Abstract
• Background and Aims Mixed reproductive strategies may have evolved as a response of plants to cope with environmental variation. One example of a mixed reproductive strategy is dimorphic cleistogamy, where a single plant produces closed, obligately self-pollinated (CL) flowers and open, potentially outcrossed (CH) flowers. Frequently, optimal environmental conditions favour production of more costly CH structures whilst economical and reliable CL structures are produced under less favourable conditions. In this study we explore (1) the effect of light and water on the reproductive phenology and (2) the effect of pollen supplementation on resource allocation to seeds in the cleistogamous weed Ruellia nudiflora.
• Methods Split-plot field experiments were carried out to assess the effect of shade (two levels: ambient light vs. a reduction of 50 %) and watering (two levels: non-watered vs. watered) on the onset, end and duration of the production of three reproductive structures: CH flowers, CH fruit and CL fruit. We also looked at the effect of these environmental factors on biomass allocation to seeds (seed weight) from obligately self-pollinated flowers (CL), open-pollinated CH flowers and pollen-supplemented CH flowers.
• Key Results CH structures were produced for a briefer period and ended earlier under shaded conditions. These conditions also resulted in an earlier production of CL fruit. Shaded conditions also produced greater biomass allocation to CH seeds receiving extra pollen.
• Conclusions Sub-optimal (shaded) conditions resulted in a briefer production period of CH structures whilst these same conditions resulted in an earlier production of CL structures. However, under sub-optimal conditions, plants also allocated more resources to seeds sired from CH flowers receiving large pollen loads. Earlier production of reproductive structures and relatively larger seed might improve subsequent success of CL and pollen-supplemented CH seeds, respectively.
Keywords: Cleistogamy, invasive plants, phenology, pollen supplementation, resource allocation, Ruellia nudiflora
INTRODUCTION
Mixed reproductive strategies in plants refers to the production of flowers which will result in progeny with contrasting genetic backgrounds (i.e. selfed vs. outcrossed), differential energetic investment or with variable morphology (Lloyd, 1984; Schoen and Lloyd, 1984; Venable, 1985; Goodwillie et al., 2005). Plants with a mixed reproductive strategy have the benefits of different mating and reproductive avenues, and may have evolved to optimize fitness in highly variable environments (Schoen and Lloyd, 1984; Goodwillie et al., 2005). Dimorphic cleistogamy (sensu Culley and Klooster, 2007) is an example of a mixed mating strategy where both obligately self-pollinated cleistogamous (CL) flowers and potentially outcrossed chasmogamous (CH) flowers are produced by a single plant. Since the production of CH and CL flowers is sensitive to different environmental cues (Culley and Klooster, 2007), dimorphic cleistogamy offers an excellent opportunity to assess how plants with mixed reproductive strategies cope with contrasting biotic and abiotic conditions.
Previous research has shown that in cleistogamous plants, CH flowers are far more energetically expensive than CL flowers (mainly because CH flowers are larger than CL fowers, and only CH flowers produce rewards for pollinators) and the balance between CH and CL flower production is environmentally determined in several plant species (Campbell et al., 1983; Culley and Klooster, 2007). Although there is evidence of the opposite (Mitchell-Olds and Waller, 1985; Berg and Redbo-Torstensson, 2000; Cheplick, 2006), most studies suggest that (owing to its reduced cost) sub-optimal conditions favour greater allocation to CL flowers whilst the production of CH flowers would be opportunistic and increase under optimal conditions (Campbell et al., 1983; Culley and Klooster, 2007).
In order for cleistogamy to evolve, progeny from CH flowers must exhibit reproductive advantages different from progeny of CL flowers under different environmental conditions (Schoen and Lloyd, 1984; Goodwillie et al., 2005). CL flowers may be part of a bet-hedging strategy since these flowers are less costly than CH flowers and because CL flowers set seeds in the absence of pollinators and under disadvantageous environmental conditions (Oakley et al., 2007). In this regard, the production of CL structures can be considered as a reproductive assurance strategy in cleistogamous plants. CL flowers may also promote local adaptations as both maternal sets of genes can be passed on to the progeny, purging deleterious alleles, though it could also lead to strong inbreeding depression (Schoen and Lloyd, 1984; Oakley et al., 2007). On the other hand, CH flowers may have some benefits because genetically variable progeny could be favoured in spatially and temporally heterogeneous habitats (Culley and Klooster, 2007; Oakley et al., 2007). Plants are not only able to re-allocate resources between different types of flowers, but also some species allocate differentially among CH flowers as a response to the quantity of pollen they receive (Knight et al., 2005, 2006). Pollen performance is influenced by pollen competitive environment, and the size of the pollen load is an important determinant of seed and fruit set (Niesenbaum, 1999). A more realistic picture of resource allocation in cleistogamous plants would emerge when the source of pollen and the size of the pollen load under a variety of environmental conditions are taken into account (Eckstein and Otte, 2005).
Some plant species alternate the production of CH and CL flowers during the same reproductive season, and some authors have suggested (without testing the hypothesis) that phenology might be indicative of optimal and sub-optimal conditions within a reproductive season (e.g. Sigrist and Sazima, 2002; but see Forrest and Thomson, 2008). Plant species with sequential production of CH and CL flowers may also regulate the production of CL fruit according to the reproductive success of CH flowers (Rebdo-Torstensson and Berg, 1995). Studies on the phenology of cleistogamous species have shown that either CH or CL flowers can be produced at first during the reproductive season and that the overlap in the production of CH and CL flowers is highly variable (Oakley et al., 2007). Some studies have qualitatively addressed the sequence and the intensity in the production of the floral types (e.g. Jasieniuk and Lechowicz, 1987; Sigrist and Sazima, 2002; Imaizumi et al., 2008) but we do not know of any quantitative study looking at the effect of environmental factors on temporal patterns in the production of CH and CL flowers or fruit under field conditions.
Cleistogamy is widespread in the genus Ruellia (Acanthaceae) (Long, 1977; Sell, 1977). However, relative to other genera (e.g. Impatiens, Oxalis and Viola), studies on cleistogamy in Ruellia are scarce (e.g. Sigrist and Sazima, 2002; De Souza-Lima et al., 2005). In this genus, production of CH and CL flowers frequently shows little or no overlap and CL flowers occur mostly during the dry season (e.g. Sigrist and Sazima, 2002); therefore, Ruellia species are good models to test for the effect of environment on allocation patterns. In this study (1) we looked at the effect of two important environmental variables (light and water) on the reproductive phenology (production of CH flowers, CH fruit and CL fruit) of Ruellia nudiflora, a perennial herb with a long reproductive season (April–October); and (2) we explored the potential effect of allogamous pollen supplementation on the biomass allocation to seeds under different environmental conditions. We predicted that optimal conditions (watered and non-light-limited sub-plots) not only favour greater investment (earlier, larger production and for longer) in CH flowers and CH fruit than CL, but also a selective allocation to those CH flowers receiving pollen supplementation. Alternatively, sub-optimal conditions (shaded, non-watered sub-plots) may also promote larger resource allocation to CH seeds from pollen-supplemented CH flowers because these may exhibit better performance under less favourable conditions than seeds from open-pollinated CH flowers.
MATERIALS AND METHODS
Study system
The study area is the locality of Molas, municipality of Mérida in central Yucatán, México (20°49′51″N, 89°36′44″W, 10 m a.s.l.). The climate is warm sub-humid with summer rains; mean annual rainfall and temperature are 850 mm and 26·2 °C, respectively (Chico-Ponce de León, 1999). Vegetation is a disturbed tropical dry forest. Dominant canopy species are Bursera simaruba (Burseraceae) and Lysiloma latisiliquum (Fabaceae), whilst Gymnopodium floribundum (Polygonaceae) and Parmentiera millspaughiana (Bignoniaceae) dominate the understorey (Flores and Espejel, 1994). Ruellia nudiflora (Fig. 1) is an invasive perennial herb that reproduces mainly by seeds (Munguía-Rosas, CINVESTAV-IPN, Mérida, México, unpubl. res.). This plant species is non-native to México; it presumably originates from southern Texas (Turner, 1991), but currently it occurs from Texas to the Caribbean (Long, 1977).
Fig. 1.
Photographs of the reproductive structures of Ruellia nudiflora: (A) chasmogamous flowers, (B) cleistogamous flowers, (C) fruit from chasmogamous flowers, and (D) fruit from cleistogamous flowers.
Ruellia nudiflora has a dimorphic cleistogamous reproductive system (sensu Culley and Klooster, 2007). CL flowers are reduced, obligately self-pollinated and never open. CH flowers open for 1 d and can be either self-pollinated (delayed selfing has been reported) or outcrossed (Abdala-Roberts et al., 2009). In the study area, the CH flowers are pollinated by some species of native and introduced bees (e.g. Apis mellifera and Trigona fulviventris) as well as butterflies (e.g. Microtia elva); there is no evidence of pollen limitation (Abdala-Roberts et al., 2009). Fruits are dry capsules that disperse seeds ballistically. CL fruits are typically smaller, fewer seeded and more basal than CH fruits (Fig. 1).
Ruellia nudiflora can be considered as a sun plant as the net assimilation rate becomes saturated only at very high levels (1200 mmol m−2 s−1) of instant photosynthetic photon flux density (PPFD); the light response curve is available online (Supplementary Data Fig. S1, available online). Seeds also have greater germination success under highly illuminated conditions (Cervera and Parra-Tabla, 2009), but it is unknown if shade affects any other aspect of the reproductive ecology of R. nudiflora.
Phenology
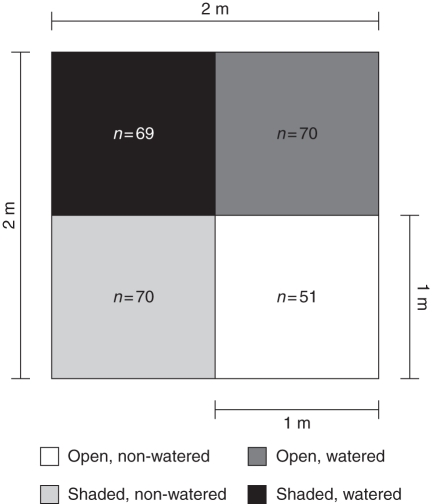
In early April 2010, we placed 11 plots (2 × 2 m) randomly in an area of about 2 ha where R. nudiflora occurs. At this stage plants had no reproductive structures, only a few leaves. The main plots were split into two sub-plots of similar size (2 ×1 m; Fig. 2), one of them was shaded with a nylon cloth placed about 90 cm above the forest floor and supported with four thin columns (2 cm diameter). The cloth is neutral in terms of light, i.e. the red:far red ratio was not statistically different (paired t-test, t8 = 1·299, P = 0·225, n = 10) between an exposed location (0·871 ± 0·004; hereafter mean ± s.e.) and under the cloth (0·864 ± 0·012). Under shaded conditions, ambient PPFD (1400–2000 mmol m−2 s−1; Cervera et al., 2007) was reduced about 50 %. The maximum photosynthetic rate for individuals receiving 50 % of total ambient PPFD can become lower than 9·06 ± 0·24 mmol CO2 m−2 s−1; that means that shaded sub-plots can lead to a 48 % decrease in the net assimilation rate. In order to avoid shading of adjacent plots, shaded sub-plots were east–west oriented and the shortest distance between plots was 1·5 m. Flower visitors moved freely beneath the shade; also, open and shaded sub-plots did not differ in CH fruit production or mass (see the Results).
Fig. 2.
Experimental split-plot design to assess the effect of shade (two levels: shaded vs. open) and watering (two levels: watered vs. non-watered) and its interaction on the reproductive phenology and resource allocation in Ruellia nudiflora. Each pattern shows a specific combination of treatments as shown in the key (control, open, non-watered). This plot was replicated 11 times; numbers inside sub-plots are the total number of plants per treatment.
Shaded and open plots were again divided into two more sub-plots of similar size (1 × 1 m); two of them (one shaded and one open) were watered twice a week until soil field capacity (about 5 L per sub-plot every time) from 10 April to 22 October 2010 (Fig. 2). Midday leaf water potential (ΨL) was optimal (–0·39 MPa) during 88·2 and 55·6 % of days for which the experiment lasted (196 d) in watered and non-watered sub-plots, respectively. Plants in sub-plots receiving only natural rainfall experienced some degree of water stress producing from moderate (ΨL: –0·76 MPa) to severe wilting (ΨL: –1·31 MPa) for 87 d, whereas plants in watered sub-plots were only slightly water stressed (ΨL –0·53) and for only 23 d (Supplementary Data Fig. S2). The terrain in the study area is flat, and the soil has excellent drainage; also, water was added at least 10 cm away from non-watered sub-plots. Therefore, water diffusion to adjacent sub-plots was unlikely. We tagged up to seven reproductive plants per sub-plot; excess plants and plants from different species were removed from experimental plots. In total we tagged 260 plants: 51 plants were in the control group (open and non-watered plots); 70 plants were shaded but not watered; 69 plants were shaded and watered; and 70 plants were watered but not shaded (Fig. 2). We counted all open CH flowers, CH fruit and CL fruit in the tagged plants once or twice a week until the growing season ended (17 December 2010). In some cases, plants were still bearing CL fruit at the end of the growing season; therefore, in these cases, the last count of CL structures was different from 0. We did not count CL flowers because, with our observation schedule, it was not possible to discriminate early CH flower buds from CL flowers and this may have produced a severe overestimation of CL flowers. CH and CL fruits are easily differentiated because CL fruits are smaller than CH fruits; also, in contrast to CL fruit, CH fruit usually bear remnants of the style (Fig. 1).
For the three different structures we counted (CH flowers, CH fruit and CL fruit) we recorded on a per-plant basis the following variables: production onset (day of the year on which we detected some reproductive structures for the first time), the end of production (the last time we saw the structures), duration of the production (the number of sampling days on which we saw the structures) and production of structures (the sum of the structures we counted during the growing season). As CH flowers open for only 1 d, the counts of CH flowers are clearly underestimates of the total number of flowers produced; however, these are useful in relative terms for treatment comparison. Since the production start and end points are the times elapsed from a date of reference to an event, these data sets do not follow a normal distribution and were analysed with generalized mixed-effects models with gamma error distribution and reciprocal link function. We used generalized mixed-effects models instead of survival analyses because in the former we can specify a hierarchal error structure of the split-plot design; gamma models produce rather more conservative results than survival analysis with constant hazard (Crawley, 2002, 2007). Since the duration of production and the number of structures are numerical counted data, we analysed these data with generalized mixed-effects models with Poisson error distribution and logit link function. In all models the fixed effects were shade and watering treatments as well as their interaction. The hierarchal structure of plots was included in the random part of the model. Analyses were performed with the glmmPQL functions from the MASS library for R 2·10·1 software (R Development Core Team, 2009) which fits generalized mixed-effects models using the penalized quasi-likelihood method (Venables and Ripley, 2002).
Pollen supplementation and biomass allocation
We collected all mature CH and CL fruit once or twice a week from the same plants used for phenological recording as described above. Also, and with the same frequency, we performed pollen supplementation of about 80 % of available CH flowers in the 11 plots described in the previous section. Every flower received supplemental pollen from only one donor flower. Some plants producing more than one flower simultaneously received pollen from more than one plant; however, even in these cases each flower was supplemented with pollen from only one flower and one donor. Pollen was placed gently on receptive stigmas of non-emasculated flowers until stigma saturation before midday (1000–1100 h). We used only one pollen donor per flower to standardize the number of donors and because the availability of plants bearing CH flowers was limited. The petioles of supplemented flowers were tagged and the fruit were harvested when ripe. We did not protect supplemented flowers to avoid disturbance of pollinator foraging behaviour. We collected the fruit (open-pollinated CH, pollen-supplemented CH and CL fruit) which were then transported to the laboratory, weighed and dissected to count seeds.
As seed number and fruit weight are highly correlated traits (rs = 0·72, P < 0·01, n = 2252), we combined these variables, expressing biomass on a per-seed basis, dividing the fruit weight by the seed number. We did not find strong evidence of a seed size–number trade-off in the study species as correlation between seed size and number was extremely weak (rs = –0·04, P = 0·03, n = 2252). Allocation on a per-seed basis has more biological meaning that on a per-fruit basis because the former is correlated with progeny performance (Richardson and Stephenson, 1992; Bernard and Toft, 2007). Only non-damaged fruits were considered in data analyses because the biomass consumed by herbivores may bias the results. Data from 2252 fruits were considered in the analysis: 553 in the control group (278 CL, 209 open-pollinated CH and 66 pollen-supplemented CH); 574 in the shaded treatment (266 CL, 211 open-pollinated CH and 97 pollen-supplemented CH); 574 in the watered treatment (283 CL, 227 open-pollinated CH and 64 pollen-supplemented CH); and 551 in the shaded and watered treatment (310 CL, 188 open-pollinated CH and 53 pollen-supplemented CH). Per-seed biomass (the response variable) was fitted to a linear mixed-effects model using the maximum likelihood method (Pinheiro and Bates, 2000) where the two treatments described above (shade and watering), type of fruit (CL as well as open-pollinated CH and pollen-supplemented CH) and all possible interactions were included as fixed factors. The random part of the model accounts for the hierarchal structure of the plots and repeated fruit collections from plants. The model was fitted with the lme function from the nlme library for R 2·10·1 software (R Development Core Team, 2009)
RESULTS
Phenology
The phenology of the three reproductive structures we considered in the study (CH flowers, CH fruit and CL fruit) were affected (P <0·05) by the shade, but the specific component of phenology affected varied (Table 1). The production of CL fruit started earlier in shaded plants (day 124 of the year: 4 May ± 1·32 d) than in plants in open sub-plots (day 130: 20 May ± 1·32 d) (Fig. 3A), the production of CH fruit ended earlier in shaded plants (day 264: 21 September ± 4·5 d) than in plants in open sub-plots (day 285: 12 October ± 4·04 d) (Fig. 3B) and, the duration in production of CH flowers was shorter in shaded plants (seen for 3·78 ± 0·23 sampling days) than in plants in open sub-plots (5·12 ± 0·34 sampling days) (Fig. 3C). However, the production of the three structures was not affected by any treatment (Fig. 3D). Watering and its interaction with shade treatment did not affect any phenological descriptor of any structure considered in this study (Table 1, Fig. 3).
Table 1.
Results of generalized mixed-effects linear models performed to assess the effect of shade (open vs. shaded), watering (watered vs. non-watered) and its interaction on the onset, end, duration and relative production of R. nudiflora chasmogamous flowers (Flowers), chamosgamous fruit and cleistogamous fruit (CH Fruit, CL Fruit)
| Structure | Descriptor | Source of variation | Statistics | P-value |
|---|---|---|---|---|
| Flowers | Onset | Water | F1,20 = 0·28 | 0·60 |
| Shade | F1,10 = 3·41 | 0·09 | ||
| Water × shade | F1,20 = 0·26 | 0·61 | ||
| End | Water | F1,20 = 1·36 | 0·26 | |
| Shade | F1,10 = 4·27 | 0·06 | ||
| Water × shade | F1,20 = 0·77 | 0·39 | ||
| Duration | Water | F1,20 = 0·67 | 0·42 | |
| Shade | F1,10 = 10·84 | <0·01 | ||
| Water × shade | F1,20 = 0·25 | 0·62 | ||
| Production | Water | F1,20 = 0·27 | 0·61 | |
| Shade | F1,10 = 4·91 | 0·05 | ||
| Water × shade | F1,20 = 0·35 | 0·56 | ||
| CH Fruit | Onset | Water | F1,20 = 0·48 | 0·50 |
| Shade | F1,10 = 4·26 | 0·06 | ||
| Water × shade | F1,20 = 0·26 | 0·61 | ||
| End | Water | F1,20 = 0·05 | 0·98 | |
| Shade | F1,10 = 5·77 | 0·03 | ||
| Water × shade | F1,20 = 0·11 | 0·75 | ||
| Duration | Water | F1,20 = 0·95 | 0·34 | |
| Shade | F1,10 = 4·87 | 0·05 | ||
| Water × shade | F1,20 = 0·089 | 0·77 | ||
| Production | Water | F1,20 = 0·66 | 0·42 | |
| Shade | F1,10 = 0·99 | 0·34 | ||
| Water × shade | F1,20 = 0·01 | 0·93 | ||
| CL Fruit | Onset | Water | F1,20 = 0·11 | 0·74 |
| Shade | F1,10 = 5·31 | 0·04 | ||
| Water × shade | F1,20 = 0·75 | 0·39 | ||
| End | Water | F1,20 = 3·77 | 0·07 | |
| Shade | F1,10 = 1·14 | 0·17 | ||
| Water × shade | F1,20 = 0·34 | 0·57 | ||
| Duration | Water | F1,20 = 0·21 | 0·65 | |
| Shade | F1,10 = 0·40 | 0·54 | ||
| Water × shade | F1,20 = 0·03 | 0·96 | ||
| Production | Water | F1,20 = 3·53 | 0·07 | |
| Shade | F1,10 = 1·39 | 0·26 | ||
| Water × shade | F1,20 = 0·34 | 0·56 |
The variables ‘onset’ and ‘end’ were fitted to gamma models, and the rest to Poisson models. Statistically significant differences are highlighted in bold.
Fig. 3.
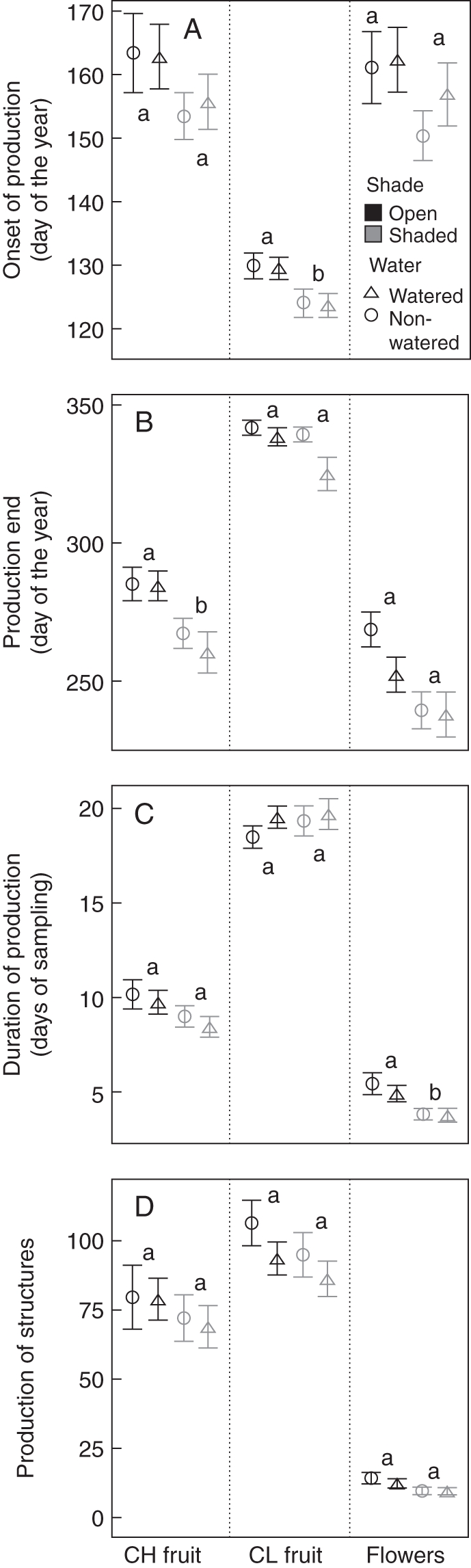
Effect of shade and watering on the onset (A), end (B), duration (C) and production (D) of R. nudiflora chasmogamous flowers (Flowers), chasmogamous fruit (CH Fruit) and cleistogamous fruit (CL Fruit). The bars show the mean ± s.e.; different letters show statistical differences between shaded plants (shaded–watered, shaded–non-watered) and plants in open plots (open–non-watered, open–watered). Differences between watered and non-watered plants as well as the interactions were not statistically significant in all cases.
Pollen supplementation and biomass allocation
Fruit types differed significantly in per-seed mass: pollen-supplemented CH fruit had the heaviest seeds (3·00 ± 0·32 mg), followed by open-pollinated CH fruit (2·59 ± 0·05 mg), and CL fruit had the lightest seeds (2·16 ± 0·05 mg) (Fig. 4). However, the interaction between type of fruit and shade treatment was significant (Table 2). The shade treatment produced a different effect on the three different types of fruit: this effect was negligible in CL fruit (open, 2·15 ± 0·03 mg; shade, 2·19 ± 0·08 mg) and open-pollinated CH fruit (open, 2·57 ± 0·05 mg; shade: 2·62 ± 0·08 mg); however, we found a large increase of the per-seed weight in pollen-supplemented CH fruit in plants under shaded conditions (open, 2·53 ± 0·06 mg; shade, 3·41 ± 0·50 mg) (Fig. 4). Watering treatment, the other second order interactions, as well as the third order interaction were all statistically non-significant (Table 2).
Fig. 4.
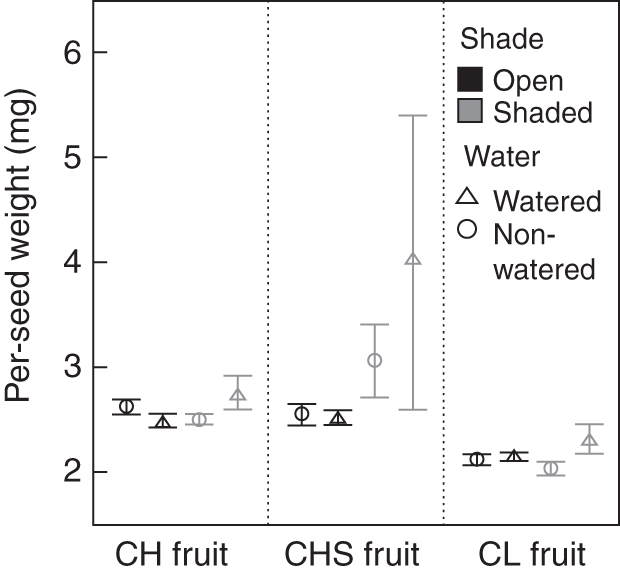
Per-seed weight of R. nudiflora under field experimental conditions to assess the effect of two treatments: shade (shaded vs. open) and water supplementation (watered vs. non-watered) on biomass allocation to three different structures: cleistogamous fruit (CL Fruit), chasmogamous fruit (CH Fruit) and chasmogamous fruit supplemented with allogamous pollen (CHS Fruit). The bars show the mean ± s.e. Per-seed weight was statistically different among structures, and the interaction treatment × structure was also significant.
Table 2.
Results of linear mixed-effects models to asses the effect of shade (shaded vs. open), watering (watered vs. non-watered) and three reproductive structures (chamosgamous flowers, chasmogamous fruit, cleistogamous fruit) on per seed mass
| Source of variation | Statistics | P-value |
|---|---|---|
| Structure | F2,1974 = 17·55 | <0·01 |
| Shade | F1,10 = 1·69 | 0·22 |
| Water | F1,20 = 2·13 | 0·16 |
| Structure × shade | F2,1974 = 4·55 | 0·01 |
| Structure × water | F2,1974 = 0·94 | 0·39 |
| Shade × water | F1,20 = 2·67 | 0·12 |
| Structure × shade × water | F2,1974 = 0·87 | 0·42 |
Statistically significant differences are highlighted in bold.
DISCUSSION
Our results suggest environmental control of phenology and differential resource allocation depending on the amount of pollen received in the cleistogamous R. nudiflora. Under shaded conditions, production of CH flowers was for a shorter period and the production of CH fruit ended earlier, whilst shade induced an earlier start to CL fruit production. However, the total numbers of CH flowers and all fruit (CH and CL) were not affected by shade. Regarding biomass allocation to seeds, sub-optimal (shaded) conditions, but not unshaded conditions, favoured allocation to CH seeds receiving pollen supplementation. These results suggest that under sub-optimal conditions, not only are resources re-allocated to produce CL structures earlier, but also plants re-allocate biomass mainly to CH seeds that developed from CH flowers receiving larger pollen loads. Thus, the cleistogamous R. nudiflora responds to sub-optimal conditions with two contrasting strategies: earlier production of CL seeds and greater biomass allocation to presumably higher quality CH seeds.
Although we did not find any effect of shade or watering on the number of CH flowers, CH fruit and CL fruit, we did identify an effect of shade on the phenology of all these structures, though on different aspects. As a response to shade, the production of CH flowers was shortened and the production of CH fruit finished earlier relative to plants in open sub-plots. On the other hand, CL fruit (and, therefore, CL flowers) were produced earlier in plants under shaded conditions than in those plants in open sub-plots. In contrast to previous studies reporting that sub-optimal conditions increase the production of CL flowers relative to CH flowers (Culley and Klooster, 2007), our results have shown that R. nudiflora produces a similar number of CH and CL flowers or fruit regardless of environmental conditions; instead, temporal patterns of CH and CL flower and fruit production are differentially influenced by light availability. An earlier production of CL flowers (and subsequently fruit) and a shorter period of production of CH flowers may be advantageous under shaded conditions even if the total number of flowers or fruit remains constant. Ruellia nudiflora starts its reproduction with CL flowers followed by production of CH flowers, and produces CL flowers once again at the end of the reproductive season. This sequence was not affected by water or light availability; therefore, an earlier production of CL flowers and fruit actually represents an earlier start of the reproductive season. Earlier flowering is usually positively correlated with reproductive success in plants (Munguía-Rosas et al., 2011) and this might be the case for our study species. On the other hand, a shorter period of production of CH flowers under shaded conditions, but in a similar quantity to those under more optimal conditions, may favour pollinator attraction, and therefore pollination success, because a larger floral display is presented. Our study also showed that R. nudiflora allocates more resources to flowers receiving larger pollen loads, and this could result in more vigorous and potentially more successful progeny in sub-optimal environments. This work reveals the importance of looking at fine scale changes in the phenology of CH and CL flowers and fruits using suitable quantitative methods. Up to now, previous studies looking at phenology of cleistogamous plants had focused only on the total production of CH and CL flowers or on qualitative temporal patterns (e.g. Jasieniuk and Lechowicz, 1987; Sigrist and Sazima, 2002; Imaizumi et al., 2008) and were unable to detect small variations in phenology in response to environmental variables.
It has been suggested that annual and perennial cleistogamous plants differ in their reproductive phenology (Oakley et al., 2007). According to these authors, in annuals CL structures are produced first (in some cases also at the end), followed by simultaneous production of CL and CH. In contrast, perennials usually sequentially produce CH and CL structures; in some cases, the production of CL structures occurs after CH but never before (Oakley et al., 2007). Ruellia nudiflora does not follow the pattern predicted for perennials: invariably CL structures are produced first, regardless of environmental conditions. Although some previous studies showed that soil moisture affects production of CH and CL flowers (e.g. Bell and Quinn, 1987), we did not identify any effect of watering on phenology or resource allocation. Our experiment was conducted under field conditions and therefore some limitations exist, such as the inability to avoid or reduce natural rainfall over specific sub-plots without disturbing light availability. Even so, we know that non-watered sub-plots were under sub-optimal or stressful conditions of water availability for a longer period of time than plants in watered sub-plots (Supplementary Data Fig. S2). However, we cannot rule out completely the possibility that the phenology of R. nudiflora may respond to an even stronger water deficit.
Sub-optimal (shaded) conditions affected not only the phenology of reproductive structures in R. nudiflora, but also patterns of resource allocation among CL fruit and CH fruit from flowers receiving larger pollen loads. Previous reviews have suggested that, in general, CL structures have priority over CH structures under sub-optimal conditions (e.g. Oakley et al., 2007). Under shaded conditions R. nudiflora plants allocated resources earlier to produce CL flowers and fruits; however, the total number of these structures and the biomass allocated to CL seeds were not affected by shade or watering. Interestingly, our results suggest that, under sub-optimal conditions, those seeds sired from CH flowers receiving extra pollen may also be prioritized in cleistogamous plants. CL seeds, as previously suggested, may ensure reproduction under disadvantageous conditions (Campbell et al., 1983; Culley and Klooster, 2007), but also, good-quality CH seeds may produce highly competitive seedlings under sub-optimal conditions. Sired seeds from larger pollen loads are not only heavier (Richardson and Stephenson, 1992), but their size is also positively correlated with performance in some species (Richardson and Stephenson, 1992; Bernard and Toft, 2007) including some cleistogamous species (e.g. Trapp and Hendrix, 1988; Berg and Redbo-Torstensson, 2000). Therefore, a superior performance of seeds sired from pollen-supplemented CH flowers is likely in R. nudiflora. Research in the study area has revealed that R. nudiflora has high genetic diversity (78 % of polymorphic loci; Ortegón-Campos, 2010) and a high outrossing rate (tm = 0·9; Marrufo, UADY, Mérida, México, unpubl. res.), which suggest that it is the size of the pollen load and not the origin (i.e. selfed vs. outcrossed) that is affecting preferential resource allocation to pollen-supplemented CH flowers. Possibly plants did not allocate more resources to pollen-supplemented CH flowers in open sub-plots because these plants are not resource limited and, therefore, these plants are able to allocate resources to CH flowers equally regardless of the size of the pollen load. It is unlikely that our results in terms of resource allocation (i.e. greater allocation to pollen-supplemented CH flowers under shaded conditions) would be an artefact introduced by limitation in pollinator service due to the shade cloth. First of all, the cloth was placed about 1 m from the soil, which allowed visitors to move freely beneath the cloth as well as fly over open sub-plots. Secondly, we did not identify any effect of shade on per-seed weight as a single factor, i.e. all non-manipulated CH flowers produced seeds of similar weight in open and shaded sub-plots (Fig. 2). Previous studies have documented that when applied to a sub-set of flowers, pollen supplementation is not indicative of pollen limitation; instead, it is useful to assess resource re-allocation (Parra-Tabla et al. 1998; Knight et al., 2005, 2006).
In conclusion, our study suggests that temporal patterns in production of CH and CL structures are affected differentially by shade; CL structures are produced earlier under shaded conditions whilst CH structures were produced for shorter periods. However, in addition to an earlier production of CL structures, R. nudiflora also preferentially allocates resources to those CH flowers receiving larger pollen loads. Earlier production of CL seeds and potentially more vigorous CH seeds can be a mixed strategy to face unpredictable environments if earlier production of CL structures and the size of the CH seeds are both positively correlated with subsequent success of the resulting seedling from each seed type. If this is the case, CL progeny may provide cheap reproductive assurance if environmental conditions improve. Instead, CH seeds sired from large pollen loads may be highly competitive if sub-optimal conditions persist. Since R. nudiflora is an invasive weed in Mexico (Villaseñor and Espinosa-García, 2004; Cervera and Parra-Tabla, 2009), our results may also be useful to understand the reproductive strategies allowing invasive species to colonize new habitats (Cheplick, 2006; Imaizumi et al., 2008). Future studies looking at the effect of environment on resource allocation in any cleistogamous plant should also consider that the size of the pollen load may produce differential allocation patterns in CH seeds. It has been suggested that cleistogamy evolved as a response to environmental heterogeneity (Schoen and Lloyd, 1984); therefore, allocation to flowers, allocation to seeds and the performance of the progeny may produce different patterns under optimal and sub-optimal conditions.
SUPPLEMENTARY DATA
ACKNOWLEDGMENTS
The first author thanks CONACyT and PROMEP for funding. M.M.-R. and V.P.-T. are also grateful to the Santander Award at the University of Northampton for providing additional funding. The authors thank L. Aldana for access to his property and logistic support. S. Mendoza, P. Tellez, N. Celaya, J. M. Pech-Canché, M. E. Jacome-Flores and B. Torales-Herrera helped with field work. UADY students from the ‘Evolutionary Ecology of Interspecific Interactions’ course helped in the laboratory. I. Ortegón-Campos, L. Salinas-Peba and D. Marrufo gave some advice with the study system and methods. L. Salinas-Peba took the pictures in Fig. 1.
LITERATURE CITED
- Abdala-Roberts L, Parra-Tabla V, Salinas-Peba L, Herrera CM. Noncorrelated effects of seed predation and pollination on the perennial herb Ruellia nudiflora remain spatially consistent. Biological Journal of the Linnean Society. 2009;96:800–807. [Google Scholar]
- Bell TJ, Quinn JA. Effects of soil moisture and light intensity on the chasmogamous and cleistogamous components of reproductive effort of Dichanthelium clandestinum populations. Canadian Journal of Botany. 1987;65:2243–2249. [Google Scholar]
- Berg H, Redbo-Torstensson P. Offspring performance in Oxalis acetosella, a cleistogamous perennial herb. Plant Biology. 2000;2:638–645. [Google Scholar]
- Bernard RB, Toft CA. Effect of seed size on seedling performance in a long-lived desert perennial shrub (Ericameria nauseosa: Asteraceae) International Journal of Plant Science. 2007;168:1027–1033. [Google Scholar]
- Campbell CS, Quinn JA, Cheplick GP, Bell TJ. Cleistogamy in grasses. Annual Review of Ecology Evolution and Systematics. 1983;14:411–441. [Google Scholar]
- Cervera JC, Parra-Tabla V. Seed germination and seedling survival traits of invasive and non-invasive congeneric Ruellia species (Acanthaceae) in Yucatan, Mexico. Plant Ecology. 2009;205:285–293. [Google Scholar]
- Cervera JC, Andrade JL, Graham EA, Durán R, Jackson PC, Simá JL. Photosynthesis and optimal light microhabitats for a rare cactus Mammillaria gaumeri, in two tropical ecosystems. Biotropica. 2007;39:620–627. [Google Scholar]
- Cheplick GP. A modular approach to biomass allocation in an invasive annual (Microstegium vimineum; Poaceae) American Journal of Botany. 2006;93:539–545. doi: 10.3732/ajb.93.4.539. [DOI] [PubMed] [Google Scholar]
- Chico-Ponce de León PA. Atlas de procesos territoriales. Mérida: Universidad Autónoma de Yucatán; 1999. [Google Scholar]
- Crawley MJ. Statistical computing: an introduction of data analysis using S-Plus. Chichester, UK: John Wiley & Sons Ltd; 2002. [Google Scholar]
- Crawley MJ. The R book. Chichester, UK: John Wiley and Sons Ltd; 2007. [Google Scholar]
- Culley TM, Klooster MR. The cleistogamous breeding system: a review of its frequency, evolution, and ecology in angiosperms. Botanical Review. 2007;73:1–30. [Google Scholar]
- De Souza-Lima NA, Faria-Vieira M, Carvalho-Okano RM, Alves-Azevedo A. Cleistogamia em Ruellia menthoides (Nees) Hiern e R. brevifolia (Pohl) C. Ezcurra (Acanthaceae) em fragmento florestal do Sudeste brasileiro. Revista Brasileira de Botânica. 2005;19:443–449. [Google Scholar]
- Eckstein RL, Otte A. Effects of cleistogamy and pollen source on seed production and offspring performance in three endangered violets. Basic and Applied Ecology. 2005;6:339–350. [Google Scholar]
- Flores S, Espejel I. Tipos de vegetación de la península de Yucatán. In: Flores S, editor. Etnoflora yucatanense. Mérida: Universidad Autónoma de Yucatán; 1994. pp. 63–70. [Google Scholar]
- Forrest J, Thomson JD. Pollen limitation and cleistogamy in subalpine Viola praemorsa. Botany. 2008;86:511–519. [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology, Evolution and Systematics. 2005;36:47–79. [Google Scholar]
- Imaizumi T, Wang G, Tominaga T. Pollination of chasmogamous flowers and the effects of light and emergence time on chasmogamy and cleistogamy in Monochoria vaginalis. Weed Biology and Management. 2008;8:260–266. [Google Scholar]
- Jasieniuk M, Lechowicz M.J. Spatial and temporal variation in chasmogamy and cleistogamy in Oxalis montana (Oxalidaceae) American Journal of Botany. 1987;74:1672–1680. [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Annual Review of Ecology, Evolution and Systematics. 2005;36:467–497. [Google Scholar]
- Knight TM, Steets JA, Ashman T-L. A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. American Journal of Botany. 2006;93:271–277. doi: 10.3732/ajb.93.2.271. [DOI] [PubMed] [Google Scholar]
- Lloyd DG. Variation strategies in heterogeneous environments. Biological Journal of the Linnean Society. 1984;21:357–385. [Google Scholar]
- Long RW. Artificial induction of obligate cleistogamy in species-hybrid in Ruellia (Acanthaceae) Bulletin of the Torrey Botanical Club. 1977;104:53–56. [Google Scholar]
- Mitchell-Olds T, Waller DM. Relative performance of selfed and outcrossed progeny in Impatiens capensis. Evolution. 1985;39:533–544. doi: 10.1111/j.1558-5646.1985.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Munguía-Rosas MA, Ollerton J, Parra-Tabla V, De Nova A. Meta-analysis of phenotypic selection on flowering phenology suggests that early flowering plants are favoured. Ecology Letters. 2011;41:511–521. doi: 10.1111/j.1461-0248.2011.01601.x. [DOI] [PubMed] [Google Scholar]
- Niesenbaum RA. The effects of pollen load size and donor diversity on pollen performance, selective abortion, and progeny vigor in Mirabilis jalapa (Nyctaginaceae) American Journal of Botany. 1999;86:261–268. [PubMed] [Google Scholar]
- Oakley CG, Maruichi KS, Winn AA. The maintenance of outcrossing in predominantly selfing species: ideas and evidence from cleistogamous species. Annual Review of Ecology, Evolution and Systematics. 2007;38:437–457. [Google Scholar]
- Ortegón-Campos I. 2010 Adaptación local y diversidad genética de Ruellia nudiflora (Acanthaceae) en el estado de Yucatán. PhD Thesis, Universidad Autónoma de Yucatán, México. [Google Scholar]
- Parra-Tabla V, Vargas MF, Eguiarte L. Is Echeveria gibbiflora (Crassulaceae) pollen limited?: an experimental test. Functional Ecology. 1998;12:591–595. [Google Scholar]
- Pinheiro CJ, Bates D. Mixed-effects models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2009. http://www.r-project.org/ [Google Scholar]
- Redbo-Torstensson P, Berg H. Seasonal cleistogamy: a conditional strategy to provide reproductive assurance. Acta Botanica Neerlandica. 1995;44:247–256. [Google Scholar]
- Richardson TE, Stephenson AG. Effect of parentage and size of the pollen load on progeny performance in Campanula americana. Evolution. 1992;46:1731–1739. doi: 10.1111/j.1558-5646.1992.tb01165.x. [DOI] [PubMed] [Google Scholar]
- Sell Y. La cléistogamie chez Ruellia lorentziana Griseb. et quelques autres Acanthacées. Berichte der Deutschen. Botanischen Gesellschaft. 1977;90:135–147. [Google Scholar]
- Sigrist MR, Sazima M. Ruellia brevifolia (Pohl) Ezcurra (Acanthaceae): fenologia da floração, biologia da polinização e reprodução. Revista Brasileira de Botânica. 2002;25:35–42. [Google Scholar]
- Schoen DJ, Lloyd DG. The selection of cleistogamy and heteromorphic diaspores. Biological Journal of the Linnean Society. 1984;23:303–322. [Google Scholar]
- Trapp EJ, Hendrix SD. Consequences of a mixed reproductive system in the hog peanut, Amphicarpaea bracteata (Fabaceae) Oecologia. 1988;75:285–290. doi: 10.1007/BF00378611. [DOI] [PubMed] [Google Scholar]
- Turner BL. Texas species of Ruellia (Acanthaceae) Phytologia. 1991;71:281–299. [Google Scholar]
- Venable DL. The evolutionary ecology of seed heteromorphism. American Naturalist. 1985;126:577–595. [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. New York: Springer; 2002. [Google Scholar]
- Villaseñor JL, Espinosa-García FJ. The alien flowering plants of Mexico. Biodiversity and Distributions. 2004;10:113–123. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.