Abstract
Objective
There is considerable epidemiological and neuropsychological evidence that attention deficit hyperactivity disorder (ADHD) is best considered dimensionally, lying at the extreme end of a continuous distribution of symptoms and underlying cognitive impairments. The authors investigated whether cortical brain development in typically developing children with symptoms of hyperactivity and impulsivity resembles that found in the syndrome of ADHD. Specifically, they examined whether a slower rate of cortical thinning during late childhood and adolescence, which they previously found in ADHD, is also linked to the severity of symptoms of hyperactivity and impulsivity in typically developing children.
Method
In a longitudinal analysis, a total of 193 typically developing children with 389 neuroanatomic magnetic resonance images and varying levels of symptoms of hyperactivity and impulsivity (measured with the Conners’ Parent Rating Scale) were contrasted with 197 children with ADHD with 337 imaging scans. The relationship between the rates of regional cortical thinning and severity of symptoms of hyperactivity/impulsivity was determined.
Results
Youth with higher levels of hyperactivity/impulsivity had a slower rate of cortical thinning, predominantly in prefrontal cortical regions, bilaterally in the middle frontal/premotor gyri, extending down the medial prefrontal wall to the anterior cingulate; the orbitofrontal cortex; and the right inferior frontal gyrus. For each increase of one point in the hyperactivity/impulsivity score, there was a decrease in the rate of regional cortical thinning of 0.0054 mm/year (SE=0.0019 mm/year). Children with ADHD had the slowest rate of cortical thinning.
Conclusions
Slower cortical thinning during adolescence characterizes the presence of both the symptoms and syndrome of ADHD, providing neurobiological evidence for dimensionality of the disorder.
There is considerable debate over whether attention deficit hyperactivity disorder (ADHD) represents a diagnostic category with distinct boundaries between the disorder and health or is better understood dimensionally, with those who have the disorder lying at the extreme end of a continuum of symptoms (1–3). Much evidence supports a dimensional view, including several taxonometric studies suggesting that symptoms of ADHD and associated neuropsychological deficits lie on a continuum (4, 5). Further support for a dimensional view would be gained from linking symptom dimensions to biological constructs such as brain development. In the present study, we used neuroanatomic imaging data acquired from typically developing children with no psychiatric diagnoses to explore whether high levels of hyperactive/impulsive symptoms are underpinned by neurodevelopmental patterns that resemble those seen in children with the syndrome of ADHD.
In this study, a dynamic measure of cortical change was used, since previous studies suggest that there are ADHD diagnostic differences in trajectories of cortical development (6–9). Using a measure of cortical thickness, we previously reported that in both typically developing children and youth with ADHD, an early childhood phase of cortical increase eventually reached a peak—attained later in those with ADHD in the fronto-temporal cortex—before giving way to a phase of cortical thinning (7). There were diagnostic differences in the phase of cortical thinning, with the velocity of thinning being consistently slower in youth with ADHD (see Results section). Given that cortical thinning dominates most of late childhood and early adolescence and most of our neuroimaging data lie within the same age range, cortical thinning emerges as a potential neuroanatomic marker for ADHD.
For the present study, we inquired whether similar differences in the rate of cortical thinning would occur in a typically developing group of children who have varying levels of hyperactive and impulsive symptoms. We used a version of the Conners’ Rating Scales (10), which includes a factor reflecting hyperactive and impulsive symptoms. This version does not include a factor reflecting purely inattentive symptoms, but rather a factor of learning difficulties, which while consisting of several items that reflect inattention and distractibility, includes more general learning difficulties. We did not include this learning problems factor and focused instead on the factor reflecting hyperactive and impulsive symptoms. We hypothesized that typically developing children with higher levels of hyperactive and impulsive symptoms would show cortical trajectories resembling those found in ADHD, specifically a slower rate of cortical thinning during late childhood and adolescence. To assess symptom specificity, the cortical changes associated with a measure of conduct problems in the same participants were determined, with the prediction of distinct cortical correlates for the different symptom domains.
Method
Participants
A total of 193 typically developing youth with no personal history of psychiatric or neurological disorders had a total of 389 neuroanatomic magnetic resonance images. Recruitment was conducted through local advertisements aimed at local parenting groups, schools, and community groups. Each participant underwent a structured diagnostic interview by a child psychiatrist to rule out any psychiatric or neurological diagnoses (11). Data from any participant who developed a mental illness during the course of the study were excluded, and all participants were free of psychotropic medications. After complete description of the study, written informed consent and assent were obtained from parents and children.
Comparison was made with a group of 197 youths with DSM-IV-defined ADHD. Diagnosis was based on the Diagnostic Interview for Children and Adolescents, Parent version (12), previously described elsewhere (6). This group of youths is a subset of our larger ADHD cohort (7), selected because their neuroanatomic data (total of 337 scans) were for the same age range as the data from participants in the present study and they had available Conners’ Rating Scales data.
All participants completed a version of the Conners’ Parent Rating Scale (10), which assesses symptoms of hyperactivity/impulsivity and conduct problems. This questionnaire is composed of 48 items, from which parents are asked to select from four possible responses based on the child’s behavior. Responses of “not at all,” “just a little,” “pretty much,” and “very much” return scores of 0 to 3, respectively. A factor analytic study (10) showed that four items load onto a factor reflecting hyperactive/impulsive symptoms, and thus the maximum score for hyperactive/impulsive symptoms is 12. Conduct problems were also measured using the same scale, with a maximum score of 24, reflecting the eight items that load onto this factor.
Neuroimaging
T1-weighted images with contiguous 1.5-mm axial slices were obtained using three-dimensional spoiled gradient recalled echo in the steady state on a 1.5-Tesla GE Signa scanner (General Electric Co., Milwaukee). Imaging parameters were as follows: echo time=5 msec, repetition time=24 msec, flip angle=45°, acquisition matrix=256×192, number of excitations=1, field of view=24 cm. The same scanner was utilized throughout the study. Native magnetic resonance imaging (MRI) scans were masked using the Brain Extraction Tool (13), registered into standardized stereotaxic space (14) using a nine-parameter linear transformation (15), corrected for nonuniformity artifacts (16) and segmented (17). The Constrained Laplacian Anatomic Segmentation using Proximity surface extraction procedure generated surface meshes representing white and gray matter interfaces (18). The root mean square thickness between corresponding nodes on the surface meshes was calculated in native space (19). Thickness measurements were aligned using surface registration to maximize thickness value correspondence between participants in terms of gyral patterning (20). A 30-mm surface blurring algorithm, which preserves cortical topologic features, was used to reduce noise in thickness measurements (21).
Phenotype
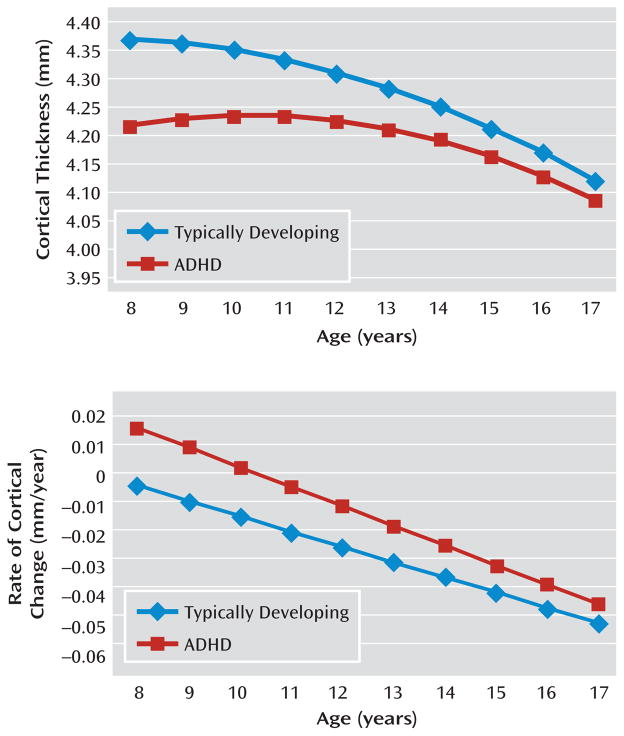
In a previous study of our entire cohort (223 youths with ADHD and age-, sex-, and IQ-matched typically developing subjects, with a total of 824 neuroanatomic scans), we demonstrated distinct trajectories of cortical growth, particularly in the prefrontal and lateral temporal cortices (7). As shown in Figure 1, which is derived from these earlier data, the ADHD group attained its peak cortical thickness later than the typically developing group throughout most of the prefrontal and lateral temporal cortices (with the exception of the superior portions of the motor strip). Additionally, by examining the rate of cortical change, the ADHD group was found to have a slower rate of cortical thinning in the regions with a delayed peak thickness, throughout all of late childhood and adolescence. Between the ages of 8 and 18 years, the estimated rate of thinning for the typically developing group was −0.027 mm/year, compared with −0.011 mm/year for the ADHD group. Thus, a slower rate of prefrontal cortical thinning during late childhood and adolescence characterizes most of the lateral prefrontal and temporal cortices in ADHD. In the present study, we examined whether a similar slowing of cortical thinning would be associated with the presence of hyperactive/impulsive symptoms in typically developing children. Data in this study were restricted to neuroanatomic scans that lay within the period of cortical thinning (≥8 years of age).
FIGURE 1.
Cortical Thickness in Typically Developing Youths With Symptoms of Hyperactivity/Impulsivity Relative to Those With ADHDa
a The top graph depicts the change in cortical thickness in the bilateral superior and middle frontal gyri in children with ADHD and in typically developing youths, from which the rate of change of cortical thickness is derived (bottom). At all ages, the rate of cortical thinning is greater for the typically developing youths.
Longitudinal Analyses
We examined whether the rate of cortical thinning varies as a function of the number of hyperactive/impulsive symptoms by regressing cortical thickness against the hyperactive/impulsive symptom score, age, and interaction between the hyperactive/impulsive symptom score and age using mixed-model regression (22). This approach was used because our data contain both multiple observations per participant, measured at different and irregular time periods, and single observations per participant. Such unbalanced longitudinal data can be explored statistically by applying mixed-effect models (23). A random effect for each individual was included to account for within-person dependence. Thus, for cortical points, the jth cortical thickness of the ith individual was modeled using the following equation, in which di is a random effects modeling within-person dependence; the intercept and β terms are fixed effects, and eij represents the residual error:
The interaction between age and hyperactive/impulsive symptoms is given by the β3 fixed effect. This parameter provides an estimate of how the relationship between cortical thickness and age varies as a function of the hyperactive/impulsive symptom score. The false discovery rate procedure was applied to control for multiple comparisons with a q value of 0.05 (24). A one-tailed adjustment was used, given the directional prediction of a slower rate of thinning in those participants with more hyperactive/impulsive symptoms. At every cortical point, t statistics were visualized through projection onto a standard brain template.
We further explored any significant interactions by splitting the typically developing sample into the following three groups: asymptomatic group (hyperactive/impulsive score=0), minimal symptoms group (hyperactive/impulsive score=1 or 2), and moderate symptoms group (hyperactive/impulsive score=3–6 [maximum score among typically developing youth]). Direct comparison was made with a group of 197 ADHD youth, with a total of 337 neuroanatomic scans, whose neuroimaging data were for the same age range as data for the typically developing youth. In these analyses, group (typically developing youth with no symptoms, minimal symptoms, and moderate symptoms and youth with ADHD) and group interaction with age were entered as fixed effects, using the aforementioned model. The β3 fixed effect was used to estimate how the relationship between cortical thickness and age varied as a function of group.
To determine the specificity of the hyperactive/impulsive symptom dimension on cortical change, we conducted parallel analyses examining the effects on cortical thickness associated with the conduct problems symptom score and interaction between this score and age.
Results
Among typically developing youth, hyperactive/impulsive symptom scores did not differ significantly by sex (boys: mean=1.43 [SD=1.59]; girls: mean=1.35 [SD=1.36]) and did not correlate with IQ (r=0.008, p=0.91). The ADHD group had greatly elevated hyperactive/impulsive symptom scores (mean=9.2 [SD=1.7]), and there was no overlap between the range of scores for the typically developing and ADHD groups (difference between groups: t=48.6, df=388, p<0.0001), in keeping with the severe phenotype of ADHD selected for this study. For the ADHD group, hyperactive/impulsive symptom scores also did not differ significantly with regard to sex (boys: mean=9.13 [SD=1.7]; girls: mean=9.3 [SD=1.7]) and did not correlate with IQ (r=0.05, p=0.53). Further details are presented in Table 1 and Table 2 of the data supplement accompanying the online version of this article, which also give the number of hyperactive/impulsive symptoms and participants’ ages at each wave of scan acquisition.
TABLE 1.
Slope Parameter Estimates for Each Brain Region in Typically Developing Youth With Hyperactive/Impulsive Symptoms and Youth With ADHD
| Brain Region | Slope Parameter (mm/year)a | Standard Error of Slope Parameter (mm/year) | t | df | p | Cluster Extent (number of vertices) | Local Maxima (x, y, z)b |
|---|---|---|---|---|---|---|---|
| Right | |||||||
| Supplementary motor and motor area, extending along the superior/middle frontal gyrus | 0.0051 | 0.0017 | 2.92 | 194 | 0.004 | 1,622 | 25, 3, 53 |
| Medial frontal gyrus extending toward anterior portions of superior frontal gyrus | 0.006 | 0.002 | 2.94 | 194 | 0.003 | 974 | 12, 39, 17 |
| Inferior frontal gyrus | 0.0062 | 0.0025 | 2.51 | 194 | 0.01 | 376 | 41, 42, 3 |
| Middle/inferior temporal gyrus | 0.0072 | 0.0025 | 2.9 | 194 | 0.004 | 700 | 65, −21, −19 |
| Precuneus | 0.0049 | 0.0018 | 2.73 | 194 | 0.006 | 331 | 5, −62, 35 |
| Parahippocampal area | 0.0064 | 0.0026 | 2.45 | 194 | 0.02 | 25 | 19, −23, −23 |
| Left | |||||||
| Middle prefrontal gyrus | 0.0052 | 0.0018 | 2.96 | 194 | 0.003 | 828 | −27, 10, 55 |
| Superior/medial prefrontal gyrus | 0.0058 | 0.0023 | 2.49 | 194 | 0.01 | 340 | −14, 46, 38 |
| Orbitofrontal gyrus | 0.0084 | 0.0027 | 3.01 | 194 | 0.003 | 1,056 | −15, 29, −26 |
| Superior temporal gyrus | 0.0088 | 0.0037 | 2.36 | 194 | 0.02 | 36 | −53, 9, −20 |
Data indicate the estimated rate of change in cortical thinning for each increase of one point in the hyperactive/impulsive symptoms score. Since throughout the age range examined, the cortex becomes thinner, positive values of the slope parameters indicate a slower rate of thinning as the number of hyperactive/impulsive symptoms increase, and therefore those with the fewest symptoms would have the highest rate of cortical thinning.
Data (Montreal Neurological Institute coordinates) indicate the cortical vortex where the greatest effect of hyperactive/impulsive symptoms is found.
For the three subgroups of typically developing youth, the mean conduct problems score was 1.6 (SD=1.9 [minimum, 0; maximum, 8]). Scores for conduct problems were higher for boys (mean=1.81 [SD=2]) than girls (mean=1.4 [SD=1.8]) but with no statistical significance. Additionally, IQ did not correlate with scores for conduct problems (r=−0.007, p=0.92).
Neuroanatomic Findings
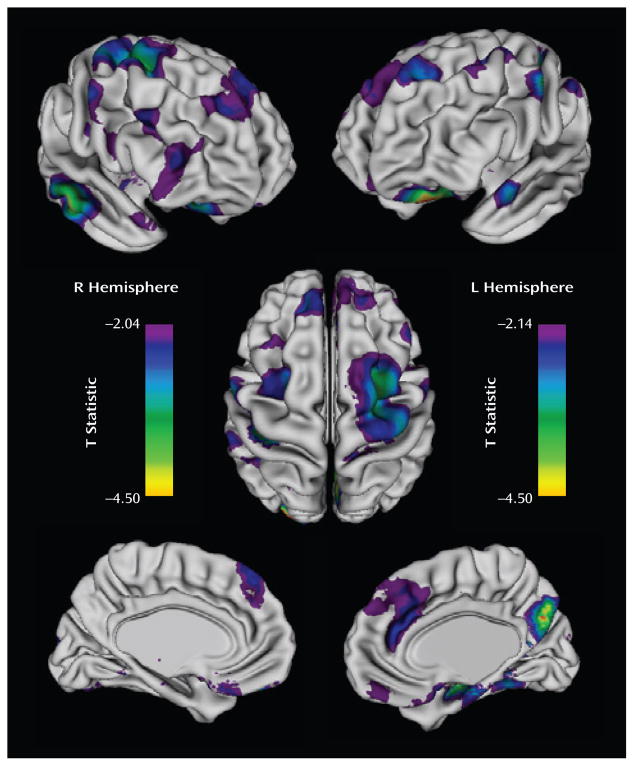
The rate of cortical thinning, predominantly in prefrontal cortical regions, varied significantly with the hyperactive/impulsive symptom score, with adjustment made for multiple comparisons using the false discovery rate procedure. There were clusters bilaterally in the middle frontal gryus, extending on the right toward the supplementary motor area and motor strip; the medial prefrontal wall, extending toward the anterior cingulate and superior frontal gyrus; the orbitofrontal cortex; and the right inferior frontal gyrus (Figure 2). More posteriorly, cortical thinning rates differed with hyperactive/impulsive symptom scores in the right middle/inferior temporal gyrus, parahippocampal gyrus, and precuneus as well as in the left superior gyrus. For each increase of one point in the hyperactive/impulsive symptom score, the estimated change in the rate of cortical thinning in the right prefrontal cortical regions, shown in Figure 2, was 0.0054 mm/year (SE=0.0019 mm/year; t=2.91, df=194, p=0.004), and the estimated change in the left prefrontal cortical regions was 0.0055 mm/year (SE=0.0019 mm/year; t=2.9, df=194, p=0.004). Extent and coordinates of the local maxima of the clusters are presented in Table 1.
FIGURE 2.
Regions Depicting Significant Differences Between Rates of Cortical Thinning and Hyperactive/Impulsive Symptoms in Typically Developing and ADHD Youtha
a The brain images depict T values for the interaction between the symptom severity score and age, which were significant following a false discovery rate procedure (right hemisphere: t>2.04, df=194, p<0.05; left hemisphere: t>−2.14, df=194, p<0.05). In all regions, an increasing number of hyperactive/impulsive symptoms was linked to slower cortical thinning. L=left; R=right.
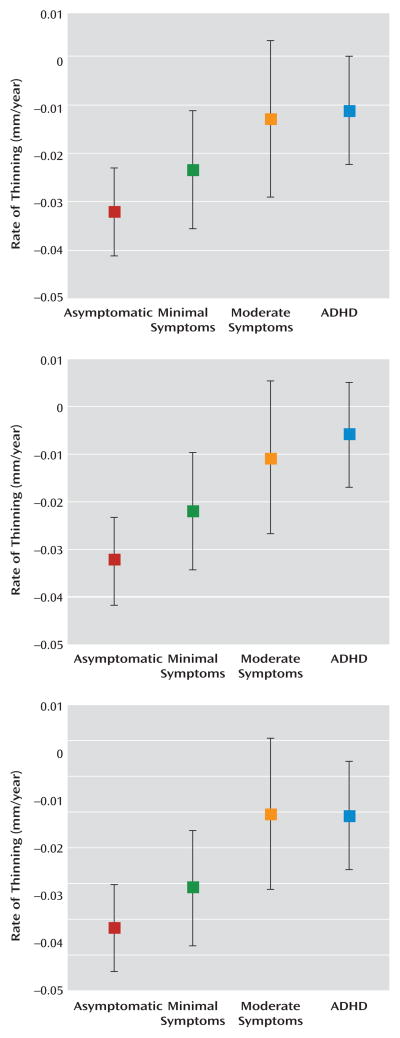
The effect of hyperactive/impulsive symptom severity can be clearly seen by comparing the rates of cortical thinning in participants who were grouped by the number of symptoms with the rates for those who had a diagnosis of ADHD (Figure 3). For both the right and left prefrontal cortical regions (Figure 2), the rate of cortical thinning changed in a stepwise manner with the degree of symptom severity. The rate of cortical thinning was slowest in participants with a diagnosis of ADHD and increased progressively in typically developing youth, with the moderate symptoms group having a higher rate than the ADHD group and the minimal symptoms group having a higher rate than those with moderate symptoms. Asymptomatic, typically developing youth had the highest rate of cortical thinning. In the right and left prefrontal cortical regions, pairwise contrasts showed that the asymptomatic group had a significantly higher rate of cortical thinning than both the moderately symptomatic group (right: t=2.4, df=194, p=0.02; left: t=2.3, df=194, p=0.02) and the clinical group with ADHD (right: t=3.5, df=194, p=0.0005; left: t=4.2, df=194, p<0.0001). Typically developing subjects with minimal symptoms had a significantly higher rate of cortical thinning than the ADHD group (right: t=2.2, df=194, p=0.03; left: t=3.1, df=194, p=0.002). For the posterior regions, the stepwise slowing of cortical change was less pronounced, since the ADHD group showed a similar rate of cortical thinning to typically developing subjects with moderate symptoms. However, participants in the ADHD group still had a significantly lower rate of cortical thinning than typically developing youth with no (t=3.2, df=194, p=0.001) or minimal (t=2.5, df=194, p=0.01) symptoms. This pattern of results remained when sex and IQ were entered as covariates.
FIGURE 3.
Estimated Rate of Cortical Thinning in Typically Developing Youth With Minimal, Moderate, and No Hyperactive/Impulsive Symptoms and Youth With ADHDa
a The graphs depict the right prefrontal region (top), left prefrontal region (center), and right temporal region (bottom) (error bars show 95% confidence intervals, derived from standard errors of the estimates).
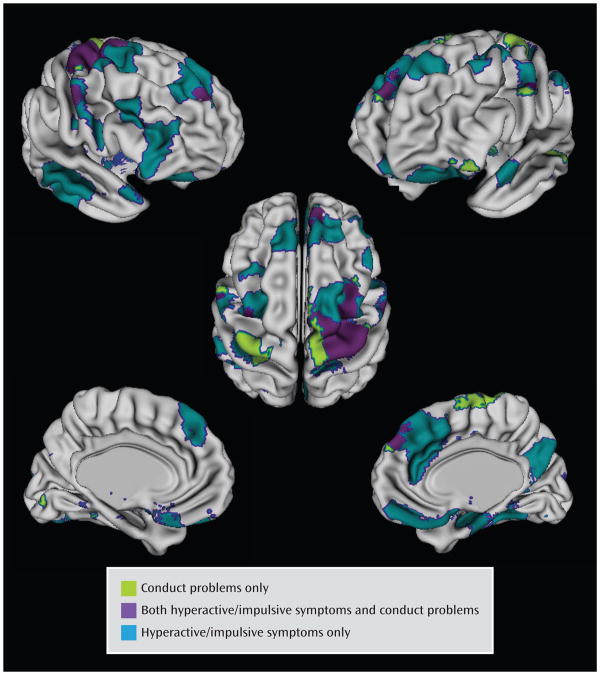
The cortical regions that varied significantly with the conduct problems score (following adjustment for multiple comparisons) were less extensive than those varying significantly with hyperactive/impulsive symptoms and centered on the superior portions of the motor and supplementary motor areas, extending into the middle prefrontal cortex on the right (Figure 4). Here, an increasing conduct problems symptom score was associated with a slower rate of cortical thinning (for each increase of one point, the rate of thinning decreased by −0.037 mm/year [SE=0.01], t=3.2, df=194, p=0.002). In the right motor/supplementary motor and middle frontal regions, growth rates were linked to both conduct problems and hyperactive/impulsive symptom severity. However, most of the regions (89%) where cortical thinning varied significantly with the hyperactive/impulsive symptom score did not vary with the conduct problems score.
FIGURE 4.
Cortical Regions Where Rate of Change Varied Significantly With Conduct Problems Only, Hyperactive/Impulsive Symptoms Only, and Both Symptom Domains
Discussion
The present study gives neurobiological support to the dimensional view of ADHD that typically developing children with no psychiatric diagnoses who have hyperactive/impulsive symptoms demonstrate neurodevelopmental changes resembling those found in youth with the syndrome of ADHD. Specifically, we found that the rate of cortical thinning during late childhood and adolescence was linked to the severity of hyperactive and impulsive symptoms. More symptoms accompanied a slower rate of cortical thinning in predominantly prefrontal cortical regions, mirroring the slower rate of adolescent cortical thinning seen in ADHD. This study also lends support to the concept of a perturbation of cortical trajectory as a fundamental deficit in the pathogenesis of ADHD, rather than reflecting features such as its treatment or comorbidities or the effects of nondisorder-specific functional impairments.
The localization of this effect may have dual significance. First, the regions overlap with cortical areas frequently reported to be structurally compromised in ADHD. Studies of gray matter density or thickness report deficits in the lateral prefrontal cortex, particularly in the right inferior and superior gyri (25–30), as well as prefrontal medial wall deficits extending to the anterior cingulate gyrus (26, 31, 32). Similarly, for some of the posterior regions showing a sensitivity to hyperactive/impulsive symptoms, structural alteration is reported, especially in the right middle/inferior temporal lobe (29, 30) and posterior cingulate/precuneus (25, 31, 33). Second, many of the cortical regions highlighted in this study are components of the neural substrates supporting certain key cognitive processes, and impairments in these domains are in turn linked to hyperactive and impulsive symptoms. These cognitive functions include the ability to inhibit responses in order to achieve later, internally represented goals (34, 35). This cognitive control skill recruits a distributed cortico-striatal network with key involvement of the lateral prefrontal cortex, particularly the right inferior frontal gyrus (36, 37). Aberrant processing of reward and punishment, with a tendency to prefer immediate over delayed but larger rewards, has also been reported in ADHD (38, 39). Studies in healthy subjects implicate a neural network incorporating interactions between the ventral striatum and the limbic system, including orbitofrontal regions (40), where we find cortical development to be linked to hyperactive/impulsive symptoms. Other investigators argue that ADHD partly results from anomalies of the spontaneous intrinsic brain activity that typifies nontask-related cognition (default-mode network), specifically its tendency to intrude into periods of active task-specific processing (41). The default mode network in healthy subjects is thought to comprise medial (medial prefrontal cortex, posterior cingulate/precuneus) and lateral (posterior parietal) brain regions. Interestingly, anomalous resting state activity of the posterior cingulate has been reported in ADHD (42, 43), a region where, again, we link severity of hyperactive/impulsive symptoms to cortical change. It is noteworthy that there was no effect of hyperactive/impulsive symptoms in the superior portions of the motor strip bilaterally. We reported in a previous study that this superior region of the motor strip had the distinction of attaining a peak cortical thickness earlier in ADHD (7) and it also did not show a diagnostic difference in the rate of adolescent cortical change. Thus, we would predict and indeed found that there was no effect of the severity of hyperactivity/impulsive symptoms on cortical change in this region. The association with symptoms was confined to the middle and inferior portions of the motor strip.
In the present study, the stepwise decrease in the rate of prefrontal cortical thinning in typically developing youth, moving from those with no symptoms to those with mild symptoms and then to those with moderate symptoms, was found to extend to youth with a diagnosis of ADHD. This is in keeping with the more severe symptoms of hyperactivity and impulsivity in the clinical group, which had slower rates of thinning than the typically developing youth, affording more support for dimensionality. In the posterior cortical region, this stepwise effect was not found because the ADHD group had rates of cortical thinning comparable to typically developing youth with mild and moderate symptoms. This is not unexpected, since we found less evidence in previous work for diagnostic differences in the properties of cortical trajectories in the posterior cortex (7).
Rates of cortical thinning also showed some links with the presence of conduct problems in typically developing youth, albeit in less extensive cortical regions. Both conduct problems and hyperactive/impulsive symptoms were linked to cortical thinning rates in the right motor/supplementary area, and some degree of overlap is unsurprising given the correlation between these symptom domains. Perhaps more striking is the degree of specificity to symptom domain: the regions where cortical thinning was linked to hyperactive/impulsive symptoms were mostly spatially distinct from those linked to conduct problems. This is congruent with reports of distinct anomalies of brain activation in children with conduct disorder and ADHD (44) and with the lack of similarity between the structural brain anomalies reported in children with conduct disorder and those most frequently reported in ADHD (45, 46).
The 48-item Conners’ Parent Rating Scale we utilized has many strengths, including its psychometric robustness, especially regarding the factor analyses used to derive the subscales for hyperactive/impulsive and conduct problem symptoms (10). During the course of this longitudinal study, the scale was updated (47), but in the interest of maximizing data we continued to use the 48-item version. Both the revised and 48-item versions are similar in their composition, particularly regarding the items loading onto the factor reflecting hyperactivity/impulsivity. As mentioned earlier, the version we used did not return a factor that reflects purely inattentive symptoms. Additionally, while there is evidence from latent class analyses of the 18 DSM-IV symptoms of ADHD that the dimensions of hyperactivity and impulsivity may be in part separable (48–50), the Conners’ Rating Scales do not allow for independent assessment of these domains nor the possibility of partly distinct neural correlates.
We expected that any differences in cortical dynamics within the typically developing youth would be subtle, and thus we focused on the age period with the greatest data density, namely late childhood and adolescence. Previous investigations have established that the dominant effect of age is linear during this age period (51, 52). Further, when our typically developing cohort was divided on the basis of symptom score, there were insufficient data to examine higher-order effects of age because of a relative lack of data for this very young age group. Future inclusion of more data from a younger age group would allow consideration of more complex growth trajectory differences. Our cohort had a relatively high socioeconomic status and IQ, reflecting the self-selected nature, the relative affluence of the geographical area surrounding the study center, and the absence of any mental illness in typically developing participants, which are factors that may limit the generalizability of the findings. We also did not systematically collect measures of potentially important environmental and lifestyle factors such as diet and tobacco, alcohol, and illicit drug use.
Alterations in cortical growth rates will be only one of the many neural changes shown to reflect or drive ADHD. Many perturbations of brain structure and function have been found in the disorder, and it is unclear whether these will also show the dimensionality we report for cortical change.
Conclusion
We demonstrate that the severity of symptoms of hyperactivity and impulsivity in typically developing children affects regional cortical trajectories in a manner similar to that found in the syndrome itself. This gives neurobiological support to the dimensionality of ADHD.
Acknowledgments
Supported by the Intramural Research Program of the National Institute of Mental Health.
Footnotes
The authors report no financial relationships with commercial interests.
References
- 1.Rutter M. Categories, dimensions, and the mental health of children and adolescents. Ann N Y Acad Sci. 2003;1008:11–21. doi: 10.1196/annals.1301.002. [DOI] [PubMed] [Google Scholar]
- 2.Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. A dimensional approach to developmental psychopathology. Int J Methods Psychiatr Res. 2007;16(suppl 1):S16–S23. doi: 10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraemer HC. DSM categories and dimensions in clinical and research contexts. Int J Methods Psychiatr Res. 2007;16(suppl 1):S8–S15. doi: 10.1002/mpr.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lubke GH, Hudziak JJ, Derks EM, van Bijsterveldt TC, Boomsma DI. Maternal ratings of attention problems in ADHD: evidence for the existence of a continuum. J Am Acad Child Adolesc Psychiatry. 2009;48:1085–1093. doi: 10.1097/CHI.0b013e3181ba3dbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polderman TJC, Derks EM, Hudziak JJ, Verhulst FC, Posthuma D, Boomsma DI. Across the continuum of attention skills: a twin study of the SWAN ADHD Rating Scale. J Child Psychol Psychiatry. 2007;48:1080–1087. doi: 10.1111/j.1469-7610.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- 6.Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 7.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAlonan GM, Cheung V, Chua SE, Oosterlaan J, Hung SF, Tang CP, Lee CC, Kwong SL, Ho TP, Cheung C, Suckling J, Leung PW. Age-related grey matter volume correlates of response inhibition and shifting in attention-deficit hyperactivity disorder. Br J Psychiatry. 2009;194:123–129. doi: 10.1192/bjp.bp.108.051359. [DOI] [PubMed] [Google Scholar]
- 9.Stanley JA, Kipp H, Greisenegger E, MacMaster FP, Panchalingam K, Keshavan MS, Bukstein OG, Pettegrew JW. Evidence of developmental alterations in cortical and subcortical regions of children with attention-deficit/hyperactivity disorder: a multivoxel in vivo phosphorus 31 spectroscopy study. Arch Gen Psychiatry. 2008;65:1419–1428. doi: 10.1001/archgenpsychiatry.2008.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyette CH, Conners CK, Ulrich RF. Normative data on revised Conners Parent and Teacher Rating Scales. J Abnorm Child Psychol. 1978;6:221–236. doi: 10.1007/BF00919127. [DOI] [PubMed] [Google Scholar]
- 11.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 12.Reich W. Diagnostic Interview for Children and Adolescents (DICA) J Am Acad Child Adolesc Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv. 2006;9(pt 2):58–66. doi: 10.1007/11866763_8. [DOI] [PubMed] [Google Scholar]
- 15.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 16.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 17.Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab’bagh Y, MacDonald D, Lee JM, Kim SI, Evans AC. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- 20.Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34:1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 22.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- 23.Diggle P, Heagerty PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford, United Kingdom: Oxford University Press; 2002. [Google Scholar]
- 24.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 25.Overmeyer S, Bullmore ET, Suckling J, Simmons A, Williams SC, Santosh PJ, Taylor E. Distributed grey and white matter deficits in hyperkinetic disorder: MRI evidence for anatomical abnormality in an attentional network. Psychol Med. 2001;31:1425–1435. doi: 10.1017/s0033291701004706. [DOI] [PubMed] [Google Scholar]
- 26.McAlonan GM, Cheung V, Cheung C, Chua SE, Murphy DG, Suckling J, Tai KS, Yip LK, Leung P, Ho TP. Mapping brain structure in attention deficit-hyperactivity disorder: a voxel-based MRI study of regional grey and white matter volume. Psychiatry Res. 2007;154:171–180. doi: 10.1016/j.pscychresns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Jiang T, Cao Q, Wang Y. Characterizing anatomic differences in boys with attention-deficit/hyperactivity disorder with the use of deformation-based morphometry. AJNR Am J Neuroradiol. 2007;28:543–547. [PMC free article] [PubMed] [Google Scholar]
- 29.Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- 30.Brieber S, Neufang S, Bruning N, Kamp-Becker I, Remschmidt H, Herpertz-Dahlmann B, Fink GR, Konrad K. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2007;48:1251–1258. doi: 10.1111/j.1469-7610.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 31.Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd JN, Castellanos FX, Rapoport JL. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 32.Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- 33.Carmona S, Vilarroya O, Bielsa A, Tremols V, Soliva JC, Rovira M, Tomas J, Raheb C, Gispert JD, Batlle S, Bulbena A. Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neurosci Lett. 2005;389:88–34. doi: 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 35.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 36.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. erratum in 6:1329. [DOI] [PubMed] [Google Scholar]
- 37.Linden DE. The working memory networks of the human brain. Neuroscientist. 2007;13:257–267. doi: 10.1177/1073858406298480. [DOI] [PubMed] [Google Scholar]
- 38.Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. erratum in 25:533. [DOI] [PubMed] [Google Scholar]
- 39.Sonuga-Barke EJ. Psychological heterogeneity in AD/HD: a dual pathway model of behaviour and cognition. Behav Brain Res. 2003;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- 40.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 41.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Rubia K, Halari R, Smith AB, Mohammed M, Scott S, Giampietro V, Taylor E, Brammer MJ. Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention deficit hyperactivity disorder. Am J Psychiatry. 2008;165:889–897. doi: 10.1176/appi.ajp.2008.07071084. [DOI] [PubMed] [Google Scholar]
- 45.De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, Hodgins S, Viding E. Size matters: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132(pt 4):843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- 46.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS–R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 48.Hudziak JJ, Heath AC, Madden PF, Reich W, Bucholz KK, Slutske W, Bierut LJ, Neuman RJ, Todd RD. Latent class and factor analysis of DSM-IV ADHD: a twin study of female adolescents. J Am Acad Child Adolesc Psychiatry. 1998;37:848–857. doi: 10.1097/00004583-199808000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Neuman RJ, Todd RD, Heath AC, Reich W, Hudziak JJ, Bucholz KK, Madden PA, Begleiter H, Porjesz B, Kuperman S, Hesselbrock V, Reich T. Evaluation of ADHD typology in three contrasting samples: a latent class approach. J Am Acad Child Adolesc Psychiatry. 1999;38:25–33. doi: 10.1097/00004583-199901000-00016. [DOI] [PubMed] [Google Scholar]
- 50.Rasmussen ER, Neuman RJ, Heath AC, Levy F, Hay DA, Todd RD. Replication of the latent class structure of attention-deficit/hyperactivity disorder (ADHD) subtypes in a sample of Australian twins. J Child Psychol Psychiatry. 2002;43:1018–1028. doi: 10.1111/1469-7610.00229. [DOI] [PubMed] [Google Scholar]
- 51.O’Donnell S, Noseworthy MD, Levine B, Dennis M. Cortical thickness of the frontopolar area in typically developing children and adolescents. Neuroimage. 2005;24:948–954. doi: 10.1016/j.neuroimage.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 52.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]