Abstract
Background and Aims
Diverse leaf forms in nature can be categorized into two groups: simple and compound. A simple leaf has a single blade unit, whilst a compound leaf is dissected into leaflets. For both simple and compound leaves, a MYB domain transcription factor PHANTASTICA (PHAN) plays an important role in establishing the adaxial domain in the leaf. Absence of PHAN in arabidopsis and antirrhinum leaves supresses blade development, and in tomato suppresses leaflet development. However, in the rachis and petiole regions of tomato leaves where PHAN and the adaxial domain coexist, it has been unclear why leaf blade and leaflets are not formed. We hypothesized that PHAN regulates the medio-lateral extent of the adaxial domain, thereby determining compound leaf architecture.
Methods
To test this hypothesis, we generated and analysed transgenic tomato plants expressing tomato PHAN (SlPHAN) under the Cauliflower mosaic virus (CaMV) 35S promoter in both sense and antisense orientations, and tobacco plants that over-express tomato SlPHAN.
Key Results
Modulations in SlPHAN resulted in a variety of leaf morphologies such as simple, ternate and compound in either a peltate or non-peltate arrangement. Measurements of the extent of the adaxial domain along the wild-type tomato leaf axis showed that the adaxial domain is narrowed in the rachis and petiole in comparison with regions where laminar tissue arises. In antiSlPHAN transgenic leaves, no blade or leaflet was formed where the adaxial domain was medio-laterally narrowed, and KNOX gene expression was correlatively upregulated. CaMV35S::SlPHAN expression led to widening of the adaxial domain and ectopic blade outgrowth in the rachis of tomato and in the petiole of tobacco. Taken together, these results suggest that SlPHAN plays a role in medio-lateral extension of the adaxial domain and contributes to final leaf morphology in tomato.
Conclusions
This study provides a novel insight into leaf architecture in tomato and highlights how changes in the expression domain of a master regulator gene such as SlPHAN can be translated into diverse final leaf morphologies.
Keywords: PHANTASTICA, KNOX genes, compound leaf, simple leaf, homology, tomato, Solanum lycopersicum, tobacco, Nicotiana tabacum, leaf evolution
INTRODUCTION
Leaves are the main photosynthetic organs and thus pivotal to plant survival. Diverse leaf forms in nature can be grouped into two categories: simple and compound. A simple leaf is composed of a single undivided blade, whereas a compound leaf is divided into subunits called leaflets. Compound leaves can be further classified into two different forms: pinnate and palmate. The leaflets of palmate compound leaves radiate from the tip of the petiole (in an ‘umbrella-like’ arrangement), while the leaflets of pinnate compound leaves are arranged along the rachis (in a ‘feather-like’ arrangement). It has been suggested that the ancestral leaf form of angiosperms was simple (Donoghue and Doyle, 1989; Taylor and Hickey, 1992), and that compound leaves have evolved independently in different monocot and dicot lineages (Goliber et al., 1999).
Leaves are generated at the shoot apical meristem (SAM). The earliest event in leaf development is the transition from indeterminate to determinate fate in a sub-set of cells at the periphery of the SAM (Poethig and Sussex, 1985). This event has been best studied in arabidopsis and involves an interplay between ASYMMETRICAL LEAVES1 (AS1), a member of the ARP (AS1-ROUGH SHEATH2-PHAN) gene family, and SHOOTMERISTEMLESS (STM), a member of the KNOX (KNOTTED-1 LIKE HOMEOBOX) gene family (Byrne et al., 2000). In simple-leaved species, STM maintains meristematic cell identity in the SAM and leaf initiation correlates with the establishment of mutually exclusive expression of STM and AS1 (Waites et al., 1998; Timmermans et al., 1999; Tsiantis et al., 1999; Byrne et al., 2000; Ori et al., 2000; Harrison et al., 2005). Plant hormones are also involved in this developmental cascade (Hay et al., 2006; Bolduc et al., 2008; Braybrook and Kuhlemeier, 2010). Positional cues from the SAM set up polarities along the adaxial–abaxial, proximal–distal and medial–lateral axes in the leaf (Sussex, 1954, 1955). Several key regulators of adaxial and abaxial cell fates in the leaf have been identified. PHANTASTICA (the antirrhinum AS1 homologue), PHABULOSA, PHAVOLUTA and REVOLUTA (REV) are involved in establishing or maintaining the adaxial domain of leaves in antirrhinum and arabidopsis (Byrne et al., 2000; Emery et al., 2003; Golz et al., 2004). The YABBY and KANADI family genes have been shown to be responsible for the abaxial cell identities (Eshed et al., 2001, 2004; Kerstetter et al., 2001; Zhang et al., 2009). MicroRNAs (miRNAs) and trans-acting siRNAs (ta-siRNAs) are also involved in establishing leaf abaxiality–adaxiality (Juarez et al., 2004; Li et al., 2005; Garcia et al., 2006; Xu et al., 2006; Nogueira et al., 2007; Yan et al., 2009). The juxtaposition of the adaxial and the abaxial domains plays an important role in subsequent leaf blade formation in simple-leaved species, and loss of either the adaxial or abaxial domains leads to an inability to form a leaf blade (McConnell and Barton, 1998; Timmermans et al., 1998; Waites et al., 1998).
In many compound-leaved species, KNOX genes are expressed in the leaf and leaflet primordia (Hareven et al., 1996; Janssen et al., 1998), presenting a different scenario from simple-leaved species where KNOX genes are excluded from the leaf. Over-expression of KNOX genes promotes leaflet formation in tomato (Hareven et al., 1996; Chen et al., 1997; Parnis et al., 1997; Janssen et al., 1998), while downregulation of Cardamine hirsuta STM led to reduced leaf complexity in C. hirsuta (Hay and Tsiantis, 2006). The role of KNOX genes in generating compound leaves appears to be conserved in independently derived compound leaves across angiosperm lineages (Bharathan et al., 2002), except in a derived clade in the legumes, where the KNOX gene role is replaced by UNIFOLIATA, a LEAFY (LFY) homologue (Hofer et al., 1997; Champagne et al., 2007; Wang et al., 2008). The function of KNOX genes in compound leaf development is antagonized by BIPPINATA (BIP), a BELL-like homeobox gene (Kimura et al., 2008). In the bip mutant, the degree of leaf complexity is increased due to the upregulation of KNOX genes (Kimura et al., 2008). The other key players in compound leaf development are the ARP genes. Downregulation of a tomato ARP, SlPHAN, led to a loss of the adaxial domain and to no leaflet formation in the abaxialized region of tomato leaves (Kim et al., 2003a). Expressing ARP genes throughout the entire adaxial side (in both proximal and distal regions) of the leaf primordium generates pinnate compound leaves, whereas restricting ARP gene expression to the distal region of the leaf primordium generates peltately palmate leaves (Kim et al., 2003a). This association between the ARP expression domain and final leaf morphologies is highly conserved in species representing diverse lineages across the angiosperms (Kim et al., 2003a). However, mutations in the pea and Cardamine ARP genes did not affect leaf complexity (Tattersall et al., 2005; Hay and Tsiantis, 2006). The regulatory relationship between ARP and KNOX genes is complex in tomato, compared with that in arabidopsis, where STM represses AS1 and AS1 in turn inhibits another KNOX gene, KNAT1 (Byrne et al., 2000). SlPHAN is downregulated in Mouse Ears, a LeT6 (the tomato STM homologue) dominant mutant that over-expresses LeT6 ectopically (Kim et al., 2003b). This suggests that LeT6 is a negative regulator of SlPHAN (Kim et al., 2003b). However, both LeT6 and SlPHAN are expressed in the compound leaf and leaflet primordium (Koltai and Bird, 2000; Kim et al., 2003b). SlPHAN activity is required for LeT6 over-expression phenotypes, and SlPHAN downregulation phenotypes are epistatic to LeT6 over-expression phenotypes in Me and Curl (Kim et al., 2003b). While lower levels of LeT6 over-expression in Me heterozygous (Me/ +) plants show LeT6 over-expression phenotypes such as increased leaf compoundness, higher levels of LeT6 upregulation in Me homozygous (Me/Me) plants lead to SlPHAN downregulation phenotypes, e.g. loss of the adaxial domain and no leaflet formation (Kim et al., 2003b). The organ boundary genes, CUP-SHAPED COTYLEDON (CUC)/NO APICAL MERISTEM (NAM), which control leaf blade margin growth and leaf serration in simple-leaved species, also determine the extent of leaf dissection in compound-leaved species (Blein et al., 2008; Hasson et al., 2011). In Aquilegia caerulea, tomato, Pisum sativum and C. hirsuta, CUC/NAM was expressed in the rachis region in which the blade margin growth is inhibited, and downregulation of CUC/NAM also led to less dissected leaves, suggesting that CUC/NAM is required for the leaf dissection in compound leaves (Blein et al., 2008).
To determine further the function of SlPHAN in compound leaf development, we analysed transgenic tomato plants expressing SlPHAN under the Cauliflower mosaic virus (CaMV) 35S promoter, in both sense and antisense orientations. We showed that along the leaf axis, not only proximal–distal regulation (shown in our previous study, Kim et al., 2003a), but also medio-lateral regulation of the SlPHAN expression and the leaf adaxial domain plays a critical role in determining the final tomato leaf morphology. In addition, an expression analysis revealed a novel SlPHAN and KNOX gene regulatory relationship in tomato. Our results illustrate that changes in the expression domain of a single key regulator such as SlPHAN can replicate a range of leaf forms (compound vs. simple, peltate vs. non-peltate, petiolated vs. sessile) seen in nature.
MATERIALS AND METHODS
Transgenic plants
CaMV35S::antisense-SlPHAN and CaMV35S::sense-SlPHAN constructs were generated by using the full-length SlPHAN cDNA (GenBank accession no. AF148934). The SlPHAN cDNA was inserted into pART7 (Gleave, 1992) in sense and antisense orientations, and then the CaMV35S promoter and cDNA region flanked by SacI and SpeI sites were cloned into the T-DNA binary vector pBIBKan (Becker, 1990). These constructs were introduced into tomato (Solanum lycopersicum ‘VF36’) and tobacco (Nicotiana tabacum) by Agrobacterium tumefaciens LBA4404 as previously described (Horsch et al., 1985; McCormick, 1991).
Scanning electron microscopy (SEM), histology and immunolocalization
Tissues for plastic sections were fixed and sectioned as described previously (Kessler et al., 2001). For hand-sectioned leaves, samples were stained with 0·5 % toluidine blue O for 5 min and destained with water. Samples were viewed with a Nikon Eclipse E600 microscope and images were collected using a SPOT (RT Color) digital camera. Samples for SEM were fixed and viewed as described previously (Kessler et al., 2001). Immunolocalization experiments with antiSlPHAN lines were performed as described (Jackson et al., 1994), using polyclonal antibodies against RS2 (Kim et al., 2003a).
Reverse transcription–PCR (RT–PCR)
RNA was extracted from about 100 mg of the shoot tips and young leaves (P4–P8) using an RNeasy mini kit (Qiagen, UK). Contaminating genomic DNA was digested with RNase-free DNase I (Promega, UK) at 37 °C for 1 h followed by an inactivation step at 95 °C for 5 min. cDNA synthesis and quantification of transcripts by real-time PCR were performed as described previously (Leutenegger et al., 1999). Primer sequences are: PHAN1, 5′ACGAGCAGCGTCTTGTTATACAACTAC3′; PHAN2, 5′CCCTTCGTCTAAATCCTTGCAGC3′; LeT6-1, 5′CTCAATTGTCAAAAGATAGGAGCTCCGCCA3′; LeT6-2, 5′CCTCGCCCAAGCAGATTCATGAG3′; TKN1-1, 5′TGATCAGTTCATGGAAGCAATATTAGAC3′; TKN1-2, 5′TTCTCCGACTCCGAAGGGTATGGC3′; LeActin-1, 5′TGGCATCATACTTTCTACAATG3′ and LeActin-2, 5′TGCTCCTGGCAGTTTCAATC3′.
RESULTS
Medio-lateral reduction of the adaxial domain inhibited leaflet and blade formation in antiSlPHAN tomato
A total of 66 independent T1 antiSlPHAN tomato transgenic plants were obtained. Among these transformants, 59 plants showed similar leaf phenotypes (Supplementary Data Table S1, available online). T2 seeds were obtained and planted from 16 lines. Three T2 families showed a 3:1 ratio of antiSlPHAN:wild type. Southern blot analysis showed the presence of the transgene only in the T2 individuals with antiSlPHAN phenotypes (data not shown). In addition, seven other families showed segregation of antiSlPHAN and wild-type phenotypes. However, these families had limited seed set, and the antiSlPHAN and wild-type phenotype did not appear to segregate in a strictly Mendelian ratio due to the small seed population tested (Supplementary Data Table S2). Individual tomato plants showing antiSlPHAN phenotypes in the three T2 families with one insertion were analysed further. In our previous study, we showed that downregulation of SlPHAN in the proximal region of the leaf led to absence of the adaxial domain in the proximal region, resulting in cup-shaped peltately simple leaves (Fig. 1D) or peltately palmate compound leaves (Fig. 1E, G) in tomato (Kim et al., 2003a). This indicates that leaflet formation requires the leaf adaxial (and SlPHAN expression) domain in tomato. However, it is still unclear why leaflets are not formed in the rachis or petiole region of wild-type tomato leaves where the adaxial domain still exists. We hypothesize that the medio-lateral regulation of the adaxial domain plays an important role for leaflet formation and that SlPHAN is involved in this process. To test this hypothesis, we further analysed non-peltate leaves of antiSlPHAN tomato lines with confirmed downregulation of SlPHAN by in situ hybridization and immunolocalization (Kim et al., 2003a).
Fig. 1.
antiSlPHAN tomato plants have medio-laterally reduced adaxial domains, which inhibit leaflet or blade formation. (A) Wild-type tomato leaf. (B) Proximal end of the wild-type petiole. (C) A transverse section of the wild-type petiole. (D–K) antiSlPHAN tomato leaves showing simple (D, H), palmate (E, G, I) and almost pinnate (J) in a peltate (D–G) or non-peltate (H–K) arrangement. A close-up of the proximal end of the adaxial domain in a peltate (F) and in a non-peltate (K) antiSlPHAN leaf. Serial sections of the petiole of a peltate (G inset) and a non-peltate (L) antiSlPHAN leaf. (M) Immunolocalization showing a gradual medio-lateral narrowing of SlPHAN expression in a non-peltate antiSlPHAN petiole. Scanning electron microscopy showing an emerging wild-type leaf (N) and a SlPHAN leaf with a medio-laterally reduced adaxial domain (O). Leaf epidermal cells of the wild type (P, Q) and antiSlPHAN (R, S), showing adaxial (P, R) and abaxial (Q, S) sides. (T) Adaxial angles of wild-type, antiSlPHAN and CaMV35S::SlPHAN tomato leaves. Abbreviations: R, rachis; P, petiole. Pu, petiolule; lf, leaflet; ad, adaxial domain; ab, abaxial domain; med, medial; lat, lateral; dis, distal; prox, proximal. Scale bars: (A, D, E, G–J) = 1 cm; (C, G, inset, L) = 400 µm; (M–S) = 40 µm.
A wild-type tomato leaf is a uni-pinnate compound leaf with 7–8 leaflets (Fig. 1A). The adaxial domain can be seen from the base to the distal end of the leaf, including the rachis and petiole (Fig. 1B). Transverse sections show distinct abaxiality–adaxiality in all parts of the wild-type leaf (Fig. 1C, T). The section of the wild-type petiole clearly showed a polar arrangement of the vascular bundle with both abaxial and adaxial domains (Fig. 1C). T2 tomato plants expressing antiSlPHAN generated leaves with reduced leaflet numbers from simple to almost wild-type leaves (Fig. 1D–K). These antiSlPHAN leaves can be categorized into two groups; peltate (Fig. 1D–G) and non-peltate (Fig. 1H–K). Peltate (Fig. 1D, E, G) and non-peltate (Fig. 1H, I, J) leaves were superficially similar. However, leaflets of a non-peltate antiSlPHAN leaf were formed in a 2-D arrangement, whereas leaflets of a peltate antiSlPHAN leaf were formed in a 3-D arrangement (due to the formation of a leaflet in a peltate position). In both peltate and non-peltate antiSlPHAN tomato leaves, the adaxial domain was confined to the distal part of the leaf, and no adaxial domain was seen in the proximal side of the leaf. Vascular bundles were radially symmetrical (abaxialized) in the region between the last proximal leaflet and the proximal end of the petiole (Fig. 1G, inset 3; and Fig. 1L, section 4). Leaflets were not formed at the proximal ends of the antiSlPHAN tomato leaf, where the adaxial domain was lost. The main difference between peltate and non-peltate antiSlPHAN leaves was the morphology of the proximal ends of the adaxial domain in a leaf. In non-peltate antiSlPHAN tomato leaves, the adaxial domain showed a gradual medio-lateral reduction (Fig. 1K). This medio-lateral reduction of the adaxial domain cannot be seen in a peltate antiSlPHAN tomato leaf because the adaxial domain of petiolules joins together in the centre of the distal end of the leaf (Fig. 1F). Serial sections along the proximal–distal leaf axis (where the adaxial domain ends) also clearly showed a gradual medio-lateral reduction (narrowing) of the adaxial domain in non-peltate antiSlPHAN leaves (Fig. 1L). Importantly, leaflets were not formed in the region with the narrow adaxial domain, suggesting that the medio-lateral width of the adaxial domain is also important for leaflet formation. In contrast, in peltate antiSlPHAN tomato leaves, serial sections along the proximal–distal leaf axis showed that the adaxial domain of petiolules merged in the centre of the petiole, and was eventually encircled by abaxial domains of petiolule and disappeared (Fig. 1G, inset). This medio-lateral reduction of the adaxial domain also affected the leaflet position in antiSlPHAN tomato leaves. In wild-type tomato leaves the adaxial domain existed between a lateral pair of two leaflets (Fig. 1N). The lateral pair of two leaflets were much closer together in some of the antiSlPHAN leaves (Fig. 1O), indicating that the adaxial domain was medio-laterally reduced in these leaves. To determine if the medio-lateral reduction of the adaxial domain in the antiSlPHAN tomato leaves is due to the SlPHAN downregulation, we performed an immunolocalization experiment using a polyclonal antibody against RS2, which also recognizes the SlPHAN protein (Kim et al., 2003a). The accumulation of SlPHAN proteins mirrored the gradual medio-lateral reduction of the adaxial domain along the proximal–distal axis of the antiSlPHAN P3 leaf (Fig. 1M), whereas SlPHAN was expressed in the entire adaxial domain of the leaf from the distal to the proximal end (Kim et al., 2003a). Taken together, the adaxial domain was reduced medio-laterally as well as proximo-distally, and this reduction of the adaxial domain affected final leaf morphology in antiSlPHAN tomato leaves. The medio-lateral width of the adaxial domain appears to be critical for leaflet formation, and the narrow adaxial domain failed to form leaflets, making non-peltate antiSlPHAN tomato leaves.
We further hypothesized that controlling the medio-lateral width of the adaxial domain plays an important role in the wild-type tomato leaf architecture. In particular, we investigated if the adaxial domain is medio-laterally narrow in the rachis and petiole region where leaflets are not formed. We measured the adaxial angle in the rachis and petiole, and compared it with that of the region with blades. In the wild-type tomato leaf, the adaxial angle of the rachis and petiole region was narrower than that of the blade area (Figs 1T and 4F). This suggests that controlling the medio-lateral size of the adaxial domain may be critical for wild-type tomato leaf architecture and that leaflets may not be formed in regions such as the rachis and petiole, where the adaxial domain is not sufficiently wide.
Fig. 4.
Increased adaxial domain in CaMV35S::SlPHAN tobacco plants produces ectopic blade outgrowth. Leaves of (A, C) wild-type tobacco and (B, D) CaMV35S::SlPHAN tobacco. Close-up of the petiole (pe) region of (C) wild-type and (D) CaMC35S:SlPHAN. (E) RT–PCR showing upregulation of SlPHAN in CaMV35S::SlPHAN tobacco plants. (F, G) Adaxial angles in tomato and tobacco leaves. Scale bars = 1 cm.
Later leaf developmental events, such as epidermal cell differentiation, were also affected in antiSlPHAN tomato leaves. Scanning electron micrographs showed that leaf epidermal cells were altered in antiSlPHAN transgenic tomato plants. In wild-type tomato leaves, adaxial epidermal cells had sparsely lobed outlines and the leaf surface was smooth with few stomata (Fig. 1P), whereas abaxial epidermal cells were irregularly shaped and more crenulated with many stomata (Fig. 1Q). In leaves of antiSlPHAN transgenic tomato plants, both adaxial and abaxial epidermal cells were crenulated and irregularly shaped with many stomata (Fig. 1R, S). In particular, the increased number of stomata of antiSlPHAN adaxial epidermal cells resembled wild-type abaxial epidermal cells, suggesting that the adaxial epidermal cells of the leaf were partially abaxialized in antiSlPHAN plants, and that SlPHAN is necessary for normal differentiation of the adaxial leaf epidermal cells.
KNOX genes were over-expressed in SlPHAN tomato leaves
Occasionally, antiSlPHAN transgenic plants produced leaves with rough surfaces and knots (Fig. 2A). Ectopic shoots were often formed on the adaxial leaf surface over the midrib region in antiSlPHAN transgenic plants (Fig. 2B arrow), resembling the KNOX-over-expressing tomato and tobacco plants (Sinha et al., 1993; Janssen et al., 1998; Kim et al., 2003b). Flowers of antiSlPHAN tomato plants also resembled those seen in plants ectopically expressing LeT6 (Janssen et al., 1998). The wild-type tomato flower has five sepals, petals, stamens and two fused carpels (Fig. 2C, inset). Petals are fused into a tubular corolla and anthers are adnate to the corolla. In antiSlPHAN tomato flowers, floral organs were narrower than those of the wild type, and the number of floral organs such as petals and anthers increased (Fig. 2C). Petals and anthers of antiSlPHAN flowers were unable to fuse properly (Fig. 2C). These phenotypes suggest that SlPHAN downregulation might lead to KNOX over-expression in the antiSlPHAN transgenic tomato plants. To test if tomato KNOX genes (TKN1 and LeT6) are over-expressed, real-time RT–PCR was performed in antiSlPHAN transgenic plants that showed a reduced leaf adaxial domain. TKN1 was upregulated in both the shoot meristem (including P1–P3 leaf primordia) and young leaf primordia (between P4 and P8) in antiSlPHAN transgenic plants relative to the wild type (Fig. 2D, E), suggesting that SlPHAN inhibits TKN1 in both the shoot meristem and young leaf primordium. On the other hand, LeT6 was downregulated in the meristem region (Fig. 2F), but upregulated in young leaf primordia (Fig. 2G) of antiSlPHAN transgenic plants. This suggests that SlPHAN regulates LeT6 positively in the meristem region, whilst SlPHAN inhibits LeT6 activity in the young leaf primordium region.
Fig. 2.
KNOX genes are over-expressed in antiSlPHAN tomato leaves. (A) Knotted leaves, (B, arrow) an ectopic shoot on the leaf, and (C) abnormal flowers of antiSlPHAN plants (inset: a wild-type tomato flower). Real-time RT–PCR data showing (D, E) TKN1 and (F, G) LeT6 in tomato antiSlPHAN meristem (D, F) and young leaf (E, G) tissues. The y-axis shows the relative level of expression. Scale bars: (A–C) = 1 cm.
CaMV35S::SlPHAN transgenic tomato plants generated leaves with an increased adaxial domain and ectopic blade formation
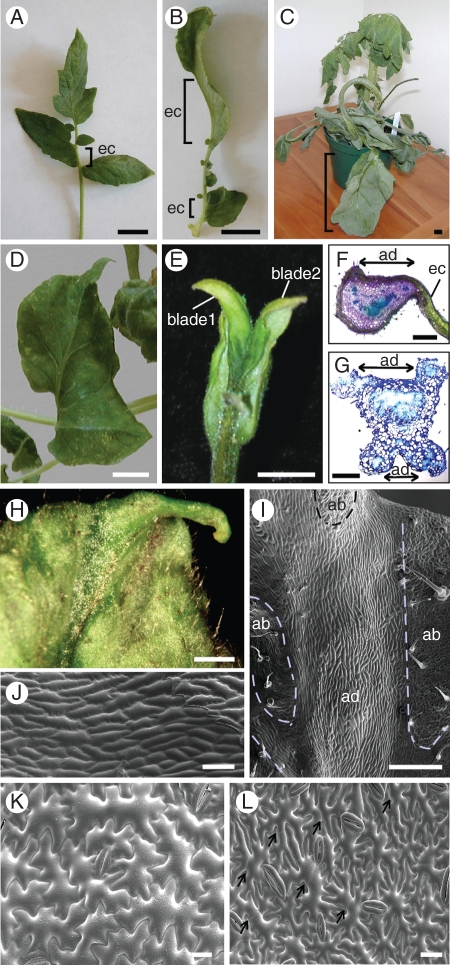
To confirm further the function of SlPHAN in leaf development, we over-expressed SlPHAN under the control of the CaMV35S promoter in tomato. We obtained 28 T1 plants and analysed ten T2 lines (Supplementary Data Table S3). The final leaf morphology was altered in several CaMV35S::SlPHAN transgenic tomato lines. In wild-type tomato leaves, no leaf blade forms in the rachis region that separates the leaflets (Fig. 1A). However, in four CaMV35S::SlPHAN lines, ectopic leaf blades grew out from the rachis regions (Fig. 3A, B, brackets), and the rachis area with the ectopic blade of these lines showed a medio-laterally broad adaxial domain (Fig. 3F). Two lines produced gigantic simple leaves, presumably by a fusion of all leaflets due to ectopic leaf blade formation in the entire rachis region (Fig. 3C, D). In one CaMV35S::SlPHAN tomato line, an ectopic leaf blade was formed on the abaxial side of the leaf, forming double leaf blades (Fig. 3E). The two blades were connected continuously in the distal region of the blades (Fig. 3E). A section of this leaf showed that an ectopic adaxial domain existed in the abaxial midvein region, allowing ectopic leaf blade formation underneath the original leaf blade (Fig. 3G). These blades had normal adaxial and abaxial epidermal cell differentiation (data not shown). Over-expression of SlPHAN also caused the production of ectopic adaxial sectors in the abaxial side of leaf blade. The adaxial side of the leaf is usually dark green, whereas the abaxial side of the leaf is pale green. One CaMV35S::SlPHAN tomato line showed dark green patches on the abaxial side (Fig. 3H). Scanning electron microscopy showed that these dark green patches had adaxial epidermal features, in that the cells were more rounded and regularly shaped with fewer stomata (Fig. 3I, J). Scanning electron microscopy also showed that CaMV35S::SlPHAN gigantic tomato leaves (Fig. 3C) underwent prolonged cell division and cell expansion. In wild-type mature tomato leaves, no traces of recent cell divisions were seen in adaxial and abaxial leaf epidermal cells (Fig. 1P, Q), whereas in the gigantic CaMV35S::SlPHAN tomato leaves (Fig. 3C), both adaxial and abaxial epidermal cells were much bigger (Fig. 3K, L) than in the wild type, and traces of recent cell divisions were evident (Fig. 3L, recent cell divisions marked by arrows).
Fig. 3.
CaMV35S::SlPHAN tomato leaves have increased adaxial domains, causing ectopic blade outgrowths. (A, B) Leaves with ectopic blades in the rachis region (brackets), (C, D) gigantic entire simple leaves and (E) a leaf with double blades. (F) A section showing the rachis region of the leaf with an ectopic blade and (G) with double blades. (H) An adaxial epidermal sector (dark green) on the abaxial side (pale green) of the leaf and (I, J) SEM showing the ectopic adaxial sector. (K) SEM showing the adaxial and (L) abaxial sides of a gigantic simple CaMV35S::SlPHAN leaf; traces of recent cell divisions are marked with arrows. Abbreviations: ad, adaxial domain; ab, abaxial domain; ec, ectopic blade. Scale bars: (A–E) = 1 cm; (F–I) = 500 µm; (J) = 100 µm; (K, L) = 40 µm.
Over-expression of tomato SlPHAN led to ectopic blade formation in the petiole of tobacco
To determine whether medio-lateral regulation of the adaxial domain also plays a role in blade formation in simple leaves, we generated CaMV35S::SlPHAN tobacco plants which over-express SlPHAN in the shoot and young leaves (Fig. 4E). Upregulation of SlPHAN promoted blade outgrowth in the tobacco petiole region (Fig. 4B, D) where no blade was normally formed in the wild-type tobacco leaves (Fig. 4A, C). Over-expression of SlPHAN also led to adaxial sectors in the abaxial side of the leaf (data not shown). To confirm that ectopic blade formation in CaMV35S::SlPHAN petioles was due to the medio-lateral increase of the adaxial domain, we measured the angle of the adaxial domain in the petiole of wild-type and CaMV35S::SlPHAN tobacco plants. The adaxial angle in the petiole was increased in CaMV35S::SlPHAN compared with that of wild-type tobacco (Fig. 4G).
DISCUSSION
The juxtaposition of the adaxial and abaxial domains is required for the formation of a blade or leaflets in simple- and compound-leaved species (McConnell and Barton, 1998; Timmermans et al., 1998; Waites et al., 1998; Kim et al., 2003a). In simple leaves, a complete adaxialization or abaxialization of the leaf led to no blade formation (McConnell and Barton, 1998; Waites et al., 1998). In tomato, abaxialization of the leaf also led to no leaflet formation (Kim et al., 2003a). One of the key regulators controlling the adaxial domain of the leaf is the ARP gene. Defects in ARP genes led to loss of the adaxial domain, suggests that ARP genes play an important role in establishing the adaxial domain (Waites et al., 1998; Kim et al., 2003a). Our results reconfirm that SlPHAN plays a role in establishing and maintaining the adaxial domain of the tomato leaf. The adaxial domain of the leaf was reduced or increased in antiSlPHAN and CaMV35S::SlPHAN plants, respectively. This role of SlPHAN in tomato is similar to that in antirrhinum, tobacco and pea, where ARP genes function in maintaining the adaxial domain (Waites et al., 1998; Tattersall et al., 2005). This function of PHAN is also conserved in arabidopsis. An as1 allele showed cup-shaped leaves with abaxialized petioles, although another as1 allele (magnifica) showed only KNOX gene over-expression phenotypes without any adaxial–abaxial polarity defects (Byrne et al., 2000; Sun et al., 2002). In maize, mutations in RS2 showed only KNOX gene over-expression phenotypes (Timmermans et al., 1999; Tsiantis et al., 1999), thus it is not clear that PHAN function in establishing or maintaining the leaf adaxial domain is conserved in monocot species such as maize. Our data showed that over-expression of SlPHAN is enough to generate ectopic adaxial domains in tomato and tobacco leaves (Figs 3F, G, H and 4B, D). Over-expression of AS1 in arabidopsis generated narrow leaves with elongate petioles but did not generate ectopic adaxial domain in the leaf (Theodoris et al., 2003). This suggests that the strong role of ARP genes in regulation of leaf abaxiality–adaxiality may be specific to the Asterids (e.g. Solanales and Lamiales), or it may be masked by other redundant or semi-redundant pathways in the rosids lineages. We only obtained 35S::SlPHAN plants generating leaves with a wide adaxial domain but did not obtain strong SlPHAN over-expressors with a complete adaxialization of the leaf. Strong over-expression SlPHAN lines are likely to be lethal because a high level of SlPHAN over-expression may cause severe KNOX downregulation (LeT6), which might result in a lethal loss of meristem. We obtained only 28 T1 plants in eight transformation experiments. This severe reduction in the transformation efficiency (compared with that of antiSlPHAN) might be because most CaMV35::SlPHAN plants were either unable to develop in culture or were extremely retarded in growth.
Our previous study showed that downregulation of SlPHAN expression in the proximal region of the leaf led to abaxialization of the petiole, and no leaflet was formed in the region where PHAN and the adaxial domain were ‘absent’ (Kim et al., 2003a). This demonstrated that SlPHAN expression (and the adaxial domain) is required for leaflet formation in tomato (Kim et al., 2003a). However, it had been unclear why leaflets were not formed in the rachis and petiole regions of a wild-type tomato leaf, where PHAN and the adaxial domain still exist. In antiSlPHAN non-peltate tomato leaves, leaflets were not formed in the region where the adaxial domain is not sufficiently wide. In CaMV35S::SlPHAN tomato and tobacco plants, upregulation of SlPHAN increased the adaxial domain in the petiole and rachis, which coincides with ectopic blade formation in these regions (Figs 3A–E and 4B, D). This suggests that medio-lateral regulation (width) of the adaxial domain (SlPHAN expression) is also crucial for leaflet formation and that a certain width of the adaxial domain is required to generate leaflets (Fig. 1T). This idea is further supported by the fact that the adaxial domain was wider in the regions where blades or leaflets were formed, compared with regions such as the rachis or petiole (Fig. 4F). This showed that both medio-lateral and proximo-distal regulation of the adaxial domain is important for the final leaflet architecture in tomato and that SlPHAN is essential for this regulation. Reduction of the adaxial domain in antiSlPHAN tomato leaves occurs sequentially, first in the proximal region, followed by the distal region. This abaxialization is almost never complete and a residual adaxial domain is always retained in the distal region. The tomato leaf primordium develops basipetally, with the first formed regions making the distal parts of the mature leaf. Thus SlPHAN activity appears more stable (e.g. resistant to suppression) in the early stage (in the distal region) than in later stages (in the proximal region) of leaf development. This preferential loss of the adaxial domain from the proximal parts of the leaf might be facilitated by a tomato locus, WIRY (W). In contrast to wiry3 (w3) and wiry6 (w6), which show a preferential loss of adaxial domain in the proximal region of the leaf, the w mutant shows a loss of the adaxial domain in the distal region (Kim et al., 2003b).
The regulatory interaction between ARP and KNOX genes is different between simple-leaved and compound-leaved species. In simple-leaved species such as arabidopsis and maize, ARP and KNOX gene expression is mutually exclusive, whilst ARP and KNOX genes are co-expressed in many compound-leaved species (Timmermans et al., 1999; Tsiantis et al., 1999; Byrne et al., 2000; Bharathan et al., 2002; Kim et al., 2003a, b; Harrison et al., 2005; Tattersall et al., 2005). In tomato, the regulatory relationship between ARP and KNOX genes appears complex. LeT6 is a negative regulator of SlPHAN and, at the same time, it requires SlPHAN to manifest its function in leaves (Kim et al., 2003b). It has been unclear if SlPHAN in turn regulates KNOX genes in tomato. Our antiSlPHAN tomato plants showed this complex regulatory relationship between SlPHAN and KNOX genes even further. Upregulation of TKN1 was seen in both the meristem and young leaf primordia of antiSlPHAN transgenic plants, suggesting that SlPHAN downregulates TKN1 in these two regions. This regulatory relationship is conserved in maize where RS2 downregulates KNOX genes (Timmermans et al., 1999; Tsiantis et al., 1999). Similarly, AS1 represses KNAT1 and KNAT2 activities in arabidopsis (Byrne et al., 2000). This upregulation of TKN1 was also seen in the tomato w6 mutants, which had reduced levels of SlPHAN expression (Kim et al., 2003b). The regulatory relationship between SlPHAN and LeT6 is spatio-temporally specific. antiSlPHAN plants showed upregulation of LeT6 in the young leaf primordia and downregulation of LeT6 in the meristem region. This suggests that SlPHAN inhibits LeT6 in the young leaf primordia, whilst SlPHAN activates LeT6 positively in the meristem region. A wide variety of expression levels of LeT6 and TKN1 were seen in antiSlPHAN plants (Fig. 2D–G). However, the expression level of KNOX genes did not correlate with the severity of phenotypes in antiSlPHAN plants. This is probably because the SlPHAN downregulation phenotype is epistatic to KNOX over-expression phenotypes (Kim et al., 2003b).
Making diverse leaf forms
Leaf forms are diverse: simple (Figs 1D, H and 5D, E) vs. compound (Figs 1A and 5G), pinnate (Figs 1A and 5G) vs. palmate (Figs 1E and 5F), peltate (Fig. 1D–F) vs. non-peltate (Fig. 1H–J) and petiolated (Fig. 5B) vs. sessile (Fig. 5C). Compound leaves can be categorized into two groups; pinnate (feather-like leaflet arrangement, Figs 1A and 5D) and palmate (umbrella-like leaflet arrangement, Fig. 1E). Previously, it was shown that juxtaposition of the adaxial and the abaxial is required for leaflet and leaf blade formation and confining the adaxial to the distal region of a leaf generates palmate compound leaves instead of pinnate leaves (Kim et al., 2003a). In antiSlPHAN tomato plants, as the adaxial domain was gradually (proximally–distally) reduced in the proximal region of the leaf, pinnate compound leaves with fewer leaflets (Fig. 1J), palmate, ternate compound leaves (Fig. 1I) and simple leaves (Fig. 1H) were produced, confirming that proximal–distal regulation of the adaxial domain is a key mechanism to generate pinnate vs. palmate leaves (Fig. 5A). Examination of antiSLPHAN and CaMV35S::SlPHAN tomato leaves showed that medio-lateral regulation of the adaxial domain is also critical for leaf architecture. Leaflets of these antiSlPHAN leaves were placed in either peltate (Fig. 1D–G) or non-peltate (Fig. 1H–K) positions. Leaf sections showed that the morphology of the proximal end of the adaxial domain in the leaf might play an important role in determining if the leaves will be peltate or non-peltate (Fig. 5A). A gradual medio-lateral reduction (narrowing) of the adaxial domain and of the SlPHAN expression in the proximal end led to non-peltate leaves (Fig. 1K, L, M). In addition, medio-lateral regulation of the adaxial domain also plays an important role in blade formation in the rachis area in tomato and in the petiole area in tobacco. It appeared that the adaxial domain was narrow in the region where leaflet and blade are not formed in wild-type tomato and tobacco. Ectopic blade was formed in the rachis and petiole region in CaMV35S::SlPHAN tomato and tobacco plants. In particular, CaMV35S::SlPHAN tobacco plants generated sessile leaves (Fig. 4B, D) instead of petiolated leaves. This suggests that medio-lateral control of the adaxial domain in the petiole region may be one of the important mechanisms to differentiate petiolated (Fig. 5B) from sessile (Fig. 5C) leaves.
Fig. 5.
(A) Schematic diagrams showing proximal–distal (p–d) and medio-lateral (m–l) regulation of the adaxial domain (the SlPHAN expression domain) and their role in final leaf morphologies. Leaflet and leaf blade formation require a boundary between the adaxial and the abaxial domains. Up- or downregulation of SlPHAN, which modulates the adaxial domain (thus the boundary between the adaxial and abaxial domains), generates diverse leaf forms including simple, pinnate compound, palmate compound, peltate, non-peltate, petiolated and sessile. While proximo-distal regulation of SlPHAN along the leaf axis was critical for determining pinnate vs. palmate, medio-lateral regulation of SlPHAN was critical for determining peltate vs. non-peltate in tomato and petiolated vs. sessile in tobacco. Medio-lateral regulation of the adaxial and the SlPHAN domain may play an important role in generating the final leaf morphology in wild-type tomato. The adaxial domain of rachis and petiole regions is medio-laterally narrower than that of blade and leaflet regions. The angle of the adaxial domain shown in sections. Leaflets in a peltate position are marked by asterisks. (B) A petiolated (Prunus sp.) and (C) a sessile leaf (Pyracantha sp.). (D–I) Homology of compound and simple leaves. A compound leaf can be made by carving a simple leaf (D–F, Acer sp.) or by reiterating many simple leaves (leaflets) along the rachis. The homeotic conversion between a leaflet and a compound leaf in Acacia sp. supports the latter (G–I). Scale bars = 1 cm.
Two hypotheses have been proposed to explain the homology of compound leaves. Kaplan and Hagemann proposed that a compound leaf is formed by dissecting or carving a simple leaf, perhaps by inhibiting blade formation in the rachis area (Kaplan, 1975; Hagemann, 1984). In many related species, gradual dissection of the leaf blade is thought to result in compound leaves (maple leaves; Fig. 5D–F). This hypothesis is supported by studies on the roles of NAM/CUC genes inhibiting leaf blade growth in the rachis region in various compound-leaved species, including tomato (Blein et al., 2008). The other hypothesis is that a compound leaf is a homeotic reiteration of simple leaves along the rachis region (Lacroix and Sattler, 1994; Rutishauser, 1995). In this view, each leaflet is homologous to a simple leaf. This hypothesis is supported by homeotic conversion between leaflet and leaf in certain species, e.g. Acacia sp. (Fig. 5G–I), and the role of KNOX genes in both meristem development and leaflet development (Hareven et al., 1996; Janssen et al., 1998; Bharathan et al., 2002; Hay and Tsiantis, 2006). Recently, it has been proposed that these two opposing hypotheses might not be mutually exclusive, since both leaflet formation and inhibition of blade outgrowth occur during compound leaf development processes (Barkoulas et al., 2008; David-Schwartz et al., 2009; Koenig et al., 2009). Our CaMV35S::SlPHAN tomato lines formed ectopic blades in the rachis region (Fig. 3A, B) and, in extreme cases, a compound leaf changed into a gigantic entire simple leaf (Fig. 3C, D). Controlling the medio-lateral size of the SlPHAN domain along the leaf primordium seems to play a critical role in sculpturing the final compound leaf morphology. Reduction in the size of the adaxial domain and thus inhibiting blade growth in the rachis region is one plausible way to make tomato leaves compound, and SlPHAN appears to be involved in this process. The connections between other factors, such as the CUC/NAM genes, auxin response and SlPHAN, in tomato leaflet formation are not fully elucidated. Interestingly, our antiSlPHAN tomato plants often generate simple leaves (Fig. 1D, H). Despite the superficial similarity, these two simple leaves of antiSlPHAN and CaMV35S::SlPHAN are not likely to be homologous. The simple leaf of CaMV35::SlPHAN is most likely to be homologous to an entire compound leaf with ectopic blade in the rachis area, whereas the simple leaf of antiSlPHAN lines is most likely to be homologous to a terminal leaflet. Analogously, two tomato mutants, Laceolate (La) and entire (e) plants, generate simple leaves. Developmental studies showed that a simple La leaf is a terminal leaflet, whereas a simple e leaf is a whole leaf with leaflets fused into one blade unit (Kessler et al., 2001; Ori et al., 2007; Zhang et al., 2007). This highlights the fact that not all simple leaves are homologous (Bharathan et al., 2002), and homology between compound and simple leaves should be resolved in the phylogenetic context of a particular clade. Our data also suggest that an evolutionary shift between simple and compound leaves can be achieved by multiple mechanisms, and the SlPHAN pathway, along with the NAM/CUC gene and KNOX gene pathways, may play a role in determining leaf architecture. Whether these mechanisms were independently and preferentially recruited in an evolutionary shift between simple and compound leaves in particular clades, or whether they somehow connect and interact to sculpt leaves, should be further investigated in the future.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Professor Neelima Sinha and members of the Sinha lab for all their great advice and help.
LITERATURE CITED
- Barkoulas M, Hay A, Kougioumoutzi E, Tsiantis M. A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nature Genetics. 2008;40:1136–1141. doi: 10.1038/ng.189. [DOI] [PubMed] [Google Scholar]
- Becker D. Binary vectors which allow the exchange of plant selectable markers and reporter genes. Nucleic Acids Research. 1990;18:203–203. doi: 10.1093/nar/18.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharathan G, Goliber TE, Moore C, Kessler S, Pham T, Sinha NR. Homologies in leaf form inferred from KNOXI gene expression during development. Science. 2002;296:1858–1860. doi: 10.1126/science.1070343. [DOI] [PubMed] [Google Scholar]
- Blein T, Pulido A, Vialette-Guiraud A, et al. A conserved molecular framework for compound leaf development. Science. 2008;322:1835–1839. doi: 10.1126/science.1166168. [DOI] [PubMed] [Google Scholar]
- Bolduc N, Hake S, Jackson D. Dual functions of the KNOTTED1 homeodomain: sequence-specific DNA binding and regulation of cell to cell transport. Science Signaling. 2008;1:28. doi: 10.1126/scisignal.123pe28. [DOI] [PubMed] [Google Scholar]
- Braybrook SA, Kuhlemeier C. How a plant builds leaves. The Plant Cell. 2010;22:1006–1018. doi: 10.1105/tpc.110.073924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ME, Barley R, Curtis M, et al. ASYMMETRIC LEAVES1 mediates leaf patterning and stem cell function in Arabidopsis. Nature. 2000;408:967–971. doi: 10.1038/35050091. [DOI] [PubMed] [Google Scholar]
- Champagne CEM, Goliber TE, Wojciechowski MF, et al. Compound leaf development and evolution in the legumes. The Plant Cell. 2007;19:3369–3378. doi: 10.1105/tpc.107.052886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Janssen BJ, Williams A, Sinha N. A gene fusion at a homeobox locus: alterations in leaf shape and implications for morphological evolution. The Plant Cell. 1997;9:1289–1304. doi: 10.1105/tpc.9.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Schwartz R, Koenig D, Sinha NR. LYRATE is a key regulator of leaflet initiation and lamina outgrowth in tomato. The Plant Cell. 2009;21:3093–3104. doi: 10.1105/tpc.109.069948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue MJ, Doyle JA. Phylogenetic analysis of angiosperms and the relationships of Hamamelidae. In: Crane PR, Blackmore S, editors. Evolution, systematics, and fossil history of the Hamamelidae. Vol. 1. Oxford: Oxford University Press; 1989. pp. 17–45. [Google Scholar]
- Emery JF, Floyd SK, Alvarez J, et al. Radial patterning of Arabidopsis shoots by class IIIHD-ZIP and KANADI genes. Current Biology. 2003;13:1768–1774. doi: 10.1016/j.cub.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Eshed Y, Baum SF, Perea JV, Bowman JL. Establishment of polarity in lateral organs of plants. Current Biology. 2001;11:1251–1260. doi: 10.1016/s0960-9822(01)00392-x. [DOI] [PubMed] [Google Scholar]
- Eshed Y, Izhaki A, Baum SF, Floyd SK, Bowman JL. Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development. 2004;131:2997–3006. doi: 10.1242/dev.01186. [DOI] [PubMed] [Google Scholar]
- Garcia D, Collier SA, Byrne ME, Martienssen RA. Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Current Biology. 2006;16:933–938. doi: 10.1016/j.cub.2006.03.064. [DOI] [PubMed] [Google Scholar]
- Gleave AP. A versatile binary vector system with a T-DNA organizational-structure conducive to efficient integration of cloned DNA into the plant genome. Plant Molecular Biology. 1992;20:1203–1207. doi: 10.1007/BF00028910. [DOI] [PubMed] [Google Scholar]
- Goliber T, Kessler S, Chen JJ, Bharathan G, Sinha N. Genetic, molecular, and morphological analysis of compound leaf development. Current Topics in Developmental Biology. 1999;43:259–290. doi: 10.1016/s0070-2153(08)60384-1. [DOI] [PubMed] [Google Scholar]
- Golz JF, Roccaro M, Kuzoff R, Hudson A. GRAMINIFOLIA promotes growth and polarity of Antirrhinum leaves. Development. 2004;131:3661–3670. doi: 10.1242/dev.01221. [DOI] [PubMed] [Google Scholar]
- Hagemann W. Morphological aspects of leaf development in ferns and angiosperms. In: White RA, Dickison WC, editors. Contemporary problems in plant anatomy. Orlando, FL: Academic Press; 1984. pp. 301–350. [Google Scholar]
- Hareven D, Gutfinger T, Parnis A, Eshed Y, Lifschitz E. The making of a compound leaf: genetic manipulation of leaf architecture in tomato. Cell. 1996;84:735–744. doi: 10.1016/s0092-8674(00)81051-x. [DOI] [PubMed] [Google Scholar]
- Harrison CJ, Corley SB, Moylan EC, Alexander DL, Scotland RW, Langdale JA. Independent recruitment of a conserved developmental mechanism during leaf evolution. Nature. 2005;434:509–514. doi: 10.1038/nature03410. [DOI] [PubMed] [Google Scholar]
- Hasson A, Plessis A, Blein T, et al. Evolution and diverse roles of the CUP-SHAPED COTYLEDON genes in Arabidopsis leaf development. The Plant Cell. 2011;23:54–68. doi: 10.1105/tpc.110.081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A, Tsiantis M. The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nature Genetics 38. 2006:942–947. doi: 10.1038/ng1835. [DOI] [PubMed] [Google Scholar]
- Hay A, Barkoulas M, Tsiantis M. ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development. 2006;133:3955–3961. doi: 10.1242/dev.02545. [DOI] [PubMed] [Google Scholar]
- Hofer J, Turner L, Hellens R, et al. UNIFOLIATA regulates leaf and flower morphogenesis in pea. Current Biology. 1997;7:581–587. doi: 10.1016/s0960-9822(06)00257-0. [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Jackson D, Veit B, Hake S. Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development. 1994;120:405–413. [Google Scholar]
- Janssen BJ, Lund L, Sinha N. Overexpression of a homeobox gene, LeT6, reveals indeterminate features in the tomato compound leaf. Plant Physiology. 1998;117:771–786. doi: 10.1104/pp.117.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez MT, Kui JS, Thomas J, Heller BA, Timmermans MCP. microRNA-mediated repression of ROLLED LEAF1 specifies maize leaf polarity. Nature. 2004;428:84–88. doi: 10.1038/nature02363. [DOI] [PubMed] [Google Scholar]
- Kaplan DR. Comparative developmental evaluation of the morphology of unifacial leaves in the monocotyledons. Botanische Jahrbuecher fuer Systematik Pflanzengeschichte und Pflanzengeographie. 1975;95:1–105. [Google Scholar]
- Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. KANADI regulates organ polarity in Arabidopsis. Nature. 2001;411:706–709. doi: 10.1038/35079629. [DOI] [PubMed] [Google Scholar]
- Kessler S, Kim M, Pham T, Weber N, Sinha N. Mutations altering leaf morphology in tomato. International Journal of Plant Sciences. 2001;162:475–492. [Google Scholar]
- Kim M, McCormick S, Timmermans M, Sinha N. The expression domain of PHANTASTICA determines leaflet placement in compound leaves. Nature. 2003a;425:102–102. doi: 10.1038/nature01820. [DOI] [PubMed] [Google Scholar]
- Kim M, Pham T, Hamidi A, McCormick S, Kuzoff RK, Sinha N. Reduced leaf complexity in tomato wiry mutants suggests a role for PHAN and KNOX genes in generating compound leaves. Development. 2003b;130:4405–4415. doi: 10.1242/dev.00655. [DOI] [PubMed] [Google Scholar]
- Kimura S, Koenig D, Kang J, Yoong FY, Sinha N. Natural variation in leaf morphology results from mutation of a novel KNOX gene. Current Biology. 2008;18:672–677. doi: 10.1016/j.cub.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Koenig D, Bayer E, Kang J, Kuhlemeier C, Sinha N. Auxin patterns Solanum lycopersicum leaf morphogenesis. Development. 2009;136:2997–3006. doi: 10.1242/dev.033811. [DOI] [PubMed] [Google Scholar]
- Koltai H, Bird DM. High throughput cellular localization of specific plant mRNAs by liquid-phase in situ reverse transcription-polymerase chain reaction of tissue sections. Plant Physiology. 2000;123:1203–1212. doi: 10.1104/pp.123.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix CR, Sattler R. Expression of shoot features in early leaf development of Murraya paniculata (Rutaceae) Canadian Journal of Botany-Revue Canadienne De Botanique. 1994;72:678–687. [Google Scholar]
- Leutenegger CM, Mislin CN, Sigrist B, Ehrengruber MU, Hofmann-Lehmann R, Lutz H. Quantitative real-time PCR for the measurement of feline cytokine mRNA. Veterinary Immunology and Immunopathology. 1999;71:291–305. doi: 10.1016/S0165-2427(99)00100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xu L, Wang H, et al. The putative RNA-dependent RNA polymerase RDR6 acts synergistically with ASYMMETRIC LEAVES1 and 2 to repress BREVIPEDICELLUS and MicroRNA165/166 in Arabidopsis leaf development. The Plant Cell. 2005;17:2157–2171. doi: 10.1105/tpc.105.033449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell JR, Barton MK. Leaf polarity and meristem formation in Arabidopsis. Development. 1998;125:2935–2942. doi: 10.1242/dev.125.15.2935. [DOI] [PubMed] [Google Scholar]
- McCormick S. Transformation of tomato with Agrobacterium tumefaciens. In. In: Lindsey K, editor. Plant tissue culture manual, fundamentals and applications. Dordrecht: Kluwer Acadenic Publishers; 1991. pp. 1–9. [Google Scholar]
- Nogueira FTS, Madi S, Chitwood DH, Juarez MT, Timmermans MCP. Two small regulatory RNAs establish opposing fates of a developmental axis. Genes and Development. 2007;21:750–755. doi: 10.1101/gad.1528607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori N, Eshed Y, Chuck G, Bowman JL, Hake S. Mechanisms that control KNOX gene expression in the Arabidopsis shoot. Development. 2000;127:5523–5532. doi: 10.1242/dev.127.24.5523. [DOI] [PubMed] [Google Scholar]
- Ori N, Cohen AR, Etzioni A, et al. Regulation of LANCEOLATE by miR319 is required for compound-leaf development in tomato. Nature Genetics. 2007;39:787–791. doi: 10.1038/ng2036. [DOI] [PubMed] [Google Scholar]
- Parnis A, Cohen O, Gutfinger T, Hareven D, Zamir D, Lifschitz E. The dominant developmental mutants of tomato, MOUSE-EAR and CURL, are associated with distinct modes of abnormal transcriptional regulation of a KNOTTED gene. The Plant Cell. 1997;9:2143–2158. doi: 10.1105/tpc.9.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig RS, Sussex IM. The cellular parameters of leaf development in tobacco – a clonal analysis. Planta. 1985;165:170–184. doi: 10.1007/BF00395039. [DOI] [PubMed] [Google Scholar]
- Rutishauser R. Developmental patterns of leaves in Podostemaceae compared with more typical flowering plants – Saltational evolution and fuzzy morphology. Canadian Journal of Botany-Revue Canadienne De Botanique. 1995;73:1305–1317. [Google Scholar]
- Sinha NR, Williams RE, Hake S. Overexpression of the maize homeo box gene, KNOTTED-1, causes a switch from determinate to indeterminate cell fates. Genes and Development. 1993;7:787–795. doi: 10.1101/gad.7.5.787. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhou QW, Zhang W, Fu YL, Huang H. ASYMMETRIC LEAVES1, an Arabidopsis gene that is involved in the control of cell differentiation in leaves. Planta. 2002;214:694–702. doi: 10.1007/s004250100673. [DOI] [PubMed] [Google Scholar]
- Sussex IM. Experiments on the cause of dorsiventrality in leaves. Nature. 1954;174:352–353. doi: 10.1038/167651a0. [DOI] [PubMed] [Google Scholar]
- Sussex IM. Morphogenesis in Solanum tuberosum L.: experimental investigation of leaf dorsiventrality and orientation in the juvenile shoot. Phytomorphology. 1955;5:286–300. [Google Scholar]
- Tattersall AD, Turner L, Knox MR, Ambrose MJ, Ellis THN, Hofer JMI. The mutant crispa reveals multiple roles for PHANTASTICA in pea compound leaf development. The Plant Cell. 2005;17:1046–1060. doi: 10.1105/tpc.104.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DW, Hickey LJ. Phylogenetic evidence for the herbaceous origin of angiosperms. Plant Systematics and Evolution. 1992;180:137–156. [Google Scholar]
- Theodoris G, Inada N, Freeling M. Conservation and molecular dissection of ROUGH SHEATH2 and ASYMMETRIC LEAVES1 function in leaf development. Proceedings of the National Academy of Sciences, USA. 2003;100:6837–6842. doi: 10.1073/pnas.1132113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans MCP, Hudson A, Becraft PW, Nelson T. ROUGH SHEATH2: a Myb protein that represses KNOX homeobox genes in maize lateral organ primordia. Science. 1999;284:151–153. doi: 10.1126/science.284.5411.151. [DOI] [PubMed] [Google Scholar]
- Timmermans MCP, Schultes NP, Jankovsky JP, Nelson T. LEAFBLADELESS1 is required for dorsoventrality of lateral organs in maize. Development. 1998;125:2813–2823. doi: 10.1242/dev.125.15.2813. [DOI] [PubMed] [Google Scholar]
- Tsiantis M, Schneeberger R, Golz JF, Freeling M, Langdale JA. The maize ROUGH SHEATH2 gene and leaf development programs in monocot and dicot plants. Science. 1999;284:154–156. doi: 10.1126/science.284.5411.154. [DOI] [PubMed] [Google Scholar]
- Waites R, Selvadurai HRN, Oliver IR, Hudson A. The PHANTASTICA gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum. Cell. 1998;93:779–789. doi: 10.1016/s0092-8674(00)81439-7. [DOI] [PubMed] [Google Scholar]
- Wang HL, Chen JH, Wen JQ, et al. Control of compound leaf development by FLORICAULA/LEAFY ortholog SINGLE LEAFLET1 in Medicago truncatula. Plant Physiology. 2008;146:1759–1772. doi: 10.1104/pp.108.117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Yang L, Pi LM, et al. Genetic interaction between the AS1–AS2 and RDR6–SGS3–AGO7 pathways for leaf morphogenesis. Plant and Cell Physiology. 2006;47:853–863. doi: 10.1093/pcp/pcj057. [DOI] [PubMed] [Google Scholar]
- Yan J, Cai XF, Luo JH, et al. The REDUCED LEAFLET genes encode key components of the trans-acting small interfering RNA pathway and regulate compound leaf and flower development in Lotus japonicus. Plant Physiology. 2009;152:797–807. doi: 10.1104/pp.109.140947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Xu Q, Zhu XD, Qian Q, Xue HW. SHALLOT-LIKE1 is a KANADI transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development. The Plant Cell. 2009;21:719–735. doi: 10.1105/tpc.108.061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen R, Xiao J, et al. A single-base deletion mutation in SlIAA9 gene causes tomato (Solanum lycopersicum) entire mutant. Journal of Plant Research. 2007;120:671–678. doi: 10.1007/s10265-007-0109-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.