Abstract
Background and Aims
Studies examining patterns and processes of speciation in South America are fewer than in North America and Europe. One of the least well documented processes has been progenitor–derivative speciation. A particularly instructive example occurs in the southern Andes in the genus Pozoa (Apiaceae, Azorelloideae), which consists of only two diploid outcrossing species, the widespread P. coriacea and the geographically and ecologically restricted P. volcanica. This paper tests the hypothesis that the latter species originated from the former through local geographical and ecological isolation by progenitor–derivative speciation.
Methods
DNA sequences were analysed from Pozoa and the related South American genera Asteriscium, Eremocharis and Gymnophyton from non-coding regions of the plastid genome, ndhF-rpl32 and rpl32-trnL, plus incorporation of previously reported rpl16 intron and trnD-trnT intergenic spacer sequences. Amplified fragment length polymorphism (AFLP) data from 105 individuals in 21 populations throughout the entire range of distribution of the genus were used for estimation of genetic diversity, divergence and SplitsTree network analysis. Ecological factors, including habitat and associated species, were also examined.
Key Results
Pozoa coriacea is more similar genetically to the outgroup genera, Asteriscium and Eremocharis, than is P. volcanica. At the population level, only P. volcanica is monophyletic, whereas P. coriacea is paraphyletic. Analyses of genetic differentiation among populations and genetic divergence and diversity of the species show highest values in P. coriacea and clear reductions in P. volcanica. Pozoa coriacea occurs in several types of high elevation habitats, whereas P. volcanica is found only in newly formed open volcanic ash zones.
Conclusions
All facts support that Pozoa represents a good example of progenitor–derivative speciation in the Andes of southern South America.
Keywords: AFLP, Andes mountains, Apiaceae, DNA sequencing, genetic diversity, geographical origin, Pozoa coriacea, P. volcanica, speciation
INTRODUCTION
Speciation relates to formation of spatial or geographical barriers and/or ecological and reproductive isolating mechanisms that allow emergence of new gene combinations in different populations, generating differences between populations that eventually produce new species (Grant, 1981; Coyne, 1992; Gavrilets, 2003; Levin, 2003). The use of information from geographical, ecological, reproductive and chromosomal studies has allowed a considerable theoretical framework to be developed over the past century to explain speciation in plants and animals. In the past two decades, the advent of molecular methods [sequencing of DNA, amplified fragment length polymorphism (AFLP) analyses, microsatellites and single nucleotide polymorphisms] has allowed a much broader understanding of the genetic patterns involved with speciation (Coyne and Orr, 2004).
Different modes of geographical speciation are known to have occurred among higher plants. Allopatric speciation takes place when a geographical boundary reduces gene flow between ancestral populations and leads to the formation of reproductive barriers (Grant, 1981; Lomolino et al., 2006). This concept includes vicariance and peripatric speciation. In the former, environmental change produces a barrier that divides the original geographical range of a species into two or more populations, reproductive isolation developing in each of them. In peripatric speciation, reproductive isolation originates by colonization of an unoccupied habitat by a few individuals (Coyne and Orr, 2004; Lomolino et al., 2006). In this case, new populations become spatially separated from the progenitor, and divergence ensues (Tauber and Tauber, 1989). Sympatric speciation involves evolution of reproductive isolation within the average dispersal distance of a single individual (Mayr, 1963; Bolnick and Fitzpatrick, 2007), and where the restriction of gene flow originates through biological characteristics of the organisms (Futuyma and Mayer, 1980).
A particular type of peripatric speciation, whereby an isolated peripheral population diverges to form a derivative species, has been called progenitor–derivative speciation (Crawford and Smith, 1982). The derivative species diverges from the ancestral condition, but the progenitor species remains almost unchanged. This differs from typical geographic allopatric speciation, whereby two populations diverge simultaneously in numerous characters and the ancestor disappears in the process (Gottlieb, 1973; Jaramillo-Correa and Bousquet, 2003). Progenitor–derivative speciation may be more recent in comparison with gradual divergence under the classical allopatric model. Thus, it should be somewhat easier to identify differences between the taxa associated with progenitor–derivative speciation as compared with those undergoing gradual divergence after speciation, given that the progenitor species presumably has changed little since the derivative species originated (Crawford, 2010).
Criteria that have been suggested for identifying cases of progenitor–derivative speciation include the following (after Crawford, 2010): (1) a close morphological and phylogenetic relationship should exist between the two taxa (i.e. they should be sister species); (2) crossability barriers between the population systems and/or observed karyotypic differences may prevail; (3) lower genetic diversity should occur in the derivative species in comparison with the progenitor; (4) the derivative populations should nest phylogenetically within those of the progenitor taxon; and (5) characteristics of the derivative populations may suggest directional evolution, such as reduction in allelic richness, breakdown of self-incompatibility, occurrence on ultramafic soils or restricted distribution at the periphery of the more broadly ranging progenitor species.
Only a few cases of progenitor–derivative speciation in plants have so far been documented, principally all in the Northern Hemisphere: Stephanomeria malheurensis from Stephanomeria exigua subsp. coronaria (Asteraceae; for a review, see Gottlieb, 2003); Clarkia lingulata from C. biloba (Onagraceae; Gottlieb, 1974); Coreopsis nuecensis from its progenitor species C. nuecensoides (Asteraceae; Crawford and Smith, 1982); Lasthenia maritima from its progenitor L. minor (Asteraceae; Crawford et al., 1985); Layia discoidea originating from L. glandulosa (Asteraceae; Gottlieb et al., 1985; Baldwin, 2005); Camassia angustata from C. scilloides (Asparagaceae; Ranker and Schnabel, 1986) and Picea rubens from P. mariana (Pinaceae; Jaramillo-Correa and Bousquet, 2003).
In South America, numerous tectonic changes and elevation of the Andes mountain chain have stimulated allopatric speciation in different plant groups. However, only a handful of papers have examined speciation by means of species-level phylogenetic analyses using molecular methods such as in Perezia (Asteraceae; Simpson, 1973; Simpson et al., 2009), Nothofagus (Nothofagaceae; Manos, 1997), Fragaria (Rosaceae; Ontivero et al., 2000), Malesherbia (Passifloraceae; Gengler-Nowak, 2002, 2003), Hypochaeris (Asteraceae, Samuel et al., 2003; Stuessy et al., 2003; Tremetsberger et al., 2005, 2006), Chaetanthera (Asteraceae, Hershkovitz et al., 2006), Tristerix (Loranthaceae, Amico et al., 2007), Drimys (Winteraceae; Ruiz et al., 2008) and Puya (Bromeliaceae; Schmidt and Systma, 2010). These studies have suggested avenues for synthesis of molecular, ecological, reproductive and biogeographical aspects, all of which are beginning to provide new understanding of evolutionary processes in the Andes of South America.
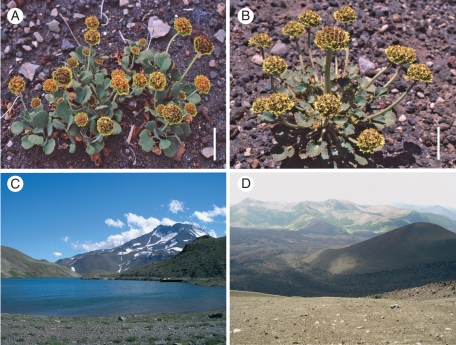
An example of possible progenitor–derivative speciation in South America occurs in the genus Pozoa (Apiaceae, Azorelloideae), endemic to the Andes of Chile and Argentina (Figs 1 and 2). This genus consists of only two species (Mathias and Constance, 1962). Pozoa coriacea is widespread at elevations between 1000 and 4000 m and distributed along the southern Andes in Chile from Coquimbo south to the Region de La Araucanía and from the Province of San Juan south to Rio Negro on the Argentinean side. Pozoa volcanica is restricted in distribution, growing between 1200 and 2400 m only in the Lonquimay region and surrounding area in southern Chile, plus the adjacent Province of Mendoza and Neuquén in Argentina (Mathias and Constance, 1962; Martínez, 2008). The restricted geographical distribution of P. volcanica near the centre of the range of P. coriacea, the similar morphology of the two species and the existence of only two species within this morphologically distinct genus suggest the hypothesis that P. volcanica arose through a process of progenitor–derivative speciation from P. coriacea.
Fig. 1.
Species of the genus Pozoa and their typical habitats in southern South America. (A) Pozoa coriacea; (B) P. volcanica; (C) Chile, Region del Maule, Laguna Teno; (D) Chile, Region de la Araucanía, Navidad cone (centre), Volcán Lonquimay. Scale bars = 3 cm.
Fig. 2.
Distribution of sampled populations of Pozoa coriacea (squares) and P. volcanica (circles) in southern South America. Generalized distributions of P. coriacea and P. volcanica are shown by dashed red and blue lines, respectively.
This paper tests the hypothesis of progenitor–derivative speciation within Pozoa with the following specific objectives: (1) confirming that Pozoa is a monophyletic genus; (2) determining if one species of Pozoa is ancestral to the other and, if so, which; and (3) investigating levels of genetic divergence and variation in the derived species in comparison with its progenitor. To complete these objectives, we have selected DNA sequencing from the plastid genome and AFLP analysis (Vos et al., 1995). The latter is particularly efficacious for revealing patterns of genetic variation in natural populations (Gaudeul et al., 2000; Nybom, 2004; Andrade et al., 2009) and for revealing genetic structure of intra- and interspecific taxa (Wooten and Tolley-Jordan, 2009). These sensitive AFLP markers, to our knowledge, have not yet been applied to examination of progenitor–derivative speciation.
MATERIALS AND METHODS
The species
Pozoa coriacea Lag. (Fig. 1A; common name ‘Anislao’ or ‘Asta de cabra’) is an outcrossing perennial herb with a large, underground stem divided into many slender lateral divisions, and with spreading-ascending to recurved terminal flowering stalks (peduncles). Leaves are ovate to orbicular–reniform or obovate, undulate or slightly to roughly dentate, usually with 3–15 shallow teeth. Umbels have 20–35 flowers, some staminate. Flowers are usually purplish or purple. Fruits are oblong–ovate to cuneate–oblong, with the mature carpels slightly compressed (Matthias and Constance, 1962). The chromosome number is 2n = 20 (Bell and Constance, 1957; Rahn, 1960).
Pozoa volcanica Mathias & Constance (Fig. 1B) is also an outcrossing perennial herb, with a large and undivided underground stem, but it has a short and enlarged terminal peduncle. Leaves are ovate–orbicular to reniform, slightly or doubly dentate, usually with 13–30 triangular teeth. Umbels have 25–45 flowers, some staminate. Flowers are usually greenish-yellow. The fruits are oblong–ovate, the mature carpels being strongly compressed. The chromosome number is also 2n = 20 (Bell and Constance, 1957; Rahn, 1960). The principal morphological differences between the two species are the stem with slender divisions in P. coriacea, the greater number of teeth on the leaves of P. volcanica and the flowers being usually greenish-yellow in P. volcanica and purplish in P. coriacea.
Sampling
Twenty-two populations of Pozoa were collected throughout the entire range of the two species (Fig. 2, Table 1), extending from Portillo in the north of Chile to La Hoya in the south of Argentina for P. coriacea (11 populations), and within the Lonquimay region in southern Chile and adjacent Mamuil Malal in Argentina for P. volcanica (11 populations). Leaves of five individuals from each population were collected in silica gel. Vouchers of each population sampled are on deposit in the herbarium of the University of Vienna (WU).
Table 1.
Collection data for populations of Pozoa and generic relatives for sequencing (S) and AFLP (A) studies
| Species | Analysis | Population | Collection number | Latitude | Longitude | Elevation (m) | EMBL accession number |
|---|---|---|---|---|---|---|---|
| Pozoa coriacea Lag. | A, S | 1: Chile, Portillo | PL et al. 2605 | 32 °50′09″S | 70 °07′43″W | 2880 | FR871934 |
| A | 2: Chile, Valle Nevado | PL et al. 2531 | 33 °20′01″S | 70 °14′49″W | 3100 | – | |
| A | 3: Chile, Embalse El Yeso | PL et al. 2601 | 33 °37′05″S | 69 °58′04″W | 2580 | – | |
| A | 4: Chile, Laguna Teno | PL et al. 2600 | 35 °09′48″S | 70 °32′17″W | 2540 | – | |
| A | 5: Chile, Baños de Colina | PL et al. 2604 | 35 °50′01″S | 69 °59′25″W | 2438 | – | |
| A | 6: Chile, Laguna del Maule | PL et al. 2593 | 36 °00′54″S | 70 °30′20″W | 2360 | – | |
| A, S | 7: Chile, Chillán, Shangri-La | PL et al. 2548 | 36 °52′34″S | 71 °27′51″W | 1550 | FR871933, FR871941 | |
| A | 8: Argentina, Volcán Copahue | KT et al. 1033 | 37 °49′53″S | 71 °06′44″W | 2120 | – | |
| A | 9: Chile, Volcán Callaquí | KT et al. 1026 | 37 °54′42″S | 71 °24′00″W | 1675 | – | |
| A | 10: Chile, Termas de Rio Blanco | PL et al. 2564 | 38 °34′34″S | 71 °37′38″W | 1035 | – | |
| A, S | 11: Argentina, La Hoya | PL et al. 2686 | 42 °49′58″S | 71 °15′26″W | 1660 | FR871940 | |
| Pozoa volcanica Math. & Const. | A | 12: Chile, Cerros de Lanco | KT et al. 135 | 38 °20′55″S | 71 °25′52″W | 1820 | – |
| A | 13: Chile, Cerro Colorado | KT et al. 16 | 38 °24′40″S | 71 °34′34″W | 1880 | – | |
| A | 14: Chile, Cordillera Las Raíces | KT et al. 1 | 38 °26′29″S | 71 °28′48″W | 1800 | – | |
| A | 15: Chile, Sierra Nevada | KT et al. 61 | 38 °37′02″S | 71 °35′50″W | 1730 | – | |
| A | 16: Chile, Pino Hachado B | PL et al. 2559 | 38 °39′20″S | 70 °55′11″W | 1770 | – | |
| A | 17: Chile, Pino Hachado A | KT et al. 130 | 38 °39′40″S | 70 °53′53″W | 1900 | – | |
| A, S | 18: Argentina, Pino Hachado C | PL et al. 2677 | 38 °40′03″S | 70 °50′46″W | 1558 | FR871943 | |
| A, S | 19: Chile, Conguillío | PL et al. 2565 | 38 °40′59″S | 71 °48′01″W | 1440 | FR871942 | |
| A | 20: Chile, Volcán Llaima | KT et al. 106 | 38 °41′26″S | 71 °47′06″W | 1725 | – | |
| A, S | 21: Argentina, Mamuil Malal | PL et al. 2680 | 39 °36′26″S | 71 °21′50″W | 980 | FR871936 | |
| S | 22: Chile, Volcán Lanin | PL et al. 2578 | 39 °35′47″S | 71 °30′20″W | 1380 | FR871935 | |
| Asteriscium chilense Cham. & Schlecht. | S | 23: Chile, Puerto Oscuro | PL et al. 2517 | 31 °21′39″S | 71 °35′21″W | 230 | FR871928, FR871938 |
| Asteriscium vidali Phil. | A, S | 25: Chile, Huasco, Cerro Negro | PL et al. 2508 | 28 °29′28″S | 71 °14′07″W | 340 | FR871929, FR871939 |
| Eremocharis fruticosa Phil. | S | 26: Chile, Quebrada Peralito | PL et al. 2505 | 25 °01′50″S | 70 °26′15″W | 710 | FR871930, FR871937 |
| Gymnophyton foliosum Phil. | S | 27: Chile, Taltal, Quebrada San Ramón | PL et al. 2504 | 25 °23′05″S | 70 °26′42″W | 40 | FR871931, FR871944 |
| Gymnophyton isatidicarpum (Presl ex DC.) Math. & Const. | A, S | 28: Chile, Vicuña | PL et al. 2509 | 30 °08′42″S | 70 °30′17″W | 1300 | FR871932 |
Vouchers are on deposit at the University of Vienna. PL, Patricio López; KT, Karin Tremetsberger.
The populations of P. coriacea grow in different substrates (Fig. 1C), such as stable volcanic soil, black or clay soil, red gravel, sand and between rocks. Genera of the high Andean vegetation that accompany P. coriacea include: Mulinum (Apiaceae); Araucaria (Araucariaceae); Baccharis, Chuquiraga, Hypochaeris, Mutisia and Nassauvia (all Asteraceae); Berberis (Berberidaceae); Empetrum (Ericaceae); Adesmia and Lathyrus (Fabaceae); Nothofagus (Fagaceae); Polygonum (Polygonaceae); Acaena (Rosaceae); Nertera (Rubiaceae); Calceolaria (Calceolariaceae); and Tropaeolum (Tropaeolaceae).
Pozoa volcanica grows in new volcanic ash (Fig. 1D), with porous rock and pebbles, and occasionally black or brown soil. Accompanying vegetation includes the genera: Baccharis, Hypochaeris, Nassauvia and Senecio (Asteraceae); Adesmia and Trifolium (Fabaceae); Loasa (Loasaceae); Chusquea (Poaceae); Polygonum and Rumex (Polygonaceae); and Acaena (Rosaceae).
Sequences
Genomic DNA was extracted from individuals in 27 populations belonging to seven species in the genera Asteriscium, Eremocharis, Gymnophyton and Pozoa (Table 1) from silica gel-dried leaf material following the cetyltrimethylammonium bromide (CTAB) method (Doyle and Doyle, 1987) with minor modifications (Tremetsberger et al., 2003). We tested several plastid sequences (3′rps16-5′trnK, trnH-psbA, matK, ndhF-rpl32 and rpl32-trnL; Shaw et al., 2007) for their polymorphism on two or three individuals of P. coriacea and P. volcanica. This primer trial suggested the two non-coding plastid regions, ndhF-rpl32 and rpl32-trnL, corresponding to the intergenic spacer and located in the small single-copy region as most informative. Amplifications were made using the following primers: ndhF (5′-GAA AGG TAT KAT CCA YGM ATA TT-3′)/rpl32-R (5′-CCA ATA TCC CTT YYT TTT CCA A-3′), and rpl32-F (5′-CAG TTC CAA AAA AAC GTA CTT C-3′)/trnL(UAG) (5′-CTG CTT CCT AAG AGC AGC GT-3′), respectively (Shaw et al., 2007).
PCRs were carried out using 0·4 mm of each primer and ReddyMix PCR Master Mix (ABgene, Vienna, Austria) including 2·5 mm MgCl2 (according to the manufacturer's instructions). Amplifications were performed in a GeneAmp PCR System 9700 (Applied Biosystems) with an initial 5 min at 80 °C followed by 36 cycles each of 30 s denaturation at 95 °C, 30 s annealing at 50 °C, an elongation phase of 4 min at 65 °C, followed by a final elongation phase of 5 min at 65 °C. PCR products were purified using 0·5 µL of exonuclease I from Escherichia coli and 1 µL of shrimp alkaline phosphatase (Fermentas) for 45 min at 37 °C followed by enzyme inactivation for 15 min at 85 °C. Cycle sequencing was performed for the forward and reverse strand with 0·7 µL of BigDye Terminator v3·1 Ready Reaction Mix (Applied Biosystems) in a 10 µL total volume filled up with water, 1 µL of forward or reverse primer and 6·8 µL of PCR product with the following conditions: 1 min at 96 °C followed by 35 cycles of 10 s at 96 °C, 5 s at 50 °C and 4 min at 60 °C. Sequencing reactions were analysed on a capillary sequencer (3730 DNA Analyzer; Applied Biosystems).
The sequences were assembled and aligned using Seqman II (DNASTAR) and Clustal (Thompson et al., 1997), followed by manual adjustments using the program BioEdit version 7·0·9·0 (Hall, 1999). Indels were treated as binary characters following the ‘simple indel coding method’ (Simmons and Ochoterena, 2000) using the program SeqState version 1·36 (Müller, 2005). A heuristic search for most parsimonious (MP) trees was performed with PAUP* version 4·0b8 (Swofford, 2002). The analyses involved 1000 replicates with stepwise random taxon addition, tree bisection–reconnection (TBR) and branch swapping saving no more than ten trees per replicate. All characters were equally weighted and treated as unordered (Fitch, 1971). Clade support was estimated using non-parametric bootstrapping (Felsenstein, 1985) with 10 000 bootstrap replicates each with ten random sequence addition replicates holding maximally ten trees per replicate, TBR branch swapping and MulTrees on. A phylogenetic supertree was built from the ndhF-rpl32 and rpl32-trnL trees using the matrix representation with parsimony method implemented in the program SuperTree 0·85b (Salamin et al., 2002) with the following options: coding scheme, Baum/Ragan; and character type, unordered. EMBL accession numbers of the DNA sequences are presented in Table 1.
AFLP fingerprinting
We scored 105 individuals of Pozoa and two individuals of the outgroup (Asteriscium and Gymnophyton) for three AFLP primer combinations. Genomic DNA was extracted from silica gel-dried leaf material following the CTAB method (Doyle and Doyle, 1987) with minor modifications (Tremetsberger et al., 2003). The AFLP protocol followed Vos et al. (1995) with modifications as indicated in Tremetsberger et al. (2003). The selective primer combinations chosen following primer trials are MseI-CTGA/EcoRI-ACT (Fam), MseI-CTT/EcoRI-ACT (Vic) and MseI-CAC/EcoRI-ACC (Ned).
The presence and absence of bands in all individuals were scored with GeneMarker ver. 1·85 by Soft Genetics. For raw data analysis of each primer combination, local southern size call algorithm, smooth peak saturation, baseline subtraction, pull-up correction and spike removal were selected. We used the range 150–510 bp for all primer combinations. The peak detection threshold was an intensity of relative fluorescent units >50, with the percentage of relative minimum intensity of allele peaks at 5 and with the same value for local region percentage. The maximum relative fluorescent units threshold of peak height for peak detection was 30 000. Size calibration was manually adjusted in some samples with values below 90 %. The electropherograms were standardized using the automatic panel editor, generating a new panel for each colour. Each primer combination generates a binary matrix, combined in one for analysis of genetic diversity and differentiation (Wooten and Tolley-Jordan, 2009).
Estimation of genetic diversity
The number of different AFLP phenotypes present in a population was counted with Arlequin ver. 3·1 (Excoffier et al., 2006). The number of private bands in each population and species was calculated using FAMD ver. 1·108 (Schlüter and Harris, 2006), and the Rarity Index, calculated by using the R-script AFLPdat (Ehrich, 2006). For each individual, each AFLP marker is divided by the total number of occurrences of this marker in the data set. These relative values are then summed to the Rarity Index for this particular individual. Population values are estimated as the average of the individual values, and species values are estimated as the average of the population values.
Genetic diversity was assessed for each population and species by using the total number of AFLP bands, percentage of polymorphic bands (by dividing the number of polymorphic bands by the total number of bands in the data set) and Shannon Diversity Index HSh = –Σ[pi × ln(pi)], where pi is the frequency of the ith band in the respective population based on all AFLP bands recorded using FAMD ver. 1·108 (Schlüter and Harris, 2006). The Pearson correlation was used to test correlation among different genetic diversity estimates using SPSS ver. 15·0 (©SPSS Inc.). The Mann–Whitney U-test was used to estimate the significance of differences of genetic divergence and diversity of populations between species using SPSS.
A Nei–Li distance matrix was calculated from the AFLP matrix, and used as input for the phylogenetic network with the NeighborNet algorithm (Bryant and Moulton, 2004), as implemented in the software SplitsTree ver. 4·10 (Hudson and Bryant, 2006). A Neighbor–Joining tree based on the Nei–Li distance matrix was condensed and midpoint-rooted using FigTree ver. 1·3·1 (Rambaut, 2006–2009).
Estimation of genetic differentiation
Genetic differentiation among species was evaluated by analysis of molecular variance (AMOVA) using Arlequin ver. 3·1 (Excoffier et al., 2006); total genetic diversity was partitioned into components among two hierarchical levels, among populations (FST) and among individuals within populations. An alternative Bayesian approach (Holsinger et al., 2002) was used to obtain an independent estimate of FST for each population. This method allows estimation of FST from dominant markers without assuming Hardy–Weinberg proportions in populations. The original data matrix was imported into Hickory ver. 1·1 (Holsinger and Lewis, 2003–2007) and used for a full model, f = 0 model, theta = 0 model and the f-free model run with default parameters (i.e. the hickory block omitted). The f-free model, which estimates theta without estimating f (thus incorporating all the uncertainty in the prior of f), is available for dominant marker data, because estimates of f derived from dominant marker data may be unreliable. The deviance information criterion (DIC; Spiegelhalter et al., 2002) was used to estimate how well a particular model fits the data and to choose between models.
RESULTS
Sequence relationships
The aligned ndhF-rpl32 region is 1231 bp long. Phylogenetic analysis shows that 23 characters are parsimony uninformative and 27 are potentially parsimony informative. The alignment resulted in 18 potentially informative indels, one of which was nested. The single most parsimonious tree [length 52, consistency index (CI) 0·962, rescaled consistency index (RC) 0·928] for the region ndhF-rpl32 among Asteriscium, Eremocharis, Gymnophyton and Pozoa (Fig. 3A) shows that Pozoa is most closely related to Gymnophyton [bootstrap (BS) = 100 %] and that P. volcanica appears to be derived from P. coriacea, but with low support. Pozoa volcanica forms a clade, but P. coriacea is paraphyletic. The aligned rpl32-trnL region is 894 bp long. Phylogenetic analysis shows that 13 characters are parsimony uninformative and 30 are potentially parsimony informative. The alignment resulted in 13 potentially informative indels. The single most parsimonious tree (length 44, CI 0·977, RC 0·958; Fig. 3B) reveals the two species of Pozoa in one clade (BS = 100 %) connecting with the clade of Asteriscium (BS = 99 %). The results of Nicolas and Plunkett (2009), using the plastid rpl16 intron and trnD-trnT intergenic spacer (Fig. 3C), revealed Pozoa as monophyletic, and related to Asteriscium and Gymnophyton. The four plastid sequences taken together, therefore, show Pozoa as a monophyletic genus, related to Gymnophyton and Asteriscium (Fig. 3C, D).
Fig. 3.
Phylogenetic relationships among the genera Asteriscium, Eremocharis, Gymnophyton and Pozoa based on plastid DNA markers (values above branches represent bootstrap values): (A) ndhF-rpl32, 50 % majority rule consensus tree of 10 000 bootstrap replicates based on maximum parsimony; (B) rpl32-trnL, 50 % majority rule consensus tree of 10 000 bootstrap replicates based on maximum parsimony; (C) rpl16 combined with trnD-trnT, redrawn from Nicolas and Plunkett (2009) (maximum likelihood and Bayesian inference); and (D) supertree using ndhF-rpl32 and rpl32-trnL trees based on maximum parsimony. Numbers after species names in A and B are collection numbers (see Table 1).
AFLP relationships
Fragment patterns
The total number of AFLP bands found in all individuals and all populations of both species of Pozoa is 406, of which 405 (99·7 %) are polymorphic. Pozoa coriacea presents a total of 355 bands, of which 354 are polymorphic, whereas P. volcanica has a total of 253 bands, with 246 being polymorphic. The number of fragments for all individuals and by species (P. coriacea/P. volcanica) are 142 (130/84) for primer MseI-CTGA/EcoRI-ACT, 165 (147/97) for MseI-CTT/EcoRI-ACT and 99 (78/72) for MseI-CAC/EcoRI-ACC. All individuals had unique AFLP phenotypes.
Genetic diversity and divergence of populations
The total number of AFLP bands is slightly higher in P. coriacea than in P. volcanica, but the percentage of polymorphic bands is higher in P. volcanica than in P. coriacea. The Shannon Diversity Index shows that both species have essentially the same levels of within-population genetic diversity. The differences in the three estimates of diversity are not significant according to the Mann–Whitney U-test (Table 2, Fig. 4A). A significant correlation was observed between these indices in both species; the Pearson correlation between the Shannon Diversity Index and the total number of bands is r = 0·899 [n = 21, significance (two-tailed) = 0·000] and between the percentage of polymorphic bands and the total number of bands it is r = 0·675 [n = 21, significance (two-tailed) = 0·001]. The correlation between the percentage of polymorphic bands and the Shannon Diversity Index is r = 0·620 [n = 21, significance (two-tailed) = 0·003]. Values of the mean for Shannon Diversity are similar in both species. Regarding estimates of genetic divergence, the number of private bands and the Rarity Index are both significantly higher in populations of P. coriacea than in P. volcanica (Mann–Whitney U-test, Table 2, Fig. 4B). Both indices are positively correlated, with Pearson correlation r = 0·809 [n = 21, significance (two-tailed) = 0·000]. Comparing values of estimates of divergence and diversity in each species, P. coriacea has considerably higher values than P. volcanica for all measures (Table 3).
Table 2.
Estimates of divergence and diversity based on AFLP analysis from five individuals in each of 21 populations of Pozoa coriacea and P. volcanica
| Species | Population | Estimates of divergence |
Estimates of diversity |
|||
|---|---|---|---|---|---|---|
| No. of private bands | Rarity Index | Total no. of bands | Percentage of polymorphic bands | Shannon Diversity Index | ||
| Pozoa coriacea | 1 | 5 | 4·70 | 93 | 14·53 | 17·13 |
| 2 | 5 | 5·16 | 120 | 19·21 | 23·42 | |
| 3 | 3 | 5·51 | 128 | 20·44 | 25·53 | |
| 4 | 3 | 5·01 | 128 | 18·23 | 24·86 | |
| 5 | 5 | 5·47 | 120 | 20·93 | 22·56 | |
| 6 | 13 | 7·63 | 152 | 26·35 | 33·32 | |
| 7 | 4 | 4·89 | 135 | 22·17 | 26·11 | |
| 8 | 4 | 2·89 | 81 | 14·78 | 18·69 | |
| 9 | 4 | 2·25 | 68 | 12·56 | 15·69 | |
| 10 | 1 | 2·16 | 78 | 12·56 | 14·58 | |
| 11 | 19 | 7·46 | 129 | 14·78 | 29·99 | |
| Mean (± s.d.) | 6·00 (± 5·25) | 4·83 (± 1·83) | 112 (± 27·33) | 17·87 (± 4·41) | 22·90 (± 5·94) | |
| Pozoa volcanica | 12 | 2 | 2·66 | 100 | 24·13 | 23·13 |
| 13 | 4 | 2·96 | 100 | 18·23 | 22·82 | |
| 14 | 1 | 2·40 | 95 | 17·49 | 20·69 | |
| 15 | 1 | 1·95 | 83 | 16·50 | 17·92 | |
| 16 | 1 | 3·32 | 122 | 21·18 | 28·55 | |
| 17 | 2 | 3·73 | 128 | 22·66 | 27·08 | |
| 18 | 4 | 3·54 | 100 | 19·95 | 24·39 | |
| 19 | 4 | 3·26 | 106 | 13·79 | 26·28 | |
| 20 | 1 | 2·30 | 90 | 21·18 | 18·85 | |
| 21 | 0 | 1·74 | 76 | 15·27 | 19·44 | |
| Mean (± s.d.) | 2·00 (± 1·49) | 2·79 (± 0·68) | 100·00 (± 15·97) | 19·04 (± 3·34) | 22·92 (± 3·67) | |
| Mann–Whitney U-test: Z (two-tailed significance) | –2·695 (0·007) | –2·394 (0·017) | –1·059 (0·289) | –0·881 (0·387) | –0·211 (0·863) | |
The Mann–Whitney U-test was used to assess the significance of difference between the two species.
Fig. 4.
Boxplots of AFLP data showing the median, 25 % and 75 % quartile (box) and non-outlier range in Pozoa coriacea and P. volcanica of (A) the total numbers of bands and Shannon Diversity and (B) the number of private bands and Rarity Index. Numbers by the circle and asterisks represent populations with middle and extreme outlier data values, respectively.
Table 3.
Estimates of divergence and diversity based on AFLP analysis of Pozoa coriacea and P. volcanica
| Species | No. of private bands | Rarity Index | Total no. of bands | Percentage of polymorphic bands | Shannon Diversity Index |
|---|---|---|---|---|---|
| Pozoa coriacea | 153 | 6·43 | 355 | 87·19 | 75·86 |
| Pozoa volcanica | 51 | 4·92 | 253 | 60·59 | 47·08 |
Genetic diversity between species
Analysis of molecular variance attributes 20·20 % variance (d.f. = 1) between species and 79·80 % variance (d.f. = 103) within populations of each species. The same analysis, but for each species, shows 54·48 % variance among populations in P. coriacea (d.f. = 10) and 45·52 % variance (d.f. = 44) within populations [95 % confidence interval (CI) = 51·4–57·4 %]. Pozoa volcanica presents 25·55 % variance among populations (d.f. = 9) and 74·45 % (d.f. = 40) variance within populations (95 % CI = 22·1–28·9 %).
The genetic variance among species using a Bayesian analysis shows the lowest DIC value with the f = 0 model (DIC value = 3242), where the theta-II value is 0·240 (95 % CI = 0·213–0·273). Among populations of P. coriacea (n = 11; pop. 1–11), using the full model (DIC value = 4576), the value of theta-II is 0·475 (95 % CI = 0·453–0·498); in P. volcanica (n = 10; pop. 12–21; DIC value = 3714) the theta-II value is 0·217 (95 % CI = 0·191–0·243).
NeighborNet analysis with SplitsTree using the whole AFLP data set shows that the sister lineage (Asteriscium/Gymnophyton) links to Pozoa between P. coriacea populations 1–10 and P. coriacea population 11 plus P. volcanica. When the SplitsTree is interpreted phylogenetically, this implies that population 11 is more closely related to P. volcanica than to the remainder of P. coriacea (Fig. 5A, B). Of the two species of Pozoa, the outgroup genera attach most closely to P. coriacea (Fig. 5B). Within P. coriacea there are two groups that correspond to a general geographical trend. The first group includes populations 1–7 of the central–north part of the range, and the second covers populations 8–11 distributed in the southern zone. Population 11 of P. coriacea (La Hoya) appears to be closely related to populations 18 and 19 of P. volcanica, which may suggest an original geographical origin of P. volcanica from populations in this southern region of P. coriacea. The populations of P. volcanica do not show a clear geographical pattern (Fig. 5B).
Fig. 5.
Phylogenetic tree and network from the AFLP data set: (A) condensed tree; values above the branches represent bootstrap values, numbers after species name are population numbers (see Table 1); (B) SplitsTree NeighborNet analysis of AFLP data showing genetic variation within and among populations of Pozoa coriacea (squares; red lines) and P. volcanica (circles; blue lines). The scale bar is percentage distance.
DISCUSSION
Monophyly of Pozoa
Before addressing the specific question of progenitor–derivative speciation in Pozoa, it is necessary to confirm that the genus is monophyletic. Although the morphology of Pozoa is unified and distinct (Fig. 1A, B), it is important to reject any consideration of biphyly involving related genera.
Previous morphological and anatomical studies have suggested which genera of Apiaceae might be the closest relatives of Pozoa. Henwood and Hart (2001) completed a cladistic analysis using morphological and anatomical data with a focus on Australian Hydrocotyloideae, but also including genera from other continents. In this study, Pozoa was generically distinct in possessing fused carpophores (free in the other genera), but it grouped most closely with Asteriscium due to shared non-inflexed petal apices. This sub-group joined next to Eremocharis, Domeykoa and Gymnophyton, constituting the ‘Pozoa clade’. Liu (2004), using 16 morphological and anatomical characters in cladistic analyses, obtained a consensus tree that showed Pozoa generically distinct by a concave dorsal fruit surface but nearest to Asteriscium and Gymnophyton.
Previous molecular studies have also suggested a relationship of Pozoa with Asteriscium and Gymnophyton of southern South America. Nicolas and Plunkett (2009) examined affinities among 40 genera of subfamily Hydrocotyloideae using plastid sequences of the rpl16 intron and trnD-trnT intergenic spacer regions. In this analysis with a combined data set (Fig. 3C), Pozoa appears sister to Asteriscium and Gymnophyton (in their paper labelled as the Gymnophyton sub-clade) with 100 % bootstrap support and a posterior probability of 1·0.
In view of the importance of confirming monophyly in Pozoa, and following the suggestions of affinities revealed from previous studies, our own sequencing efforts focused initially on examining relationships among Pozoa, Asteriscium, Domeykoa, Eremocharis and Gymnophyton. Primer trials recommended employment of the plastid markers ndhF-rpl32 and rpl32-trnL. Results of the former (Fig. 3A) showed the closest relative to be Gymnophyton (100 % BS), and of the latter (Fig. 3B) to be Asteriscium (99 %). The studies of Nicolas and Plunkett (2009; Fig. 3C), using the rpl16 intron and trnD-trnT intergenic spacer, also showed a strong tie of Pozoa (100 % BS) to the genera Asteriscium and Gymnophyton. These two genera, therefore, were selected as outgroups for more detailed AFLP population-level analyses. All molecular data also point to Pozoa being monophyletic. The previous studies (Fig. 3C) of Nicolas and Plunkett (2009) placed P. coriacea and P. volcanica together (>95 % BS), as do our own results (Fig. 3A, B). AFLP analyses (Fig. 5) further support monophyly of Pozoa. NeighborNet analysis using SplitsTree of the many populations of both Pozoa spp., and including representatives of Asteriscium and Gymnophyton, show substantial degrees of divergence of these genera in attachment to populations of P. coriacea. All data, therefore, support Pozoa as being monophyletic.
Ancestry of the species of Pozoa
In the context of monophyly of Pozoa, the next consideration is the specific ancestry of the two included species. There are three likely alternatives: (1) origin of both species from a common, now extinct, ancestor; (2) origin of P. coriacea from P. volcanica; or (3) origin of P. volcanica from P. coriacea. Choosing among these alternatives involves examining data from geography, ecology and patterns of genetic variation. The DNA sequences as a whole are not very informative on this question, with the exception that with ndhF-rpl32 (Fig. 3A) the monophyletic P. volcanica appears more derived.
The geography (Fig. 2) and ecology of P. coriacea and P. volcanica (Fig. 1C, D) suggest strongly that the latter was derived from the former. The distributional range of P. coriacea is broad, ranging along the Andean mountain chain. Pozoa volcanica, on the other hand, is restricted to the volcanic region near Volcán Lonquimay in southern Chile. Complex alternative hypotheses might be formulated, obviously, to suggest that P. volcanica might have been the original progenitor that survived in refugia during Pleistocene glaciation, followed by derivative speciation into P. coriacea and subsequent extensive range expansion north and south. The broader level of genetic variation in P. coriacea, however, in contrast to that in P. volcanica, argues against this possibility (see below). The range of ecological tolerance of P. coriacea is also much broader than that of P. volcanica. The former is found in numerous habitats in and around Nothofagus and Araucaria forests, in varying types of substrate, including organic soils. Pozoa volcanica, on the other hand, is restricted to open sites in the active volcanic region around Volcán Lonquimay. Similar differences in the habitat occur in other cases of progenitor–derivative speciation: Layia glandulosa (progenitor species) lives on sandy soils and L. discoidea (derivative) on serpentine soils (Baldwin, 2005); Mimulus cupriphilus (derivative from M. guttatus) grows near copper mines (MacNair et al., 1989; Macnair and Gardner, 1998). Impetus for the present project, in fact, came from noting that P. volcanica was one of the early colonizers of the fresh bare volcanic ash in the explosion zone of the Navidad cone of Lonquimay, which erupted in 1988 (González-Ferrán, 1994). The more open and uniform habitat in which P. volcanica occurs, therefore, argues for this species being a populational derivative into a unique ecological zone from P. coriacea rather than the reverse.
Populational genetic data from AFLP analyses (Fig. 5) also argue for P. volcanica being derived from P. coriacea. First, SplitsTree analysis places the outgroup representatives of Asteriscium and Gymnophyton within populations of P. coriacea and not in P. volcanica. Secondly, and more compelling, is that the degree of genetic variation among populations of P. coriacea is much greater than that of P. volcanica (see also Fig. 4). The total number of bands, number of private bands and the Rarity Index all support a reduced genetic profile in P. volcanica. This is what would be expected to occur with a founder effect origin of a derivative peripheral population system from a more genetically (and ecologically) diverse progenitor.
Levels of genetic variation in progenitor and derivative species
The genetic characteristics that a species must have to establish progenitor–derivative origins are (from Crawford et al., 1985; Perron et al., 2000; Jaramillo-Correa and Bousquet, 2003): (1) high genetic similarity between the two species; (2) less genetic variation in the derivative species; (3) absence of alleles present in the progenitor, often in low frequencies, and (4) few or no unique alleles in the derivative species.
AFLP data from P. coriacea and P. volcanica indicate a low FST value between the species (FST = 0·2019), and hence a high degree of genetic similarity due to a high proportion of similar alleles between them. The number of private bands for P. coriacea is three times higher than that in P. volcanica (Table 3). This is concordant with the idea of reduced genetic variability via recent origin of the taxon in the context of a founder effect (Purps and Kadereit, 1998).
This same trend of loss of genetic variation in the derivative species has been documented in other species pairs. Perron et al. (2000) and Jaramillo-Correa (2003), investigating black spruce (Picea mariana) and red spruce (P. rubens), showed that the genetic diversity of the derivative species was a subset of that observed in the progenitor. Gottlieb (1974) found a reduced allelic diversity in the derivative Clarkia lingulata in comparison with C. biloba when analysed for electrophoretic variation specified by eight loci. Crawford and Smith (1982), using allozymes, showed a decrease in genetic variation in the derivative species Coreopsis nuecensis in relation to the progenitor species C. nuecensoides. The same general trend has been documented in (progenitor species given first): Lasthenia minor and L. maritima (Crawford et al., 1985), Camassia scilloides and C. angusta (Ranker and Schnabel, 1986), Erythronium albidum and E. propullans (Pleasants and Wendel, 1989), and Senecio nebrodensis and S. viscosus (Kadereit et al., 1995; Purps and Kadereit, 1998). The causes of decline in genetic diversity in the derivative taxon are several, such as origin from a small number of individuals (bottleneck), and limited gene flow from parental populations.
In conclusion, P. volcanica represents a species recently derived from its progenitor P. coriacea. The volcanic activity in and around Volcán Lonquimay has provided an opportunity for establishment of peripheral populations from P. coriacea through dispersal into new open habitats, and subsequent divergence in isolation. The lower level of unique alleles in P. volcanica is consistent with the hypothesis of a founder effect. Biogeographically, it is likely that the origin of P. volcanica occurred after Pleistocene glaciation. Local glaciers along the Andean chain (Ortiz-Jaureguizar and Cladera, 2006), which resulted in a cooler climate, had the effect of shifting the vegetation to lower elevations, with the flora rebounding upwards only after the glaciers receded (Simpson, 1983). This may have coincided with volcanic activity, so frequent along the Chilean cordillera (González-Ferrán, 1994), which provided even more new ecological opportunities. These events may have been responsible for stimulating speciation within Pozoa as well as within other genera that inhabit the southern Andean mountain chain.
ACKNOWLEDGEMENTS
We are grateful to J. Cáceres-Chamizo, C. López Cáceres, I. Yañez, P. Arias, A. Jiménez, S. Gómez-González, M. J. Parra and R. Hössinger for help with collecting population samples; Departamento de Botánica, Universidad de Concepción, Chile, for logistic support; Corporación Nacional Forestal (CONAF) for permission to collect samples in Chilean National Parks; Administración de Parques Nacionales, Argentina, for permission to make collections in Argentina; and D. J. Crawford for helpful comments on an earlier draft of the manuscript. This project was supported by the Fonds zur Förderung der Wissenschaftlichen Forschung, Austria (FWF grant P18446 to T.F.S.).
LITERATURE CITED
- Amico GC, Vidal Russell R, Nickrent DL. Phylogenetic relationships and ecological speciation in the mistletoe Tristerix (Loranthaceae): the influence of pollinators, dispersers, and hosts. American Journal of Botany. 2007;94:558–567. doi: 10.3732/ajb.94.4.558. [DOI] [PubMed] [Google Scholar]
- Andrade IM, Mayo SJ, van den Berg C, Fay MF, Chester M, Lexer C, Kirkup D. Genetic variation in natural populations of Anthurium sinuatum and A. pentaphyllum var. pentaphyllum (Araceae) from north-east Brazil using AFLP molecular markers. Botanical Journal of the Linnean Society. 2009;159:88–105. [Google Scholar]
- Baldwin BG. Origin of the serpentine-endemic herb Layia discoidea from the widespread L. glandulosa (Compositae) Evolution. 2005;59:2473–2479. [PubMed] [Google Scholar]
- Bell CR, Constance L. Chromosome numbers in Umbelliferae. I. American Journal of Botany. 1957;44:565–572. [Google Scholar]
- Bolnick DI, Fitzpatrick BM. Sympatric speciation: models and empirical evidence. Annual Review of Ecology, Evolution and Systematics. 2007;38:459–487. [Google Scholar]
- Bryant D, Moulton V. Neighbor-Net: an agglomerative method for the construction of phylogenetic networks. Molecular Biology and Evolution. 2004;21:255–265. doi: 10.1093/molbev/msh018. [DOI] [PubMed] [Google Scholar]
- Coyne JA. Genetics and speciation. Nature. 1992;355:511–515. doi: 10.1038/355511a0. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- Crawford DJ. Progenitor–derivative species pairs and plant speciation. Taxon. 2010;59:1413–1423. [Google Scholar]
- Crawford DJ, Smith EB. Allozyme variation in Coreopsis nuecensoides and C. nuecensis (Compositae), a progenitor–derivative species pair. Evolution. 1982;36:379–386. doi: 10.1111/j.1558-5646.1982.tb05054.x. [DOI] [PubMed] [Google Scholar]
- Crawford DJ, Ornduff R, Vasey MC. Allozyme variation within and between Lasthenia minor and its derivative species. L. maritima. American Journal of Botany. 1985;72:1177–1184. [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin, Botanical Society of America. 1987;19:11–15. [Google Scholar]
- Ehrich D. AFLPdat: a collection of R functions for convenient handling of AFLP data. Molecular Ecology Notes. 2006;6:603–604. [Google Scholar]
- Excoffier L, Laval G, Schneider S. Arlequin ver. 3·11: an integrated software package for population genetics data analysis. 2006 Computational and Molecular Population Genetics Lab., University of Berne, Berne, Switzerland. Website: http://cmpg.unibe.ch/software/arlequin3 . [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fitch WM. Toward defining the course of evolution: minimal change for a specific tree topology. Systematic Zoology. 1971;20:406–416. [Google Scholar]
- Futuyma DJ, Mayer GC. Non-allopatric speciation in animals. Systematic Zoology. 1980;29:254–271. [Google Scholar]
- Gaudeul M, Taberlet P, Till-Bottraud I. Genetic diversity in an endangered alpine plant, Eryngium alpinum L. (Apiaceae), inferred from amplified fragment length polymorphism markers. Molecular Ecology. 2000;9:1625–1637. doi: 10.1046/j.1365-294x.2000.01063.x. [DOI] [PubMed] [Google Scholar]
- Gavrilets S. Perspective: models of speciation: what have we learned in 40 years? Evolution. 2003;57:2197–2215. doi: 10.1111/j.0014-3820.2003.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Gengler-Nowak KM. Reconstruction of the biogeographical history of Malesherbiaceae. Botanical Review. 2002;68:171–188. [Google Scholar]
- Gengler-Nowak KM. Molecular phylogeny and taxonomy of Malesherbiaceae. Systematic Botany. 2003;28:333–344. [Google Scholar]
- González-Ferrán O. Volcanes de Chile. Santiago: Instituto Geográfico Militar; 1994. [Google Scholar]
- Gottlieb LD. Genetic differentation, sympatric speciation, and the origin of a diploid species of Stephanomeria. American Journal of Botany. 1973;60:545–553. [Google Scholar]
- Gottlieb LD. Genetic confirmation of the origin of Clarkia lingulata. Evolution. 1974;28:244–250. doi: 10.1111/j.1558-5646.1974.tb00743.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb LD. Rethinking classic examples of recent speciation in plants. New Phytologist. 2003;161:71–82. [Google Scholar]
- Gottlieb LD, Warwick SI, Ford VS. Morphological and electrophoretical divergence between Layia discoidea and L. glandulosa. Systematic Botany. 1985;10:484–495. [Google Scholar]
- Grant V. Plant speciation. 2nd edn. New York: Columbia University Press; 1981. [Google Scholar]
- Hall T. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Henwood MJ, Hart JM. Toward an understanding of the phylogenetic relationships of Australian Hydrocotyloideae (Apiaceae) Edinburgh Journal of Botany. 2001;58:269–289. [Google Scholar]
- Hershkovitz MA, Arroyo MTK, Bell C, Hinojosa LF. Phylogeny of Chaetanthera (Asteraceae: Mutisieae) reveals both ancient and recent origins of the high elevation lineages. Molecular Phylogenetics and Evolution. 2006;41:594–605. doi: 10.1016/j.ympev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Holsinger KE, Lewis PO. Hickory: a package for analysis of population genetic data. 2003–2007 v1·1. Website: http://darwin.eeb.uconn.edu/hickory/hickory.html . [Google Scholar]
- Holsinger KE, Lewis PO, Dey DK. A Bayesian approach to inferring population structure from dominant markers. Molecular Ecology. 2002;11:1157–1164. doi: 10.1046/j.1365-294x.2002.01512.x. [DOI] [PubMed] [Google Scholar]
- Hudson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Jaramillo-Correa JP, Bousquet J. New evidence from mitochondrial DNA of a progenitor–derivative species relationship between black spruce and red spruce (Pinaceae) American Journal of Botany. 2003;90:1801–1806. doi: 10.3732/ajb.90.12.1801. [DOI] [PubMed] [Google Scholar]
- Kadereit JW, Comes HP, Curnow DJ, Irwin JA, Abbott RJ. Chloroplast DNA and isozyme analysis of the progenitor–derivative species relationship between Senecio nebrodensis and S. viscosus (Asteraceae) American Journal of Botany. 1995;82:1179–1185. [Google Scholar]
- Levin D. The ecological transition in speciation. New Phytologist. 2003;161:91–96. [Google Scholar]
- Liu MR. A taxonomic evaluation of fruit structure in the family Apiaceae. 2004. PhD Thesis, Rand Afrikaans University, South Africa. [Google Scholar]
- Lomolino MV, Riddle BR, Brown JH. Biogeography. 3rd edn. Sunderland, MA: Sinauer Associates; 2006. [Google Scholar]
- MacNair MR, Gardner M. The evolution of edaphic endemics. In: Howard DJ, Berlocher SH, editors. Endless forms: species and speciation. Oxford: Oxford University Press; 1998. pp. 157–171. [Google Scholar]
- MacNair MR, MacNair VE, Martin BE. Adaptive speciation in Mimulus: an ecological comparison of M. cupriphilus with its presumed progenitor, M. guttatus. New Phytologist. 1989;112:269–279. [Google Scholar]
- Manos PS. Systematics of Nothofagus (Nothofagaceae) based on rDNA spacer sequences (ITS): taxonomic congruence with morphology and plastid sequences. American Journal of Botany. 1997;84:1137–1155. [PubMed] [Google Scholar]
- Martínez S. Zuloaga FO, Morrone O, Belgrano MJ, editors. Apiaceae. Catálogo de las plantas vasculares del Cono Sur (Argentina, Sur de Brasil, Chile, Paraguay y Uruguay). Monographs in Systematic Botany from the Missouri Botanical Garden. 2008;107:1056–1090. [Google Scholar]
- Mathias ME, Constance L. A revision of Asteriscium and some related hydrocotyloid Umbelliferae. University of California Publications in Botany. 1962;33:99–184. [Google Scholar]
- Mayr E. Animal species and evolution. Cambridge, MA: Harvard University Press; 1963. [Google Scholar]
- Müller K. SeqState – primer design and sequence statistics for phylogenetic DNA data sets. Applied Bioinformatics. 2005;4:65–69. doi: 10.2165/00822942-200504010-00008. [DOI] [PubMed] [Google Scholar]
- Nicolas AN, Plunkett GM. The demise of subfamily Hydrocotyloideae (Apiaceae) and the re-alignment of its genera across the entire order Apiales. Molecular Phylogenetics and Evolution. 2009;53:134–151. doi: 10.1016/j.ympev.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Nybom H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Molecular Ecology. 2004;13:1143–1155. doi: 10.1111/j.1365-294X.2004.02141.x. [DOI] [PubMed] [Google Scholar]
- Ontivero M, Arias M, Ricci JD, Babot J, Albornoz P, Castagnaro A. Analysis of genetic similarities among species of Fragaria, Potentilla, and Duchesnea found in northwest Argentina by using morphological, anatomical, and molecular characters. Canadian Journal of Botany. 2000;78:547–556. [Google Scholar]
- Ortiz-Jaureguizar E, Cladera GA. Paleoenviromental evolution of southern South America during the Cenozoic. Journal of Arid Environments. 2006;66:498–532. [Google Scholar]
- Perron M, Perry DJ, Andalo C, Bousquet J. Evidence from sequence-tagged-site markers of a recent progenitor–derivative species pair in conifers. Proceeding of the National Academy of Sciences, USA. 2000;97:11331–11336. doi: 10.1073/pnas.200417097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasants JM, Wendel JF. Genetic diversity in a clonal narrow endemic, Erythronium propullans, and in its widespread progenitor, Erythronium albidum. American Journal of Botany. 1989;76:1136–1151. [Google Scholar]
- Purps DM, Kadereit JW. RAPD evidence for a sister group relationship of the presumed progenitor–derivative species pair Senecio nebrodensis and S. viscosus (Asteraceae) Plant Systematics and Evolution. 1998;211:57–70. [Google Scholar]
- Rahn K. Chromosome numbers in some South American angiosperms. Botanisk Tidsskrift. 1960;56:177–127. [Google Scholar]
- Rambaut A. FigTree v1·3·1. 2006–2009 http://tree.bio.ed.ac.uk/software/figtree. (April 2011) [Google Scholar]
- Ranker TA, Schnabel AF. Allozymic and morphological evidence for a progenitor–derivative species pair in Camassia (Liliaceae) Systematic Botany. 1986;11:433–445. [Google Scholar]
- Ruiz E, Toro O, Crawford DJ, et al. Phylogenetic relationships among Chilean species of Drimys (Winteraceae) based on its sequences and insertion/deletion events. Gayana, Botanica. 2008;65:220–228. [Google Scholar]
- Salamin N, Hodkinson TR, Savolainen V. Building supertrees: an empirical assessment using the grass family (Poaceae) Systematic Biology. 2002;51:136–150. doi: 10.1080/106351502753475916. [DOI] [PubMed] [Google Scholar]
- Samuel R, Stuessy TF, Tremetsberger K, Baeza CM, Siljak-Yakovlev S. Phylogenetic relationships among species of Hypochaeris (Asteraceae, Cichorieae) based on ITS, plastid trnL intron, trnL-F spacer, and matK sequences. American Journal of Botany. 2003;90:496–507. doi: 10.3732/ajb.90.3.496. [DOI] [PubMed] [Google Scholar]
- Schlüter PM, Harris SA. Analysis of multilocus fingerprinting data sets containing missing data. Molecular Ecology Notes. 2006;6:569–572. [Google Scholar]
- Schmidt R, Systma KJ. Phylogenetics of Puya (Bromeliaceae): placement, major lineages, and evolution of Chilean species. American Journal of Botany. 2010;97:337–356. doi: 10.3732/ajb.0900107. [DOI] [PubMed] [Google Scholar]
- Shaw J, Lickey EB, Schilling EE, Small RL. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. American Journal of Botany. 2007;94:275–288. doi: 10.3732/ajb.94.3.275. [DOI] [PubMed] [Google Scholar]
- Simmons MP, Ochoterena H. Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology. 2000;49:369–381. [PubMed] [Google Scholar]
- Simpson BB. Contrasting modes of evolution in two groups of Perezia (Mutisieae; Compositae) of southern South America. Taxon. 1973;22:525–536. [Google Scholar]
- Simpson BB. An historical phytogeography of the high Andean flora. Revista Chilena de Historia Natural. 1983;56:109–122. [Google Scholar]
- Simpson BB, Arroyo MK, Sipe S, Dias M, McDill J. Phylogeny and evolution of Perezia (Asteraceae: Mutisieae: Nassauviinae) Journal of Systematics and Evolution. 2009;47:431–443. [Google Scholar]
- Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society: Series B. 2002;64:583–639. [Google Scholar]
- Stuessy TF, Tremetsberger K, Müllner AN, et al. The melding of systematics and biogeography through investigations at the populational level: examples from the genus Hypochaeris (Asteraceae) Basic and Applied Ecology. 2003;4:287–296. [Google Scholar]
- Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony and other methods. ver. 4·0b8. Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- Tauber CA, Tauber MJ. Sympatric speciation in insects: perception and perspective. In: Otte D, Endler JA, editors. Speciation and its consequences. Sunderland, MA: Sinauer Associates; 1989. pp. 307–344. [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgings DG. The Clustal X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremetsberger K, Stuessy TF, Samuel RM, Baeza CM, Fay MF. Genetics of colonization in Hypochaeris tenuifolia (Asteraceae, Lactuceae) on Volcán Lonquimay, Chile. Molecular Ecology. 2003;12:2649–2659. doi: 10.1046/j.1365-294x.2003.01956.x. [DOI] [PubMed] [Google Scholar]
- Tremetsberger K, Weiss-Schneeweiss H, Stuessy T, et al. Nuclear ribosomal DNA and karyotypes indicate a NW African origin of South American Hypochaeris (Asteraceae, Cichorieae) Molecular Phylogenetics and Evolution. 2005;35:102–116. doi: 10.1016/j.ympev.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Tremetsberger K, Stuessy TF, Kadlec G, et al. AFLP phylogeny of South American species of Hypochaeris (Asteraceae, Lactuceae) Systematic Botany. 2006;31:610–626. [Google Scholar]
- Vos P, Hogers R, Bleeker M, et al. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Research. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten JA, Tolley-Jordan LR. Validation of phylogenetic signals in amplified fragment length data: testing the utility and reliability in closely related taxa. BMC Research Notes. 2009;2:26. doi: 10.1186/1756-0500-2-26. http://dx.doi.org/10.1186/1756-0500-2-26 . [DOI] [PMC free article] [PubMed] [Google Scholar]