Abstract
Magnetic resonance imaging studies have begun to map effects of genetic variation on trajectories of brain development. Longitudinal studies of children and adolescents demonstrate a general pattern of childhood peaks of gray matter followed by adolescent declines, functional and structural increases in connectivity and integrative processing, and a changing balance between limbic/subcortical and frontal lobe functions, which extends well into young adulthood. Twin studies have demonstrated that genetic factors are responsible for a significant amount of variation in pediatric brain morphometry. Longitudinal studies have shown specific genetic polymorphisms affect rates of cortical changes associated with maturation. Although over-interpretation and premature application of neuroimaging findings for diagnostic purposes remains a risk, converging data from multiple imaging modalities is beginning to elucidate the influences of genetic factors on brain development and implications of maturational changes for cognition, emotion, and behavior.
Keywords: Magnetic resonance imaging, Brain, Development, Genes, Twins
The “Nature versus Nurture” question of biology has long been put to rest in favor of the recognition that each individual’s unique phenotype emerges from a developmentally sensitive interaction of genes and environment. The advent of magnetic resonance imaging (MRI) has provided unprecedented ability to look past the protective layers of skull, fluid and dura to capture exquisitely accurate pictures of brain anatomy and physiology. Because MRI does not use ionizing radiation it can be used not only to scan children, but to scan them repeatedly during the course of development. This capacity to acquire longitudinal brain maturation data safely has launched a new era of child and adolescent neuroscience, including the ability to explore the relative effects of genetic and environmental factors on the trajectory of brain development.
In this review we will begin by describing the longitudinal changes in brain structure occurring in typically developing children and adolescents. The next section will discuss the use of twin studies to delineate the relative effects of genes and environment. Finally, we will highlight recent work looking at effects of specific genetic polymorphisms. We will emphasize data from the typically developing individuals participating in a large-scale longitudinal study carried out at the Child Psychiatry Branch of the National Institutes of Health in order to provide an integrated overview of varying influences on brain development from scans of subjects obtained and analyzed using the same methods. In this study, begun by Markus Krusei in 1989, participants between the ages of 3 and 30 years come to the campus in Bethesda, MD at approximately 2 year intervals for MRI scans, neuropsychological testing, and genetic analysis. Currently the data base consists of over 6,000 scans from over 2,000 subjects, approximately half from clinical populations such as ADHD, Autism, and Childhood onset Schizophrenia, and half from typically developing individuals, including over 800 scans from twins.
Mapping Developmental Anatomic Trajectories During Typical Childhood and Adolescence
Data taken from the Child Psychiatry Branch (CPB) cohort has shown several striking findings. One is the degree of variability of brain structures across individuals. For example, as seen in Fig. 1 two healthy 10 year old boys can have a nearly two-fold differences in total brain size. This high variability extends to measures of brain substructures as well and has important implications for the interpretation and utility of brain imaging results. Second, the development of total cerebral volume and many subcortical regions follows an inverted U shaped trajectory. These trajectories were estimated using statistical methods which derive an overall developmental curve for a population, including an approximate age of peak volume for the population as a whole (see Fig. 2) (Lenroot et al. 2007). A third key finding is the difference in developmental trajectories for males and females. Throughout childhood and adolescence in this sample the group average brain size for males is approximately 10% larger than for females. This 10% differences has also been found in hundreds of adult neuroimaging and postmortem studies, frequently attributed to the larger body size of males. However, in our pediatric subjects the boys’ bodies are not larger than girl’s until after puberty, and girls are actually somewhat taller from ages 10–13 in both our samples and data from the CDC because of their earlier pubertal growth spurt.
Fig. 1.
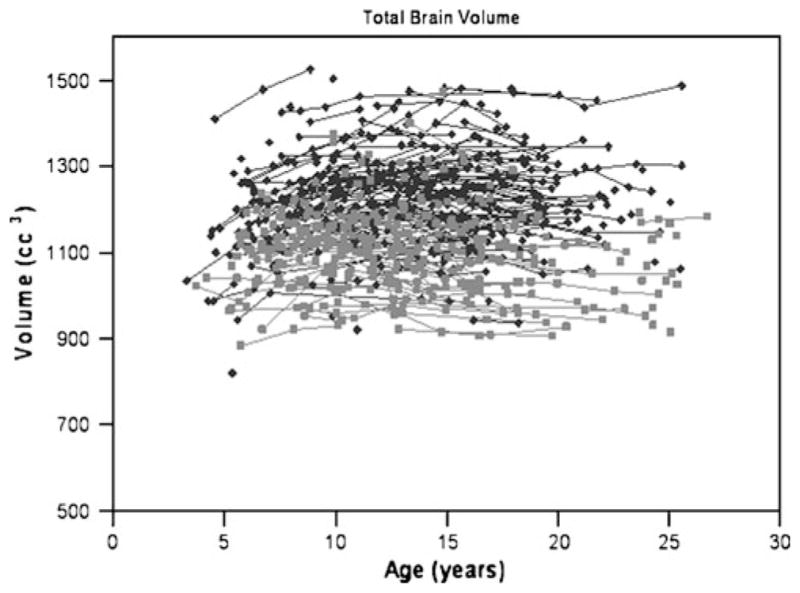
Scatterplot of longitudinal measurements of total brain volume for males (N=475 scans, shown in dark gray) and females (N=354 scans, shown in light gray) (Lenroot et al. 2007)
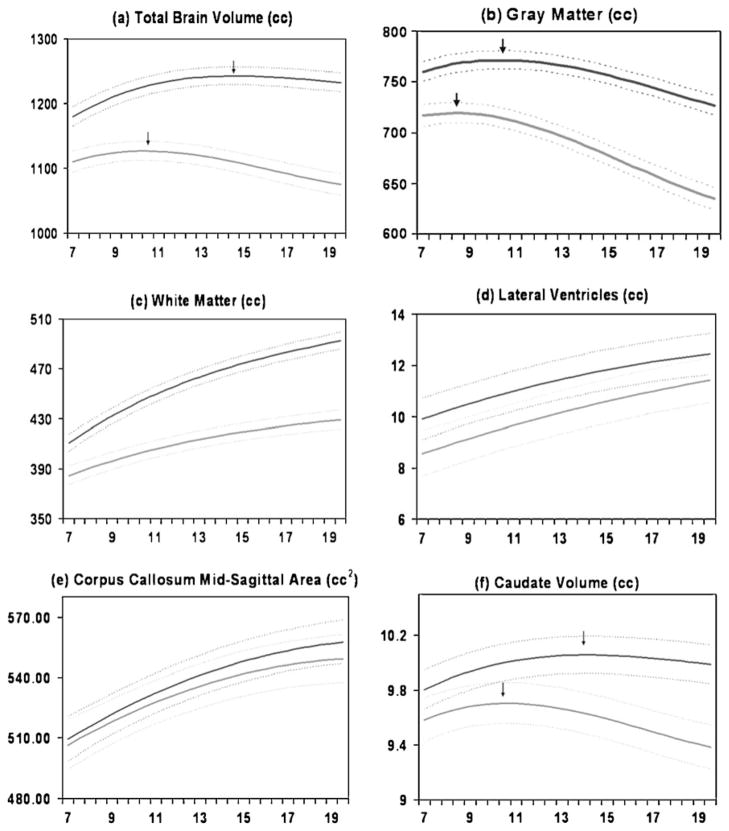
Fig. 2.
Mean volume by age in years for males (N=475 scans) and females (N=354 scans). Middle lines in each set of three lines represent mean values, and upper and lower lines represent upper and lower 95% confidence intervals. a Total brain volume, b Gray matter volume, c White matter volume, d Lateral ventricle volume, e Mid-sagittal area of the corpus callosum, f Caudate volume (Lenroot et al. 2007)
These findings highlight one of the most important principles emerging from neuroimaging research, which is that differences in brain size cannot be straightforwardly interpreted as imparting any sort of functional advantage or disadvantage. Male/female differences and the striking variability of brain volumes in the presence of equal intellectual capacity demonstrate the lack of a linear relationship, while the inverted U shape of brain trajectories indicates the dissociation between ongoing maturational improvement in cognitive ability while brain volumes have already begun to fall during mid-late adolescence. Features such as neuronal connectivity and receptor density may confer functional differences that are not captured with gross structural measures.
Trajectories of Brain Volumes
Brain volume follows an inverted U shape trajectory, which peaks at approximately age 10.5 in girls and 14.5 in boys (see Fig. 2). Tissue classified as gray matter (GM) by MRI consists mostly of cell bodies, dendrites, and dendritic processes, with contributions from axons, glia, blood vessels, and extracellular space (Braitenberg 2001). White matter (WM) is composed primarily of myelinated axons of neurons with associated vasculature and glia. Although GM and WM are bound by lifelong reciprocal relationships they have different developmental trajectories. GM developmental trajectories follow an inverted U shaped curve with peak sizes occurring at different times in different regions. For instance, in the frontal lobes peak cortical GM volume occurs at 9.5 in girls and 10.5 years in boys; in the temporal lobes at 10.0 in girls and 11.0 years in boys; and in the parietal lobes at 7.5 in girls and 9 years in boys.
White matter volumes instead increase throughout childhood and adolescence (Fig. 2c). At lobar levels (i.e. frontal, temporal, and parietal lobes) the white matter trajectories are roughly similar. However, for smaller regions the growth rates can be quite dynamic with as much as a 50% change over a 2-year period (Thompson et al. 2000)
The corpus callosum (CC) is the most prominent white matter structure and easily visualized on mid sagittal MR images. The CC consists of approximately 200 million mostly myelinated axons connecting homologous areas of the left and right cerebral hemispheres. The functions of the CC can generally be thought of as integrating the activities of the left and right cerebral hemispheres, including functions related to the unification of sensory fields (Berlucchi 1981; Shanks et al. 1975), memory storage and retrieval (Zaidel and Sperry 1974), attention and arousal (Levy 1985) and enhancement of language and auditory functions (Cook 1986). In agreement with several studies that have indicated increasing CC size during adolescence (Allen et al. 1991; Cowell et al. 1992; Pujol et al. 1993; Rauch and Jinkins 1994; Thompson et al. 2000), total midsagittal CC area increased robustly from ages 4–20 years in the CPB sample (Fig. 2e).
Increasing white matter during childhood and adolescence allows for greater integration of disparate neural circuitry, the hallmark of many maturational changes in brain function. Neurons integrate information from other neurons by summing excitatory and inhibitory input. If excitatory input exceeds a certain threshold, the receiving neuron fires and initiates a series of molecular changes that strengthens the synapses, or connections, from the input neurons, or as described by Donald Hebb in 1940, “cells that fire together wire together.” The summation of input depends on exquisite timing of signals coming from neurons that may be nearby or more distant. Myelin participates in the fine-tuning of this timing, which encodes the basis for thought, consciousness and meaning in the brain. The dynamic activity of myelination during adolescence reflects how much new wiring is occurring. Myelination also plays a central role in developmental changes in the brains ability to change in response to its environment by inhibiting axon sprouting and the creation of new synapses (Fields 2008).
Recognition of the importance of white matter development for brain function has stimulated the development of new imaging techniques such as diffusion tensor imaging (DTI) and magnetization transfer (MT) which can be used to assess myelination and coherence of white matter tracts. These new techniques further confirm an increase in white matter organization during adolescence, which correlates in specific brain regions with improvements in language (Nagy et al. 2004), reading (Deutsch et al. 2005), ability to inhibit a response (Liston et al. 2006) and memory (Nagy et al. 2004).
Subcortical Structures
Basal Ganglia
The basal ganglia are a collection of subcortical nuclei (caudate, putamen, globus pallidus, subthalamic nucleus, and substantia nigra) that are involved in circuits mediating movement, higher cognitive functions, attention, and affective states. Basal ganglia anomalies have been reported for almost all neuropsychiatric disorders that have been investigated by neuroimaging (Giedd et al. 2006). Because of the small size and ambiguity of MR signal contrast of the borders defining the structures, only the caudate, putamen, and globus pallidus are readily quantifiable by MRI, and reliable automated techniques have only been established for the caudate. Like cortical GM the caudate follows an inverted U shape developmental trajectory, peaking at age 10.5 years in girls and 14.0 years in boys (see Fig. 2f). The shape of the caudate developmental trajectory is more similar to that of frontal and parietal gray matter than temporal supporting the notion that brain regions that share extensive connections also share similar developmental courses.
Amygdala and Hippocampus
The temporal lobes, amygdala, and hippocampus are integral players in the arenas of emotion, language, and memory (Nolte 1993). Human capacity for these functions changes markedly between the ages of 4 and 18 years (Diener et al. 1985; Jerslid 1963; Wechsler 1974), although the relationship between the development of these capacities and morphological changes in the structures subserving these functions is poorly understood.
Description of the amygdala and hippocampus has been performed using manual tracing by expert raters, due to concerns regarding validity of existing automated methods for quantification of these structures, and is not yet completed for the longitudinal sample. In a previous report from a cross-sectional sample subset of the NIMH sample, amygdala volume increased significantly during adolescence only in males and hippocampal volume increased significantly only in females (Giedd et al. 1996). This pattern of gender-specific maturational volumetric changes is consistent with nonhuman primate studies indicating a relatively high number of androgen receptors in the amygdala (Clark et al. 1988) and a relatively higher number of estrogen receptors in the hippocampus (Morse et al. 1986).
Lateral Ventricles
The lateral ventricles are distinctive as a brain morphometry measure, as they are compartments filled with cerebrospinal fluid, rather than gray or white matter structures. Lateral ventricle size measures are usually interpreted as an indirect assessment of loss of the tissue from the neighboring structures that define its borders. The increasing size of lateral ventricle volume is shown in Fig. 2d. That ventricular volume increases so robustly during typical child and adolescent development should be considered when interpreting the many reports of increased ventricular volumes in a broad range of neuropsychiatric conditions.
Cerebellum
Although only about 1/9 the volume of the cerebrum, the cerebellum (Latin for “little brain”) actually contains more brain cells than the cerebrum. The function of the cerebellum has traditionally been described as related to motor control, but it is now commonly accepted that the cerebellum is also involved in emotional processing and other higher cognitive functions that mature throughout adolescence (Riva and Giorgi 2000; Schmahmann 2004, 2010). Similarly to the cerebrum, the cerebellum is made up of subunits that arise from different embryologic precursors and appear to have evolved at different times. In cross section the anatomy of the cerebellum resembles a butterfly shape with the central body part corresponding to the cerebellar vermis and the wings corresponding to more recently evolved cerebellar hemispheric lobes. Developmental curves of total cerebellum size follow an inverted U shaped developmental trajectory with peak size occurring at 11.3 in girls and 15.6 in boys, similar to the cerebrum. However, these different subregions appear to follow different developmental trajectories. In contrast to the evolutionarily more recent cerebellar hemispheric lobes that followed the inverted U shaped developmental trajectory, cerebellar vermis size did not change across the age span covered in this study (5–24 years) (Tiemeier et al. 2009).
Regional Differences in Cortical Thickness
Although some functional implications may be gleaned by examining GM at the lobar level, a significant advance was gained with the development of techniques able to measure cortical thickness at a high level of resolution, making it possible to describe structural differences with a greater degree of spatial detail potentially more suitable for relating to functional characteristics. Challenges to measurement of cortical thickness include accurate removal of overlying skull, modeling the cortical surface in regions where opposing sulcal surfaces may touch, and individual differences in cortical morphology.
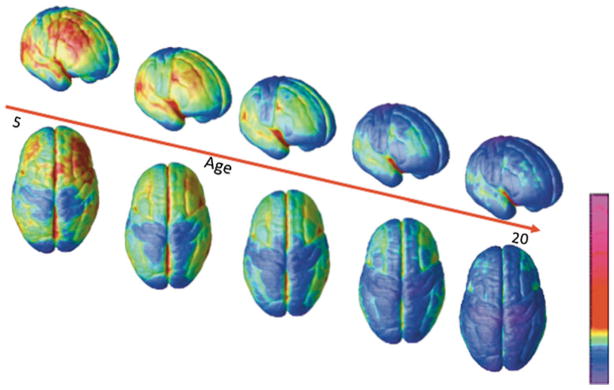
We have created movies of changes in cortical thickness over development by analyzing scans acquired longitudinally from the same individuals. One such animation derived from scans of 13 subjects who had each undergone scanning four times at approximately 2-year intervals between the ages of four and 22 is available at http://www.nimh.nih.gov/videos/press/prbrainmaturing.mpeg. Still images of the movie at different ages are seen in Fig. 3.
Fig. 3.
Right lateral and top views of the dynamic sequence of GM maturation over the cortical surface. The side bar shows a color representation in units of GM volume (Gogtay et al. 2004)
The thinning associated with cortical maturation appears to occur first in primary sensorimotor areas and latest in higher order association areas such as the dorsolateral prefrontal cortex, inferior parietal, and superior temporal gyrus. Postmortem studies suggest that part of the GM changes may be related to synaptic proliferation and pruning (Huttenlocher 1994). The connection between GM decreases and synaptic pruning is indirectly supported by an MRI/quantified EEG study of 138 healthy 10–30 year old subjects that found curvelinear reductions in frontal and parietal GM were matched by similar curvilinear reductions in EEG power of the corresponding regions (Whitford et al. 2006). As EEG power reflects synaptic activity, the temporally linked EEG power and GM changes suggests that the GM volume reductions are accompanied by reductions in the number of synapses.
Another potential contributor to decreased GM volume and cortical thickness is the ongoing myelination of small axons at the interior cortical border. This would lead to voxels changing classification from GM to WM along this border, resulting in cortical thinning as assessed by MR volumetrics, without necessarily entailing changes in synaptic density (Sowell et al. 2001). At present it is thought both factors are likely involved, but a definitive answer awaits studies such as imaging of nonhuman primates with post-mortem validation.
Genetic and Environmental Influences on Brain Development: Quantitative Genetics
Individual variation in the trajectory of brain development arises from the interaction of genetic and environmental factors. Statistical quantitative genetics, developed by the work of Fisher, Wright, and others (Fisher 1918; Wright 1968) provides a method of estimating the relative contributions of these factors based on the similarity of a given trait within individuals of different degrees of relatedness. In this model, phenotypic variation can be expressed as the addition of genetic, shared environmental, and unique environmental factors, plus their interactions. Heritability, or h, is defined as fraction of total variation due to genetic factors. Statistical quantitative genetics provides a broad estimation of what types of factors affect variation in a trait, without knowing what the specific relevant genetic or environmental factors actually are, particularly useful in complex traits to which it is likely a large number of genetic or environmental factors may each be contributing a small component. One of the most commonly used and powerful study designs within quantitative genetics is the twin study.
In the classical twin model, it is assumed that the amount of genetic material shared by a pair of monozygotic (MZ) twins is 100% and by dizygotic (DZ) twins is 50% (Neale and Maes 2005). One of the strengths of the twin model above other types of familial relatedness is the potential to separate out similarity due to shared genes from similarity due to being raised in the same environment. When twins are raised together, the environment is assumed to be identical for both, whether MZ or DZ. Therefore increased similarity between MZ twins compared to DZ for a given trait suggest that variation in the trait is being affected more strongly by genetic factors (A), while increased similarity between DZ twins suggests a prominent effect of the shared environment (C). Non-additive genetic effects will also tend to further increase the degree of similarity between MZ twins compared to DZ, providing an estimate of dominance (D). The environmental factors unique to each individual and measurement error are combined in the residual variance term (E).
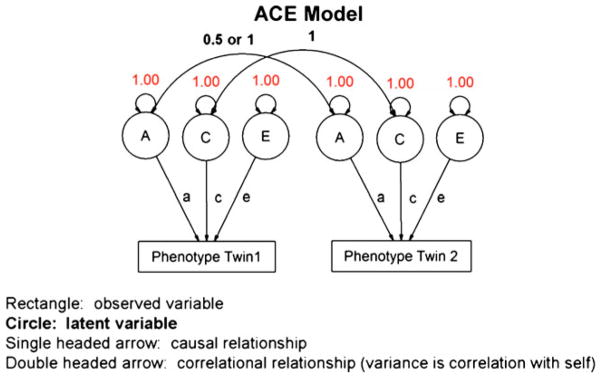
A classical twin study containing MZ and DZ twin pairs that have been raised together allows estimation of A, C or D, and E. C and D cannot be determined simultaneously because these terms are negatively confounded with each other: dominance effects increase the correlation between MZ twins, while shared environment factors increase the correlation between DZ twins (Anderson et al. 2002). Interaction terms such as gene-environment correlation can be estimated with more complex models including populations such as children of twins, an extension made possible through the use of more sophisticated techniques such as structural equation modeling (SEM), and its visual analogue, path analysis (see Fig. 4). While heritability can be estimated using simple equations based on correlations, SEM allows the creation of broader models including other family members such as siblings, or estimating the effects of variance components with factors such as age. SEM also makes it possible to systematically test whether age or other specific variance components are contributing significantly to improve the fit of the overall model (Neale and Maes 2005).
Fig. 4.
Graphic representation of structural equation modeling with genetically informative data
Most brain volumes in adults are significantly heritable (Baare et al. 2001; Posthuma et al. 2000; Tramo et al. 1998; Wright et al. 2002). Variation of cortical thickness in some regions such as the frontal and superior temporal lobes appears have a significant genetic contribution, while in other regions environmental factors are more pronounced (Rimol et al. 2010; Thompson et al. 2001). Topological features such as gyrification, by contrast, have thus far appeared to show stronger environmental influences (White et al. 2002).
The CPB twin study and others have explored whether estimates of heritability of brain structure and function change over the course of development.
The first report from the CPB study in a large group of healthy pediatric twins found that heritability values ranged from 70% to 90% for brain volumes for all structures except the ventricles and cerebellum (Wallace et al. 2006) similar to values previously reported in adults. High heritability values for regional gray and white matter volumes was also reported using a voxel-based morphometry method in a large group of pediatric twins aged 9–10 years from an ongoing longitudinal study in the Netherlands (Peper et al. 2009)
Heritability of cortical thickness in pediatric subjects from the CPB study varied by region (Lenroot et al. 2009). A striking overlap with previous findings in adults was seen, with areas in the dorsolateral frontal and superior temporal regions demonstrating the strongest genetic contribution, as did regions associated with language in the left parieto-temporal junction. Non-genetic factors were the chief contributors to variance over substantial areas of the cortex, including those regions associated with primary motor and sensory functions. Shared environmental factors were nonsignificant. Interestingly, although the areas of high heritability were similar to what had previously been reported in adults (Thompson et al. 2001) the heritability values were lower, raising the question of whether the lower values were related to the difference in age between the populations.
When the interaction of age with variance components was examined, developmental changes were seen in both brain volumes and in cortical thickness. Variance due to genetic effects increased over childhood and adolescence for both gray and white matter volumes. However, environmental variance decreased for white matter volumes, while it increased for gray matter volumes, leading to an overall increase in white matter heritability and decrease in gray matter heritability (Wallace et al. 2006).
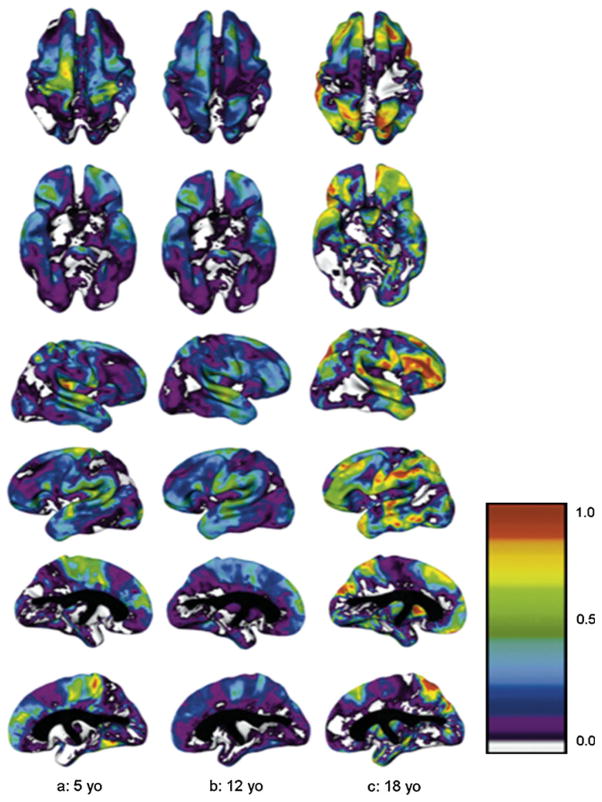
Age-related changes in heritability of cortical thickness also varied by brain region (see Fig. 5). Regions such as the primary motor and sensory cortices were significantly heritable in younger children but became progressively less so with time, such that genetic factors were not significant in young adults. Conversely, variation in regions such as the dorsal prefrontal and temporal regions was more environmentally driven in younger children, and became more strongly heritable with maturation (Lenroot et al. 2009).
Fig. 5.
Heritability at ages 5, 12, and 18 years for superior, inferior, right and left cortical surfaces. Colorbar shows scale of heritability values from 0.0 to 1.0 (Lenroot et al. 2009)
Multivariate statistical genetic analyses provide an estimate of the degree to which the same genetic or environmental factors contribute to multiple neuroanatomic structures, such as distributed neural networks or ontologically related regions. In a large multivariate analysis of the CPB data, a single genetic factor accounted for 60% of variability in cortical thickness across the brain (Schmitt et al. 2007). Six factors account for 58% of the remaining variance, with five groups of structures strongly influenced by the same underlying genetic factors. These findings are consistent with the radial unit hypothesis of neocortical expansion proposed by Rakic (Rakic and Caviness 1995) and with hypotheses that global, genetically mediated differences in cell division were the driving force behind interspecies differences in total brain volume (Darlington et al. 1999; Finlay and Darlington 1995; Fishell 1997)
Multivariate analyses can also be used to determine whether similar genetic or environmental factors may be contributing to a brain structure and a cognitive or behavioral phenotype. Previous studies in adults had found that shared genetic influences contributed to both brain volume and IQ (Hulshoff Pol et al. 2006; Posthuma et al. 2002). We carried out a multivariate analysis of brain structures with verbal and non-verbal IQ measures in the CPB pediatric twin sample to determine what kind of relationship might exist during this period of rapid cognitive development. Interestingly, a common environmental factor affected variation in both verbal IQ and gray matter volume. Nonverbal IQ, on the other hand, shared a common genetic factor with regional variation in both gray and white matter volumes. These results suggested that different mechanisms underlie the relationship of specific subcomponents of intelligence to brain structure, at least in this younger cohort (Raznahan et al. 2010; Wallace et al. 2010)
The mechanisms underlying the increasing genetic contributions to variation for some traits over development are not well understood. One likely factor is changes in gene expression, for example as triggered by pubertal processes. The presence of developmental changes in gene expression in the brain has been well established in animal models (Stead et al. 2006; Sun et al. 2005). A growing number of genes whose expression in the brain changes over development is being documented in humans as well, including genes related to the dopamine transporter (DAT), BDNF, its receptor, tyrosine kinase B (trkB), and others (Webster et al. 2006; Weickert et al. 2007).
Another possibility is that the change in heritability arises from the changing interaction of genes and environment. While gene-environment interactions can be modeled within statistical quantitative genetics as one of the terms contributing to phenotypic variance, typical twin study designs lack the power to adequately describe them. They are therefore usually not modeled as separate factors, but may still contribute to the additive genetic component as an expression of genetic factors influencing environmental variance.
Gene-environment correlation in particular is a promising candidate for explaining increasing heritability with age (Scarr and McCartney 1983). Gene-environmental correlations are theorized to occur through three different processes: passive, active, and evocative. In the first type, a child’s genes and his or her environment are correlated because the parents providing the genes are also shaping the child’s environment. In active gene-environment correlation, the child is helping to create his or her environment through actively choosing activities or other factors which complement inherited capacities. Evocative gene-environment correlation occurs when genetically mediated traits in the child stimulates particular reactions in other people which again influence the child’s environment. Scarr and McCartney and others (McGue 2010; Rutter 2007) have proposed that increasing heritability with age may be related to the increasing ability of a child to choose their own environment as they mature and gain autonomy, and thus the increasing contribution of active gene-environment correlations to heritability values.
The assumptions and limitations of the method should be kept in mind. One assumption that is often questioned is whether the environment in which DZ twins are reared is really the same as their identical MZ counterparts. While work done thus far has not shown differences in MZ and DZ familial environment to be so significant a factor as to invalidate twin studies (Kendler et al. 1994), the potential must be kept in mind for each particular trait. A second limitation is that a given set of variability estimates can only be considered valid for a specific population and environment. One of the most challenging limitations, however, is the limited power of this model to describe gene-environment interactions. One aspect of this is the limited power to detect GxE and rGE interactions as contributors to variance. The other is a more fundamental challenge to the methodology: whether acting as if genetic and environmental factors can actually be separated and added together as independent factors is inherently problematic, given that genes are linked to phenotypes only through their interaction with environmental factors (Lewontin 1974; Meaney 2010; Tabery 2009; Vreeke 2000).
Despite these limitations, quantitative statistical genetic methods have made significant contributions to demonstrating the relative roles of genetic and environment factors across different stages of development. Among these is establishing that brain structural characteristics measurable with MRI are heritable traits and thus viable endophenotypes for studies of the effects of specific genes.
Specific Genes
As with any quantifiable behavioral or physical parameter, individuals can be categorized into groups based on genotype. Brain images of individuals in the different genotype groups can then be averaged and compared statistically. The CPB study has begun examining effects of genetic polymorphisms known to be relevant to major psychiatric disorders on the trajectories of brain development in healthy children and adolescents.
In adult populations, one of the most frequently studied genes has been apolipoprotein E (apoE), which modulates risk for Alzheimer’s disease. Carriers of the four allele of apoE have increased risk, whereas carriers of the two allele are possibly at decreased risk. To explore whether apoE alleles have distinct neuroanatomic signatures identifiable in childhood and adolescence, we examined 529 scans from 239 healthy subjects aged 4–20 years (Shaw et al. 2007). Although there were no significant IQ–genotype interactions, there was a step wise effect on cortical thickness in the entorhinal and right hippocamapal regions, with the four group exhibiting the thinnest, the three homozygotes in the middle range, and the two group the thickest. These data suggest that pediatric assessments might 1 day be informative for adult-onset disorders.
The catechol-O-methyltransferase (COMT) gene has received extensive attention due to its effects on dopamine metabolism and links to disorders such as schizophrenia. A polymorphism within COMT results in a valine (Val) to methionine (Met) substitution with a lower enzymatic activity and resulting higher dopamine levels in prefrontal cortical regions where the gene is densely expressed (Tunbridge et al. 2007). A comparison of cortical thickness in 209 healthy children and adolescents from the CPB study with the Val/Val, Val/Met, and Met/Met alleles found a stepwise increase in cortical thickness in the right inferior frontal and right superior/middle temporal gyrus with each additional Met allele (Shaw et al. 2009).
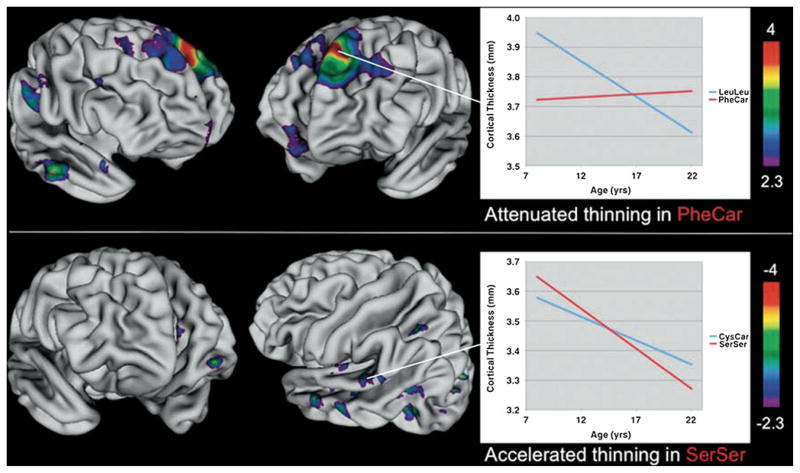
A longitudinal study of the effects of polymorphisms in Disrupted-in-schizophrenia-1 (DISC1) found that rates of cortical thinning associated with maturation varied by genotype (see Fig. 6) (Raznahan et al. 2010). Leu607Phe and Ser704Cys are two common polymorphisms within DISC1 that have been specifically linked both to cortical structure, and to disorders such as schizophrenia and autism that are related to neurodevelopmental abnormalities. In this analysis, 598 scans were obtained from 255 typically developing individuals between the ages of nine and 22 years. The Phe607Car genotype showed less cortical thinning than the LeuLeu genotype in superior frontal and temporal regions, while the Ser704Ser phenotype had accelerated thinning compared to the Ser704Cys in areas within the temporal lobes. Both genotypes also showed fixed effects on cortical thickness that did not vary by age across broad ranges, with almost 20% of the cortex being affected by polymorphisms in one or the other or both sites.
Fig. 6.
Vertex maps of areas showing statistically significant differences in the rate of cortical thinning between genotype groups. Pheu607Leu top panel, Ser704Cys bottom panel. Phe carriers (PheCar) showed a significant attenuation of cortical thinning relative to Leu homozygotes (LeuLeu) in the colored regions shown. The inset plot illustrates estimated genotype group trajectories for the left superior frontal focus. Ser homozygotes (SerSer) showed a significant acceleration of cortical thinning in the colored regions shown. The inset plot illustrates estimated genotype group trajectories for the left posterior superior temporal focus. In all instances ‘Warmer’ colors indicate thickness trajectory differences of greater statistical significance (Raznahan et al. 2010)
In each of these cases, polymorphisms of genes associated with psychiatric disorders have resulted in differences in cortical thickness in typically developing children and adolescents. Moreover, the regions affected have tended to fall within regions important for the psychiatric disorder or cognitive function associated with that gene. These findings support the roles of these various genes on structural brain development. In addition they suggest that the cortical differences seen in these disorders may be at least in part due to heritable neurodevelopmental differences associated with a specific genotype, rather than downstream effects of having the clinical condition.
Summary/Discussion
Anatomic brain MRI measures show high individual variability. The high variability and substantial overlap of most measures for most groups being compared has profound implications for the diagnostic utility of psychiatric neuroimaging and the sensitivity/specificity in using neuroimaging to make predictions about behavior or ability in a particular individual. For example, although group average anatomic MRI differences have been reported for all major psychiatric disorders MRI is not currently indicated for the routine diagnosis of any. Going from group average differences to individual use is one of the preeminent challenges of neuroimaging. A more immediate use of neuroimaging may be to provide endophenotypes, biologic markers that are intermediate between genes and behavior. Neuroimaging endophenotypes have the potential to define biologically meaningful subtypes of disorders that may respond to different interventions.
Despite high individual variation several statistically robust patterns of average maturational changes are evident. Specifically, WM volumes increase and GM volumes follow an inverted U developmental trajectory with peaks latest in high association areas such as dorsolateral prefrontal cortex.
These anatomic changes are consistent with electroencephalographic, functional MRI, postmortem, and neuropsychological studies indicating an increasing “connectivity” in the developing brain. “Connectivity” characterizes several neuroscience concepts. In anatomic studies connectivity can mean a physical link between areas of the brain that share common developmental trajectories. In studies of brain function, connectivity describes the relationship between different parts of the brain that activate together during a task. In genetic studies it refers to different regions that are influenced by the same genetic or environmental factors. All of these types of connectivity increase during adolescence. A linguistic metaphor would be to consider the maturational changes not so much as adding new letters to the alphabet as combining existing letters into words, those words into sentences, and the sentences into paragraphs. Characterizing developing neural circuitry and the changing relationships amongst disparate brain components is one of the most active areas of neuroimaging research utilizing graph theory to quantify such things as small world network properties of the brain.
Relatively late maturation of the dorsolateral prefrontal cortex, which is prominently involved in neural circuitry involved in judgment, decision making and impulse control, has prominently entered discourse affecting the social, legislative, judicial, parenting and educational realms. It is also consistent with a growing body of literature indicating a changing balance between earlier-maturing limbic system networks (the seat of emotion), and later maturing frontal systems. The frontal/limbic relationship is highly dynamic. Appreciating the interplay between limbic and cognitive systems is imperative for understanding decision making during adolescence. Psychological tests are usually conducted under conditions of “cold cognition”—hypothetical, low-emotion situations. However, real world decision making often occurs under conditions of “hot cognition”—high arousal, with peer pressure and real consequences. Neuroimaging investigations continue to discern the different biological circuitry involved in hot and cold cognition and are beginning to map how the parts of the brain involved in decision making mature.
Another prominent challenge for linking brain imaging findings to behavior/cognition/emotion is that behaviors emanate from the integrated activity of distributed networks not individual structures. Further complicating brain/behavior investigations is the growing realization that differences in the trajectories of development may in some cases be more informative than the final adult differences. For instance, in our longitudinal study looking at the relationship between cortical thickness and IQ differences in age by cortical thickness developmental curves were more predictive of IQ than differences in cortical thickness at age 20 years (Shaw et al. 2006). Trajectories are also more discriminating than static measures for sexual dimorphism and clinical investigations. The idea that in neuroimaging, as in life, the journey is often as important as the destination is becoming an accepted tenet of pediatric neuroimaging.
The “journey as well as destination” tenet also highlights the fundamentally dynamic nature of brain development, one of the most striking features of the human brain illuminated by pediatric neuroimaging. The ability of our brains to adapt and change based on the demands of environment is the hallmark of our species. Understanding the mechanisms and influences responsible for these changes may help us to harness the brain’s plasticity towards interventions for clinical disorders and optimizing the path to healthy development.
Acknowledgments
This research was supported by the Intramural Program of the National Institute of Mental Health, National Institutes of Health.
Contributor Information
Jay N. Giedd, Email: jg@nih.gov, Child Psychiatry Branch, National Institutes of Mental Health, Bethesda, MD, USA. Brain Imaging Unit Child Psychiatry Branch, NIMH, Building 10, Room 4C110, MSC 1367, BethesdaMD 20892, USA
Michael Stockman, Child Psychiatry Branch, National Institutes of Mental Health, Bethesda, MD, USA.
Catherine Weddle, Child Psychiatry Branch, National Institutes of Mental Health, Bethesda, MD, USA.
Maria Liverpool, Child Psychiatry Branch, National Institutes of Mental Health, Bethesda, MD, USA.
Aaron Alexander-Bloch, Child Psychiatry Branch, National Institutes of Mental Health, Bethesda, MD, USA.
Gregory L. Wallace, Child Psychiatry Branch, National Institutes of Mental Health, Bethesda, MD, USA
Nancy R. Lee, Child Psychiatry Branch, National Institutes of Mental Health, Bethesda, MD, USA
Francois Lalonde, Child Psychiatry Branch, National Institutes of Mental Health, Bethesda, MD, USA.
Rhoshel K. Lenroot, Child Psychiatry Branch, National Institutes of Mental Health, Bethesda, MD, USA. University of New South Wales and Neuroscience Research Australia, Sydney, Australia
References
- Allen LS, Richey MF, Chai YM, Gorski RA. Sex differences in the corpus callosum of the living human being. The Journal of Neuroscience. 1991;11:933–942. doi: 10.1523/JNEUROSCI.11-04-00933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BJ, Eckburg PB, Relucio KI. Alterations in the thickness of motor cortical subregions after motor-skill learning and exercise. Learning & Memory. 2002;9(1):1–9. doi: 10.1101/lm.43402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baare WF, Hulshoff Pol HE, Boomsma DI, Posthuma D, de Geus EJ, Schnack HG, et al. Quantitative genetic modeling of variation in human brain morphology. Cerebral Cortex. 2001;11(9):816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- Berlucchi G. Interhemispheric asymmetries in visual discrimination: a neurophysiological hypothesisDocumenta ophthalmologica. Proceedings series. 1981;30:87–93. [Google Scholar]
- Braitenberg V. Brain size and number of neurons: an exercise in synthetic neuroanatomy. Journal of Computational Neuroscience. 2001;10(1):71–77. doi: 10.1023/a:1008920127052. [DOI] [PubMed] [Google Scholar]
- Clark AS, MacLusky NJ, Goldman-Rakic PS. Androgen binding and metabolism in the cerebral cortex of the deveoping rhesus monkey. Endocrinology. 1988;123:932–940. doi: 10.1210/endo-123-2-932. [DOI] [PubMed] [Google Scholar]
- Cook ND. Mechanisms of information transfer and the role of the corpus callosum. London: Methuen; 1986. The brain code. [Google Scholar]
- Cowell PE, Allen LS, Zalatimo NS, Denenberg VH. A developmental study of sex and age interactions in the human corpus callosum. Developmental brain research. 1992;66:187–192. doi: 10.1016/0165-3806(92)90079-c. [DOI] [PubMed] [Google Scholar]
- Darlington RB, Dunlop SA, Finlay BL. Neural development in metatherian and eutherian mammals: variation and constraint. The Journal of Comparative Neurology. 1999;411(3):359–368. [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41(3):354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Diener E, Sandvik E, Larsen RF. Age and sex effects for affect intensity. Developmental Psychology. 1985;21:542–546. [Google Scholar]
- Fields R. Oligodendrocytes changing the rules: action potentials in glia and oligodendrocytes controlling action potentials. The Neuroscientist. 2008;14(6):540–543. doi: 10.1177/1073858408320294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Fishell G. Regionalization in the mammalian telencephalon. Current Opinion in Neurobiology. 1997;7(1):62–69. doi: 10.1016/s0959-4388(97)80121-3. [DOI] [PubMed] [Google Scholar]
- Fisher RA. The correlation between relatives on the supposition of Mendelian inheritance. Transactions of the Royal Society of Edinburgh. 1918;52:399–433. [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Shaw P, Wallace G, Gogtay N, Lenroot RK. Anatomic brain imaging studies of normal and abnormal brain development in children and adolescents. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology. 2. Vol. 2. Hoboken: Wiley; 2006. pp. 127–194. [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RC, Baare WF, van Oel C, et al. Genetic contributions to human brain morphology and intelligence. The Journal of Neuroscience. 2006;26(40):10235–10242. doi: 10.1523/JNEUROSCI.1312-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptogenesis in human cerebral cortex. In: Dawson G, Fischer K, editors. Human behavior and the developing brain. New York: Guilford; 1994. pp. 137–152. [Google Scholar]
- Jerslid AT. The psychology of adolescence. 2. New York: Macmillan; 1963. [Google Scholar]
- Kendler KS, Walters EE, Truett KR, Heath AC, Neale MC, Martin NG, et al. Sources of individual differences in depressive symptoms: analysis of two samples of twins and their families. The American Journal of Psychiatry. 1994;151(11):1605–1614. doi: 10.1176/ajp.151.11.1605. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, et al. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Human Brain Mapping. 2009;30(1):163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. Interhemispheric collaboration: Single mindedness in the asymmetric brain. In: Best CT, editor. Hemisphere function and collaboration in the child. New York: Academic; 1985. pp. 11–32. [Google Scholar]
- Lewontin RC. Analysis of variance and analysis of causes. American Journal of Human Genetics. 1974;26(3):400–411. [PMC free article] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- McGue M. The end of behavioral genetics? Behavior Genetics. 2010;40(3):284–296. doi: 10.1007/s10519-010-9354-0. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene × environment interactions. [Review] Child Development. 2010;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Morse JK, Scheff SW, DeKosky ST. Gonadal steroids influence axonal sprouting in the hippocampal dentate gyrus: a sexually dimorphic response. Experimental Neurology. 1986;94:649–658. doi: 10.1016/0014-4886(86)90244-x. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Neale MC, Maes HHM. Methodology for genetic studies of twins and families. 2005. [Google Scholar]
- Nolte J. Olfactory and limbic systems. In: Farrell R, editor. The human brain. An introduction to its functional anatomy. 3. St. Louis: Mosby-Year Book; 1993. pp. 397–413. [Google Scholar]
- Peper JS, Brouwer RM, Van Baal GC, Schnack HG, Van Leeuwen M, Boomsma DI, et al. Does having a twin-brother make for a bigger brain? European Journal of Endocrinology. 2009 doi: 10.1530/EJE-08-0915. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus E, Neale M, Hulshoff Pol H, Baare W, Kahn R, et al. Multivariate genetic analysis of brain structure in an extended twin design. Behavior Genetics. 2000;30(4):311–319. doi: 10.1023/a:1026501501434. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJ, Baare WF, Hulshoff Pol HE, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nature Neuroscience. 2002;5(2):83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Annals of Neurology. 1993;34:71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Rakic P, Caviness VS., Jr Cortical development: view from neurological mutants two decades later. Neuron. 1995;14(6):1101–1104. doi: 10.1016/0896-6273(95)90258-9. [DOI] [PubMed] [Google Scholar]
- Rauch RA, Jinkins JR. Analysis of cross-sectional area measurements of the corpus callosum adjusted for brain size in male and female subjects from childhood to adulthood. Behavioural Brain Research. 1994;64:65–78. doi: 10.1016/0166-4328(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Long R, Greenstein D, Clasen L, Addington A, et al. Common functional polymorphisms of DISC1 and cortical maturation in typically developing children and adolescents. Molecular Psychiatry. 2010 doi: 10.1038/mp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Panizzon MS, Fennema-Notestine C, Eyler LT, Fischl B, Franz CE, et al. Cortical thickness is influenced by regionally specific genetic factors. Biological Psychiatry. 2010;67(5):493–499. doi: 10.1016/j.biopsych.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(Pt 5):1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Rutter M. Gene-environment interdependence. Developmental Science. 2007;10(1):12–18. doi: 10.1111/j.1467-7687.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: a theory of genotype greater than environment effects. Child Development. 1983;54(2):424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. The Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16(3):367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychology Review. 2010;20(3):236–260. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- Schmitt J, Lenroot R, Wallace G, Ordaz S, Taylor K, Kabani N, et al. Identification of genetically mediated cortical networks: a multivariate study of pediatric twins and siblings. Cerebral Cortex. 2007;18(8):1737–1747. doi: 10.1093/cercor/bhm211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks MF, Rockel AJ, Powel TPS. The commissural fiber connections of the primary somatic sensory cortex. Brain Research. 1975;98:166–171. doi: 10.1016/0006-8993(75)90516-8. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63(5):540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, et al. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurology. 2007;6(6):494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- Shaw P, Wallace GL, Addington A, Evans A, Rapoport J, Giedd JN. Effects of the Val158Met catechol-O-methyltransferase polymorphism on cortical structure in children and adolescents. Molecular Psychiatry. 2009;14(4):348–349. doi: 10.1038/mp.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. The Journal of Neuroscience. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead JD, Neal C, Meng F, Wang Y, Evans S, Vazquez DM, et al. Transcriptional profiling of the developing rat brain reveals that the most dramatic regional differentiation in gene expression occurs postpartum. The Journal of Neuroscience. 2006;26(1):345–353. doi: 10.1523/JNEUROSCI.2755-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Patoine C, Abu-Khalil A, Visvader J, Sum E, Cherry TJ, et al. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308(5729):1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabery J. Interactive predispositions: gene-environment interactions-from IQ controversy to genetic screening. Behavior Genetics. 2009;39(6):683–683. [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404(6774):190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, et al. Genetic influences on brain structure. Nature Neuroscience. 2001;4(12):1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramo MJ, Loftus WC, Stukel TA, Green RL, Weaver JB, Gazzaniga MS. Brain size, head size, and intelligence quotient in monozygotic twins. Neurology. 1998;50(5):1246–1252. doi: 10.1212/wnl.50.5.1246. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Weickert CS, Kleinman JE, Herman MM, Chen J, Kolachana BS, et al. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cerebral Cortex. 2007;17(5):1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- Vreeke GJ. Nature, nurture and the future of the analysis of variance. Human Development. 2000;43(1):32–45. [Google Scholar]
- Wallace G, Eric Schmitt J, Lenroot R, Viding E, Ordaz S, Rosenthal M, et al. A pediatric twin study of brain morphometry. Journal of child psychology and psychiatry. 2006;47(10):987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Lee NR, Prom-Wormley EC, Medland SE, Lenroot RK, Clasen LS, et al. A bivariate twin study of regional brain volumes and verbal and nonverbal intellectual skills during childhood and adolescence. Behavior Genetics. 2010;40(2):125–134. doi: 10.1007/s10519-009-9329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MJ, Herman MM, Kleinman JE, Shannon Weickert C. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expression Patterns. 2006;6(8):941–951. doi: 10.1016/j.modgep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children—revised. New York: Psychological; 1974. [Google Scholar]
- Weickert CS, Webster MJ, Gondipalli P, Rothmond D, Fatula RJ, Herman MM, et al. Postnatal alterations in dopaminergic markers in the human prefrontal cortex. Neuroscience. 2007;144(3):1109–1119. doi: 10.1016/j.neuroscience.2006.10.009. [DOI] [PubMed] [Google Scholar]
- White T, Andreasen N, Nopoulos P. Brain volumes and surface morphology in monozygotic twins. Cerebral Cortex. 2002;12(5):486. doi: 10.1093/cercor/12.5.486. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Human Brain Mapping. 2006 doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. Genetic and biometric foundations. Chicago: University of Chicago Press; 1968. Evolution and the genetics of populations. I. [Google Scholar]
- Wright IC, Sham P, Murray RM, Weinberger DR, Bullmore ET. Genetic contributions to regional variability in human brain structure: methods and preliminary results. Neuroimage. 2002;17(1):256–271. doi: 10.1006/nimg.2002.1163. [DOI] [PubMed] [Google Scholar]
- Zaidel D, Sperry RW. Memory impairment after commissurotomy in man. Brain. 1974;97:263–272. doi: 10.1093/brain/97.1.263. [DOI] [PubMed] [Google Scholar]