Abstract
Coronary arterial disease, one of the leading causes of adult mortality, is triggered by atherosclerosis. A disease with complex etiology, atherosclerosis results from the progressive long-term combination of atherogenesis, the accumulation of modified lipoproteins within blood vessel walls, along with vascular and systemic inflammatory processes. The management of atherosclerosis is challenged by the localized flare-up of several multipronged signaling interactions between activated monocytes, atherogenic macrophages and inflamed or dysfunctional endothelial cells. A new generation of approaches is now emerging founded on multifocal, targeted therapies that seek to reverse or ameliorate the athero-inflammatory cascade within the vascular intima. This article reviews the various classes and primary examples of bioactive configurations of nanoscale assemblies. Of specific interest are polymer-based or polymer-lipid micellar assemblies designed as multimodal receptor-targeted blockers or drug carriers whose activity can be tuned by variations in polymer hydrophobicity, charge, and architecture. Also reviewed are emerging reports on multifunctional nanoassemblies and nanoparticles for improved circulation and enhanced targeting to athero-inflammatory lesions and atherosclerotic plaques.
Atherosclerosis: Scope and Challenges
Cardiovascular disease (CVD) is the leading cause of death in the developed world. An estimated 81 million people in the United States (more than one in three) have one or more types of CVDs. CVD also causes nearly 50% of all deaths in westernized countries including over 1 million American adults a year, and is the leading cause of mortality among diabetics, with overall yearly costs exceeding US $360 billion (AHA, 2010). Atherosclerosis, the inflammatory vascular wall disease serves as a major trigger for coronary artery disease, a critical component of the pathologies underlying CVD. Atherosclerosis is characterized by the build-up of lipid-rich plaques within the blood vessel walls of large arteries, and underlies the clinical conditions of myocardial infarction, chronic stable angina, stroke and peripheral vascular disease.1 Moreover, this chronic condition does not just afflict seniors, rather, atherosclerosis is evident as early as the second and third decades in life, indicating that beyond lifestyle modification, drug therapy targeted at individuals with sub-clinical disease could have revolutionary impact.2 The American Heart Association (AHA) aims at a 20% reduction in deaths caused by CVDs through the encouragement of sensible life style changes for the prevention of the disease as well as applying novel technologies for diagnosis and treatment.3
The complexity of treating atherosclerosis relates to the multi-step combination of atherogenesis (accumulation of oxidized low density lipoprotein or LDL within the blood vessel wall) and an ensuing inflammatory cascade, leading to later stages of plaque development and thrombosis that are difficult to reverse. Since atherosclerosis evolves over several years and is comprised of several complex stages, the disease can often go undetected until later stages. As a result, the treatment or management of the disease, especially at early stages, proves difficult. Despite the complexity of this disease, it offers several targetable biomarkers that can be exploited for directing therapeutic, diagnostic, or hybrid carriers to the lesion sites. A brief summary of the molecular and cellular events underlying atherosclerosis is discussed next.
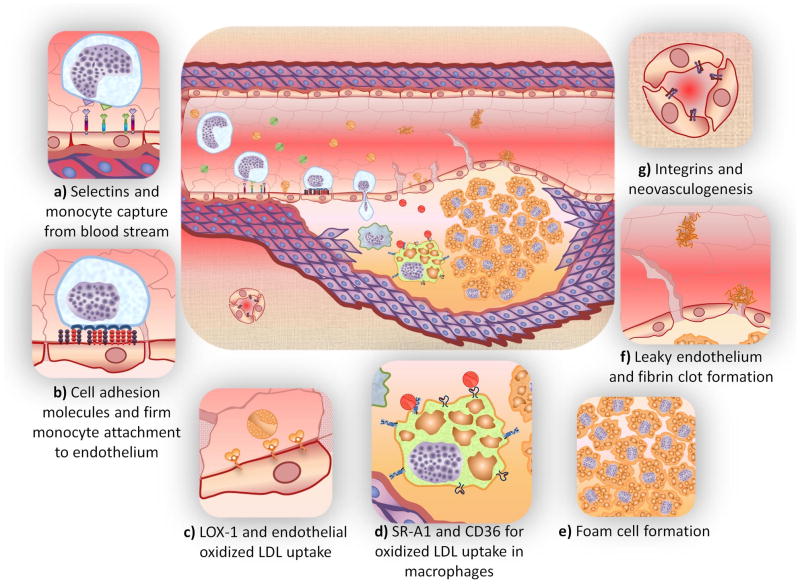
As shown in Figure 1, hyperlipidemia (excessive circulating levels of low density lipoproteins, LDL) leads to the sequestration of LDL within the arterial wall and subsequent LDL oxidation by matrix glycosoaminoglycans. Oxidized LDL (oxLDL) causes chronic injury to the endothelial cell layer, which in turn triggers an inflammatory response defined by upregulated cytokine and adhesion molecules that promote monocyte recruitment. Following recruitment monocytes are transported through the endothelial membrane and differentiate into macrophages, which in turn mediate unregulated uptake of oxLDL via scavenger receptors (SR) leading to the formation of lipid-filled foam cells. Foam cells further express inflammatory cytokines continuing the cycle of inflammation and lipoprotein modification. Following the accumulation of lipid laden macrophages, smooth muscle cells migrate into the lipid layer. Significant buildup leads to a necrotic lipid core surrounded by a fibrous cap. Degradation of the cap and subsequent rupture can lead to myocardial infarction or stroke. The inflammatory component of the disease is mediated by macrophages, primarily through scavenger receptor interactions with oxLDL. How the disease progresses and manifests symptoms (i.e., heart attack or stable angina) are governed by the critical relationship between atherogenesis and inflammation throughout the lifetime of the disease.4
Figure 1. Key cellular and molecular interactions that trigger the onset of atherosclerosis.
Hyperlipidemia and intimal retention of LDL promote inflammation of endothelial cells lining the blood vessels. Endothelial cell adhesion molecules [selectins (a) and IgG-type (b)] enhance the recruitment of circulating monocytes, which make the endothelium more permeable, in turn, facilitating LDL transport to the extravascular space. Endothelial cells continually internalize oxLDL [mediated by receptors such as LOX-1 (c)] while monocytes differentiate into macrophages that internalize oxLDL [mediated by SR-1 and CD36 receptors, (d)] and become lipid laden macrophages called “foam cells” (e). The build-up of oxidized lipids triggers the secretion of a range of cytokines and engenders a more inflammatory phenotype within all vascular cells. Compromised endothelia expose lipid bodies to thrombosis, forming fibrin clots (f). Further, to fulfill the increased metabolic demand of the cells in growing plaques, new blood vessels start to form in the media and extend into the intima (g). As the lesion progresses, endothelium becomes dysfunctional, smooth muscle cells start to migrate, making the lesion a dynamic mass protruding inside the lumen of the vessel, reducing blood flow to vital organs downstream.
Therapeutic approach
Conventional pharmacologic approaches
Even though treatments for clinical manifestations of atherosclerosis are available, they tend to be plagued by several inherent drawbacks. Many pharmaceutical candidates exhibit off target effects and have low efficacy at tolerated doses, which results in theoretically cardioprotective drugs falling short in a clinical setting. A characteristic example is that PPARγ agonists, thought to have anti-inflammatory and anti-atherogenic effects, have been shown to cause weight gain, edema, fluid retention and increased risk of cardiac failure. 5, 6 Edema is primarily caused by PPARγ off-target action in the kidney nephrons causing increases in sodium and water reabsorption.7
Out of all cholesterol lowering drugs, only statins have demonstrated a reduction of mortality from coronary heart disease. However, statins are unsuccessful at rescuing from acute ischemic events and preventing cellular uptake of oxidatively modified LDL.8, 9 Anti-cytokine antibodies and cytokine secretion inhibitors can reduce inflammatory responses that progress atherogenesis, but trials have not shown efficacy in reducing clinical endpoints. Additionally, the underlying cause of athero-inflammation is not addressed by these antibodies and inhibitors.10 Anti-chemokine biologics interfere with macrophage recruitment and inflammation initiation. However, the complex interplay between chemokines and receptors has limited development of such therapeutics.11 Lipid oxidation is a primary reason for inflammation, but antioxidants have failed clinical trials due to the difficulty in reversing decades of oxidative damage. 9, 12 Finally, anti-platelet therapies prevent clot formation and aids clot breakup, but only addresses the latter stages of cardiovascular disease when clots are more likely to form. 13–15 The poor efficacy of the above atherosclerosis therapies demonstrates the need for the development of alternative strategies and platforms. Nanoassemblies offer a promising option for developing novel approaches to manage the disease and have potential to transform current atherosclerotic therapies.
How can nanotechnology help?
Nanomaterials, specifically molecular assemblies and organic or inorganic nanoparticles, are emerging as attractive candidates for therapeutic applications.16 Nanosized carriers, in the range of 10 to 200 nm, are suitable for cellular level therapies as are small enough to interact with receptor targets with high specificity and avidity, but are large enough to transport small molecule drugs and protect them from metabolic deactivation, avoid renal clearance, and provide high surface areas that can be decorated with targeting ligands.17 In addition, these nanoassemblies can be functionalized to allow tracking their distribution and thus serve as diagnostic agents. Nanotechnology and the design of synthetic nanoassemblies have the opportunity to advance atherosclerosis therapies by 1) increasing systemic circulation time of the carrier, 2) lowering drug cytotoxicity, 3) enhancing drug solubility, 4) reducing the required dosage, 5) combining imaging and therapeutic agents for inspection of disease progression, and 6) increasing specific tissue accumulation through active or passive targeting. Further development of nanoassemblies may also lead to the identification of novel molecular targets in the disease and elucidation of their biological role.
Molecular assemblies or nanoparticles are typically metal-, polymer- or lipid-based or a combination thereof. Several formulation methods have been employed to design such nanoassemblies. These include ligand exchange with inorganic nanoparticles with polymers or lipids, in situ reduction of metal salts in the presence of a steric stabilizer (i.e., ligands or polymer),16, 18, 19 surface modification through non- or covalent linkages,16, 20, 21 mini-emulsions,17, 22 nanoprecipitation into a non-solvent,23–25 as well as self-assembly of amphiphilic small molecules, polymers or lipids. Although, several nanoscale structures have been explored for therapeutic and diagnostic delivery, this review will primarily address the use of nanoscale, micellar assemblies for managing or diagnosing atherosclerosis. Micelles are composed of amphiphilic molecules that self-assemble due to the energy minimization associated with hydrophobic sequestration. Typical micellar nanoassemblies that are pertinent to atherosclerosis therapies or diagnoses are depicted in Figure 2. The modular nature of micelles allows individual amphiphilic molecules (unimers) with distinct functionalities to be assembled to address several barriers that currently plague conventional delivery methods. The formulation of multifunctional micelles with targeting, imaging and therapeutic features is made possible by the wide range of available compositions, which in turn allows tailoring of micelle properties and/or parameters. Furthermore, the core of micelles can be exploited to solubilize hydrophobic drugs. Inorganic nanoparticle cores, necessary for in vivo imaging, can also be engineered by either reacting or adsorbing unimers to the particle surface. The range of molecular compositions and facile modifications to the unimer structure has made molecular assembly of polymer therapeutics a versatile platform for elucidating, benchmarking, and developing new classes of therapies and diagnostics for atherosclerosis.
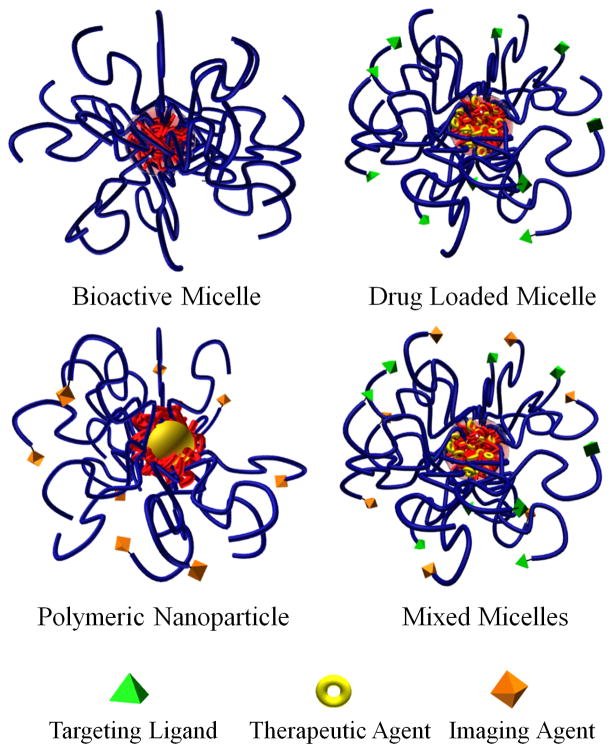
Figure 2.
Nanoassemblies for the management or diagnosis of atherosclerosis can be classified into four broad categories: (a) Bioactive micelle with inherent therapeutic capabilities; (b) Drug loaded micelle with targeting ligands for cell-specific delivery; (c) Polymer modified nanoparticle (i.e. gold, super paramagnetic iron oxide, quantum dots, etc.) for imaging applications; (d) Mixed micelles with both therapeutic and diagnostic capabilities. Blue - hydrophilic polymer and red - hydrophobic polymer or lipid.
The design of micellar systems is often motivated by the need to modify the pharmacokinetics and pharmacodynamics of established drugs. Optimal performance can be achieved if the therapeutic agent can be directed to a specific site requiring therapy, thus reducing dose frequency. Site specific delivery can be accomplished through either passive or active targeting. The in vivo performance of micellar systems is controlled by numerous factors including pathophysiological and physiochemical interactions that depend on the size distribution, shape, density, deformability, and surface properties.26 These properties also influence how the nanoassembly may flows throughout the circulatory system in addition to determining the interactions with serum proteins and cells. 27 Unfortunately, due to the complex, dynamic nature of both the nanoassembly, pharmacokinetic profiles typically cannot be represented by straightforward models.
The circulation time and tissue distribution are determined by biological effects such as phagocytotic/endocytotic recognition and ingestion, the immune responsiveness, and vascular escape routes.28 As micelles circulate, plasma proteins associate with the micellar surface supporting interactions with macrophage receptors (opsonization). These binding proteins include immunoglobulins, components of the complement system, fibronectin, C-reactive protein (CRP) and the von Willebrand factor. The micelle characteristics (i.e., conjugated elements, size, steric stability), concentration and circulation time collectively determine the degree of protein-micelle interactions.29 This interaction is a primary reason for micelle removal from circulation and has clear implications on the pharmacokinetics of the system, as rapid protein adsorption to the nanoassembly surfaces will typically led to faster clearance by mononuclear phagocyte system (MPS). For example, macrophages concentrated in the liver sinuses recognize protein coated materials, rapidly clearing these if not adequately shielded. This clearance diminishes therapeutic efficacy as the particle concentration is lowered. Furthermore, protein adsorption supports aggregation and these newly formed agglomerates can become trapped in capillary beds.
Micelles exhibit slower clearance rates since they tend to not agglomerate and resist protein binding due to their flexibility and hydrophilic corona. However, the high sensitivity of the complement system can result in different clearance rates for similar formulations. Micelle size, net charge and charge distribution presented at the surface play a role in the rate of opsonization. Anionic particles are usually cleared via the classical complement pathway, whereas cationically charged particles activate the alternative complement system and subsequently cleared. In contrast, zwitterionic or neutral particles display longer circulation time and are most likely cleared via CRP binding. Although it is simple in theory to design particles that will avoid rapid MPS clearance, developing a system that is able to specifically deliver a therapy is more complex.
Most delivery vehicles have been designed to focus on ways to prevent associations with blood and plasma proteins. Opsonization effects can be controlled through surface modifications, such as providing the particle with a hydrophilic corona. This coronal layer imparts steric stabilization to the micelle and thus inhibits protein adsorption by interfering with the binding of macrophage complement receptors. Masking a particle’s cargo requires a simple conjugation, but the amount and length of polymer chain attached can significantly alter the in vivo efficacy.30 Studies utilizing a library of various particle shapes and sizes have provided guidelines for designing delivery systems that can either enhance or avoid macrophage binding.31 Delivery of gene or RNAi therapy typically necessitates using cationic polymers to bind the anionic nucleic acids, which will attract more protein binding. 30
Characteristics and Design Criteria for Polymeric Micelles
Although several types of supramolecular assemblies have been utilized for the treatment and detection of various pathologies,32–34 including atherosclerosis,35–41 perhaps the most promising nanoassemblies are those based on synthetic polymers. Due to the wide variety and various properties of (co)polymers, as well as the diversity and flexibility of available polymer chemistries,42–51 multimodal nanomaterials with requisites for treating, detecting, and/or targeting cardiovascular diseases can be potentially realized. Of particular interest, is the functionalization of polymers with hydrophilic-hydrophobic motifs that promote self-assembly when introduced into an aqueous environment. The facile nature of incorporating reactive moieties to either the polymer chain ends or side chains is especially advantageous for realizing biorelevant conjugates for targeted delivery therapies or diagnostics. Polymeric architectures suited to drug or gene delivery include functional homo- or copolymers, di- or triblock, graft and star copolymers, as well as “dendrimers”. Another current area of high interest, not only for atherosclerosis but other CVDs, is the use of polymer modified inorganic nanoparticles for developing novel diagnostic or imaging technologies. For more details the reader is directed to recent reviews by Broz et al.35 and Fayad and coworkers. 36, 37
Since the onset and progression of atherosclerosis are consistent with the cellular uptake of natural and/or modified (i.e., oxidized) LDL,52, 53 polymeric biomaterials for cardiovascular therapies have been designed to emulate these naturally occurring lipophilic assemblies or inhibit cellular uptake of modified LDL.35, 38, 40, 41, 54–60 Amphiphilic polymers having the appropriate hydrophilic-hydrophobic balance can self-assemble just as small molecule amphiphiles above the critical micelle concentration (CMC) or critical aggregation concentration (CAC).61–65 The most common polymeric assembled structures reported are micelles, but other assemblies, including worm-like micelles and vesicles, are possible through variation of the hydrophobic to hydrophilic mass balance.62, 63, 66 Amphiphilic polymers can be prepared through the incorporation of both hydrophobic and hydrophilic monomers in either a random or di-/triblock architecture. The latter is more widespread, whereas the former has been used to form unimolecular micelles under dilute solution.67 The hydrophilic versus hydrophobic block lengths (i.e. number of repeat units) or weight fractions can determine the nature of supramolecular structure formed; therefore, careful consideration should be given to this structural feature prior to polymer design. For example, tuning the length or incorporating branching of hydrophilic or hydrophobic functionalities not only dictates the assembled structure (e.g., micelle, vesicle, cylindrical micelles), but also the hydrodynamic size and steric stability of the micelle. A reduction in hydrophilic moieties may lead to unwanted aggregation and eventual precipitation, while too many may yield thermodynamically unstable assemblies due to amplified hydrophilicity, thus causing dissolution. In addition to block length, architecture and composition, other polymer characteristics to consider, in regards to developing an atherosclerosis therapy, include net ionic charge, charge type, charge and reactive functionality placement, stereochemistry, and available chemistries, all the while maintaining biocompatibility.
If carefully designed, polymeric micelles or other polymer based biomaterials, used for therapeutic or diagnostic delivery, have several inherent strengths. These include 1) facile encapsulation of aqueous insoluble therapeutics and contrast agents in the hydrophobic core, 2) retarded dissolution or enhanced thermodynamic stability in comparison to small molecular amphiphiles due to depressed CMC values (<10−5 M versus ~ 10−3 M)61, 64, 68, 69 3) coronal steric stabilization that prevents inter-micellar bridging and unwanted associations with blood opsonins, thus circumventing premature circulatory clearance by the MPS,45, 70, 71 and 4) the formation of nanosized assemblies (10–200 nm) that evade renal clearance and increase circulation half-life. In addition to these inherent strengths, the inclusion of amphiphilic polymers decorated with targeting ligands can be employed to direct the micellar assembly, along with its cargo, to a diseased site. Furthermore, in principle, the release rate of the encapsulated pharmaceutics can be further controlled by integrating reactive sites along the polymer backbone that are cross-linked following micellar assembly (i.e. shell cross-linked micelles).50, 72–74
Given the numerous reports and variants of (co)polymers utilized for micellar assemblies, only the essential polymeric features and notable structures are mentioned. For more detailed reports, the reader is referred to several reviews that discuss not only the polymeric structure required for micellar assemblies, but also recent advances made in stimuli-responsive block copolymers and shell cross-linked micelles with therapeutic delivery applications in mind.50, 61, 64, 67, 68, 70, 74, 75 A wide range of hydrophilic polymers are suitable when designing amphiphilic macromolecules, but the most commonly employed are poly(ethylene glycol) (PEG) or poly(ethylene oxide) (PEO) and poly(N-(2-hydroxypropyl)methacrylamide) (PHPMA). It should be noted that PEG and PEO have identical repeat structures and are named according to polymerization method, condensation and ring-opening, respectively. The popularity of these water-soluble polymers is undoubtedly a result of the commercial availability of monofunctional or homo- and heterobifunctional derivatives, cytocompatibility, regulatory approval, facile conjugation to biorelevant molecules, poor protein adsorptivity, and widespread knowledge of solution behavior in vitro as well as in vivo.
An important component of unimer design for micellar assembly is the chain extension of hydrophilic polymers with hydrophobic monomers or coupling to other hydrophobic motifs. Commonly studied hydrophobic macromolecules exploited for synthesizing block copolymers include, but are not limited to, poly(styrene), poly(meth)acrylates, poly(lactic acid), poly(butadiene), poly(propylene oxide) and poly(caprolactone).65 Amphiphilicty can also be established through the direct coupling of hydrophobes to hydrophilic polymers. For example, the laboratories of Moghe and Uhrich et al. 38, 40, 41, 59, 60, 76 have synthesized several derivatives of PEG-hydrophobe constructs through the alkylation of mucic acid followed by PEGylation. The resulting material was able to micellize and inhibit uptake of oxLDL, which is known to exacerbate atherogenesis. Structural features for this system are displayed and discussed in the Therapeutic Applications of Polymeric Micelles section below. Micellization for hydrophilic-block-hydrophobic copolymers or other amphiphiles can be induced by either first dissolving the macromolecule in an organic solvent (i.e. THF, DMF, DMSO, etc.), conducive for both the hydrophilic and hydrophobic moieties, followed by dialysis against water or direct dissolution of the amphiphile into water, which promotes self-assembly.
In addition to the use of block copolymers there are several literature reports that employ micellar assemblies from polymer-lipid mimics to detect or alleviate the progression of atherosclerosis.35–37, 54, 56–58 Generally, hydrophilic homopolymers, most notably PEG, are covalently attached to naturally occurring phospholipids, such as phosphatidylethanolamine (PE), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) and phosphatidylcholines, or other synthetically derived lipid-mimic compounds. Just as hydrophilic-block-hydrophobic copolymers, polymer-lipid mimics can micellize above a CMC, solubilize hydrophobic motifs, and be decorated with ligands for targeted therapeutic/diagnostic delivery applications (Figure 3). For example, Fayad and coworkers,54, 77, 78 in addition to other laboratories,58 have mixed PEGylated-DSPE in conjunction with other lipid-targeting ligand and/or lipid-diagnostic/therapeutic agent conjugates to construct multimodal micelles for imaging and treating atherosclerosis. Additional highlights of these research works will be elaborated upon in subsequent sections. An alternate strategy to those discussed above would be the use of hydrophilic-block-stimuli responsive copolymers where one block changes hydrophilicity based on a specific stimulus (i.e. acid/base, salt or temperature).50, 74, 79, 80 This method provides self-assembly and reversibility in an aqueous environment allowing the transition between unimers to micelles simply by introducing and removing an external stimulus. This approach offers opportunities to design novel micellar configurations for site selective efficacy in atherosclerotic lesions.
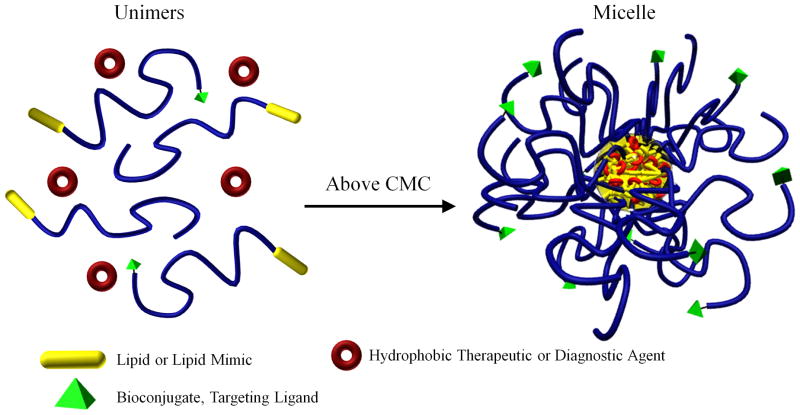
Figure 3.
Unimer to micelle transition above the critical micelle concentration (CMC) in the presence of a therapeutic or diagnostic agent. Blue represents hydrophilic polymer.
Targeting the disease
The management of atherosclerosis utilizing small molecule pharmaceutics and conventional delivery methods is challenged by several biological barriers. Efficient targeting strategies are critically required since nonspecific nanosystems can be readily cleared by the body’s inherent filters (i.e., liver, lymph nodes, and kidneys) or invoke adverse side effects systemically. Site-specific delivery through the conjugation of ligands provides routes to bypass problems associated with traditional therapeutic approaches. Knowledge of atherosclerotic markers and attachment of complementary ligands to the nanocarrier system can guide and concentrate the therapeutic agent at the site of action. Alternatively, inflammation and angiogenesis within diseased arteries enable the use of passive targeting via the enhanced permeability and retention (EPR) effect 81. A review of various nanocarrier compositions and targeting approaches for atherosclerosis is tabulated below (Table 1).
Table 1.
Current targeted nanoassemblies for the treatment and/or diagnosis of atherosclerosis.
| Configuration | Composition | Targeting moiety | Therapeutic/Diagnostic modality | Reference |
|---|---|---|---|---|
| Nanoparticle | CLIO – crosslinked iron oxide | VCAM-1 targeting cyclic peptide CVHSPNKKC | CLIO (crosslinked iron oxide) – MRI image contrast | 96 |
| Nanoparticle | CLIO – crosslinked iron oxide | VCAM-1 targeting linear peptide VHPKQHR | CLIO – MRI image contrast | 97 |
| Micelle | PEGylated lipids | Fibrin monoclonal antibody | Gd-DTPA amphiphile – MRI signal enhancement | 104 |
| Micelle | PEGylated lipids | Peptidomimetic αvβ3 integrin antagonist | Gd-DTPA amphiphile – MRI signal enhancement | 136 |
| Micelle | PEGylated lipids | Peptidomimetic αvβ3 integrin antagonist | Fumagillin – antiangiogenic drug Gd-DTPA amphiphile – MRI signal enhancement |
103 |
| Micelle | PEGylated lipids | Tyrosine – for targeting lipid-rich areas of plaques | Gd-DTPA amphiphile – MRI signal enhancement | 78 |
| Micelle | PEGylated lipids | Clot binding peptide CREKA | Hirulog – anticoagulant drug | 58 |
| Polymer vesicle | PMOXA-PDMS-PMOXA | Polyguanylic acid (PolyG) – for targeting SR-A1 | Pravastatin – HMG-CoA reductase inhibitor | 55 |
| Nanoparticle | Graft polymer with PNTBA main chain and PEG side chains | Evans blue analog recognizing endothelium dysfunction | Doxorubicin | 122 |
| Micelle | PEGylated lipids | SR-A1 antibody | Gd-DTPA amphiphile – MRI signal enhancement | 54 |
| Micelle | PEGylated lipids | SR-A1 antibody | Gd-DTPA amphiphile – MRI signal enhancement | 56 |
| Micelle | PEGylated lipids | Annexin A5 – for targeting PS exposed on the membrane of apoptotic cells | Gd-DTPA amphiphile – MRI signal enhancement | 57 |
| Nanoparticle | MION – monocrytalline iron oxide inside a dextran shell | Dextran – for macrophage targeting (specificly dextran receptor SIGNR1) | MION (monocrytalline iron oxide nanoparticle) – MRI image contrast TPC – photosensitizer for photodynamic therapy | 115 |
| Nanoparticle | MION – monocrytalline iron oxide inside a dextran shell | Dextran – for macrophage targeting (specifically dextran receptor SIGNR1) | MION (monocrytalline iron oxide nanoparticle) – MRI image contrast Cu64-DTPA – radiotracing in PET-CT imaging | 137 |
| Micelle | PEGylated lipids | Antibodies for oxidation specific epitopes | Gd-DTPA amphiphile – MRI signal enhancement | 77 |
| Nanoparticle | PLA-Paclitaxel conjugate inside a lipid-PEGylated lipid combination corona | Collagen-IV targeting peptide KLWVLPK | Paclitaxel conjugated to PLA core – inhibition of vascular smooth muscle proliferation following percutaneous angioplasty | 105 |
Passive targeting
Propagating lesions in atherosclerosis leads to neovasculogenesis similar to that seen in cancerous tumor growth. High metabolic activity of the building plaque requires elevated nutrition and oxygen supply to the underlying cells. To fulfill this nutritional need, endothelial cells rapidly proliferate and form atypical blood vessels that are defective and immature. This state changes the dynamics of macromolecular transport to and from the lesion and is known as the EPR effect. Compromised or “leaky” vasculature allows macromolecules or nanoassemblies to pass into the interstitial tissue, while an undeveloped lymphatic drainage system promotes accumulation, and hence a higher local therapeutic concentration.36, 82 In addition to neovascularization, tissue inflammation causes incessant leukocyte recruitment through the release of proinflammatory cytokines. This condition also increases endothelial permeability and allows for selective delivery of therapeutic carriers in the inflamed area 83. Balloon angioplasty of arteries can injure the endothelium and permeabilize it to systemically delivered nanoassemblies via EPR.84
Active targeting
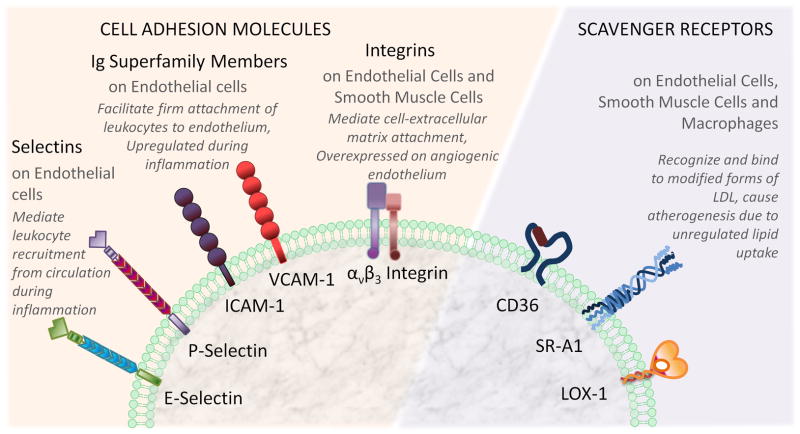
Polymeric systems that are deficient in intrinsic bioactivity need to be modified with ligands to confer selectivity and specificity for athero-inflammatory lesions. The vascular endothelium serves as a natural candidate for targeting as it is strategically located between the circulating blood and the growing lesion, in addition to its multiple roles in pathogenesis 85. At the early stages of the disease, the endothelium alters its cell surface protein expression from basal to proinflammatory, which in turn initiates the recruitment of inflammatory cells from the blood stream to the intima. Ligands that recognize these proinflammatory cell surface proteins may thus serve as appropriate markers for guiding novel diagnostic and therapeutic systems. For example, cell adhesion molecules (CAMs) are of special interest for targeted delivery because of their important roles in leukocyte recruitment and internalizing receptor bound ligands via CAM mediated endocytosis 85, 86. Current cell-surface receptors that play a role in the pathogenicity underlying atherosclerosis and also exemplify suitable interventional targets are depicted in Figure 4.
Figure 4.
Targetable cell surface receptors for diagnostic and theraputic applications of atherosclerosis. See text for details.
The intercellular cell adhesion molecule-1 (ICAM-1) is a member of the CAM immunoglobulin superfamily of glycoproteins. ICAM-1 is normally expressed on the luminal side of the endothelium and significantly (~20–50 fold 87) upregulated following inflammation. Immunohistochemical analysis of human ex-vivo lesions showed strong ICAM-1 expression in vascular cells comprising the atheroma, thus establishing its role in the progression of the disease 88. Antibodies 87, 89–91 and small peptide sequences 92–94 derived from endogenous ICAM-1 ligands have been employed for developing targeting strategies to treat inflammatory diseases, including atherosclerosis.
Vascular cell adhesion molecule (VCAM-1), similar to ICAM-1, is also attractive for targeting delivery applications. VCAM-1 is expressed on endothelial surfaces under pathological conditions but also prior to the onset of visible lesions 95. Nanoparticles conjugated to VCAM-1 targeting peptide sequences (derived from known ligands of VCAM-1 by phage display) were shown to be effective for imaging the initial progression of the disease in ApoE knock-out mice 96, 97. Aside from therapeutic or diagnostic uses, nanosystems decorated with CAM targeted ligands offer an added benefit for atherosclerosis since such ligands can also attenuate the leukocyte-endothelium adhesion and consequently reduce athero-inflammation.
Selectin (E- and P- selectin) receptors are also considered as potential endothelial targets 98, 99 considering their important roles in inflammation. Unfortunately, their transient expression and lower cell surface density, even at maximal activation, limit their use in targeting atherosclerosis. Moreover, therapeutic vehicles targeted to P-Selectin might not be specific to plaque endothelium since this receptor is also expressed in activated platelets 95. However, dual targeting of P-selectin and VCAM-1 with micron sized iron oxide particles has been used as a successful strategy for MR imaging of atherosclerotic plaques in ApoE knock-out mice. In this study, it was shown that dual targeting results in 5 to 7 fold increase in binding of the contrast agent to the lesion compared to singly targeted counterparts.100
Integrins are one of the most important subclasses of cell adhesion molecules for targeted cancer therapy due to their significant upregulation in angiogenic endothelium during tumor growth. As mentioned previously, neovascularization has also been shown to be associated with atherosclerosis.81, 101, 102 In addition to causing the EPR effect, newly formed blood vessels surrounding the plaque also display an increase in αvβ3 integrin expression. αvβ3 Integrin targeted paramagnetic nanoparticles incorporating an angiostatic drug were used as a “theranostic” systems in cholesterol fed rabbits.103 This integrated design demonstrated the potential of nanosystems to not only deliver a therapeutic, but also simultaneously deliver a contrast agent, thus providing a means to visualize the local response of the atherosclerotic lesion over time.
In addition to endothelial cell surface proteins, macrophage specific scavenger receptors 54, 56, oxidation specific epitopes 77, fibrin 58, 104 and extracellular matrix proteins 105 are potential candidates for the development of targeting ligands. For instance, targeting endothelial or macrophage scavenger receptors can block the initiation of proinflammatory signaling since these receptors mediate oxLDL uptake and establish the inflammation cascade. SR-A1 and CD36 scavenger receptors, predominately expressed on macrophages, are both participants of atherogenesis and atheroinflammation.106 LOX-1, an endothelial scavenger receptor, internalizes oxLDL and has been shown to cause endothelial dysfunction causing further disease progression.107 Nanocarriers targeting these receptors have utilized the attachment of complementary ligands, 54, 56 similar to other cell surface receptor targeted carriers, as well as utilizing electrostatic charge based interactions.38, 108, 109 Specific oxidation residues of phosphocholine are recognized by CD36 and can be incorporated in micelles to target nanosystems to lesion macrophages.110 The discovery of new antagonists and ligands 111, 112 for these receptors will facilitate more efficient targeting strategies in the near future.
Decorating nanosystems with ligands to lesion targets while limiting unspecific protein adsorption (i.e. through PEGylation) can effectively increase the local concentration of imaging or therapeutic agents at the lesion site. Constituents used for specific targeting purposes include antibodies, peptides and polysaccharides. Antibodies, despite their high specificity and affinity towards their targets, are limited by large hydrodynamic sizes, and undesired immunogenic responses. Alternatively, antibody fragments and the even more effective small peptide sequences offer advantages over antibodies as long as in vivo stability is sustained.83 For example, with the development of phage display technology, the number of small peptide sequences, that are highly selective towards their complementary receptor, has increased rapidly over recent years.113 This technology offers immense potential to discover and subsequently utilize peptides as targeting ligands in the rational design of therapeutic carriers. Polysaccharide ligands, for therapies aimed at macrophages, are appealing as they are avidly internalized via macrophages.114, 115 Advantages of polysaccharide targeting ligands include their widespread availability and high biocompatibility; however, their specificity needs to be evaluated based on the specific need of carrier system.
Therapeutic Applications of Polymeric Micelles
The development of micelles for therapeutic management of atherosclerosis is a rapidly growing field and several different approaches have been utilized. While some micellar systems possess intrinstic bioactivity others rely on solubilization of pharmacologic cargo for therapeutic activity.
Bioactive Micelles
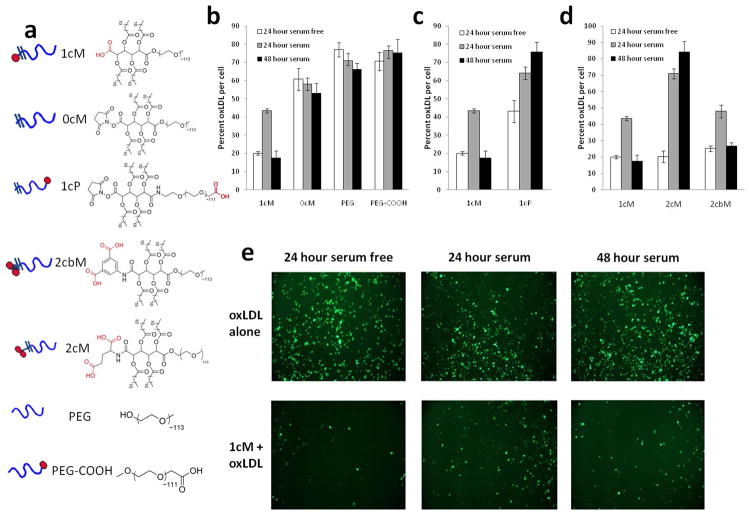
Most work on micelles, in terms of atherosclerosis therapies, have utilized (co)polymers designed to mimic the amphilicity and polyanionic charge distribution seen in oxLDL.116 The aforementioned amphiphilic polymers, first designed for drug delivery applications by Uhrich and coworkers,76 comprised of a lauroyl modified mucic acid and a 5 kDa PEG chain demonstrated self-assembly behavior in water with a CMC near 10−7 M and a hydrodynamic diameter between 15–20 nm. The architecture of these polymers allows for selective charge placement through the addition of carboxylic acids or amine functionalities. Moghe and coworkers found that polymers containing a single carboxylate anion on the hydrophobic terminus, termed 1cM (Figure 5), yielded micelles that could sequester unmodified LDL and mildly oxLDL, but not highly oxLDL, whereas neutral polymers had no affinity towards either unmodified or oxidized forms LDL. 60
Figure 5.
Structure function relationship of the bioactive nanopolymers designed to reduce oxLDL uptake in macrophages. Degree of oxLDL inhibition with serum-free and serum conditions in the presence of polymers show the importance of carrier design criteria for the individual unimers that form micelles. a) Schematic represantations and chemical structures of polymeric unimers b) Effect of amphiphilicity and anionic charge c) Effect of anionic charge location, d) Effect of number and rotational freedom of the anionic charge located in the hydrophobic domain, e) Micrographs showing the internalization of BODIPY labeled oxLDL in the absence or presence of 1cM (Reprinted with the permission of 38 Copyright 2010 Elsevier, Ltd.).
In vitro studies indicated high levels of polymer binding, determined via fluorolabeled polymers, to macrophage scavenger receptors in contrast to endothelial and smooth muscle cells.117 Polymer binding to the scavenger receptors SR-A1 and CD36 was confirmed utilizing an antibody blocking assay. Upon introducing antibodies complementary to SR-A1 or CD36, the binding affinity of the anionic polymer, 1cM, was reduced. This suggests that the oxLDL binding domain of scavenger receptors have some degree of specificity towards 1cM. Above the CMC, the polymers displayed a dose dependent effect in reducing oxLDL uptake, with the greatest decrease coming from 1cM. Controls using only the hydrophobic or hydrophilic portion of the polymer functionalized with a carboxylate had no effect. 40
In a subsequent study, polymers with differing charge number, placement, and/or rotational flexibility as well as various PEG lengths or architectures (i.e., linear or branched) were synthesized in order to determine the influence that polymer structure has on oxLDL uptake in macrophages.38, 108 Inhibition of oxLDL uptake in monocyte derived macrophages for several of the reported polymeric structures are shown in Figure 5. The highest level of uptake inhibition was seen with 1cM, a polymer containing a single carboxylate anion with restricted rotational mobility. The degree of PEG branching and/or additional anionic charges did not appear to have a significant effect on oxLDL uptake.
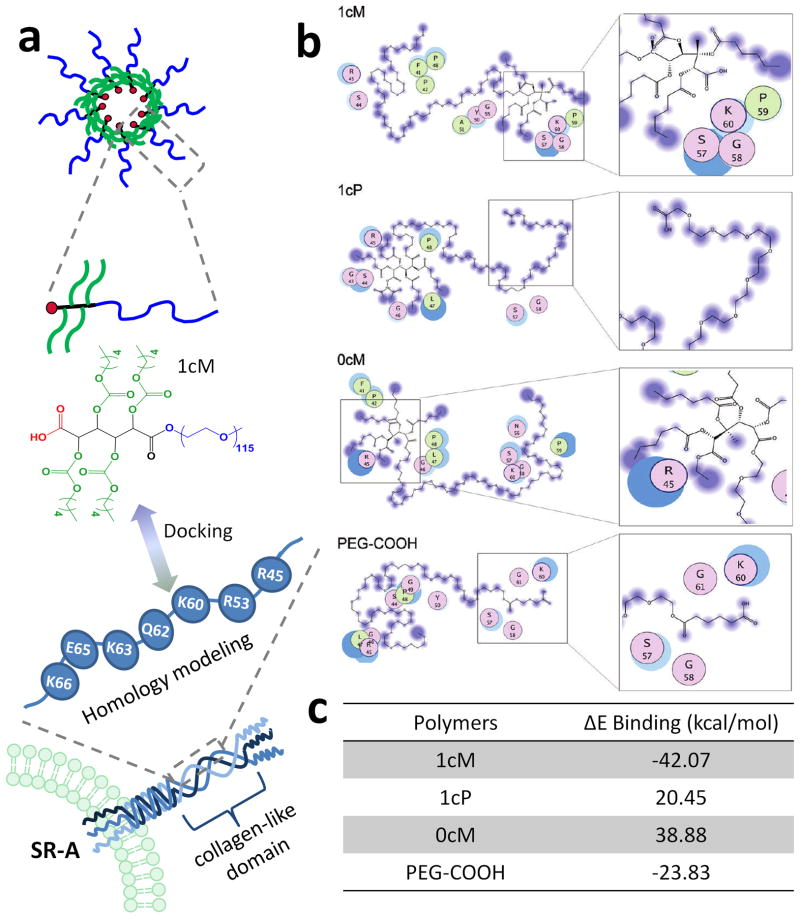
The importance of minor structural changes for the above studied polymers was previously demonstrated through computer modeling by Moghe and coworkers.109 The use of molecular dynamics docking was utilized to determine the key polymeric features required for the most favored interactions between the polymer and the oxLDL binding domain of SR-A1. Molecular simulations on polymers with various structural manipulations correlated well with the experimental findings discussed in the previous paragraph. The simulations revealed that the polymer-lipid mimic 1cM had the most favorable binding energy to the modeled SR-A1 collagen-like domain. Simulation results are shown in Figure 6. In addition, it was also found that the presence of cationic residues in the SR-A1 binding pocket (specifically Lys60, Lys63 and Lys66) were critical for polymer-receptor binding efficacy.
Figure 6.
a) Idealized representation of the structures and interactions between the amphiphilic polymer and SR-A1 receptor that were utilized in molecular modeling simulations. b) Schematic representation of the docked interactions of SR-A1 collagen-like domain homology model residues (as seen in the colored circles) with 1cM, 1cP, 0cM, and PEG-COOH. Residue characteristics are illustrated through color: purple: polar, green: hydrophobic, blue border: basic and red border: acidic. c) Binding energy values calculated from polymer models docked to SR-A1 collagen like domain homology model (Reprinted with the permission of 41 Copyright 2010 American Chemical Society).
Another therapeutic approach exploited the cholesterol receiving tendency of phosphatidylcholine micelles 118. Reverse cholesterol transport by the ABCA1 pathway to HDL provides a clear mechanism for reducing the intracellular cholesterol content of macrophages and thus reducing the rate of atherogenesis. However, using lipid depleted serum for this function could be highly immunogenic. To avoid this problem, Nikitini et al. created mixed micelles of polyunsaturated phosphatidylcholine and a plant-derived glycoside. This lowered the accumulation of cholesterol in cells incubated with atherogenic serum and caused a further decrease in intracellular cholesterol levels relative to control studies.
One of the earliest examples of therapeutic micelles used varying structures of the polymer surfactant polyoxyethylene alkyl phenol formaldehyde to mitigate atherosclerosis in rabbits 119. Atherosclerosis was initiated in rabbits by a diet supplemented with cholesterol and trans-isomerized olive oil 84 days. 20 and 30 t-octyl phenol formaldehyde were able to reduce the severity of aortic lesions relative to control animals.
Micelles for Drug and Gene Delivery
Hydrophobic drugs, proteins and nucleic acids are excellent candidates for micelle encapsulation, conjugation, or electrostatic complexation. Receptor and extracellular matrix targeted polymeric carriers offer the added advantage of delivering an optimal concentration of therapeutics in contrast to other delivery methods. The poor aqueous solubility of numerous drugs often necessitates high dosing, which in turn results in toxicity and off target effects. On the other hand, while free proteins or nucleic acids are aqueous soluble they are readily inactivated by metabolic degradation or cleared from the circulatory system through opsonization. Micelle encapsulation can negate many of these adverse outcomes by solubilizing, protecting and specifically delivering biologically susceptible therapeutics. The loading efficiency can vary broadly depending on the properties of the polymer, micellar assembly, and the packaged therapeutic agent.
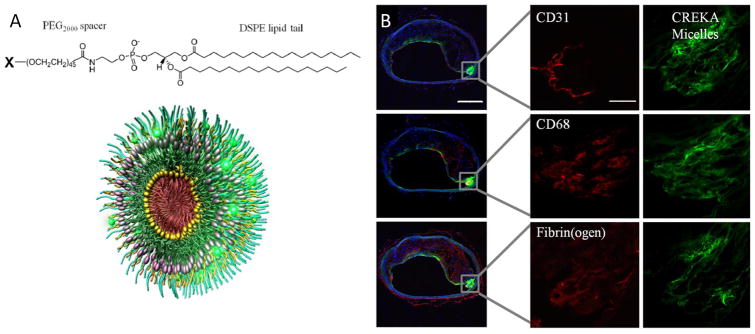
To avoid issues with low entrapment efficiency and allow incorporation of hydrophilic drugs, polymer-drug conjugates utilize covalent linkers can be synthesized prior to micellization. Ruoslahti et al. 58 created a micellar system with polymeric unimers comprised of DSPE-PEG2000 and variable head groups that contained a blood clot binding peptide (CREKA), a fluorophore or the anticoagulant synthetic peptide, hirulog. Hirulog was able to directly inhibit the clotting protein thrombin, even after binding fibrin. The micelles showed strong localization, as indicated through fluorescence techniques, to the shoulders of plaques and were able to exert antithrombin activity, see Figure 7.
Figure 7.
A) Chemical structure of the unimers with variable X- group (targeting peptide, fluorophore or drug molecule) and 3D representation of mixed micelles combining targeting, tracking and therapeutic modalities. B) Localization of fibrin targeting peptide conjugated micelles in atherosclerotic plaques. Serial cross sections stained with endothelial (CD31), macrophage (CD68) and fibrin(ogen) specific antibodies illustrate show that micelles bind to entire surface of the plaque and penetrate under endothelium in the shoulder region. (Reprinted with the permission of 58 Copyright 2009 National Academy of Sciences, USA).
Drug eluting stents have been used to combat restenosis following angioplasty by incorporating anti-proliferatives that retard smooth muscle cell growth. However, local overdose toxicity can cause damage to the tissue surrounding the stent, while systemically administered drugs suffer from a low percentage of the initial dose reaching the site of action. 120 Several micellar systems have been developed to address these issues. Farokhzad et al. have designed multilayered polymer-lipid nanoparticles with paclitaxel conjugated to the PLA core via hydrolysable ester bonds. (105) The drug-polymer core was surrounded by a PEGylated-lipid/lipid monolayer with targeting peptides specific to collagen IV, a key basement membrane matrix protein within blood vessel walls. In vivo studies with balloon injured arteries showed that the targeting peptide enabled spatial distribution of the nanoparticles on the basement membrane exposed after the percutaneous angioplasty injury. It was also seen that the hydrolysis and slow elution of paclitaxel from the core inhibited the vascular smooth muscle cell proliferation commonly seen after this procedure. As another approach to mitigate the dosage problems following stenting, Joner et al. developed a polymer liposome targeted to chondroitin sulfate proteoglycans that encapsulated glucocorticoid prednisolone. This nanoassembly was administered to atherosclerotic rabbits following stent injury. The drug preferentially localized at the injured arteries and was capable of reducing the degree of stenosis relative to control studies, demonstrating the utility of targeted delivery systems.121 Ikuta et al. developed a polymer micelle with encapsulated Evans blue dye and doxorubicin that increased drug delivery to injured porcine aortas. 122 Rather than attaching a specific targeting ligand, some micelle systems have natural affinity to lesion sites, possibly due to increased endothelial permeability after injury. Uwatoku et al. injected untargeed micelle forming doxorubicin-polyaspartic acid-PEG conjugates after single and double balloon injury in rats. 84 Evans blue staining demonstrated increased vascular permeability and the polymer conjugates had much higher delivery compared to free doxorubicin, which resulted in reduced neointimal formation.
Perfluorocarbon nanoassemblies have been employed in several studies that combine imaging with targeted drug delivery in an attempt to mitigate drug toxicity. 123 When the water soluble formulation of the antiangiogenic drug fumagillin was administered at high doses, adverse neurocognitive effects were seen. Therefore, a surfactant emulsion was formulated to effectively deliver fumagillin and iron oxide nanoparticles for MRI.103 These multifunctional particles enabled the imaging αvβ3 integrin expression and concurrent delivery of a hydrophobic drug at 50,000 fold lower concentration than previous studies with oral doses. Fumagillin lowered the expression of αvβ3 integrin, a key marker of angiogenic growth of the vasa vasorum. Further studies with αvβ3 fumagillin micelles in conjunction with atorvastatin demonstrated sustained anti-angiogenic behavior over 8 weeks. 124
Polymeric carriers are also favorable as they provide an enhanced circulation half-life in comparison to free drug delivery. Extended circulation times allow the therapeutic agent to be used preferentially due to more infrequent dosing regimens. A polyamidoamine (PAMAM) dendrimer-PEG construct was developed to entrap and deliver low molecular weight heparin (LMWH), a widely used anti-thrombotic.125 The entrapped LMWH showed significantly higher pulmonary absorption and had ~60% of the bioavailability of subcutaneous heparin. More importantly, the half-life of the dendrimer-LMWH was increased 2.4-fold relative to subcutaneous delivery in saline. This resulted in similar reductions in thrombus weight when dosed at half the frequency of subcutaneous LMWH in a rodent model. While longer circulation half-life is ideal, it is only part of the drug delivery challenge as there also needs to be cell specific delivery of the active.
Cationic micelles offer a suitable alternative to viral vectors for gene and siRNA delivery. Negatively charged nucleic acids typically require cationic carriers to complex and carry to desired cellular target. Akagi et al synthesized PEG-b-P[Asp(DET)] polymers to deliver plasmid DNA to rabbit carotid arteries through polyion complex formation. 126 The polyplex micelles were stable in blood proteins and had a dual effect of inducing luciferase reporter expression and preventing the thrombus formation that occurred with non-micelle polymer controls.
Nanosystems have also been used to probe the function of composition in interactions with cells and atherosclerotic tissue. Although not directly used for therapies, these have developed mechanisms of the targeting and LDL uptake. Synthetic nanoemulsions with a structure resembling LDL were shown to have distinct fates in atherosclerotic rabbits depending on if they contain free cholesterol or cholesterol esters. Free cholesterol was cleared faster than cholesterol esters in cholesterol fed rabbits, but not healthy ones. This demonstrated the significant differences in cholesterol metabolism. 127 C-reactive protein (CRP) is involved in the aggregation and uptake of oxidized LDL. LDL mimetic polymer lipid coated nanoparticles were used to elucidate mechanisms of CRP binding to curved lipid membranes. 128 Buono et al demonstrated that fluorescent PEGylated nanoparticles that were similarly sized to LDL were able to model LDL uptake by fluid phase pinocytosis in macrophages. They found that these displayed accumulation in aortic arch atherosclerotic lesions in ApoE mice. 129
Tracking the Distribution of Polymeric Micelles
Many therapeutic systems are designed with a reporter to develop and track their fate. 130 The design flexibility of micelles allows for either direct conjugation of small molecules or as encapsulation and solubilization agents for hydrophobic dyes or particles. Individual imaging methods each have distinct advantages and drawbacks, but they are rarely used alone. For example, Mulder et al created a mixed micelle system that incorporated the MRI contrast agent gadolinium with flourophore or quantum dot labeled monomers. 54
Many different types of micellar formulations have been made using magnetic resonance contrast agents as the reporter molecule. 36 Gadolinium is often used as a MRI contrast agent in micellar systems due to its ability to sharply reduce T1 relaxation times. It is easily conjugated by covalent attachment of a chelator such as DTPA that reduces the inherent toxicity of free gadolinium. 131 Super paramagnetic iron oxide nanoparticles (SPIO) can also be used to image plaques, but amphiphilic surfactants are needed to stabilize the SPIO particles, leading to micelles with the particle at the core. Monocytes and macrophages have high affinity for surfactant stabilized SPIO particles and can result in T2* weighted signal loss at the site of the lesion relative to the blood-pool. Since macrophage uptake can correlate to the rate of lesion growth and its instability, it may be a particularly useful marker. 132, 133
PET and SPECT rely on radioactive isotopes and CT contrast agents rely on iodinated compounds, all of which can be conjugated within micellar systems. Hyafil et al developed an iodinated contrast agent (N1177) that showed preferential uptake by macrophages relative to traditional contrast agent; however it needed to be solubilized with a polymer surfactant to prevent agglomeration. 134
Quantum dots and organic fluorophores offer the ability to easily visualize localization ex vivo or in small animals. While their utility is limited for clinical diagnostic use due to poor tissue penetration of visible light, many micellar systems incorporate a fluorophore for development and characterization. Near infrared (NIR) fluorescent quantum dots can mitigate this problem due to the increased penetration depth of NIR light. 135 Quantum dots can be encapsulated within the micelle core if coated with a hydrophobic surface or covalently conjugated to polymer monomers.
Summary and Conclusions
Polymer and polymer-lipid based micellar nanoassemblies are emerging as promising new candidates for the potential management of atherosclerosis. Intelligent design and composition flexibility in conjunction with improved targeting and tunable pharmacokinetic properties are some of the salient attributes of polymeric nanoassemblies. To date, a large portion of the systems developed have used polymers that mimic modified LDL to competitively inhibit oxLDL uptake by receptors involved in lesion lipoprotein uptake and inflammation. As a result, most of these systems employ polymer-lipid or lipid mimic constructs, thus leaving amphiphilic block copolymers relatively unexplored. The facile synthesis of well-defined block copolymers, thanks to controlled polymerization methods, and the wide variety of polymerizable monomers offers a unique opportunity to design novel multifunctional nanoassemblies that can rival delivery platforms currently under development.
One of the areas that is rapidly evolving is the elucidation of intracellular signaling pathways, which parallels the identification of novel molecular targets for better disease targeting and cell-based delivery. Advanced techniques, such as phage display, are leading to the rapid discovery of novel ligands that effectively bind to these targets. Utilizing highly specific, non-immunogenic and small size ligands, including peptides and peptidomimetics, and incorporation within nanocarriers will allow for the development of more selective and efficient nanosystems. Computer simulations of molecular docking and micellar lipoprotein mimetics can guide the mechanistic design of polymer-target receptor interactions, screen for optimal micelle configurations and probe possible intervention of lesion development. Since atherosclerosis is a focal disease, it is especially important to have localized therapy in order to slow down and possibly reverse the disease progression. With the advent of controlled release drugs and nanocarriers that can prevent vascular remodeling leading to blood vessel occlusion, new alternatives could be envisioned that replace drug-eluting stents. However several challenges will need to be overcome, including the barriers for intravascular administration and effective targeting that can evade premature clearance. Micellar systems may have key advantages: 1) increased circulation half-life and 2) resistance to clearance that allow widely spaced dosing while maintaining therapeutic efficacy. Additionally, drug carrying micelles are often designed with the intent to deliver after an ischemic event or cardiovascular intervention. These target angiogenesis in growing lesions or restenosis following stenting by inhibiting cell proliferation.
Inflammatory signaling plays a key role in the intensification of atherosclerosis and is one of the most promising targets for future nanosystems. Designing nanoscale drug/polymer systems to interrupt the signaling cascade at multiple nodes would be an effective way to inhibit disease progression. Specific targeting to growing lesions could avoid some of the increased susceptibility to infection seen with anti-cytokine therapies. Additionally, using RNA interference to knockdown gene expression could regulate the pro-inflammatory cytokine and monocyte recruitment proteins without compromising systemic immune responsiveness. The somewhat disparate fields of atherogenesis and inflammation need to be integratively addressed in order to design effective methods for integrative inhibition of atheroinflammatory cascades that underlie the complex etiology of atherosclerosis.
Acknowledgments
Authors want to acknowledge the funding from NIH R21 HL 093753, NIH R01 HL 107913, NIH T32 EB005583 (AWY), and NIH T32 GM008339 (DRL) as well as invaluable collaborations of Dr. Kathryn Uhrich at Rutgers and Dr. William Welsh at UMDNJ for the work related to bioactive nanopolymers.
Contributor Information
Daniel R. Lewis, Department of Chemical & Biochemical Engineering, Rutgers University
Kubra Kamisoglu, Department of Chemical & Biochemical Engineering, Rutgers University
Adam York, Department of Biomedical Engineering, New Jersey Center for Biomaterials
Prabhas V. Moghe, Email: Moghe@rutgers.edu, Department of Biomedical Engineering, Department of Chemical and Biochemical Engineering, Rutgers University
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.McGill HC, McMahan CA. Determinants of atherosclerosis in the young. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Am J Cardiol. 1998;82:30T–36T. doi: 10.1016/s0002-9149(98)00720-6. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Executive Summary: Heart Disease and Stroke Statistics--2010 Update: A Report From the American Heart Association. Circulation. 2010;121(7):948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Coronary artery injury and the biology of atherosclerosis: inflammation, thrombosis, and stabilization. Am J Cardiol. 2000;86(8B):3J–8J. doi: 10.1016/s0002-9149(00)01339-4. discussion 8J–9J. [DOI] [PubMed] [Google Scholar]
- 5.Rubenstrunk A, Hanf R, Hum DW, Fruchart JC, Staels B. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta. 2007;1771(8):1065–81. doi: 10.1016/j.bbalip.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Zinn A, Felson S, Fisher E, Schwartzbard A. Reassessing the cardiovascular risks and benefits of thiazolidinediones. Clinical Cardiology. 2008;31(9):397–403. doi: 10.1002/clc.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staels B. Fluid retention mediated by renal PPAR[gamma] Cell Metabolism. 2005;2(2):77–78. doi: 10.1016/j.cmet.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Bucher HC, Griffith LE, Guyatt GH. Systematic Review on the Risk and Benefit of Different Cholesterol-Lowering Interventions. Arterioscler Thromb Vasc Biol. 1999;19(2):187–195. doi: 10.1161/01.atv.19.2.187. [DOI] [PubMed] [Google Scholar]
- 9.Gotto AM. Antioxidants, statins, and atherosclerosis. Journal of the American College of Cardiology. 2003;41(7):1205–1210. doi: 10.1016/s0735-1097(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 10.Klingenberg R, Hansson GrK. Treating inflammation in atherosclerotic cardiovascular disease: emerging therapies. European Heart Journal. 2009;30(23):2838–2844. doi: 10.1093/eurheartj/ehp477. [DOI] [PubMed] [Google Scholar]
- 11.Sheikine YA, Hansson GK. Chemokines as Potential Therapeutic Targets in Atherosclerosis. Current Drug Targets. 2006;7(1):13–28. doi: 10.2174/138945006775270240. [DOI] [PubMed] [Google Scholar]
- 12.Steinhubl SR. Why Have Antioxidants Failed in Clinical Trials? The American Journal of Cardiology. 2008;101(10, Supplement 1):S14–S19. doi: 10.1016/j.amjcard.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Krotz F, Sohn HY, Klauss V. Antiplatelet drugs in cardiological practice: established strategies and new developments. Vasc Health Risk Manag. 2008;4(3):637–45. doi: 10.2147/vhrm.s2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez ER. Antiplatelet therapy in atherosclerotic cardiovascular disease. Clin Ther. 1998;20 (Suppl B):B18–41. doi: 10.1016/s0149-2918(98)80028-3. [DOI] [PubMed] [Google Scholar]
- 15.Siu D. A new way of targeting to treat coronary artery disease. Journal of Cardiovascular Medicine. 2010;11(1):1–6. doi: 10.2459/JCM.0b013e32832e0af3. [DOI] [PubMed] [Google Scholar]
- 16.Caruso F. Nanoengineering of Particle Surfaces. Adv Mater. 2001;13(1):11–22. [Google Scholar]
- 17.Mailänder V, Landfester K. Interaction of Nanoparticles with Cells. Biomacromolecules. 2009;10(9):2379–2400. doi: 10.1021/bm900266r. [DOI] [PubMed] [Google Scholar]
- 18.Lowe AB, Sumerlin BS, Donovan MS, McCormick CL. Facile preparation of transition metal nanoparticles stabilized by well-defined (co)polymers synthesized via aqueous reversible addition-fragmentation chain transfer polymerization. J Am Chem Soc. 2002;124:11562–11563. doi: 10.1021/ja020556h. [DOI] [PubMed] [Google Scholar]
- 19.Sumerlin BS, Lowe AB, Stroud PA, Zhang P, Urban MW, McCormick CL. Modification of gold surfaces with water-soluble (co)polymers prepared via aqueous reversible addition-fragmentation chain transfer (RAFT) polymerization. Langmuir. 2003;19:5559–5562. [Google Scholar]
- 20.Ranjan R, Brittain WJ. Combination of living radical polymerization and click chemistry for surface modification. Macromolecules. 2007;40(17):6217–6223. [Google Scholar]
- 21.Balazs AC, Emrick T, Russell TP. Nanoparticle Polymer Composites: Where Two Small Worlds Meet. Science. 2006;314(5802):1107–1110. doi: 10.1126/science.1130557. [DOI] [PubMed] [Google Scholar]
- 22.Anton N, Benoit J-P, Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates--A review. J Control Release. 2008;128(3):185–199. doi: 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Hornig S, Heinze T, Becer CR, Schubert US. Synthetic polymeric nanoparticles by nanoprecipitation. J Mater Chem. 2009;19(23):3838–3840. [Google Scholar]
- 24.Johnson BK, Prud’homme RK. Flash NanoPrecipitation of Organic Actives and Block Copolymers using a Confined Impinging Jets Mixer. Aust J Chem. 2003;56(10):1021–1024. [Google Scholar]
- 25.Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55(1):R1–R4. [Google Scholar]
- 26.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–15. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorganic & Medicinal Chemistry. 2009;17(8):2950–2962. doi: 10.1016/j.bmc.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 28.Moghimi SM, Hunter AC, Murray JC. Long-Circulating and Target-Specific Nanoparticles: Theory to Practice. Pharmacological Reviews. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 29.Chonn A, Semple SC, Cullis PR. Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes. Journal of Biological Chemistry. 1992;267(26):18759–18765. [PubMed] [Google Scholar]
- 30.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Progress in Lipid Research. 2003;42(6):463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 31.Sharma G, Valenta DT, Altman Y, Harvey S, Xie H, Mitragotri S, Smith JW. Polymer particle shape independently influences binding and internalization by macrophages. Journal of Controlled Release. 2010;147(3):408–412. doi: 10.1016/j.jconrel.2010.07.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehn J-M. Supramolecular polymer chemistry—scope and perspectives. Polym Int. 2002;51(10):825–839. [Google Scholar]
- 33.Haag R. Supramolecular Drug-Delivery Systems Based on Polymeric Core–Shell Architectures. Angew Chem Int Ed. 2004;43(3):278–282. doi: 10.1002/anie.200301694. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu T, Masuda M, Minamikawa H. Supramolecular Nanotube Architectures Based on Amphiphilic Molecules. Chem Rev. 2005;105(4):1401–1444. doi: 10.1021/cr030072j. [DOI] [PubMed] [Google Scholar]
- 35.Broz P, Marsch S, Hunzikel P. Targeting of Vulnerable Plaque Macrophages with Polymer-Based Nanostructures. Trends Cardiovas Med. 2007;17(6):190–196. doi: 10.1016/j.tcm.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Cormode DP, Fayad ZA, Mulder WJM. Nanoparticles as magnetic resonance imaging contrast agents for vascular and cardiac diseases. WIREs Nanomed Nanobiotechnol. 2010 doi: 10.1002/wnan.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulder WJM, Strijkers GJ, van Tilborg GAF, Cormode DP, Fayad ZA, Nicolay K. Nanoparticulate Assemblies of Amphiphiles and Diagnostically Active Materials for Multimodality Imaging. Acc Chem Res. 2009;42(7):904–914. doi: 10.1021/ar800223c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iverson NM, Sparks SM, Demirdirek B, Uhrich KE, Moghe PV. Controllable inhibition of cellular uptake of oxidized low-density lipoprotein: structure-function relationships for nanoscale amphiphilic polymers. Acta Biomater. 2010;6(8):3081–3091. doi: 10.1016/j.actbio.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iverson N, Plourde N, Chnari E, Nackman GB, Moghe PV. Convergence of nanotechnology and cardiovascular medicine: progress and emerging prospects. BioDrugs. 2008;22(1):1–10. doi: 10.2165/00063030-200822010-00001. [DOI] [PubMed] [Google Scholar]
- 40.Chnari E, Nikitczuk JS, Uhrich KE, Moghe PV. Nanoscale anionic macromolecules can inhibit cellular uptake of differentially oxidized LDL. Biomacromolecules. 2006;7(2):597–603. doi: 10.1021/bm0506905. [DOI] [PubMed] [Google Scholar]
- 41.Plourde NM, Kortagere S, Welsh W, Moghe PV. Structure-activity relations of nanolipoblockers with the atherogenic domain of human macrophage scavenger receptor A. Biomacromolecules. 2009;10(6):1381–91. doi: 10.1021/bm8014522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kearney AS. Prodrugs and targeted delivery. Adv Drug Deliv Rev. 1996;19:225–239. [Google Scholar]
- 43.Kabanov AV, Kabanov VA. Interpolyelectrolyte and block ionomer complexes for gene delivery: physico-chemical aspects. Adv Drug Deliv Rev. 1998;30(1–3):49–60. doi: 10.1016/s0169-409x(97)00106-3. [DOI] [PubMed] [Google Scholar]
- 44.Christie RJ, Grainger DW. Design strategies to improve soluble macromolecular delivery constructs. Adv Drug Delivery Rev. 2003;55:421–437. doi: 10.1016/s0169-409x(02)00229-6. [DOI] [PubMed] [Google Scholar]
- 45.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discovery. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 46.D’Emanuele A, Attwood D. Dendrimer–drug interactions. Adv Drug Delivery Rev. 2005;57:2147–2162. doi: 10.1016/j.addr.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Nori A, Kopecek J. Intracellular targeting of polymer-bound drugs for cancer chemotherapy. Adv Drug Delivery Rev. 2005;57:609–636. doi: 10.1016/j.addr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Svenson S, Tomalia DA. Dendrimers in biomedical applications—reflections on the field. Adv Drug Delivery Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 49.Wong SY, Pelet JM, Putnam D. Polymer systems for gene delivery -Past, present, and future. Prog Polym Sci. 2007;32:799–837. [Google Scholar]
- 50.York AW, Kirkland SE, McCormick CL. Advances in the synthesis of amphiphilic block copolymers via RAFT polymerization: Stimuli-responsive drug and gene delivery. Adv Drug Deliv Rev. 2008;60(9):1018–1036. doi: 10.1016/j.addr.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Lutz J-F, Börner HG. Modern trends in polymer bioconjugates design. Prog Polym Sci. 2008;33:1–39. [Google Scholar]
- 52.Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. P Natl Acad Sci USA. 1979;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mulder WJM, Strijkers GJ, Briley-Saboe KC, Frias JC, Aguinaldo JGS, Vucic E, Amirbekian V, Tang C, Chin PTK, Nicolay K, Fayad ZA. Molecular imaging of macrophages in atherosclerotic plaques using bimodal PEG-micelles. Magn Reson Med. 2007;58(6):1164–1170. doi: 10.1002/mrm.21315. [DOI] [PubMed] [Google Scholar]
- 55.Broz P, Ben-Haim N, Grzelakowski M, Marsch S, Meier W, Hunziker P. Inhibition of Macrophage Phagocytotic Activity by a Receptor-targeted Polymer Vesicle-based Drug Delivery Formulation of Pravastatin. Journal of Cardiovascular Pharmacology. 2008;51(3):246–252. doi: 10.1097/FJC.0b013e3181624aed. [DOI] [PubMed] [Google Scholar]
- 56.Amirbekian V, Lipinski MJ, Briley-Saebo KC, Amirbekian S, Aguinaldo JGS, Weinreb DB, Vucic E, Frias JC, Hyafil F, Mani V, Fisher EA, Fayad ZA. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. P Natl Acad Sci USA. 2007;104(3):961–966. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Tilborg GAF, Vucic E, Strijkers GJ, Cormode DP, Mani V, Skajaa T, Reutelingsperger CPM, Fayad ZA, Mulder WJM, Nicolay K. Annexin A5-Functionalized Bimodal Nanoparticles for MRI and Fluorescence Imaging of Atherosclerotic Plaques. Bioconjugate Chem. 2010;21(10):1794–1803. doi: 10.1021/bc100091q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters D, Kastantin M, Kotamraju VR, Karmali PP, Gujraty K, Tirrell M, Ruoslahti E. Targeting atherosclerosis by using modular, multifunctional micelles. P Natl Acad Sci USA. 2009;106:9815–9819. doi: 10.1073/pnas.0903369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chnari E, Nikitczuk JS, Wang J, Uhrich KE, Moghe PV. Engineered polymeric nanoparticles for receptor-targeted blockage of oxidized low density lipoprotein uptake and atherogenesis in macrophages. Biomacromolecules. 2006;7(6):1796–805. doi: 10.1021/bm0600872. [DOI] [PubMed] [Google Scholar]
- 60.Chnari E, Lari HB, Tian L, Uhrich KE, Moghe PV. Nanoscale anionic macromolecules for selective retention of low-density lipoproteins. Biomaterials. 2005;26(17):3749–58. doi: 10.1016/j.biomaterials.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 61.Jones M-C, Leroux J-C. Polymeric micelles - a new generation of colloidal drug carriers. Eur J Pharm Biopharm. 1999;48(2):101–111. doi: 10.1016/s0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 62.Discher DE, Eisenberg A. Polymer Vesicles. Science. 2002;297(5583):967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 63.Discher BM, Won Y-Y, Ege DS, Lee JCM, Bates FS, Discher DE, Hammer DA. Polymersomes: Tough Vesicles Made from Diblock Copolymers. Science. 1999;284(5417):1143–1146. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 64.Kwon GS, Okano T. Polymeric micelles as new drug carriers. Adv Drug Deliv Rev. 1996;21(2):107–116. [Google Scholar]
- 65.Riess G. Micellization of block copolymers. Prog Polym Sci. 2003;28(7):1107–1170. [Google Scholar]
- 66.Discher DE, Ahmed F. Polymersomes. Annu Rev Biomed Eng. 2006;8:323–341. doi: 10.1146/annurev.bioeng.8.061505.095838. [DOI] [PubMed] [Google Scholar]
- 67.Morishima Y, Nomura S, Ikeda T, Seki M, Kamachi M. Characterization of Unimolecular Micelles of Random Copolymers of Sodium 2-(Acrylamido)-2-methylpropanesulfonate and Methacrylamides Bearing Bulky Hydrophobic Substituents. Macromolecules. 1995;28(8):2874–2881. [Google Scholar]
- 68.Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev. 2004;56(9):1273–1289. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Li F, Danquah M, Mahato RI. Synthesis and Characterization of Amphiphilic Lipopolymers for Micellar Drug Delivery. Biomacromolecules. 2010;11(10):2610–2620. doi: 10.1021/bm100561v. [DOI] [PubMed] [Google Scholar]
- 70.Nishiyama N, Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Therapeut. 2006;112(3):630–648. doi: 10.1016/j.pharmthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 71.Dash PR, Read ML, Barrett LB, Wolfert MA, Seymour LW. Factors affecting blood clearance and in vivo distribution of polyelectrolyte complexes for gene delivery. Gene Ther. 1999;6:643–650. doi: 10.1038/sj.gt.3300843. [DOI] [PubMed] [Google Scholar]
- 72.Li Y, Lokitz BS, Armes S, McCormick CL. Synthesis of reversible shell cross-linked micelles for controlled release of bioactive agents. Macromolecules. 2006;39(8):2726–2728. [Google Scholar]
- 73.Wooley KL. Shell crosslinked polymer assemblies: Nanoscale constructs inspired from biological systems. Journal of Polymer Science Part A: Polymer Chemistry. 2000;38(9):1397–1407. [Google Scholar]
- 74.Read ES, Armes SP. Recent advances in shell cross-linked micelles. Chem Commun. 2007:3021–3035. doi: 10.1039/b701217a. [DOI] [PubMed] [Google Scholar]
- 75.Torchilin VP. Lipid-Core Micelles for Targeted Drug Delivery. Current Drug Delivery. 2005;2:319–327. doi: 10.2174/156720105774370221. [DOI] [PubMed] [Google Scholar]
- 76.Tian L, Yam L, Zhou N, Tat H, Uhrich KE. Amphiphilic Scorpion-like Macromolecules: Design, Synthesis, and Characterization. Macromolecules. 2004;37(2):538–543. [Google Scholar]
- 77.Briley-Saebo KC, Shaw PX, Mulder WJM, Choi S-H, Vucic E, Aguinaldo JGS, Witztum JL, Fuster V, Tsimikas S, Fayad ZA. Targeted Molecular Probes for Imaging Atherosclerotic Lesions With Magnetic Resonance Using Antibodies That Recognize Oxidation-Specific Epitopes. Circulation. 2008;117(25):3206–3215. doi: 10.1161/CIRCULATIONAHA.107.757120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beilvert A, Cormode DP, Chaubet F, Briley-Saebo KC, Mani V, Mulder WJ, Vucic E, Toussaint JF, Letourneur D, Fayad ZA. Tyrosine polyethylene glycol (PEG)-micelle magnetic resonance contrast agent for the detection of lipid rich areas in atherosclerotic plaque. Magnetic Resonance in Medicine. 2009;62(5):1195–1201. doi: 10.1002/mrm.22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCormick CL, Sumerlin BS, Lokitz BS, Stempka JE. RAFT-synthesized diblock and triblock copolymers: thermally-induced supramolecular assembly in aqueous media. Soft Matter. 2008;4:1760–1773. [Google Scholar]
- 80.McCormick CL, York AW, Kirkland SE. Synthetic routes to stimuli-responsive micelles, vesicles, and surfaces via controlled/living radical polymerization. Poly Rev. 2006;46:421–443. [Google Scholar]
- 81.Doyle B, Caplice N. Plaque Neovascularization and Antiangiogenic Therapy for Atherosclerosis. Journal of the American College of Cardiology. 2007;49(21):2073–2080. doi: 10.1016/j.jacc.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 82.Yokoyama M. Drug targeting with nano-sized carrier systems. J Artif Organs. 2005;8:77–84. doi: 10.1007/s10047-005-0285-0. [DOI] [PubMed] [Google Scholar]
- 83.Vasir JK, Reddy MK, Labhasetwar VD. Nanosystems in Drug Targeting: Opportunities and Challenges. Current Nanoscience. 2005;1:47–64. [Google Scholar]
- 84.Uwatoku T, Shimokawa H, Abe K, Matsumoto Y, Hattori T, Oi K, Matsuda T, Kataoka K, Takeshita A. Application of Nanoparticle Technology for the Prevention of Restenosis After Balloon Injury in Rats. Circ Res. 2003;92(7):e62–69. doi: 10.1161/01.RES.0000069021.56380.E2. [DOI] [PubMed] [Google Scholar]
- 85.Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Advanced drug delivery systems that target the vascular endothelium. Mol Interv. 2006;6(2):98–112. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- 86.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116(8):1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 87.Muro S, Cui XM, Gajewski C, Murciano JC, Muzykantov VR, Koval M. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. Am J Physiol-Cell Ph. 2003;285(5):C1339–C1347. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- 88.Poston RN, Haskard DO, Coucher JR, Gall NP, Johnson-Tidey RR. Expression of intercellular adhesion molecule-1 in atherosclerotic plaques. Am J Pathol. 1992;140(3):665–673. [PMC free article] [PubMed] [Google Scholar]
- 89.Garnacho C, Dhami R, Simone E, Dziubla T, Leferovich J, Schuchman EH, Muzykantov V, Muro S. Delivery of acid sphingomyelinase in normal and Niemann-Pick disease mice using intercellular adhesion molecule-1-targeted polymer nanocarriers. J Pharmacol Exp Ther. 2008;325(2):400–408. doi: 10.1124/jpet.107.133298. [DOI] [PubMed] [Google Scholar]
- 90.Muro S, Dziubla T, Qiu WN, Leferovich J, Cui X, Berk E, Muzykantov VR. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. J Pharmacol Exp Ther. 2006;317(3):1161–1169. doi: 10.1124/jpet.105.098970. [DOI] [PubMed] [Google Scholar]
- 91.Murciano JC, Muro S, Koniaris L, Christofidou-Solomiclou M, Harshaw DW, Albelda SM, Granger DN, Cines DB, Muzykantov VR. ICAM-directed vascular immunotargeting of antithrombotic agents to the endothelial luminal surface. Blood. 2003;101(10):3977–3984. doi: 10.1182/blood-2002-09-2853. [DOI] [PubMed] [Google Scholar]
- 92.Zhang N, Chittasupho C, Duangrat C, Siahaan TJ, Berkland C. PLGA nanoparticle-peptide conjugate effectively targets intercellular cell-adhesion molecule-1. Bioconjugate Chemistry. 2008;19(1):145–152. doi: 10.1021/bc700227z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tibbetts SA, Jois DSS, Siahaan TJ, Benedict SH, Chan MA. Linear and cyclic LFA-1 and ICAM-1 peptides inhibit T cell adhesion and function. Peptides. 2000;21(8):1161–1167. doi: 10.1016/s0196-9781(00)00255-2. [DOI] [PubMed] [Google Scholar]
- 94.Yusuf-Makagiansar H, Siahaan TJ. Binding and Internalization of an LFA-1-Derived Cyclic Peptide by ICAM Receptors on Activated Lymphocyte: A Potential Ligand for Drug Targeting to ICAM-1-Expressing Cells. Pharmaceutical Research. 2001;18(3):329–335. doi: 10.1023/a:1011007014510. [DOI] [PubMed] [Google Scholar]
- 95.Simone E, Ding BS, Muzykantov V. Targeted delivery of therapeutics to endothelium. Cell and Tissue Research. 2009;335(1):283–300. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res. 2005;96(3):327–36. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 97.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114(14):1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 98.Molenaar TJM, Twisk J, de Haas SAM, Peterse N, Vogelaar BJCP, van Leeuwen SH, Michon IN, van Berkel TJC, Kuiper J, Biessen EAL. P-selectin as a candidate target in atherosclerosis. Biochemical Pharmacology. 2003;66(5):859–866. doi: 10.1016/s0006-2952(03)00387-3. [DOI] [PubMed] [Google Scholar]
- 99.Kang HW, Torres D, Wald L, Weissleder R, Bogdanov AA. Targeted imaging of human endothelial-specific marker in a model of adoptive cell transfer. Lab Invest. 2006;86(6):599–609. doi: 10.1038/labinvest.3700421. [DOI] [PubMed] [Google Scholar]
- 100.McAteer MA, Schneider JE, Ali ZA, Warrick N, Bursill CA, von zur Muhlen C, Greaves DR, Neubauer S, Channon KM, Choudhury RP. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol. 2008;28(1):77–83. doi: 10.1161/ATVBAHA.107.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kolodgie FD, Narula J, Yuan C, Burke AP, Finn AV, Virmani R. Elimination of Neoangiogenesis for Plaque Stabilization: Is There a Role for Local Drug Therapy? Journal of the American College of Cardiology. 2007;49(21):2093–2101. doi: 10.1016/j.jacc.2006.10.083. [DOI] [PubMed] [Google Scholar]
- 102.Ribatti D, Levi-Schaffer F, Kovanen PT. Inflammatory angiogenesis in atherogenesis- a double-edged sword. Annals of Medicine. 2008;40(8):606–621. doi: 10.1080/07853890802186913. [DOI] [PubMed] [Google Scholar]
- 103.Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA, Schmieder AH, Hu G, Allen JS, Lacy EK, Zhang H, Wickline SA, Lanza GM. Endothelial {alpha}{nu}{beta}3 Integrin-Targeted Fumagillin Nanoparticles Inhibit Angiogenesis in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26(9):2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 104.Flacke S, Fischer S, Scott MJ, Fuhrhop RJ, Allen JS, McLean M, Winter P, Sicard GA, Gaffney PJ, Wickline SA, Lanza GM. Novel MRI Contrast Agent for Molecular Imaging of Fibrin: Implications for Detecting Vulnerable Plaques. Circulation. 2001;104(11):1280–1285. doi: 10.1161/hc3601.094303. [DOI] [PubMed] [Google Scholar]
- 105.Chan JM, Zhang L, Tong R, Ghosh D, Gao W, Liao G, Yuet KP, Gray D, Rhee J-W, Cheng J, Golomb G, Libby P, Langer R, Farokhzad OC. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proceedings of the National Academy of Sciences. 2010;107(5):2213–2218. doi: 10.1073/pnas.0914585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moore KJ, Freeman MW. Scavenger Receptors in Atherosclerosis: Beyond Lipid Uptake. Arterioscler Thromb Vasc Biol. 2006;26(8):1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 107.Ogura S, Kakino A, Sato Y, Fujita Y, Iwamoto S, Otsui K, Yoshimoto R, Sawamura T. LOX-1: The Multifunctional Receptor Underlying Cardiovascular Dysfunction. Circulation Journal. 2009;73(11):1993–1999. doi: 10.1253/circj.cj-09-0587. [DOI] [PubMed] [Google Scholar]
- 108.Wang J, Plourde NM, Iverson N, Moghe PV, Uhrich KE. Nanoscale amphiphilic macromolecules as lipoprotein inhibitors: the role of charge and architecture. Int J Nanomedicine. 2007;2(4):697–705. [PMC free article] [PubMed] [Google Scholar]
- 109.Plourde NM, Kortagere S, Welsh W, Moghe PV. Structure Activity Relations of Nanolipoblockers with the Atherogenic Domain of Human Macrophage Scavenger Receptor A. Biomacromolecules. 2009;10(6):1381–1391. doi: 10.1021/bm8014522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boullier A, Bird DA, Chang MK, Dennis EA, Friedman P, Gillotre-Taylor K, Horkko S, Palinski W, Quehenberger O, Shaw P, Steinberg D, Terpstra V, Witztum JL. Scavenger receptors, oxidized LDL, and atherosclerosis. Ann N Y Acad Sci. 2001;947:214–22. doi: 10.1111/j.1749-6632.2001.tb03943.x. discussion 222–3. [DOI] [PubMed] [Google Scholar]
- 111.White SJ, Nicklin SA, Sawamura T, Baker AH. Identification of Peptides That Target the Endothelial Cell-Specific LOX-1 Receptor. Hypertension. 2001;37(2):449–455. doi: 10.1161/01.hyp.37.2.449. [DOI] [PubMed] [Google Scholar]
- 112.Wang L, Bao Y, Yang Y, Wu Y, Chen X, Si S, Hong B. Discovery of Antagonists for Human Scavenger Receptor CD36 via an ELISA-Like High-Throughput Screening Assay. J Biomol Screen. 2010;15(3):239–250. doi: 10.1177/1087057109359686. [DOI] [PubMed] [Google Scholar]
- 113.Azzazy HME, Highsmith WE. Phage display technology: clinical applications and recent innovations. Clinical Biochemistry. 2002;35(6):425–445. doi: 10.1016/s0009-9120(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 114.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT Imaging of Macrophages in Inflammatory Atherosclerosis. Circulation. 2008;117(3):379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McCarthy J, Jaffer F, Weissleder R. A Macrophage-Targeted Theranostic Nanoparticle for Biomedical Applications. Small. 2006;2(8–9):983–987. doi: 10.1002/smll.200600139. [DOI] [PubMed] [Google Scholar]
- 116.Bijsterbosch MK, van Berkel TJC. Native and modified lipoproteins as drug delivery systems. Advanced Drug Delivery Reviews. 1990;5(3):231–251. [Google Scholar]
- 117.Chnari E, Nikitczuk JS, Wang JZ, Uhrich KE, Moghe PV. Engineered polymeric nanoparticles for receptor-targeted blockage of oxidized low density lipoprotein uptake and atherogenesis in macrophages. Biomacromolecules. 2006;7(6):1796–1805. doi: 10.1021/bm0600872. [DOI] [PubMed] [Google Scholar]
- 118.Nikitina N, Khalilov E, Torkhovskaya T, Tertov V, Orekhov A. Polyunsaturated phosphatidylcholine micelle-induced decrease of atherogenicity of the serum in vitro. Bulletin of Experimental Biology and Medicine. 1995;119(5):480–483. [PubMed] [Google Scholar]
- 119.Weigensberg BI. Effects of four variations of a surfactant polymer on experimental atherosclerosis in rabbits. Journal of Atherosclerosis Research. 1969;10(3):291–301. doi: 10.1016/s0368-1319(69)80033-5. [DOI] [PubMed] [Google Scholar]
- 120.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of Drug-Eluting Stents in Humans: Delayed Healing and Late Thrombotic Risk. J Am Coll Cardiol. 2006;48(1):193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 121.Joner M, Morimoto K, Kasukawa H, Steigerwald K, Merl S, Nakazawa G, John MC, Finn AV, Acampado E, Kolodgie FD, Gold HK, Virmani R. Site-Specific Targeting of Nanoparticle Prednisolone Reduces In-Stent Restenosis in a Rabbit Model of Established Atheroma. Arterioscler Thromb Vasc Biol. 2008;28(11):1960–1966. doi: 10.1161/ATVBAHA.108.170662. [DOI] [PubMed] [Google Scholar]
- 122.Ikuta K, Mori T, Yamamoto T, Niidome T, Shimokawa H, Katayama Y. Development of polymeric drug delivery system for recognizing vascular endothelial dysfunction. Bioorganic & Medicinal Chemistry. 2008;16(6):2811–2818. doi: 10.1016/j.bmc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 123.Lanza G, Winter P, Caruthers S, Hughes M, Hu G, Schmieder A, Wickline S. Theragnostics for tumor and plaque angiogenesis with perfluorocarbon nanoemulsions. Angiogenesis. 2010;13(2):189–202. doi: 10.1007/s10456-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Winter PM, Caruthers SD, Zhang H, Williams TA, Wickline SA, Lanza GM. Antiangiogenic Synergism of Integrin-Targeted Fumagillin Nanoparticles and Atorvastatin in Atherosclerosis. J Am Coll Cardiol Img. 2008;1(5):624–634. doi: 10.1016/j.jcmg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bai S, Ahsan F. Synthesis and evaluation of pegylated dendrimeric nanocarrier for pulmonary delivery of low molecular weight heparin. Pharm Res. 2009;26(3):539–48. doi: 10.1007/s11095-008-9769-y. [DOI] [PubMed] [Google Scholar]
- 126.Akagi D, Oba M, Koyama H, Nishiyama N, Fukushima S, Miyata T, Nagawa H, Kataoka K. Biocompatible micellar nanovectors achieve efficient gene transfer to vascular lesions without cytotoxicity and thrombus formation. Gene Ther. 2007;14(13):1029–1038. doi: 10.1038/sj.gt.3302945. [DOI] [PubMed] [Google Scholar]
- 127.Padoveze AF, Maniero F, Oliveira TV, Vitorio TS, Couto RD, Maranhão RC. Effect of a cholesterol-rich diet on the metabolism of the free and esterified cholesterol components of a nanoemulsion that resembles LDL in rabbits. Brazilian Journal of Medical and Biological Research. 2009;42:172–178. doi: 10.1590/s0100-879x2009000200005. [DOI] [PubMed] [Google Scholar]
- 128.Mackiewicz MR, Hodges HL, Reed SM. C-Reactive Protein Induced Rearrangement of Phosphatidylcholine on Nanoparticle Mimics of Lipoprotein Particles. The Journal of Physical Chemistry B. 2010;114(16):5556–5562. doi: 10.1021/jp911617q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Buono C, Anzinger JJ, Amar M, Kruth HS. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. The Journal of Clinical Investigation. 2009;119(5):1373–1381. doi: 10.1172/JCI35548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Choudhury RP, Fuster V, Fayad ZA. Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov. 2004;3(11):913–25. doi: 10.1038/nrd1548. [DOI] [PubMed] [Google Scholar]
- 131.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chemical Reviews. 1999;99(9):2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 132.Schmitz SA, Coupland SE, Gust R, Winterhalter S, Wagner S, Kresse M, Semmler W, Wolf K-J. Superparamagnetic Iron Oxide-Enhanced MRI of Atherosclerotic Plaques in Watanabe Hereditable Hyperlipidemic Rabbits. Investigative Radiology. 2000;35(8):460–471. doi: 10.1097/00004424-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 133.Fayad ZA, Razzouk L, Briley-Saebo KC, Mani V. Iron Oxide Magnetic Resonance Imaging for Atherosclerosis Therapeutic Evaluation: Still “Rusty?”. J Am Coll Cardiol. 2009;53(22):2051–2052. doi: 10.1016/j.jacc.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hyafil F, Cornily JC, Feig JE, Gordon R, Vucic E, Amirbekian V, Fisher EA, Fuster V, Feldman LJ, Fayad ZA. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007;13(5):636–41. doi: 10.1038/nm1571. [DOI] [PubMed] [Google Scholar]
- 135.Smith AM, Mancini MC, Nie S. Bioimaging: second window for in vivo imaging. Nat Nanotechnol. 2009;4(11):710–1. doi: 10.1038/nnano.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, Wickline SA. Molecular Imaging of Angiogenesis in Early-Stage Atherosclerosis With {alpha}v{beta}3-Integrin-Targeted Nanoparticles. Circulation. 2003;108(18):2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 137.Nahrendorf M, Zhang HW, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117(3):379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]