Abstract
The Deinococcus radiodurans bacterium exhibits an extreme resistance to ionizing radiation. Here, we investigated the in vivo role of DdrB, a radiation-induced Deinococcus specific protein that was previously shown to exhibit some in vitro properties akin to those of SSB protein from E. coli but also to promote annealing of single stranded DNA. First we report that the deletion of the C-terminal motif of the DdrB protein, which is similar to the SSB C-terminal motif involved in recruitment to DNA of repair proteins, did neither affect cell radioresistance nor DNA binding properties of purified DdrB protein. We show that, in spite of their different quaternary structure, DdrB and SSB occlude the same amount of ssDNA in vitro. We also showed that DdrB is recruited early and transiently after irradiation into the nucleoid to form discrete foci. Absence of DdrB increased the lag phase of the extended synthesis-dependent strand annealing (ESDSA) process, affecting neither the rate of DNA synthesis nor the efficiency of fragment reassembly, as indicated by monitoring DNA synthesis and genome reconstitution in cells exposed to a sub-lethal ionizing radiation dose. Moreover, cells devoid of DdrB were affected in the establishment of plasmid DNA during natural transformation, a process that requires pairing of internalized plasmid single stranded DNA fragments, whereas they were proficient in transformation by a chromosomal DNA marker that integrates into the host chromosome through homologous recombination. Our data are consistent with a model in which DdrB participates in an early step of DNA double strand break repair in cells exposed to very high radiation doses. DdrB might facilitate the accurate assembly of the myriad of small fragments generated by extreme radiation exposure through a single strand annealing (SSA) process to generate suitable substrates for subsequent ESDSA-promoted genome reconstitution.
Keywords: Deinococcus radiodurans, DdrB, single-strand binding protein SSB, ESDSA, SSA, DNA transformation
1- Introduction
The bacterium D. radiodurans is known for its exceptional ability to withstand the lethal effects of DNA damaging agents and to reconstruct a functional genome from hundreds of radiation-induced chromosomal fragments. This resistance to extremely high doses of ionizing radiation is thought to result from a combination of active and passive mechanisms, such as efficient protection of proteins against oxidation, efficient DNA double strand break repair, and a condensed nucleoid structure favoring genome reassembly (for review see [1–4]).
Transcriptome analysis identified a small subset of Deinococcus genus-specific genes up-regulated in response to desiccation and ionizing radiation exposure and required for radioresistance [5]. Among these genes, ddrB encodes a protein that was shown to bind in vitro to ssDNA but not to duplex DNA and to exhibit some biochemical properties similar to those of the E. coli SSB protein, leading to the proposal that DdrB is a specialized SSB-like protein required for recovery from extreme ionizing radiation exposure [6,7].
The primary activity of SSB proteins is to bind with high affinity to single-stranded DNA independently of the DNA sequence, protecting it from degradation and from the formation of secondary structures [8]. In addition SSB proteins, through their conserved C-terminal region, also act as recruiting scaffold for targeting other proteins to DNA (for review, see [8]). Interestingly, the recent elucidation of the Deinococcus geothermalis DdrB structure [7] has shown that the structured residues of the D. geothermalis protein form a pentameric ring and revealed a novel fold that is structurally distinct from the OB-folds (oligonucleotide/oligosaccharide-binding domain) generally used by proteins to bind ssDNA [9]. This structured domain of DdrB is followed by a putative flexible arm ending in the deinococcal DdrB protein with the EETPF motif, very similar to the highly conserved C-terminal motif of the bacterial SSB proteins (DDIPF in E. coli SSB and DDLPF in D. radiodurans SSB) [8], raising the question whether the disordered C-terminal region of DdrB plays a role in radioresistance.
It has been shown recently that DdrB stimulates annealing of complementary single-stranded DNA in vitro and it has been suggested that this protein might be involved in the RecA-independent single strand annealing (SSA) DNA double strand break repair pathway [10]. SSA has been proposed to occur at early times in irradiated deinococcal cells to account for the observation that part of the radiation-induced double strand breaks can be mended in a recombination-defective recA mutant [10,11]. After exonuclease-catalysed resection of DNA ends, single-stranded overhangs are produced. If the overhangs contain complementary sequences, they can anneal. Then, single-stranded regions present in the sealed fragments are filled in by DNA synthesis. Single strand annealing activity was also shown to be required for establishment of plasmid DNA to pair internalized complementary plasmid DNA fragments in order to reconstitute a circular replicon in naturally transformable bacteria such as Streptococcus pneumoniae and Bacillus subtilis, raising the question of a putative role of the DdrB protein in D. radiodurans plasmid transformation [12,13].
Both the ss-DNA binding and the single strand annealing activities of DdrB protein might also play important roles in the extended synthesis-dependent strand annealing (ESDSA) pathway, a major DNA double strand break repair process in which long tracts of newly synthesized single-stranded DNA are generated [14]. According to the ESDSA model, after an initial phase involving processing of the ends of the DNA fragments to produce 3′ overhangs, the resected fragments invade other fragments with overlapping sequence homology to prime DNA synthesis. The newly synthesized strands then dissociate and anneal with each other to form large genome fragments that subsequently recombine to restore circular chromosomes [14,15].
In this study, we aimed to dissect further the role of DdrB in D. radiodurans radioresistance. We showed that the C-terminal disordered arm of DdrB was not required for radioresistance or binding to ss-DNA. We report that inactivation of DdrB protein did not affect the efficiency of fragment assembly and the rate of DNA synthesis but only delayed these two processes. Moreover, the DdrB protein was recruited early and transiently to the nucleoid during post-irradiation incubation before genome reconstitution and accompanying DNA synthesis took place. We also showed that DdrB is involved in transformation by plasmid DNA but not by chromosomal DNA suggesting an important role of DdrB in pairing of plasmid single-stranded DNA fragments required for the establishment of the plasmid in the recipient cell.
Taking into account all the results, we propose that a single strand annealing process, requiring the DdrB protein, plays a major role in the early step of DNA double strand break repair when a myriad of small fragments are generated by extreme radiation exposure.
2. Materials and Methods
2.1. Bacterial strains, plasmids, oligonucleotides, media
Bacterial strains and plasmids are listed in Table 1. The Escherichia coli strain DH5α was used as the general cloning host, strain SCS110 was used to propagate plasmids prior to introduction into D. radiodurans via transformation [16] and the Rosetta strain was used to express proteins before purification. All D. radiodurans strains were derivatives of the wild-type strain R1 ATCC 13939. Alleles ΔddrBΩkan, ddrBΔ5::kan, ddrBΔ41::kan,and, ddrB::spa::cat were constructed by the tripartite ligation method [17]. The genetic structure and the purity of the mutants were checked by PCR. All oligonucleotides used are listed in Table S1.
Table 1.
Bacterial strains and plasmids
| Bacterial strains | Description | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | supE44 ΔlacU(φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | laboratory stock |
| SCS110 | endA dam dcm supE44 Δ (lac-proAB) (F’traD36 proAB lacIqZΔM15) | laboratory stock |
| Rosetta | F− ompT hsdSB(RB− mB−) gal dcm λ(DE3) pRARE (camR) | Novagen |
| D. radiodurans | ||
| R1 | ATCC 13939 | laboratory stock |
| GY11733 | rpoBΔ1250–1258, [RifR] | [17] |
| GY11944 | R1/p11520 | [38] |
| GY12830 | ddrB::spa::cat | this work |
| GY12835 | ΔddrBΩkan | this work |
| GY12966 | ΔrecOΩhph | [36] |
| GY12968 | ΔrecAΩkan | [36] |
| GY13378 | ΔddrBΩkan/p11520 | this work |
| GY13384 | ΔddrBΩkan/p13421(ddrB+) | this work |
| GY13928 | ddrBΔ5::kan | this work |
| GY13930 | ddrBΔ41::kan | this work |
|
| ||
| plasmids | ||
|
| ||
| p11086 | Source of kanamycin cassette in D. radiodurans | laboratory stock |
| p11520 | Shuttle vector E. coli/D radiodurans, SpcR | laboratory stock |
| P11559 | Shuttle vector E. coli/D radiodurans, SpcR | laboratory stock |
| p12723 | Source of flag-tag chloramphenicol cassette | [19] |
| p13421 | p11520 with a PCR fragment encoding ddrB | this work |
| pET21a | T7 expression vector | Novagen |
| pEAW571 | pET21a (NdeI/HindIII) with a PCR fragment encoding ddrB | [6] |
| pEAW588 | pET21a (NdeI/HindIII) with a PCR fragment encoding ddrBΔ41 | this work |
D. radiodurans strains were grown at 30°C in TGY2X (1% tryptone, 0.2% dextrose, 0.6% yeast extract) or in TGYA (0,5% tryptone, 0,2% dextrose, 0,15 % yeast extract) or plated on TGY1X containing 1,5% agar and E. coli strains were grown at 37°C in Luria Broth. When necessary, media were supplemented with the appropriate antibiotics used at the following final concentrations: kanamycin, 6 μg/mL; chloramphenicol, 3 μg/mL; hygromycin, 50 μg/mL; rifampicin, 25 μg/mL; spectinomycin, 75 μg/mL for D. radiodurans and 40 μg/mL for E. coli; ampicillin, 100 μg/mL for E. coli.
2.2. Transformation of D. radiodurans
To prepare competent cells, exponentially growing bacteria were harvested by centrifugation, resuspended at 5×108 cells/mL in TGY2X medium supplemented with 30 mM CaCl2 and 10% (V/V) glycerol, and stored at −80°C. For transformation, aliquots (100 μL) of competent cells were thawed on ice and mixed with an equal volume of TGY-CaCl2 before DNA (genomic DNA or plasmid DNA) was added. After 20 min at 0°C and 60 min at 30°C, 800 μL of TGY2X were added and the cells were incubated for a further 5 h to allow expression of rifampicin (transformation by genomic DNA) or spectinomycin (transformation by plasmid DNA) resistance. Diluted samples were plated on TGY plates containing appropriate antibiotics.
2.3. Expression and purification of D. radiodurans DdrB and DdrBΔ41 proteins
Wild type DdrB and mutant DdrBΔ41 proteins were expressed in the Rosetta host (Novagen) from plasmids pEAW571 and pEAW588, respectively. DdrB and DdrBΔ41 proteins were purified as described previously [6] except for the following variations introduced in the protocol of purification of DdrBΔ41 protein. The DdrBΔ41 protein was eluted from the butyl Sepharose (Amersham Biosciences) column after the flow through during the wash using R buffer (20 mM Tris-Cl 80% cations, 100 μM EDTA, and 10% w/v glycerol) containing 1 M NH4(SO4)2. The protein solution was then dialyzed against R buffer and loaded on a SP Sepharose (Amersham) column. DdrBΔ41 was recovered in the flow through. Fractions containing > 99% pure protein (as estimated from SDS-PAGE) were pooled, snap frozen in liquid nitrogen, and stored at −80°C.
2.4. Fluorescence titration
Titrations monitoring the tryptophan fluorescence of D. radiodurans DdrB proteins were performed with an SLM 8000 spectrofluorometer. The excitation wavelength was 295 nm (2 nm band-pass), and fluorescence was monitored at 350 nm (4 nm band-pass). The experiments were performed at 25°C. A 1.5 ml solution of 1μM DdrB (0.2μM in pentamer) in 10 mM Tris pH 8, 1 mM EDTA and 1 or 300 mM NaCl in a 4.0-ml quartz fluorescence cell was constantly stirred with a magnetic stir bar while the nucleic acid solution in the same buffer containing an identical concentration of the protein was titrated in 20- to 30-μl aliquots (increasing with total volume), corresponding each time to a DNA concentration increase of 5 nucleotides per protein complex. The excitation shutter remained closed during a 2-minute equilibration of the sample after each aliquot addition and was opened only for 7 to 9 s, allowing two 3 s (integrated time) data acquisitions, in order to minimize photobleaching of the sample. The fluorescence measurements were corrected for photobleaching and inner filter effects using the following equation: Fi,corr = Fi,obs * (f0/fi) * (1/C), where Fi,obs and Fi,corr are the observed (uncorrected) and corrected fluorescence readings, respectively, after the ith aliquot of nucleic acid, f0 is the initial fluorescence of the control protein solutions, and fi the fluorescence of the control solution which has been titrated by a solution devoid of DNA but has been exposed to the excitation beam for the same length of time after the ith aliquot (photobleaching correction). Photobleaching was determined under each set of buffer conditions that were used in the actual titrations. The inner filter effect was corrected using C = (1 − 10−Ai)/2.303Ai, where Ai is the sum of the OD295nm of DdrB and of the DNA after the ith aliquot addition. Data points were analyzed using the model of Schwarz and Watanabe with n (binding site), qK (cooperative binding affinity) and Qf (fluorescence quench) as parameters [18]. The poly(dT) oligodeoxynucleotides were obtained from Midland Certified Reagent Co. (Midland, TX). Concentrations were determined using the following extinction coefficients: ε280nm of 26500 M−1cm−1 for DdrB, and ε265nm of 8600 M−1cm−1 for poly(dT).
2.5. Electrophoretic mobility shift assay (EMSA)
The 5′-6FAM-OCN324 fluorescent oligonucleotide (50μM in nucleotides) was incubated with increasing concentrations of DdrB or DdrBΔ41 ranging from 10 nM to 10 μM in 50 mM Tris-Cl 80% cation (pH 7.8), 5 mM MgCl2, 20 mM KCl, and 3% glycerol for 10 minutes at 37°C. The samples were then run on a 4% native PAGE for 2 hours at 150V at 4°C. The positions of the fluorescent oligonucleotides were analyzed using a Typhoon scanner (GE Healthcare).
2.6. Treatment of D. radiodurans with γ-irradiation
Exponential cultures were concentrated to an A650 = 20 in TGY2X and irradiated on ice with a 137Cs irradiation system (Institut Curie, Orsay, France) at a dose rate of 41.8 Gy/min. Following irradiation, diluted samples were plated on TGY plates. Colonies were counted after 3–4 days incubation at 30°C.
2.7. Western blot analysis
Non-irradiated or irradiated (3.8 kGy) cultures were diluted in TGY2X to an A650 = 0.2 and incubated at 30°C. At different post-irradiation times, 20 mL of culture were centrifuged. The pellets were resuspended in 150 μL of SSC1X buffer and the cells were disrupted as described previously [19]. After centrifugation, 5 μg of the cell extracts were subjected to electrophoresis through a 15% SDS-PAGE and the proteins were transferred on to a PVDF (polyvinylidene difluoride) membrane. The membrane was blocked with TBS containing 5% milk, 0.05% Tween 20 before being incubated with a 1:5000 dilution of monoclonal mouse anti-flag antibodies (Sigma-Aldrich) in TBS containing 3% milk, 0.05% Tween 20 overnight at 4°C. After extensive washes in TBS- 0.05% Tween 20, the membrane was incubated with anti-mouse IgG alkaline phosphatase conjugate used as secondary antibody and revealed by a colorimetric reaction.
2.8. Kinetics of DNA repair measured by pulse-field gel electrophoresis
Non-irradiated or irradiated (3.8 kGy) cultures were diluted in TGY2X to an A650 = 0.2 and incubated at 30°C. At different post-irradiation recovery times, culture aliquots (5mL) were removed to prepare DNA plugs as described previously [20]. The embedded cells plugs were lysed, treated with NotI restriction enzyme and subjected to pulsed field gel electrophoresis as described previously [21].
2.9. Rate of DNA synthesis measured by DNA pulse labelling
The rate of DNA synthesis was measured according to a modified protocol from Zahradka et al [14]. Exponential cultures, grown in TGYA, were concentrated to an A650 = 20 in TGYA and irradiated as described previously. Non-irradiated or irradiated cultures (3.8 kGy) were diluted in TGYA to an A650 = 0.2 and incubated at 30°C. At different times 0.5mL samples were taken and mixed with 0.1mL pre-warmed TGYA containing 4.8μCi [methyl-3H]thymidine (PerkinElmer, specific activity 70–90 Ci/mmol). Radioactive pulses of 15 min were terminated by addition of 2 mL ice-cold 10% TCA. Samples were kept on ice for at least 1h, and then collected by vacuum filtration onto Whatman GF/C filters followed by washing twice with 5mL 5% TCA and twice with 5mL 96% ethanol. Filters were dried for 10 min under a heat source and placed in 4 mL scintillation liquid. The precipitated counts were measured in a liquid scintillation counter (Packard, TRI- carb 1600 TR).
2.10. Immunofluorescence labeling and microscopy
Bacterial strains were grown exponentially in TGY 2X, concentrated to an A650 = 20 in TGY 2X, and irradiated as described above. Non-irradiated or irradiated cultures (3.8 kGy) were diluted in TGY 2X to an A650 = 0.2 and incubated at 30°C. Aliquots of 0.5 ml of the exponential phase culture were taken and fixed by addition of 1/10 volume of 37% formaldehyde in the culture medium and incubation 2h at 4°C. The cell pellet was subsequently washed once in 1 x PBS. In order to permeabilize the cell envelope, the cells were treated with 2 mg/ml lysozyme for 30 min at 37°C followed by incubation with 0.1 % Triton X-100 in PBS for 5 min at room temperature. Finally, the cells were washed in PBS and resuspended in 40 μl of PBS. A 3 μl aliquot was applied to a poly-L-lysine pre-treated slide spot, allowed to air dry and fixed by incubating in 4% formaldehyde or 20 min at 37°C. Cells were then blocked in 2% BSA in PBS-T (0.05% Tween 20 in PBS) and incubated for 2 hours at 37°C with a monoclonal mouse anti-FLAG antibody (Sigma-Aldrich) diluted 1/700 in blocking solution. After 20 min washing in PBS-T, the cells were incubated for 1 hour at 37°C with a fluorescein isothyocyanate (FITC) conjugated goat anti-mouse antibody (Jackson Immunoresearch Laboratories) diluted 1/250 in blocking solution and washed for 20 min in PBS-T. Cells were finally stained with DAPI 10 μg/ml for 10 min at room temperature. After a final wash in PBS-T slides were mounted using fluoromount G as a mounting medium (Fluoprobes). The stained cells were observed using a Leica DM RXA microscope. Images were captured with a CDD camera 5 MHz Micromax 1300Y (Roper Instruments). The final reconstructed images were obtained by deconvoluting Z-series with the Metamorph software (Universal Imaging Corp.).
3. Results
3.1. The C-terminal domain of DdrB is not required for radioresistance
It has been shown that the disordered C-terminal region of the E. coli or the B. subtilis SSB proteins is essential for mediating interactions with numerous proteins involved in DNA metabolism (for review, see [8]). To test a possible role of the disordered C-terminal domain of DdrB in radioresistance, we determined whether deletion of the C-terminal 5 (DdrBΔ5) or 41 (DdrBΔ41) residues of DdrB protein sensitizes the cells to γ-irradiation. For this purpose, we constructed mutants expressing the truncated forms of DdrB protein as well as a ΔddrB deletion mutant by allelic replacement of the wild type ddrB gene.
The ΔddrB mutant displayed a radiosensitive phenotype showing an increased sensitivity to γ-rays, as compared to the wild type at doses exceeding 5 kGy (Figure 1, [5]). Expression in trans of the DdrB protein inΔddrB bacteria restored a wild-type level of γ-ray resistance, indicating that the radio-sensitive phenotype was due to the lack of the DdrB protein and not to a polar effect of the construct on the expression of downstream genes (Figure S1). Moreover, strains expressing the truncated DdrB proteins were as resistant to γ-rays as the wild type (Figure 1, panel A), indicating that the C-terminal domain of DdrB is not essential for radioresistance and, likely, plays no crucial role in the recruitment of repair proteins at the sites of DNA lesions.
Figure 1. The C-terminal domain of DdrB is dispensable for radioresistance and binding to single-stranded DNA.
(A) Radioresistance of mutants expressing truncated or C-terminal fused DdrB proteins. Survival curves of wild-type (filled squares), GY12835: ΔddrB (filled circles), GY13928: ddrBΔ5 (open triangles), GY13930: ddrBΔ41 (open circles) are shown. (B) Binding affinities of DdrB and DdrBΔ41 proteins to ssDNA were measured by electrophoretic mobility shift assay. Increasing protein concentrations (from 10 nM to 10 μM) were incubated with 50 μM fluorescent oligonucleotides and loaded onto a native 4% PAGE. Protein to total nucleotides ratios are indicated on the top of each lane.
Then, we tested the ability of purified DdrBΔ41 protein to bind single-stranded DNA using a gel mobility shift assay. As shown in Figure 1 (panel B), DdrBΔ41 interacted with the ssDNA substrate at the same protein to DNA ratio as the wild-type protein. The difference of migration of the ssDNA bound DdrBΔ41 versus the full length protein is most likely due to the smaller size of the truncated protein. However the fact that DdrBΔ41 is expected to present a more globular shape than wild-type DdrB could also contribute to this difference of gel shift. Thus, the loss of the 41 last residues of DdrB, which constitute its C-terminal unstructured tail, does not affect DdrB single-stranded DNA binding properties.
3.2. DdrB occludes the same amount of ssDNA as D. radiodurans SSB
To characterize DdrB ssDNA binding properties and compare them with those of D. radiodurans SSB [22], we measured by inverse fluorescence titration D. radiodurans DdrB interaction with poly(dT). As previously observed with SSB proteins [23], we could verify that DdrB presents some tryptophan fluorescence, which is quenched by the addition of ssDNA. The linear decrease in relative fluorescence indicates stoichiometric binding of DdrB to poly(dT) (Figure 2). This allows the determination of the amount of nucleotides occluded by each protein complex when saturation by DNA is achieved. The stoichiometry (n) of nucleotides bound per DdrB pentamer depends on the salt concentration and varies from n = 45 ± 4 nucleotides per pentamer under low salt condition (0.001 M NaCl) to n = 53 ± 3 nucleotides per pentamer under high salt condition (0.3 M NaCl). The tryptophan fluorescence change (Qf) upon saturation with ssDNA was 58% ± 5% at 1 mM NaCl and 64 % ± 4% at 0.3 M NaCl. Thus, as has already been reported for various SSB proteins [22, 23], DdrB interaction with ssDNA presents multiple binding modes that are dependent upon the salt concentration. Furthermore, Witte et al. [22] previously reported stoichiometries of n = 47.5 nucleotides at 1 mM NaCl and n = 53.9 nucleotides at 0.3 M NaCl for D. radiodurans SSB (dimers) on poly(dT), indicating that D. radiodurans DdrB pentamers and SSB dimers interact with equivalent amounts of ssDNA under the same salt concentrations.
Figure 2. Determination of the stoechiometry of nucleotides bound per DdrB pentamer.

The size of the DdrB binding site was determined by fluorescence titrations of DdrB with poly(dT) at different salt concentrations. Reactions were carried out at 25°C with 1 μM D. radiodurans DdrB (0.2 μM in pentamer) in 10 mM Tris-HCl pH 8, 1 mM EDTA and 1 mM NaCl (circles) or 300 mM NaCl (triangles). The data points represent the mean value of three independent experiments. Solid lines represent theoretical binding isotherms that best fit data points. The binding isotherms were calculated using the model of Schwarz and Watanabe [18] with n (binding site), qK (cooperative binding affinity) and Qf (fluorescence quench) as parameters. Circle isotherm parameters: n = 45 ± 4, Qf = 58% ± 5%, qK = 7.5×109 M−1. Triangle isotherm parameters: n = 52 ± 2, Qf = 64 % ± 4%, qK = 4.2×109 M−1. Control experiments performed in the same conditions with E. coli SSB provided equivalent results to those previously reported in [22, 23 ] (data not shown).
3.3. ESDSA-associated DNA synthesis is delayed in a ΔddrB mutant
Given the strong affinity of DdrB protein for single-stranded DNA, we tested whether a DdrB deficiency would affect ESDSA, a major DNA double strand break repair process in which extensive regions of single-stranded DNA are generated [14,15]. For this purpose, we examined whether the ΔddrB mutant was affected in the massive DNA synthesis which took place during the ESDSA process. In parallel, we examined the kinetics of the reassembly of broken DNA fragments. Cells were exposed to 3.8 kGy γ-irradiation, a dose that introduces approximately 100 DNA double strand breaks per genome equivalent in a D. radiodurans cell [24] but only marginally affects the survival of ΔddrB bacteria (Figure 1). de novo DNA synthesis was measured by labelling DNA with a 15 min 3H-thymidine pulse at different post-irradiation times and recovery from DNA damage was monitored by the appearance of the complete pattern of 11 resolvable genomic DNA fragments generated by NotI digestion.
As seen in Figure 3, the wild type parental strain exhibited a classical biphasic DNA repair kinetics in which, after a lag, fragment assembly took place quickly (panel B) and this process was accompanied by massive DNA synthesis (panel A). In the ΔddrB mutant, the lag before DNA synthesis increased by about one hour (Figure 3, panel A) but the rate of DNA synthesis was roughly the same as in the wild type. This delay in DNA synthesis was, as expected, associated with an increased lag for the beginning of fragment reassembly (Figure 3, panel B). This suggests that DdrB is involved in a very early step of DNA double strand break repair preceding ESDSA associated DNA synthesis but not in annealing of the long tracts of newly synthesized single-stranded DNA required for genome reconstitution by ESDSA pathway.
Figure 3. DNA synthesis in ΔddrB mutant and DNA repair.
(A) Rate of DNA synthesis in wild type and ΔddrB mutant. Incorporation of [3H]thymidine during 15 min pulse labelling measures the global rate of DNA synthesis in 3.8 kGy irradiated (filled circles) and unirradiated (open circles) bacteria. (B) Kinetics of double strand break repair in wild type and ΔddrB mutant followed by pulse-field gel electrophoresis (PFGE). PFGE shows NotI treated DNA from unirradiated cells (lane pre-irradiation) and from irradiated cells (3.8 kGy) immediately after irradiation (0) and at the indicated incubation times (hours). The data shown are from a single experiment, and matched those obtained in the two other independent assays.
3.4. DdrB is transiently recruited into the nucleoid upon induction of DNA double strand breaks
To confirm the early DdrB involvement in DNA double strand break repair, we determined the kinetics of DdrB recruitment to the D. radiodurans nucleoid in cells recovering from DNA damage. For this purpose, the chromosomal ddrB gene was replaced by a tagged gene expressing a DdrB-SPA protein. This SPA tag [25] contains a 3 × FLAG epitope that is recognized with high affinity by commercially available antibodies that can be used for western blot analyses and immuno-fluorescence microscopy. Cells expressing the DdrB-SPA protein were as resistant to γ-rays as the wild type cells indicating that the tagged protein is functional (Figure S1). Western blot analysis showed that the DdrB-SPA protein was present at a basal level before irradiation and was induced when cells were exposed to a dose of 3.8 kGy γ-irradiation, the maximum of induction being observed after 60 min post-irradiation incubation corresponding to about four fold increase over the basal level (Figure S2).
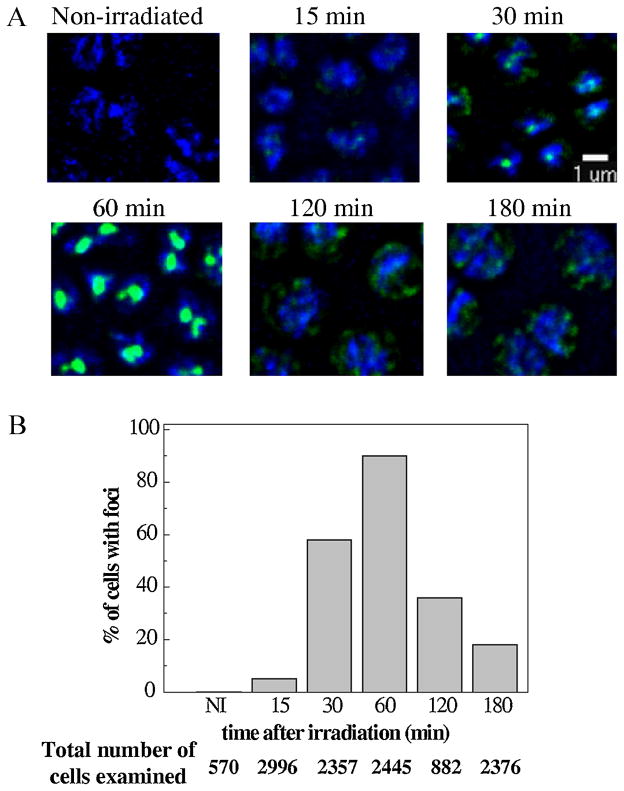
The subcellular localization of DdrB-SPA was investigated by immuno-fluorescence microscopy (Figure 4). In the absence of irradiation, the DdrB-SPA protein concentration was below the detection threshold and was hardly detectable in situ. At 15 min after irradiation, fluorescent signals were already detectable in the nucleoid of 5% of the cells. Rather than diffuse fluorescence, the signals appeared to be concentrated in few small spots. The proportion of cells containing fluorescence signals inside the nucleoid increased with the post-irradiation incubation time to reach 58% at 30 min and 90% at 60 min (Figure 4, panels A and B). The spots also became quite large and intense at the 60 min timepoint, suggesting an important increase in the concentration of the tagged DdrB protein recruited to the nucleoid. The recruitment of the DdrB-SPA protein to the nucleoid after irradiation took place early and was transient as, with further incubation, the proportion of cells containing fluorescent signals dropped to 25% at 120 min and 17% at 180 min. This decrease was not due to a decay of the DdrB-SPA protein, as shown by western blot analysis (Figure S2) but the protein was detectable as a diffuse signal outside the nucleoid (Figure 4). The kinetics of DdrB-SPA recruitment to DNA confirms an involvement of DdrB in an early process preceding massive DNA synthesis and extensive fragment reassembly.
Figure 4. Kinetics of recruitment of DdrB to nucleoid after γ-irradiation.
(A) GY12830 cells (ddrB::spa) exposed to 3.8 kGyγ-irradiation were incubated for the indicated time periods and probed with anti-Flag primary antibody followed by a FITC secondary antibody (green) and with DAPI (blue). Overlays of the FITC and DAPI images are shown. As a control a picture of non-irradiated exponentially growing ddrB::spa bacteria is also shown. The scale bar 1μm is applicable to all the cells in the panel. (B) Number of cells examined and % of cells with nuclear FITC florescence signals for each condition.
3.5. The DdrB protein plays a major role in transformation by plasmid DNA
Establishment of plasmid DNA requires host single strand pairing activity to pair internalized complementary plasmid DNA fragments in order to reconstitute a circular replicon in naturally transformable bacteria such as S. pneumoniae and B. subtilis [12,13]. Since DdrB was shown to possess an in vitro single strand annealing activity [10], we tested whether a DdrB deficiency would affect plasmid transformation in D. radiodurans. For this purpose, we measured the efficiencies of transformation of wild type and ΔddrB bacteria by plasmid or chromosomal DNA. Whereas the absence of DdrB had no effect on the efficiency of transformation by chromosomal DNA, it strongly affected the efficiency of transformation by plasmid DNA that dropped 100-fold in cells devoid of DdrB as compared to the wild type (Figure 5). This reduction was not related to a defect in plasmid maintenance since, once established, the transforming plasmid was maintained at the same level in ΔddrB and in wild type cells during 30 generations of growth without selective pressure (data not shown). The absence of the disordered C-terminal region of DdrB had only a modest effect on the efficiency of transformation by plasmid DNA of the ddrBΔ5 and ddrBΔ41mutants (Figure 5). As expected, cells devoid of the RecA protein exhibited a 1000-fold reduction in the frequency of transformation by chromosomal DNA whereas the frequency of transformation by plasmid DNA was only slightly affected (4-fold reduction). The deinococcal RecO protein was previously shown to exhibit a weak in vitro single strand pairing activity compared with the E. coli RecO protein [26]. Interestingly, a deinococcal mutant devoid of the RecO protein exhibited a 6-fold reduction in plasmid transformation and a 3 fold reduction in chromosomal transformation as compared to a wild type recipient (Figure 5). These results suggest that DdrB protein plays a major role in plasmid transformation, likely through its single strand annealing activity.
Figure 5. Frequencies of plasmid and chromosomal transformation in ddrB, recA and recO mutant bacteria.
D.radiodurans bacteria were transformed with 50 ng p11559 plasmid (conferring spectinomycin resistance) (A) or chromosomal DNA purified from GY11733 (conferring rifampicin resistance) (B) and appropriate dilutions were plated on TGY plates to measure numbers of viable cells and on TGY plates supplemented with spectinomycin or rifampicin to select transformants. Transformation frequencies were expressed as the number of transformants divided by the total number of viable cells in the transformation mixture. The values obtained were normalized relative to that of the wild type strain, taken as 100. The results are the average of at least three independent experiments. The same experiments performed using 200 ng of plasmid or chromosomal DNA gave the same level of transformation inhibition in cells devoid of DdrB, RecA or RecO proteins. The frequencies of transformation of the wild type bacteria by chromosomal and plasmid DNA, used as reference 100, were 5.7 × 105 and 7.5 × 103 transformants per 108 recipient cells respectively.
4. Discussion
The D. radiodurans bacterium is known for its exceptional ability to tolerate massive DNA damage and to efficiently repair hundreds of radiation induced DNA double strand breaks. DdrB, a single-strand DNA binding protein specific to Deinococcaceae, was shown to be induced after irradiation and to be required for radioresistance [5]. In vitro, DdrB exhibits functional properties similar to those of the SSB protein [6] but also stimulates annealing of single-stranded DNA, even in the presence of SSB [10].
The recent elucidation of the structure of DdrB from Deinococcus geothermalis shows that DdrB comprises a novel fold [7] structurally distinct from the OB-fold used by all other SSB homologues to interact with ssDNA [27]. Moreover, the quaternary structures of DdrB (pentameric ring), and SSB (tetramer for E. coli SSB and dimer for D. radiodurans SSB) are very different suggesting that their modes of DNA association might be different. Namely, DdrB might bind to single-stranded DNA more tightly than SSB as suggested by measuring RecA protein binding to SSB or DdrB-coated single-stranded DNA using the RecA ATPase activity as reporter [6]. Here, we show that DdrB pentamers occlude the same amount of ssDNA as D. radiodurans SSB dimers. Furthermore, the two proteins present the same binding mode variations at low and high salt concentrations. Interestingly, DdrB and DrSSB were shown to interact in vitro [10]. This raises the possibility that the DdrB protein and the SSB protein could swap on a DrSSB/DdrB coated ssDNA filament.
The DdrB protein contains like SSB, a flexible region at its C-terminal tail ending with an EETPF motif very similar to the highly conserved C-terminus motif of the bacterial SSB proteins [6,8]. It has been shown that the disordered C-terminal region of the E. coli or the B. subtilis SSB proteins is essential for mediating interactions with numerous proteins involved in DNA metabolism (for review, see [8]). More than a dozen of B. subtilis proteins involved in DNA replication, DNA recombination, DNA replication restart and DNA repair have been shown to bind SSB and it was proposed that SSB plays a crucial role for their recruitment to DNA [28]. In E. coli, mutations within the SSB C-terminus confers temperature sensitivity [29,30] and deletion of 10 amino acids from the C-terminus renders the E. coli cells unviable [31]. In B. subtilis, deletion of 35 or 6 amino acids from the C-terminus of SSB is not lethal but mutant cells exhibit a 5 to 10 fold lower plating efficiency and are nearly as sensitive to UV-irradiation as cells devoid of RecA [28]. In this report, we show that deletion of the 41 C-terminal residues of the D. radiodurans DdrB protein does neither affect DdrBΔ41 single-stranded DNA binding properties nor radioresistance of the mutant cells. Taking into account the possibility of formation of mixed DdrB/SSB coated single-stranded DNA complexes, we cannot exclude that the presence of SSB masks the absence of the C-terminal end of DdrB in cells expressing truncated DdrB proteins. However, SSB alone, even when expressed in trans from an expression vector in addition from the chromosomal SSB gene, was unable to restore radioresistance in cells devoid of the DdrB protein (data not shown); conversely, DdrB was unable, even when overexpressed, to functionally replace SSB for cell viability (data not shown). Thus, DdrB, induced in response to γ-irradiation, may function as a specialized SSB specifically involved in DNA repair, but our results are not in favor of its involvement in the recruitment of repair proteins and assembly repair complexes at the sites of the lesions via its C-terminal tail.
The naturally transformable S. pneumoniae and B. subtilis bacteria have two single-stranded DNA-binding proteins, one essential for cell viability, SsbA, the counterpart of the E. coli SSB protein, and the other, SsbB, that is a smaller protein specifically induced during natural transformation [32]. S. pneumoniae SsbB protein was in vitro shown to bind to ssDNA with an affinity that is similar or higher than that of the SsbA protein [33] and was identified in vivo as the major protein component of the pneumococcal eclipse complex [34].
The naturally transformable D. radiodurans does not contain a second SSB, apart from the SSB-like DdrB protein. However, in this study, we have shown that DdrB is dispensable for transformation by chromosomal DNA. This suggests that the deinococcal SSB, together with RecA and a DprA homolog present in D. radiodurans, are sufficient for the protection of the incoming transforming ss-DNA. In contrast, our data indicate that DdrB is essential for efficient plasmid transformation in D. radiodurans. In B. subtilis, plasmid transformation requires RecO protein to anneal complementary plasmid ssDNA molecules in the presence of SsbA [13]. In D. radiodurans, the RecO protein seems to play only a minor role in plasmid transformation when DdrB is present in the cells, according with the low in vitro DNA single strand pairing activity of the Deinococcal RecO protein [26]. DdrB protein stimulates annealing of complementary single-stranded DNA in vitro [10]. It was also shown that D. radiodurans can be transformed by monomeric plasmid DNA [35]. Moreover, transformation by unirradiated or in vitro irradiated plasmid DNA showed no difference in efficiency between wild-type and recombination-deficient rec30 D. radiodurans bacteria [35]. Together, these results suggest that (1) RecA does not participate in repair of exogenously damaged plasmid upon transformation (2) a RecA-independent single strand annealing process may play a major role in the reconstitution of an intact double stranded circular replicon as previously shown for the establishment of plasmid DNA during transformation of B. subtilis. Taking into account all these results, we propose that DdrB might be specifically involved in plasmid transformation through its single-strand annealing activity.
Then, we investigated the involvement of DdrB in ESDSA, the major pathway of DNA double strand break repair in D. radiodurans. We found that, in cells devoid of the DdrB protein, the lag phase preceding fragment reassembly and accompanied DNA synthesis was increased but the efficiency of fragment assembly and the rate of DNA synthesis were not affected. Moreover, our data on the subcellular localization of a functional tagged DdrB protein indicate that DdrB is recruited to the nucleoid soon after irradiation, Thus, DdrB could be involved in the protection of the early-generated single-stranded DNA overhangs required, through RecA- and RadA-mediated strand invasion, to prime DNA synthesis on overlapping fragments [15]. The DdrB protein may act in conjunction with the DdrA protein, previously shown to bind in vitro to the 3′ single-stranded DNA overhangs and to protect the ends from nuclease degradation [20]. Interestingly, the concentration of DdrB inside the nucleoid begun to decrease 120 min after irradiation, suggesting that DdrB was displaced from single-stranded DNA, probably upon the RecFOR-mediated recruitement of RecA on SSB- and/or DdrB-coated DNA. This rules out an involvement of DdrB in the protection and in the annealing of the long tracts of newly synthesized DNA generated by ESDSA.
The survival of cells devoid of the DdrB protein but proficient for RecA activity was only marginally affected by gamma irradiation doses below 5 kGy. In contrast, when the cells were exposed to doses exceeding 14 kGy, their survival decreased approximately 10,000-fold as compared to the wild type, indicating that DdrB plays a key role in DNA repair only in heavily irradiated cells. This phenotype is very different from those of cells devoid of RecA, RecF, RecO or RecR that are highly sensitive to gamma irradiation. These bacteria, in contrast to the ΔddrB mutant, are totally deficient in ESDSA and exhibit a very slow and partial fragment reassembly after irradiation [36].
The possible roles of DdrB protein in DNA double strand break repair are illustrated in the model depicted in Figure 6. According to our model, after limited resection of the DNA ends, DdrB binds very rapidly to the early-generated single-stranded DNA tails and may act in conjunction with the DdrA protein to protect them from nuclease degradation. Then, DdrB through its single strand annealing activity patches together the small resected fragments. This process is particularly efficient in heavily irradiated cells in which the radiation-induced breaks are sufficiently close so that the likelihood of the occurrence of complementary single-stranded overhangs among the resected fragments is elevated. In these cells, the action of DdrB results in a reduction in the number of small DNA fragments that are poor substrate for repair via ESDSA or homologous recombination. In wild type cells, this first step of DNA repair is followed by DNA double strand break repair through the ESDSA pathway that involves a more extensive resection of the DNA ends (probably involving UvrD and RecJ activities), loading of RecA on single stranded DNA tails via the RecFOR mediator proteins, and RecA-promoted invasion of a double-stranded homologous DNA to prime Pol III- and Pol I-dependent DNA synthesis. Then, the long tracts of newly synthesized DNA, generated by ESDSA, anneal to complementary single-stranded extensions to form long DNA double-stranded intermediates which are assembled into intact circular chromosomes by RecA-mediated homologous recombination [14,15,36].
Figure 6. Model of DNA double strand break repair through SSA and ESDSA in heavily irradiated D. radiodurans cells.
Adapted from [2,15]. The fragments generated by γ-irradiation are represented in black or red. Green lines indicated DNA newly synthesized during DNA double strand break repair. For details, see the main text
The model (Figure 6) is supported by several lines of evidence: (1) a partial mending of radiation-induced DNA double strand breaks takes place in the absence of a functional RecA protein through single strand annealing (SSA) [11] (2) this RecA-independent fragment reassembly is abolished in the double ΔrecA ΔddrB mutant [10], (3) the DdrB protein possesses a single strand DNA annealing activity in vitro [10], and, in vivo, as suggested by our finding that DdrB is required for the establishment of plasmid DNA during transformation (4) the kinetics of recruitement of the DdrB protein into the nucleoid (transient localization of DdrB preceeding the beginning of DNA synthesis) and (6) the mild radiation-sensitive phenotype of DdrB-deficient bacteria, apparent only at elevated ionizing radiation doses, suggest that DdrB participates in a backup repair pathway that operates in heavily irradiated cells.
In conclusion, we propose that the DdrB protein (1) belongs to the large family of single-strand annealing proteins (SSAP) [37] including the E. coli RecT and the eukaryotic Rad52 proteins (2) is involved in plasmid transformation through its single-strand annealing activity, and (3) plays a major role in an early SSA DNA double strand break repair pathway in heavily irradiated cells.
Supplementary Material
Increased sensitivity of cells devoid of the ddrB gene and complementation ofΔddrB mutant. GY11944 (wild type/p11520, filled squares), GY13378 (ΔddrB/p11520, filled circles), GY13384 (ΔddrB/p13421:ddrB+, open triangles), GY12830 (ddrB::spa, open triangles) bacteria were exposed to γ-irradiation at doses indicated on the abscissa and cell survival was measured as described in the materials and methods.
Expression of DdrB in D. radiodurans. D. radiodurans GY12830 (ddrB::spaΩcat) cell extracts from unirradiated (pre-irradiation) or irradiated cells exposed to 3.8 kGy gamma-irradiation and incubated during the indicated periods (min) were subjected to 15% SDS-PAGE and analyzed by western blot with anti-FLAG antibodies. 5μg of protein were loaded on each well. Cell extract from R1 was used as a negative control.
Overview of primers used for construction of mutant strains, cloning and diagnostic PCR experiments.
Acknowledgments
We thank Adriana Bailone for critical reading of the manuscript, Charlie Dulberger for help with the gel shift assay, Mahel Zeghouf for the gift of the plasmid with SPA tag, the Institut Curie for the use of the 137Cs irradiation system and Vincent Favaudon for help with γ-irradiation.
Funding
This work was supported by the Centre National de la Recherche Scientifique, the University Paris-Sud 11, the Commissariat à l’Energie Atomique (CEA LRC42V), Electricité de France (RB2007-11), the Agence Nationale de la Recherche (ANR-07-BLAN-0106), and the National Institutes of General Medical Sciences (USA)(GM032335).
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest
References
- 1.Cox MM, Battista JR. Deinococcus radiodurans - the consummate survivor. Nat Rev Microbiol. 2005;3:882–892. doi: 10.1038/nrmicro1264. [DOI] [PubMed] [Google Scholar]
- 2.Blasius M, Hubscher U, Sommer S. Deinococcus radiodurans: what belongs to the survival kit? Crit Rev Biochem Mol Biol. 2008;43:221–238. doi: 10.1080/10409230802122274. [DOI] [PubMed] [Google Scholar]
- 3.Daly MJ. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat Rev Microbiol. 2009;7:237–245. doi: 10.1038/nrmicro2073. [DOI] [PubMed] [Google Scholar]
- 4.Slade D, Radman M. Oxidative Stress Resistance in Deinococcus radiodurans. Microbiol Mol Biol Rev. 2011;75:133–191. doi: 10.1128/MMBR.00015-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka M, Earl AM, Howell HA, Park MJ, Eisen JA, Peterson SN, Battista JR. Analysis of Deinococcus radiodurans’s transcriptional response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics. 2004;168:21–33. doi: 10.1534/genetics.104.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norais CA, Chitteni-Pattu S, Wood EA, Inman RB, Cox MM. DdrB protein, an alternative Deinococcus radiodurans SSB induced by ionizing radiation. J Biol Chem. 2009;284:21402–21411. doi: 10.1074/jbc.M109.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugiman-Marangos S, Junop MS. The structure of DdrB from Deinococcus: a new fold for single-stranded DNA binding proteins. Nucleic Acids Res. 2010;38:3432–3440. doi: 10.1093/nar/gkq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shereda RD, Kozlov AG, Lohman TM, Cox MM, Keck JL. SSB as an organizer/mobilizer of genome maintenance complexes. Crit Rev Biochem Mol Biol. 2008;43:289–318. doi: 10.1080/10409230802341296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murzin AG. OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J. 1993;12:861–867. doi: 10.1002/j.1460-2075.1993.tb05726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu G, Lu H, Wang L, Chen H, Xu Z, Hu Y, Tian B, Hua Y. DdrB stimulates single-stranded DNA annealing and facilitates RecA-independent DNA repair in Deinococcus radiodurans. DNA Repair (Amst) 2010;9:805–812. doi: 10.1016/j.dnarep.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Daly MJ, Minton KW. An alternative pathway of recombination of chromosomal fragments precedes recA-dependent recombination in the radioresistant bacterium Deinococcus radiodurans. J Bacteriol. 1996;178:4461–4471. doi: 10.1128/jb.178.15.4461-4471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders CW, Guild WR. Pathway of plasmid transformation in Pneumococcus: open circular and linear molecules are active. J Bacteriol. 1981;146:517–526. doi: 10.1128/jb.146.2.517-526.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidane D, Carrasco B, Manfredi C, Rothmaier K, Ayora S, Tadesse S, Alonso JC, Graumann PL. Evidence for different pathways during horizontal gene transfer in competent Bacillus subtilis cells. PLoS Genet. 2009;5:e1000630. doi: 10.1371/journal.pgen.1000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zahradka K, Slade D, Bailone A, Sommer S, Averbeck D, Petranovic M, Lindner AB, Radman M. Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature. 2006;443:569–573. doi: 10.1038/nature05160. Epub 2006 Sep 2027. [DOI] [PubMed] [Google Scholar]
- 15.Slade D, Lindner AB, Paul G, Radman M. Recombination and replication in DNA repair of heavily irradiated Deinococcus radiodurans. Cell. 2009;136:1044–1055. doi: 10.1016/j.cell.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Meima R, Rothfuss HM, Gewin L, Lidstrom ME. Promoter cloning in the radioresistant bacterium Deinococcus radiodurans. J Bacteriol. 2001;183:3169–3175. doi: 10.1128/JB.183.10.3169-3175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mennecier S, Coste G, Servant P, Bailone A, Sommer S. Mismatch repair ensures fidelity of replication and recombination in the radioresistant organism Deinococcus radiodurans. Mol Genet Genomics. 2004;272:460–469. doi: 10.1007/s00438-004-1077-6. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz G, Watanabe F. Thermodynamics and kinetics of co-operative protein-nucleic acid binding. I. General aspects of analysis of data. J Mol Biol. 1983;163:467–484. doi: 10.1016/0022-2836(83)90069-4. [DOI] [PubMed] [Google Scholar]
- 19.Bouthier de la Tour C, Toueille M, Jolivet E, Nguyen HH, Servant P, Vannier F, Sommer S. The Deinococcus radiodurans SMC protein is dispensable for cell viability yet plays a role in DNA folding. Extremophiles. 2009;13:827–837. doi: 10.1007/s00792-009-0270-2. [DOI] [PubMed] [Google Scholar]
- 20.Harris DR, Tanaka M, Saveliev SV, Jolivet E, Earl AM, Cox MM, Battista JR. Preserving genome integrity: the DdrA protein of Deinococcus radiodurans R1. PLoS Biol. 2004;2:e304. doi: 10.1371/journal.pbio.0020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecointe F, Shevelev IV, Bailone A, Sommer S, Hubscher U. Involvement of an X family DNA polymerase in double-stranded break repair in the radioresistant organism Deinococcus radiodurans. Mol Microbiol. 2004;53:1721–1730. doi: 10.1111/j.1365-2958.2004.04233.x. [DOI] [PubMed] [Google Scholar]
- 22.Witte G, Urbanke C, Curth U. Single-stranded DNA-binding protein of Deinococcus radiodurans: a biophysical characterization. Nucleic Acids Res. 2005;33:1662–1670. doi: 10.1093/nar/gki310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohman TM, Overman LB, Datta S. Salt-dependent changes in the DNA binding co-operativity of Escherichia coli single strand binding protein. J Mol Biol. 1986;187:603–615. doi: 10.1016/0022-2836(86)90338-4. [DOI] [PubMed] [Google Scholar]
- 24.Battista JR. Against all odds: the survival strategies of Deinococcus radiodurans. Annu Rev Microbiol. 1997;51:203–224. doi: 10.1146/annurev.micro.51.1.203. [DOI] [PubMed] [Google Scholar]
- 25.Zeghouf M, Li J, Butland G, Borkowska A, Canadien V, Richards D, Beattie B, Emili A, Greenblatt JF. Sequential Peptide Affinity (SPA) system for the identification of mammalian and bacterial protein complexes. J Proteome Res. 2004;3:463–468. doi: 10.1021/pr034084x. [DOI] [PubMed] [Google Scholar]
- 26.Makharashvili N, Koroleva O, Bera S, Grandgenett DP, Korolev S. A novel structure of DNA repair protein RecO from Deinococcus radiodurans. Structure. 2004;12:1881–1889. doi: 10.1016/j.str.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Theobald DL, Mitton-Fry RM, Wuttke DS. Nucleic acid recognition by OB-fold proteins. Annu Rev Biophys Biomol Struct. 2003;32:115–133. doi: 10.1146/annurev.biophys.32.110601.142506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costes A, Lecointe F, McGovern S, Quevillon-Cheruel S, Polard P. The C-terminal domain of the bacterial SSB protein acts as a DNA maintenance hub at active chromosome replication forks. PLoS Genet. 2010;6:e1001238. doi: 10.1371/journal.pgen.1001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chase JW, L’Italien JJ, Murphy JB, Spicer EK, Williams KR. Characterization of the Escherichia coli SSB-113 mutant single-stranded DNA-binding protein. Cloning of the gene, DNA and protein sequence analysis, high pressure liquid chromatography peptide mapping, and DNA-binding studies. J Biol Chem. 1984;259:805–814. [PubMed] [Google Scholar]
- 30.Wang TC, Smith KC. Effects of the ssb-1 and ssb-113 mutations on survival and DNA repair in UV-irradiated delta uvrB strains of Escherichia coli K-12. J Bacteriol. 1982;151:186–192. doi: 10.1128/jb.151.1.186-192.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curth U, Genschel J, Urbanke C, Greipel J. In vitro and in vivo function of the C-terminus of Escherichia coli single-stranded DNA binding protein. Nucleic Acids Res. 1996;24:2706–2711. doi: 10.1093/nar/24.14.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson SN, Sung CK, Cline R, Desai BV, Snesrud EC, Luo P, Walling J, Li H, Mintz M, Tsegaye G, Burr PC, Do Y, Ahn S, Gilbert J, Fleischmann RD, Morrison DA. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol Microbiol. 2004;51:1051–1070. doi: 10.1046/j.1365-2958.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 33.Grove DE, Willcox S, Griffith JD, Bryant FR. Differential single-stranded DNA binding properties of the paralogous SsbA and SsbB proteins from Streptococcus pneumoniae. J Biol Chem. 2005;280:11067–11073. doi: 10.1074/jbc.M414057200. [DOI] [PubMed] [Google Scholar]
- 34.Morrison DA, Mortier-Barriere I, Attaiech L, Claverys JP. Identification of the major protein component of the pneumococcal eclipse complex. J Bacteriol. 2007;189:6497–6500. doi: 10.1128/JB.00687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daly MJ, Ouyang L, Fuchs P, Minton KW. In vivo damage and recA-dependent repair of plasmid and chromosomal DNA in the radiation-resistant bacterium Deinococcus radiodurans. J Bacteriol. 1994;176:3508–3517. doi: 10.1128/jb.176.12.3508-3517.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bentchikou E, Servant P, Coste G, Sommer S. A major role of the RecFOR pathway in DNA double-strand-break repair through ESDSA in Deinococcus radiodurans. PLoS Genet. 2010;6:e1000774. doi: 10.1371/journal.pgen.1000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iyer LM, Koonin EV, Aravind L. Classification and evolutionary history of the single-strand annealing proteins, RecT, Redbeta, ERF and RAD52. BMC Genomics. 2002;3:8. doi: 10.1186/1471-2164-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Servant P, Jolivet E, Bentchikou E, Mennecier S, Bailone A, Sommer S. The ClpPX protease is required for radioresistance and regulates cell division after gamma-irradiation in Deinococcus radiodurans. Mol Microbiol. 2007;66:1231–1239. doi: 10.1111/j.1365-2958.2007.06003.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Increased sensitivity of cells devoid of the ddrB gene and complementation ofΔddrB mutant. GY11944 (wild type/p11520, filled squares), GY13378 (ΔddrB/p11520, filled circles), GY13384 (ΔddrB/p13421:ddrB+, open triangles), GY12830 (ddrB::spa, open triangles) bacteria were exposed to γ-irradiation at doses indicated on the abscissa and cell survival was measured as described in the materials and methods.
Expression of DdrB in D. radiodurans. D. radiodurans GY12830 (ddrB::spaΩcat) cell extracts from unirradiated (pre-irradiation) or irradiated cells exposed to 3.8 kGy gamma-irradiation and incubated during the indicated periods (min) were subjected to 15% SDS-PAGE and analyzed by western blot with anti-FLAG antibodies. 5μg of protein were loaded on each well. Cell extract from R1 was used as a negative control.
Overview of primers used for construction of mutant strains, cloning and diagnostic PCR experiments.