Abstract
Background and Aims
Fine root decomposition is an important determinant of nutrient and carbon cycling in grasslands; however, little is known about the factors controlling root decomposition among species. Our aim was to investigate whether interspecific variation in the potential decomposition rate of fine roots could be accounted for by root chemical and morphological traits, life history and taxonomic affiliation. We also investigated the co-ordinated variation in root and leaf traits and potential decomposition rates.
Methods
We analysed potential decomposition rates and the chemical and morphological traits of fine roots on 18 Mediterranean herbaceous species grown in controlled conditions. The results were compared with those obtained for leaves in a previous study conducted on similar species.
Key Results
Differences in the potential decomposition rates of fine roots between species were accounted for by root chemical composition, but not by morphological traits. The root potential decomposition rate varied with taxonomy, but not with life history. Poaceae, with high cellulose concentration and low concentrations of soluble compounds and phosphorus, decomposed more slowly than Asteraceae and Fabaceae. Patterns of root traits, including decomposition rate, mirrored those of leaf traits, resulting in a similar species clustering.
Conclusions
The highly co-ordinated variation of roots and leaves in terms of traits and potential decomposition rate suggests that changes in the functional composition of communities in response to anthropogenic changes will strongly affect biogeochemical cycles at the ecosystem level.
Keywords: Above-ground–below-ground interaction, chemical composition, interspecific variation, leaf decomposition, life history, Mediterranean species, morphology, plant functional traits, taxonomic families, root decomposition
INTRODUCTION
The decomposition of plant tissues is a key process in terrestrial ecosystems, as it regulates the release of carbon (C) and nutrients in the soil (Berg and Laskowski, 2006) and constitutes a major source of atmospheric CO2 (Gholz et al., 2000). Fine root decomposition may account for up to 53 % of total plant tissue turnover (Gill and Jackson, 2000), but only 2 % of studies on plant decomposition have focused on roots (Zhang et al., 2008), and the factors playing a critical role in the determination of root decomposition rate among species have not yet been clearly identified.
Among three major types of factor influencing plant decomposition (i.e. climate, edaphic factors and litter quality), root quality, which is often assessed by determining chemical composition, has been reported to be a major factor determining root decomposition rates (Heal et al., 1997; Silver and Miya, 2001; Zhang et al., 2008; Prescott, 2010). In most studies, root decomposition has been shown to be favoured by high concentrations of nitrogen (N) (Silver and Miya, 2001; Vivanco et al., 2006) and soluble compounds (Hobbie et al., 2010) in the roots and to be decreased by a high root lignin concentration or a high C/N ratio (Silver and Miya, 2001). Other root characteristics, such as root order and pigmentation, have recently been reported to influence root decomposition (Fan and Guo, 2010; Goebel et al., 2011). The effect of root morphology on decomposition rates has seldom been investigated. This is surprising given that the morphological traits of roots, particularly those determining the length of root produced per unit of root mass [i.e. specific root length (SRL)], and its two components, diameter and tissue density, are thought to influence decomposition, because they determine the exchange surface between the root and soil decomposers, together with root toughness and tensile strength (Pohl et al., 2011). Strong relationships have also been found between leaf litter decomposition and leaf morphological traits, such as specific leaf area (SLA) and leaf dry matter content (LDMC; Gallardo and Merino, 1993; Cornelissen, 1996; Cornelissen and Thompson, 1997; Kazakou et al., 2006; Cornwell et al., 2008; Fortunel et al., 2009; Kazakou et al., 2009). Root morphology and chemistry differ widely between species (Craine et al., 2001; Roumet et al., 2006; Pohl et al., 2011), so we expected to find extensive interspecific variation in root decomposition rates. Our first objective was thus to assess the effects of root chemical composition and morphological traits on root decomposition.
Comparative studies based on leaves have shown that the variation in leaf decomposition rate is associated with functional or phylogenetic groups and with the ecological strategy employed by the species for carbon acquisition and growth (Cornwell et al., 2008). Among herbaceous species, the leaves of forbs have been shown to decompose more rapidly than those of graminoids, and those of N-fixers have been shown to decompose more rapidly than those of non-N-fixers (Cornwell et al., 2008). On the other hand, Wardle et al. (2004) hypothesized that species with resource acquisition strategies, such as rapidly growing species and annuals, produce high-quality tissues favouring soil food web activities and, thus, rapid decomposition, whereas species with resource conservation strategies, such as slow-growing and perennial species, produce long-lived, nutrient-poor, recalcitrant tissues that decompose slowly. This hypothesis has been confirmed for leaves (Cornelissen, 1996; Wardle et al., 1998; Kazakou et al., 2006; Cornwell et al., 2008; Kazakou et al., 2009), but remains to be tested for roots. The second objective of this study was therefore to investigate whether root decomposition rates differ between taxonomic groups and between annual and perennial species, and to determine whether the decomposition rate is part of the acquisition–conservation trade-off.
The possibility that root and leaf traits are subject to the same trade-offs is a topical issue in plant ecology since roots and leaves would have a major, cumulative impact on ecosystem functioning (Tjoelker et al., 2005; Kerkhoff et al., 2006; Withington et al., 2006; Freschet et al., 2010a; Hobbie et al., 2010; Liu et al., 2010). There is some evidence in favour of co-ordinated variation of root and leaf traits – such as for N and phosphorus (P) concentrations, two important traits for decomposition (Kerkhoff et al., 2006; Reich et al., 2008). For other pairs of traits, such as root and leaf morphology, and lignin concentration, the results obtained to date are fragmented and partly inconsistent. Few relationships have been demonstrated between leaf and root decomposition, and those that have been described relate to only a few species, mainly trees (Hobbie et al., 2010; Wang et al., 2010; Freschet et al., 2011). Co-ordinated variation of root and leaf decomposition rates together with a large number of chemical and morphological traits have never been investigated in herbaceous species. Our third objective was therefore to investigate the possible existence of correlated groups of leaf and root traits and decomposition rate as part of plant resource economy in herbaceous species.
In this study, we compared the potential decomposition rates of fine roots – the rates of decomposition measured under standard conditions – of 18 Mediterranean herbaceous species. We studied annual and perennial species from contrasting plant families (Asteraceae, Fabaceae, Lamiales and Poaceae), to include a large range of traits (Craine et al., 2001; Roumet et al., 2006, 2008). We measured fine root potential decomposition rate, and ten chemical and morphological root traits, and compared the results obtained with analogous data for leaves obtained for the same species in a previous study (Kazakou et al., 2009).
We hypothesized that (1) the contribution of fine root morphology to differences in potential decomposition rates between species would be almost as great as that of chemical composition; (2) the fine roots of annual species (resource acquisition strategy) would decompose more rapidly than those of perennial species (conservation strategy); and (3) root traits and decomposition patterns would mirror those of leaves and would contribute to the acquisition–conservation trade-off.
MATERIALS AND METHODS
Species and plant growth
Eighteen herbaceous species representative of plant communities from French Mediterranean old-field succession were studied (Garnier et al., 2004; Table 1). The species selected for study had contrasting life histories (eight annuals, two biennials and eight perennials) and represented different taxonomic groups (Poaceae, Fabaceae, Lamiales and Asteraceae; Table 1).
Table 1.
List of the species which have been studied at both the root (this study) and leaf level (Kazakou et al., 2009)
| Species | Abbrev. | Life history | Family/taxonomic group |
|---|---|---|---|
| Arenaria serpillyfolia | As | Annual | Caryophyllaceae |
| Bromus madritensis | Bm | Annual | Poaceae (1) |
| Crepis foetida | Cf | Annual | Asteraceae (2) |
| Geranium rotundifolium | Gr | Annual | Geraniaceae |
| Medicago minima | Mm | Annual | Fabaceae (3) |
| Veronica persica | Vp | Annual | Scrophulariaceae (4) |
| Trifolium angustifolium | Ta | Annual | Fabaceae (3) |
| Tordylium maximum | Tm | Annual | Apiaceae |
| Daucus carota | Dc | Biennual | Apiaceae |
| Picris hieracioides | Ph | Biennual | Asteraceae (2) |
| Calamintha nepeta | Cn | Perennial | Lamiaceae (4) |
| Dactylis glomerata | Dg | Perennial | Poaceae (1) |
| Brachypodium phoenicoides | Be | Perennial | Poaceae |
| Bromus erectus | Bp | Perennial | Poaceae (1) |
| Inula conyza | Ic | Perennial | Asteraceae (2) |
| Psoralea bituminosa | Pb | Perennial | Fabaceae (3) |
| Rubia peregrina | Rp | Perennial | Rubiaceae |
| Teucrium chamaedrys | Tc | Perennial | Lamiaceae (4) |
Species' abbreviations (Abbrev.) correspond to the first letter of the genus name followed by the first letter of the species name. Four taxonomic groups were considered (numbered 1–4), based on the sequences of three genes: 18S rDNA, rbcL and atpB (Soltis et al., 2000). Taxonomic groups are (1) Poaceae; (2) Asteraceae; (3) Fabaceae and (4) Lamiales. Nomenclature follows Tutin et al. (1968–1980).
Species were grown for 9 months (from October 2007 to June 2008) in a greenhouse at the ‘Centre d'Ecologie Fonctionnelle et Evolutive’ in Montpellier, France (43 °59′N, 3 °51′E). Seeds (annual or biennial species) or ramets (perennial species) were collected from a common garden experiment in which species were grown in monoculture (Hummel et al., 2007; Kazakou et al., 2009). Once they had reached an appropriate size, the seedlings were transplanted into 2 L pots (one plant per pot) filled with soil from the common garden experiment; this soil contained, on average, 14·5 g C kg−1, 1·4 g N kg−1, 42 % silt, 33 % clay and 25 % sand, and it had a pH of 7·8. We prepared 15–50 pots for each species, to obtain a final root dry mass of 10–15 g per species, the amount required for the decomposition experiment. Pots were watered weekly and the plants were harvested at the peak of vegetative growth (April–June, according to species). The whole root system of each individual was washed with water to remove all soil and then frozen until the decomposition experiment.
Preparation of the root decomposition bags
For each species, we sorted the roots to obtain live, fine roots (diameter <2 mm) with no sign of senescence for decomposition experiments. These roots therefore cannot be considered to constitute root litter. We used live roots because it was impossible to identify and collect large enough quantities of dead roots, particularly from perennial species. The features of living and decomposing roots form a continuum (Hobbie et al., 2010), and most studies have reported little or no difference in nutrient content between live and dead roots (e.g. McClaugherty et al., 1982; Nambiar, 1987; Aerts, 1990; Freschet et al., 2010b). A root sub-sample was selected for morphological analyses; the rest of the sample was carefully spread on filter paper and air-dried for 4 d. For each species, 18 air-dried root samples (500 ± 0·1 mg) were enclosed in a nylon root decomposition bag (Northen Mesh, Oldham, UK) (12 × 8 cm, 2 mm mesh) closed with staples. We used only 14 root decomposition bags for Trifolium angustifolia and 16 for Arenaria serpillyfolia, because we were unable to collect sufficient root material to constitute 18 samples. Four additional root subsamples per species were weighed, oven dried for 48 h at 60 °C and reweighed to determine their initial root mass (Rootmass,i) and chemical composition.
Potential rate of decomposition of fine roots in microcosms
The root potential decomposition rate (root Kpot) was determined according to the protocol described by Taylor and Parkinson (1988), as modified by Ibrahima et al. (1995). Roots were incubated for 12 weeks in controlled conditions, in microcosms. The use of microcosms made it possible to study root decomposition under standard temperature, humidity and soil conditions, in the presence of similar decomposer populations in each case. The microcosm used consisted of a polyvinylchloride pipe, 15 cm in diameter and 15 cm high, fitted with a lid and with a sealed bottom. A grid, 2 cm above the bottom, divided the chamber into two unequal parts: a usable space with a capacity of 1·5 L into which we placed 1 kg of soil, and a 300 mL drainage compartment. The soil (pH = 8·2, C = 13·9 g kg−1, N = 1·32 g kg−1, P = 0·03 g kg−1) was a 3:1 mixture of soil from the common garden experiment and the surface organic horizon. Within each microcosm, we buried a root decomposition bag horizontally in the soil, at a depth of 3 cm. The microcosms were kept in the dark at 22 ± 0·01 °C throughout the experiment and were watered once per week to keep soil humidity at 80 % of field capacity.
Three (or two) bags per species were removed from the microcosms after 1, 2, 4, 6, 8 and 12 weeks of incubation. Each bag was opened and soil particles were carefully removed from the samples by washing roots with water in a sieve with a 0·2 mm mesh, to ensure that all the root fragments were retained. Washed roots were oven-dried for 48 h at 60 °C and weighed to determine the root mass remaining at each harvest (Rootmass,t). Corrections for inorganic contaminants (mostly soil particles) were made after sample combustion at 550 °C (3 h at 350 °C then 3 h at 550 °C) in a muffle furnace (LE14 Nabertherm, Lilienthal, Germany) for determination of the root biomass on an ash-free basis (Rootash-mass,t). The percentage of the initial mass remaining after incubation (MR, %) was calculated as:
with Rootmass,i the initial dry root mass at the beginning of incubation and Rootash-mass,i the initial root ash-free biomass.
For each species, the proportion of the initial mass remaining (MR, %) over time t (d) (n = 18) was fitted with the single negative exponential model proposed by Olson (1963):
| (1) |
where Kpot (g g−1 d−1) is the potential decomposition rate constant. For the comparison of root Kpot with the leaf Kpot determined for the same species in a previous study (Kazakou et al., 2009), rate constants were multiplied by 103 and are expressed in g kg−1 d−1.
Root traits
Determination of root chemical composition was conducted on four ground replicates per species. The C and N concentrations were determined with an elemental analyser (CHN model EA 1108; Carlo Erba Instruments, Milan, Italy). The P concentration was determined by digestion with sulfuric acid and hydrogen peroxide for 35 min at 100 °C and 2 h at 360 °C. The P concentration was determined colorimetrically, by the molybdenum blue method (Grimshaw et al., 1989), with an autoanalyser (Evolution II, Alliance Instrument, Frépillon, France). The P concentration was determined for all species other than Arenaria serpyllifolia, for which we were unable to collect sufficient amounts of root material. The concentrations of water-soluble compounds, hemicellulose, cellulose and lignin were obtained by the Van Soest method (Van Soest, 1963), and with a Fibersac 24 fiber analyser (Ankom, Macedon, NJ, USA).
For each species, fine root morphological traits were determined on three fresh replicates. Roots were stained with methylene blue (5 g L−1), to increase contrast during scanning, rinsed, spread out on a transparent sheet and scanned at a resolution of 400 dpi. A digital image analysis system (Winrhizo, version 2003b, Regent Instrument, Québec, Canada) was used to determine root length (L), volume (V, as the sum of the volumes in the different diameter classes) and diameter. Roots were then oven-dried for 48 h at 60 °C and weighed to determine their dry mass (DM). Root tissue density (g cm−3) was calculated as the ratio DM/V, and SRL (m g−1) was calculated as the ratio L/DM.
Leaf potential decomposition rate and traits
Leaf Kpot and trait data were taken from Kazakou et al. (2007, 2009). Leaf traits and Kpot were measured on the same species as used here for the root experiment. Plants were grown in monocultures in a common garden experiment. Litter was collected at the season of maximal leaf senescence for each species. Leaf litter Kpot was determined with the same protocol as for root Kpot, by incubating the litter in microcosms for 8 weeks (for more details, see Kazakou et al., 2009). Leaf traits were measured on green leaves harvested at peak vegetative growth, by standardized protocols (Cornelissen et al., 2003). The SLA and LDMC, a surrogate for leaf tissue density (Garnier et al., 1999), were calculated as the ratio of leaf area to leaf dry mass, and the ratio of dry mass to saturated fresh mass, respectively. Leaf P concentration was determined by the same method as used for root P determinations (see above). A sub-sample of leaf litter was ground and scanned with a near-infrared reflectance spectrophotometer (NIRS; NIRS systems 6500, Foss NIRSystems, Raamsdonksveer, The Netherlands), to determine litter soluble compound, cellulose, hemicellulose and lignin concentrations.
Data analyses
For all the variables measured, the distribution of values was tested for normality (Shapiro–Wilk test, α = 0·05) and log-transformed when necessary (N, C/N and cellulose concentrations, SRL and diameter). Differences in Kpot (one fitted data point per species) between life history or taxonomic groups were assessed by one-way analysis of variance (ANOVA). Differences in root traits between species, or between life history or taxonomic groups were tested by two-way ANOVA. The models included one of the two fixed factors of interest (i.e. life history or taxonomic group), with species nested within these factors. Post hoc tests [Student–Newman–Keuls (SNK) comparisons] were performed to identify significant differences between life history or taxonomic groups. We assessed the relative importance of the effects of factors on the variables measured by calculating effect sizes (η2) by retrospective power analyses (Faul et al., 2007; at α = 0·05, power >0·90 in all instances). Effect size was calculated as η2 = SS factor/(SS factor + SS residual) (Weiner et al., 1997; Cohen, 1988), and corresponds to the proportion of the variance of the dependent variable that can be attributed to the factor concerned. Bivariate correlations between variables were evaluated by calculating Pearson's correlation coefficient. Two principal component analyses (PCAs) were carried out. The first included seven root variables: Kpot and the root traits with the largest effect sizes (η2) in ANOVA (N, P, soluble compounds, cellulose, SRL and diameter). The second PCA was conducted with six pairs of analogous leaf and root traits (root and leaf Kpot, P, soluble compound and cellulose concentrations, SRL and SLA, root tissue density and LDMC). These variables were selected on the basis of their contribution to root or leaf Kpot. One-way ANOVA was used to assess the effect of life history and taxonomic group on species axis scores.
Analyses were carried out with Statistical Analysis System (SAS Institute, Cary, NC, USA, version 8) and R software. Retrospective power analyses for ANOVA were conducted with G*Power V3 software (Faul et al., 2007).
RESULTS
Differences in root potential decomposition rate and root traits between species and groups
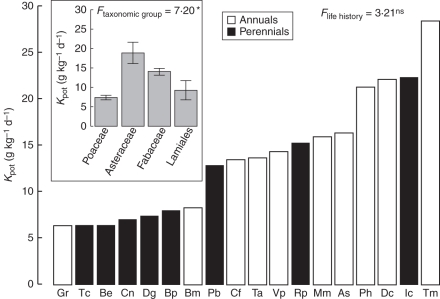
The proportion of the fine root mass remaining after 12 weeks of incubation in microcosms differed significantly between species (F = 59, P < 0·001), ranging from 6·6 % (Daucus carota) to 66·6 % (Teucrium chamaedrys; Supplementary Data Table S1, available online). For all species, a single exponential decay model accurately fitted the data for the mass remaining over time (P < 0·001). The potential rate of decomposition (Kpot), which ranged from 6·3 g kg d−1 (Geranium rotundifolium) to 28·4 g kg d−1 (Tordylium maximum; Fig. 1), did not differ significantly between life histories (Fig. 1, Table 2). The roots of Poaceae and Lamiales decomposed more slowly (7·3 and 9·1 g kg d−1, respectively) than those of Fabaceae (14 g kg d−1) and Asteraceae (18·9 g kg d−1; Fig. 1, Table 2).
Fig. 1.
Potential decomposition rate of fine roots (Kpot) for 18 herbaceous species. For each species, Kpot was estimated by adjusting 18 points (6 harvesting dates × 3 replicates), P < 0·001 (Wald test). See Table 1 for species' abbreviations; annuals and perennials as indicated in the key. The inset box indicates the mean Kpot (± s.e.) of the four taxonomic groups considered. The F-values of the ANOVAs comparing life histories (n = 18) and taxonomic groups (n = 12) are given: *P < 0·05; ns, non-significant.
Table 2.
Root decomposability (Kpot), chemical and morphological traits (means ± s.e.) for 18 species belonging to different life histories and taxonomic groups
| Root trait | Kpot (g kg−1 d−1) | N (mg g−1) | P (mg g−1) | C/N | Soluble (mg g−1) | Hemicel. (mg g−1) | Cellulose (mg g−1) | Lignin (mg g−1) | Tissue density (g cm−3) | SRL (m g−1) | Diameter (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Life history | |||||||||||
| Annual | 15·9 ± 2·2 | 14·0 ±1·2 | 1·9 ± 0·09 | 40·4 ± 3·4 | 336 ± 141 | 259 ± 12 | 241 ± 8 | 163 ± 10 | 0·09 ± 0·004 | 325 ± 25 | 0·25 ± 0·005 |
| Perennial | 13·6 ± 2·2 | 11·4 ± 0·9 | 1·6 ± 0·08 | 47·0 ± 4·0 | 273 ± 17 | 319 ± 14 | 262 ± 11 | 146 ± 9 | 0·12 ± 0·005 | 154 ± 11 | 0·29 ± 0·008 |
| F-value | 3·21ns | 137*** | 78·5*** | 99·6*** | 78·9*** | 87·9*** | 34·9*** | 9·11** | 35·0*** | 366*** | 113*** |
| η2 | – | 0·72 | 0·63 | 0·65 | 0·61 | 0·64 | 0·41 | 0·15 | 0·39 | 0·88 | 0·68 |
| Taxonomic group | |||||||||||
| Poaceae | 7·3 ± 0·6b | 5·8 ± 0·3d | 1·1 ± 0·03b | 77·6 ± 3·6a | 182 ± 8d | 411 ± 7a | 318 ± 8a | 89 ± 6c | 0·12 ± 0·008a | 294 ± 47a | 0·23 ± 0·01a |
| Asteraceae | 19·0 ± 3·4a | 11·9 ± 0·09c | 2·2 ± 0·16a | 40·9 ± 4·2b | 390 ± 11a | 245 ± 9c | 208 ± 5d | 157 ± 10b | 0·09 ± 0·007b | 234 ± 14b | 0·27 ± 0·01c |
| Fabaceae | 14·1 ± 1·1ab | 24·1 ± 0·2a | 1·8 ± 0·06a | 18·4 ± 1·3d | 334 ± 18b | 263 ± 14b | 245 ± 12c | 158 ± 6b | 0·10 ± 0·006a | 172 ± 13c | 0·30 ± 0·01d |
| Lamiales | 9·2 ± 3·1b | 14·1 ± 0·1b | 1·9 ± 0·1a | 34·8 ± 3·7c | 274 ± 14c | 240 ± 4c | 274 ± 10b | 212 ± 9a | 0·11 ± 0·01a | 213 ± 45c | 0·26 ± 0·009b |
| F-value | 7·20* | 779*** | 91·7*** | 479*** | 209*** | 255*** | 144*** | 245*** | 8·67** | 13·3*** | 62·9*** |
| η2 | 0·27 | 0·98 | 0·89 | 0·98 | 0·94 | 0·96 | 0·93 | 0·96 | 0·43 | 0·57 | 0·85 |
Kpot, root potential decomposition rate; N, nitrogen concentration; P, phosphorus concentration; Soluble, water-soluble compound concentration; Hemicel., hemicellulose concentration; Cellulose, cellulose concentration; SRL, specific root length.
F-values for ANOVAs assessing the effects of life history and taxonomic group are given, with their level of significance. Different letters indicate the results of post hoc tests (Student–Newman–Keuls). The effect of species was highly significant for each trait (P > 0·001). Effect sizes (η2) indicate the relative importance of a factor. *P < 0·05; **P < 0·01; ***P < 0·001; ns, non-significant.
All root traits differed significantly between species, life history and taxonomic groups (Table 2). The most variable traits were N concentration and SRL, which varied by a factor of six between species, followed by lignin concentration (4-fold variation), P and soluble compound concentrations (3-fold variation), with the lowest level of variation observed for hemicellulose and cellulose concentrations, tissue density and diameter (2-fold variation; Supplementary Data Table S1). The fine roots of annual species had high N, P, soluble compound and lignin concentrations, whereas those of perennial species had higher C/N ratios, hemicellulose and cellulose concentrations (Table 2). Annual species had a higher SRL and lower root diameter and tissue density than perennial species (Table 2). Power analysis showed that the SRL was the variable most strongly influenced by life history (η2 = 0·88; Table 2). The fine roots of the Poaceae had the highest C/N ratio, and hemicellulose and cellulose concentrations, whereas those of the Asteraceae had the highest soluble compound concentration and those of the Fabaceae the highest N concentration (Table 2). Poaceae also had the highest root tissue density and SRL, but the lowest diameter (Table 2). Chemical traits were the most strongly influenced by taxonomic group (0·89 < η2 < 0·98; Table 2).
Relationship between root potential decomposition rate and root traits
Root Kpot was correlated with three chemical traits – P, soluble compound and cellulose concentrations – but it was not correlated with any of the morphological traits (Table 3). Species with high root soluble compound and P concentrations tended to decompose faster than species with low soluble compound and P concentrations. In contrast, Kpot was negatively correlated with cellulose concentration.
Table 3.
Correlation matrix for Pearson's coefficients, for the root traits and decomposition of 18 herbaceous species
| N | P | C/N | Soluble | Hemicel. | Cellulose | Lignin | Tissue density | SRL | Diameter | |
|---|---|---|---|---|---|---|---|---|---|---|
| Kpot | ns | 0·49* | ns | 0·71** | ns | –0·60* | ns | ns | ns | ns |
| N | 0·53* | –1*** | ns | –0·49* | ns | ns | ns | ns | ns | |
| P | –0·77*** | 0·55* | –0·67** | –0·54* | 0·57* | ns | ns | ns | ||
| C/N | ns | 0·49* | ns | ns | ns | ns | ns | |||
| Soluble | –0·75*** | –0·94*** | ns | ns | ns | –0·48* | ||||
| Hemicellulose | 0·64** | –0·85*** | ns | ns | ns | |||||
| Cellulose | ns | ns | ns | –0·50* | ||||||
| Lignin | ns | ns | ns | |||||||
| Tissue density | –0·64** | ns | ||||||||
| SRL | –0·77*** |
n = 18. For abbreviations, see Table 2.
*P < 0·05; **P < 0·01; ***P < 0·001; ns, non-significant; ns, marginally significant results (0·05 < P < 0·10).
Chemical traits were not correlated with morphological traits, with the exception of soluble compound and cellulose concentrations, which were negatively correlated with root diameter (Table 3). SRL was strongly negatively correlated with root diameter and tissue density (Table 3).
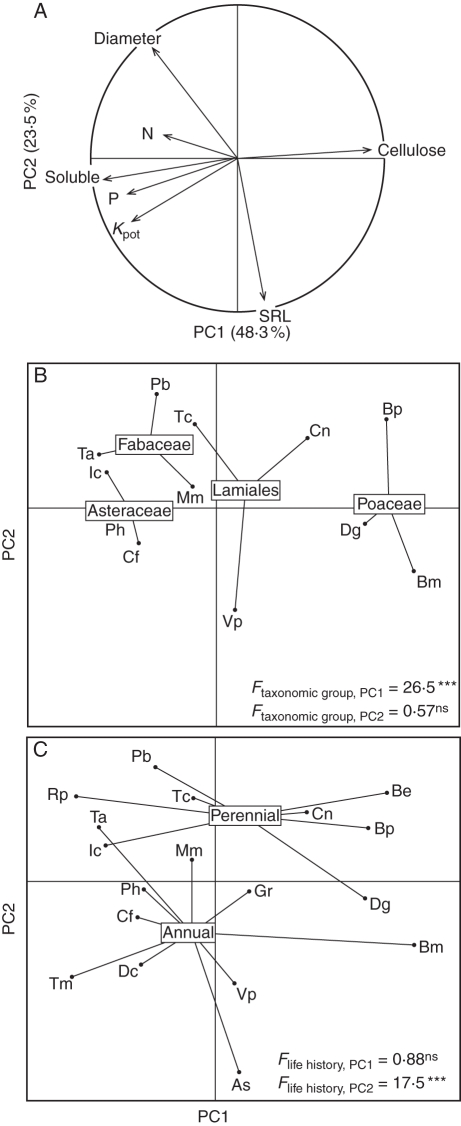
The first two axes of the PCA performed with six root traits and Kpot accounted for 71·8 % of the variance (Fig. 2). The first PCA axis (PC1) accounted for 48·3 % of the variance and was defined by chemical traits and Kpot: as expected from the correlation coefficients (Table 3), it opposed traits related to Kpot, P and soluble compound concentrations, and to the concentration of cellulose, a more recalcitrant compound (Fig. 2A). The second PCA axis (PC2), which accounted for 23·5 % of the variance, was a morphological axis opposing SRL and root diameter (Fig. 2A). Root N concentration was on the third axis. The ANOVAs performed on the two main PCA axes showed that PC1 discriminated between species from different taxonomic groups (Fig. 2B). The Poaceae had a higher cellulose concentration but lower Kpot, P and soluble compound concentrations than the Asteraceae and Fabaceae; the Lamiales gave intermediate results (SNK post hoc test, not shown). PC2 discriminated between species from different life histories (Fig. 2C). Annual species had a higher SRL and a lower diameter than perennial species.
Fig. 2.
Principal component analysis of root traits and potential decomposition rates for the 18 species studied. Projection of variables (A) and species as a function of taxonomic group (B) and life history (C). Abbreviations for root traits: Kpot, root potential decomposition rate; N, nitrogen concentration; P, phosphorus concentration; Soluble, water-soluble compound concentration; Cellulose, cellulose concentration; SRL, specific root length. For species' abbreviations, see Table 1. F- and P-values from ANOVAs evaluating the effects of taxonomic group and life history on species' axis scores (PC1 and PC2) are given in (B) and (C), respectively. ***P < 0·001; ns, non-significant.
Relationship between root and leaf Kpot and traits
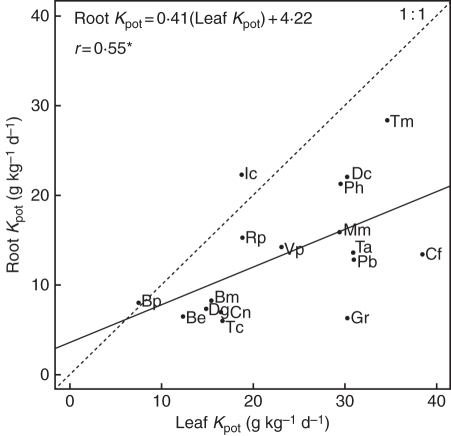
On average, roots decomposed at half the rates reported for leaves, and the decomposition rates of these two organs were positively correlated (Root Kpot = 0·41(Leaf Kpot) + 4·22, r = 0·55, Fig. 3). The only exceptions to this common pattern were Geranium rotundifolium, the roots of which decomposed at a rate one-fifth that for the leaves of the same species, and Inula conyza, the roots of which decomposed more rapidly than the leaves.
Fig. 3.
Relationship between root and leaf potential decomposition rates (Kpot); the 1:1 ratio is indicated. Each point represents a species; for abbreviations see Table 1. r is the Pearson's correlation coefficient for the relationship between root and leaf potential decomposition rates (*P < 0·05).
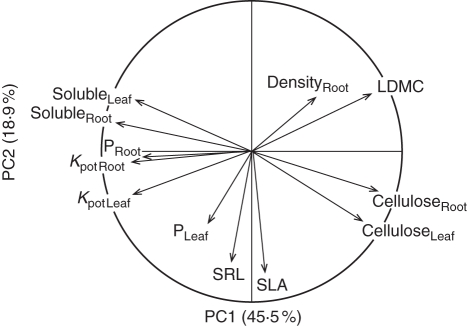
A number of pairs of analogue chemical root and leaf traits covaried. This was the case for soluble compound, hemicellulose and cellulose concentrations; SRL was also positively correlated with the analogous trait in leaves, SLA (Table 4). The PCA conducted with six pairs of analogue root and leaf variables (Fig. 4) confirmed trait convergence for most of the pairs of root and leaf traits, because pairs of analogue root and leaf traits (soluble compound and cellulose concentrations, SRL and SLA) were closely grouped on the PCA. The overall location of variables in the multivariate space (Fig. 4) was similar to that in the PCA for root traits and Kpot (Fig. 2A). The first axis of the PCA (PC1) accounted for 45·5 % of the variance and opposed root and leaf Kpot, soluble compound concentrations and root P concentration to root and leaf cellulose concentrations and LDMC (Fig. 4). The second PCA axis (PC2) accounted for 18·9 % of the variance and corresponded principally to SRL and SLA (Fig. 4). Root tissue density and leaf P concentration were not located on the two main axes. As in the previous root PCA, PC1 discriminated between species from different taxonomic groups (F = 30·3; P < 0·001), whereas PC2 discriminated between species with different life histories (F = 15·6; P < 0·01).
Table 4.
Matrix of Pearson's correlation coefficients for the relationships between leaf traits and analogous root traits and root potential decomposition rate (Root Kpot)
| Leaf trait | Analogous root trait |
Root Kpot |
||
|---|---|---|---|---|
| r | P-value | r | P-value | |
| Kpot | 0·55 | * | 0·55 | * |
| N | ns | ns | ||
| P | ns | ns | ||
| C/N | ns | ns | ||
| Soluble | 0·69 | ** | 0·61 | * |
| Hemicellulose | 0·80 | *** | –0·50 | * |
| Cellulose | 0·65 | ** | –0·54 | * |
| Lignin | ns | ns | ||
| LDMC | ns | –0·59 | * | |
| SLA | 0·59 | * | ns | |
The leaf trait data were obtained from Kazakou et al. (2009).
The analogous root traits are: root Kpot; root N concentration; root P concentration; root C/N ratio; water-soluble compound concentration; hemicellulose, cellulose and lignin concentrations, root tissue density and specific root length (SRL). LDMC, leaf dry matter content; SLA, specific leaf area.
r is Pearson's correlation coefficient, *P < 0·05; **P < 0·01; ***P < 0·001; ns, non-significant.
Fig. 4.
Principal component analysis of root and leaf traits and potential decomposition rates for the 18 species studied. Abbreviations for traits: Leaf refers to leaf traits and Root to root traits; Kpot, potential decomposition rate; P, phosphorus concentration; Soluble, water-soluble compound concentration; Cellulose, cellulose concentration; SRL, specific root length; SLA, specific leaf area; Density, tissue density; LDMC, leaf dry matter content. For species' abbreviations, see Table 1.
We investigated whether root Kpot could be predicted from leaf traits, by investigating the relationship between root Kpot and leaf variables (Table 4). Root Kpot was positively correlated with leaf soluble compound concentration and negatively correlated with leaf hemicellulose and cellulose concentrations and LDMC.
DISCUSSION
Fine root Kpot is dependent on root chemical composition but not on morphology
Our results demonstrated that only chemical composition accounted for differences in fine root Kpot between species. Root Kpot increases with the concentration of soluble compounds in the root in both herbaceous (this study) and woody (Lemma et al., 2007; Lindedam et al., 2009; Hobbie et al., 2010) species, mostly because soluble compounds are rapidly leached and constitute a labile energy source for decomposers (Berg and Laskowski, 2006). In contrast, Kpot decreased with increasing cellulose concentration, cellulose being a more recalcitrant cell wall component. Consistent with another study on herbaceous species (Vivanco and Austin, 2006) but contrasting with recent studies on herbaceous species (Freschet et al., 2011; Aulen et al., 2012) and meta-analyses (Silver and Miya, 2001; Zhang et al., 2008), lignin concentration did not affect root Kpot. This may be explained by the narrower range of lignin concentrations in our species (6–26 %) as compared to studies including both woody and herbaceous species (5–50 %; Zhang et al., 2008), or by the short period of decomposition experienced (12 weeks). Lignin has indeed been reported to affect decomposition rates in the longer term (Heal et al., 1997). In this study, Kpot was correlated with P concentration, but not with N concentration or C/N ratio, in contrast to previous reports (Jensen, 1929, cited by Heal et al., 1997; Silver and Miya, 2001; Zhang et al., 2008). This probably reflects the presence of limited concentrations of P in the soil (N/P = 39), leading soil micro-organisms to have a preference for species with high root P concentrations.
Contrary to our initial hypothesis, morphological traits did not explain differences in decomposition rate between species. For instance, two species with similar root potential decomposition rates (Arenaria serpillyfolia and Rubia peregrina) had very different morphological traits: A. serpillyfolia had the highest SRL and the lowest root tissue density and root diameter, whereas R. peregrina has the lowest SRL and the highest root tissue density. The absence of an effect of SRL on Kpot was surprising, because a high SRL maximizes the surface area for exchange between roots and decomposers, which has been shown to facilitate decomposition (Wardle et al., 1998). However, this hypothesis has not been demonstrated, either in tree species (Hobbie et al., 2010; Aulen et al., 2012) or in the herbaceous species studied here, despite the large differences in SRL between species (130–653 m g−1). Similarly, root diameter and tissue density had no effect on Kpot, despite these traits being related to toughness and being expected to increase the proportion of resistant compounds (Fitter, 1985) and hence the time required for penetration by fungal hyphae (Foster and Lang, 1982; Berg, 1984). Consistent with our findings, root decomposition has been shown to be unrelated to the root density of 11 species (Freschet et al., 2011), and to decrease only when root diameter is >5 mm (Silver and Miya, 2001). The lack of correspondence between Kpot and morphology may result from the use of bulk fine roots (<2 mm) from young plants (9 months old) in particular for perennials which were in their first year of growth. This would have limited the range of variation, particularly for root diameter and tissue density (0·21–0·34 mm and 0·067–0·143 g cm−3, respectively), resulting in lower levels of variation than reported in previous studies in which traits were measured on whole-root systems on adult plants (Roumet et al., 2006). Furthermore, morphological data may be biased due to differences between species in the relative contribution of different root orders that are known to differ in morphology (Pregitzer et al., 2002; Withington et al., 2006; Goebel et al., 2011).
Our results suggest that decomposer activity is more strongly influenced by the chemical composition than by the morphology of fine roots. However, the consistency of these results should be tested on a larger number of species, to provide a wider range of morphological and chemical traits.
Fine root Kpot is not involved in the acquisition–conservation trade-off
Root decomposition rates differed between taxonomic groups, but not between annual and perennial species. PCA revealed the existence of two independent root trait patterns between these groups. The first pattern discriminated between taxonomic groups and was associated with Kpot and chemical traits (Fig. 2). Poaceae roots decomposed 2·6 times more slowly than Asteraceae roots and 1·9 times more slowly than Fabaceae roots. This slower decomposition can be accounted for by their higher cellulose concentration and lower N, P and soluble compound concentrations than other dicots. The unique status of the Poaceae may also reflect their particular architecture (fasciculate, herringbone root system) and anatomy, characterized by a high structural investment in recalcitrant tissue, such as lignified xylem rings (Lindedam et al., 2009), and their high proportion of xylem (Wahl and Ryser, 2000; Hummel et al., 2007). In contrast Fabaceae showed the highest N concentration and diameter of roots, probably owing to their symbiotic association with N-fixing bacteria which had been reported to lead to a high tissue N concentration (Gebauer et al., 1988; Del Pozo et al., 2000) and is supposed to require less investment in root foraging by fine roots. The second pattern discriminated between species with different life histories and was associated with morphological traits. Annual species, occurring in disturbed, fertile habitats, had a high SRL, this trait being related to resource acquisition and foraging (Reich et al., 1998; Hodge, 2004), as it maximizes the area for exchange with soil, thereby providing rapid access to mineral resources. In contrast, perennial species had coarse, dense roots, these two traits being associated with resource conservation and reflecting adaptation to infertile habitats. Annuals also produced roots that were richer in N, P and soluble compounds than perennials. However, these differences were smaller than those between taxonomic groups. As reported in previous studies comparing species chemical and morphological traits of leaves (Garnier, 1992) and roots (Roumet et al., 2006), we found that annuals had a greater resource acquisition strategy than perennials. However, this study show that this did not lead to more rapid root decomposition, because the morphological traits involved in nutrient acquisition did not influence the rate of decomposition (see above). Similarly, a recent study on 11 species demonstrated that the fine root economics spectrum did not drive root decomposability (Freschet et al., 2011). The decomposition rate of roots therefore cannot be considered to be involved in the acquisition–conservation trade-off as suggested by conceptual frameworks (Wardle et al., 2004) and by results for leaves showing that the potential rate of leaf decomposition of a species is consistently correlated with the ecological strategy of that species (Cornwell et al., 2008).
Co-ordinated variation of root and leaf traits
Root and leaf potential decomposition rates have seldom been investigated together, on the same species, with respect to other root and leaf traits. In this study, we provide the first demonstration that root and leaf Kpot are positively correlated in herbaceous species. We also show that the rate of decomposition of roots is about half that of leaves. There is a correspondence between leaf and root Kpot values because traits influencing root decomposition, such as soluble compound, cellulose and P concentrations, also influence leaf decomposition (Kazakou et al., 2009). In addition, root and leaf Kpot values have similar regression relationships with the concentrations of cellulose (r = –0·60, P < 0·001, n = 34) and soluble compounds (r = 0·72, P < 0·001, n = 32). This accounts for the slower decomposition rates of fine roots, which have a higher cellulose and lower soluble compound concentration than of leaves, and confirms previous findings of lower rates of decomposition for fine roots than for leaves (see Vivanco and Austin, 2006; Lemma et al., 2007; Wang et al., 2010). Other traits had different effects on root and leaf Kpot: tissue density did not affect root decomposition, whereas LDMC, a surrogate for leaf density, has been reported to be a strong determinant of leaf Kpot (Kazakou et al., 2006, 2009).
A consideration of root and leaf decomposition together with root and leaf traits demonstrated that root traits and leaf traits displayed similar patterns. We found consistent patterns for pairs of analogous root and leaf traits. Five of the ten pairs of analogous root and leaf traits examined covaried (potential decomposition rate, cellulose, hemicellulose and soluble compound concentrations, and SRL/SLA), resulting in similar trade-offs and groupings of species. This suggests that evolutionary and habitat constraints have similar effects above- and below-ground, with potential major and cumulative implications for ecosystem processes. These results contrast with those reported for 11 temperate trees (Hobbie et al., 2010a), showing a lack of correspondence between root and leaf chemical traits and potential decomposition rates. Differences in trait patterns between herbaceous and woody species might reflect the differences in plant size (Freschet et al., 2010a) or mycorrhizal status (Hobbie et al., 2010), both of which influence the decomposition rate (Cornelissen et al., 2001) and root traits (Zangaro et al., 2008). Our results also demonstrate that root Kpot can be predicted from leaf traits, such as leaf soluble compound, hemicellulose and cellulose concentrations and LDMC (Table 4). This finding, like those of Freschet et al. (2010a), suggests it may be possible to predict below-ground functions from much more accessible, and easier to measure, above-ground traits.
This study suggests that, in herbaceous species, the potential rate of fine root decomposition depends on root chemical composition rather than root morphology. It is also heavily dependent on taxonomic group, with the Poaceae decomposing more slowly than dicots. These patterns observed at the root level were found to be conserved and accentuated when leaf traits and potential decomposition rate were also considered, suggesting a consistency of the pattern at the whole-plant scale. These results may have important implications for studies of the effects of changes in biodiversity on ecosystem processes. Potential shifts in the relative abundance of plant species or in the distribution of traits in response to anthropogenic changes may have a major effect on the decomposition of both roots and leaves, thereby also strongly affecting nutrient and C cycling. For example, an increase in the predominance of Poaceae species would lead to lower rates of decomposition and an impoverishment of the ecosystem due to lower levels of nutrient restitution. Conversely, it might also lead to an increase in soil carbon storage.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank the staff of the CEFE experimental field station and the CEFE chemical analysis service for their invaluable assistance. We also thank Virginie Pons and Stéphanie Saussure for assistance in the collection, monitoring and analysis of root samples. We thank Eric Garnier and Cyrille Violle and two anonymous reviewers for their pertinent comments and suggestions on the manuscript. This is a publication from the ‘Groupement De Recherche TRAITS’ (GDR 2574, CNRS, France). During the writing, M.B. was supported by fellowships from the ‘Agence de l'Environnement et de la Maîtrise de l'Energie (ADEME)’ and the ‘Centre International d'études supérieures en sciences agronomiques (Montpellier SupAgro)’. The research was supported by the FRB RESPIRS CT 054045 grant, from the ‘Fondation de la Recherche sur la Biodiversité’.
LITERATURE CITED
- Aerts R. Nutrient use efficiency in evergreen and deciduous species from heathlands. Oecologia. 1990;84:391–397. doi: 10.1007/BF00329765. [DOI] [PubMed] [Google Scholar]
- Aulen M, Shipley B, Bradley R. Prediction of in situ root decomposition rates in an interspecific context from chemical and morphological traits. Annals of Botany. 2012;109:287–297. doi: 10.1093/aob/mcr259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg B. Decomposition of root litter and some factors regulating the process: long-term root litter decomposition in a Scots pine forest. Soil Biology and Biochemistry. 1984;16:609–617. [Google Scholar]
- Berg B, Laskowski R. Litter decomposition: a guide to carbon and nutrient turnover. San Diego: Elsevier Academic Press; 2006. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Cornelissen J. An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. Journal of Ecology. 1996;84:573–582. [Google Scholar]
- Cornelissen J, Thompson K. Functional leaf attributes predict litter decomposition rate in herbaceous plants. New Phytologist. 1997;135:109–114. doi: 10.1046/j.1469-8137.1997.00628.x. [DOI] [PubMed] [Google Scholar]
- Cornelissen J, Aerts R, Cerabolini B, Werger M, Van Der Heijden M. Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia. 2001;129:611–619. doi: 10.1007/s004420100752. [DOI] [PubMed] [Google Scholar]
- Cornelissen J, Lavorel S, Garnier E, et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- Cornwell WK, Cornelissen JHC, Amatangelo K, et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecology Letters. 2008;11:1065–1071. doi: 10.1111/j.1461-0248.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- Craine JM, Froehle J, Tilman DG, Wedin DA, Chapin FS., III The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos. 2001;93:274–285. [Google Scholar]
- Del Pozo A, Garnier E, Aronson J. Contrasted nitrogen utilization in annual C3 grass and legume crops: physiological explorations and ecological considerations. Acta Oecologica. 2000;21:79–89. [Google Scholar]
- Fan P, Guo D. Slow decomposition of lower order roots: a key mechanism of root carbon and nutrient retention in the soil. Oecologia. 2010;163:509–515. doi: 10.1007/s00442-009-1541-4. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fitter A. Functional significance of root morphology and root system architecture. Oxford: Blackwell Scientific Publications; 1985. [Google Scholar]
- Fortunel C, Garnier E, Joffre R, et al. Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology. 2009;90:598–611. doi: 10.1890/08-0418.1. [DOI] [PubMed] [Google Scholar]
- Foster J, Lang G. Decomposition of red spruce and balsam fir boles in the White Mountains of New Hampshire. Canadian Journal of Forest Research. 1982;12:617–626. [Google Scholar]
- Freschet GT, Cornelissen JHC, van Logtestijn RSP, Aerts R. Evidence of the ‘plant economics spectrum’ in a subarctic flora. Journal of Ecology. 2010a;98:362–373. [Google Scholar]
- Freschet GT, Cornelissen JHC, van Logtestijn RSP, Aerts R. Substantial nutrient resorption from leaves, stems and roots in a subarctic flora: what is the link with other resource economics traits? New Phytologist. 2010b;186:879–889. doi: 10.1111/j.1469-8137.2010.03228.x. [DOI] [PubMed] [Google Scholar]
- Freschet GT, Aerts R, Cornelissen JHC. A plant economics spectrum of litter decomposability. Functional Ecology. 2011 in press. http://dx.doi.org/10.1111/j.1365-2435.2011.01913.x . [Google Scholar]
- Gallardo A, Merino J. Leaf decomposition in two Mediterranean ecosystems of southwest Spain: influence of substrate quality. Ecology. 1993;74:152–161. [Google Scholar]
- Garnier E. Growth analysis of congeneric annual and perennial grass species. Journal of Ecology. 1992;80:665. [Google Scholar]
- Garnier E, Salager J-L, Laurent G, Sonie L. Relationships between photosynthesis, nitrogen and leaf structure in 14 grass species and their dependence on the basis of expression. New Phytologist. 1999;143:119–129. [Google Scholar]
- Garnier E, Cortez J, Billès G, et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004;85:2630–2637. [Google Scholar]
- Gebauer G, Rehder H, Wollenweber B. Nitrate, nitrate reduction and organic nitrogen in plants from different ecological and taxonomic groups of Central Europe. Oecologia. 1988;75:371–385. doi: 10.1007/BF00376940. [DOI] [PubMed] [Google Scholar]
- Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ. Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Global Change Biology. 2000;6:751–765. [Google Scholar]
- Gill RA, Jackson RB. Global patterns of root turnover for terrestrial ecosystems. New Phytologist. 2000;147:13–31. [Google Scholar]
- Goebel M, Hobbie SE, Bulaj B, et al. Decomposition of the finest root branching orders: linking belowground dynamics to fine-root function and structure. Ecological Monographs. 2011;81:89–102. [Google Scholar]
- Grimshaw H, Allen S, Parkinson J. Nutrient elements. Oxford: Blackwell Scientific Publications; 1989. [Google Scholar]
- Heal O, Anderson J, Swift M. Plant litter quality and decomposition: an historical overview. In: Cadisch G, Giller KE, editors. Driven by nature: plant litter quality and decomposition. Wallingford, UK: CAB International; 1997. pp. 3–32. [Google Scholar]
- Hobbie SE, Oleksyn J, Eissenstat DM, Reich PB. Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia. 2010;162:505–513. doi: 10.1007/s00442-009-1479-6. [DOI] [PubMed] [Google Scholar]
- Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist. 2004;162:9–24. [Google Scholar]
- Hummel I, Vile D, Violle C, et al. Relating root structure and anatomy to whole-plant functioning in 14 herbaceous Mediterranean species. New Phytologist. 2007;173:313–321. doi: 10.1111/j.1469-8137.2006.01912.x. [DOI] [PubMed] [Google Scholar]
- Ibrahima A, Joffre R, Gillon D. Changes in litter during the initial leaching phase: an experiment on the leaf litter of Mediterranean species. Soil Biology and Biochemistry. 1995;27:931–939. [Google Scholar]
- Kazakou E, Garnier E, Navas M-L, Roumet C, Collin C, Laurent G. Components of nutrient residence time and the leaf economics spectrum in species from Mediterranean old-fields differing in successional status. Functional Ecology. 2007;21:235–245. [Google Scholar]
- Kazakou E, Vile D, Shipley B, Gallet C, Garnier E. Co-variations in litter decomposition, leaf traits and plant growth in species from a Mediterranean old-field succession. Functional Ecology. 2006;20:21–30. [Google Scholar]
- Kazakou E, Violle C, Roumet C, Pintor C, Gimenez O, Garnier E. Litter quality and decomposability of species from a Mediterranean succession depend on leaf traits but not on nitrogen supply. Annals of Botany. 2009;104:1151–1161. doi: 10.1093/aob/mcp202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoff AJ, Fagan WF, Elser JJ, Enquist BJ. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. American Naturalist. 2006;168:103–122. doi: 10.1086/507879. [DOI] [PubMed] [Google Scholar]
- Lemma B, Nilsson I, Kleja D, Olsson M, Knicker H. Decomposition and substrate quality of leaf litters and fine roots from three exotic plantations and a native forest in the southwestern highlands of Ethiopia. Soil Biology and Biochemistry. 2007;39:2317–2328. [Google Scholar]
- Lindedam J, Magid J, Poulsen P, Luxhøi J. Tissue architecture and soil fertility controls on decomposer communities and decomposition of roots. Soil Biology and Biochemistry. 2009;41:1040–1049. [Google Scholar]
- Liu G, Freschet GT, Pan X, Cornelissen JHC, Li Y, Dong M. Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytologist. 2010;188:543–553. doi: 10.1111/j.1469-8137.2010.03388.x. [DOI] [PubMed] [Google Scholar]
- McClaugherty CA, Aber JD, Melillo JM. The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology. 1982;63:1481–1490. [Google Scholar]
- Nambiar EKS. Do nutrients retranslocate from fine roots? Canadian Journal of Forest Research – Revue Canadienne De Recherche Forestiere. 1987;17:913–918. [Google Scholar]
- Olson JS. Energy storage and the balance of producers and decomposers in ecological systems. Ecology. 1963;44:322–331. [Google Scholar]
- Pohl M, Stroude R, Buttler A, Rixen C. Functional traits and root morphology of alpine plants. Annals of Botany. 2011;108:537–545. doi: 10.1093/aob/mcr169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL. Fine root architecture of nine North American trees. Ecological Monographs. 2002;72:293–309. [Google Scholar]
- Prescott CE. Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry. 2010;101:133–149. [Google Scholar]
- Reich P, Tjoelker M, Walters M, Vanderklein D, Buschena C. Close association of RGR, leaf and root morphology, seed mass and shade tolerance in seedlings of nine boreal tree species grown in high and low light. Functional Ecology. 1998;12:327–338. [Google Scholar]
- Reich PB, Tjoelker MG, Pregitzer KS, Wright IJ, Oleksyn J, Machado J-L. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecology Letters. 2008;11:793–801. doi: 10.1111/j.1461-0248.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- Roumet C, Lafont F, Sari M, Warembourg F, Garnier E. Root traits and taxonomic affiliation of nine herbaceous species grown in glasshouse conditions. Plant and Soil. 2008;312:69–83. [Google Scholar]
- Roumet C, Urcelay C, Díaz S. Suites of root traits differ between annual and perennial species growing in the field. New Phytologist. 2006;170:357–368. doi: 10.1111/j.1469-8137.2006.01667.x. [DOI] [PubMed] [Google Scholar]
- Silver WL, Miya RK. Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia. 2001;129:407–419. doi: 10.1007/s004420100740. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, et al. Angiosperm phylogeny inferred from a combined data set of 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society. 2000;133:381–461. [Google Scholar]
- Taylor B, Parkinson D. A new microcosm approach to litter decomposition studies. Canadian Journal of Botany. 1988;66:1933–1939. [Google Scholar]
- Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D. Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytologist. 2005;167:493–508. doi: 10.1111/j.1469-8137.2005.01428.x. [DOI] [PubMed] [Google Scholar]
- Tutin TG, Heywood VH, Burges NA, et al. Flora Europaea. vols 2–5. Cambridge: Cambridge University Press; 1968–1980. [Google Scholar]
- Van Soest PJ. Use of detergents in the analysis of fibrous feeds. A rapid method for the determination of fiber and lignin. Journal of the Association of Official Analytical Chemists. 1963;46:829–835. [Google Scholar]
- Vivanco L, Austin AT. Intrinsic effects of species on leaf litter and root decomposition: a comparison of temperate grasses from North and South America. Oecologia. 2006;150:97–107. doi: 10.1007/s00442-006-0495-z. [DOI] [PubMed] [Google Scholar]
- Wahl S, Ryser P. Root tissue structure is linked to ecological strategies of grasses. New Phytologist. 2000;148:459–471. doi: 10.1046/j.1469-8137.2000.00775.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu S, Mo J. Correlation between leaf litter and fine root decomposition among subtropical tree species. Plant and Soil. 2010;335:289–298. [Google Scholar]
- Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, Wall DH. Ecological linkages between aboveground and belowground biota. Science. 2004;304:1629–1633. doi: 10.1126/science.1094875. [DOI] [PubMed] [Google Scholar]
- Wardle DA, Barker GM, Bonner KI, Nicholson KS. Can comparative approaches based on plant ecophysiological traits predict the nature of biotic interactions and individual plant species effects in ecosystems? Journal of Ecology. 1998;86:405–420. [Google Scholar]
- Weiner J, Martinez S, Muller-Scharer H, Stoll P, Schmid B. How important are environmental maternal effects in plants? A study with Centaurea maculosa. Journal of Ecology. 1997;85:133–142. [Google Scholar]
- Withington JM, Reich PB, Oleksyn J, Eissenstat DM. Comparisons of structure and life span in roots and leaves among temperate trees. Ecological Monographs. 2006;76:381–397. [Google Scholar]
- Zangaro W, Assis RL, Rostirola LV, et al. Changes in arbuscular mycorrhizal associations and fine root traits in sites under different plant successional phases in southern Brazil. Mycorrhiza. 2008;19:37–45. doi: 10.1007/s00572-008-0202-5. [DOI] [PubMed] [Google Scholar]
- Zhang D, Hui D, Luo Y, Zhou G. Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. Journal of Plant Ecology. 2008;1:85–93. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.