Abstract
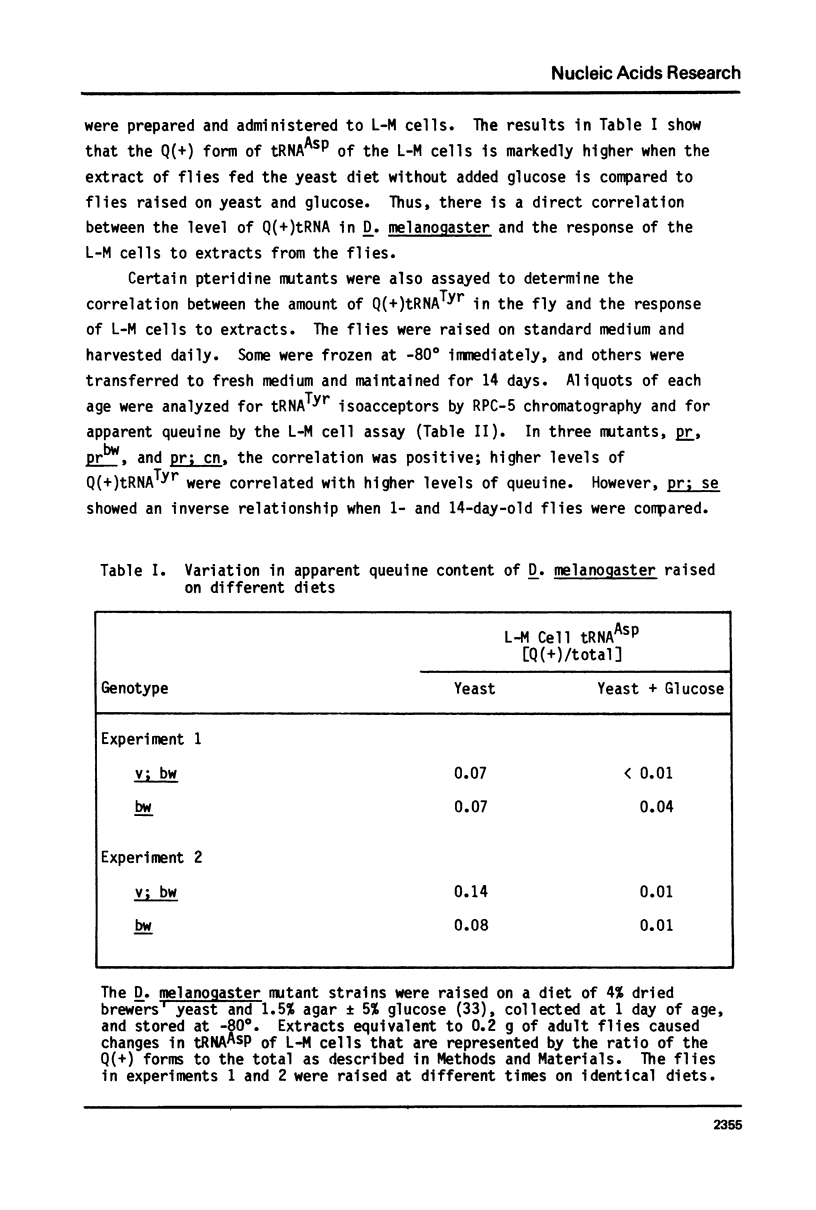
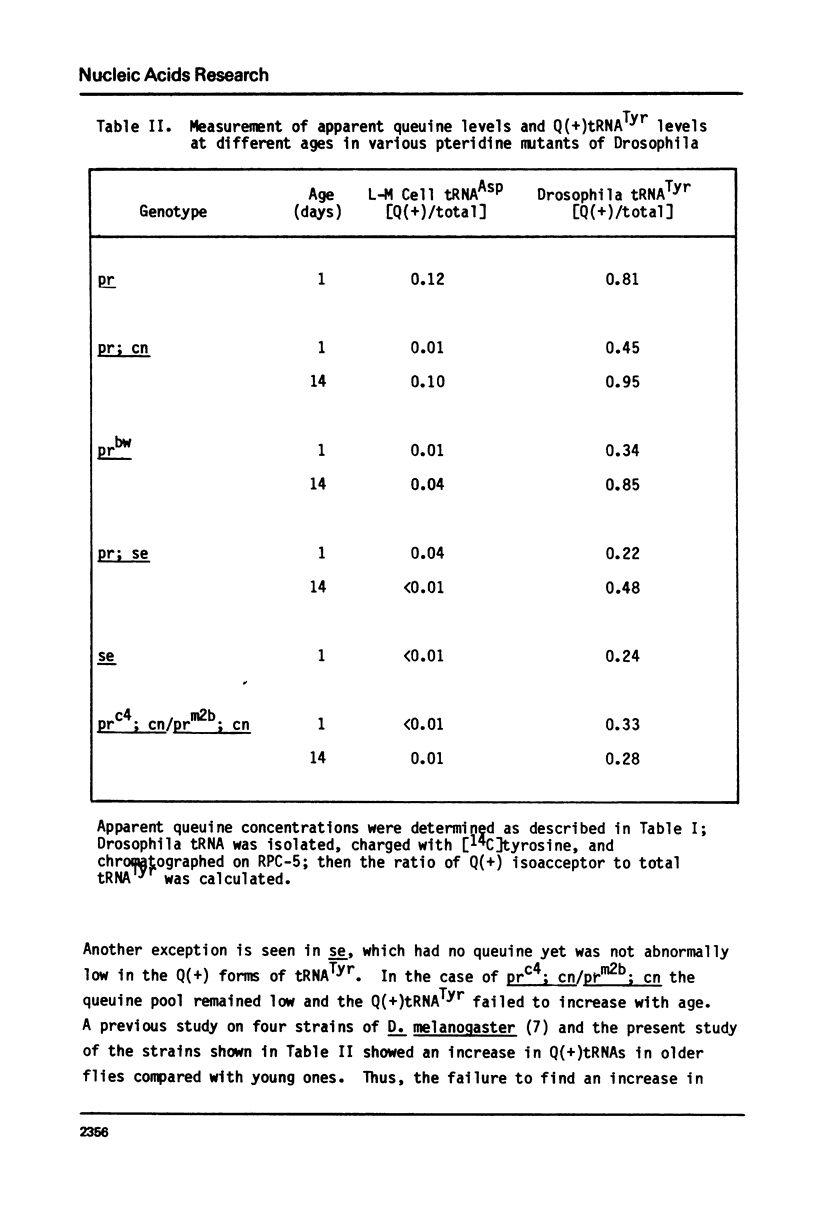
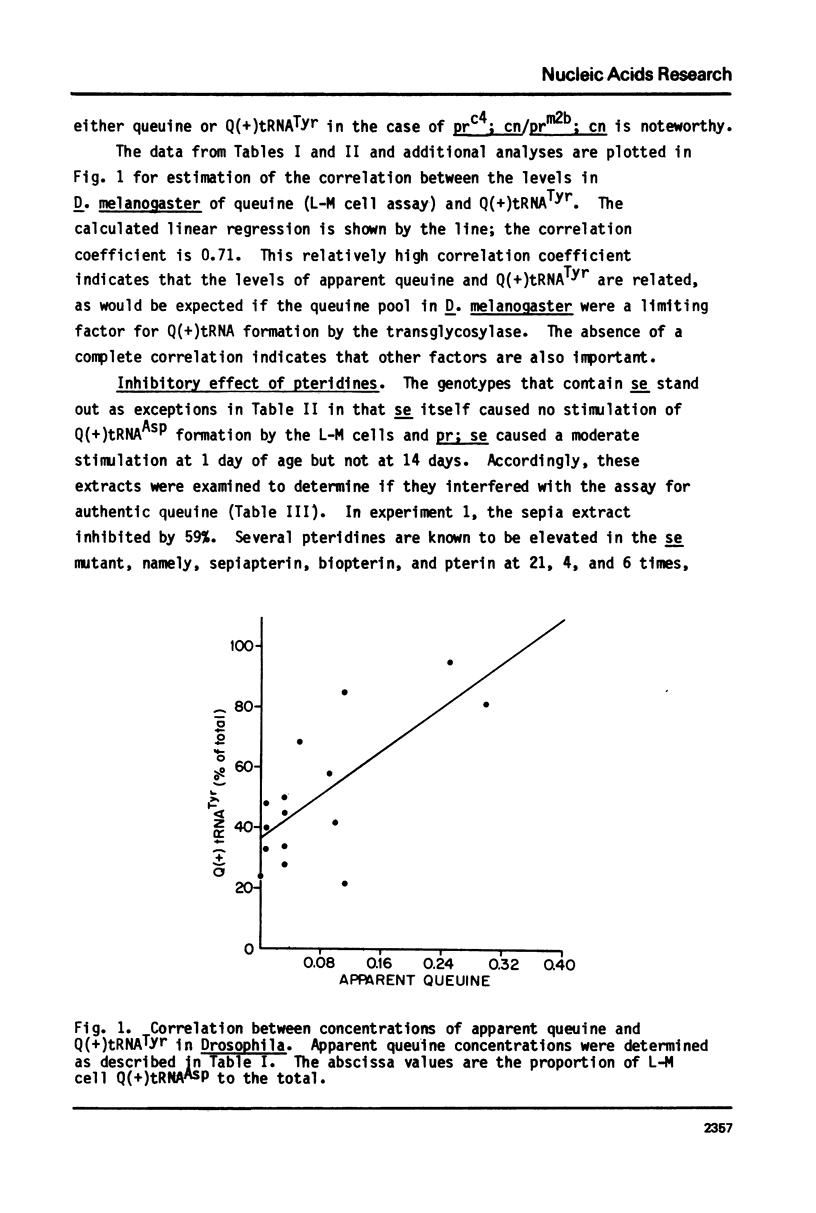
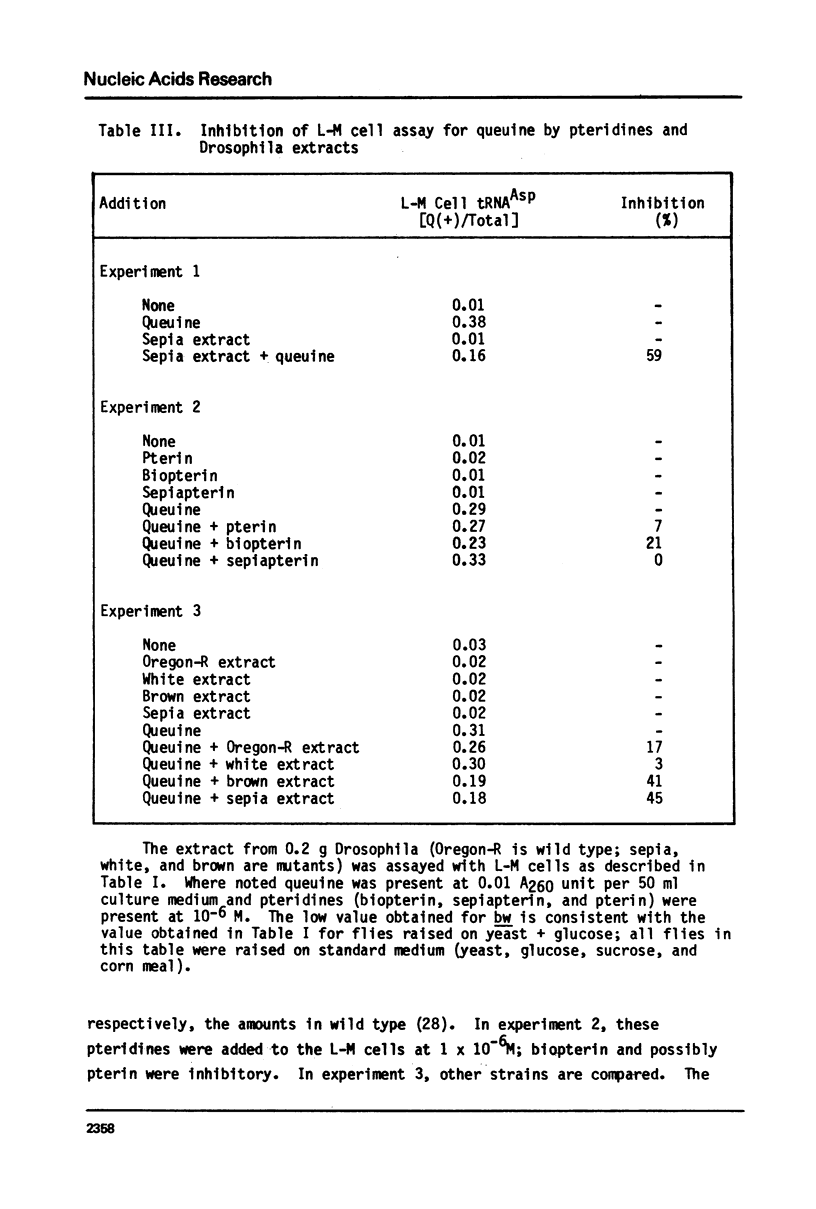
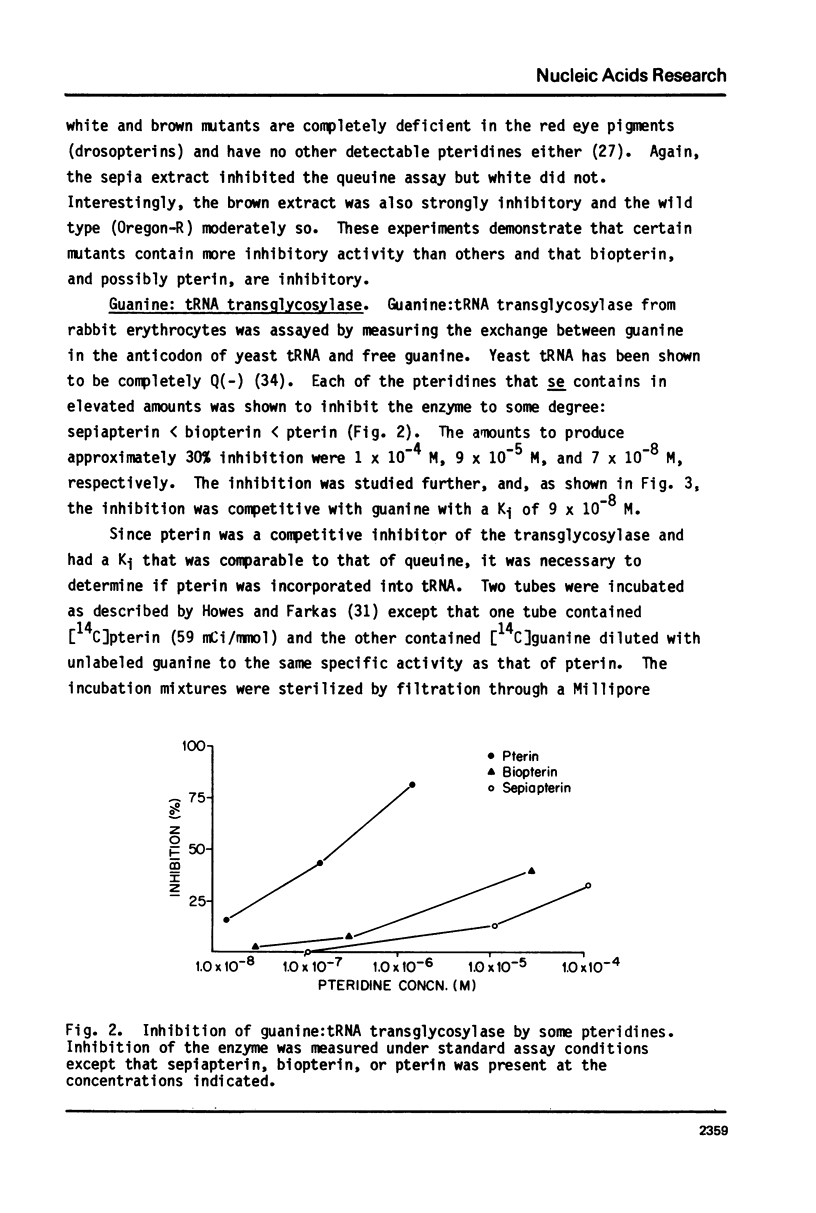
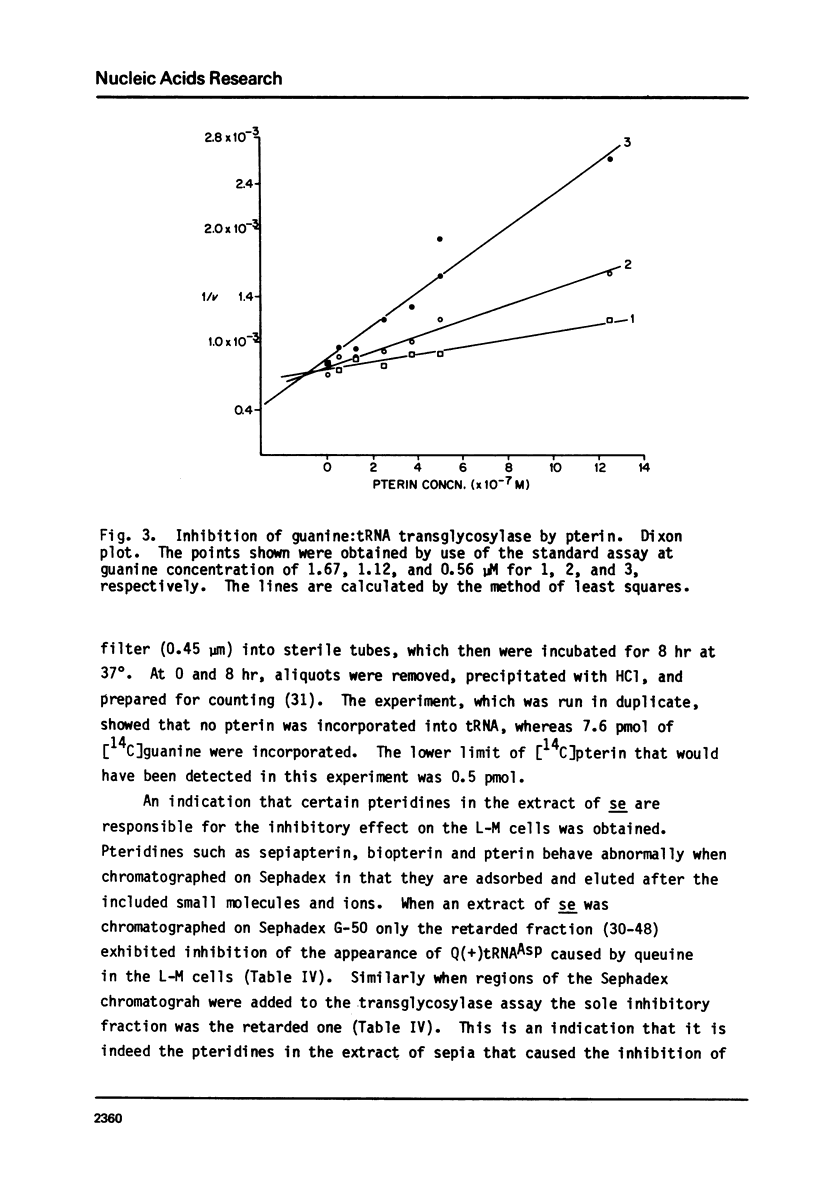
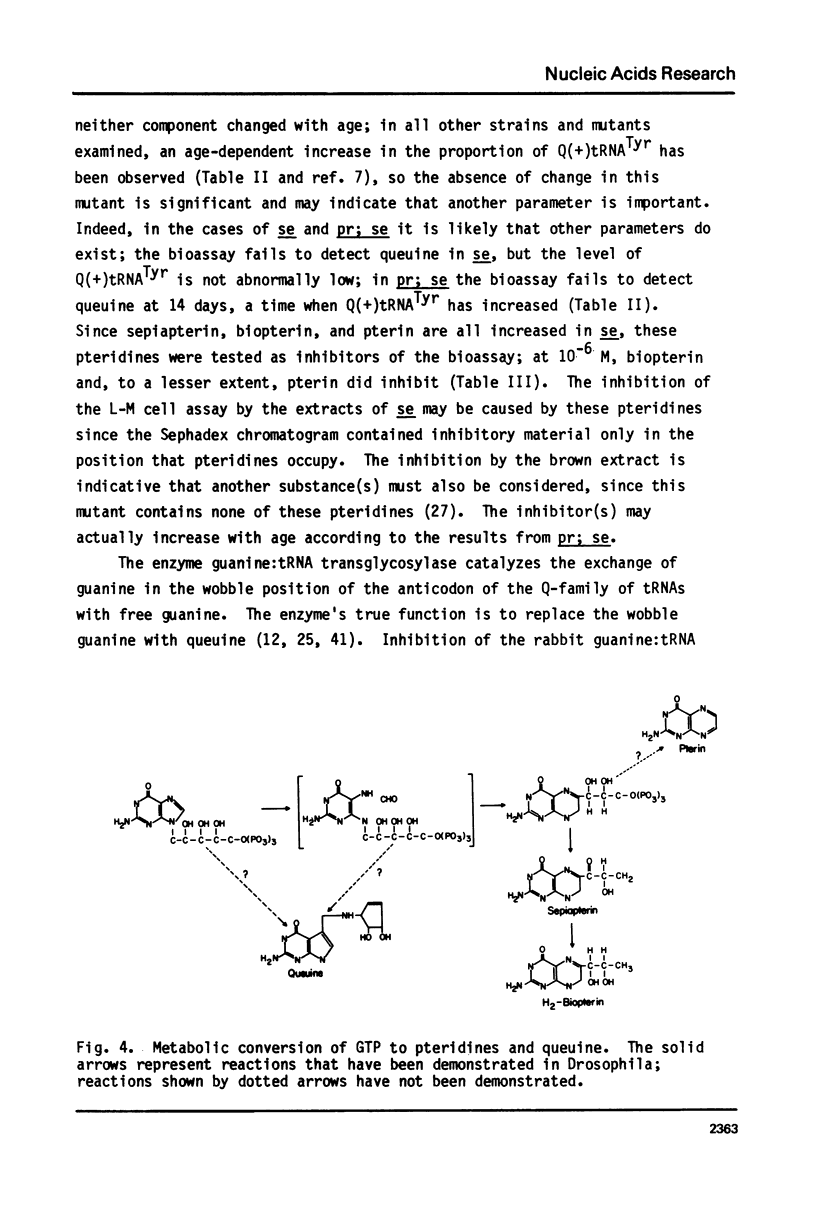
Queuine, a modified form of 7-deazaguanine present in certain transfer RNAs, is shown to occur in Drosophila melanogaster adults in a free form and its concentration varies as a function of age, nutrition and genotype. In several, but not all mutant strains, the concentrations of queuine and the Q(+) (queuine-containing) form of tRNATyr are correlated. The bioassay employs L-M cells which respond to the presence of queuine by an increase in their Q(+)tRNAAsp that is accompanied by a decrease in the Q(-)tRNAAsp isoacceptors. The increase in Q(+)tRNATyr in Drosophila that occurs on a yeast diet is accompanied by an increase in queuine. Similarly the increase of Q(+)tRNAs with age also is accompanied by an increase in free queuine. In two mutants, brown and sepia, these correlations were either diminished or failed to occur. Indeed, the extract of both mutants inhibited the response of the L-M cells to authentic queuine. When the pteridines that occur at abnormally high levels in sepia were used at 1 x 10(-6)M, the inhibition of the L-M cell assay occurred in the order biopterin greater than pterin greater than sepiapterin. These pteridines were also inhibitory for the purified guanine:tRNA transglycosylase from rabbit but the relative effectiveness then was pterin greater than biopterin greater than sepiapterin. Pterin was competitive with guanine in the enzyme reaction with Ki = 0.9 x 10(-7)M. Also when an extract of sepia was chromatographed on Sephadex G-50, the pteridine-containing fractions only were inhibitory toward the L-M cell assay or the enzyme assay. These results indicate that free queuine occurs in Drosophila but also that certain pteridines may interfere with the incorporation of queuine into RNA.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briscoe W. T., Griffin A. C., McBride C., Bowen J. M. The distribution and properties of aspartyl transfer RNA in human and animal tumors. Cancer Res. 1975 Sep;35(9):2586–2593. [PubMed] [Google Scholar]
- Burg A. W., Brown G. M. The biosynthesis of folic acid. 8. Purification and properties of the enzyme that catalyzes the production of formate from carbon atom 8 of guanosine triphosphate. J Biol Chem. 1968 May 10;243(9):2349–2358. [PubMed] [Google Scholar]
- Crain P. F., Sethi S. K., Katze J. R., McCloskey J. A. Structure of an amniotic fluid component, 7-(4,5-cis-dihydroxy-1-cyclopenten-3-ylaminomethyl)-7-deazaguanine (queuine), a substrate for tRNA: guanine transglycosylase. J Biol Chem. 1980 Sep 25;255(18):8405–8407. [PubMed] [Google Scholar]
- Dubrul E. F., Farkas W. R. Partial purification and properties of the reticulocyte guanylating enzyme. Biochim Biophys Acta. 1976 Sep 6;442(3):379–390. doi: 10.1016/0005-2787(76)90312-9. [DOI] [PubMed] [Google Scholar]
- Evans B. A., Howells A. J. Control of drosopterin synthesis in Drosophila melanogaster: mutants showing an altered pattern of GTP cyclohydrolase activity during development. Biochem Genet. 1978 Feb;16(1-2):13–26. doi: 10.1007/BF00484381. [DOI] [PubMed] [Google Scholar]
- Fan C. L., Hall L. M., Skrinska A. J., Brown G. M. Correlation of guanosine triphosphate cyclohydrolase activity and the synthesis of pterins in Drosophila melanogaster. Biochem Genet. 1976 Apr;14(3-4):271–280. doi: 10.1007/BF00484766. [DOI] [PubMed] [Google Scholar]
- Farkas W. R. Effect of diet on the queuosine family of tRNAs of germ-free mice. J Biol Chem. 1980 Jul 25;255(14):6832–6835. [PubMed] [Google Scholar]
- Farkas W. R., Singh R. D. Guanylation of transfer ribonucleic acid by a cell-free lysate of rabbit reticulocytes. J Biol Chem. 1973 Nov 25;248(22):7780–7785. [PubMed] [Google Scholar]
- Gregg T G, Smucker L A. Pteridines and Gene Homologies in the Eye Color Mutants of DROSOPHILA HYDEI and DROSOPHILA MELANOGASTER. Genetics. 1965 Nov;52(5):1023–1034. doi: 10.1093/genetics/52.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins W. D., Farkas W. R. Guanylation of transfer RNA by rabbit reticulocytes. Biochim Biophys Acta. 1970 Jul 16;213(1):77–89. doi: 10.1016/0005-2787(70)90009-2. [DOI] [PubMed] [Google Scholar]
- Hosbach H. A., Kubli E. Transfer RNA in aging Drosophila: II. Isoacceptor patterns. Mech Ageing Dev. 1979 Apr;10(1-2):141–149. doi: 10.1016/0047-6374(79)90077-0. [DOI] [PubMed] [Google Scholar]
- Howes N. K., Farkas W. R. Studies with a homogeneous enzyme from rabbit erythrocytes catalyzing the insertion of guanine into tRNA. J Biol Chem. 1978 Dec 25;253(24):9082–9087. [PubMed] [Google Scholar]
- Jackson C. D., Irving C. C., Sells B. H. Changes in rat liver transfer RNA following growth hormone administration and in regenerating liver. Biochim Biophys Acta. 1970 Sep 17;217(1):64–71. doi: 10.1016/0005-2787(70)90123-1. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B., Calviño J. F., Murphy J. B., Warner C. K. Mechanism of suppression in Drosophila. H. Enzymatic discrimination of wild-type and suppressor tyrosine transfer RNA. J Mol Biol. 1975 Mar 25;93(1):89–97. doi: 10.1016/0022-2836(75)90362-9. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B. Mechanism of suppression in Drosophila. VII. Correlation between disappearance of an isoacceptor of tyrosine tRNA and activation of the vermilion locus. Nucleic Acids Res. 1978 Jul;5(7):2391–2404. doi: 10.1093/nar/5.7.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Kuchino Y., Nihei K., Nishimura S. Distribution of the modified nucleoside Q and its derivatives in animal and plant transfer RNA's. Nucleic Acids Res. 1975 Oct;2(10):1931–1939. doi: 10.1093/nar/2.10.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Oashi Z., Harada F., Nishimura S., Oppenheimer N. J., Crain P. F., Liehr J. G., von Minden D. L., McCloskey J. A. Structure of the modified nucleoside Q isolated from Escherichia coli transfer ribonucleic acid. 7-(4,5-cis-Dihydroxy-1-cyclopenten-3-ylaminomethyl)-7-deazaguanosine. Biochemistry. 1975 Sep 23;14(19):4198–4208. doi: 10.1021/bi00690a008. [DOI] [PubMed] [Google Scholar]
- Katze J. R., Beck W. T. Administration of queuine to mice relieves modified nucleoside queuosine deficiency in Ehrlich ascites tumor tRNA. Biochem Biophys Res Commun. 1980 Sep 16;96(1):313–319. doi: 10.1016/0006-291x(80)91216-4. [DOI] [PubMed] [Google Scholar]
- Katze J. R., Farkas W. R. A factor in serum and amniotic fluid is a substrate for the tRNA-modifying enzyme tRNA-guanine transferase. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3271–3275. doi: 10.1073/pnas.76.7.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze J. R. Q-factor: a serum component required for the appearance of nucleoside Q in tRNA in tissue culture. Biochem Biophys Res Commun. 1978 Sep 29;84(2):527–535. doi: 10.1016/0006-291x(78)90201-2. [DOI] [PubMed] [Google Scholar]
- Krivi G. G., Brown G. M. Purification and properties of the enzymes from Drosophila melanogaster that catalyze the synthesis of sepiapterin from dihydroneopterin triphosphate. Biochem Genet. 1979 Apr;17(3-4):371–390. doi: 10.1007/BF00498976. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Kasai H., Nihei K., Nishimura S. Biosynthesis of the modified nucleoside Q in transfer RNA. Nucleic Acids Res. 1976 Feb;3(2):393–398. doi: 10.1093/nar/3.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landin R. M., Boisnard M., Petrissant G. Correlation between the presence of tRNA His GUG and the erythropoietic function in foetal sheep liver. Nucleic Acids Res. 1979 Nov 24;7(6):1635–1648. doi: 10.1093/nar/7.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini M., Mushinski J. F. Transfer ribonucleic acids from eleven immunoglobulin-secreting mouse plasmacytomas. Constant and variable chromatographic profiles compared with the myeloma protein sequences. Biochim Biophys Acta. 1979 Apr 26;562(2):252–270. doi: 10.1016/0005-2787(79)90171-0. [DOI] [PubMed] [Google Scholar]
- McKinnon R. D., Wosnick M. A., White B. N. The role of the guanine insertion enzyme in O-biosynthesis in Drosophila melanogaster. Nucleic Acids Res. 1978 Dec;5(12):4865–4876. doi: 10.1093/nar/5.12.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara A. L., Smith D. W. The function of the histidine tRNA isoaccepting species in hemoglobin synthesis. J Biol Chem. 1978 Sep 10;253(17):5964–5970. [PubMed] [Google Scholar]
- Okada N., Shindo-Okada N., Sato S., Itoh Y. H., Oda K., Nishimura S. Detection of unique tRNA species in tumor tissues by Escherichia coli guanine insertion enzyme. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4247–4251. doi: 10.1073/pnas.75.9.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C. E., Penhoet E. E. Chromatographic and functional comparison of human placenta and HeLa cell tyrosine transfer ribonucleic acids. Biochemistry. 1976 Oct 19;15(21):4649–4654. doi: 10.1021/bi00666a016. [DOI] [PubMed] [Google Scholar]
- Owenby R. K., Stulberg M. P., Jacobson K. B. Alteration of the Q family of transfer RNAs in adult Drosophila melanogaster as a function of age, nutrition, and genotype. Mech Ageing Dev. 1979 Sep;11(2):91–103. doi: 10.1016/0047-6374(79)90027-7. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Stankiewicz A. F., Rizi H. L., Weisz C., DiLauro M. N., Pike D., Chen C. Y., Chen E. Y. Comparison of rat liver and Walker 256 carcinosarcoma tRNAs. Nucleic Acids Res. 1979 Feb;6(2):673–688. doi: 10.1093/nar/6.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler J. E., Yim J. J., Grell E. H., Jacobson K. B. Developmental changes of sepiapterin synthase activity associated with a variegated purple gene in Drosophila melanogaster. Biochem Genet. 1979 Feb;17(1-2):197–206. doi: 10.1007/BF00484485. [DOI] [PubMed] [Google Scholar]
- White B. N., Tener G. M. Activity of a transfer RNA modifying enzyme during the development of Drosophila and its relationship to the su(s) locus. J Mol Biol. 1973 Mar 15;74(4):635–651. doi: 10.1016/0022-2836(73)90054-5. [DOI] [PubMed] [Google Scholar]
- Wilson T. G., Jacobson K. B. Isolation and characterization of pteridines from heads of Drosophila melanogaster by a modified thin-layer chromatography procedure. Biochem Genet. 1977 Apr;15(3-4):307–319. doi: 10.1007/BF00484462. [DOI] [PubMed] [Google Scholar]
- Wilson T. G., Jacobson K. B. Mechanism of suppression in Drosophila. V. Localization of the purple mutant of Drosophila melanogaster in the pteridine biosynthetic pathway. Biochem Genet. 1977 Apr;15(3-4):321–332. doi: 10.1007/BF00484463. [DOI] [PubMed] [Google Scholar]
- Yim J. J., Brown G. M. Characteristics of guanosine triphosphate cyclohydrolase I purified from Escherichia coli. J Biol Chem. 1976 Aug 25;251(16):5087–5094. [PubMed] [Google Scholar]
- Yim J. J., Grell E. H., Jacobson K. B. Mechanism of suppression in Drosophila: control of sepiapterin synthase at the purple locus. Science. 1977 Dec 16;198(4322):1168–1170. doi: 10.1126/science.412253. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Miyazawa T., Iitaka Y., Yamaizumi Z., Kasai H., Nishimura S. Three-dimensional structure of hyper-modified nucleoside Q located in the wobbling position of tRNA. Nature. 1979 Nov 1;282(5734):107–109. doi: 10.1038/282107a0. [DOI] [PubMed] [Google Scholar]


