Abstract
The role of withdrawal-related phenomena in development and maintenance of alcohol addiction remains under debate. A “self-medication” framework postulates that emotional changes are induced by a history of alcohol use, persist into abstinence, and are a major factor in maintaining alcoholism. This view initially focused on negative emotional states during early withdrawal: these are pronounced, occur in the vast majority of alcohol dependent patients, and are characterized by depressed mood and elevated anxiety. This concept lost popularity with the realization that, in most patients, these symptoms abate over 3 – 6 weeks of abstinence, while relapse risk persists long beyond this period. More recently, animal data have established that a prolonged history of alcohol dependence induces more subtle neuroadaptations. These confer altered emotional processing that persists long into protracted abstinence. The resulting behavioral phenotype is characterized by excessive voluntary alcohol intake and increased behavioral sensitivity to stress. Emerging human data support the clinical relevance of negative emotionality for protracted abstinence and relapse. These developments prompt a series of research questions: 1) Are processes observed during acute withdrawal, while transient in nature, mechanistically related to those that remain during protracted abstinence? 2) Is susceptibility to negative emotionality in acute withdrawal in part due to heritable factors, similar to what animal models have indicated for susceptibility to physical aspects of withdrawal? 3) To what extent is susceptibility to negative affect that persists into protracted abstinence heritable?
Keywords: alcoholism, withdrawal, neuroadaptation, stress
1. Introduction
Symptoms that emerge when prolonged and excessive alcohol consumption is discontinued are characteristic of the alcohol dependence syndrome, or alcoholism (Edwards & Gross 1976). Although neither necessary nor sufficient for a diagnosis, withdrawal symptoms belong to consensus diagnostic criteria for this disease (American Psychiatric Association et al. 1994). Despite this central role, understanding the role of withdrawal symptoms for excessive drinking or relapse remains challenging.
“Self-medication” views of addiction posit that a desire to avoid or relieve pre-existing or withdrawal-related aversive states is a key motivation behind excessive drug use and relapse (Markou et al. 1998). Pharmacologically based theories such as those of Wikler (Wikler 1948) and Dole (Dole et al. 1966; Dole 1965) focused on relief from drug withdrawal symptoms as the basis for continued drug use. This perspective contrasts with subsequent views emphasizing the positive reinforcing actions of drugs (Wise & Bozarth 1985; Koob et al. 1987; Di Chiara & Imperato 1988). The distinction has important therapeutic implications. For instance, the most successful treatment for an addictive disorder to date, methadone maintenance for heroin dependence, was developed based on the hypothesis that relieving withdrawal symptoms would diminish the desire to continue heroin use (Dole 1965). The main objective of the present paper is to examine the nature and relevance of alcohol withdrawal symptoms for maintaining alcoholism, from the specific perspective of evaluating how well we model them in animals and identifying their genetic and neurobiological underpinnings. While there are interesting data from many species, we confine our analysis to the rodent species used in the vast majority of studies.
Withdrawal is a diverse and temporally dynamic process. When its different aspects are assessed clinically, a summary rating of withdrawal severity is routinely derived (Sullivan et al. 1989), but different withdrawal symptoms follow different time courses. Traditionally, the focus is on physical manifestations. However, as will be discussed below, psychological changes, e.g. increased anxiety, low mood and attenuated ability to experience pleasure from natural rewards, are also key components of the withdrawal syndrome. It remains unclear which, if any, of these diverse withdrawal manifestations that are linked to motivational processes are involved in maintaining alcoholism. The objective of the present paper is to identify conceptual and methodological issues that need to be resolved to address this question more effectively.
2. Historical perspective
2.1 A central role for physical dependence
Although specific symptoms differ, alcohol shares a general pattern of pharmacodynamic tolerance (i.e., reduced sensitivity to alcohol’s effects) and physical withdrawal with other addictive drugs. The presumption historically has been that postponement or avoidance of early, physical withdrawal symptoms such as tremors, autonomic nervous system hyperactivity and seizures, is key to sustaining heavy alcohol use. This thinking was influenced by research on opiate dependence. For instance, Dole (Dole 1965) largely focused on the physical symptoms of acute opiate withdrawal, and considered the desire to relieve the “sick” feeling of acute withdrawal to be a major motivation for drug seeking and use. Wikler and O’Brien (Wikler 1948; O’Brien et al. 1977) observed that long after acute physical withdrawal symptoms subsided in a treatment setting, detoxified heroin addicts began to experience physical withdrawal symptoms and craving when exposed to drug-associated stimuli. Groundbreaking operant drug self-administration studies were performed under the assumption that morphine self-administration would not be sustained in animals unless they were first made physically dependent (Spragg 1940; Headlee et al. 1955; Weeks 1962; Thompson & Schuster 1964).
2.2 Drugs as positive reinforcers
Over time, it became clear that physical dependence and withdrawal are not required for sustaining drug use. Clinical studies noted that while 70 – 80% of subjects with any addiction relapsed within the first year, the vast majority of relapses occurred long after acute physical withdrawal symptoms had resolved (Hunt et al. 1971). Also, while acute alcohol withdrawal became easily managed with benzodiazepines (Mayo-Smith 1997), relapse rates were not reduced by these treatments. Finally, with the spread of psychostimulant use, it became clear that an addictive process can share core clinical characteristics of excessive use and frequent relapse in the absence of physical dependence.
In laboratory studies, morphine self-administration was eventually observed in animals with no prior history of morphine exposure (Deneau et al. 1969). Drugs which do not produce evident signs of physical dependence, such as the psychomotor stimulants, were also readily self-administered by laboratory animals (Pickens & Thompson 1968; Balster & Schuster 1973). In addition, the relationship between physical withdrawal symptoms and ethanol self-administration was found to be complex, with some studies reporting diminished alcohol self-administration in the presence of severe acute physical withdrawal signs, while others observed increased drinking when withdrawal symptoms were less severe (see below).
In light of these observations, acute withdrawal and its dramatic physical symptoms were concluded to be of lesser relevance for understanding the addictive process. Instead, addiction was explained in terms of positive reinforcement principles. In this view, drug use occurs to the exclusion of other behaviors because drug reinforcers are more potent than alternative positive reinforcers. The contingency relationship between behavior and its consequences is viewed as more important than drug-receptor actions in defining this effect of drugs, as animals will work to reject or receive the same highly addictive drug, depending on the experimental conditions (Spealman 1979).
A highly productive research line emphasized drug actions upon neural substrates of reward and positive reinforcement, primarily focusing on mesolimbic dopamine circuitry (Wise & Bozarth 1985; Koob et al. 1987; Di Chiara & Imperato 1988). Over the course of three decades, the view of addiction shifted markedly. Opiates, with their distinct physiological withdrawal syndrome, were once viewed as the quintessential addictive drugs. Following the cocaine epidemic, psychomotor stimulants with dopaminergic action began to define the prototypical properties of an addictive substance. This view also emphasized similarities rather than differences between addictive drugs. There is now clear evidence for a role of the mesolimbic dopamine pathway in mediating the acute positive reinforcing properties of alcohol (Di Chiara et al. 1996; Ahlenius et al. 1973; Weiss et al. 1996; Weiss et al. 1993; Tanda & Di Chiara 1998; Spanagel & Weiss 1999), but it is noteworthy that consumption and self-administration of alcohol does not rely on the integrity of this system (Rassnick et al. 1993b; Kiianmaa et al. 1979). Indeed, a review of 9 mouse studies comparing null mutants for dopamine D1, D2, D3, and D4 receptors, the dopamine transporter DAT, or the vesicular monoamine transporter VMAT with their respective wild types revealed a wide range of effects (or lack thereof) on ethanol preference drinking (Crabbe et al. 2006)
Rather than directly mediating the rewarding effects of natural and drug reinforcers, more recent hypotheses focus on the role of mesolimbic dopamine as a motivational learning signal (Spanagel & Weiss 1999), a signal of pathological associative learning in addiction (Di Chiara 2002), a neural substrate of incentive salience (Robinson & Berridge 2003), or a signal informing about the predictability of reward related to cues associated with drug availability (Fiorillo et al. 2003). To what extent these phenomena contribute to clinical alcoholism is currently unknown.
2.3 Withdrawal revisited: focus on changes in affective processing
Most recently, there has been a return to emphasis on chronic alcohol use and the role of dependence in vulnerability to relapse and in sustaining substance use. Among the several factors which have led to this shift, three are of particular relevance for the present paper:
Recognition that affective (psychological) components of withdrawal and protracted abstinence influence alcohol/drug use rather than simply avoidance of physiological symptoms of withdrawal.
Advances in research approaches that enable detecting changes in affective components of withdrawal, e.g. human functional brain imaging and laboratory procedures; and animal behavioral and neurochemical/molecular procedures (Koob 2009).
Refinement of animal models that better reflect the course of alcohol/drug use from initiation and sustained use to the development of dependence.
The renewed focus on processes related to chronic alcohol use, dependence and withdrawal takes into account the limitations of early views centered upon physical manifestations of acute withdrawal, as pointed out e.g. in (Robinson & Berridge 2003). Instead, a neuroadaptive view of alcoholism focuses on long-term plasticity that leads to a persistent negative affective state and altered function of key motivational systems as the proximal cause of relapse and excessive alcohol drinking. In this scenario, phenomena encountered during acute withdrawal and early abstinence are of interest primarily if they reflect events that are critical to the induction of long-term plasticity. Against this background, it becomes critical to define the different phases that follow discontinuation of alcohol use.
3. The withdrawal process
3.1 Time course of acute withdrawal, early abstinence and protracted abstinence
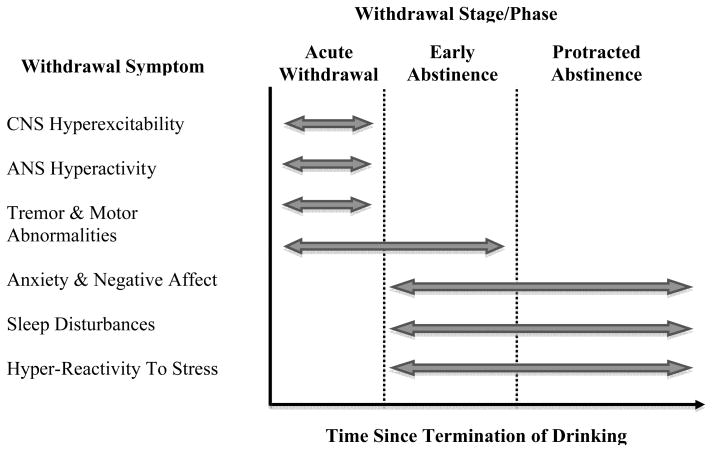
Clinically, alcohol withdrawal follows a characteristic temporal course (See Figure 1). In the absence of co-morbid conditions, other drug use or treatment, three relatively distinct phases are observed:
Figure 1. Alcohol Withdrawal Syndrome: Temporal Pattern of Withdrawal Symptoms.
Acute Withdrawal Phase: Humans (48–72 hours); Animals (24–48 hours)
Early Abstinence Phase: Humans (3–6 weeks); Animals (1–2 weeks)
Protracted Abstinence Phase: Humans (>3 months); Animals (>1 month)
Acute withdrawal reflects generalized nervous system hyperexcitability, and is dominated by tremors, autonomic nervous system hyperactivity, risk for delirium tremens and seizures. Seizures typically occur within the first 48h following discontinued consumption, with a peak around 24h; tremor follows a similar time course; while delirium tremens typically peaks around 72h. After the first week, symptoms in the acute category are rarely encountered. During acute withdrawal, treatment focus is on controlled return from generalized hyperexcitability, and prevention of seizures and delirium tremens (Victor & Adams 1953; Mayo-Smith 1997).
Early abstinence
We use this term in reference to an intermediate period that follows, during which anxiety, low mood and disturbed sleep continue, but are now expressed in the absence of acute physical symptoms. The elevated anxiety resolves over 3 – 6 weeks after discontinued alcohol use (Schuckit & Hesselbrock 1994), somewhat more slowly in women than in men (Bokström et al. 1991).
Protracted abstinence
During this final phase, elevated anxiety and dysphoria are not necessarily detected by standard assessment methods, but patients continue to report what will here be called a shift in affective processing. During this phase, small, normally insignificant challenges provoke negative affect, craving and relapse (Sinha & Li 2007). An interesting corollary is an attenuation or absence of expected positive responses to normally pleasurable events. This shift can be captured by functional brain imaging, and is associated with craving and relapse risk (Heinz et al. 2007; George et al. 2008; Gilman & Hommer 2008). Whether these negative affective changes are a key component of protracted abstinence in any given individual may be related to heterogeneity of alcoholics. Such heterogeneity was critically captured by classical adoption studies (Cloninger 1987; Sigvardsson et al. 1996; Cloninger et al. 1981). This is highlighted by more recent human data, in which alcoholics without antisocial personality disorder (ASPD) show increased potentiation of the startle response by negative affective stimuli when compared to normal non-alcoholic controls. In contrast, alcoholics with co-morbid ASPD in fact have a lower degree of startle potentiation (Miranda, Jr. et al. 2003; Miranda, Jr. et al. 2002). The former group clearly represents an internalizing (Cloninger – Bohman type I) form of the disease, while the latter is at the extreme of an externalizing subtype (Cloninger – Bohman type II).
3.2 Acute withdrawal vs. protracted abstinence – relation to relapse
Renewed intake of alcohol for relief of acute withdrawal symptoms is one of the diagnostic criteria for alcoholism (American Psychiatric Association et al. 1994). It is, however, unlikely that acute withdrawal, dominated by physical symptoms, is a major motivational factor for inducing relapse by the time that patients seek treatment. One indication that this is not the case is provided by the observation that concurrent use of benzodiazepines, unless provided under the controlled conditions of a detox unit, will not only be ineffective in preventing return to heavy drinking, but in fact increase the risk for it [see e.g. (Malcolm 2003)].
In contrast, protracted abstinence and the affective shift that remains during this phase are emerging as major factors behind craving and relapse (Heinz et al. 2003; Breese et al. 2005a; Sinha & Li 2007; Heilig & Koob 2007). The accumulated evidence for prolonged pathological activation of brain systems mediating negative affective responses have been captured in an intuitively attractive term as “relief craving” (Heinz et al. 2003). In classical learning theory, this represents negative reinforcement, i.e., the elimination or avoidance of a negative situation.
While affective dysregulation in protracted abstinence is likely to be of immediate relevance for relapse to excessive alcohol use, the link between the early withdrawal phenomena and subsequent affective dysregulation remains unclear. If such a link exists, it would provide a strong rationale for revisiting the biology and genetics of acute withdrawal symptoms.
4. Modeling human withdrawal phenotypes in animals
4.1 Methods to Induce Dependence
A major limitation in modeling clinical withdrawal phenomena has for a long time been that most laboratory animals will not voluntarily consume sufficient amounts of alcohol to produce dependence. Although several lines of rats and mice have been developed through selective breeding to exhibit high avidity for alcohol [for reviews see (Ciccocioppo & Hyytia 2006; Ciccocioppo et al. 2006; Colombo et al. 2006; Sommer et al. 2006; Quintanilla et al. 2006; Murphy et al. 2002)], few studies have focused on whether voluntary oral consumption of alcohol in these models leads to dependence and, in particular, emergence of withdrawal upon termination of drinking. One exception involves a genetic model, in which a subgroup of P (Preferring) rats selectively bred for high alcohol preference were given unlimited access to alcohol for a period of time (15–20 weeks). This subgroup was chosen for further study because they ingested more than 5 g/kg ethanol/day and showed greater than 67% preference for the 10% ethanol solution vs water. These highest-drinking P rats were shown to exhibit withdrawal signs upon termination of drinking (Waller et al. 1982). In a more recent study, P rats drinking 10% ethanol by choice for 10 weeks and then withdrawn were shown to have 30% reductions in threshold sensitivity to bicucculine seizures (Kampov-Polevoy et al. 2000). It is also clear that both duration and pattern of voluntary ethanol intake contribute to whether signs of withdrawal will emerge (Holter et al. 2000).
Most animal models of physical alcohol dependence involve experimenter-administered alcohol. Numerous procedures can be used to induce dependence, with three approaches being most common (Becker 2000). Repeated gastric intubations (Majchrowicz 1975) avoid problems of injecting large volumes or high alcohol concentrations intraperitoneally, and allow the dose and timing to be controlled. They also may avoid issues of taste aversion, which develops if oral consumption of unmasked alcohol is forced (Wise 1975). Alternatively, alcohol consumption can be forced by provision in a liquid diet offered as the sole source of fluid and calories. With this procedure, the investigator controls the duration of exposure, but the animal determines the amount and pattern of alcohol consumption. Finally, chronic inhalation of alcohol vapor offers the tightest control over dose and timing of exposure (Rogers et al. 1979; Goldstein & Pal 1971).
It is well established in both animal and clinical studies that the amount and duration of alcohol exposure play a significant role for the development of dependence and expression of withdrawal (Finn & Crabbe 1997). Additionally, the pattern of chronic alcohol exposure has been recognized as an important variable in defining the severity of the withdrawal reaction (Becker 1998). Greater severity of withdrawal symptoms has consistently been found when chronic alcohol exposure is delivered in an intermittent rather than continuous fashion (Becker 1999). As discussed below, a growing body of evidence suggests that “kindling” or sensitization of alcohol withdrawal severity may significantly contribute to the negative emotional state that endures into the protracted abstinence phase, as well as enhance relapse vulnerability (Becker 2008; Breese et al. 2005a; Heilig & Koob 2007).
4.2 Withdrawal-Related Phenotypes
Regardless of the methods used to induce dependence, animal models have effectively demonstrated numerous physiological and behavioral measures of withdrawal when exposure to alcohol is terminated or significantly reduced [for reviews see (Becker 2000; Deitrich et al. 1996; Finn & Crabbe 1997; Friedman 1980; Metten & Crabbe 1996)]. Moreover, these withdrawal responses to a great extent correspond to withdrawal signs and symptoms observed in humans. The following is an overview of alcohol withdrawal-related phenotypes identified in rodent models and the extent to which they correspond to clinical signs and symptoms of the human alcohol withdrawal syndrome. Special attention is given to withdrawal phenotypes that are purported to most strongly affect the vulnerability to relapse and perpetuation of excessive drinking that is characteristic of alcohol dependence.
4.2.1 Measures of Central Nervous System (CNS) Hyperexcitability
A hallmark feature of alcohol withdrawal is CNS hyperexcitability. Because it is relatively easy to measure, withdrawal-related seizures are well documented in animals and humans by electrophysiological as well as behavioral measures (Becker 2000; Deitrich et al. 1996; Porjesz & Begleiter 1996; Victor 1970). These include increased frequency of spontaneous as well as evoked perturbations in EEG activity that include spike and sharp wave epileptiform activity and more global synchronized high-voltage spindling activity. Motor convulsions may occur spontaneously, but can be more readily elicited by exposure to sensory stimuli (e.g., audiogenic), handling manipulation, electroconvulsive stimulation, and various chemoconvulsant agents. While the time course for spontaneous and various experimentally induced convulsions may differ, the general time-dependent waxing and waning of seizure susceptibility is very similar in time course to that observed clinically (Finn & Crabbe 1997; Becker 2000). Further, both electrographic and behavioral measures of seizure activity in animals are highly sensitive to clinically effective anticonvulsants (Becker & Veatch 2002; Becker et al. 2006; Crabbe 1992; Veatch & Becker 2005; Watson et al. 1997). Thus, withdrawal seizure susceptibility is extensively characterized and clinically relevant. What is uncertain is whether it represents manifestations of those neuroadaptations that will affect motivational changes occurring later in withdrawal to engender high risk for relapse and harmful drinking.
Enhanced sensory reactivity is another measure thought to reflect heightened CNS excitability during withdrawal in human alcoholics [e.g., (Grillon et al. 1994; Krystal et al. 1997). A number of studies have been conducted in rats and mice examining the startle response to auditory or tactile (air puff) stimuli. An appealing aspect of this work is that methodology and outcome measures are similar for human and animal studies. However, only a few controlled studies have been conducted in humans, and results from animal studies are generally mixed (i.e., startle reactivity is reported to be increased, decreased, or unchanged during alcohol withdrawal) (Chester et al. 2005; Chester & Barrenha 2007; Macey et al. 1996; Ponomarev & Crabbe 1999; Rassnick et al. 1992; Vandergriff et al. 2000). Interestingly, modulation of the acoustic startle response has been shown to be altered in human alcoholics (Miranda, Jr. et al. 2003) as well as in those with a genetic predisposition for the disease (Miranda, Jr. et al. 2002), depending on the emotional valence of presented visual cues. This suggests that the acoustic startle response may be a useful tool for unveiling a shift in affective processing in which a negative emotional state is predominantly associated with alcohol dependence. This possibility has not been examined in animal models, and stress-induced modulation of the startle response during alcohol withdrawal may be a fruitful approach. Conversely, it has recently been shown in animals that neutral stimuli that have been associated with primary reinforcers suppress acoustic startle, presumably providing a measure of hedonic reactivity (Schneider & Spanagel 2008). This is an approach that presumably could be adapted for use with humans.
4.2.2 Measures of Autonomic Nervous System (ANS) Hyperactivity
Physiological symptoms commonly experienced during acute alcohol withdrawal include those that reflect heightened ANS activation and, in particular, increased sympathetic activity [e.g., (Hawley et al. 1994; King et al. 1994). These include tachycardia (increased heart rate), increased blood pressure, diaphoresis (heavy sweating), body temperature dysregulation, and gastrointestinal disturbances (nausea, vomiting). Animal models demonstrate many of these classic sympathomimetic withdrawal symptoms, including altered cardiovascular function, central and behavioral thermal dysregulation, diarrhea, and reduced food and water intake (Crawshaw et al. 1994; Friedman 1980; Rasmussen et al. 2006). However, given the complex and dynamic nature of variables that influence autonomic function, the reliability and practical utility of these measures is questionable (Bar et al. 2006). Further, most of these symptoms resolve within the acute to early phase of abstinence. Thus, the relevance of autonomic perturbations for triggering relapse and sustained heavy drinking is also questionable.
4.2.3 Motor Abnormalities
Tremor is a frequent symptom of alcohol withdrawal. It has all the characteristics of essential (postural) tremor and is thought to emerge as a manifestation of sympathetic hyperactivity (Koller et al. 1985; Charles et al. 1999). Animal studies have used subjective rating scores (Frye et al. 1983) as well as more quantitative measures (Macey et al. 1996; Meert et al. 1992) to demonstrate increased tremor during withdrawal. Most of this work has been conducted in rats and, to our knowledge, only one study has been performed using mice. Withdrawal Seizure-Prone mice, bred for severe convulsions following chronic ethanol vapor inhalation, were shown to have greater tremor during withdrawal than Withdrawal Seizure-Resistant mice, bred for low convulsion severity (Kosobud & Crabbe 1986).
The time course for withdrawal-related tremor appears to be similar in both animal and clinical studies, predominantly manifesting during acute abstinence, although in humans tremor can extend into the early abstinence phase. Interestingly, although anecdotal, alcoholics often report resumption of drinking linked to a desire to self-medicate the “shakes” (tremor). Given that tremor can be measured objectively in both humans and animals along with its potential relevance to relapse, this might be a withdrawal phenotype worthy of further investigation. However, none of the animal studies have examined time points longer than 12hr following withdrawal, so it is unknown how long tremors persist, or whether they can be exacerbated by the application of stress. A recent study reported that the motor performance of mice on a rotarod is compromised for up to 24 hr following withdrawal from chronic vapor inhalation, but is normalized by one week. These studies indicate a genetic contribution to the phenotype, since effects observed differed as a function of genetic background, and suggest that they are more likely due to compromised motor capacity than to compromised ability to acquire this task, which also involves motor learning (Philibin et al. 2008).
4.2.4 Anxiety and negative affect
Increased anxiety represents a significant component of the alcohol withdrawal syndrome. In humans, anxiety emerges during the early abstinence phase and in many cases lingers for an extended period of time. Of particular relevance to this article, the psychological discomfort associated with anxiety experienced during abstinence, even after most acute physical symptoms have subsided, has been suggested to play a prominent role in increasing risk for relapse as well as perpetuating continued use/abuse of alcohol (Becker 1999; Driessen et al. 2001; Koob 2003; Roelofs 1985). Indeed, both preclinical and clinical studies suggest a link between anxiety and propensity to self-administer alcohol (Henniger et al. 2002; Willinger et al. 2002; Ciccocioppo et al. 2006; Barrenha & Chester 2007; Spanagel et al. 1995; Holter et al. 1998), although this link is by no means simple either in human alcoholics or in animals (Schuckit & Hesselbrock 1994; Henniger et al. 2002).
Of note, while numerous studies reveal comorbid anxiety and depression in human alcoholics, other studies demonstrate a link between alcohol dependence and externalizing psychopathology, which is characterized by a pathological absence of anxiety. Not only do these studies illustrate the heterogeneity of alcoholism, but they also suggest that alcohol dependence can arise from susceptibilities anchored to either extreme of the anxiety spectrum. This has implications for animal studies seeking to examine the relationship between anxiety and alcohol drinking.
Several animal models have been used to demonstrate increased anxiety-like behavior during withdrawal from alcohol (Becker 2000; Kliethermes 2005). Many of these models involve procedures that exploit the natural tendency of rodents to avoid exposure to bright and/or open spaces during free exploration. For example, increased withdrawal anxiety-like behavior has been reported in rats using the elevated plus-maze procedure following chronic alcohol exposure in a liquid diet (Baldwin et al. 1991; Rassnick et al. 1993a) and following withdrawal from a single large alcohol dose (presumably reflecting “hangover anxiety”) (Doremus et al. 2003; Gehlert et al. 2007; Prediger et al. 2006). Interestingly, both these phenotypes are reversed by blockade of CRH receptors, presumably of the CRH1 subtype within the amygdala (Rassnick et al. 1993a; Baldwin et al. 1991; Gehlert et al. 2007), while increased release of CRH within the central amygdala has been shown during alcohol withdrawal (Pich et al. 1995). These findings are in agreement with the observation that homozygous null-mutation for the CRH1 receptor gene blocks anxiety-like behavior caused by ethanol withdrawal (Timpl et al. 1998). Based on a principle similar to the plus-maze, mice have been shown to exhibit increased avoidance (reduced exploration) of a brightly illuminated portion of a two-compartment chamber when testing is conducted during alcohol withdrawal (Hascoet et al. 2001). Another test, used to assess anxiety-like behavior in rats after withdrawal from extended alcohol exposure (Overstreet et al. 2002) and in an acute (“hangover anxiety”) model (Varlinskaya & Spear 2004), involves detecting a reduction in social interaction behavior in a novel environment.
All of these models have some degree of pharmacological validation, i.e. the anxiety-like behavior during withdrawal is restored to normal by drugs that are effective anxiolytics in humans. They are relatively simple to conduct and, in some instances, allow automated monitoring of behavioral measures. However, while findings of withdrawal anxiety using these procedures have been generally consistent in rats (Heilig & Koob 2007), results in mice have been more variable (for review, see (Kliethermes 2005)). This may in part be related to the labile nature of the behavioral outcomes measured and their sensitivity to environmental testing conditions (Crabbe et al. 1999). A more challenging issue relates to the confound of non-specific reduction in activity during early alcohol withdrawal. Operant procedures have been employed to train animals to discriminate between subjective (interoceptive) cues associated with an anxiogenic state experienced during withdrawal (Gauvin et al. 1992). This approach circumvents confounds from general activity suppression, but it requires extensive training prior to testing, and the extent to which discriminative stimulus effects arising during withdrawal correspond to other behavioral manifestations of anxiety must be considered.
Given that anxiety is a complex construct that requires operational definition in animal studies, it may be advantageous to employ several tasks to draw firm conclusions about the anxiety component of alcohol withdrawal in rodent models. On the other hand, a recent study compared multiple inbred mouse strains on three common behavioral assays of anxiety-like behavior: the elevated zero maze, an open field, and a light/dark box. This study found little differentiation between behavioral indices of “anxiety” and those thought to assess “activity,” either within a task or across tasks. This suggests that simply adding additional tasks may be insufficient to resolve the effects of withdrawal on anxiety from those on activity (Milner & Crabbe 2008). Also, as is the case for tremor and other ANS signs, the temporal course of anxiety-like behavior during withdrawal has not been extensively studied. Although some longer term effects have been reported (Valdez et al. 2002; Sommer et al. 2008), most studies examine only a few hours or at most a few days following withdrawal (Kliethermes 2005). A recent paper reported that depression-like behaviors (swim test immobility) and anxiety-like behaviors (in an elevated plus maze) emerged at distinct time points following abstinence from voluntary alcohol drinking in mice (Stevenson et al. 2008). Clearly, this is an area of research that would benefit from more study, especially in relating withdrawal anxiety to relapse.
4.2.5 Sleep Disturbances
Sleep disturbances are common during early withdrawal and often extend into protracted abstinence (Brower 2001; Landolt & Gillin 2001). Clinical studies have noted a self-reported link between disrupted sleep during abstinence and increased risk of relapse (Clark et al. 1998; Drummond et al. 1998; Feige et al. 2007; Malcolm et al. 2007). Clinical studies have recently begun to evaluate treatment strategies for addressing this aspect of the abstinence syndrome, especially in the context of relapse prevention ((Brower et al. 2008; Le Bon et al. 2003; Malcolm et al. 2007; Staner et al. 2006). Despite their potential clinical significance, there have been surprisingly few animal studies focused on studying alcohol withdrawal-related sleep disturbances. Analysis of EEG in freely moving rats (Slawecki et al. 2000) and mice (Veatch 2006) undergoing alcohol withdrawal has revealed significant and long-lasting alterations in sleep architecture. Use of animal models involving objective measures of abstinence-related sleep disturbances may prove beneficial for elucidating mechanisms underlying the problem, and its potential relation to relapse.
4.2.6 Increased Sensitivity to Stress and Discomfort
Beyond the early stages of withdrawal, overt anxiety subsides both in patients and experimental animals. However, as recently reported in a variety of models, increased reactivity to stress (i.e., exaggerated anxiety responses to stressful stimuli/events) persists for a protracted period of time (Valdez et al. 2003; Valdez et al. 2002; Sommer et al. 2008; Breese et al. 2005a). Although complaints about irritability and increased sensitivity to every day stressors have long been recognized clinically in alcoholic patients, corresponding human data are just beginning to emerge. For instance, recent neuroimaging (fMRI) studies have revealed selectively exaggerated brain responses to negative affective stimuli (George et al. 2008; Gilman & Hommer 2008), and an influence of stress on craving and relapse is beginning to be recognized (Fox et al. 2007; Sinha & Li 2007; Sinha 2009).
Both clinical and experimental studies have documented profound disturbances in endocrine stress responses involving the hypothalamic-pituitary-adrenocortical (HPA) axis following chronic alcohol exposure and withdrawal (Rivier 2000; Wand 2000). For example, in humans and rodents, heightened HPA axis activation associated with alcohol withdrawal usually resolves within a few days, but blunted HPA axis responsiveness to stress challenges appears to persist for a protracted period of time (Adinoff et al. 1990; Adinoff et al. 1991; Costa et al. 1996; Lovallo et al. 2000). However, one animal study suggests that stress-induced relapse is independent of HPA-axis function (Le et al. 2000). Rather, HPA-axis changes may be a key signal for the neuroadaptive processes that drive increased sensitivity to stress and relapse vulnerability through extra-hypothalamic effects. In addition to HPA axis related effects, chronic alcohol alters CRH activity independent from the HPA axis (Heilig & Koob 2007; Koob & Le Moal 2001).
Animal studies have shown that increased CRH release in brain structures intimately tied to reward and stress pathways plays a key role in mediating withdrawal-related anxiety and dysphoria both in the acute phase (Rassnick et al. 1993a; Baldwin et al. 1991; Pich et al. 1995) and during their emergence in protracted abstinence (Breese et al. 2005b; Sommer et al. 2008; Valdez et al. 2002). CRH expression within the amygdala complex and the Bed Nucleus of Stria Terminalis (BNST) is under positive transcriptional control by glucocorticoids, in contrast to hypothalamic CRH (Makino et al. 2002). This offers a potential mechanistic link through which increased glucocorticoid release (HPA axis activation) during cycles of withdrawal drives activation of extra-hypothalamic stress circuitry that is at the core of motivational neuroadaptations in alcoholism. Thus, chronic alcohol exposure and withdrawal experience can be viewed as potent stressors that disrupt the functional integrity of the HPA axis along with recruiting extra-hypothalamic CRH systems, and this perturbation in brain/neuroendocrine stress axes may have significant implications regarding motivation for alcohol self-administration behavior.
CRH activity plays a key role in mediating relapse provoked by stressors, as well as excessive drinking in dependent animals (Funk et al. 2006; Funk et al. 2007; Gehlert et al. 2007; Liu & Weiss 2002; Valdez et al. 2002; Le et al. 2000). These actions of CRH are independent of HPA axis activity (Le et al. 2000; Gehlert et al. 2007). Furthermore, repeated cycles of chronic alcohol exposure and withdrawal lead to not only an exacerbation of physiological symptoms of withdrawal, but also enhanced susceptibility to relapse. For instance, such treatment was found to enhance sensitivity to various stressors, as measured by their ability to activate the HPA axis (Becker 1999), produce anxiety-like behavior (Breese et al. 2005b; Breese et al. 2005a; Sommer et al. 2008), and trigger relapse-like behavior (Ciccocioppo et al. 2003). In all these cases increased CRH transmission was a critical mediating factor, lending support to the idea that enhanced CRF activity represents a key neuroadaptive change that is fueled by repeated withdrawal experience, and this drives (at least in part) motivation to drink as well as amplifies responsiveness to stimuli/events that provoke relapse (Heilig & Koob 2007; Koob & Le Moal 2008).
Other neuropeptides that interact with the CRH circuitry have also been implicated in ethanol drinking. One of these is urocortin 1, a member of the CRH family (Ryabinin & Weitemier 2006). Another player is neuropeptide Y (NPY), an endogenous anti-stress mediator that counteracts the effects of CRH system activations (Heilig et al. 1994). Activation of NPY transmission selectively suppresses excessive alcohol drinking in the post-dependent state (Rimondini et al. 2005; Gilpin et al. 2008), although it remains unclear whether this reflects plasticity within the NPY circuitry itself.
While the CRH actions described above are mediated through extra-hypothalamic circuits and do not directly involve glucocorticoid effects, activation of the HPA axis associated with alcohol dependence has also been postulated to contribute directly to amplified motivation to drink. For example, animal studies have indicated that elevation of corticosteroid hormones may enhance propensity to drink through an interaction with the mesocorticolimbic reward circuitry (Fahlke et al. 1994b; Fahlke et al. 1994a; Piazza & Le 1997).
Taken together, there is a substantial body of evidence suggestig that phenomena associated with alcohol dependence, particularly the withdrawal experience, are stressful events that activate brain/neuroendocrine stress pathways. This may directly and/or through mediating withdrawal-related anxiety and stress/dysphoria responses influence motivation to engage in alcohol self-administration behavior. While it is generally acknowledged that stressful life events play a prominent role in influencing alcohol drinking and, in particular, triggering relapse ((Brady & Sonne 1999; Sillaber & Henniger 2004; Sinha 2001; Sinha & Li 2007; Weiss 2005), the circumstances and manner in which stress influences drinking behavior is complex and not well understood. Future research efforts are needed to further elucidate how dependence/withdrawal modulates stress responsiveness and how such an interaction affects the course of alcohol addiction.
4.2.7 Anhedonia/Dysphoria
Another common feature of the alcohol abstinence syndrome relates to anhedonia, i.e., a reduced ability to derive pleasure from events/stimuli typically perceived as rewarding. Although anecdotal reports have long noted the significance of this aspect of the abstinence syndrome, recent clinical studies have used a number of assessment instruments to better quantify the subjective anhedonic/dysphoric state that endures throughout the protracted abstinence phase (Janiri et al. 2005; Martinotti et al. 2008; Pozzi et al. 2008). Animal studies examining this topic are quite limited. In one study, withdrawal-related anhedonia was demonstrated using an intracranial self-stimulation (ICSS) model. During withdrawal from chronic alcohol exposure, rats evidenced significant increases in ICSS threshold (i.e., the minimal amount of electrical stimulation delivered to reward pathways in the brain that is perceived as rewarding) (Schulteis et al. 1995). To our knowledge, similar studies have not been conducted in mouse models of alcohol dependence and withdrawal. It has been suggested that a dopamine hypofunctional state may underlie the anhedonia/dysphoria associated with protracted withdrawal (Bailey et al. 2001; Diana et al. 1996; Heinz et al. 1995; Weiss et al. 1996), although other neuroadaptations may contribute [e.g., (Shippenberg et al. 2007)]. It is relatively difficult to study this aspect of withdrawal in animals and development of other models would be beneficial. For example, conditioning models (e.g., conditioned place learning, suppression of acoustic startle by conditioned reinforcers) may be useful to study the aversive/dysphoric aspect of alcohol withdrawal. Again, to the extent that individuals may resume drinking in an attempt to self-medicate the anhedonia/dysphoria associated with abstinence, further investigation into this may be valuable for elucidating the underlying mechanisms and time course, as well as for evaluating therapeutic agents that may be used for relapse prevention.
4.2.8 Dependence/Withdrawal vs Relapse and Excessive Alcohol Intake
The phenotypes most directly relevant for linking withdrawal processes with maintenance of alcoholic drinking are excessive voluntary alcohol intake/self-administration, and propensity for relapse. Over several decades, numerous rodent and primate models have been employed to study the relationship between alcohol dependence, experience with withdrawal, and subsequent self-administration behavior (Cappell & LeBlanc 1981; Grant 1995). Early studies generally yielded equivocal findings (Begleiter 1975; Deutsch & Koopmans 1973; Hunter et al. 1974; Myers et al. 1972; Numan 1981; Samson & Falk 1974; Schulteis et al. 1996; Winger 1988), but this was most likely due to procedures that did not sufficiently establish the reinforcing effects of alcohol prior to dependence induction. In these situations, subjects had minimal opportunities to associate alcohol drinking with its withdrawal-alleviating consequences (Meisch 1983; Meisch & Stewart 1994). Incorporating these procedural considerations, more recent studies in mice (Becker & Lopez 2004; Chu et al. 2007; Dhaher et al. 2008; Finn et al. 2007; Lopez & Becker 2005) and rats (O’Dell et al. 2004; Rimondini et al. 2002; Roberts et al. 2000; Sommer et al. 2008; Valdez et al. 2002) have demonstrated increased alcohol responding and/or drinking in dependent compared to non-dependent subjects. In some studies, the enhanced alcohol consumption during withdrawal in dependent animals was shown to produce blood and brain alcohol levels that nearly reached the levels attained during the forced chronic alcohol exposure that produced the dependent state (Griffin et al. 2009; Roberts et al. 2000). Also, just as demonstrated in clinical studies, animals with a history of alcohol dependence exhibit exaggerated sensitivity to the effect of alcohol-related cues and stressors to enhance alcohol-seeking behavior (Gehlert et al. 2007; Liu & Weiss 2002; Sommer et al. 2008). These effects have been observed up to several months after termination of the alcohol exposure (Lopez & Becker 2005; Valdez et al. 2002; Sommer et al. 2008), supporting the notion that during protracted abstinence there is a latent (subclinical) enhanced vulnerability to relapse that perpetuates the addiction. Recent findings suggest that an additional component of this vulnerability may be a long-lasting tolerance to alcohol following a history of dependence (Rimondini et al. 2008).
A convergent body of preclinical and clinical evidence has demonstrated that a history of multiple detoxification (withdrawal) experiences can result in “kindling” or sensitization of the alcohol withdrawal syndrome (Ballenger & Post 1978; Becker 1998; Becker & Littleton 1996; Breese et al. 2005a; Heilig & Koob 2007). As reviewed above, animal studies have shown that experience with repeated cycles of chronic alcohol exposure and withdrawal leads to not only an exacerbation of physical symptoms of withdrawal (e.g., seizures), but also enhanced severity of symptoms that constitute psychological components of the withdrawal syndrome. These models have suggested a link between induction of dependence with repeated withdrawal experiences, and elevated alcohol consumption or self-administration as a consequence, both in rats (Roberts et al. 2000; Rimondini et al. 2002; Rimondini et al. 2003; O’Dell et al. 2004; Sommer et al. 2008) and in mice (Becker & Lopez 2004; Dhaher et al. 2008; Finn et al. 2007; Lopez & Becker 2005; Chu et al. 2007; Lopez et al. 2008). Further support for such a link is provided by findings that increased self-administration or consumption in this type of model is selectively sensitive to CRH1 antagonists (Funk et al. 2007; Funk et al. 2006), just as is the increased behavioral responses to stress in the post-dependent state. Interestingly, the alpha-2 adrenoceptor antagonist yohimbine, which potently induced anxiety responses in alcoholics (Krystal et al. 1996), also reinstates alcohol seeking and induces excessive alcohol self-administration in a CRH1 dependent manner (Marinelli et al. 2007).
5. Conclusions
A growing literature indicates that a history of physical dependence on alcohol that involves repeated cycles of withdrawal is accompanied by increased motivation to self-administer alcohol and propensity to relapse. Robust measures of alcohol withdrawal in humans and experimental animals have long been available, but have mainly focused on physical withdrawal phenomena, and the early stages of the withdrawal process. It remains unknown whether these commonly studied withdrawal phenomena reflect pathophysiological processes that give rise to the motivational aspects of the post-dependent state, or whether they are merely correlative. Human studies are to some extent further along in defining this relationship, through the demonstration that elevated stress-reactivity during early withdrawal from both cocaine and alcohol predicts relapse (Sinha 2008; Sinha et al. 2006). Our paper discusses several withdrawal-related phenotypes that could be studied in animals, and would potentially be more closely related to motivational adaptations relevant for maintaining alcoholism. These types of models would allow studies to determine whether shared or distinct pathophysiology underlies physical and motivational consequences of repeated alcohol withdrawal.
Acknowledgments
The authors wish to acknowledge support by the NIAAA intramural research program (MH), NIH grants AA10760, AA13519, and a grant from the US Department of Veterans Affairs (JC), and NIH grants AA010761, AA014095 and VA Medical Research (HB).
Footnotes
disclosures
Author HB has received consulting fees from Eli Lilly & Co.
Reference List
- Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, Moss HB, Eskay R, Linnoila M, Gold PW. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry. 1990;47:325–330. doi: 10.1001/archpsyc.1990.01810160025004. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Risher-Flowers D, De JJ, Ravitz B, Bone GH, Nutt DJ, Roehrich L, Martin PR, Linnoila M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiatry. 1991;148:1023–1025. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- Ahlenius S, Carlsson A, Engel J, Svensson T, Sodersten P. Antagonism by alpha methyltyrosine of the ethanol-induced stimulation and euphoria in man. Clinical Pharmacology & Therapeutics. 1973;14:586–591. doi: 10.1002/cpt1973144part1586. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, American Psychiatric Association and Task Force on DSM-IV. Diagnostic and Statistical Manual of Mental Disorders DSM-IV. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Bailey CP, O’Callaghan MJ, Croft AP, Manley SJ, Little HJ. Alterations in mesolimbic dopamine function during the abstinence period following chronic ethanol consumption. Neuropharmacology. 2001;41:989–999. doi: 10.1016/s0028-3908(01)00146-0. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton TK. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR. Fixed-Interval Schedule of Cocaine Reinforcement -Effect of Dose and Infusion Duration. J Exp Anal Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar KJ, Boettger MK, Neubauer R, Groteluschen M, Jochum T, Baier V, Sauer H, Voss A. Heart rate variability and sympathetic skin response in male patients suffering from acute alcohol withdrawal syndrome. Alcohol Clin Exp Res. 2006;30:1592–1598. doi: 10.1111/j.1530-0277.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- Barrenha GD, Chester JA. Genetic correlation between innate alcohol preference and fear-potentiated startle in selected mouse lines. Alcoholism: Clinical & Experimental Research. 2007;31(7):1081–8. doi: 10.1111/j.1530-0277.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- Becker HC. Kindling in alcohol withdrawal. Alcohol Health Res World. 1998;22:25–33. [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Alcohol Withdrawal: Neuroadaptation and Sensitization. CNS Spectrums. 1999;4:38–65. [Google Scholar]
- Becker HC. Animal models of alcohol withdrawal. Alcohol Research & Health: the Journal of the National Institute on Alcohol Abuse & Alcoholism. 2000;24(2):105–13. [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Alcohol Dependence, Withdrawal and Relapse. Alcohol Res Health. 2008 in press. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Littleton JM. The Alcohol Withdrawal “Kindling” Phenomenon: Clinical and Experimental Findings. Alcoholism: Clinical and Experimental Research. 1996;20:121–124. doi: 10.1111/j.1530-0277.1996.tb01760.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Becker HC, Myrick H, Veatch LM. Pregabalin is effective against behavioral and electrographic seizures during alcohol withdrawal. Alcohol Alcohol. 2006;41:399–406. doi: 10.1093/alcalc/agl029. [DOI] [PubMed] [Google Scholar]
- Becker HC, Veatch LM. Effects of lorazepam treatment for multiple ethanol withdrawals in mice. Alcohol Clin Exp Res. 2002;26:371–380. [PubMed] [Google Scholar]
- Begleiter H. Ethanol consumption subsequent to physical dependence. Adv Exp Med Biol. 1975;59:373–378. doi: 10.1007/978-1-4757-0632-1_26. [DOI] [PubMed] [Google Scholar]
- Bokström K, Balldin J, Långström G. Individual mood profiles in alcohol withdrawal. Alcoholism Clinical & Experimental Research. 1991;15:508–513. doi: 10.1111/j.1530-0277.1991.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne SC. The role of stress in alcohol use, alcoholism treatment, and relapse. Alcohol Res Health. 1999;23:263–271. [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a “ kindling”/stress hypothesis. Psychopharmacology (Berl) 2005a;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005b;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Myra KH, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32:1429–1438. doi: 10.1111/j.1530-0277.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE. Tolerance and physical dependence: Do they play a role in alcohol and drug self-administration. Addiction Research Foundation; Toronto: 1981. pp. 159–196. [Google Scholar]
- Charles PD, Esper GJ, Davis TL, Maciunas RJ, Robertson D. Classification of tremor and update on treatment. Am Fam Physician. 1999;59:1565–1572. [PubMed] [Google Scholar]
- Chester JA, Barrenha GD. Acoustic startle at baseline and during acute alcohol withdrawal in replicate mouse lines selectively bred for high or low alcohol preference. Alcohol Clin Exp Res. 2007;31:1633–1644. doi: 10.1111/j.1530-0277.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Effects of chronic alcohol treatment on acoustic startle reactivity during withdrawal and subsequent alcohol intake in high and low alcohol drinking rats. Alcohol Alcohol. 2005;40:379–387. doi: 10.1093/alcalc/agh172. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF(1) receptor antagonist antalarmin and by CRF(1) receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Hyytia P. The genetic of alcoholism: learning from 50 years of research. Addict Biol. 2006;11:193–194. doi: 10.1111/j.1369-1600.2006.00028.x. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- Clark CP, Gillin JC, Golshan S, Demodena A, Smith TL, Danowski S, Irwin M, Schuckit M. Increased REM sleep density at admission predicts relapse by three months in primary alcoholics with a lifetime diagnosis of secondary depression. Biol Psychiatry. 1998;43:601–607. doi: 10.1016/s0006-3223(97)00457-5. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Carai MA, Gessa GL. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and -non-preferring (sNP) rats. Addict Biol. 2006;11:324–338. doi: 10.1111/j.1369-1600.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- Costa A, Bono G, Martignoni E, Merlo P, Sances G, Nappi G. An assessment of hypothalamo-pituitary-adrenal axis functioning in non-depressed, early abstinent alcoholics. Psychoneuroendocrinology. 1996;21:263–275. doi: 10.1016/0306-4530(96)00001-7. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Antagonism of ethanol withdrawal convulsions in Withdrawal Seizure Prone mice by diazepam and abecarnil. Eur J Pharmacol. 1992;221:85–90. doi: 10.1016/0014-2999(92)90775-y. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crawshaw LI, O’Connor CS, Crabbe JC, Hayteas DL. Temperature regulation in mice during withdrawal from ethanol dependence. Am J Physiol. 1994;267:R929–R934. doi: 10.1152/ajpregu.1994.267.4.R929. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Radcliffe R, Erwin VG. The Pharmacology of Alcohol and Alcohol Dependence. Oxford University Press, USA; New York: 1996. Pharmacological effects in the development of physiological tolerance and physical dependence; pp. 431–176. [Google Scholar]
- Deneau G, Yanagita T, Seevers MH. Self-Administration of Psychoactive Substances by Monkey - A Measure of Psychological Dependence. Psychopharmacologia. 1969;16:30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- Deutsch JA, Koopmans HS. Preference enhancement for alcohol by passive exposure. Science. 1973;179:1242–1243. doi: 10.1126/science.179.4079.1242. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Finn D, Snelling C, Hitzemann R. Lesions of the extended amygdala in C57BL/6J mice do not block the intermittent ethanol vapor-induced increase in ethanol consumption. Alcohol Clin Exp Res. 2008;32:197–208. doi: 10.1111/j.1530-0277.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: Differential roles in associative learning and drug addiction. Behav Pharmacol. 2002;13:481–482. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Acquas E, Tanda G. Ethanol as a neurochemical surrogate of conventional reinforcers: the dopamine-opioid link. Alcohol. 1996;13:13–17. doi: 10.1016/0741-8329(95)02034-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations int he mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Pistis M, Muntoni A, Gessa G. Mesolimbic dopaminergic reduction outlasts ethanol withdrawal syndrome: evidence of protracted abstinence. Neuroscience. 1996;71:411–415. doi: 10.1016/0306-4522(95)00482-3. [DOI] [PubMed] [Google Scholar]
- Dole VP. Thoughts on narcotics addiction. Bulletin of the New York Academy of Medicine. 1965;41:211–3. [PMC free article] [PubMed] [Google Scholar]
- Dole VP, Nyswander ME, Kreek MJ. Narcotic blockade. Arch Intern Med. 1966;118:304–309. [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36:249–255. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, Demodena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22:1796–1802. [PubMed] [Google Scholar]
- Edwards G, Gross MM. Alcohol dependence: provisional description of a clinical syndrome. Br Med J. 1976;1:1058–1061. doi: 10.1136/bmj.1.6017.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Engel JA, Eriksson CJ, Hard E, Soderpalm B. Involvement of corticosterone in the modulation of ethanol consumption in the rat. Alcohol. 1994a Jun;11(3):195–202. doi: 10.1016/0741-8329(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Thomasson R, Engel JA, Hansen S. Metyrapone-induced suppression of corticosterone synthesis reduces ethanol consumption in high-preferring rats. Pharmacology, Biochemistry & Behavior. 1994b;48(4):977–81. doi: 10.1016/0091-3057(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Feige B, Scaal S, Hornyak M, Gann H, Riemann D. Sleep electroencephalographic spectral power after withdrawal from alcohol in alcohol-dependent patients. Alcohol Clin Exp Res. 2007;31:19–27. doi: 10.1111/j.1530-0277.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Crabbe JC. Exploring alcohol withdrawal syndrome. Alcohol Health & Research World. 1997;21(2):149–56. [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcoholism: Clinical & Experimental Research. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Friedman HJ. Assessment of physical dependence on and withdrawal from ethanol in animals. In: Rigter H, Crabbe JC, editors. Alcohol Tolerance and Dependence. Elsevier/North-Holland Biomedical Press; Amsterdam: 1980. pp. 93–121. [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Differential sensitivity of ethanol withdrawal signs in the rat to gamma-aminobutyric acid (GABA)mimetics: blockade of audiogenic seizures but not forelimb tremors. J Pharmacol Exp Ther. 1983;226:720–725. [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin DV, Youngblood BD, Holloway FA. The Discriminative Stimulus Properties of Acute Ethanol Withdrawal (Hangover) in Rats. Alcoholism-Clinical and Experimental Research. 1992;16:336–341. doi: 10.1111/j.1530-0277.1992.tb01387.x. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl- imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 Receptor Antagonism as a Possible Therapy for Alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Hommer DW. Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addict Biol. 2008;13:423–434. doi: 10.1111/j.1369-1600.2008.00111.x. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav. 2008;90:475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol Dependence Produced in Mice by Inhalation of Ethanol - Grading Withdrawal Reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Grant KA. Animal models of the alcohol addiction process. In: Kranzler HR, editor. The Pharmacology of Alcohol Abuse. Springer-Verlag; Berlin: 1995. pp. 185–229. [Google Scholar]
- Griffin WC, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 2009;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Sinha R, O’Malley SS. Effects of ethanol on the acoustic startle reflex in humans. Psychopharmacology (Berl) 1994;114:167–171. doi: 10.1007/BF02245459. [DOI] [PubMed] [Google Scholar]
- Hascoet M, Bourin M, Dhonnchadha BAN. The mouse light-dark paradigm: A review. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2001;25:141–166. doi: 10.1016/s0278-5846(00)00151-2. [DOI] [PubMed] [Google Scholar]
- Hawley RJ, Nemeroff CB, Bissette G, Guidotti A, Rawlings R, Linnoila M. Neurochemical correlates of sympathetic activation during severe alcohol withdrawal. Alcohol Clin Exp Res. 1994;18:1312–1316. doi: 10.1111/j.1530-0277.1994.tb01429.x. [DOI] [PubMed] [Google Scholar]
- Headlee CP, Coppock HW, Nichols JR. Apparatus and Technique Involved in A Laboratory Method of Detecting the Addictiveness of Drugs. J Am Pharm Assoc (Wash) 1955;44:229–231. doi: 10.1002/jps.3030440415. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17:80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Heinz A, Lichtenberg-Kraag B, Baum SS, Graf K, Kruger F, Dettling M, Rommelspacher H. Evidence for prolonged recovery of dopaminergic transmission after detoxification in alcoholics with poor treatment outcome. J Neural Transm Gen Sect. 1995;102:149–157. doi: 10.1007/BF01276510. [DOI] [PubMed] [Google Scholar]
- Heinz A, Lober S, Georgi A, Wrase J, Hermann D, Rey ER, Wellek S, Mann K. Reward craving and withdrawal relief craving: assessment of different motivational pathways to alcohol intake. Alcohol Alcohol. 2003;38:35–39. doi: 10.1093/alcalc/agg005. [DOI] [PubMed] [Google Scholar]
- Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grusser SM, Kienast T, Smolka MN, Flor H, Mann K. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol Clin Exp Res. 2007;31:1138–1147. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- Henniger MS, Spanagel R, Wigger A, Landgraf R, Holter SM. Alcohol self-administration in two rat lines selectively bred for extremes in anxiety-related behavior. Neuropsychopharmacology. 2002;26:729–736. doi: 10.1016/S0893-133X(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Holter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav Pharmacol. 1998;9:41–48. [PubMed] [Google Scholar]
- Holter SM, Linthorst AC, Reul JM, Spanagel R. Withdrawal symptoms in a long-term model of voluntary alcohol drinking in Wistar rats. Pharmacology, Biochemistry & Behavior. 2000;66:143–151. doi: 10.1016/s0091-3057(00)00196-9. [DOI] [PubMed] [Google Scholar]
- Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. Journal of Clinical Psychology. 1971;27(4):455–6. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hunter BE, Walker DW, Riley JN. Dissociation between physical dependence and volitional ethanol consumption: role of multiple withdrawal episodes. Pharmacol Biochem Behav. 1974;2:523–529. doi: 10.1016/0091-3057(74)90013-6. [DOI] [PubMed] [Google Scholar]
- Janiri L, Martinotti G, Dario T, Reina D, Paparello F, Pozzi G, Addolorato G, Di GM, De RS. Anhedonia and substance-related symptoms in detoxified substance-dependent subjects: a correlation study. Neuropsychobiology. 2005;52:37–44. doi: 10.1159/000086176. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: Changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcoholism-Clinical and Experimental Research. 2000;24:278–284. [PubMed] [Google Scholar]
- Kiianmaa K, Andersson K, Fuxe K. On the role of ascending dopamine systems in the control of voluntary ethanol intake and ethanol intoxication. Pharmacol Biochem Behav. 1979;10:603–608. doi: 10.1016/0091-3057(79)90240-5. [DOI] [PubMed] [Google Scholar]
- King AC, Parsons OA, Bernardy NC, Lovallo WR. Drinking history is related to persistent blood pressure dysregulation in postwithdrawal alcoholics. Alcohol Clin Exp Res. 1994;18:1172–1176. doi: 10.1111/j.1530-0277.1994.tb00100.x. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Koller W, O’Hara R, Dorus W, Bauer J. Tremor in chronic alcoholism. Neurology. 1985;35:1660–1662. doi: 10.1212/wnl.35.11.1660. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF. New dimensions in human laboratory models of addiction. Addiction Biology. 2009;14:1–8. doi: 10.1111/j.1369-1600.2008.00127.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the Brain Antireward System. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Vaccarino F, Amalric M, Bloom FE. Positive reinforcement properties of drugs: Search for neural substrates. In: Engel J, Oreland L, editors. Brain Reward Systems and Abuse. Raven Press; New York: 1987. pp. 35–50. [Google Scholar]
- Kosobud A, Crabbe JC. Ethanol Withdrawal in Mice Bred to be Genetically Prone Or Resistant to Ethanol Withdrawal Seizures. J Pharmacol Exp Ther. 1986;238:170–177. [PubMed] [Google Scholar]
- Krystal JH, Webb E, Cooney NL, Kranzler HR, Southwick SW, Heninger GR, Charney DS. Serotonergic and noradrenergic dysregulation in alcoholism: m-chlorophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatry. 1996;153:83–92. doi: 10.1176/ajp.153.1.83. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan CA, Southwick SM, Davis M, Charney DS. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorophenylpiperazine (mCPP) Psychopharmacology (Berl) 1997;131:207–215. doi: 10.1007/s002130050285. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Gillin JC. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiology and management. CNS Drugs. 2001;15:413–425. doi: 10.2165/00023210-200115050-00006. [DOI] [PubMed] [Google Scholar]
- Le Bon O, Murphy JR, Staner L, Hoffmann G, Kormoss N, Kentos M, Dupont P, Lion K, Pelc I, Verbanck P. Double-blind, placebo-controlled study of the efficacy of trazodone in alcohol post-withdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol. 2003;23:377–383. doi: 10.1097/01.jcp.0000085411.08426.d3. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure increase both self-administration and the reinforcing value of ethanol in C57BL/6J mice. Alcoholism-Clinical and Experimental Research. 2008;32:163A. doi: 10.1111/j.1530-0277.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–658. [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav. 2002;73:147–158. doi: 10.1016/s0091-3057(02)00791-8. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Myrick LH, Veatch LM, Boyle E, Randall PK. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3:24–32. [PubMed] [Google Scholar]
- Malcolm RJ. GABA systems, benzodiazepines, and substance dependence. J Clin Psychiatry. 2003;64:36–40. [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Nicola MD, Reina D, Andreoli S, Foca F, Cunniff A, Tonioni F, Bria P, Janiri L. Alcohol protracted withdrawal syndrome: the role of anhedonia. Subst Use Misuse. 2008;43:271–284. doi: 10.1080/10826080701202429. [DOI] [PubMed] [Google Scholar]
- Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278:144–151. doi: 10.1001/jama.278.2.144. [DOI] [PubMed] [Google Scholar]
- Meert TF, Rassnick S, Huysmans H, Peeters J, Clincke GH. Quantification of tremor sensitivity and inhibition of exploratory behaviour during alcohol withdrawal in rats. Behav Pharmacol. 1992;3:601–607. [PubMed] [Google Scholar]
- Meisch RA. Relationship between physical dependence on ethanol and reinforcing properties of ethanol in animals. NIAAA Research Monographs. 1983;13:27–32. [Google Scholar]
- Meisch RA, Stewart RB. Ethanol as a reinforcer: a review of laboratory studies of non-human primates. Behav Pharmacol. 1994;5:425–440. [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Dependence and withdrawal. In: Deitrich RA, Erwin EG, editors. Pharmacological Effects of Ethanol on the Nervous System. CRC Press; New York: 1996. pp. 269–290. [Google Scholar]
- Milner LC, Crabbe JC. Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain and Behavior. 2008;7:496–505. doi: 10.1111/j.1601-183X.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Miranda R, Jr, Meyerson LA, Buchanan TW, Lovallo WR. Altered emotion-modulated startle in young adults with a family history of alcoholism. Alcoholism: Clinical & Experimental Research. 2002;26(4):441–8. [PubMed] [Google Scholar]
- Miranda R, Jr, Meyerson LA, Myers RR, Lovallo WR. Altered affective modulation of the startle reflex in alcoholics with antisocial personality disorder. Alcoholism: Clinical & Experimental Research. 2003;27(12):1901–11. doi: 10.1097/01.ALC.0000099263.71214.F9. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Myers RD, Stoltman WP, Martin GE. Effects of ethanol dependence induced artificially in the rhesus monkey on the subsequent preference for ethyl alcohol. Physiol Behav. 1972;9:43–48. doi: 10.1016/0031-9384(72)90262-4. [DOI] [PubMed] [Google Scholar]
- Numan R. Multiple exposures to ethanol facilitate intravenous self-administration of ethanol by rats. Pharmacol Biochem Behav. 1981;15:101–108. doi: 10.1016/0091-3057(81)90346-4. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Testa T, O’Brien TJ, Brady JP, Wells B. Conditioned narcotic withdrawal in humans. Science. 1977;195:1000–1002. doi: 10.1126/science.841320. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibin SD, Cameron AJ, Metten P, Crabbe JC. Motor impairment: a new ethanol withdrawal phenotype in mice. Behav Pharmacol. 2008;19:604–614. doi: 10.1097/FBP.0b013e32830ded27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le MM. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Research - Brain Research Reviews. 1997;25(3):359–72. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Pich EM, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. Journal of Pharmacology & Experimental Therapeutics. 1968;161(1):122–9. [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC. Genetic association between chronic ethanol withdrawal severity and acoustic startle parameters WSP and WSR mice. Alcohol Clin Exp Res. 1999;23:1730–1735. [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Effects of alcohol on electrophysiological activity of the brain. In: Begleiter H, Kissin B, editors. The Pharmacology of Alcohol and Alcohol Dependence. Oxford University Press; USA: New York: 1996. pp. 207–247. [Google Scholar]
- Pozzi G, Martinotti G, Reina D, Dario T, Frustaci A, Janiri L, Bria P. The assessment of post-detoxification anhedonia: influence of clinical and psychosocial variables. Subst Use Misuse. 2008;43:722–732. doi: 10.1080/00952990701202954. [DOI] [PubMed] [Google Scholar]
- Prediger RDS, da Silva GE, Batista LC, Bittencourt AL, Takahashi RN. Activation of adenosine A(1) receptors reduces anxiety-like behavior during acute ethanol withdrawal (hangover) in mice. Neuropsychopharmacology. 2006;31:2210–2220. doi: 10.1038/sj.npp.1301001. [DOI] [PubMed] [Google Scholar]
- Quintanilla ME, Israel Y, Sapag A, Tampier L. The UChA and UChB rat lines: metabolic and genetic differences influencing ethanol intake. Addict Biol. 2006:11. doi: 10.1111/j.1369-1600.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Wilkinson CW, Raskind MA. Chronic daily ethanol and withdrawal: 6. Effects on rat sympathoadrenal activity during “abstinence”. Alcohol. 2006;38:173–177. doi: 10.1016/j.alcohol.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993a;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Koob GF, Geyer MA. Responding to acoustic startle during chronic ethanol intoxication and withdrawal. Psychopharmacology (Berl) 1992;106:351–358. doi: 10.1007/BF02245417. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self- administration of ethanol in the rat. Brain Res. 1993b;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol. 2003;64:445–449. doi: 10.15288/jsa.2003.64.445. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer WH, Dall’olio R, Heilig M. Long-lasting tolerance to alcohol following a history of dependence. Addict Biol. 2008;13:26–30. doi: 10.1111/j.1369-1600.2007.00079.x. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Thorsell A, Heilig M. Suppression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BIIE0246: evidence for sensitization in rats with a history of dependence. Neurosci Lett. 2005;375:129–133. doi: 10.1016/j.neulet.2004.10.084. [DOI] [PubMed] [Google Scholar]
- Rivier C. Effects of alcohol on the neuroendocrine system. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio: NIAAA Research Monograph No. 34. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000. pp. 61–81. [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Roelofs SM. Hyperventilation, anxiety, craving for alcohol: a subacute alcohol withdrawal syndrome. Alcohol. 1985;2:501–505. doi: 10.1016/0741-8329(85)90123-5. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behavioral & Neural Biology. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Weitemier AZ. The urocortin 1 neurocircuit: Ethanol-sensitivity and potential involvement in alcohol consumption. Brain Research Reviews. 2006;52:368–380. doi: 10.1016/j.brainresrev.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Samson HH, Falk JL. Alteration of fluid preference in ethanol-dependent animals. J Pharmacol Exp Ther. 1974;190:365–376. [PubMed] [Google Scholar]
- Schneider M, Spanagel R. Appetitive odor-cue conditioning attenuates the acoustic startle response in rats. Behav Brain Res. 2008;189:226–230. doi: 10.1016/j.bbr.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Hesselbrock V. Alcohol dependence and anxiety disorders: what is the relationship? Am J Psychiatry. 1994;151:1723–1734. doi: 10.1176/ajp.151.12.1723. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Hyytia P, Heinrichs SC, Koob GF. Effects of chronic ethanol exposure on oral self-administration of ethanol or saccharin by Wistar rats. Alcohol Clin Exp Res. 1996;20:164–171. doi: 10.1111/j.1530-0277.1996.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigvardsson S, Bohman M, Cloninger CR. Replication of the Stockholm adoption study of alcoholism - confirmatory cross-fostering analysis. Arch Gen Psychiatry. 1996;53:681–687. doi: 10.1001/archpsyc.1996.01830080033007. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Henniger MS. Stress and alcohol drinking. Ann Med. 2004;36:596–605. doi: 10.1080/07853890410018862. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic alcohol-related neuroadaptation in stress responses: Effects on compulsive alcohol seeking and relapse outcomes. Alcoholism-Clinical and Experimental Research. 2008;32:341A. [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction Biology. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug & Alcohol Review. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Somes C, Ehlers CL. Effects of prolonged ethanol exposure on neurophysiological measures during an associative learning paradigm. Drug Alcohol Depend. 2000 doi: 10.1016/s0376-8716(99)00072-1. [DOI] [PubMed] [Google Scholar]
- Sommer W, Hyytia P, Kiianmaa K. The alcohol-preferring AA and alcohol-avoiding ANA rats: neurobiology of the regulation of alcohol drinking. Addict Biol. 2006;11:289–309. doi: 10.1111/j.1369-1600.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig M. Upregulation of Voluntary Alcohol Intake, Behavioral Sensitivity to Stress, and Amygdala Crhr1 Expression Following a History of Dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F, Landgraf R. Anxiety - a potential predictor of vulnerability to the initiation of ethanol self administration in rats. Psychopharmacology (Berl) 1995;122:369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Behavior maintained by termination of a schedule of self-administered cocaine. Science. 1979;204:1231–1233. doi: 10.1126/science.109920. [DOI] [PubMed] [Google Scholar]
- Spragg SDS. Morphine addiction in chimpanzees. Comparative Psychology Monographs. 1940;15:1–132. [Google Scholar]
- Staner L, Boeijinga P, Danel T, Gendre I, Muzet M, Landron F, Luthringer R. Effects of acamprosate on sleep during alcohol withdrawal: A double-blind placebo-controlled polysomnographic study in alcohol-dependent subjects. Alcohol Clin Exp Res. 2006;30:1492–1499. doi: 10.1111/j.1530-0277.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Schroeder JP, Nixon K, Besheer J, Crews FT, Hodge CW. Abstinence Following Alcohol Drinking Produces Depression-Like Behavior and Reduced Hippocampal Neurogenesis in Mice. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.90. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tanda GL, Di Chiara G. A dopamine mu(1) opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and non-psychostimulant drugs of abuse. Eur J Neurosci. 1998;10:1179–1187. doi: 10.1046/j.1460-9568.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- Thompson T, Schuster CR. Morphine Self-Administration, Food-Reinforced, and Avoidance Behaviors in Rhesus-Monkeys. Psychopharmacologia. 1964;5:87–94. doi: 10.1007/BF00413045. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Vandergriff J, Kallman MJ, Rasmussen K. Moxonidine, a selective imidazoline-1 receptor agonist, suppresses the effects of ethanol withdrawal on the acoustic startle response in rats. Biol Psychiatry. 2000;47:874–879. doi: 10.1016/s0006-3223(00)00229-8. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcoholism-Clinical and Experimental Research. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Veatch LM. Disruptions in sleep time and sleep architecture in a mouse model of repeated ethanol withdrawal. Alcohol Clin Exp Res. 2006;30:1214–1222. doi: 10.1111/j.1530-0277.2006.00134.x. [DOI] [PubMed] [Google Scholar]
- Veatch LM, Becker HC. Lorazepam and MK-801 effects on behavioral and electrographic indices of alcohol withdrawal sensitization. Brain Res. 2005;1065:92–106. doi: 10.1016/j.brainres.2005.10.047. [DOI] [PubMed] [Google Scholar]
- Victor M, Adams RD. Effects of alcohol on the nervous system. In: Merritt HH, Hare CC, editors. Metabolic and Toxic Diseases of the Nervous System. Williams and Wilkins; Baltimore: 1953. pp. 526–573. [Google Scholar]
- Victor M. The alcohol withdrawal syndrome: theory and practice. Postgrad Med. 1970;47:68–72. doi: 10.1080/00325481.1970.11697437. [DOI] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Lumeng L, Li TK. Induction of Dependence on Ethanol by Free-Choice Drinking in Alcohol-Preferring Rats. Pharmacology Biochemistry and Behavior. 1982;16:501–507. doi: 10.1016/0091-3057(82)90459-2. [DOI] [PubMed] [Google Scholar]
- Wand G. Hypothalamic-pituitary-adrenal axis: Changes and risk for alcoholism. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio: NIAAA Research Monograph No. 34. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000. pp. 397–415. [Google Scholar]
- Watson WP, Robinson E, Little HJ. The novel anticonvulsant, gabapentin, protects against both convulsant and anxiogenic aspects of the ethanol withdrawal syndrome. Neuropharmacology. 1997;36:1369–1375. doi: 10.1016/s0028-3908(97)00118-4. [DOI] [PubMed] [Google Scholar]
- Weeks JR. Experimental Morphine Addiction - Method for Automatic Intravenous Injections in Unrestrained Rats. Science. 1962;138:143. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. Recent progress in research on the neurophysiological basis of morphine addiction. Am J Psychiatry. 1948;105:329–338. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]
- Willinger U, Lenzinger E, Hornik K, Fischer G, Schonbeck G, Aschauer HN, Meszaros K. Anxiety as a predictor of relapse in detoxified alcohol-dependent patients. Alcohol Alcohol. 2002;37:609–612. doi: 10.1093/alcalc/37.6.609. [DOI] [PubMed] [Google Scholar]
- Winger G. Effects of ethanol withdrawal on ethanol-reinforced responding in rhesus monkeys. Drug & Alcohol Dependence. 1988;22(3):235–40. doi: 10.1016/0376-8716(88)90023-3. [DOI] [PubMed] [Google Scholar]
- Wise RA. Maximization of ethanol intake in the rat. Advances in Experimental Medicine & Biology. 1975;59:279–294. doi: 10.1007/978-1-4757-0632-1_19. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatric Medicine. 1985;3(4):445–60. [PubMed] [Google Scholar]