Abstract
N-methyl-D-aspartate (NMDA) receptor subunit-specific probes were used to characterize developmental changes in the distribution of excitatory amino acid receptors in the chicken’s auditory brainstem nuclei. Although NR1 subunit expression does not change greatly during the development of the cochlear nuclei in the chicken (Tang and Carr [2004] Hear. Res 191:79 – 89), there are significant developmental changes in NR2 subunit expression. We used in situ hybridization against NR1, NR2A, NR2B, NR2C, and NR2D to compare NR1 and NR2 expression during development. All five NMDA subunits were expressed in the auditory brainstem before embryonic day (E) 10, when electrical activity and synaptic responses appear in the nucleus magnocellularis (NM) and the nucleus laminaris (NL). At this time, the dominant form of the receptor appeared to contain NR1 and NR2B. NR2A appeared to replace NR2B by E14, a time that coincides with synaptic refinement and evoked auditory responses. NR2C did not change greatly during auditory development, whereas NR2D increased from E10 and remained at fairly high levels into adulthood. Thus changes in NMDA NR2 receptor subunits may contribute to the development of auditory brainstem responses in the chick.
Indexing terms: cochlear nucleus, magnocellularis, laminaris, angularis, tonotopic gradient
Physiological studies have shown that the auditory system is characterized by fast, accurate encoding of auditory information (Warchol and Dallos, 1990; Zhang and Trussell, 1994a,b; Fukui and Ohmori, 2003). We have therefore investigated the distribution of subtypes of N-methyl-D-aspartate (NMDA) receptors (NMDAR) in the brainstem auditory nuclei of the chicken to determine whether changes in receptors may reflect the development of specializations for temporal processing. NMDAR can contain two classes of subunits, NR1 and NR2 (A–D). The third class of subunits (NR3), containing NR3A and NR3B, is thought to act as modulators of the NMDAR (Ciabarra et al., 1995; Nishi et al., 2001; for review see Cull-Candy, 2001). A functional NMDAR appears to consist of two NR1 subunits and two or three NR2 subunits. NR1 is the fundamental subunit necessary for the NMDAR complex, and its expression is spatiotemporally ubiquitous compared with that of NR2 subunits in vertebrate brain (Watanabe et al., 1992; Wada et al., 2004).
Changes in NMDAR composition characterize the development of forebrain circuits. NR2B has been shown to be necessary for synapse formation and plasticity (Tang et al., 1999; Slutsky et al., 2004), whereas NR2A expression is associated with synaptic maturation (Fu et al., 2005). NMDAR made up of NR2B and NR1 subunits are the dominant form during the “critical period” window for plasticity in mouse visual cortex (Quinlan et al., 1999a) and barrel cortex (Lu et al., 2001) and in vocal learning regions of songbirds (Basham et al., 1999; Singh et al., 2000; Ding and Perkel, 2004). Near the end of the critical period, there is a gradual increase in the contribution of NR2A subunits in parallel with changes in NMDAR-mediated currents. These processes can be rapidly modulated by environmental stimuli (Quinlan et al., 1999b; Berardi et al., 2000).
NR1 expression increases in early development, then falls to and/or remains at an intermediate level into adulthood in rat (Akazawa et al., 1994) and mouse (Watanabe et al., 1992) as well as in chick auditory brainstem (Tang and Carr, 2004). NR2 subunits also show developmental changes in their expression. In rodent cortex, NR2B is highly expressed before birth and remains roughly constant into adulthood, whereas NR2A is expressed after birth and increases with age (Watanabe et al., 1992; Sheng et al., 1994; Wenzel et al., 1997). During postnatal development, NR2C is expressed at low levels in cortex but at high levels in cerebellum, whereas NR2D is expressed at high levels in brainstem of mice (Watanabe et al., 1992), rats (Wenzel et al., 1997), and zebra finches (Wada et al., 2004).
NMDAR contribute to auditory nerve responses during development (Zhou and Parks, 1992; Zhang and Trussell, 1994a,b; Futai et al., 2001; Joshi and Wang, 2002; Hoffpauir et al., 2006). In the chick vestibulocochlear nuclei, NMDAR emerge at E6, with functional synapses apparently generated at E7 (Sato and Momose-Sato, 2003). Zhou and Parks (1992) provided pharmacological evidence of age-dependent decreases in NMDAR responses in the auditory neurons in chicks. Replacement of NMDAR by AMPA receptors in the auditory brainstem is correlated with the maturation of functional synapses in both mouse and chick (Kubke and Carr, 1998; Parks, 2000; Futai et al., 2001).
In the present study, we made quantitative measurements of changes in NMDAR subunit expression (NR1, NR2A–D) in chick cochlear nuclei by using in situ mRNA hybridization. NMDAR composition changes with the transition from synapse formation to the emergence of specialized temporal coding in chicken (Tang and Carr, 2004). NR2A replaces NR2B expression after synapse formation, when auditory signals first reach the cochlear nuclei. Both NR1 and NR2D RNA levels increase during development and remain at moderate to high levels into adulthood.
MATERIALS AND METHODS
Probes
DNA templates of the five NMDAR subunits were provided by Drs. Wada and Jarvis (Wada et al., 2004). DNA fragments spanning 400 –500 bp for NR1, NR2A, NR2B, NR2C, and NR2D were amplified by RT-PCR from zebra finch (Taeniopygia guttata) brain total RNA. The NR1 probe contained 417 base pairs (bp; GenBank accession No.: AB042756), corresponding to human 705– 843 amino acids (aa), and extended from the extracellular loop between putative transmembrane regions III and IV to part of the intracellular C-terminal (Sprengel and Seeburg, 1994). The chicken NR1 gene has recently been sequenced and shows 85% homology to mammalian NR1 (Zarain-Herzberg et al., 2005). The NR2A probe (GenBank accession No.: AB042757) contained 521 bp, corresponding to rodent 647– 819 aa, and included the extracellular loop between transmembrane regions III and IV (Sprengel and Seeburg, 1994). The NR2B probe (GenBank accession No.: AB107125) contained 413 bp, corresponding to rodent 801–937 aa, and extended from the extracellular loop between putative transmembrane regions III and IV to the intracellular C-terminal (Sprengel and Seeburg, 1994). The NR2C probe (GenBank accession No.: AB042758) contained 521 bp, corresponding to rodent 645– 817 aa, and comprises whole extracellular loop between transmembrane regions III and IV (Sprengel and Seeburg, 1994). The NR2D probe (GenBank accession No.: AB042759) contained 521 bps, corresponding to rodent 675– 847 aa and including a region similar to that of NR2C (Sprengel and Seeburg, 1994). The cDNA probes were constructed in pGEM-T Easy Vector (Promega, Madison, WI). The SP6 and T7 promoters in the construct were used to synthesize the digoxigenin- and 35S-labeled sense and antisense RNA probes, which were extracted with phenol/chloroform (1:1) and then precipitated twice with ethanol to remove the unincorporated nucleotides. The purified cRNA probes were dissolved in DEPC-water and kept at −80°C.
Animals and tissue preparation
Chickens (white leghorn) were purchased from a local breeder (CBT Farms). Chick ages (number of embryos or chickens) were as follows: E7 (10), E10 (16); E12 (12), E14 (12), E18 (8), P0 (12), P16 (4), and adult (older than 4 months; 3). Numbers of animals in which we carried out in situ hybridization studies with 35S-labeled probes and counted silver grains were: E10, 4 – 6; E12, 4 –5; E14, 4 –5; E18, 4 –5; P0, 4; P16, 3; adult, 3. Numbers varied because the cochlear nucleus was so small that we could not always obtain enough sections of the central regions of NM and NL from one embryo for hybridization with probes against all five subunits.
The time points were selected to correspond to developmental events (see Discussion). Fertilized eggs were incubated in a Marsh automatic incubator (Lyon Electric Company, Chula Vista, CA) at 37°C and 60% humidity. All animal procedures were approved by the University of Maryland Animal Care and Use Committee and followed NIH guidelines.
Embryos up to E18 were anesthetized by cooling and P0 to adult chickens by halothane inhalation, followed by injection of euthasol (Delmarva Laboratories, Inc.). Animals were perfused intracardially with a saline solution (0.9% NaCl), followed by 4% paraformaldehyde in 50 mM phosphate-buffered saline (PBS) for 15 minutes. Dissected brains were postfixed in 4% paraformaldehyde for 8 –10 hours. Brains were placed in 30% sucrose solution in 50 mM PBS for 48 hours at 4°C, then embedded in O.C.T. (Sakura Finetek), and stored at −80°C.
In situ hybridization
Twenty-micrometer coronal or oblique (parallel to the frequency axis) sections were cut on a Leica CM 1850 cryostat and mounted on silanated (amine) nuclease-free slides (CEL Associates, Inc.). To make hybridization comparable across all ages, sections from different ages were put on the same slide such that a single slide contained 10 sections with two sections of E10, E12, and E14, and one section of E18, P0, P16, and adult, respectively. All sections were cut and placed on slides, which were stored desiccated at −80°C. We controlled hybridization and autoradiographic conditions to allow accurate comparisons between batches of sections. In all cases, hybridization experiments were carried out with identical probe concentration and strength, solution volumes and hybridization conditions. Similarly, all autoradiography had identical exposure time and development conditions.
For in situ hybridization, sections were digested with 10 μg/ml proteinase K at 37°C for 10 minutes and fixed in 4% paraformaldehyde for 5 minutes. The sections were then washed three times in PBS, pH 7.5, for 5 minutes each, and acetylated in 0.25% acetic anhydride and 0.1 M tri-ethanolamine in 0.9% NaCl for 10 minutes. After dehydration in a series of graded ethanols, the slides were placed in chloroform for 10 minutes and then in a prehybridization solution containing 50% formamide, 10% dextran sulfate, 10 mM Tris, pH 8.0, 0.3 M NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1× Denhardt’s solution, and 0.5 mg/ml tRNA at 52°C for 2 hours. Subsequently, the sections were incubated in the hybridization solution containing 0.5 μg digoxigenin-labeled probe/ml or 1.0 × 106 cpm/100 μl for radiolabeled probes at 55°C for 16 –18 hours. After briefly rinsing in SSC, the sections were washed twice with SSC for 15 minutes each and incubated in RNase solution with a final concentration of 0.02 mg/ml for 45 minutes. The slides were washed again in 1 × SSC and 0.1 × SSC for 15 minutes at 42°C. Subsequent procedures for digoxigenin-labeled probes hybridization after SSC washing followed a standard method (Tang et al., 2001). For 35S-labeled probe hybridization, the slides were air dried and dipped in NTB2 emulsion diluted 1:1 in water. Sections were exposed at 4°C for 2–3 weeks and then developed with Kodak D-19. Sections were counterstained with 1.0% eosin Y for 15 seconds, followed by 2.0% thionine for 30 minutes. Proteinase K digestion, prehybridization, hybridization, digoxigenin-antibody reactions, and signal development were carried out in a humid environment.
Analyses
Sections were examined by light microscopy in bright-and darkfield. Digital photomicrographs were captured via Neurolucida system (MicroBrightField, Colchester, VT) and processed in Photoshop CS2 (Adobe Systems) to adjust for brightness and contrast. For each probe, the localization of NMDAR mRNA was verified by comparison of sections hybridized to antisense probe with those hybridized to sense probe in the initial three experiments carried out with new probes. No specific signal was obtained when the sections were treated with sense RNA probes. Silver grains were counted above all well-defined cell bodies, identified by the presence of a clear neuronal profile in both NM and NL. In the NL of E10 and E12 embryos, distinct clusters of silver grains over less well defined cytoplasm in the NL monolayer were also identified as cell bodies, because ribonuclease eliminated most substances stained by thionine and eosin Y in young embryos. For each section, we also counted silver grains in 10 circles just outside each brain section near the cochlear nucleus region. These circles were equal in size to the average NM or NL neuron, and they provided background counts for NM and NL neuronal grain counts. For NM and NL, we counted silver grain density in neurons from chickens killed at seven ages (E10, E12, E14, E18, P0, P16, and adult) for NR1, NR2A, and NR2B and at four ages (E10, E14, P0, and adult) for NR2C and NR2D.
Data were normalized by subtracting background grain counts from neuronal grain counts. We then normalized for increasing cell body size during development by measuring cytoplasm area, obtained from digoxigenin material, and using that area to provide silver grain counts per area of labeled cytoplasm. Because there is tonotopic variation in NMDAR expression during development, all counts were taken from the central region of NM and NL. Counts from the same nucleus were pooled within each animal and presented as means ± SD of the grain density per 100 μm2 of cytoplasm, except for the measures of tonotopic variation within a nucleus (see below). All data from grain counts were statistically analyzed by one-way ANOVA. For post hoc comparisons, Sidak tests were performed (SPSS Inc.). Paired comparisons were made among all age groups. Age pairs that showed a significant difference are presented in the text. All neighboring age groups, such as E10 with E12, were compared. If two successive comparisons showed no significant difference, we compared additional age groups. For example, if there were no significant changes between P0 and P16 and from P16 to adult, we compared the difference between P0 and adult. To show the relative expression levels of NMDAR subunits between NM and NL, grain counts of NM and NL at the same developmental age are presented side by side. Otherwise, data are presented by nucleus and describe NR1, NR2A, NR2B, NR2C, and NR2D expression for each structure.
Tonotopic variation in NR1 hybridization was quantified by measuring silver grain density at E14, because our previous study had shown a tonotopic variation in anti-NR1 expression in NM at this age (Tang and Carr, 2004). Oblique sections through NM and NL (parallel to the isofrequency axis) were divided into three approximately equal regions, and grain densities in the cells in the rostromedial, central, and caudolateral regions were determined and analyzed as described above.
RESULTS
There were significant developmental changes in the composition of NMDAR in the chicken NM and NL (Fig. 1). Both NR1 and NR2 subunit mRNAs were present by at least E10. NR2B levels were high early in development but decreased during synaptogenesis, whereas NR2A levels increased. NR2C levels were generally unchanged during development, whereas NR2D became the dominant NR2 subunit after hatching.
Fig. 1.
Auditory nuclei in the hindbrain. Sections of the hindbrain of a P16 chick were stained with antibodies against NR1 (Tang and Carr, 2004). The auditory nerve projects to both NM and NA. All nuclei are tonotopically organized, with high best frequencies rostromedial and low best frequencies caudolateral in NM and NL (Rubel and Parks, 1975, 1988; Warchol and Dallos, 1990). NL receives projections from NM such that ipsilateral axons project to NL dorsal dendrites and contralateral axons to the ventral dendrites (Parks and Rubel, 1975; Young and Rubel, 1986). Scale bar = 500 μm.
Specificity of the probes
The probes used in the present study hybridized specifically to the avian NMDAR subunit mRNAs (Wada et al., 2004). The digoxigenin-labeled probes stained the neuronal cytoplasm (Fig. 2A,B), and the 35S-labeled probes produced clusters of silver grains within neurons (Fig. 2C,D). Both digoxigenin- and 35S-labeled probes displayed consistent patterns of gene expression for all five NMDAR subunits. For example, the NR2D probe conjugated with digoxigenin yielded the heaviest staining in NM and the lightest in NA, with NL intermediate, in adult chicken (Fig. 6F), whereas the 35S-labeled probe hybridization resulted in an average of 16.6 grains/cell in NM, 11.9 grains/cell in NL, and 7.8 grains/cell in NA in parallel sections. We present in situ hybridization results from both probe types and have used the 35S material for quantitation.
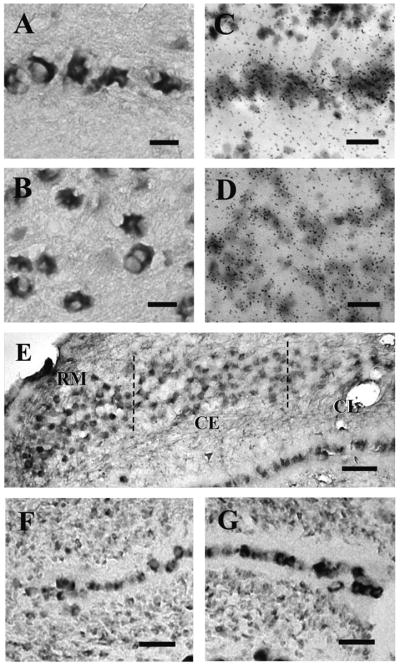
Fig. 2.
Examples of digoxigenin and autoradiographic labeling in chicken NM and NL. A,B: NR1 mRNA expressed in the cytoplasm of adult NL (A) and NM (B), detected by an antibody against digoxigenin. C,D: High levels of NR1 mRNA in E14 NL (C) and NM (D) marked by silver grains overlying the cytoplasm. E–G: NR1 mRNA displayed a gradient of expression in both NM and NL at E14. E: Rostromedial (RM) NM cells expressed higher levels of NR1 mRNA than caudolateral (CL) cells. At E14, central (CE) NM expresses similar levels to rostromedial NM F: Rostromedial NL with lower levels of hybridization. G: Caudolateral NL region with higher level of hybridization. Scale bars = 20 μm in A–C; 40 μm in D; 50 μm in E; 20 μm in F,G.
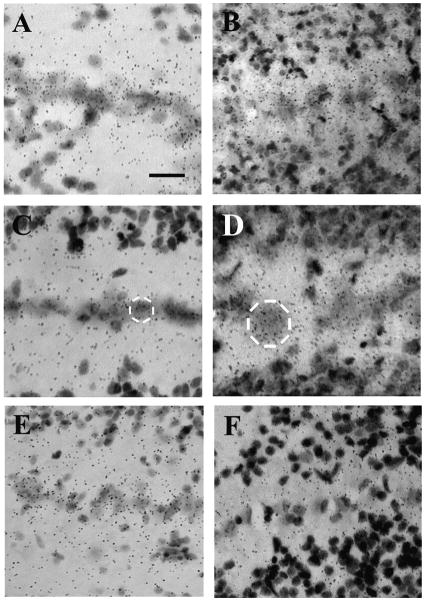
Fig. 6.
NR2C mRNA hybridization in NM and NL (A, E10; C, P0; E, adult). NR2D mRNA increased in both NM and NL during development (B, E10; D, P0; F, adult). A low-power photomicrograph (F) shows the relative strengths of NR2D mRNA expression in NL, NM, and NA. Scale bars = 50 μm in A,C,D,E; 20 μm in B; 300 μm in F. Note change in scale for F.
We also obtained hybridization in Purkinje cells with mRNA probes to NR1 (data not shown), similar to the pattern in mice (Watanabe et al., 1992) and to our previous immunohistochemical study in which we employed the Purkinje cells as positive control (Tang and Carr, 2004). Note that, in contrast to the case in mouse, in which no NR2C is expressed in the Purkinje cells, we found that NR2C was expressed strongly in chick Purkinje cells as well as granule cells.
General expression patterns
The auditory nerve projects to both NM and NA (Fig. 1). NM neurons encode the timing of the auditory stimulus in a pathway that contributes to sound localization based on interaural timing differences (Overholt et al., 1992; Kuba et al., 2002), whereas NA encodes other auditory cues (Carr and Soares, 2002; Fukui and Ohmori, 2003; MacLeod and Carr, 2005). NL receives projections from NM such that ipsilateral axons project to NL dorsal dendrites and contralateral axons to the ventral dendrites (Parks and Rubel, 1975; Young and Rubel, 1986).
The general expression patterns of NMDAR mRNAs were compared in NM, NL, and NA at four ages: E10, E14, P0, and adult (Figs. 2, 3, 6, 7). Other ages were also examined, for a more complete description of developmental changes (Fig. 4). For NR1, NR2A, and NR2B, hybridization signals in NL were greater than those in NM at almost all ages investigated, and signals in NA were intermediate. For NR2C and NR2D, hybridization in NM was greater than that in NL, with NA showing the lowest levels of hybridization. Because NA is both physiologically and morphologically heterogeneous (Soares et al., 2002; Fukui and Ohmori, 2003; Koppl and Carr, 2003; Wada et al., 2004) and is difficult to classify in in situ hybridization material, we did not further describe its cell types in this study.
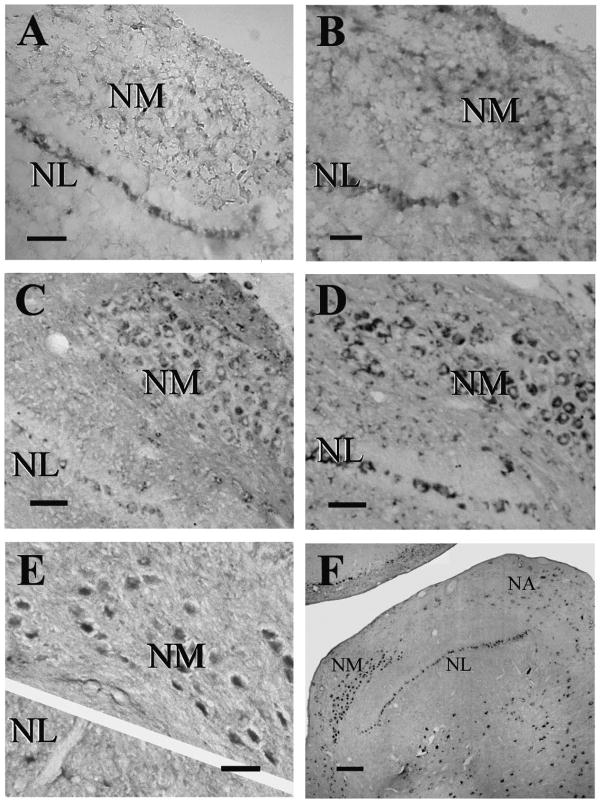
Fig. 3.
Expression patterns of NR1, NR2A, and NR2B mRNA in embryonic NM. NR1 mRNA was moderate in E10 NM (A) and increased by E18 (B). NR2A mRNA was low at E10 (C) and increased by E14 (D). NR2B mRNA was expressed at high levels at E10 (E) and greatly decreased by E14 (F). NM is encircled by dots and NL marked by dashed lines below the cell body layer. Scale bars = 200 μm for A–F; 10 μm for insets.
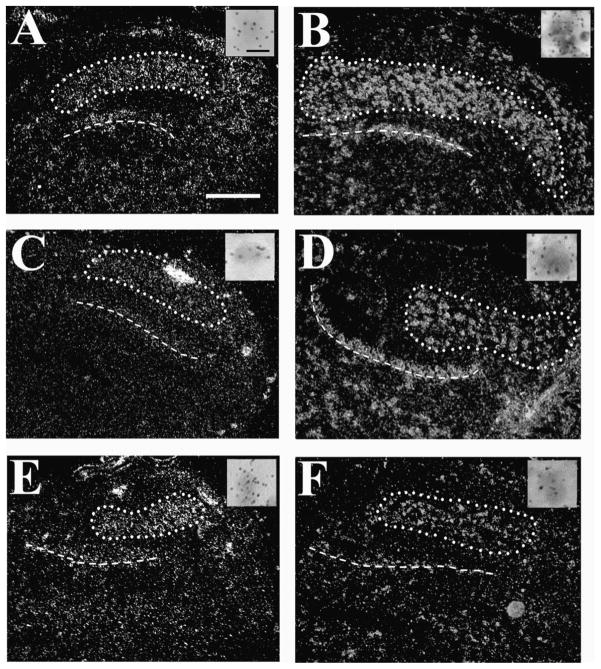
Fig. 7.
Expression patterns of NR1, NR2A, and NR2B mRNA in embryonic NL. NR1 mRNA hybridization levels were moderate in E10 neurons (A) and increased to high levels in E18 (B). NR2A mRNA hybridization was low in E12 (C) and increased by E18 (D; encircled). NR2B mRNA was highly expressed in E10 (E) and decreased greatly by E14 (F). Scale bar = 20 μm.
Fig. 4.
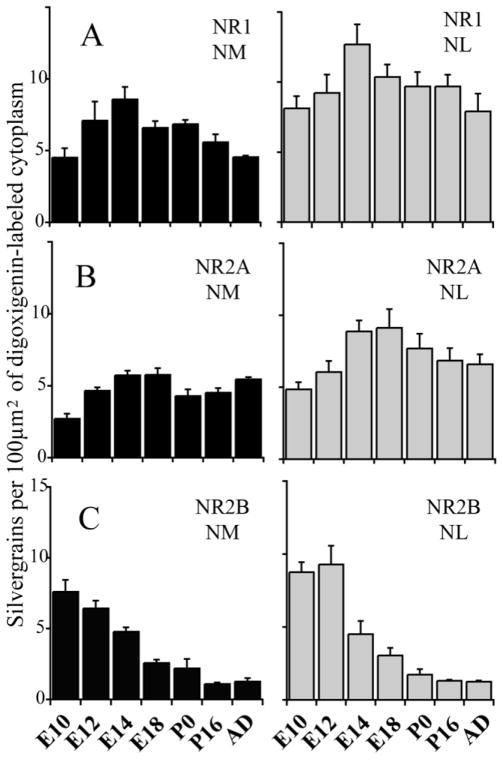
A: NR1 mRNA levels increased between E10 and E14 and then remained at relatively high levels in NL and at moderate levels in NM throughout development. B: NR2A mRNA increased in both NM and NL between E10 and E18 and remained at moderate levels in NL and NM after hatching. C: NR2B mRNA was expressed at high levels at E10 and E12, and declined between E12 and E18. Afterward, NR2B remained at low or negligible levels. Each bar is the normalized mean grain count for all labeled neurons in sections through NM or NL, pooled across animals. Error bars show standard deviations of the mean.
At E10, the earliest age we examined quantitatively, NR1 and NR2B were expressed at moderate levels, and NR2A levels were very low in NM and NL. At about E14, NR2A appeared to replace NR2B in both NM and NL at a time that coincides with synaptic refinement. Around hatching (P0), NR1 was strongly expressed and NR2B expression was weak or undetectable, whereas NR2A, NR2C, and NR2D were found at moderate levels, commensurate with NR1 expression. In the adult NM, NR1 and NR2D were highly expressed, and NR2A and NR2C were expressed at moderate levels. In adult NL and NA, NR2D was expressed at a moderate levels, and the other subunits showed patterns similar to those of NM.
Nucleus magnocellularis (NM)
NM is tonotopically organized, with high best frequencies rostromedial and low best frequencies caudolateral (Rubel and Parks, 1975; Warchol and Dallos, 1990). NM neurons receive auditory nerve input, and excitatory postsynaptic currents mediated by NMDAR peak at about E14 and then decrease (Zhou and Parks, 1992; Lu and Trussell, 2002).
NR1
NR1 mRNA was detected in NM and NL at E7– 8, the earliest age in our study. We could not, however, measure NR1 mRNA quantitatively in these sections because of the low signal-to-noise ratio. NR1 mRNA levels increased steadily from E10 to E14 in NM (Figs. 2D, 3A, 4A) and reached a peak at E14. Afterward, NR1 was expressed at moderate levels in NM into adulthood, although some fluctuations in level were observed (Figs. 2B, 3B, 4A). A one-way ANOVA indicated significant differences with age.
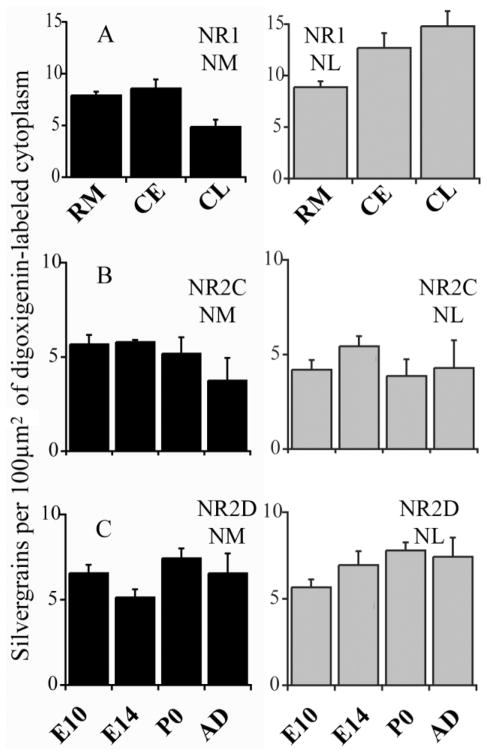
Early in development, NR1 mRNA was expressed in a tonotopic gradient in NM (Fig. 2E). Oblique sections through NM, orthogonal to the tonotopic axis, were used to show a wide frequency range in single sections (Tang and Carr, 2004). At E14, the expression pattern in NM was analyzed by dividing NM into three parts, from rostromedial to caudolateral (Fig. 2E). Higher levels of NR1 mRNA hybridization were found within the rostromedial one-third of NM, the future high best frequency region, than in the caudolateral one-third, the future low best frequency region (one-way ANOVA, Sidak test, P < 0.01). NR1 mRNA levels in the central region of NM were similar to those in the rostromedial one-third (rostromedial and central comparison, Sidak test, P > 0.05), whereas the difference between central and caudolateral regions was significant (Sidak test, P < 0.01; Fig. 5A). The gradient in NR1 mRNA expression was almost gone by E18, similar to our previous study, which showed that the NR1 protein expression gradient in NM appeared at E12, peaked at E14 –15, and was no longer detected at E19 (Tang and Carr, 2004).
Fig. 5.
A: NR1 mRNA levels in rostromedial NM and NL were similar, whereasd levels were significantly higher in caudolateral NL than that in NM. In NM, NR1 mRNA levels were significantly lower in the caudolateral regions than in the rostromedial and central regions; in NL, NR1 hybridization levels were higher in caudolateral than rostromedial regions. RM, rostromedial; Ce, central; CL, caudolateral. B: Changes of NR2C mRNA in NM decreased slightly after hatching and generally decreased in NL. C: NR2D mRNA in both NM and NL displayed a slight increase with age. Error bars show standard deviations.
NR2A and NR2B
Each NR2 subunit may impart different characteristics to functional NMDAR (Kutsuwada et al., 1992; Loftis and Janowsky, 2003), and changes in NMDAR subunit composition might have consequences for activity-dependent development (Perez-Otano and Ehlers, 2005). Expression of NR2A mRNA in NM increased from E10 (Fig. 3C) to E14 (Fig. 3D; see also Fig. 4B) and decreased from E18 to P0. After hatching, NR2A mRNA remained at low to moderate levels (Fig. 4B). A multiple-comparisons test indicated a significant difference with development (one-way ANOVA, P < 0.01). Post hoc Sidak tests showed that NR2A mRNA increased significantly from E10 to E12 and from E12 to E14 (P < 0.01).
NR2B mRNA was expressed strongly in NM at E10 (Fig. 3E) and E12 (Fig. 4C). This expression decreased greatly between E14 and E18 (Figs. 3F, 4C) and was correlated with an increase in NR2A hybridization (compare Fig. 4B and C). After hatching, NR2B mRNA was either absent or expressed at very low levels. Post hoc Sidak tests showed highly significant decreases from E12 to E14 and from E14 to E18.
NR2C and NR2D
In NM, NR2C mRNA levels were generally unchanged during development (Fig. 6, left; see also Fig. 5B). NR2D mRNA hybridization increased significantly between E14 and P0 (P < 0.01; Fig. 6 right; see also Fig. 5C). Even though NR2D expression decreased slightly in adults, the expression levels in NM were the highest among all subunits examined.
Nucleus laminaris (NL)
NL neurons act as coincidence detectors and compute sensitivity to interaural time differences (Overholt et al., 1992; Kuba et al., 2002). Chicken NL is largely composed of a monolayer of cell bodies with symmetrical dorsal and ventral dendritic arbors (Smith, 1981; Jhaveri and Morest, 1982a,b). NL has a tonotopic organization similar to that of NM (Fig. 1; Rubel and Parks, 1975). NR1 immunoreactivity first appears in NL cell bodies, sandwiched by largely unstained neuropil, and later is expressed in both cell bodies and neuropil (Tang and Carr, 2004).
NR1
NR1 hybridization in NL was greater than in NM for each age sampled (Fig. 4A). In NL, NR1 mRNA increased from E10 to E14 and remained at moderate levels into adulthood (Fig. 2C, 7A). The total difference among the sampled ages was significant (one-way ANOVA, P < 0.01). NR1 expression in NL changed significantly during embryonic stages and gradually after post-hatch (Fig. 4A), with significant changes from E12 and E14 and from E14 to E18 (Sidak test, P < 0.01).
As in NM, NR1 mRNA was expressed in a tonotopic gradient in NL at E14. Contrary to our findings in NM, however, the gradient was in the opposite direction, with the highest NR1 levels in the lateral, future low best frequency region. More NR1 mRNA was found within the caudolateral third (Fig. 2F) of NL than in the rostromedial one-third (Fig. 2G), the future high best frequency region. The mean level of NR1 mRNA in the central region of NL was lower than that in the caudolateral region (one-way ANOVA, Sidak test, P < 0.01) and higher than that in rostromedial region (Sidak test, P < 0.01; Fig. 5A). This gradient in mRNA expression was clearly visible at E14, could still be observed at E18, and was not detected at hatching. It should be noted that our immunohistochemical investigation of NR1 revealed heterogeneous staining in NR1 protein in NL during the corresponding developmental stages but no gradient (Tang and Carr, 2004).
NR2A and NR2B
As in NM, NR2A mRNA levels increased between E10 and E18 in NL, whereas NR2B levels decreased (Figs. 4, 7C,D). There was a large increase in NR2A hybridization between E12 and E14. NR2A expression remained at high levels at both E14 and E18 and decreased around and after hatching (Fig. 4B). There was significant variance with development (ANOVA, P < 0.01). NR2B mRNA expression levels in NL were high at both E10 and E12 (Fig. 4C) and declined steeply after E12 (Fig. 7E,F). Significant decreases continued between E12 and E14 and between E14 and E18 (Sidak test, P < 0.01). As in NM, NR2B mRNA levels were either very low or absent after hatching.
NR2C and NR2D
There was no significant difference in NR2C mRNA levels among age groups in NL (Fig. 5B). NR2C mRNA in NL decreased slightly around the time of hatching (Fig. 6, left). Unlike NR2C, NR2D mRNA expression in NL displayed a steady increase from E10 to hatching (Fig. 5C). After NR1, NR2D was the most prominent subunit in NL after hatching.
DISCUSSION
Early NMDAR expression is correlated with the appearance of electrical activity and synaptic responses in NM and NL. NR1 was detected in E7– 8 sections, at a time when Sato and Momose-Sato (2003) recorded responses in the vestibulocochlear nuclei evoked by cochlear or vestibular nerve stimulation, components of which were abolished by NMDAR antagonists (Sato and Momose-Sato, 2003). NR2A and NR2B mRNA levels changed significantly during the period of synaptogenesis, suggesting a role for NMDAR in formation of auditory brainstem circuits.
For investigation of mRNA expression, a significant technical issue is the possibility of a discrepancy between in situ hybridization and immunohistochemical results; e.g., protein expression levels are not necessarily correlated with mRNA expression levels (Sucher et al., 1993). We compared our in situ hybridization data with our previous immunohistochemical data (Tang and Carr, 2004) and found only slight differences between the two data sets. Specifically, we had found that NR1 immunoreactivity decreases after E18 and remains at moderate levels into adulthood. We had also observed a tonotopic gradient of NR1 expression in NM from E12 to E18 (Tang and Carr, 2004). Finally, we found that NR2A subunit immunoreactivity increases, whereas NR2B subunit immunoreactivity decreases from E12 to E18 (Tang et al., 2004). These results are in agreement with the present in situ hybridization study. Thus it appears that the general developmental trends for mRNA and protein were similar in chicken auditory brainstem.
Glutamate receptors in mammalian and avian auditory development
At age E10, NR1 and NR2B are the dominant subunits in NM. This is also the age when Jackson et al. (1982) were first able to record the eighth nerve afferent volley from the surface of the brainstem above NM. By E12, high levels of NR2B expression coincide with changes in NM dendrites and with the formation of terminal axonal arbors in the dorsal and ventral neuropil of NL (Jackson et al., 1982; Jhaveri and Morest, 1982a,b). We suggest that NMDAR containing NR1 and NR2B contribute to the evoked responses to intense acoustic stimuli first recorded from the brainstem at E11–12 (Saunders et al., 1973).
At about E14, the chick embryo begins to respond behaviorally to environmental sounds (Jackson and Rubel, 1978). Also at this time, levels of NR1 mRNA reach their expression peak, and NR2B subunits are replaced by NR2A subunits. Several developmental events are correlated with this switch in NMDAR subunit expression. First, there is a reduction in the number of cochlear nerve axons innervating individual NM neurons, and a transformation of preterminal branches into calycine endbulb terminals (Jackson and Parks, 1982; Jhaveri and Morest, 1982a). Second, EPSCs mediated by NMDAR peak at about E14 and decrease by 2.5-fold 2 days later (Lu and Trussell, 2002). Third, rhythmic bursting begins at E14 and gives way to an adult-like, steady level of firing on E19, 2 days prior to hatching (Lippe, 1995).
The NMDA component of the response in NM evoked by auditory nerve stimulation continues to decrease with maturation of the auditory system. In E18 chick NM, EPSPs and EPSCs are composed of a large, brief, AMPA receptor-mediated component and a smaller, slowly decaying NMDAR-mediated component (Zhang and Trussell, 1994a,b). Our results suggest that the NMDAR consists largely of NR1, NR2A, and NR2D at this time.
Maturation of the auditory nerve synapse continues after hatching. Posthatch chicks (P1–11) exhibit a higher probability of firing a well-timed postsynaptic action potential in comparison with E18 synapses during high-frequency stimulation of the auditory nerve (Brenowitz and Trussell, 2001). Improvements in the reliability and timing of postsynaptic spikes in chick NM are accompanied by a developmental increase in steady-state EPSCs during stimulus trains and a decline in synaptic depression (Brenowitz and Trussell, 2001). These changes are attributed, at least in part, to reduced AMPA receptor desensitization but might also be affected by change in NMDAR expression. Similarly, in the mouse, where the early postnatal developmental period corresponds to a late embryonic stage in chick (Kubke and Carr, 2000), synaptogenesis in the calyx of Held is marked by both AMPA-and NMDAR-mediated currents (Hoffpauier et al., 2006). The mean amplitude of NMDA EPSCs in calicine terminals in the mouse medial nucleus of the trapezoid body (MNTB) decreases in a stepwise fashion between P5 and P13, whereas the mean amplitude of AMPA EPSCs increases steadily from P5 to P15 (Futai et al., 2001; Joshi and Wang, 2002; Youssoufian et al., 2005). In rat MNTB, AMPA receptor-mediated EPSCs as well as quantal synaptic currents acquire progressively faster kinetics, whereas NMDAR-mediated EPSCs diminish with age, as indicated by a 50% reduction in mean amplitude and faster decay kinetics from P5 to P14 (Taschenberger and von Gersdorff, 2000). The mean conductance of both spontaneous and evoked NMDA-EPSCs in the endbulb-bushy cell synapse decreased by more than half between P4 –11 and P12–22 (Isaacson and Walmsley, 1995; Bellingham et al., 1998). Thus in both MNTB and cochlear nucleus, AMPA-receptor-mediated evoked synaptic responses become larger and faster, and NMDAR-mediated EPSCs diminish with age. Pharmacological studies in chicks further support the observation that NMDAR decrease in number and/or efficacy with age (Zhou and Parks, 1992).
Although both physiological and pharmacological studies show that the importance of NMDAR-mediated EPSCs decreases with age, we found that NR1 and NR2D remained at moderate levels into adulthood. Posthatch NMDAR might include two or three types: one containing NR1/NR2A, the second NR1/NR2D and/or NR1/NR2A/NR2D. NR2C is present at lower levels than NR2A and NR2D in adult and may be incorporated into NMDAR only with low efficiency. Similar to the NR2A and NR2D expression patterns in chick, both the NR2A and the NR2D subunits were expressed in all cochlear nuclei and superior olivary complex in wild-type mice (Munemoto et al., 1998). Significant threshold elevation of the auditory brainstem response in the NR2D mutant mice implies that NR2D plays a critical but as yet unknown role in auditory brainstem function (Munemoto et al., 1998). The role of NR2C in the development of auditory brainstem is also not known, although NR2C expression increases in cerebellar granule cells during development (Akazawa et al., 1994).
In rodents, investigations of both protein and RNA levels also show little, if any, age-related decline in NMDAR expression in the auditory brainstem (Bilak et al., 1996; Caicedo and Eybalin, 1999). The cochlear and vestibular ganglia of the rat exhibit high levels of NR1, moderate levels of NR2B and NR2D, and lower levels of NR2A and NR2C (Niedzielski and Wenthold, 1995). Furthermore, NR1 staining of neuronal somata in some regions of the gerbil cochlear nucleus increases from P7 to P28 (Joelson and Schwartz, 1998). Even in the adult rat, heavy labeling of NR1 mRNA is observed in all major cochlear nucleus neuronal types (Sato et al., 1998). These results from rodents are generally similar to our previous immunohistochemical study (Tang and Carr, 2004) and our current in situ hybridization results in chickens and barn owl (Tyto alba; data not shown). NMDAR expression in the chicken is not identical to that in the rat, however, where NR2D expression in the superior olivary complex was generally lower than that of NR2A and NR2C (Sato et al., 1998).
The reasons for the discrepancy between the anatomical and physiological studies remain to be determined. Studies in cell culture, cortex, and hippocampus show that NMDAR may regulate AMPA receptor trafficking with interacting proteins and function through its phosphorylation (Esteban, 2003; Sheng and Lee, 2001; Song and Huganir, 2002). NR2A and NR2B may both regulate AMPA receptor distribution in mature neurons. For example, NR2A-containing NMDAR promote, whereas NR2B-containing NMDAR inhibit, the surface expression of GluR1 (Kim et al., 2005). In addition, a third NMDAR subunit, NR3, could regulate NMDAR transmission in mammalian motoneurons (Ciabarra et al., 1995; Nishi et al., 2001). It is not known whether NR3 is found outside mammals.
NMDAR subunit changes and development of the circuit for detection of interaural time differences
NMDAR containing NR2B and NR1 subunits may play a role in the formation of the early postsynaptic responses in the projection from NM to NL. The NM axons act as delay lines to construct a map of interaural time differences in NL (Carr and Konishi, 1990; Overholt et al., 1992; Kuba et al., 2002). NR1 and NR2B mRNA first appear in NL before E10, when the NL cells are aligned in a compact layer. Between E10 and E11, NM axons arrive above NL, and the first synaptic connections form between NM and NL (Jackson et al., 1982; Young and Rubel, 1986). NL responses could be elicited by direct stimulation of the contralateral NM at E11, prior to the first NL responses to eighth nerve stimulation, which were recorded at E12 (Jackson et al., 1982).
The timing of NR2A expression in NL is consistent with a role in the maturation of synaptic connections. Between E12 and E14, most NR2B containing receptors in NL were replaced by receptors containing NR2A and/or NR2D. This transition may be important in the development of the NM-NL circuit. Rhythmic bursting in the auditory nerve begins as early as E14, and Lippe (1995) has proposed that the pattern of spontaneous discharges could provide developmental cues for the spatial ordering of auditory projections (Lippe, 1995). E14 is also when responses to loud environmental sound signals may be recorded in the cochlear nuclei (Saunders et al., 1973; Jackson and Rubel, 1978).
The refinement of the axonal synaptic connections from NM to NL takes place between E14 and E16, when both the dorsal and the ventral terminal fields of NM axons form narrow bands across NL (Smith, 1981; Young and Rubel, 1986; Parks et al., 1987). Synaptophysin and syntaxin immunoreactivity also increase in NM and NL between E12 and E16 (Alladi et al., 2002). Modeling studies in the barn owl have proposed that formation of a computational map for interaural time differences in NL is mediated by the action of homosynaptic spike-based Hebbian learning (Kempter et al., 2001; Leibold et al., 2001). NR2A and NR2B subunits are of central importance in models of Hebbian learning and memory (Lisman et al., 2002; Liu et al., 2004; Massey et al., 2004), and we propose that early expression of NR2B contributes to synapse formation in the circuit for detection of interaural time differences.
The transition from NR2B to NR2A coincides with the change in the spontaneous firing of NL neurons from an immature rhythmic bursting pattern at E14 to an adult-like steady firing pattern at E19 (Lippe, 1995). The accuracy of coincidence detection also improved between E17 and the first week after hatching (Kuba et al., 2002). After E18, NR2A, NR2D, and NR1 are the dominant components in both NM and NL and may contribute to the maturation of responses in NL (Rubel et al., 1976).
The transition from NR2B to NR2A is similar to the results from studies of developing cortex and songbird forebrain. Forebrain NMDAR are composed of NR2B and NR1 subunits during the critical period, followed by a progressive inclusion of the NR2A subunit in parallel with changes in NMDAR-mediated currents (Stocca and Vicini, 1998; Basham et al., 1999; Quinlan et al., 1999a; Singh et al., 2000; Ding and Perkel, 2004; Liu et al., 2004). Later in development, the proportion of NMDAR consisting of NR2B and NR1 in visual cortex may be altered by changing visual experience (Quinlan et al., 1999b; Philpot et al., 2001).
Gradients in NMDAR distribution
NR1 displayed a gradient in expression during development. In NM, the gradient was first observed at E12, peaked between E14 and E15, and was no longer detected at E19 (Tang and Carr, 2004). GluR2/3 and GluR4 AMPA receptors exhibit a similar development gradient in the barn owl NM (Kubke and Carr, 1998). The gradients in both mRNA and protein expression suggest that a developmental wave of excitatory synaptic consolidation and refinement progressed along the future tonotopic axis in avian NM. NM neurons also show a gradient in the loss of dendrites between E13 and E17 (Parks and Jackson, 1984). Whether developmental gradients in NR1 and other subunits expressed in NM reflect a gradient in the functional development of eighth nerve-NM synapses, a developmental gradient in the excitability of eighth nerve axons, or a gradient in excitability of NM cells remains to be examined.
NL showed a gradient in NR1 mRNA expression in a direction opposite that in NM. The highest NR1 mRNA levels were found in the caudolateral region and lowest in the rostromedial, whereas the central part of NL showed a level similar to that in the caudolateral region. The size gradient in NL dendritic length begins to form at about E14, with a progressive decrease in dendritic length beginning in the rostromedial region (Smith, 1981). Our previous immunohistochemical study did not, however, show consistent gradients in NL during late embryonic development, suggesting that posttranslation modification may regulate NMDAR expression in NL (Tang and Carr, 2004).
Possible subunit components in NMDAR in chick cochlear nuclei
The precise subunit stoichiometry of the NMDAR remains unclear. There is evidence for both a tetrameric assembly (Laube et al., 1998; Schorge and Colquhoun, 2003) and a pentameric assembly (Premkumar and Auerbach, 1997; Hawkins et al., 1999; Opella et al., 1999). Most studies show two NR1 subunits in a functional NMDAR complex (Behe et al., 1995; Chazot and Stephenson, 1997).
In chick auditory brainstem before E14, NMDAR in the cochlear nuclei appear to be made up of both NR1 and NR2 subunits, most likely NR2B. After environmental sounds reach the cochlear nucleus, there is a gradual increase in the contribution of NR2A subunits to the NMDAR. The NMDAR stoichiometry may change to include more NR2A (Wafford et al., 1993; Luo et al., 1997). In addition, there also is the possibility that some NMDAR are composed of only two subunits, NR1/NR2A or NR1/NR2B (Blahos and Wenthold, 1996; Chazot and Stephenson, 1997). During posthatch development, NR2D is the dominant NR2 subunit, by which time the major NMDAR may consist of NR1, NR2D, and/or NR2A (Dunah et al., 1998). NR2C may be inefficiently recruited into NMDAR (Chazot et al., 1994). NR2D has been shown to contribute to auditory brainstem responses, insofar as thresholds were elevated in mice lacking this subunit (Munemoto et al., 1998).
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: DC00436 (to C.E.C.); Grant number: P30 DC0466 (to the University of Maryland Center for the Evolutionary Biology of Hearing).
We thank Drs. E. Jarvis and K. Wada for providing probes and suggestions on the manuscript and Drs. T. Finger, K. MacLeod, and H. Adler for helpful comments.
LITERATURE CITED
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-D-aspartate receptor subunit mR-NAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347:150–160. doi: 10.1002/cne.903470112. [DOI] [PubMed] [Google Scholar]
- Alladi PA, Wadhwa S, Singh N. Effect of prenatal auditory enrichment on developmental expression of synaptophysin and syntaxin 1 in chick brainstem auditory nuclei. Neuroscience. 2002;114:577–590. doi: 10.1016/s0306-4522(02)00319-6. [DOI] [PubMed] [Google Scholar]
- Basham ME, Sohrabji F, Singh TD, Nordeen EJ, Nordeen KW. Developmental regulation of NMDA receptor 2B subunit mRNA and ifenprodil binding in the zebra finch anterior forebrain. J Neurobiol. 1999;39:155–167. [PubMed] [Google Scholar]
- Behe P, Stern P, Wyllie DJ, Nassar M, Schoepfer R, Colquhoun D. Determination of NMDA NR1 subunit copy number in recombinant NMDA receptors. Proc Biol Sci. 1995;262:205–213. doi: 10.1098/rspb.1995.0197. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Lim R, Walmsley B. Developmental changes in EPSC quantal size and quantal content at a central glutamatergic synapse in rat. J Physiol. 1998;511:861–869. doi: 10.1111/j.1469-7793.1998.861bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Bilak MM, Bilak SR, Morest DK. Differential expression of N-methyl-D-aspartate receptor in the cochlear nucleus of the mouse. Neuroscience. 1996;75:1075–1097. doi: 10.1016/0306-4522(96)00197-2. [DOI] [PubMed] [Google Scholar]
- Blahos J, 2nd, Wenthold RJ. Relationship between N-methyl-D-aspartate receptor NR1 splice variants and NR2 subunits. J Biol Chem. 1996;271:15669–15674. doi: 10.1074/jbc.271.26.15669. [DOI] [PubMed] [Google Scholar]
- Brenowitz S, Trussell LO. Maturation of synaptic transmission at end-bulb synapses of the cochlear nucleus. J Neurosci. 2001;21:9487–9498. doi: 10.1523/JNEUROSCI.21-23-09487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Eybalin M. Glutamate receptor phenotypes in the auditory brainstem and mid-brain of the developing rat. Eur J Neurosci. 1999;11:51–74. doi: 10.1046/j.1460-9568.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci. 1990;10:3227–3246. doi: 10.1523/JNEUROSCI.10-10-03227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CE, Soares D. Evolutionary convergence and shared computational principles in the auditory system. Brain Behav Evol. 2002;59:294–311. doi: 10.1159/000063565. [DOI] [PubMed] [Google Scholar]
- Chazot PL, Stephenson FA. Molecular dissection of native mammalian forebrain NMDA receptors containing the NR1 C2 exon: direct demonstration of NMDA receptors comprising NR1, NR2A, and NR2B subunits within the same complex. J Neurochem. 1997;69:2138–2144. doi: 10.1046/j.1471-4159.1997.69052138.x. [DOI] [PubMed] [Google Scholar]
- Chazot PL, Coleman SK, Cike M, Stephenson FA. Molecular characterization of N-methyl-D-aspartate receptors expressed in mammalian cells yields evidence for the coexistence of three subunit types within a discrete receptor molecule. J Biol Chem. 1994;269:24403–24409. [PubMed] [Google Scholar]
- Ciabarra AM, Sullivan JM, Gahn LG, Pecht G, Heinemann S, Sevarino KA. Cloning and characterization of x-1: a developmentally regulated member of a noval class of the ionotropic glutamate receptor family. J Neurosci. 1995;15:6498–6508. doi: 10.1523/JNEUROSCI.15-10-06498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Current Opinion in Neurobiology. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Ding L, Perkel DJ. Long-term potentiation in an avian basal ganglia nucleus essential for vocal learning. J Neurosci. 2004;24:488–494. doi: 10.1523/JNEUROSCI.4358-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Luo J, Wang YH, Yasuda RP, Wolfe BB. Subunit composition of N-methyl-D-aspartate receptors in the central nervous system that contain the NR2D subunit. Mol Pharmacol. 1998;53:429–437. doi: 10.1124/mol.53.3.429. [DOI] [PubMed] [Google Scholar]
- Esteban JA. AMPA receptor trafficking: a road map for synaptic plasticity. Mol Intervent. 2003;3:375–385. doi: 10.1124/mi.3.7.375. [DOI] [PubMed] [Google Scholar]
- Fu Z, Logan SM, Vicini S. Deletion of the NR2A subunit prevents developmental changes of NMDA-mEPSCs in cultured mouse cerebellar granule neurones. J Physiol. 2005;563:867–881. doi: 10.1113/jphysiol.2004.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui I, Ohmori H. Developmental changes in membrane excitability and morphology of neurons in the nucleus angularis of the chicken. J Physiol. 2003;548:219–232. doi: 10.1113/jphysiol.2002.036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai K, Okada M, Matsuyama K, Takahashi T. High-fidelity transmission acquired via a developmental decrease in NMDA receptor expression at an auditory synapse. J Neurosci. 2001;21:3342–3349. doi: 10.1523/JNEUROSCI.21-10-03342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins LM, Chazot PL, Stephenson FA. Biochemical evidence for the co-association of three N-methyl-D-aspartate (NMDA) R2 subunits in recombinant NMDA receptors. J Biol Chem. 1999;274:27211–27218. doi: 10.1074/jbc.274.38.27211. [DOI] [PubMed] [Google Scholar]
- Hoffpauir BK, Grimes JL, Mathers PH, Spirou GA. Synaptogenesis of the calyx of Held: rapid onset of function and one-to-one morphological innervation. J Neurosci. 2006;26:5511–5523. doi: 10.1523/JNEUROSCI.5525-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Receptors underlying excitatory synaptic transmission in slices of the rat anteroventral cochlear nucleus. J Neurophysiol. 1995;73:964–973. doi: 10.1152/jn.1995.73.3.964. [DOI] [PubMed] [Google Scholar]
- Jackson H, Parks TN. Functional synapse elimination in the developing avian cochlear nucleus with simultaneous reduction in cochlear nerve axon branching. J Neurosci. 1982;2:1736–1743. doi: 10.1523/JNEUROSCI.02-12-01736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson H, Rubel EW. Ontogeny of behavioral responsiveness to sound in the chick embryo as indicated by electrical recordings of motility. J Comp Physiol Psychol. 1978;92:682–696. doi: 10.1037/h0077496. [DOI] [PubMed] [Google Scholar]
- Jackson H, Hackett JT, Rubel EW. Organization and development of brain stem auditory nuclei in the chick: ontogeny of postsynaptic responses. J Comp Neurol. 1982;210:80–86. doi: 10.1002/cne.902100109. [DOI] [PubMed] [Google Scholar]
- Jhaveri S, Morest DK. Sequential alterations of neuronal architecture in nucleus magnocellularis of the developing chicken: a Golgi study. Neuroscience. 1982a;7:837–853. doi: 10.1016/0306-4522(82)90046-x. [DOI] [PubMed] [Google Scholar]
- Jhaveri S, Morest DK. Neuronal architecture in nucleus magnocellularis of the chicken auditory system with observations on nucleus laminaris: a light and electron microscope study. Neuroscience. 1982b;7:809–836. doi: 10.1016/0306-4522(82)90045-8. [DOI] [PubMed] [Google Scholar]
- Joelson D, Schwartz IR. Development of N-methyl-D-aspartate receptor subunit immunoreactivity in the neonatal gerbil cochlear nucleus. Microsc Res Techniq. 1998;41:246–262. doi: 10.1002/(SICI)1097-0029(19980501)41:3<246::AID-JEMT8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Joshi I, Wang LY. Developmental profiles of glutamate receptors and synaptic transmission at a single synapse in the mouse auditory brainstem. J Physiol. 2002;540:861–873. doi: 10.1113/jphysiol.2001.013506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempter R, Leibold C, Wagner H, van Hemmen JL. Formation of temporal-feature maps by axonal propagation of synaptic learning. Proc Natl Acad Sci U S A. 2001;98:4166–4171. doi: 10.1073/pnas.061369698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A-and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Koppl C, Carr CE. Computational diversity in the cochlear nucleus angularis of the barn owl. J Neurophysiol. 2003;89:2313–2329. doi: 10.1152/jn.00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba H, Koyano K, Ohmori H. Development of membrane conductance improves coincidence detection in the nucleus laminaris of the chicken. J Physiol. 2002;540:529–542. doi: 10.1113/jphysiol.2001.013365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubke MF, Carr CE. Development of AMPA-selective glutamate receptors in the auditory brainstem of the barn owl. Microsc Res Techniq. 1998;41:176–186. doi: 10.1002/(SICI)1097-0029(19980501)41:3<176::AID-JEMT2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kubke MF, Carr CE. Development of the auditory brainstem of birds: comparison between barn owls and chickens. Hear Res. 2000;147:1–20. doi: 10.1016/s0378-5955(00)00116-7. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, et al. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci. 1998;18:2954–2961. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold C, Kempter R, van Hemmen JL. Temporal map formation in the barn owl’s brain. Phys Rev Lett. 2001;87:248101. doi: 10.1103/PhysRevLett.87.248101. [DOI] [PubMed] [Google Scholar]
- Lippe WR. Relationship between frequency of spontaneous bursting and tonotopic position in the developing avian auditory system. Brain Res. 1995;703:205–213. doi: 10.1016/0006-8993(95)01096-3. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- Lu HC, Gonzalez E, Crair MC. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron. 2001;32:619–634. doi: 10.1016/s0896-6273(01)00501-3. [DOI] [PubMed] [Google Scholar]
- Lu T, Trussell LO. Program No. 146.1. 2002 Abstract viewer and itinerary planner. Washington, DC: Society for Neuroscience; 2002. The early development of glutamatergic synapses in nucleus magnocellularis; p. 2002. [CD-ROM] [Google Scholar]
- Luo J, Wang Y, Yasuda RP, Dunah AW, Wolfe BB. The majority of N-methyl-D-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B) Mol Pharmacol. 1997;51:79–86. doi: 10.1124/mol.51.1.79. [DOI] [PubMed] [Google Scholar]
- MacLeod KM, Carr CE. Synaptic physiology in the cochlear nucleus angularis of the chick. J Neurophysiol. 2005;93:2520–2529. doi: 10.1152/jn.00898.2004. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemoto Y, Kuriyama H, Doi T, Sato K, Matsumoto A, Sugatani J, Cho H, Komeda M, Altschuler RA, Kitajiri M, Mishina M, Yamashita T. Auditory pathway and auditory brainstem response in mice lacking NMDA receptor epsilon 1 and epsilon 4 subunits. Neurosci Lett. 1998;251:101–104. doi: 10.1016/s0304-3940(98)00509-6. [DOI] [PubMed] [Google Scholar]
- Niedzielski AS, Wenthold RJ. Expression of AMPA, kainate, and NMDA receptor subunits in cochlear and vestibular ganglia. J Neurosci. 1995;15:2338–2353. doi: 10.1523/JNEUROSCI.15-03-02338.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Hinds H, Lu HP, Kawata M, Hayashi Y. Motoneuron-specific expression of NR3B, a noval NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J Neurosci. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-23-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opella SJ, Marassi FM, Gesell JJ, Valente AP, Kim Y, Oblatt-Montal M, Montal M. Structures of the M2 channel-lining segments from nicotinic acetylcholine and NMDA receptors by NMR spectroscopy. Nat Struct Biol. 1999;6:374–379. doi: 10.1038/7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholt EM, Rubel EW, Hyson RL. A circuit for coding interaural time differences in the chick brainstem. J Neurosci. 1992;12:1698–1708. doi: 10.1523/JNEUROSCI.12-05-01698.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks TN. The AMPA receptors of auditory neurons. Hear Res. 2000;147:77–91. doi: 10.1016/s0378-5955(00)00122-2. [DOI] [PubMed] [Google Scholar]
- Parks TN, Jackson H. A developmental gradient of dendritic loss in the avian cochlear nucleus occurring independently of primary afferents. J Comp Neurol. 1984;227:459–466. doi: 10.1002/cne.902270315. [DOI] [PubMed] [Google Scholar]
- Parks TN, Rubel EW. Organization and development of brain stem auditory nuclei of the chicken: organization of projections from n. magnocellularis to n. laminaris. J Comp Neurol. 1975;164:435–448. doi: 10.1002/cne.901640404. [DOI] [PubMed] [Google Scholar]
- Parks TN, Gill SS, Jackson H. Experience-independent development of dendritic organization in the avian nucleus laminaris. J Comp Neurol. 1987;260:312–319. doi: 10.1002/cne.902600211. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Auerbach A. Stoichiometry of recombinant N-methyl-D-aspartate receptor channels inferred from single-channel current patterns. J Gen Physiol. 1997;110:485–502. doi: 10.1085/jgp.110.5.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci U S A. 1999a;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999b;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Parks TN. Organization and development of brain stem auditory nuclei of the chicken: tonotopic organization of n. magnocellularis and n. laminaris. J Comp Neurol. 1975;164:411–433. doi: 10.1002/cne.901640403. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Parks TN. Organization and development of the avian brain-stem auditory system. In: Edelman GM, Einar Gall W, Maxwell Cowan W, editors. Brain function. New York: John Wiley & Sons; 1988. pp. 3–92. [Google Scholar]
- Rubel EW, Smith DJ, Miller LC. Organization and development of brain stem auditory nuclei of the chicken: ontogeny of n. magnocellularis and n. laminaris. J Comp Neurol. 1976;166:469–489. doi: 10.1002/cne.901660408. [DOI] [PubMed] [Google Scholar]
- Sato K, Momose-Sato Y. Optical detection of developmental origin of synaptic function in the embryonic chick vestibulocochlear nuclei. J Neurophysiol. 2003;89:3215–3224. doi: 10.1152/jn.01169.2002. [DOI] [PubMed] [Google Scholar]
- Sato K, Kuriyama H, Altschuler RA. Differential distribution of NMDA receptor subunit mRNA in the rat cochlear nucleus. Microsc Res Techniq. 1998;41:217–223. doi: 10.1002/(SICI)1097-0029(19980501)41:3<217::AID-JEMT5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Coles RB, Gates GR. The development of auditory evoked responses in the cochlea and cochlear nuclei of the chick. Brain Res. 1973;63:59–74. doi: 10.1016/0006-8993(73)90076-0. [DOI] [PubMed] [Google Scholar]
- Schorge S, Colquhoun D. Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J Neurosci. 2003;23:1151–1158. doi: 10.1523/JNEUROSCI.23-04-01151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Lee SH. AMPA receptor trafficking and the control of synaptic transmission. Cell. 2001;105:825–828. doi: 10.1016/s0092-8674(01)00406-8. [DOI] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Singh TD, Basham ME, Nordeen EJ, Nordeen KW. Early sensory and hormonal experience modulate age-related changes in NR2B mRNA within a forebrain region controlling avian vocal learning. J Neurobiol. 2000;44:82–94. doi: 10.1002/1097-4695(200007)44:1<82::aid-neu8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Slutsky I, Sadeghpour S, Li B, Liu G. Enhancement of synaptic plasticity through chronically reduced Ca2+ flux during uncorrelated activity. Neuron. 2004;44:835–849. doi: 10.1016/j.neuron.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Smith ZD. Organization and development of brain stem auditory nuclei of the chicken: dendritic development in n. laminaris. J Comp Neurol. 1981;203:309–333. doi: 10.1002/cne.902030302. [DOI] [PubMed] [Google Scholar]
- Soares D, Chitwood RA, Hyson RL, Carr CE. Intrinsic neuronal properties of the chick nucleus angularis. J Neurophysiol. 2002;88:152–162. doi: 10.1152/jn.2002.88.1.152. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Sprengel R, Seeburg PH. Ionotropic glutamate receptors. In: North RA, editor. Handbook of receptors and channels, vol 2: ligand and voltage-gated ion channels. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher NJ, Brose N, Deitcher DL, Awobuluyi M, Gasic GP, Bading H, Cepko CL, Greenberg ME, Jahn R, Heimemann SF. Expression of endogenous NMDAR1 transcripts without receptor protein suggests post-transcriptional control in PC12 cells. J Biol Chem. 1993;268:22299–22304. [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tang YZ, Carr CE. Development of NMDA R1 expression in chicken auditory brainstem. Hear Res. 2004;191:79–89. doi: 10.1016/j.heares.2004.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YZ, Piao YS, Zhuang LZ, Wang ZW. Expression of androgen receptor mRNA in the brain of Gekko gecko: implications for understanding the role of androgens in controlling auditory and vocal processes. J Comp Neurol. 2001;438:136–147. doi: 10.1002/cne.1305. [DOI] [PubMed] [Google Scholar]
- Tang YZ, Yan K, Carr CE. Program No. 304.3. Society for Neuroscience; 2004. Changes in NMDA receptor subunit 2A and 2B expression in chick cochlear nucleus during embryonic development. [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Sakaguchi H, Jarvis ED, Hagiwara M. Differential expression of glutamate receptors in avian neural pathways for learned vocalization. J Comp Neurol. 2004;476:44–64. doi: 10.1002/cne.20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Bain CJ, Le Bourdelles B, Whiting PJ, Kemp JA. Preferential co-assembly of recombinant NMDA receptors composed of three different subunits. Neuroreport. 1993;4:1347–1349. doi: 10.1097/00001756-199309150-00015. [DOI] [PubMed] [Google Scholar]
- Warchol ME, Dallos P. Neural coding in the chick cochlear nucleus. J Comp Physiol. 1990;A166:721–734. doi: 10.1007/BF00240021. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 1997;68:469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- Young SR, Rubel EW. Embryogenesis of arborization pattern and topography of individual axons in n. laminaris of the chicken brain stem. J Comp Neurol. 1986;254:425–459. doi: 10.1002/cne.902540402. [DOI] [PubMed] [Google Scholar]
- Youssoufian M, Oleskevich S, Walmsley B. Development of a robust central auditory synapse in congenital deafness. J Neurophysiol. 2005;94:3168–3180. doi: 10.1152/jn.00342.2005. [DOI] [PubMed] [Google Scholar]
- Zarain-Herzberg A, Lee-Rivera I, Rodriguez G, Lopez-Colome AM. Cloning and characterization of the chick NMDA receptor subunit-1 gene. Brain Res Mol Brain Res. 2005;137:235–251. doi: 10.1016/j.molbrainres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Zhang S, Trussell LO. A characterization of excitatory postsynaptic potentials in the avian nucleus magnocellularis. J Neurophysiol. 1994a;72:705–718. doi: 10.1152/jn.1994.72.2.705. [DOI] [PubMed] [Google Scholar]
- Zhang S, Trussell LO. Voltage clamp analysis of excitatory synaptic transmission in the avian nucleus magnocellularis. J Physiol. 1994b;480:123–136. doi: 10.1113/jphysiol.1994.sp020346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Parks TN. Developmental changes in the effects of drugs acting at NMDA or non-NMDA receptors on synaptic transmission in the chick cochlear nucleus (nuc. magnocellularis) Brain Res Dev Brain Res. 1992;67:145–152. doi: 10.1016/0165-3806(92)90215-i. [DOI] [PubMed] [Google Scholar]