved S6 kinase (S6K) phosphorylation. Surprisingly, rapamycin effectively inhibits Arabidopsis TOR-S6K1 signaling and retards glucose-mediated root and leaf growth, mimicking estradiol-inducible tor mutants. Rapamycin inhibition is relieved in transgenic plants deficient in Arabidopsis FK506-binding protein 12 (FKP12), whereas FKP12 overexpression dramatically enhances rapamycin sensitivity. The role of Arabidopsis FKP12 is highly specific as overexpression of seven closely related FKP proteins fails to increase rapamycin sensitivity. Rapamycin exerts TOR inhibition by inducing direct interaction between the TOR-FRB (FKP-rapamycin binding) domain and FKP12 in plant cells. We suggest that variable endogenous FKP12 protein levels may underlie the molecular explanation for longstanding enigmatic observations on inconsistent rapamycin resistance in plants and in various mammalian cell lines or diverse animal cell types. Integrative analyses with rapamycin and conditional tor and fkp12 mutants also reveal a central role of glucose-TOR signaling in root hair formation. Our studies demonstrate the power of chemical genetic approaches in the discovery of previously unknown and pivotal functions of glucose-TOR signaling in governing the growth of cotyledons, true leaves, petioles, and primary and secondary roots and root hairs.
Introduction
The evolutionarily conserved protein kinase (PK), target of rapamycin (TOR),3 is encoded by orthologous genes from yeast to plants and humans and likely plays central roles in integrating nutrients and energy availability with other environmental signals to coordinate survival, growth and development (1–4). In Saccharomyces cerevisiae, TOR is activated under favorable conditions to promote cell growth by maintaining a robust rate of ribosome biogenesis, translation initiation, and nutrient import (4). In mammals, TOR operates as a hub of the signal transduction network that controls metabolism, growth, and division by diverse signals including nutrient, energy, stress, hormones, and mitogens (2–7). Modulation of TOR expression levels suggests a correlation with the cell and organ size, seed yield, and stress resistance in Arabidopsis (8, 9). Studies of the Arabidopsis TOR interaction partner RAPTOR and a downstream effector TAP46 also suggest their vital roles in growth and development, stress adaptation, autophagy, and nitrogen mobilization (10–12). Despite the importance of TOR functions in eukaryotes, little is known about the plant TOR signaling network and its upstream regulators due to the lack of molecular and biochemical assays for endogenous TOR PK activity and the embryo lethality of null Arabidopsis tor mutants (1).
Rapamycin, a natural antibiotic produced by the soil bacterium Streptomyces hyproscopicus, can specifically inactivate TOR PK in yeast and mammals. The inhibitory effect of rapamycin is mediated by the formation of a specific protein complex, in which rapamycin forms noncovalent bonds between FKP12 and the FRB domain of TOR proteins (4). Recent data suggest that FKP12-rapamycin inhibition of TOR PK function is achieved by causing dissociation of RAPTOR from TOR (13) or by blocking a specific subset of TOR complex1 (TORC1) substrates, but not TORC2 functions (14). In contrast to yeasts and mammals, studies over the past decade with land plants generated the prevailing view that plants are insensitive to rapamycin because this drug does not inhibit Arabidopsis growth at concentrations that are effective in yeast and mammalian cells (1, 15). Yeast two-hybrid studies suggested that Arabidopsis FKP12 is unable to form a complex with rapamycin and TOR, whereas the Arabidopsis TOR-FRB can still bind to yeast or human FKP12 in the presence of rapamycin (15–17). It was proposed that Arabidopsis FKP12 had evolved structural changes to prevent the formation of the inhibitory complex with TOR and rapamycin (1, 15).
A main obstacle in elucidating the plant TOR signaling network is the lack of convenient and reliable molecular and biochemical assays to monitor plant TOR PK activities. The embryo lethality of null tor mutants (1, 15) further limits the molecular dissection of TOR functions in higher plants in the past decade. A key substrate and mediator of TOR PK is S6K, which is evolutionarily conserved in plants and humans (16). We report here that site-specific phosphorylation of Arabidopsis S6Ks can serve as a reliable and sensitive molecular and biochemical marker to monitor endogenous TOR PK activity in Arabidopsis. Unexpectedly, using this sensitive assay we found that rapamycin can effectively inhibit Arabidopsis TOR PK activation by glucose. Rigorous genetic analyses using independent transgenic Arabidopsis plants and cellular assays with reduced or increased FKP12 expression provide compelling evidence for the specific role of endogenous FKP12 protein in mediating rapamycin inactivation of TOR PK activity. The establishment of the S6K1 Thr-449 phosphorylation-based TOR PK activity in vivo assay, the conditional tor mutants, and the discovery of the effectiveness of rapamycin in Arabidopsis unravel the central roles of glucose-TOR signaling in diverse plant cells and organs and open new possibilities to molecular dissect the TOR signaling networks in plants.
EXPERIMENTAL PROCEDURES
Plant Materials and Growth Conditions
Col-0 wild-type (WT) Arabidopsis plants were used in this study, and all transgenic plants generated are in the Col-0 background. Plants were grown at 23 °C/20 °C, 65% humidity, and 75 μmol m−2 s−1 light intensity under a 12-h light/12-h dark photoperiod condition. Plants were grown in soil for 4 weeks for mesophyll protoplast isolation. For phenotypic analysis of rapamycin effects on seedling growth, seeds were germinated and grown in 6-well plates containing 1 ml of liquid medium (0.5 × MS and 0.5% sucrose, adjusted to pH 5.7 with KOH) with 1–10 μm rapamycin. In glucose experiments for seedling and root hair growth, 0.5% sucrose was replaced without or with 30 mm glucose. For long term rapamycin treatments, the medium was changed with fresh rapamycin every 2 days to ensure the rapamycin effect.
Plasmid Constructs
For S6K1 (At3g08730) and S6K2 (At3g08720) constructs: cDNAs were amplified from leaf RNA, fused to the FLAG tag, and cloned between a 35S-driven promoter and NOS terminator (18, 19). The S6K1 mutant (T449A) and S6K2 mutant (T455A) were generated by PCR-based site-specific mutagenesis (20). For FKP12 (At5g64350), FKP15.1 (At3g25220), FKP15.2 (At5g48580), FKP20.1 (At3g55520), FKP42 (At3g21640), FKP62 (At3g25230), FKP65 (At5g48570), and FKP72 (At3g54010) constructs, cDNAs were amplified from leaf RNA, fused to the HA tag, and cloned between a 35S-driven promoter and NOS terminator. All primers used are listed in supplemental Table 1.
Protoplast Transient Expression Assay
Protoplast transient expression assays were carried out as described previously (21). Data were generated from at least three independent experiments with consistent results. Protoplasts (1–4 × 104) were incubated for 10 h in 1 ml of mannitol (0.5 m) and KCl (20 mm) buffer (4 mm MES, pH 5.7) in 6-well tissue culture plates (1-mm depth) and treated with rapamycin for 30 min in the indicated concentrations. Protoplasts were harvested by centrifugation and suspended in SDS sample buffer (62.5 mm Tris-HCl (pH 6.8), 2% w/v SDS, 10% glycerol, and 50 mm DTT) for SDS-PAGE and protein blot analysis.
Antibodies and Protein Blot Analysis
Phospho-p70 S6 kinase (Thr(P)-389) polyclonal antibody (Cell Signaling, catalog no. 9205) was used to detect TOR PK phosphorylation of Thr(P)-449 in S6K1 and Thr(P)-455 in S6K2. HA- or FLAG-tagged proteins were detected by anti-HA (Roche Applied Science) or anti-FLAG (Sigma) monoclonal antibodies using standard techniques. Polyclonal Arabidopsis TOR antibody was generated using a synthetic peptide, CTLNRVIADLCSRGNPKEGAP. The antibody was affinity-purified using a SulfoLink matrix (Pierce).
Split Luciferase Assay
Protoplasts were co-transfected with NLUC-FRB and CLUC-FKP12s for 8 h, then incubated for 30 min with luciferin (0.25 μg/μl) and finally incubated for 5 min with rapamycin. Samples were transferred to 96-well plates, and the output was read using a modulus microplate reader (Turner Biosystems).
RT-PCR Analysis
Total RNA was isolated with TRIzol reagent (Invitrogen). First-strand cDNA was synthesized from 1 μg of total RNA with MuLV reverse transcriptase (Promega). All quantitative RT-PCR analyses were performed by CFX96 real time PCR detection system with iQ SYBR Green supermix (Bio-Rad). TUB4 (AT5G44340) was used as a control gene.
Transgenic Plants
To generate transgenic FKP12 and S6K1 overexpression lines, the coding region was cloned into an expression vector derived from the pCB302 minibinary vector (19). To generate the fkp12 RNAi lines, the last 332 bp of the coding region were cloned in two opposite orientations in the pHANNIBAL plasmid (22) under the control of the 35S-driven promoter. To generate the estradiol-inducible tor RNAi lines, the 480-bp coding region (560–1040) was cloned in two opposite orientations in the pHANNIBAL plasmid, and the whole silencing cassette was shifted to estradiol-inducible vector pLB12 (23). Estradiol (10 μm) was used to induce gene silencing effects.
Phylogenetic Tree
Protein sequences were aligned with ClustalW, using the SDSC Workbench, to generate the phylogenetic tree.
RESULTS
Sensitive Cell-based Assay to Monitor Endogenous Arabidopsis TOR PK Activity
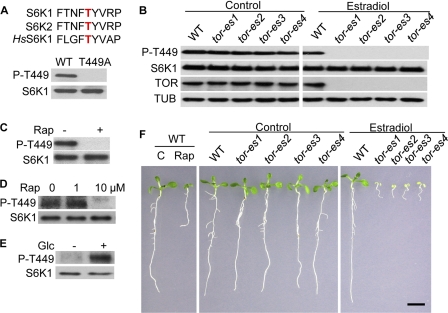
To investigate the molecular and biochemical mechanisms underlying the regulations and functions of Arabidopsis TOR, we first established a sensitive cell-based assay to monitor TOR PK activity. Arabidopsis TOR shares high amino acid sequence similarity with human TOR, especially for the PK domain (supplemental Fig. S1), suggesting that they may have similar PK properties and share protein substrates. The direct TOR substrate and major effector S6K are highly conserved in Arabidopsis and humans. Intriguingly, the TOR phosphorylation motif FLGFTYVAP in human S6K1 appears to be conserved in the Arabidopsis orthologs, S6K1 and S6K2 (Fig. 1A and supplemental Fig. S2). Using a specific antibody against the Thr(P)-389 peptide in human S6K1 phosphorylated by TOR (4), we developed a cell assay to monitor endogenous Arabidopsis TOR PK activity based on the conserved S6K phosphorylation, Thr-449 and Thr-455 in Arabidopsis S6K1 and S6K2, respectively, as molecular and biochemical markers (Fig. 1A). With this sensitive assay, the endogenous PK activity for S6K phosphorylation is clearly detected in nondividing and fully differentiated leaf cells expressing the WT S6K1 or S6K2, but not the mutant S6K1(T449A) or S6K2(T455A) (Fig. 1A and supplemental Fig. S3A). To provide direct genetic and biochemical evidence for the endogenous TOR PK activity, we generated estradiol-inducible tor transgenic Arabidopsis plants (tor-es) to circumvent the embryo lethality of null tor mutants and produced a peptide antibody that specifically recognizes Arabidopsis TOR. Depletion of TOR expression by estradiol treatment eliminated Thr-449 and Thr-455 phosphorylation of S6K1 and S6K2, respectively (Fig. 1B and supplemental Fig. S3B). These studies provide the first evidence that S6K1 and S6K2 are true Arabidopsis TOR substrates and that Thr-449 phosphorylation of S6K1 or Thr-455 phosphorylation of S6K2 can be used to monitor the TOR PK activity in plants. The data reveal active TOR signaling in nondividing and fully differentiated and expanded leaf cells, which is not previously recognized.
FIGURE 1.
Monitor TOR activity based on Thr-449 phosphorylation of S6K1. A, conserved TOR phosphorylation of S6K proteins: conserved TOR phosphorylation motif in human and Arabidopsis S6Ks (upper) and Thr-449 phosphorylation specificity of S6K1 (lower). FLAG-tagged WT or mutant S6K1 (T449A) was transiently expressed in protoplasts. Total proteins were analyzed by protein blot analysis using anti-Thr(P)-T389 (P-T455) or anti-FLAG (S6K1) antibody. B, Thr-449 phosphorylation abolished in tor mutants. WT or estradiol-inducible tor mutants (tor-es) protoplasts expressing FLAG-tagged S6K1 were treated without or with estradiol (10 μm). Total proteins were analyzed by protein blotting probed with anti-TOR (TOR), anti-Thr(P)-389 (P-T449), or anti-FLAG (S6K1) antibody. Tubulin (TUB) was used as a loading control. C, inhibition of Thr-449 phosphorylation by rapamycin in leaf cells. WT protoplasts expressing FLAG-tagged S6K1 were treated with rapamycin (Rap, 1 μm) for 30 min. D, inhibition of Thr-449 phosphorylation by rapamycin in seedlings. Transgenic S6K1-overexpressing seedlings were treated with rapamycin (1 or 10 μm) for 30 min. E, activation of Thr-449 phosphorylation by glucose. Transgenic S6K1-overexpressing seedlings were grown with or without glucose (Glc, 30 mm) for 4 days. F, rapamycin and tor mutants retarding seedling growth. Rapamycin (10 μm) or estradiol (10 μm) was added at the time of germination for 9 days. Scale bar, 5 mm.
Rapamycin Inhibits TOR-S6K Signaling in Arabidopsis
Rapamycin, as a specific inhibitor of TOR PK, has been an instrumental tool in dissecting the complex TOR signaling network in yeasts and animals (2, 3). Research during the past decade generated the prevailing view that land plants are resistant to rapamycin due to evolutionary changes in the Arabidopsis FKP12 gene (1, 15). We reexamined this hypothesis using the new sensitive cell assay. Surprisingly, we discovered that rapamycin effectively inactivates endogenous TOR PK activity revealed by Thr-449 and Thr-455 phosphorylation of S6K1 and S6K2, respectively (Fig. 1C and supplemental Fig. S3C), although at a higher concentration than was shown in mammalian and yeast cells (100–1000 nm versus 10–50 nm). Although S6K phosphorylation was clearly detected using the antibody against the Thr(P)-389 peptide in human S6K1 when overexpressed in Arabidopsis leaf cells, we failed to detect a reliable signal for the endogenous S6K1. It is possible that the endogenous S6K protein level was too low for efficient analysis in intact plants, which explained the difficulty in generating any Arabidopsis TOR PK assay in vivo in the past decade. Also, the antibody probably has low affinity for the Arabidopsis S6K proteins as it was generated against the Thr(P)-389 peptide motif in human S6K1, which has three amino acid variations in Arabidopsis S6Ks (Fig. 1A). As an alternative approach to detect endogenous TOR PK activity in planta, we generated transgenic S6K1-overexpressing plants and demonstrated that rapamycin also effectively inactivates Thr-449 phosphorylation of S6K1 in Arabidopsis seedlings (Fig. 1D). Because rapamycin completely blocked the phosphorylation of overexpressed S6K1 in cells and seedlings, it is suggested that the endogenous S6K1 is similarly inhibited in planta.
Glucose-TOR Signaling in Arabidopsis Seedling Growth
Using S6K1 Thr-449 phosphorylation-based TOR PK activity assay, we screened for potential upstream nutrient signals and discovered that glucose strongly activates TOR PK activity in Arabidopsis seedlings (Fig. 1E). Because our previous studies have indicated that the growth of Arabidopsis seedlings strictly relies on glucose after germination and photomorphogenesis in the liquid culture medium (24), we showed that Arabidopsis seedlings are arrested in standard liquid culture medium without any sugar, whereas 30 mm glucose promotes vigorous growth (supplemental Fig. S4). We examined the role of glucose-TOR signaling in the growth of diverse organs in the presence of rapamycin. As shown in Fig. 1F, rapamycin strongly retards many key aspects of organ growth in seedlings after germination, including cotyledon expansion, true leaf development, petiole elongation, as well as primary and lateral root growth. Importantly, these phenotypes closely resemble the inducible tor mutant plant grown in the presence of glucose (Fig. 1F), supporting pivotal roles of glucose-TOR signaling in the growth of diverse plant organs. Interestingly, TOR activity may not be required for germination and photomorphogenesis because WT seedlings without or with rapamycin and tor-es seedlings look alike in the absence of sugar (Fig. 1F and supplemental Fig. S4). Contrary to previous studies and conclusions, these data strongly indicate that rapamycin can effectively inhibit Arabidopsis growth by suppressing TOR PK activity, which is not limited to embryo, endosperm, and meristems (15).
Inhibitory Effect of Rapamycin Requires Endogenous FKP12
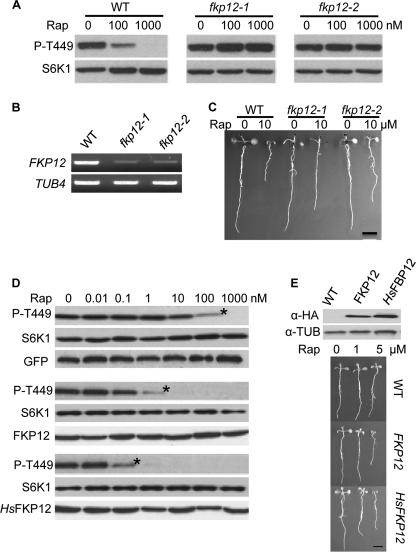
It has been shown that rapamycin forms an inhibitory complex involving FKP12 and the FRB domain of TOR (25). To determine whether the inhibitory effect of rapamycin observed in Arabidopsis seedlings and leaf cells is dependent on FKP12, multiple fkp12 transgenic lines were generated using RNA interference (RNAi) (Fig. 2B). In fkp12 leaf cells, the inhibitory effect of rapamycin on Thr-449 phosphorylation of S6K1 is severely compromised compared with that in WT leaf cells (Fig. 2A). The inhibitory effect of rapamycin in whole seedling growth is also much reduced in fkp12 plants (Fig. 2C and supplemental Fig. S5A). These studies strongly support the specific role of endogenous FKP12 protein in mediating rapamycin inactivation of TOR PK activity.
FIGURE 2.
A crucial role of FKP12 for rapamycin inactivation of TOR. A, FKP12 requirement for inhibition of Thr-449 phosphorylation by rapamycin (Rap). WT or fkp12 mutant protoplasts expressing FLAG-tagged S6K1 were treated with rapamycin. Total proteins were analyzed by protein blotting probed with anti-Thr(P)-389 (P-T449) or anti-FLAG (S6K1) antibody. B, RT-PCR analysis of FKP12 expression. C, FKP12 requirement for inhibition of seedling growth by rapamycin. Rapamycin was added at the time of germination for 9 days. D, overexpression of Arabidopsis or human FKP12 enhancing the rapamycin inhibition effect on Thr-449 phosphorylation in leaf cells. FLAG-tagged S6K1 was co-expressed with HA-tagged FKP12s or GFP in protoplasts and treated with rapamycin for 30 min. E, overexpression of Arabidopsis or human FKP12 enhancing rapamycin inhibition effect on seedling growth. Rapamycin was added at the time of germination for 9 days. Scale bar, 5 mm.
Overexpression of Arabidopsis or Human FKP12 Significantly Enhances Rapamycin Efficacy
Arabidopsis was thought to be resistant to rapamycin for a lack of the FKP12 binding to TOR-FRB in a rapamycin-dependent manner observed in yeast and human FKP12 (1). To facilitate the study of TOR functions using the rapamycin-based chemical genetics in Arabidopsis, we intended to genetically engineer the rapamycin sensitivity in Arabidopsis leaf cells by ectopic expression of human FKP12 (HsFKP12). Arabidopsis FKP12 was also overexpressed in leaf cells to serve originally as a negative control. Unexpectedly, we found that overexpression of either Arabidopsis FKP12 or HsFKP12 dramatically enhances cell sensitivity to rapamycin 100–1000-fold (Fig. 2D). Transgenic Arabidopsis seedlings with elevated Arabidopsis or human FKP12 expression also exhibit enhanced growth retardation at lower rapamycin concentrations (Fig. 2E and supplemental Fig. S5B). These data suggest that, in contrast to previous studies, rapamycin inhibits Arabidopsis TOR PK activity and this inhibition can be further enhanced by overexpressing Arabidopsis or human FKP12 protein.
Action of Arabidopsis FKP12 Is Highly Specific
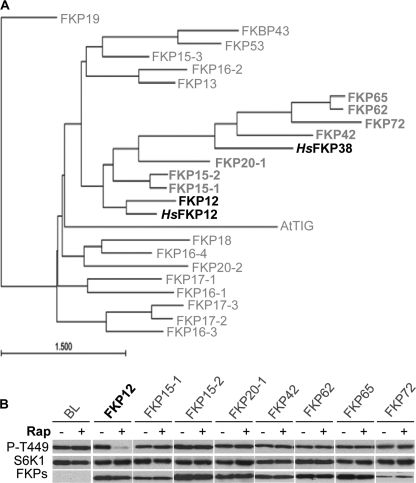
In addition to FKP12, it has been suggested that human FKP38 (HsFKP38) is also involved in TOR regulation (26). We further investigated the function of seven closely related Arabidopsis FKP proteins (Fig. 3A) in mediating the inhibitory effect of rapamycin on the endogenous Arabidopsis TOR PK activity. Despite equal level of protein expression, none of these seven FKPs can increase rapamycin sensitivity or display function similar to that of HsFKP38 to inhibit TOR PK activity directly (Fig. 3B). The findings demonstrate the specificity of Arabidopsis and human FKP12 in rapamycin-dependent TOR PK inhibition.
FIGURE 3.
Specificity of FKP12 in rapamycin-dependent TOR inhibition. A, phylogenetic comparison of Arabidopsis FKPs with human FKP12 and FKP38. The nomenclature is based on Ref. 34. B, unique role of FKP12 in enhancing Arabidopsis leaf cell sensitivity to rapamycin. HA-tagged FKPs and FLAG-tagged S6K1 were co-expressed in protoplasts and treated with rapamycin (Rap, 1 nm) for 30 min. Total proteins were analyzed by protein blotting probed with anti-Thr(P)-389 (P-T449), anti-FLAG (S6K1), or anti-HA (FKPs) antibody.
Rapamycin Induces Interaction between Arabidopsis TOR-FRB Domain and FKP12s in Plant Cells
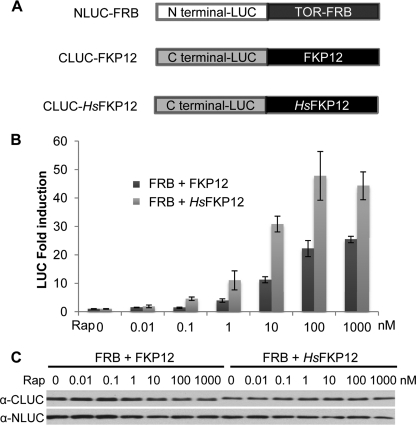
Comparison of FKP12 coding sequences and the FRB domains between human TOR and Arabidopsis TOR reveals high sequence conservation (supplemental Fig. S6). Because our comprehensive analyses have suggested that the inhibitory effect of rapamycin depends on FKP12, we next examined whether FKP12 associates with Arabidopsis TOR-FRB domain in a rapamycin-dependent manner by employing the split luciferase complementation assay for protein-protein interaction in intact and active Arabidopsis leaf cells (27). The N- and C-terminal fragments of firefly luciferase (NLUC and CLUC) were translationally fused to TOR-FRB and FKP12, respectively (Fig. 4A), and the constructs were co-expressed in leaf protoplasts. The emitted luminescence reflects the intensity of protein interaction. As shown in Fig. 4B, there is only minimal background luminescence signal in leaf cells co-transfected with NLUC-FRB and CLUC-FKP12 or empty control vectors in the absence of rapamycin. In contrast, rapamycin even at 1 nm (a low concentration level) can quickly and significantly trigger the luminescence intensity increase, which reaches the maximum at 1 μm, correlating with complete inhibition of TOR phosphorylation of S6K1 by rapamycin (Fig. 2A). Rapamycin does not affect the expression of NLUC-FRB and CLUC-FKP12 proteins (Fig. 4C). These results strongly indicate that rapamycin induces the direct interactions between the Arabidopsis TOR-FRB domain and both Arabidopsis and human FKP12 in a concentration-dependent manner in plant cells.
FIGURE 4.
Rapamycin-dependent interactions between Arabidopsis TOR-FRB domain and FKP12s. A, NLUC-FRB and CLUC-FKP12 constructs. B, relative interaction intensity between TOR-FRB domain and FKP12s. NLUC-FRB and CLUC-FKP12 or CLUC-HsFKP12 were co-expressed in protoplasts for 6 h and treated with rapamycin (Rap) for 5 min. Rapamycin induces the direct interactions between Arabidopsis TOR-FRB domain and both Arabidopsis and human FKP12 in a concentration-dependent manner. Results represent means ± S.D. (error bars). n = 3. C, protein expression level of NLUC-FRB and CLUC-FKP12s.
Glucose-TOR Signaling Is Central to Root Hair Development
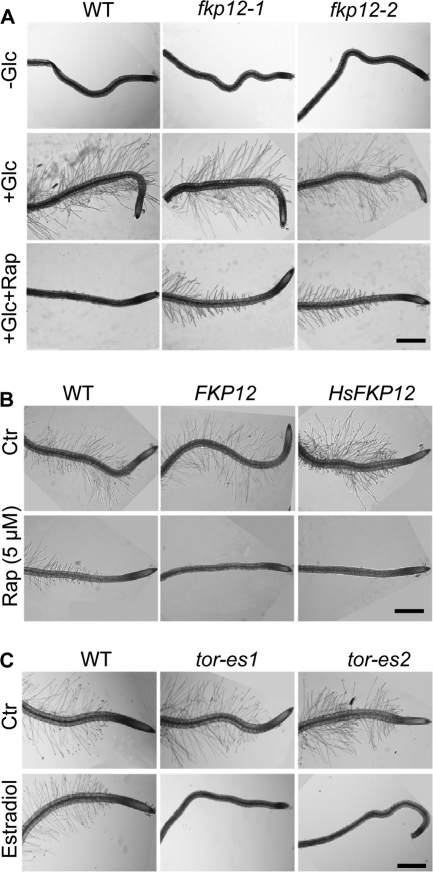
To explore other possible roles of glucose-TOR signaling further, we thoroughly examined Arabidopsis seedlings grown in the presence or absence of glucose and found profound differences in root hair patterns (Fig. 5A). Root hairs are long tubular-shaped extensions from single epidermal cells to aid plants in absorbing nutrients and water, interacting with microorganisms, and physically anchoring plants to the soil. Although it is established that root hair development is controlled by hormones and other signals, the effect of glucose is previously unknown (28). Importantly, rapamycin completely prevents root hair growth in WT seedlings in the presence of glucose, but the inhibitory effect of rapamycin is diminished in fkp12-deficient seedlings (Fig. 5A). Consistently, FKP12-overexpressing transgenic seedlings are hypersensitive to rapamycin (Fig. 5B), and the estradiol-inducible tor-es mutants abolish root hair growth mediated by glucose-TOR signaling (Fig. 5C). Because this glucose regulation is unaffected in the hexokinase 1 glucose sensor mutant gin2 (supplemental Fig. S7), glucose-TOR signaling in controlling root hair development acts in the hexokinase 1-independent pathway (24, 29).
FIGURE 5.
A pivotal role of glucose-TOR signaling in root hair formation. A, rapamycin inhibition of glucose-mediated root hair growth. Seedlings were germinated with or without glucose (30 mm) for 4 days. Rapamycin (Rap, 10 μm). B, enhancing the rapamycin inhibition effect on root hair formation by overexpressing Arabidopsis or human FKP12. Seedlings were germinated with 30 mm glucose for 4 days. C, retardation of root hair growth in tor mutants. Seedlings were germinated with 30 mm glucose for 4 days. Scale bar, 500 μm.
DISCUSSION
TOR PK is a central integrator of nutrient sensing and signaling in all eukaryotes (1–4). The TOR signaling network is involved in various growth-related processes by mostly unknown mechanisms in plants (1). Although the Arabidopsis TOR transcript is detected in most tissues, it has been reported that the expression patterns of Arabidopsis TOR::GUS fusion protein are limited to proliferating cells in embryo, endosperm, and primary meristems (15). This raised the question for how nonproliferating cells regulate their growth-related processes through TOR signaling or whether TOR has any functions in fully differentiated cells in plants. The analyses with conditional tor mutant plants and an antibody to a specific Arabidopsis TOR peptide presented here provide compelling evidence that the TOR protein is expressed and active in fully differentiated leaves, and its PK activity is responsible for Thr-449 and Thr-455 phosphorylation of S6K1 and S6K2, respectively (Fig. 1 and supplemental Fig. S3). Thus, TOR may have distinct functions tailored and integrated in specific cellular contexts and environments in both dividing and nondividing cells. The use of liquid culture in protoplast transient expression and seedling assays facilitates the uptake and replenishment of rapamycin for effective and flexible TOR PK manipulation in future exploration of the plant TOR signaling network.
Although structural studies have demonstrated the formation of an inhibitory complex involving rapamycin, FKP12 and TOR-FRB, several studies using either the yeast two-hybrid method or direct binding assay had failed to show that Arabidopsis FKP12 can interact with TOR-FRB (15–17). The use of in vivo split luciferase complementation assay clearly illustrates that it is possible to detect physiologically relevant, direct, and quick interactions between the Arabidopsis TOR-FRB domain and Arabidopsis or human FKP12 induced by rapamycin in a concentration-dependent manner (Fig. 4). These results suggest that the interaction between FKP12 and TOR-FRB may be transient and weak or need posttranslational modification, making it difficult to be detected using in vitro, nonphysiological, or nonplant assays.
Previous studies have suggested successful “restoration” of the rapamycin susceptibility by ectopic expression of Saccharomyces FKP12 in Arabidopsis (17). Our results clearly suggest that overexpression of FKP12 actually enhances rather than restores the rapamycin sensitivity. Therefore, variable endogenous FKP12 protein levels may offer a molecular explanation underlying varied rapamycin resistance at low rapamycin concentrations (nanomolar) and provide a feasible strategy to improve rapamycin efficacy for clarifying TOR functions and advancing TOR research in plants, various mammalian cell lines, or diverse animal cell types and model systems (2, 3).
Glucose is the preferred energy source in all organisms from bacteria, yeasts, plants, to humans. In plants, glucose has emerged as the key regulators of many vital processes, including root, stem and shoot growth; reproduction; stress responses; and senescence (29, 30). How glucose modulates complex responses remains largely enigmatic. Integrative analyses with TOR-S6K phosphorylation, rapamycin, and conditional tor and fkp mutants uncover the central roles of glucose-TOR signaling in controlling the growth of diverse plant organs and root hairs. In addition to glucose, plant organ growth and root hair development are also controlled by many genetic, nutritional, hormonal, and environmental factors (28–31). Future studies will determine how the glucose-TOR signaling is coordinated and integrate with these multiple signals to control plant organ and root hair growth.
Taking advantage of chemical genetic tools and approaches, the established cellular and seedlings assays will facilitate the understanding of molecular and biochemical mechanisms of TOR signaling in plants, circumventing limitations inherent to lethal and conditional mutants. A new generation of mammalian TOR (mTOR) PK active site inhibitors (e.g. PP242 and Torin1) has recently been developed (32, 33). These inhibitors directly target the ATP site of mTOR PK and have shown to be effective for suppression of animal cell growth and proliferation. Unlike rapamycin, which is suggested to be specific for TORC1, PP242 and Torin1 inhibit a broader spectrum of mTOR functions through inactivation of both TORC1 and TORC2 (32, 33). It will be of great interest to combine and compare the effective range and specificity of these distinct inhibitors in future molecular dissections of the plant TOR PK signaling network.
Supplementary Material
Acknowledgments
We thank N. Dai and J. Avruch for S6K antibody and advice; J. F. Li for the LUC system; M. D. Curtis and Y. J. Niu for the estradiol-inducible binary vector; M. McCormack for the TOR peptide antibody design; and J. F. Li, H. Lee, L. Li, and M. Ramon for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 GM60493 (to J. S.). This work was also supported by National Science Foundation Grant IOS-0843244 (to J. S.).

This article contains supplemental Table 1, Figs. S1–S7, and an additional reference.
- TOR
- target of rapamycin
- CLUC
- C-terminal firefly luciferase
- FKP12
- FK506-binding protein 12
- FRB domain
- FKP-rapamycin binding domain
- mTOR
- mammalian TOR
- NLUC
- N-terminal firefly luciferase
- RAPTOR
- regulatory associated protein of TOR
- S6K
- S6 kinase
- TORC1
- TOR complex 1
- TORC2
- TOR complex 2.
REFERENCES
- 1. Dobrenel T., Marchive C., Sormani R., Moreau M., Mozzo M., Montané M. H., Menand B., Robaglia C., Meyer C. (2011) Regulation of plant growth and metabolism by the TOR kinase. Biochem. Soc. Trans. 39, 477–481 [DOI] [PubMed] [Google Scholar]
- 2. Zoncu R., Efeyan A., Sabatini D. M. (2011) mTOR: from growth signal integration to cancer, diabetes, and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Russell R. C., Fang C., Guan K. L. (2011) An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development 138, 3343–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wullschleger S., Loewith R., Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 5. Inoki K., Li Y., Xu T., Guan K. L. (2003) Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 17, 1829–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao X., Zhang Y., Arrazola P., Hino O., Kobayashi T., Yeung R. S., Ru B., Pan D. (2002) Tsc tumor suppressor proteins antagonize amino-acid-TOR signaling. Nat. Cell Biol. 4, 699–704 [DOI] [PubMed] [Google Scholar]
- 7. Brugarolas J., Lei K., Hurley R. L., Manning B. D., Reiling J. H., Hafen E., Witters L. A., Ellisen L. W., Kaelin W. G., Jr. (2004) Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18, 2893–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deprost D., Yao L., Sormani R., Moreau M., Leterreux G., Nicolaï M., Bedu M., Robaglia C., Meyer C. (2007) The Arabidopsis TOR kinase links plant growth, yield, stress resistance, and mRNA translation. EMBO Rep. 8, 864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ren M., Qiu S., Venglat P., Xiang D., Feng L., Selvaraj G., Datla R. (2011) Target of rapamycin regulates development and ribosomal RNA expression through kinase domain in Arabidopsis. Plant Physiol. 155, 1367–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deprost D., Truong H. N., Robaglia C., Meyer C. (2005) An Arabidopsis homolog of RAPTOR/KOG1 is essential for early embryo development. Biochem. Biophys. Res. Commun. 326, 844–850 [DOI] [PubMed] [Google Scholar]
- 11. Anderson G. H., Veit B., Hanson M. R. (2005) The Arabidopsis AtRaptor genes are essential for post-embryonic plant growth. BMC Biol. 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahn C. S., Han J. A., Lee H. S., Lee S., Pai H. S. (2011) The PP2A regulatory subunit Tap46, a component of the TOR signaling pathway, modulates growth and metabolism in plants. Plant Cell 23, 185–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oshiro N., Yoshino K., Hidayat S., Tokunaga C., Hara K., Eguchi S., Avruch J., Yonezawa K. (2004) Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells 9, 359–366 [DOI] [PubMed] [Google Scholar]
- 14. Yip C. K., Murata K., Walz T., Sabatini D. M., Kang S. A. (2010) Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol. Cell 38, 768–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Menand B., Desnos T., Nussaume L., Berger F., Bouchez D., Meyer C., Robaglia C. (2002) Expression and disruption of the Arabidopsis TOR (target of rapamycin) gene. Proc. Natl. Acad. Sci. U.S.A. 99, 6422–6427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahfouz M. M., Kim S., Delauney A. J., Verma D. P. (2006) Arabidopsis target of rapamycin interacts with RAPTOR, which regulates the activity of S6 kinase in response to osmotic stress signals. Plant Cell 18, 477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sormani R., Yao L., Menand B., Ennar N., Lecampion C., Meyer C., Robaglia C. (2007) Saccharomyces cerevisiae FKBP12 binds Arabidopsis thaliana TOR, and its expression in plants leads to rapamycin susceptibility. BMC Plant Biol. 7, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kovtun Y., Chiu W. L., Zeng W., Sheen J. (1998) Suppression of auxin signal transduction by a MAPK cascade in higher plants. Nature 395, 716–720 [DOI] [PubMed] [Google Scholar]
- 19. Hwang I., Sheen J. (2001) Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413, 383–389 [DOI] [PubMed] [Google Scholar]
- 20. Asai T., Tena G., Plotnikova J., Willmann M. R., Chiu W. L., Gomez-Gomez L., Boller T., Ausubel F. M., Sheen J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415, 977–983 [DOI] [PubMed] [Google Scholar]
- 21. Yoo S. D., Cho Y. H., Sheen J. (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572 [DOI] [PubMed] [Google Scholar]
- 22. Wesley S. V., Helliwell C. A., Smith N. A., Wang M. B., Rouse D. T., Liu Q., Gooding P. S., Singh S. P., Abbott D., Stoutjesdijk P. A., Robinson S. P., Gleave A. P., Green A. G., Waterhouse P. M. (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J. 27, 581–590 [DOI] [PubMed] [Google Scholar]
- 23. Brand L., Hörler M., Nüesch E., Vassalli S., Barrell P., Yang W., Jefferson R. A., Grossniklaus U., Curtis M. D. (2006) A versatile and reliable two-component system for tissue-specific gene induction in Arabidopsis. Plant Physiol. 141, 1194–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xiao W., Sheen J., Jang J. C. (2000) The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol. Biol. 44, 451–461 [DOI] [PubMed] [Google Scholar]
- 25. Choi J., Chen J., Schreiber S. L., Clardy J. (1996) Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science 273, 239–242 [DOI] [PubMed] [Google Scholar]
- 26. Bai X., Ma D., Liu A., Shen X., Wang Q. J., Liu Y., Jiang Y. (2007) Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science 318, 977–980 [DOI] [PubMed] [Google Scholar]
- 27. Li J. F., Bush J., Xiong Y., Li L., McCormack M. (2011) Large-scale protein-protein interaction analysis in Arabidopsis mesophyll protoplasts by split firefly luciferase complementation. PLoS ONE 6, e27364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishida T., Kurata T., Okada K., Wada T. (2008) A genetic regulatory network in the development of trichomes and root hairs. Annu. Rev. Plant Biol. 59, 365–386 [DOI] [PubMed] [Google Scholar]
- 29. Moore B., Zhou L., Rolland F., Hall Q., Cheng W. H., Liu Y. X., Hwang I., Jones T., Sheen J. (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300, 332–336 [DOI] [PubMed] [Google Scholar]
- 30. Rolland F., Baena-Gonzalez E., Sheen J. (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu. Rev. Plant Biol. 57, 675–709 [DOI] [PubMed] [Google Scholar]
- 31. Baena-González E., Sheen J. (2008) Convergent energy and stress signaling. Trends Plant Sci. 13, 474–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feldman M. E., Apsel B., Uotila A., Loewith R., Knight Z. A., Ruggero D., Shokat K. M. (2009) Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 7, e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thoreen C. C., Kang S. A., Chang J. W., Liu Q., Zhang J., Gao Y., Reichling L. J., Sim T., Sabatini D. M., Gray N. S. (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 284, 8023–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He Z., Li L., Luan S. (2004) Immunophilins and parvulins: superfamily of peptidyl prolyl isomerases in Arabidopsis. Plant Physiol. 134, 1248–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.